Abstract
Aortic stenosis (AS) is the most common valvular disease that can lead to increased afterload, left ventricular (LV) remodeling, and myocardial fibrosis. We reviewed the literature addressing the impact of transcatheter aortic valve replacement (TAVR) on LV remodeling and patients’ outcomes by elimination of AS-related high afterload. TAVR reduces afterload and improves LV remodeling recovery. However, myocardial fibrosis may not completely reverse after the TAVR. The LV diastolic dysfunction (LVDD) induced by AS is an independent predictor of post-TAVR mortality, and mortality increases with severity of LVDD. The impact of diastolic dysfunction on patient outcomes emerges at 30 days but continues to persist during mid-term follow-up. Based on severity of the baseline LVDD, some patients may tolerate post-TAVR aortic regurgitation (AR), but even minimal post-TAVR AR in patients with severe baseline LVDD can have an additive negative impact on survival. It is crucial to consider TAVR prior to development of advanced LVDD. Appropriate device selection and deployment technique are important in improvement of TAVR outcomes via elimination of AR.
Keywords: Aortic regurgitation, Aortic stenosis, Heart valve prosthesis, Heart valve replacement, Left ventricular afterload, Left ventricular remodeling, Myocardial fibrosis
Introduction
Aortic valve stenosis (AS) is the most common valvular disease in developed countries. AS is a progressive disease and once it becomes symptomatic, the mortality rate can be as high as 68% at 2 years in patients who receive medical therapy or balloon aortic valvuloplasty with no valve replacement [1]. Patients with AS usually have a long asymptomatic phase and then develop a short symptomatic phase. It was reported that the outcome of AS patients is significantly associated with timing of afterload elimination [2]. Aortic valve replacement is not usually considered for patients with asymptomatic AS, while left ventricle (LV) remodeling and myocardial fibrosis secondary to AS can begin in the asymptomatic phase. The LV remodeling leads to diastolic dysfunction (LVDD), which affects the outcome of patients with AS who undergo aortic valve replacement [3]. Transcatheter aortic valve replacement (TAVR) is accepted as an appropriate treatment approach for inoperable, high-risk, and intermediate risk patients who are not eligible for surgical aortic valve replacement [4, 5]. TAVR was shown to improve cardiac function and patients’ outcomes, but not all effects of prolonged AS on LV [6]. In this article, we aimed to review the clinical aspects of AS-related LVDD and its impact on patients’ outcomes. We will also review the role of TAVR in the recovery of LVDD and procedural factors that can influence patients’ outcomes.
Methods
The current study is based on literature review, and no direct human or animal intervention was performed for this report. We searched PubMed, Google Scholar, and Google to find appropriate studies. Search terms were ‘transcatheter aortic valve replacement’ or ‘TAVR’ in combination with ‘left ventricular diastolic dysfunction’ or ‘LVDD.’ There was no time or geographic limit in the search strategy and articles that were published by the end of December 2018 were eligible. All available studies were reviewed, and applicable results were used for the current review article. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
AS and Diastolic Dysfunction
Mechanical obstruction of LV secondary to AS increases LV afterload pressure, leading to compensatory cardiomyocyte hypertrophy and collagen network abnormality, which result in myocardial fibrosis and eventually LVDD [7–9]. As the duration of high afterload pressure prolongs, severity of LVDD progresses and myocardial fibrosis becomes more prominent, which increases the risk of irreversibility of unfavorable LV remodeling [10]. It was shown that LV remodeling-induced myocardial fibrosis is the main component of transition from compensatory hypertrophy to heart failure (HF) in AS patients [8, 9]. In addition to lower ejection fraction (EF), higher pulmonary artery pressure and more mitral or tricuspid valve regurgitation were also found among AS patients with LVDD [11, 12]. The myocardial fibrosis itself was found to be an independent predictor of mortality in AS patients [8]. It was reported that up to 67% of patients with severe AS who undergo TAVR have some degree of LVDD [11]. Although TAVR was suggested to be an effective approach for afterload and wall stress reduction [13], some degree of LVDD may persist after aortic valve replacement [3].
LVDD Improvement After TAVR
Elimination of AS-induced afterload by TAVR can improve cardiac function in an acute phase and also reverse LV remodeling in a slower process [13]. In general, myocardium hypertrophy regresses much faster than the fibrotic tissue, and fibrosis reduction may happen during the delayed phase, if at all [14]. In a study using magnetic resonance imaging, it was reported that myocardial fibrosis does not recover until 9 months after the afterload elimination [15]. Consistently, significant LV mass reduction was seen at 6 and 12 months after TAVR [16, 17]. Patients with higher LV mass regression were found to have a 50% lower readmission rate within the first year after TAVR [18]. However, the LV mass reduction does not necessarily lead to complete LV diastolic function improvement because LV mass decrease is slow and continues in a nonlinear fashion. The fibrotic component of the LV mass may take several years to regress after the TAVR and can even become permanent [14, 19, 20].
There are controversial reports about changes in diastolic function parameters (lateral e′ velocity, E/lateral e′, and left atrium volume index (LAVI), septal e′, and E/A ratio, and LV mass) after TAVR [21, 22]. Pre-procedural E/e′ was found to be a good measure of LV end diastolic pressure (LVEDP) and an excellent predictor of poor outcome and cardiac function in patients who underwent surgical aortic valve replacement [21]. This ratio might not be generalizable to TAVR patients due to a greater age-related mitral annulus calcification. Asami et al. have shown that despite improvement of individual diastolic parameters within 6–18 months after TAVR, the overall LVDD grade does not change in at least 50% of patients [11]. Although in a study by Blair et al. the number of patients with grade III LVDD at 30 days after TAVR was less than that at baseline, some (lateral e’ velocity, E/lateral e′, and LAVI) but not all (LV mass, septal e′, and E/A ratio) diastolic dysfunction parameters improved after TAVR [12]. The authors suggested that in patients with prolonged severe AS, LVDD can improve after TAVR but does not normalize due to sustained LV stiffness from myocardial fibrosis. Even after TAVR, the myocardial fibrosis can increase the risk of arrhythmia and sudden cardiac death [23]. This indicates the importance of timing for elimination of afterload by TAVR in high-risk and inoperable patients. Early TAVR, especially in patients with baseline LVDD, might prevent additional LV fibrosis and increase the odds of potential LV recovery.
TAVR can decrease left atrium volume and improve transmitral filling and mitral annular tissue Doppler velocity in early diastolic phase [12]. Severe mitral-valve regurgitation was reported in up to 49% of AS patients before TAVR and up to 29% of patients after TAVR [24]. A prospective study showed that afterload elimination by aortic valve replacement improves mitral valve regurgitation, especially in those with functional mitral regurgitation rather than myxomatous degeneration. The lower EF and larger LV mass were found to be associated with post-procedure reduction in degree of mitral regurgitation [25, 26]. Despite improvement in diastolic parameters, the degree of mitral regurgitation did not improve after TAVR in the study by Blair et al. possibly because of the advanced age of the study patients [12].
LVDD and Post-TAVR Outcome
Hospital readmission within 1 year after TAVR was found to be significantly associated with mortality. The most common reason for hospitalization after TAVR was HF [27, 28]. Baseline LVDD was found to play an important role in sustaining HF after TAVR [10]. Hospitalization duration increases with worsening of LVDD grade [11]. A higher rate of mortality was seen at 30 days in patients with LVDD, but the difference in cardiovascular mortality rate continues to be significant at mid-term follow-up, regardless of patients’ LVEF [11]. Blair et al. found that patients with grade 1a LVDD are not at increased risk of mortality after TAVR, and poor outcome emerges when LVDD grade progresses to grade 2 [12]. However, the grade 1a only existed when diastolic dysfunction was classified based on the Kuwaki et al. grading system (Table 1) [29, 30]. Baseline LVDD grade 3 was reported to be the strongest predictor of all-cause mortality at 1 year [11]. Chin et al. found that a higher mortality rate in patients with worse LVDD grade is due to a higher component of myocardial fibrosis in these patients [8]. Importantly, it was reported that 1-year mortality increases by 16.3% for each LVDD grade worsening [12]. Kampaktsis and colleagues also found a higher mortality rate in patients with severe LVDD (29%) versus those with moderate or mild LVDD (19%), but the difference in mortality rates was not statistically significant in their study [31]. Although LVDD grade can change after TAVR, no significant association was found between post-TAVR LVDD and mortality [12].
Table 1.
Diastolic dysfunction classification based on Kuwaki et al. and American Society of Echocardiography (ASE) and European Association of Cardiovascular Imaging (EACVI) systems
| Grading system | Grade 0 | Grade 1 | Grade 1a | Grade 2 | Grade 3 |
|---|---|---|---|---|---|
| Kuwaki et al. | |||||
| E/A | 0.75 < to < 1.5 | ≤0.75 | ≤0.75 | 0.75 < to < 1.5 | ≥ 1.5 |
| DT | > 140 ms | > 140 ms | > 140 ms | > 140 ms | ≤ 140 ms |
| E/e′ | < 10 | < 10 | ≥ 10 | ≥ 10 | ≥ 10 |
| ASE/EACVI | – | ||||
| Septal e′ | ≥ 8 | < 8 | < 8 | < 8 | |
| Lateral e′ | ≥ 10 | < 10 | < 10 | < 10 | |
| Left atrium volume | < 34 ml/m2 | ≥ 34 ml/m2 | ≥ 34 ml/m2 | ≥ 34 ml/m2 | |
| E/A | – | < 0.8 | 0.8–1.5 | ≥ 2 | |
| DT | – | > 200 ms | 160–200 ms | < 160 ms | |
| Av E/e′ | – | ≤ 8 | 9–12 | ≥ 13 | |
| Ar-A | – | < 0 ms | ≥ 30 ms | ≥ 30 ms | |
| Val ∆E/A | – | < 0.5 | ≥ 0.5 | ≥0.5 |
E early mitral inflow velocity, A duration of the pulmonary flow reversal, DT E wave velocity deceleration time, e′ early diastolic mitral annular velocity, Av average, Ar pulmonary venous atrial flow reversal, Val Valsalva maneuver
Muratori et al. did not find any association between baseline LVDD and 1-year mortality, despite improvement in New York Heart Association (NYHA) class and LVDD following TAVR [32]. Initial improvement in NYHA class may not persist, as the study found a higher proportion of patients with NYHA class III and IV at 3 years compared with 1 year after TAVR. The worsening of NYHA class can originate from patients’ old age and other baseline comorbidities [33, 34].
Impact of Prosthesis-Patient Mismatch and Aortic Regurgitation on LVDD and Patients’ Outcomes
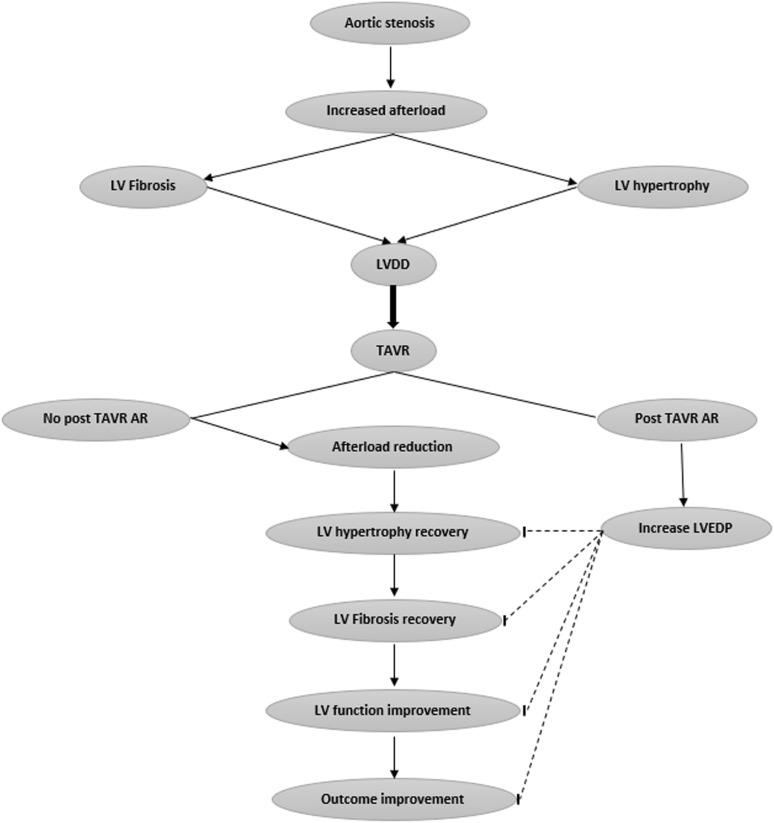
Prevalence of post-TAVR paravalvular regurgitation (PVR) was estimated as high as 100%, with up to 39% moderate to severe PVR among patients who underwent TAVR using an early generation valve [35, 36]. Fortunately, the rate of PVR ≥ moderate has decreased significantly, even to 0%, with newer- generation valves [37]. PVR increases LVEDP, leading to greater hemodynamic decompensation [38]. The LV of patients with diastolic dysfunction does not have the ability to increase dimensions and compliance for acute post-TAVR PVR, leading to a very high elevation in LVEDP [39]. Hence, post-TAVR PVR can exacerbate the baseline HF, adversely affect LV remodeling, and have an additional negative impact on mortality after TAVR (Fig. 1). Controversial studies were reported about the impact of different degrees of PVR on outcomes after TAVR [31, 40]. Sato et al. found increased LVEDP among patients who died within 1 year after TAVR, and presence of post-TAVR aortic regurgitation was the only independent predictor of mortality at 1 year [20]. Halkin et al. did not find any significant association between mild PVR and post-TAVR mortality, but moderate to severe PVR was shown to be an independent predictor of all-cause mortality at mid-term follow-up (30 months) [22]. They found that deceleration time (DT) of early filling velocity < 160 ms (suggestive of severely impaired LV diastolic filling) is an independent predictor of mortality in patients with mild and moderate to severe PVR. It was suggested that baseline LVDD plays an important role in exacerbation of post-TAVR PVR-related volume overload, and makes PVR an independent predictor of mortality [4]. The presence of PVR ≥ mild after TAVR is associated with increased mortality, with up to four times increased risk of mortality at 2 years among those with severe baseline LVDD. The degree of LVDD alone (without post-TAVR PVR) was not found to be significantly associated with mortality [31]. The difference in impact of various degrees of PVR on TAVR outcome originates from baseline LVDD. In patients with severe LVDD, even trace PVR can increase LVEDP and deteriorate heart function, increasing mortality, but those with more compliant LV may better tolerate higher degrees of PVL [40]. Post-TAVR PVR was found to be related to device size and form, patient native valve and LV outflow tract anatomy, and technical issues [22]. Therefore, it is important to use the most appropriate TAVR device with the lowest reported degree of PVL. It is also crucial to assess baseline diastolic function before TAVR and have a meticulous deployment technique to prevent any PVL. Post-deployment ballooning may be beneficial in elimination of observed PVL in selected cases.
Fig. 1.
Impact of transcatheter aortic valve replacement (TAVR) and aortic regurgitation (AR) on left ventricular diastolic dysfunction (LVDD) and patients’ outcomes. LVEDP left ventricular end diastolic pressure. The arrows show stimulatory effect and the flat heads show inhibitory effect
Post-TAVR prosthesis–patient mismatch (PPM) incidence was reported up to 42%, with a severe PPM rate of 9% [6, 41]. A negative impact of PPM on LV remodeling and function was reported. Although a higher rate of post-TAVR PVR was found in patients without PPM than in those with PPM (41 versus 17%, p = 0.01), higher diastolic function improvement and more LV mass regression was found in the no-PPM group versus the PPM group. However, no significant difference was found in midterm survival rates between PPM and no-PPM groups [41]. In one report, PPM occurred most commonly in patients who underwent TAVR with smaller-size prosthetics (Sapein and CoreValve size < 29 mm) [41]. On the other hand, it was suggested that patients with a larger aortic annulus can have suboptimal valve deployment due to less prosthesis–annulus congruence, leading to post-TARV PVR [42]. The presence of PVR after TAVR restrains any benefits of PPM absence in improvement of LVDD [43]. As TAVR use is expanding to low-risk and young patients, elimination of both PPM and AR by appropriate valve selection and deployment technique is ideal. However, since post-TAVR PVR is an independent predictor of mortality in patients with baseline LVDD, and PPM is not, prevention of AR might outweigh prevention of PPM.
Conclusions
LVDD plays an important role in patient outcomes with TAVR. Progression of LVDD to an advanced stage in patients with AS increases fibrotic tissue in the LV and decreases chance of LV recovery after the TAVR. Further, patients with severe LVDD may not tolerate minimal PVR and deteriorate after TAVR, but those with close to normal LV diastolic function can tolerate significant amounts of PVR. Therefore, evaluation of LVDD severity along with AS assessment, and consideration of TAVR prior to significant LVDD development with an appropriate valve and deployment technique, can potentially eliminate post-TAVR PVR and improve patient outcomes.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Seyed Hossein Aalaei-Andabili has nothing to disclose. Anthony A. Bavry has received honoraria from the American College of Cardiology and Edwards Lifesciences, and is a member of the journal’s Editorial Board.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced digital features
To view enhanced digital features for this article go to 10.6084/m9.figshare.7751420.
References
- 1.Makkar RR, Fontana GP, Jilaihawi H, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366(18):1696–1704. doi: 10.1056/NEJMoa1202277. [DOI] [PubMed] [Google Scholar]
- 2.Mihaljevic T, Nowicki ER, Rajeswaran J, et al. Survival after valve replacement for aortic stenosis: implications for decision making. J Thorac Cardiovasc Surg. 2008;135(6):1270–1278. doi: 10.1016/j.jtcvs.2007.12.042. [DOI] [PubMed] [Google Scholar]
- 3.Beach JM, Mihaljevic T, Rajeswaran J, et al. Ventricular hypertrophy and left atrial dilatation persist and are associated with reduced survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg. 2014;147(1):362.e8–369.e8. doi: 10.1016/j.jtcvs.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374(17):1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 5.Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376(14):1321–1331. doi: 10.1056/NEJMoa1700456. [DOI] [PubMed] [Google Scholar]
- 6.Hahn RT, Pibarot P, Stewart WJ, et al. Comparison of transcatheter and surgical aortic valve replacement in severe aortic stenosis: a longitudinal study of echocardiography parameters in cohort A of the PARTNER trial (placement of aortic transcatheter valves) J Am Coll Cardiol. 2013;61(25):2514–2521. doi: 10.1016/j.jacc.2013.02.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaid RR, Barker CM, Little SH, Nagueh SF. Pre- and post-operative diastolic dysfunction in patients with valvular heart disease: diagnosis and therapeutic implications. J Am Coll Cardiol. 2013;62(21):1922–1930. doi: 10.1016/j.jacc.2013.08.1619. [DOI] [PubMed] [Google Scholar]
- 8.Chin CWL, Everett RJ, Kwiecinski J, et al. Myocardial fibrosis and cardiac decompensation in aortic stenosis. JACC Cardiovasc Imaging. 2017;10(11):1320–1333. doi: 10.1016/j.jcmg.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hein S, Arnon E, Kostin S, et al. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107(7):984–991. doi: 10.1161/01.CIR.0000051865.66123.B7. [DOI] [PubMed] [Google Scholar]
- 10.Villari B, Vassalli G, Monrad ES, Chiariello M, Turina M, Hess OM. Normalization of diastolic dysfunction in aortic stenosis late after valve replacement. Circulation. 1995;91(9):2353–2358. doi: 10.1161/01.CIR.91.9.2353. [DOI] [PubMed] [Google Scholar]
- 11.Asami M, Lanz J, Stortecky S, et al. The impact of left ventricular diastolic dysfunction on clinical outcomes after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2018;11(6):593–601. doi: 10.1016/j.jcin.2018.01.240. [DOI] [PubMed] [Google Scholar]
- 12.Blair JEA, Atri P, Friedman JL, et al. Diastolic function and transcatheter aortic valve replacement. J Am Soc Echocardiogr. 2017;30(6):541–551. doi: 10.1016/j.echo.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Loncar S, Perlman G, Planer D, et al. Acute aortic regurgitation and hemodynamic collapse after balloon aortic valvuloplasty. Int J Cardiol. 2014;171(1):88–89. doi: 10.1016/j.ijcard.2013.11.063. [DOI] [PubMed] [Google Scholar]
- 14.Krayenbuehl HP, Hess OM, Monrad ES, Schneider J, Mall G, Turina M. Left ventricular myocardial structure in aortic valve disease before, intermediate, and late after aortic valve replacement. Circulation. 1989;79(4):744–755. doi: 10.1161/01.CIR.79.4.744. [DOI] [PubMed] [Google Scholar]
- 15.Weidemann F, Herrmann S, Stork S, et al. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120(7):577–584. doi: 10.1161/CIRCULATIONAHA.108.847772. [DOI] [PubMed] [Google Scholar]
- 16.Tzikas A, Geleijnse ML, Van Mieghem NM, et al. Left ventricular mass regression one year after transcatheter aortic valve implantation. Ann Thorac Surg. 2011;91(3):685–691. doi: 10.1016/j.athoracsur.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 17.Vizzardi E, D’Aloia A, Fiorina C, et al. Early regression of left ventricular mass associated with diastolic improvement after transcatheter aortic valve implantation. J Am Soc Echocardiogr. 2012;25(10):1091–1098. doi: 10.1016/j.echo.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Lindman BR, Stewart WJ, Pibarot P, et al. Early regression of severe left ventricular hypertrophy after transcatheter aortic valve replacement is associated with decreased hospitalizations. JACC Cardiovasc Interv. 2014;7(6):662–673. doi: 10.1016/j.jcin.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zakkar M, Alassar A, Lopez-Perez M, et al. Left ventricular remodeling after transcatheter aortic valve implantation: one-year follow-up study. Innovations (Phila). 2015;10(1):44–47. doi: 10.1097/IMI.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 20.Sato K, Kumar A, Jones BM, et al. Reversibility of cardiac function predicts outcome after transcatheter aortic valve replacement in patients with severe aortic stenosis. J Am Heart Assoc. 2017 doi: 10.1161/JAHA.117.005798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang SA, Park PW, Sung K, et al. Noninvasive estimate of left ventricular filling pressure correlated with early and midterm postoperative cardiovascular events after isolated aortic valve replacement in patients with severe aortic stenosis. J Thorac Cardiovasc Surg. 2010;140(6):1361–1366. doi: 10.1016/j.jtcvs.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Halkin A, Steinvil A, Aviram G, et al. Aortic regurgitation following transcatheter aortic valve replacement: impact of preprocedural left ventricular diastolic filling patterns on late clinical outcomes. Catheter Cardiovasc Interv. 2016;87(6):1156–1163. doi: 10.1002/ccd.26298. [DOI] [PubMed] [Google Scholar]
- 23.Rajesh GN, Thottian JJ, Subramaniam G, Desabandhu V, Sajeev CG, Krishnan MN. Prevalence and prognostic significance of left ventricular myocardial late gadolinium enhancement in severe aortic stenosis. Indian Heart J. 2017;69(6):742–750. doi: 10.1016/j.ihj.2017.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gotzmann M, Bojara W, Lindstaedt M, et al. One-year results of transcatheter aortic valve implantation in severe symptomatic aortic valve stenosis. Am J Cardiol. 2011;107(11):1687–1692. doi: 10.1016/j.amjcard.2011.01.058. [DOI] [PubMed] [Google Scholar]
- 25.Unger P, Plein D, Van Camp G, et al. Effects of valve replacement for aortic stenosis on mitral regurgitation. Am J Cardiol. 2008;102(10):1378–1382. doi: 10.1016/j.amjcard.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 26.Barreiro CJ, Patel ND, Fitton TP, et al. Aortic valve replacement and concomitant mitral valve regurgitation in the elderly: impact on survival and functional outcome. Circulation. 2005;112(9 Suppl):I443–I447. doi: 10.1161/CIRCULATIONAHA.104.526046. [DOI] [PubMed] [Google Scholar]
- 27.Barbanti M, Petronio AS, Ettori F, et al. 5-Year outcomes after transcatheter aortic valve implantation with CoreValve prosthesis. JACC Cardiovasc Interv. 2015;8(8):1084–1091. doi: 10.1016/j.jcin.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 28.Franzone A, Pilgrim T, Arnold N, et al. Rates and predictors of hospital readmission after transcatheter aortic valve implantation. Eur Heart J. 2017;38(28):2211–2217. doi: 10.1093/eurheartj/ehx182. [DOI] [PubMed] [Google Scholar]
- 29.Kuwaki H, Takeuchi M, Chien-Chia WuV, et al. Redefining diastolic dysfunction grading: combination of E/A </=0.75 and deceleration time > 140 ms and E/epsilon’ >/=10. JACC Cardiovasc Imaging. 2014;7(8):749–758. doi: 10.1016/j.jcmg.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Kampaktsis PN, Bang CN, Chiu Wong S, et al. Prognostic importance of diastolic dysfunction in relation to post procedural aortic insufficiency in patients undergoing transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2017;89(3):445–451. doi: 10.1002/ccd.26582. [DOI] [PubMed] [Google Scholar]
- 32.Muratori M, Fusini L, Tamborini G, et al. Sustained favourable haemodynamics 1 year after TAVI: improvement in NYHA functional class related to improvement of left ventricular diastolic function. Eur Heart J Cardiovasc Imaging. 2016;17(11):1269–1278. doi: 10.1093/ehjci/jev306. [DOI] [PubMed] [Google Scholar]
- 33.Bleiziffer S, Mazzitelli D, Opitz A, et al. Beyond the short-term: clinical outcome and valve performance 2 years after transcatheter aortic valve implantation in 227 patients. J Thorac Cardiovasc Surg. 2012;143(2):310–317. doi: 10.1016/j.jtcvs.2011.10.060. [DOI] [PubMed] [Google Scholar]
- 34.Bleiziffer S, Bosmans J, Brecker S, et al. Insights on mid-term TAVR performance: 3-year clinical and echocardiographic results from the CoreValve ADVANCE study. Clin Res Cardiol. 2017;106(10):784–795. doi: 10.1007/s00392-017-1120-3. [DOI] [PubMed] [Google Scholar]
- 35.Nombela-Franco L, Ruel M, Radhakrishnan S, et al. Comparison of hemodynamic performance of self-expandable CoreValve versus balloon-expandable Edwards SAPIEN aortic valves inserted by catheter for aortic stenosis. Am J Cardiol. 2013;111(7):1026–1033. doi: 10.1016/j.amjcard.2012.11.063. [DOI] [PubMed] [Google Scholar]
- 36.Cribier A, Eltchaninoff H, Tron C, et al. Treatment of calcific aortic stenosis with the percutaneous heart valve: mid-term follow-up from the initial feasibility studies: the French experience. J Am Coll Cardiol. 2006;47(6):1214–1223. doi: 10.1016/j.jacc.2006.01.049. [DOI] [PubMed] [Google Scholar]
- 37.Tang GHL, Zaid S, Schnittman SR, et al. Novel predictors of mild paravalvular aortic regurgitation in SAPIEN 3 transcatheter aortic valve implantation. EuroIntervention. 2018;14(1):58–68. doi: 10.4244/EIJ-D-18-00005. [DOI] [PubMed] [Google Scholar]
- 38.Sinning JM, Vasa-Nicotera M, Chin D, et al. Evaluation and management of paravalvular aortic regurgitation after transcatheter aortic valve replacement. J Am Coll Cardiol. 2013;62(1):11–20. doi: 10.1016/j.jacc.2013.02.088. [DOI] [PubMed] [Google Scholar]
- 39.Gotzmann M, Lindstaedt M, Mugge A. From pressure overload to volume overload: aortic regurgitation after transcatheter aortic valve implantation. Am Heart J. 2012;163(6):903–911. doi: 10.1016/j.ahj.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 40.Okafor I, Raghav V, Midha P, Kumar G, Yoganathan A. The hemodynamic effects of acute aortic regurgitation into a stiffened left ventricle resulting from chronic aortic stenosis. Am J Physiol Heart Circ Physiol. 2016;310(11):H1801–H1807. doi: 10.1152/ajpheart.00161.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poulin F, Yingchoncharoen T, Wilson WM, et al. Impact of prosthesis-patient mismatch on left ventricular myocardial mechanics after transcatheter aortic valve replacement. J Am Heart Assoc. 2016;5(2):e002866. doi: 10.1161/JAHA.115.002866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Detaint D, Lepage L, Himbert D, et al. Determinants of significant paravalvular regurgitation after transcatheter aortic valve: implantation impact of device and annulus discongruence. JACC Cardiovasc Interv. 2009;2(9):821–827. doi: 10.1016/j.jcin.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Poulin F, Carasso S, Horlick EM, et al. Recovery of left ventricular mechanics after transcatheter aortic valve implantation: effects of baseline ventricular function and postprocedural aortic regurgitation. J Am Soc Echocardiogr. 2014;27(11):1133–1142. doi: 10.1016/j.echo.2014.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.