Abstract
PRRT2 pathogenic variants have been described in benign familial infantile epilepsy, episodic ataxia, paroxysmal kinesigenic dyskinesia, and hemiplegic migraines.
We describe a patient with compound heterozygous variants, infantile epilepsy with status epilepticus, paroxysmal dyskinesia and episodic ataxia.
Testing revealed a pathogenic PRRT2 duplication (c.649dupC), and a likely pathogenic missense variant (c.916G>A).
His presentation meets the severe phenotypic category with a combination of at least 3 neurological symptoms: seizures and status epilepticus, prolonged episodic ataxia, and paroxysmal dyskinesia. This further expands the clinical findings related to PRRT2, and suggests that compound heterozygous variants could confer a severe phenotype.
Keywords: Epilepsy, Kinesigenic dyskinesia, Ataxia, PRRT2, Genetic epilepsies
Note:
Throughout this manuscript, we have adopted the use of the term “genetic variant,” rather than referring to DNA changes as “mutations,” in keeping with the most recent guidelines of the American College of Medical Genetics and Genomics (ACMG) [1]. Depending on many factors, including predicted effect on the corresponding protein, conservation of the amino acid involved, and presence or absence in published control populations, genetic variants may be considered to be pathogenic, likely pathogenic, benign, and likely benign. Variants of unknown significance are variants with intermediate predictions that have not been established the normal population nor in a specific disease setting.
1. Introduction
Pathogenic variants (previously referred to conventionally as mutations) in the proline-rich transmembrane protein (PRRT2) gene were first described in individuals with paroxysmal kinesigenic dyskinesia (PKD) [2], [3]. Shortly afterward, heterozygous PRRT2 variants were associated with benign familial infantile epilepsy (BFIE) and infantile convulsions with choreoathetosis (ICCA) [4]. The phenotypic spectrum of PRRT2 was further expanded by the report of isolated BFIE without co-occurring movement disorders, making PKD and BFIE “two faces of the same coin” [5]. Other reported PRRT2-related phenomena, whether in isolation or in combination with PKD and/or BFIE, include paroxysmal torticollis, hemiplegic migraine, episodic ataxia, and fever-related infantile seizures [6], [7].
The PRRT2 gene is located in the 16p11.2 chromosome region. Copy number variations in this region are associated with neuropsychiatric symptoms, including autism spectrum disorders [8]. There is an ongoing study of the presence of seizures and movement disorders in patients with autism who have deletions and duplications of 16p11.2 (Unpublished, Rosen AR. et al., American Epilepsy Society Meeting, 2013).
In the vast majority of PRRT2-related cases with epilepsy, the seizure course is reported to be relatively benign, with brief seizures and resolution within the first 2–3 years of life. However, there is variability in seizure severity. For example, in a report of 5 Spanish families, 36% of individuals with PRRT2 variants had an atypical course with either neonatal onset, recurrent seizures into adulthood, learning difficulties, or mild hemiparesis [9]. Two patients with homozygous variants in PRRT2 (c.649dupC) have been reported in the literature to have a more severe phenotype than their family members with the c.649dupC variant in the heterozygous state, with a combination of phenotypes including BFIE, PKD, episodic ataxia, and developmental delay [10]. More recently, another study reported 5 additional patients, 3 with homozygous c.649dupC, one patient with a homozygous missense variant (c.913G>A), as well as one patient with concurrent c.649dupC and whole gene deletion of PRRT2 [[11]. These patients all had relatively severe neurological manifestations, including at least three forms of paroxysmal neurological disorders within the same patient, longer than usual episodes of ataxia, or permanent neurological disorders including learning difficulties (4 patients) and cerebellar atrophy (2 patients).
We report the case of a 4-year-old patient with compound heterozygous PRRT2 variants, one maternally and one paternally inherited. He presented with an initially severe course consisting of infantile onset epilepsy, including status epilepticus, with prolonged episodes of post-ictal ataxia. He later went on to experience ataxia after minor head trauma, paroxysmal dyskinesia, yet remarkably preserved neurodevelopment.
The purpose of this case report is to highlight the pleiotropy and variability of phenotypic presentation and severity associated with variants of the PRRT2 gene, and to provide additional data regarding the phenotypic spectrum of compound heterozygous variants. Additionally, this case provides an example of both the importance and potential pitfalls of genetic testing.
2. Case description
2.1. Past medical and family history
The patient was born at full term after an uncomplicated pregnancy. He attained early developmental milestones on time.
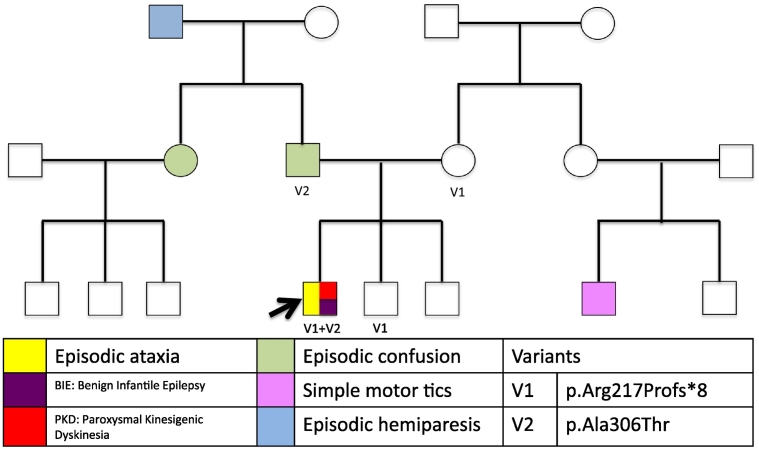
The family history is positive for episodes of aphasia with a feeling of doom in his father; these episodes have not been more precisely characterized though seizure vs. panic disorder has been suspected. There was a history of episodes of recurrent transient right-sided weakness in his paternal grandfather and tic disorder in a maternal cousin. The patient's mother and two younger brothers are healthy (Fig. 1).
Fig. 1.
Pedigree, including the different phenotypes, and the variants on all tested individuals.
2.2. Presenting symptoms: focal epilepsy with status epilepticus
The patient was previously well and presented with his first seizure at 3 months of age. He presented to the emergency department after two events of head and eye deviation to the right followed by right facial twitching, right arm twitching, and bilateral upper extremity stiffening, each lasting for 2 min. He received intravenous lorazepam on arrival, but he had two more events shortly afterwards. He was started on phenobarbital at an initial dose of 3 mg/kg/day. He had a single seizure at 4 months of age in the setting of a low phenobarbital level (21 mcg/ml); the dose was increased to 4.5 mg/kg/day with levels of 30 mcg/ml on average. Over the following 20 months, he had 4 more seizure clusters (3–4 seizures each), in addition to an isolated seizure at 20 months of age, all seemingly provoked (following immunization, viral gastroenteritis, upper respiratory infection, pneumonia, and minor head trauma). On at least 3 occasions, these met the criteria for status epilepticus as he did not return to baseline between seizures and required admission to the intensive care unit. While his initial seizure semiology consisted of right sided facial twitching followed by right-sided arm and leg clonic movements, followed by right-sided transient weakness, at least one seizure was reported as left sided predominant twitching. He had his last seizure at age 2 years. He was maintained on phenobarbital throughout the first 2 years of life; levetiracetam was added after one hospital stay for seizures at 16 months of age and ultimately increased to 77 mg/kg/day, with a maximum level of 40 mcg/ml.
During one of his admissions for breakthrough seizures, he developed unilateral 6th cranial nerve palsy and had an elevated opening pressure on his lumbar puncture of 44 cm H20, and a closing pressure of 15 cm H2O. He was therefore started on acetazolamide 100 mg BID with resolution of the 6th nerve palsy within days. He has had no other signs of increased intracranial pressure.
2.3. Ataxia provoked by seizures and head trauma
In addition to seizures and dyskinesia, he experienced episodes of prolonged ataxia that started at nearly 2 years of age either occurring spontaneously or following clusters of seizures, or minor head trauma. The episodes consisted of ataxia and inability to walk, with or without nausea and vomiting, with the ataxia being continuous or paroxysmal at times. On average, the episodes lasted for one to 3 weeks.
2.4. Paroxysmal dyskinesia
At 3 years of age, he developed frequent episodes of eye deviation with asymmetric facial contortion, occurring in clusters and concerning clinically for asymmetric epileptic spasms. There was no epileptiform correlate on EEG. As the episodes evolved, they appeared to be clinically consistent paroxysmal dyskinesia. He was started on oxcarbazepine, with near-total resolution of these episodes. His medication regimen currently consists of oxcarbazepine alone (28 mg/kg/day). Phenobarbital was discontinued at 2 years of age. Levetiracetam and acetazolamide were discontinued at 2 and 2.5 years of age respectively.
2.5. Development
Our patient had normal development upon his initial presentation and has continued to meet his developmental milestones appropriately. At five years of age, he is doing well in preschool, with no services required. He can recognize letters, identify colors, count to 20, and write his name.
2.6. Investigations
He underwent an extensive initial work up including a brain MRI, which showed no cortical abnormalities and an incidental left cerebellar developmental venous anomaly. MRI has been stable over time, and has been performed at the following ages: 3 months, 16 months and 21 months with MR angiography, and lastly at 4 years of age. The vast majority of his EEGs have been normal. No seizures have been captured on EEG. On one occasion, left fronto-central spikes were found at 2 years of age, and on another occasion, he had continuous generalized slowing the same year, however this was in the post-ictal setting after he had received multiple doses of a benzodiazepine.
A comprehensive metabolic work up, including urine organic acids, serum, urine and CSF amino acids, serum and CSF lactic acid, serum acylcarnitine profile, urine oligosaccharides and glycan screening, CSF 5-methyltetrahydrofolate was unrevealing. CSF to serum glucose ratio was 0.79, and the absolute CSF glucose was 73. Initial genetic testing with chromosomal microarray and familial hemiplegic migraine panel (CACNA1A, ATP1A2 and SCN1A) was negative. Ultimately, an infantile epilepsy panel utilizing next generation sequencing as well as comparative genomic hybridization revealed 4 variants (2 in the PRRT2 gene, and one in each of RELN and SCN9A genes). The missense variant in RELN gene (c.9340A>G, p.Ile3114Val), is of unknown clinical significance. This variant is reported in the heterozygous and homozygous forms in at least 47 and 1 individual respectively in the ExAC database (http://exac.broadinstitute.org/, last accessed 01/24/2016). Homozygous variants in this gene cause lissencephaly, though a recent report suggests a possible phenotype of lateral temporal lobe epilepsy in individuals with heterozygous RELN variants [12]. The isoleucine to valine substitution is better tolerated as both are branched chain amino acids. The isoleucine, however, is in a highly conserved position across species. The missense variant in SCN9A (c.2215A>G, p.Ile739Val), was reported in the heterozygous state in at least 168 healthy individuals in the ExAC database, but also reported in one individual with small fiber neuropathy (evs.gs.washington.edu/EVS). The isoleucine to valine substitution is better tolerated as both are branched chain amino acids. The isoleucine, however, is in a highly conserved position across species. Base on the available information these two variants were considered not to be of clinical significance.
The two variants found in the PRRT2 gene were of clinical interest and therefore were tested in the patient's parents (Table 1).
Table 1.
Details of the PRRT2 variants found on the epilepsy gene panel:
| Gene | Variant | Amino acid change | Carriers |
|---|---|---|---|
| PRRT2 | Ex2 c.649dupC1 | p.Arg217Profs*8 | Mother, brother |
| PRRT2 | Ex3 c.916G > A2 | p.Ala306Thr | Father |
1-This is a frame-shift variant resulting in protein truncation, leading to loss of the trans-membrane segment, and is a well-established pathogenic variant [2].
2-This is a missense variant, the alanine to threonine substitution is likely tolerated given similar physical and chemical properties. The amino acid is in a highly conserved position across species [14]. This variant was not found in the Exome Aggregation Consortium (ExAC, http://exac.broadinstitute.org/, last accessed 01/24/2016) data and therefore is not a known polymorphism.
3. Discussion
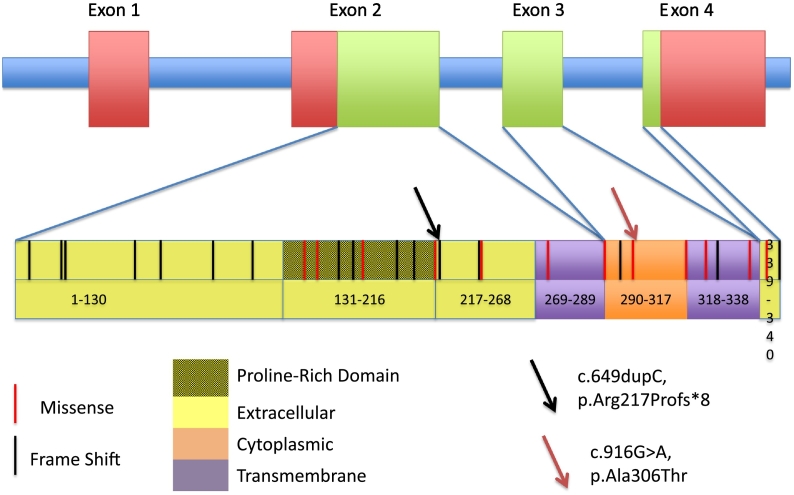
The phenotypes associated to PRRT2 are typically associated with heterozygous variants that can be inherited with an autosomal dominant pattern or arise de novo. The PRRT2 gene is located on the short (p) arm of chromosome 16 and consists of 4 exons encoding a 340-amino acid protein, forming two extracellular domains (exons 2 and 4), two transmembrane domains (exons 2&3) and one cytoplasmic domain (exon 3) (Fig. 2). PRRT2 mRNA is highly expressed in the nervous system, but the exact function of the protein is unknown.
Fig. 2.
Representation of the PRRT2 gene and protein, all mutations reported to date, and the location of the patient's pathogenic variant (p.Arg217Profs*8) and likely pathogenic variant p.Ala306Thr.
Our patient had two inherited PRRT2 variants, including one that has been reported many times in symptomatic individuals. His constellation of features can be considered severe, with the following three major PRRT2-related disorders present: (1) recurrent episodes of status epilepticus, akin to the infantile convulsions presentation reported; (2) episodic ataxia with prolonged episodes lasting on average one to three weeks before ambulation is recovered, typically occurring after seizures or minor head trauma; and (3) paroxysmal dyskinesia that has responded well to low-dose oxcarbazepine. Notably, despite having two variants and this constellation of many PRRT2-related symptoms, his developmental progress has been excellent.
Our patient has a maternally inherited PRRT2 variant (c.649dupC), a published variant reported as a disease-causing mutation in the literature. His additional PRRT2 variant (c.916G>A) is inherited from his father. A similar variant with the same amino acid change, (c.916G>C, p.Ala306Thr) has been reported in a patient and her mother who both had BFIE, but functional analysis is not available [13]. Given the conservation of the amino acid and the rarity of this variant, we consider this variant as a likely pathogenic variant.
While a single variant, particularly the c.649dupC previously reported in the literature, might be sufficient to explain our patient's multiple symptoms, we hypothesize that the multiplicity and severity of his symptoms is related to the presence of the second variant in PRRT2. Our case illustrates the many syndromes that can be associated with PRRT2, a pleotropic gene. This also suggests that compound heterozygosity of pathogenic variants in this gene might confer a more severe phenotype, as seen in the previously discussed case series reporting on 5 additional patients with relatively severe neurological manifestations, 4 with homozygous pathogenic variant and one with concurrent c.649dupC and whole gene deletion of PRRT2 [[11].
It is unclear why, while intellectual disability was reported in that case series, our patient has completely preserved development. It may be that our patient has some additional protective factor(s) preserving his intellectual function. At this point, we can only speculate that this may be due to the particular unique combination of variants and their effect on the PRRT2 protein vs. additional non-PRRT2-related factors.
Our findings highlight the importance of genetic testing on many different levels. Knowledge of the patient's etiology led to the use and optimization of oxcarbazepine with an improvement in his dyskinesia, as response to carbamazepine in patients with PRRT2-related paroxysmal dyskinesia has been reported [14]. Oxcarbazepine and carbamazepine share structural and therapeutic indication similarities, but the first was chosen over the latter in this situation as it has a more tolerated side effect profile. On another level, the genetic diagnosis provided more information regarding the expected range of clinical symptoms including the patient's recurrent episodic ataxia and paroxysmal dyskinesia, and prevented multiple unnecessary and at times invasive procedures such as lumbar punctures and MRIs under sedation as well as exposure to additional anti-seizure medications. Knowing that the symptoms he experienced, however severe at the time, had been seen before in the context of PRRT2 and that recovery could be expected from the paroxysmal seizures and ataxia provided the ability to be optimistic in prognosis.
Our case leaves some unanswered questions. While the c.649dupC variant is an established pathogenic variant, the patient's mother and 2-year-old brother are both seemingly unaffected carriers. While incomplete penetrance is not infrequently encountered in dominantly inherited epilepsies and other paroxysmal disorders, it raises the question of whether this variant in this family's genetic background is sufficient to produce any of the PRRT2-related phenotypes. We thus invoke the hypothesis that the second PRRT2 variant is functioning as a “second hit,” inherited from our patient's father whose phenotype suggests the possibility of a paroxysmal disorder. The lack of precision in his father's diagnosis and the inability to ascertain the paternal grandfather's genotype reflect the complexities and realities of phenotype-genotype correlation in epilepsy genetics.
Finally, our patient's fully preserved development presents an important lesson in terms of prognosis. Given his early presentation with status epilepticus, prolonged episodes of ataxia, and multiple presentations with dyskinesia, there was early concern about developmental prognosis, even prior to a genetic diagnosis. Nonetheless, by history and regular follow-up, his normal developmental trajectory bodes well without any indication of regression or stagnation of development. Once a genetic diagnosis was made, in the face of an expanding PRRT2 literature, particularly with two variants, concern for developmental consequences as reported in the literature was again tempered by his continued normal developmental progress. This feature, a relief certainly to his family and clinicians, cannot be overemphasized. Thus, while we extoll the benefits of having a genetic diagnosis for all of the reasons discussed above, we must add that knowing that he had a non-progressive genetic disorder and that he continued to make normal developmental progress allowed us to maintain an optimistic and yet cautious outlook for his ongoing cognitive and motor development.
Contributor Information
Christelle Moufawad El Achkar, Email: christelle.achkar@childrens.harvard.edu.
Beth Rosen Sheidley, Email: beth.sheidley@childrens.harvard.edu.
Declan O'Rourke, Email: declan.orourke@cuh.ie.
Masanori Takeoka, Email: masanori.takeoka@childrens.harvard.edu.
Annapurna Poduri, Email: annapurna.poduri@childrens.harvard.edu.
References
- 1.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Committee ALQA Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen W.J., Lin Y., Xiong Z.Q., Wei W., Ni W., Tan G.H. Exome sequencing identifies truncating mutations in PRRT2 that cause paroxysmal kinesigenic dyskinesia. Nat Genet. 2011;43:1252–1255. doi: 10.1038/ng.1008. [DOI] [PubMed] [Google Scholar]
- 3.Wang J.L., Cao L., Li X.H., Hu Z.M., Li J.D., Zhang J.G. Identification of PRRT2 as the causative gene of paroxysmal kinesigenic dyskinesias. Brain. 2011;134:3493–3501. doi: 10.1093/brain/awr289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heron S.E., Grinton B.E., Kivity S., Afawi Z., Zuberi S.M., Hughes J.N. PRRT2 mutations cause benign familial infantile epilepsy and infantile convulsions with choreoathetosis syndrome. Am J Hum Genet. 2012;90:152–160. doi: 10.1016/j.ajhg.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt A., Kumar K.R., Redyk K., Grünewald A., Leben M., Münchau A. Two faces of the same coin: benign familial infantile seizures and paroxysmal kinesigenic dyskinesia caused by PRRT2 mutations. Arch Neurol. 2012;69:668–670. doi: 10.1001/archneurol.2012.187. [DOI] [PubMed] [Google Scholar]
- 6.Gardiner A.R., Bhatia K.P., Stamelou M., Dale R.C., Kurian M.A., Schneider S.A. PRRT2 gene mutations: from paroxysmal dyskinesia to episodic ataxia and hemiplegic migraine. Neurology. 2012;79:2115–2121. doi: 10.1212/WNL.0b013e3182752c5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheffer I.E., Grinton B.E., Heron S.E., Kivity S., Afawi Z., Iona X. PRRT2 phenotypic spectrum includes sporadic and fever-related infantile seizures. Neurology. 2012;79:2104–2108. doi: 10.1212/WNL.0b013e3182752c6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Angelo D., Lebon S., Chen Q., Martin-Brevet S., Snyder L.G., Hippolyte L. Cardiff University experiences of children with copy number variants (ECHO) study tpEC, and the Simons variation in individuals project (VIP) consortium. Defining the effect of the 16p11.2 duplication on cognition, behavior, and medical comorbidities. JAMA Psychiat. 2016;73:20–30. doi: 10.1001/jamapsychiatry.2015.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guerrero-López R., Ortega-Moreno L., Giráldez B.G., Alarcón-Morcillo C., Sánchez-Martín G., Nieto-Barrera M. Atypical course in individuals from Spanish families with benign familial infantile seizures and mutations in the PRRT2 gene. Epilepsy Res. 2014;108:1274–1278. doi: 10.1016/j.eplepsyres.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Labate A., Tarantino P., Viri M., Mumoli L., Gagliardi M., Romeo A. Homozygous c.649dupC mutation in PRRT2 worsens the BFIS/PKD phenotype with mental retardation, episodic ataxia, and absences. Epilepsia. 2012;53:e196–e199. doi: 10.1111/epi.12009. [DOI] [PubMed] [Google Scholar]
- 11.Delcourt M., Riant F., Mancini J., Milh M., Navarro V., Roze E. Severe phenotypic spectrum of biallelic mutations in PRRT2 gene. J Neurol Neurosurg Psychiatry. 2015;86:782–785. doi: 10.1136/jnnp-2014-309025. [DOI] [PubMed] [Google Scholar]
- 12.Dazzo E., Fanciulli M., Serioli E., Minervini G., Pulitano P., Binelli S. Heterozygous reelin mutations cause autosomal-dominant lateral temporal epilepsy. Am J Hum Genet. 2015;96:992–1000. doi: 10.1016/j.ajhg.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marini C., Conti V., Mei D., Battaglia D., Lettori D., Losito E. PRRT2 mutations in familial infantile seizures, paroxysmal dyskinesia, and hemiplegic migraine. Neurology. 2012;79:2109–2114. doi: 10.1212/WNL.0b013e3182752ca2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H.F., Chen W.J., Ni W., Wang K.Y., Liu G.L., Wang N. PRRT2 mutation correlated with phenotype of paroxysmal kinesigenic dyskinesia and drug response. Neurology. 2013;80:1534–1535. doi: 10.1212/WNL.0b013e31828cf7e1. [DOI] [PubMed] [Google Scholar]