Abstract
A third of patients with triple negative breast cancer (TNBC) have relapsed disease within 2–5 years from initial diagnosis, leaving an unmet need for therapeutic targets. TNBC frequently harbors alterations of the PI3K/AKT/mTOR pathway, but single agent PI3K/AKT/mTOR inhibitors have not shown marked efficacy. In this study, we investigated a strategy to improve efficacy of PI3K-α inhibitor BYL719 (alpelisib) in TNBC. While BYL719 is effective at inhibiting cell proliferation in T47D, a triple positive cell line, it had limited activity in TNBC. This may be partially due to persistent phosphorylation of RB, and incomplete inhibition of p-S6 in TNBC, since the inhibitory effect of BYL719 on p-RB and p-S6 was significantly reduced in TNBC compared to T47D cells. Addition of the CDK4/6 inhibitor LEE011 to BYL719 caused a simultaneous reduction of p-RB and p-S6, and a more complete inhibition of p-S6, leading to decreased expression of the pro-survival protein MCL-1, an induction of apoptosis, and an enhanced reduction of tumor growth in a PDX model of TNBC. These findings suggest that inhibition of p-RB and p-S6 is important for an effective response to the treatment of TNBC, and provides a strong rationale for clinical development of combination therapy with BYL719 and LEE011 for treatment of metastatic TNBC with intact RB.
Presentation: This study was presented in part as an abstract at the 2016 San Antonio Breast Cancer Symposium (P3-03-15) and the 2018 Cancer Research and Targeted Therapy in London.
Subject terms: Targeted therapies, Growth factor signalling
Introduction
Triple negative breast cancer is an aggressive subtype of breast cancer with limited treatment options and very poor prognosis for metastatic disease. Median progression-free survival with chemotherapy ranges from 2.5 to 4 months, and an average overall survival of 13 months in the metastatic setting1,2. The disproportionately high rate of mortality compared with hormone receptor positive breast cancer or HER2 positive breast cancer is largely due to the lack of effective targeted therapies, with the exception of tumors with germline BRCA1/2 mutation3. Early trials using the VEGF inhibitor4, c-Kit inhibitor5, EGFR inhibitor6,7, and histone deacetylase inhibitor8 failed to show efficacy. Androgen receptor (AR) targeted therapies such as bicalutamide and enzalutamide showed limited efficacy in the subpopulation of AR+ TNBCs9,10. Recent clinical trial data shows the potential utility of immune check point inhibitors; however, single agent response rates are modest, ranging between 5–19%11–14. These results highlight the unmet need to develop effective targeted therapy combinations for treatment of metastatic TNBC.
TNBC is a heterogeneous disease with at least 7 subtypes identified through mRNA expression profiling by the Vanderbilt Classification15: basal like-1 (BL-1); basal like-2 (BL-2); mesenchymal (M); mesenchymal stem-like (MSL); immunomodulatory (IM); luminal androgen receptor (LAR) and unstable (UNS) subtypes. The complexities of TNBC at the genomic level predict no single treatment approach will produce universal benefit across all subtypes, and combination therapy is likely to yield a better outcome. Individualized therapeutic approaches are needed based on the subtypes; however, the clinical utility of the molecular classifiers in guiding treatment decisions is still unclear.
The Cancer Genome Atlas (TCGA) analysis demonstrated that the most frequent tumor genomic alterations in TNBC involve genes associated with DNA damage repair, phosphatidylinositol 3-kinase (PI3K) signaling pathway16, and RAS/RAF/MEK pathways. The PIK3A-AKT-mTOR pathway alteration occurs in 40% of TNBCs. Non-basal subtypes (LAR, M and MSL) have demonstrated relatively high PIK3CA –activating mutations, and exhibit sensitivity to PI3K inhibitors in vitro17. Nevertheless, use of PI3K inhibitors as single agent therapy has proven minimally effective due to multiple feedback mechanisms18. Combination therapies with chemotherapy agents have shown synergy but are associated with more toxicities19,20. Recently, combination of PI3K inhibitor with CDK4/6 inhibitor has been shown to have a synergistic effect in tumor suppression in ER positive breast cancer21,22. Dysregulation of multiple CDK family members occurs commonly in human cancer; in particular, the cyclin D-CDK4/6-retinoblastoma protein (RB)-INK4 axis is universally disrupted, facilitating cancer cell proliferation23,24. CDK4/6 inhibitors (palbociclib, ribociclib and abemaciclib) in combination with hormone therapies are currently approved by the FDA to treat patients with estrogen receptor positive (ER+) and HER 2 negative breast cancers. Palbociclib has substantial activity in breast cancer cell lines of the LAR sub-type of TNBC, with similar levels of activity to ER positive (ER+) breast cancer cell lines25.
The current study was designed to investigate the efficacy of the PI3K-α inhibitor BYL719 (alpelisib), either alone or in combination with the FDA approved CDK4/6 inhibitor LEE011 (ribociclib) in TNBC. BYL719 is an α-isoform selective PI3K inhibitor, which has demonstrated clinical activity as a single agent and in combination with hormone therapy in patients with advanced HR+ BC. We hypothesize that combining PI3K-α inhibitor BYL719 and CDK4/6 inhibitor LEE011 may overcome single agent resistance, and provide synergistic suppression in TNBC.
Results
Effects of single agent BYL719 and LEE011 on cell viability of TNBC cell lines
The anti-tumor activity of single agent BYL719 and LEE011 was tested in TNBC cell lines representative of each molecular subtype through cell viability analysis. As shown in Table 1, the concentration that gave 50% inhibition (IC50) of cell viability for single agent BYL719 ranged from 3.68 μM to 26 μM. The IC50 for LEE011 was 13.1 μM in MDA-MB-231 cells, and was >40 μM in most other cells. These IC50 values for BYL719 were higher than the IC50 for T47D cell, a triple positive cell line, which is sensitive to BYL719.
Table 1.
IC50 for BYL719, either alone or in combination, in TNBC15.
| Cell line | Subtype | RB status | Mutations | Single Agent | Combination BYL719 + LEE011 | ||
|---|---|---|---|---|---|---|---|
| BYL719 IC50 (μM) | LEE011 IC50 (μM) | BYL719 IC50 (μM) | IC50 Fold Reduction | ||||
| HCC38 | BL1 | WT | CDKN2A; TP53 | 26.01 | >40 | 11.88 | 2.19 |
| MDA-MB-468 | BL1 | Mut | PTEN;SMAD4;TP53 | 11.28 | >40 | 13.46 | 0.84 |
| HCC1806 | BL2 | WT | CDKN2A;TP53;UTX | 8.18 | >40 | 5.75 | 1.42 |
| HCC1187 | IM | WT | TP53;CTNNA1;DDX18;HUWE1;NFKBIA | 14.41 | >10 | 11.04 | 1.30 |
| BT549 | M | Mut | PTEN;TP53 | 17.37 | >40 | 14.61 | 1.19 |
| Hs578T | MSL | WT | CDKN2A;TP53 | 19.84 | >40 | 8.99 | 2.21 |
| MDA-MB-231 | MSL | WT | BRAF;CDKN2A;KRAS;NF2;TP53;PDGFRA | 19.29 | 13.01 | 6.80 | 2.83 |
| MFM223 | LAR | WT | PIK3CA;TP53 | 3.68 | >20 | 0.79 | 4.66 |
| T47D | ER+/PR+/HER2+ | WT | PIK3CA;TP53 | 0.26 | 25.24 | ||
Effects of combining BYL719 and LEE011 on signaling pathways
To understand the limited sensitivity of TNBC cell lines to BYL719 compared to T47D, the effect of BYL719 on the PI3K/AKT/mTOR pathway was analyzed in TNBCs. The inhibition of p-RB has previously been reported to be correlated with the sensitivity of cells to PI3K inhibitors21. As shown in Fig. 1, treatment with BYL719 alone not only significantly reduced the levels of p-AKT, p-S6K and p-S6, but also p-RB, in T47D cells. In contrast, treatment with BYL719 alone had little effect on p-RB in MDA-MB-231 and Hs578T cells. Although BYL719 was able to inhibit p-S6K and p-S6 in MDA-MB-231 and Hs578T cells, the inhibition was more significantly reduced in these two cells than in T47D cells, indicating that TNBC cells exhibit less inhibition of mTORC1 (as indicated by p-S6) than in sensitive cells, such as T47D. These findings are consistent with previous reports in other cancer cells that the sensitivity of cells to BYL719 may be dependent on the ability of BYL719 to suppress p-RB and mTORC121,26–28.
Figure 1.
The combination of BYL719 and LEE011 leads to a simultaneous reduction of p-RB and p-S6 and a more complete inhibition of mTORC1 in TNBC cell lines. TNBC cells (MDA-MB-231 and Hs578T), as well as a triple positive cell (T47D), were treated with vehicle (DMSO), BYL719, LEE011, or BYL719 + LEE011 at various concentrations for 24 hours. (A) Whole-cell lysates were prepared and analyzed by Western blot for expression of signaling molecules. (B) Relative expression of p-AKT, p-S6K, and p-S6 was determined by measuring the density of each band and normalizing to the corresponding total protein. Relative expression of RB protein was normalized to β-actin since total RB protein was not detectable in these cells. Images are representative of results from three independent experiments. *P < 0.05, combination vs. vehicle, BYL719 or LEE011 alone. Data are mean ± S.D. from 3 experiments.
Treatment with BYL719 alone led to the inhibition of the p-S6 pathway, but not the p-RB pathway in MDA-MB-231 and Hs578T cells. In contrast treatment with LEE011 alone led to inhibition of the p-RB pathway, but not the p-S6 pathway, in these two cell lines. However, the combination of LEE011 and BYL719 caused the suppression of both p-RB and p-S6K/p-S6 pathways, and a greater inhibition of p-S6K and p-S6 in MDA-MB-231 and Hs578T cells. Also, a stronger inhibition of p-RB was found in MDA-MB-231 cells treated with both BYL719 and LEE011. Taken together, these results demonstrate that the combined targeting of both PI3K and CDK4/6 pathways can inhibit multiple survival pathways, and results in a more complete inhibition of mTORC1.
Synergistic effect of combined treatment with BYL719 and LEE011 on cell viability
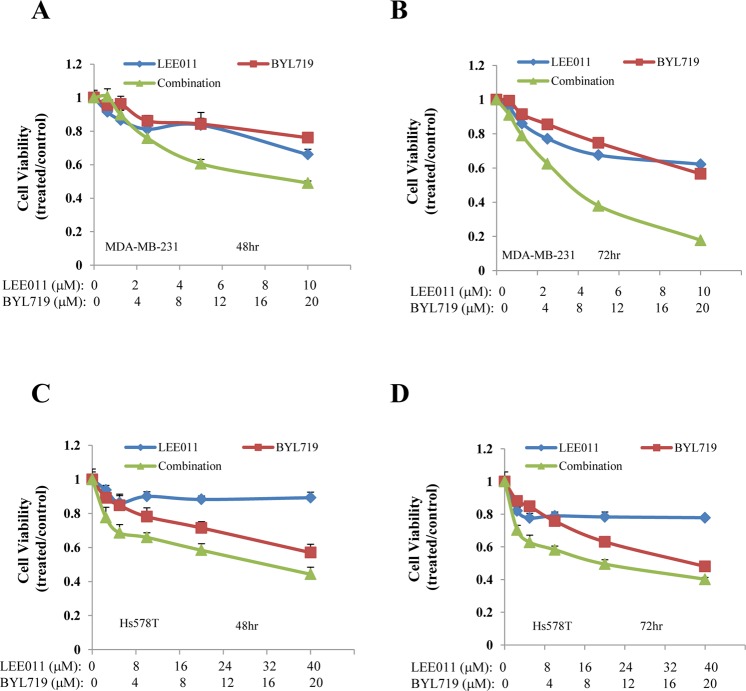
Given that BYL719 in combination with LEE011 led to a simultaneous inhibition of p-RB and p-AKT/p-S6K/p-S6, we next evaluated whether this combination resulted in reduced cell viability in TNBC cell lines. MDA-MB-231 and Hs578T cells were treated with BYL719 and LEE011 alone or in combination for 48 hours and 72 hours. As shown in Fig. 2, the combination treatment decreased cell viability much more robustly than either agent alone in both cell lines, with stronger activity seen at 72 hours.
Figure 2.
The combination of BYL719 and LEE011 decreases cell viability in TNBC cell lines. MDA-MB-231 (A,B) and Hs578T (C,D) cells were treated with BYL719 or LEE011, either alone or in combination, at various concentrations in a fixed molar ratio. Cell viability was determined after 48 hours (A,C) and after 72 hours (B,D). Data are mean of triplicates.
To further understand whether this reduced cell viability could be reproduced in other TNBC cells, multiple TNBC cell lines representing several molecular subtypes were treated with BYL719 and LEE011 alone or in combination. As shown in Table 1, the concentrations of BYL719 that gave 50% inhibition (IC50) were significantly reduced by including LEE011 in the treatment of TNBC cell lines with intact RB (HCC38, HCC1806, HCC1187,Hs578T, MDA-MB-231 and MFM223), but not in cell lines with mutant RB (MDA-MB-468 and BT549). This reduced cell viability by combination treatment was observed in a number of representative cell lines, including BL1 (HCC38 and MDA-MB-468), BL2 (HCC1806), IM (HCC1187), M (BT549), MSL (MDA-MB-231 and Hs578T), and LAR (MFM223). It is important to note that in MFM223 (LAR TNBC subtype), the fold reduction of BYL719 IC50 induced by LEE011 was significantly higher (4.66 fold).
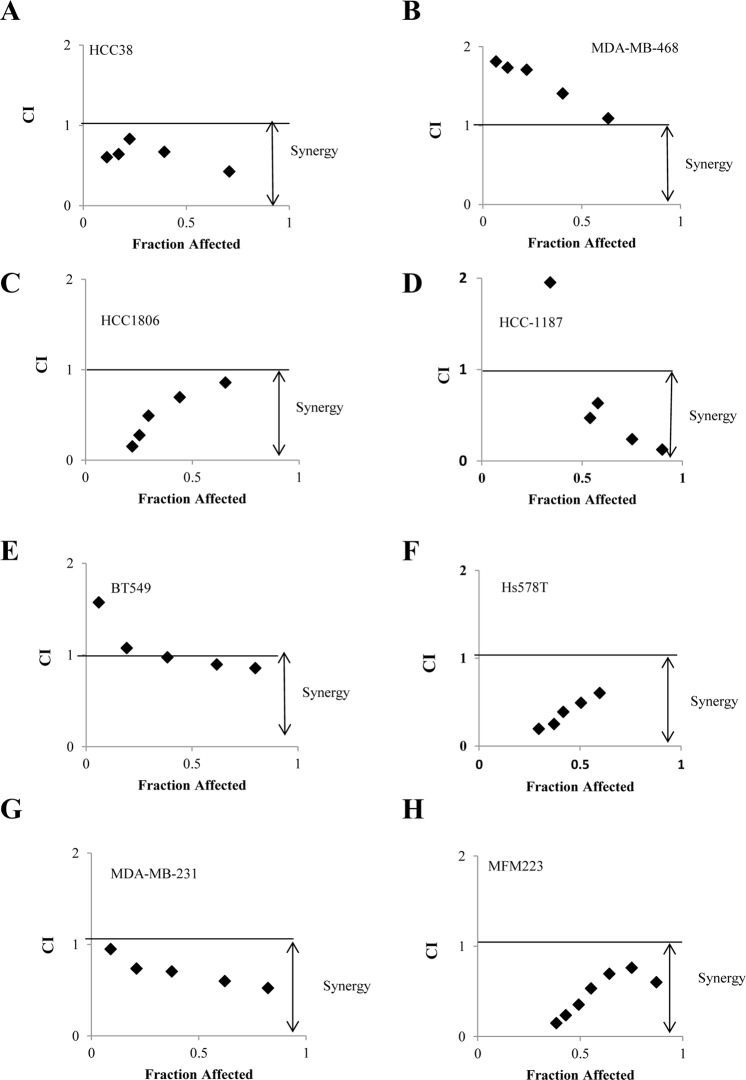
To understand whether the increased activity was additive or synergistic, the Chou-Talalay method was used to determine the combination index (CI) (CI = 1, additive effect; CI < 1, synergism; CI > 1 antagonism)29. As shown in Fig. 3, the combination treatment produced strong to very strong synergism in a number of TNBC cell lines with intact RB (MDA-MB 231, HS578T, HCC-1187, HCC38, HCC-1806, MFM223), but not in TNBCs with mutant RB (BT 549 and MD-MB-468), providing evidence that this synergistic effect is associated with RB status.
Figure 3.
Synergistic interaction is observed between BYL719 and LEE011 in TNBC cells. (A–H) MDA-MB-231, Hs578T, HCC 1187, HCC38, HCC1806, MFM 223, BT548 and MDA-MB-468 cells were treated with BYL719 or LEE011 either alone or in combination at various concentrations. Cell viability was determined after 72 hours. The combination index (CI) was determined by Chou-Talalay method using the Calcusyn software.
Effect of combined treatment with BYL719 and LEE011 on apoptosis
To determine whether the reduced cell viability could be due to the induction of apoptosis, cells were treated with BYL719 and LEE011 alone or in combination for 48 hours, and the number of apoptotic cells was determined by Annexin V staining. As shown in Fig. 4, BYL719-induced apoptosis increased from 4.27% to 20.39% when combined with LEE011 in MDA-MB-231 cells (Fig. 4A), and from 14.5% to 31.9% in Hs578T cells (Fig. 4B). Consistent with the Annexin V staining results, the increase of cleaved caspase 3, an apoptotic marker, and reduction of MCL-1, an anti-apoptotic protein, were also found in the cells that were treated with both BYL719 and LEE011 (Fig. 4C). These results indicate that inhibition of both PI3K and CDK4/6 pathways effectively suppresses cell survival in TNBC cells by promoting apoptosis.
Figure 4.

The combination of BYL719 and LEE011 induces apoptosis in MDA-MB-231 and Hs578T cells. Cells were treated with BYL719 and LEE011, either alone or in combination for 48 hours. Apoptosis was determined by (A,B) flow cytometry using Annexin V and PI staining or (C) Western blot analysis of cleaved caspase-3 and MCL-1. *P < 0.05, **P < 0.005, combination vs. vehicle, BYL719 or LEE011 alone. NS: not significant, BYL719 or LEE011 vs. vehicle. Data represents the mean ± SD of three preparations.
Anti-tumor activities of BYL719 and LEE011 in PDX models of human TNBC
To test the treatment effect of the combination therapy of BYL719 and LEE011, a PDX model of chemotherapy-resistant TNBC was used. Exome sequencing of the tumor identified the following genomic alterations: PIK3CA E542K mutation, PTEN loss, CDK4 amplification, TP53 H179R mutation, KIT amplification, PDGFRA amplification, KDR amplification, and NOTCH2 exon 3 truncation. The combination of BYL719 and LEE011 showed synergistic tumor suppression, which was statistically significant compared to either agent alone (Fig. 5A). No toxicity was observed in mice with any of the treatments, whether the drugs were used alone or in combination, as indicated by absence of significant (>5%) change in body weight (Fig. 5B). Tumor weight at the end of treatment showed significant reduction in the combination therapy compared to single agents (Fig. 5C). Tumor weight decreased from 2.041 g to 0.476 g with BYL719 alone at a daily dose of 30 mg/kg, and from 2.041 g to 0.787 g with LEE011 alone at a daily dose 75 mg/kg. The combination treatment further decreased the tumor weight to 0.191 g.
Figure 5.
The combination of BYL719 and LEE011 induces anti-tumor activity in a TNBC PDX model. PDX tumors were surgically implanted into mammary fat pad of 6- to 8- week-old female NSG mice. Mice were treated daily by oral gavage with vehicle, BYL719 (30 mg/kg), LEE011 (75 mg/kg) or combination of both. (A–C) Tumor volume and body weight were measured 1–2 times per week, and tumor weight was measured at the end of the treatment. (D) Effect of BYL719 and LEE011 on the expression of signaling molecules in tumors was analyzed by Western blot. Whole-cell tumor lysates were prepared and analyzed by Western blot for expression of phosphorylated RB, AKT, and S6. (E) Relative expression of p-RB, p-AKT, and p-S6 was determined by measuring the density of each band and normalizing to GAPDH. Data represents the mean ± SD (n = 5–10). *P < 0.05; **P < 0.005; ***P < 0.0005, ****P < 0.0001, ns, not significant.
To examine the effect of therapy on the downstream molecular targets, tumor tissue lysates were analyzed for the expression of p-RB, p-AKT, AKT, p-S6 and S6 by Western blot analysis. As shown in Fig. 5D,E, the combination of BYL719 with LEE011 led to the suppression of both p-RB and mTORC1 pathways and a greater inhibition of p-RB, which was consistent with the in vitro results shown in Fig. 1. Overall, these results support a consistent synergistic effect of PI3K-α and CDK4/6 inhibition in TNBC in vitro and in vivo in RB-intact tumors.
Discussion
A third of patients with TNBC have relapsed disease within 2–5 years from initial diagnosis. This highlights the unmet need to develop effective targeted therapies in this population2,30. TNBC frequently harbors alterations of the PI3K/AKT/mTOR pathway, but single agent PI3K/AKT/mTOR inhibitors are not effective31. In this study, we showed that dual-targeting of PI3K-α and CDK4/6 provided synergistic suppression of TNBC cell lines and a PDX mouse model in an RB-dependent and subtype-independent manner. We found that the limited activity of BYL719 in TNBC may be partially due to persistent phosphorylation of RB and incomplete inhibition of the PI3K/AKT/mTOR pathway (as indicated by p-S6) in TNBC. Addition of the CDK4/6 inhibitor LEE011 to BYL719 caused a simultaneous reduction of p-RB and p-S6, and a more complete inhibition of mTORC1. This led to the decreased expression of pro-survival protein MCL-1, a synergistic inhibition of cell survival, and the reduction of tumor growth in this PDX model of TNBC.
The cyclinD:CDK4/6:RB axis is dysregulated in a variety of human cancers24,32–34. Targeting this pathway has proven to be a successful therapeutic approach in ER+ breast cancer with three CDK4/6 inhibitors currently used in the clinic35. CDK4/6 inhibitors halt cell cycle progression and induce G1 cell cycle arrest33. The CDK4/6 inhibitor LEE011 (ribociclib) has been approved for treatment of ER+ metastatic breast cancer. It is believed that intact RB is required in order for a CDK4/6 inhibitor to be effective in ER+ breast cancer, but cyclin D1 overexpression or loss of p16INK4A did not predict response to palbociclib in the PALOMA-1 study36. Loss of RB expression has been reported in 20–30% of total breast cancers and approximately 40% of TNBCs37. O’Brien et al. identified RB-dependence of abemaciclib in multiple TNBC cell lines38. TNBC cell lines with high baseline total and phosphorylated p-RB, accompanied by low P16 level are among the most sensitive to abemaciclib38. Consistent with this report, our study has shown that CDK4/6 inhibition can sensitize TNBC cancer cells to PI3K inhibition, producing a greater reduction of cell viability in an RB-dependent and subtype-independent manner39,40.
Although our results suggest a possibility that the synergistic effect by combining BYL719 and LEE011 is associated with RB status, the two cell lines with RB mutated in our study that did not have synergistic effect (MDA-MB-468 and BT549) are also PTEN-deficient lines. PTEN is a tumor suppressor that de-phosphorylates phosphatidylinositol (3,4,5)-triphosphate (PIP), reversing the activation of PI3-kinase. PTEN also functions independently of its phosphatase in the nucleus, where it mediates cell proliferation, genome stabilization, and regulation of DNA repair41. PTEN deficient cell lines have been previous shown to depend more on p110-β instead of p110-α42–45. In comparison, both the MFM223 cell line and the PDX model have PIK3CA mutations that likely depend on p110α, and respond well to the single agent BYL719 treatment. Recent clinical trials suggested that many PTEN-deficient tumors don’t respond to PI3K inhibitors46. It is possible that the pan-PI3K inhibitors currently in trials are less effective on p110β compared to p110α; however, it is also possible that PTEN loss activates more than the PI3K pathway. PTEN loss may be more resistant to PI3K inhibition than tumors driven by p110α activation. Further study is needed to investigate the therapeutic potential of p110β-specific inhibitors in combination of CDK4/6 inhibitors for the treatment of PTEN-deficient TNBC.
The capability of the CDK4/6 inhibitor to increase the activity of PI3K was previously shown in TNBC carrying PIK3CA mutations treated with the CDK 4/6 inhibitor palbociclib and the pan-PI3K inhibitor taselisib47. Teo et al. also showed that dual blockade of PI3Kα and CDK4/6 is synergistic and immunogenic in multiple RB-wildtype TNBC models48. Our current finding is consistent with these previous reports. In addition, we demonstrated that combined inhibition of CDK4/6 and PI3Kα caused a simultaneous reduction of p-RB and p-S6 and a more complete inhibition of the PI3K/AKT/mTOR pathway, leading to a decreased expression of pro-survival protein MCL-1 and the induction of apoptosis. These results suggest that p-RB and p-S6 may act synergistically to control cancer cell survival and suppression of both pathways may be needed for an optimal anti-tumor activity of BYL719 in TNBCs. Furthermore, our results agree with previous findings that complete inhibition of p-S6 is important for sensitivity of PI3K inhibition in melanoma cells and ER+ breast cancer cells21,26–28. In addition to breast cancer, the synergistic anti-tumor effect of dual inhibition of the PI3K/AKT/mTOR pathway and CDK4/6 has also been demonstrated in malignant pleural mesothelioma, T-cell acute lymphoblastic leukemia, and mantle cell lymphomas49–51.
Other co-targeting strategies such as combining CDK4/6 inhibitors with chemotherapy have been tested. Palbociclib was found to antagonize the cytotoxic effect of doxorubicin and taxanes in the RB-intact TNBC cell lines MDA-MB-231 and Hs578T52,53. Increasing evidence suggests that the timing of palbociclib and paclitaxel use is critical. Increased paclitaxel cytotoxicity was found when cells were pre-exposed to palbociclib for synchronization53. A phase I trial of palbociclib and paclitaxel showed safety and early efficacy54. CDK4/6 inhibition with abemaciclib combined with anti-mitotic agents, both in vitro and in vivo, did not antagonize the effects of either agent. Co-administration of docetaxel with either palbociclib or ribociclib after CDK4/6-inhibitor pre-treatment blocked apoptosis induction, whereas co-treatment with abemaciclib did not38.
Asghar et al. has identified that TNBC cell lines of the luminal-androgen receptor (LAR) and mesenchymal-stem like (MSL) subsets were sensitive to palbociclib, and sensitivity was associated with the expression of androgen receptor and the absence or low levels of cyclin E147. They also found that PI3K inhibition was synergistic with palbociclib in PIK3CA-mutated TNBC cell lines, with a greater effect in LAR/MSL subgroups compared to M/basal subgroups. LAR cell lines represent a TNBC subgroup that may benefit from CDK4/6 inhibition. This is consistent with our current results, that adding LEE011 to BYL719 leads to a significant 4.66 fold reduction of BYL719 IC50. This confirms the significant efficacy of CDK4/6 inhibitor in combination with PI3K-α inhibition in the LAR subtype of TNBC. TNBC subtype-dependent inhibition was not clearly demonstrated in the other cell lines.
In our study, synergy of BYL719 and LEE011 was observed in a PDX model of TNBC with genomic mutations in both the PI3K and CDK4/6 pathways: PIK3CA (E542K), PTEN loss, CDK4 amplification, TP53 (H179R), KIT amplification, PDGFRA amplification, KDR amplification, and NOTCH2 truncation exon 3. Whether tumors that harbor these mutations are necessary to elicit a response to the combination is currently unknown. Zhang et al. developed a panel of PDX models representing multiple TNBC subtypes (IM, BL1, BL2, and M) in order to test the preclinical drug efficacy of mTOR inhibitors55. Ideally PDX models representing each subtype should be used to verify the efficacy of combination BYL719 and LEE011 treatment.
Conclusion
This study demonstrates the synergistic effect of dual-targeting of the PI3K-α and CDK4/6 pathways in RB-intact TNBC. Given the data from the current study, as well as other published work, there is a strong rationale for clinical development of combination therapy with BYL719 and LEE011 for treatment of metastatic TNBC with intact RB. The synergistic effect is particularly notable in the LAR subtype of TNBC, and further studies are needed to address the significance of this result.
Materials and Methods
Cell lines, cell culture, and reagents
The human breast cancer cell lines used in this analysis, including T47D, MDA-MB-468, BT549, HCC38, HCC1187, and Hs578T were obtained from American Type Culture Collection (Rockville, MD). MDA-MB-231 was kindly provided by Dr. Emily Wang (City of Hope). BT549, HCC38 and HCC1187 were cultured in RPMI 1640 medium (Mediatech Inc., Manassas, VA) supplemented with 10% fetal bovine serum (FBS, Atlanta Biologicals, Norcross, GA) and 1% penicillin/streptomycin. MDA-MB-468 cells were propagated in 1:1 DMEM/F12 (1:1) (Gibco, Invitrogen) supplemented with 10% FBS and 1% penicillin/streptomycin. MDA-MB-231 and Hs578T were cultured in DMEM medium (Mediatech Inc., Manassas, VA) supplemented with10% fetal bovine serum and 1% penicillin/streptomycin.
All cells were incubated at 37 °C at 5% CO2. BYL719 (alpelisib, selective PI3K-α inhibitor) and LEE011 (ribociclib, highly specific CDK4/6 inhibitor) were obtained under a Material Transfer Agreement with Novartis (Basel, Switzerland).
Proliferation assays and multiple drug effects analysis
Cells (4000 per well) were plated in 96-well plate format in 100 μl growth medium. Cells were incubated with DMSO or increasing concentrations of BYL719 and LEE011, and cell viability was determined 72 hours later. The following starting doses were used: MDA-MB-468, HCC1187, and MDA-MB-231: (1.25 μM BYL719 and 0.625 μM LEE011); BT549 (2.5 μM BYL719 and 2.5 μM LEE011); HCC38 and Hs578T (1.25 μM BYL719 and 2.5 μM LEE011). Viable cells were determined by the MTT assay according to manufacturer’s instruction (Promega, Madison, WI, USA). Briefly, after treatment, the media was removed and 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye was added to each well and incubated for 4 hours. The formazan crystals were dissolved in dimethyl sulfoxide (DMSO) after removing the media. Absorbance was read at 570 nm. The IC50 was determined using the CalcuSyn software (Biosoft, Ferguson, MO). The combination index (CI) was determined by Chou-Talalay method using CalcuSyn software (Biosoft, MO)29.
A CI < 1 indicates synergy, CI > 1 indicates antagonistic interactions, and a CI value = 1 indicates additive effects.
Western blot analysis
Cells were grown in complete medium overnight and treated with DMSO or drugs at recorded concentrations and times. Cells were lysed in RIPA lysis buffer containing Halt protease and phosphatase inhibitors (Thermo Scientific Inc.). Equal amounts of protein were separated by SDS-polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride membranes and incubated with total and phosphorylated protein-specific antibodies. Antibodies against p-RB (catalog no. 8180; 1:800 dilution), p-AKT (S473) (catalog no. 9271; 1;1000 dilution), AKT (catalog no. 9272; 1;1000 dilution), p-S6K1 (catalog no. 9234; 1;1000 dilution), S6K1 (catalog no. 2708; 1;1000 dilution), p-S6 (catalog no. 2211; 1;3000 dilution), S6 (catalog no. 2217; 1;3000 dilution), and β-actin (catalog no. 4970; 1;3000 dilution) were obtained from Cell Signaling Technology (Danvers, MA). Binding of the primary antibody was detected using a horseradish peroxidase (HRP)-conjugated secondary antibody and chemiluminescent substrates (Thermo Scientific Inc.). Signal was detected by exposing standard X-ray film. Films were scanned using Epson Perfection V750 pro Scanner. The density of each protein band was quantified by Image J software (NIH). The density of phosphoprotein was normalized to the corresponding total proteins.
Annexin V staining
Annexin V apoptosis detection kit (BD bioscience) was used to measure apoptosis. Breast cancer cells were treated with BYL719, LEE011, or both at various concentrations for 48 hours. All cells, including floating and attached cells, were collected and stained with FITC-Annexin V and PI. The staining intensity was quantified using fluorescence-activated cell sorting (FACS).
TNBC patient-derived xenograft (PDX) model
The combination of BYL719 and LEE011 was tested in vivo using a TNBC patient-derived xenograft (PDX) based upon the molecular profile of the tumor. After obtaining informed written patient consent, a triple negative breast tumor sample was obtained from the patient at the time of surgery at City of Hope under protocol approved by Institutional Review Board (IRB). All methods were performed in accordance with the relevant guidelines and regulations. Fresh primary tumor tissues (2-3 mm in diameter) were surgically implanted into mammary fat pad of 6- to 8- week-old female NOD/SCID/IL2Rgamma null (NSG) mice. Once the xenograft was established, the tumor was removed, cut into small fragments, surgically implanted into mammary fat pad of mice to expand the xenograft numbers. When the xenografts were palpable, animals were randomized into 4 groups and treated by oral gavage with vehicle (30% Solutol HS15 + 0.5% methycellulose, daily), BYL719 (30 mg/kg, daily), LEE011 (75 mg/kg, daily), or a combination of both agents. Tumor volumes were assessed using calipers 1-2 times per week and determined using the formula (width)2 × length × 0.52. Body weight was monitored weekly as an indicator of drug-induced toxicity and overall health of the mice. Tumor was harvested and measured for the weight at day 23 of the experiment. All animal studies were carried out under protocols approved by the Institutional Animal Care and Use Committee (IACUC) at City of Hope in accordance with all applicable federal, state, and local requirements and institutional guidelines.
Statistical methods
Data are presented as mean ± S.D from 3 experiments. All the experiments were carried out in triplicate or more. Student’s t-test was used to compare the mean of two groups. ANOVA was used to compare the difference for the multiple groups. A value of p < 0.05 was considered statistically significant.
Acknowledgements
We thank Dr. Jun Wu and the Animal Tumor Model Core, Lucy Brown and the Analytical Cytometry Core, as well as the Parvin Animal Facility for their technical assistance. This study was supported by the National Cancer Institute of the National Institutes of Health under award number K12CA001727 (Joanne Mortimer, MD), STOP Cancer Foundation (Yuan Yuan, MD PhD), Concern/Save the Ta-Tas Foundations (Yuan Yuan, MD, PhD), City of Hope Board of Governors (John Yim, MD) and the Panda Charitable Foundation (John Yim, MD). Research reported in this publication included work performed in the cores supported by the National Cancer Institute of the National Institutes of Health under award number P30CA033572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions
Y.Y. and J.H.Y. developed the research idea, designed the experiments, analyzed the data and wrote the manuscript. W.W. designed and performed experiments, analyzed the data and wrote the manuscript. S.E.Y. analyzed the data and wrote the manuscript. Q.X. and J.Y. performed the experiments and analyzed the data. E.S.H. and J.M. analyzed and interpreted data. All authors approved the final manuscript.
Competing Interests
W.W., S.E.Y., Q.X., J.Y., E.S.H., J.M. and J.H.Y. declare no competing interests. Y.Y. has contracted clinical trials and research projects sponsored by Merck, Eisai, Novartis, Genentech, and Pfizer.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yuan Yuan, Email: yuyuan@coh.org.
John H. Yim, Email: jyim@coh.org
References
- 1.Kassam F, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clinical breast cancer. 2009;9:29–33. doi: 10.3816/CBC.2009.n.005. [DOI] [PubMed] [Google Scholar]
- 2.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 3.Robson Mark, Im Seock-Ah, Senkus Elżbieta, Xu Binghe, Domchek Susan M., Masuda Norikazu, Delaloge Suzette, Li Wei, Tung Nadine, Armstrong Anne, Wu Wenting, Goessl Carsten, Runswick Sarah, Conte Pierfranco. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. New England Journal of Medicine. 2017;377(6):523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 4.Sikov WM, et al. Impact of the Addition of Carboplatin and/or Bevacizumab to Neoadjuvant Once-per-Week Paclitaxel Followed by Dose-Dense Doxorubicin and Cyclophosphamide on Pathologic Complete Response Rates in Stage II to III Triple-Negative Breast Cancer: CALGB 40603 (Alliance) Journal of Clinical Oncology. 2015;33:13–21. doi: 10.1200/jco.2014.57.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Modi S, et al. A phase II trial of imatinib mesylate monotherapy in patients with metastatic breast cancer. Breast cancer research and treatment. 2005;90:157–163. doi: 10.1007/s10549-004-3974-0. [DOI] [PubMed] [Google Scholar]
- 6.O’shaughnessy J, et al. Preliminary results of a randomized phase II study of weekly irinotecan/carboplatin with or without cetuximab in patients with metastatic breast cancer. Breast Cancer Research and Treatment. 2007;106:S32–S33. [Google Scholar]
- 7.Baselga J, et al. Phase II and tumor pharmacodynamic study of gefitinib in patients with advanced breast cancer. Journal of Clinical Oncology. 2005;23:5323–5333. doi: 10.1200/JCO.2005.08.326. [DOI] [PubMed] [Google Scholar]
- 8.Finn RS, et al. Dasatinib as a single agent in triple-negative breast cancer: results of an open-label phase 2 study. Clinical Cancer Research. 2011;17:6905–6913. doi: 10.1158/1078-0432.CCR-11-0288. [DOI] [PubMed] [Google Scholar]
- 9.Traina, T. A. et al. Results from a phase 2 study of enzalutamide (ENZA), an androgen receptor (AR) inhibitor, in advanced AR+ triple-negative breast cancer (TNBC). Journal of Clinical Oncology33(15_suppl), 1003-1003 (2015).
- 10.Gucalp A, et al. Phase II trial of bicalutamide in patients with androgen receptor–positive, estrogen receptor–negative metastatic breast cancer. Clinical cancer research. 2013;19:5505–5512. doi: 10.1158/1078-0432.CCR-12-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nanda R, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. Journal of Clinical Oncology. 2016;34:2460–2467. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams S, Diamond J, Hamilton E, Pohlmann P, Tolaney S, Molinero L, Zou W, Liu B, Waterkamp D, Funke R, Powderly J. Abstract P2-11-06: Safety and clinical activity of atezolizumab (anti-PDL1) in combination with nab-paclitaxel in patients with metastatic triple-negative breast cancer. Cancer Research. 2016;76(4 Supplement):P2-11-06-P2-11-06. [Google Scholar]
- 13.Dirix Luc Y., Takacs Istvan, Jerusalem Guy, Nikolinakos Petros, Arkenau Hendrik-Tobias, Forero-Torres Andres, Boccia Ralph, Lippman Marc E., Somer Robert, Smakal Martin, Emens Leisha A., Hrinczenko Borys, Edenfield William, Gurtler Jayne, von Heydebreck Anja, Grote Hans Juergen, Chin Kevin, Hamilton Erika P. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Research and Treatment. 2017;167(3):671–686. doi: 10.1007/s10549-017-4537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams S, et al. Phase 2 study of pembrolizumab (pembro) monotherapy for previously treated metastatic triple-negative breast cancer (mTNBC): KEYNOTE-086 cohort A. Journal of Clinical Oncology. 2017;35:1008–1008. doi: 10.1200/JCO.2017.35.15_suppl.1008. [DOI] [Google Scholar]
- 15.Lehmann BD, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koboldt Daniel C., Fulton Robert S., McLellan Michael D., Schmidt Heather, Kalicki-Veizer Joelle, McMichael Joshua F., Fulton Lucinda L., Dooling David J., Ding Li, Mardis Elaine R., Wilson Richard K., Ally Adrian, Balasundaram Miruna, Butterfield Yaron S. N., Carlsen Rebecca, Carter Candace, Chu Andy, Chuah Eric, Chun Hye-Jung E., Coope Robin J. N., Dhalla Noreen, Guin Ranabir, Hirst Carrie, Hirst Martin, Holt Robert A., Lee Darlene, Li Haiyan I., Mayo Michael, Moore Richard A., Mungall Andrew J., Pleasance Erin, Gordon Robertson A., Schein Jacqueline E., Shafiei Arash, Sipahimalani Payal, Slobodan Jared R., Stoll Dominik, Tam Angela, Thiessen Nina, Varhol Richard J., Wye Natasja, Zeng Thomas, Zhao Yongjun, Birol Inanc, Jones Steven J. M., Marra Marco A., Cherniack Andrew D., Saksena Gordon, Onofrio Robert C., Pho Nam H., Carter Scott L., Schumacher Steven E., Tabak Barbara, Hernandez Bryan, Gentry Jeff, Nguyen Huy, Crenshaw Andrew, Ardlie Kristin, Beroukhim Rameen, Winckler Wendy, Getz Gad, Gabriel Stacey B., Meyerson Matthew, Chin Lynda, Park Peter J., Kucherlapati Raju, Hoadley Katherine A., Todd Auman J., Fan Cheng, Turman Yidi J., Shi Yan, Li Ling, Topal Michael D., He Xiaping, Chao Hann-Hsiang, Prat Aleix, Silva Grace O., Iglesia Michael D., Zhao Wei, Usary Jerry, Berg Jonathan S., Adams Michael, Booker Jessica, Wu Junyuan, Gulabani Anisha, Bodenheimer Tom, Hoyle Alan P., Simons Janae V., Soloway Matthew G., Mose Lisle E., Jefferys Stuart R., Balu Saianand, Parker Joel S., Neil Hayes D., Perou Charles M., Malik Simeen, Mahurkar Swapna, Shen Hui, Weisenberger Daniel J., Triche Jr Timothy, Lai Phillip H., Bootwalla Moiz S., Maglinte Dennis T., Berman Benjamin P., Van Den Berg David J., Baylin Stephen B., Laird Peter W., Creighton Chad J., Donehower Lawrence A., Getz Gad, Noble Michael, Voet Doug, Saksena Gordon, Gehlenborg Nils, DiCara Daniel, Zhang Juinhua, Zhang Hailei, Wu Chang-Jiun, Yingchun Liu Spring, Lawrence Michael S., Zou Lihua, Sivachenko Andrey, Lin Pei, Stojanov Petar, Jing Rui, Cho Juok, Sinha Raktim, Park Richard W., Nazaire Marc-Danie, Robinson Jim, Thorvaldsdottir Helga, Mesirov Jill, Park Peter J., Chin Lynda, Reynolds Sheila, Kreisberg Richard B., Bernard Brady, Bressler Ryan, Erkkila Timo, Lin Jake, Thorsson Vesteinn, Zhang Wei, Shmulevich Ilya, Ciriello Giovanni, Weinhold Nils, Schultz Nikolaus, Gao Jianjiong, Cerami Ethan, Gross Benjamin, Jacobsen Anders, Sinha Rileen, Arman Aksoy B., Antipin Yevgeniy, Reva Boris, Shen Ronglai, Taylor Barry S., Ladanyi Marc, Sander Chris, Anur Pavana, Spellman Paul T., Lu Yiling, Liu Wenbin, Verhaak Roel R. G., Mills Gordon B., Akbani Rehan, Zhang Nianxiang, Broom Bradley M., Casasent Tod D., Wakefield Chris, Unruh Anna K., Baggerly Keith, Coombes Kevin, Weinstein John N., Haussler David, Benz Christopher C., Stuart Joshua M., Benz Stephen C., Zhu Jingchun, Szeto Christopher C., Scott Gary K., Yau Christina, Paull Evan O., Carlin Daniel, Wong Christopher, Sokolov Artem, Thusberg Janita, Mooney Sean, Ng Sam, Goldstein Theodore C., Ellrott Kyle, Grifford Mia, Wilks Christopher, Ma Singer, Craft Brian, Yan Chunhua, Hu Ying, Meerzaman Daoud, Gastier-Foster Julie M., Bowen Jay, Ramirez Nilsa C., Black Aaron D., XPATH ERROR: unknown variable "tname". Robert E., White Peter, Zmuda Erik J., Frick Jessica, Lichtenberg Tara M., Brookens Robin, George Myra M., Gerken Mark A., Harper Hollie A., Leraas Kristen M., Wise Lisa J., Tabler Teresa R., McAllister Cynthia, Barr Thomas, Hart-Kothari Melissa, Tarvin Katie, Saller Charles, Sandusky George, Mitchell Colleen, Iacocca Mary V., Brown Jennifer, Rabeno Brenda, Czerwinski Christine, Petrelli Nicholas, Dolzhansky Oleg, Abramov Mikhail, Voronina Olga, Potapova Olga, Marks Jeffrey R., Suchorska Wiktoria M., Murawa Dawid, Kycler Witold, Ibbs Matthew, Korski Konstanty, Spychała Arkadiusz, Murawa Paweł, Brzeziński Jacek J., Perz Hanna, Łaźniak Radosław, Teresiak Marek, Tatka Honorata, Leporowska Ewa, Bogusz-Czerniewicz Marta, Malicki Julian, Mackiewicz Andrzej, Wiznerowicz Maciej, Van Le Xuan, Kohl Bernard, Viet Tien Nguyen, Thorp Richard, Van Bang Nguyen, Sussman Howard, Duc Phu Bui, Hajek Richard, Phi Hung Nguyen, Viet The Phuong Tran, Quyet Thang Huynh, Zaki Khan Khurram, Penny Robert, Mallery David, Curley Erin, Shelton Candace, Yena Peggy, Ingle James N., Couch Fergus J., Lingle Wilma L., King Tari A., Maria Gonzalez-Angulo Ana, Mills Gordon B., Dyer Mary D., Liu Shuying, Meng Xiaolong, Patangan Modesto, Waldman Frederic, Stöppler Hubert, Kimryn Rathmell W., Thorne Leigh, Huang Mei, Boice Lori, Hill Ashley, Morrison Carl, Gaudioso Carmelo, Bshara Wiam, Daily Kelly, Egea Sophie C., Pegram Mark D., Gomez-Fernandez Carmen, Dhir Rajiv, Bhargava Rohit, Brufsky Adam, Shriver Craig D., Hooke Jeffrey A., Leigh Campbell Jamie, Mural Richard J., Hu Hai, Somiari Stella, Larson Caroline, Deyarmin Brenda, Kvecher Leonid, Kovatich Albert J., Ellis Matthew J., King Tari A., Hu Hai, Couch Fergus J., Mural Richard J., Stricker Thomas, White Kevin, Olopade Olufunmilayo, Ingle James N., Luo Chunqing, Chen Yaqin, Marks Jeffrey R., Waldman Frederic, Wiznerowicz Maciej, Bose Ron, Chang Li-Wei, Beck Andrew H., Maria Gonzalez-Angulo Ana, Pihl Todd, Jensen Mark, Sfeir Robert, Kahn Ari, Chu Anna, Kothiyal Prachi, Wang Zhining, Snyder Eric, Pontius Joan, Ayala Brenda, Backus Mark, Walton Jessica, Baboud Julien, Berton Dominique, Nicholls Matthew, Srinivasan Deepak, Raman Rohini, Girshik Stanley, Kigonya Peter, Alonso Shelley, Sanbhadti Rashmi, Barletta Sean, Pot David, Sheth Margi, Demchok John A., Mills Shaw Kenna R., Yang Liming, Eley Greg, Ferguson Martin L., Tarnuzzer Roy W., Zhang Jiashan, Dillon Laura A. L., Buetow Kenneth, Fielding Peter, Ozenberger Bradley A., Guyer Mark S., Sofia Heidi J., Palchik Jacqueline D. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abramson VG, Lehmann BD, Ballinger TJ, Pietenpol JA. Subtyping of triple-negative breast cancer: implications for therapy. Cancer. 2015;121:8–16. doi: 10.1002/cncr.28914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon V, Banerji S. Molecular pathways: PI3K pathway targets in triple-negative breast cancers. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19:3738–3744. doi: 10.1158/1078-0432.CCR-12-0274. [DOI] [PubMed] [Google Scholar]
- 19.Luyimbazi, D. et al. Combination of eribulin and PI3K inhibitors in triple negative and HER2 expressing breast cancer cell lines results in synergistic growth inhibition and enhanced inhibition of the PI3K pathway. Cancer Res73(24 Suppl): Abstract nr P3-03-07 (2013).
- 20.Luyimbazi, D. et al. A comparison of PI3K inhibition by eribulin, other microtubule targeting agents and a DNA-damaging chemotherapeutic in triple negative and HER2 expressing breast cancer cell lines. Cancer Res73(24 Suppl): Abstract nr P3-03-08 (2013).
- 21.Vora SR, et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell. 2014;26:136–149. doi: 10.1016/j.ccr.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michaloglou C, et al. Combined Inhibition of mTOR and CDK4/6 Is Required for Optimal Blockade of E2F Function and Long-term Growth Inhibition in Estrogen Receptor–positive Breast Cancer. Molecular Cancer Therapeutics. 2018;17:908–920. doi: 10.1158/1535-7163.mct-17-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson N, Shapiro GI. Cyclin-dependent kinase 4/6 inhibition in cancer therapy. Cell Cycle. 2012;11:3913. doi: 10.4161/cc.22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13:417–430. doi: 10.1038/nrclinonc.2016.26. [DOI] [PubMed] [Google Scholar]
- 25.Asghar U, et al. Identification of subtypes of triple negative breast cancer (TNBC) that are sensitive to CDK4/6 inhibition. Journal of Clinical Oncology. 2015;33:11098–11098. doi: 10.1200/jco.2015.33.15_suppl.11098. [DOI] [Google Scholar]
- 26.Hosford SR, et al. Combined Inhibition of Both p110alpha and p110beta Isoforms of Phosphatidylinositol 3-Kinase Is Required for Sustained Therapeutic Effect in PTEN-Deficient, ER(+) Breast Cancer. Clin Cancer Res. 2017;23:2795–2805. doi: 10.1158/1078-0432.CCR-15-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elkabets M, et al. mTORC1 inhibition is required for sensitivity to PI3K p110alpha inhibitors in PIK3CA-mutant breast cancer. Science translational medicine. 2013;5:196–199. doi: 10.1126/scitranslmed.3005747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romano G, et al. A Preexisting Rare PIK3CAE545K Subpopulation Confers Clinical Resistance to MEK plus CDK4/6 Inhibition in NRAS Melanoma and Is Dependent on S6K1 Signaling. Cancer Discovery. 2018;8:556–567. doi: 10.1158/2159-8290.cd-17-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 30.Tan DS, et al. Triple negative breast cancer: molecular profiling and prognostic impact in adjuvant anthracycline-treated patients. Breast Cancer Res Treat. 2008;111:27–44. doi: 10.1007/s10549-007-9756-8. [DOI] [PubMed] [Google Scholar]
- 31.Costa Ricardo L. B., Han Hyo Sook, Gradishar William J. Targeting the PI3K/AKT/mTOR pathway in triple-negative breast cancer: a review. Breast Cancer Research and Treatment. 2018;169(3):397–406. doi: 10.1007/s10549-018-4697-y. [DOI] [PubMed] [Google Scholar]
- 32.Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. Journal of clinical oncology. 2006;24:1770–1783. doi: 10.1200/JCO.2005.03.7689. [DOI] [PubMed] [Google Scholar]
- 33.Lee HJ, et al. A selective cyclin-dependent kinase 4, 6 dual inhibitor, Ribociclib (LEE011) inhibits cell proliferation and induces apoptosis in aggressive thyroid cancer. Cancer Letters. 2018;417:131–140. doi: 10.1016/j.canlet.2017.12.037. [DOI] [PubMed] [Google Scholar]
- 34.Zhou J, et al. Palbociclib, a selective CDK4/6 inhibitor, enhances the effect of selumetinib in RAS-driven non-small cell lung cancer. Cancer Lett. 2017;408:130–137. doi: 10.1016/j.canlet.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton E, Infante JR. Targeting CDK4/6 in patients with cancer. Cancer treatment reviews. 2016;45:129–138. doi: 10.1016/j.ctrv.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Joseph RW, et al. PD-1 and PD-L1 Expression in Renal Cell Carcinoma with Sarcomatoid Differentiation. Cancer immunology research. 2015;3:1303–1307. doi: 10.1158/2326-6066.CIR-15-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Witkiewicz AK, Knudsen ES. Retinoblastoma tumor suppressor pathway in breast cancer: prognosis, precision medicine, and therapeutic interventions. Breast Cancer Res. 2014;16:207. doi: 10.1186/bcr3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Brien, N. et al. Preclinical activity of abemaciclib alone or in combination with anti-mitotic and targeted therapies in breast cancer. Molecular cancer therapeutics, molcanther. 0290.2017 (2018). [DOI] [PubMed]
- 39.Yuan Y, Mortimer J, Xing Q, Yan J, Wen W, Han E, Yim JH. Abstract P3-03-15: Synergistic suppression of triple negative breast cancer with the combination of PI3K inhibitor (alpelisib, BYL719) and CDK inhibitor (ribociclib, LEE011) Cancer Research. 2017;77(4 Supplement):P3-03-15-P3-03-15. [Google Scholar]
- 40.Yuan, Y et al. Abstract: CDK 4/6 Inhibitors Beyond Estrogen Receptor Positive Breast Cancer: Synergistic Effect of PI3K and CDK4/6 in Triple Negative Breast Cancer. Cancer Research and Targeted Therapy-2018, 42 (2018).
- 41.Chalhoub N, Baker SJ. PTEN and the PI3-Kinase Pathway in Cancer. Annual Review of Pathology: Mechanisms of Disease. 2009;4:127–150. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wee S, et al. PTEN-deficient cancers depend on PIK3CB. Proceedings of the National Academy of Sciences. 2008;105:13057–13062. doi: 10.1073/pnas.0802655105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jia Shidong, Liu Zhenning, Zhang Sen, Liu Pixu, Zhang Lei, Lee Sang Hyun, Zhang Jing, Signoretti Sabina, Loda Massimo, Roberts Thomas M., Zhao Jean J. Essential roles of PI(3)K–p110β in cell growth, metabolism and tumorigenesis. Nature. 2008;454(7205):776–779. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J, et al. CRKL Mediates p110β-Dependent PI3K Signaling in PTEN-Deficient Cancer Cells. Cell Reports. 2017;20:549–557. doi: 10.1016/j.celrep.2017.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwartz S, et al. Feedback suppression of PI3Kα signaling in PTEN-mutated tumors is relieved by selective inhibition of PI3Kβ. Cancer cell. 2015;27:109–122. doi: 10.1016/j.ccell.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yap TA, Bjerke L, Clarke PA, Workman P. Drugging PI3K in cancer: refining targets and therapeutic strategies. Current Opinion in Pharmacology. 2015;23:98–107. doi: 10.1016/j.coph.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Asghar US, et al. Single-cell dynamics determines response to CDK4/6 inhibition in triple-negative breast cancer. Clinical Cancer Research. 2017;23:5561–5572. doi: 10.1158/1078-0432.CCR-17-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teo Zhi Ling, Versaci Stephanie, Dushyanthen Sathana, Caramia Franco, Savas Peter, Mintoff Chris P., Zethoven Magnus, Virassamy Balaji, Luen Stephen J., McArthur Grant A., Phillips Wayne A., Darcy Phillip K., Loi Sherene. Combined CDK4/6 and PI3Kα Inhibition Is Synergistic and Immunogenic in Triple-Negative Breast Cancer. Cancer Research. 2017;77(22):6340–6352. doi: 10.1158/0008-5472.CAN-17-2210. [DOI] [PubMed] [Google Scholar]
- 49.Bonelli MA, et al. Combined Inhibition of CDK4/6 and PI3K/AKT/mTOR Pathways Induces a Synergistic Anti-Tumor Effect in Malignant Pleural Mesothelioma Cells. Neoplasia. 2017;19:637–648. doi: 10.1016/j.neo.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pikman Y, et al. Synergistic Drug Combinations with a CDK4/6 Inhibitor in T-cell Acute Lymphoblastic Leukemia. Clin Cancer Res. 2017;23:1012–1024. doi: 10.1158/1078-0432.CCR-15-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Divakar SKA, et al. Dual inhibition of CDK4/Rb and PI3K/AKT/mTOR pathways by ON123300 induces synthetic lethality in mantle cell lymphomas. Leukemia. 2016;30:86–93. doi: 10.1038/leu.2015.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu Jing, Steeg Patricia S., Price Janet E., Krishnamurthy Savitri, Mani Sendurai A., Reuben James, Cristofanilli Massimo, Dontu Gabriela, Bidaut Luc, Valero Vicente, Hortobagyi Gabriel N., Yu Dihua. Breast Cancer Metastasis: Challenges and Opportunities. Cancer Research. 2009;69(12):4951–4953. doi: 10.1158/0008-5472.CAN-09-0099. [DOI] [PubMed] [Google Scholar]
- 53.Dean JL, McClendon AK, Knudsen ES. Modification of the DNA damage response by therapeutic CDK4/6 inhibition. Journal of Biological Chemistry. 2012;287:29075–29087. doi: 10.1074/jbc.M112.365494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeMichele A, et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clinical Cancer Research. 2015;21:995–1001. doi: 10.1158/1078-0432.CCR-14-2258. [DOI] [PubMed] [Google Scholar]
- 55.Zhang H, et al. Patient-derived xenografts of triple-negative breast cancer reproduce molecular features of patient tumors and respond to mTOR inhibition. Breast cancer research: BCR. 2014;16:R36. doi: 10.1186/bcr3640. [DOI] [PMC free article] [PubMed] [Google Scholar]