Abstract
In vitro and animal models suggest that obstructive sleep apnea (OSA) increases cancer risk. However, the impact of OSA severity on cancer risk is poorly understood.
We conducted a case-cohort study (a variant of the case-control study design), nested in a cohort of patients with a clinical diagnosis of OSA. OSA patients diagnosed between 2005 and 2013 were linked to a population-based cancer registry to identify cancers diagnosed subsequent to OSA between 2005 and 2015. Medical records were reviewed for a representative sample of 1162 OSA patients from this cohort (including 24 with subsequent cancer), and for an additional 304 OSA patients diagnosed with cancer; information regarding OSA severity indicators, including apnea-hypopnea index (AHI) was abstracted from these records. Adjusted Cox proportional hazards regression were used to calculate hazard ratios (HR) and 95% confidence intervals (CI) for associations of OSA severity indicators on cancer incidence.
Compared with individuals in the lowest AHI category (5–14.9), indicating mild OSA, the adjusted HR (95% CI) for cancer incidence associated with having moderate (15–29.9) or severe (30+) OSA were 0.72 (0.40–1.29) and 0.87 (0.52–1.45) respectively. Associations with other severity indicators were not significantly associated with cancer. However, the proportion of patients with severe OSA (AHI ≥30) was consistently higher across numerous cancer sites relative to the subcohort, suggesting increased cancer risk relative to patients with less severe OSA.
The absence of significant associations with OSA severity measures suggest that the underlying mechanisms deserve further investigation.
Keywords: Epidemiology, Intermittent hypoxia, Cancer development
1. Introduction
Obstructive sleep apnea (OSA) is a sleep-related breathing disorder affecting 25 million US adults, and is characterized by recurrent upper airway collapse during sleep, leading to episodic hypoxia and recurrent arousals (Peppard et al., 2013). OSA is strongly associated with systemic hypertension (Konecny et al., 2014) and is also independently associated with increased risk for Type 2 diabetes, cardiovascular disease, depression, motor vehicle accidents, and substantially elevated healthcare utilization and costs (Konecny et al., 2014; Acker et al., 2016; Barger et al., 2015; Kendzerska et al., 2014a; Kao et al., 2015).
The potential existence of a significant relationship between OSA and cancer has gained increasing attention in the last decade, with evidence suggesting that OSA is both a risk factor for cancer development (Toffoli and Michiels, 2008; Nanduri & Prabhakar, 2014; Almendros et al., 2012a) and a marker of adverse cancer prognosis (Akbarpour et al., 2017; Khalyfa et al., 2016; Santamaria-Martos et al., 2018; Cubillos-Zapata et al., 2017; Almendros et al., 2014; Li et al., 2016; Miao et al., 2014). Animal and in-vitro cancer models have shown that controlled exposures to chronic intermittent hypoxia and sleep fragmentation can trigger oxidative stress and systemic inflammation, and can promote enhanced activity of oncogenic pathways (Santamaria-Martos et al., 2018; Li et al., 2016; Gozal et al., 2015; Wan et al., 2011).
The Wisconsin Sleep Cohort (Nieto et al., 2012) was the first human study to note an elevation in cancer mortality among individuals with severe OSA relative to those without sleep disordered breathing. Subsequently, both a community-based (Marshall et al., 2014) and clinic-based (Martinez-Garcia et al., 2014a) cohort reported elevated cancer mortality in those diagnosed with OSA. The relationship between OSA and cancer incidence in humans, however, remains inconclusive. While four prior studies found higher overall cancer incidence rates among those patients with OSA versus those without OSA (Marshall et al., 2014; Fang et al., 2015; Campos-Rodriguez et al., 2013; Christensen et al., 2013), two more recent studies showed no evidence of elevated risk (Gozal et al., 2016; Kendzerska et al., 2014b). We recently reported our findings suggesting an elevated cancer burden, particularly at certain organ sites, among individuals with a diagnosis of sleep apnea (Sillah et al., 2018). In another recent study, the severity of OSA was shown to be independently associated with greater aggressiveness of cutaneous melanoma in a cohort of patients evaluated for OSA at the time of cancer diagnosis, suggesting a dose-response relationship between OSA severity and cancer progression and outcomes (Martinez-Garcia et al., 2018).
Building on aforementioned work, we evaluated the potential relationship of OSA severity and treatment with cancer incidence in a cohort of individuals initially diagnosed with OSA. To this effect, we explored five OSA severity indicators to investigate the hypothesis that exposure to severe OSA would be associated with increased cancer risk.
2. Methods
2.1. Study design
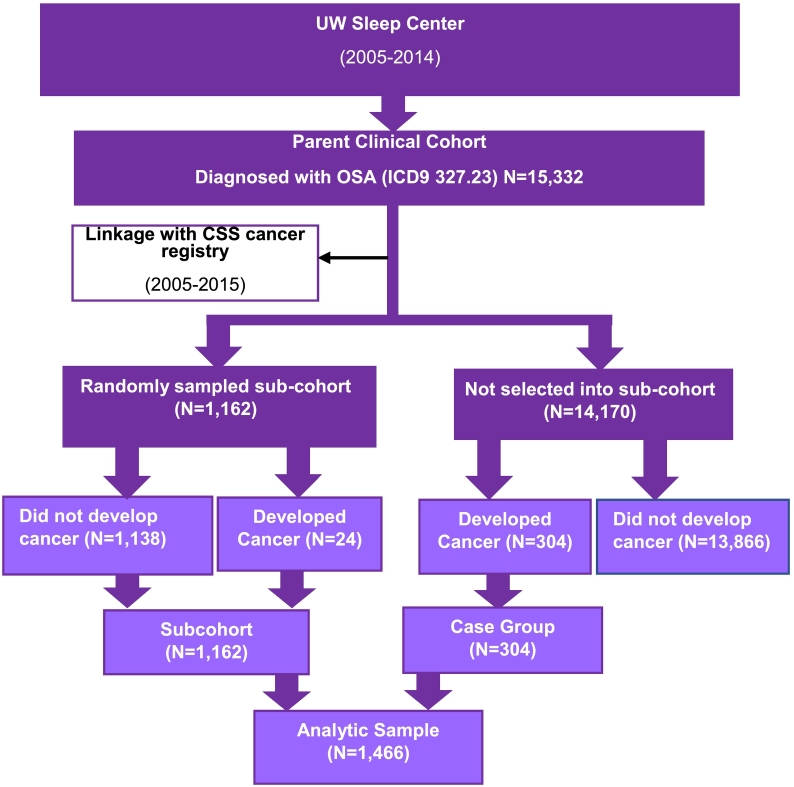
We conducted a case-cohort study (a variant of the case-control study design) (Langholz, 2014; Ernster, 1994), nested within a cohort of individuals identified as suffering from OSA, based on the recording of an ICD diagnostic code, in the University of Washington Medicine (UWM) system (Fig. 1). As described elsewhere, we linked this cohort with a population-based cancer registry covering the same geographic region as the UWM system in Western Washington State (Sillah et al., 2018). For the present nested study, we selected a random sample of the overall OSA cohort (free of cancer at time of OSA diagnosis, N = 1162); 24 OSA patients selected into the subcohort were diagnosed with cancer after their OSA diagnosis. We then included all other individuals within our parent OSA cohort who had received a cancer diagnosis subsequent to their OSA diagnosis (i.e., cases, n = 304) resulting in 328 total subsequent cancer cases. Consistent with the composition of the case group, random sampling of the subcohort was prioritized to individuals with OSA diagnostic codes recorded from encounters within the sleep medicine facilities of UWM system (n = 205 cases, n = 784 subcohort), followed by those with OSA diagnostic codes recorded from encounters with otolaryngology, pulmonary, cardiology, or neurology clinics (n = 99 cases, n = 378 subcohort). We prioritized sampling from the UWM Sleep Center because these patients were more likely to have a polysomnography (PSG) or home sleep apnea test report included in their medical records. As described below, detailed medical record abstraction was conducted for all members of the case-cohort study population. The case-cohort approach is efficient since not all members of the parent cohort (n = 15,332 vs study sample, n = 1466) required detailed medical record abstraction; use of this strategy also reduced selection and information bias since the cancer cases and randomly selected subcohort comes from the same population.
Fig. 1.
Case-cohort design of linked SEER population database and OSA clinical cohort.
2.2. Medical record abstraction
All study participants had a diagnosis code for OSA based on International Classification of Diseases, Ninth Revision, Clinical Modification codes [ICD-9-CM] (codes 327.23) recorded in medical records between 2005 and 2014 within UWM system. We reviewed medical records from the time of OSA diagnosis date to retrieve PSG reports related to the diagnosis of OSA. From these reports, we extracted information on five commonly used OSA severity indicators that are routinely collected on PSG (Table 1). Severity on each of these metrics was categorized into mild, moderate, or severe, based on clinical guidelines and prior publications (Anon, 1999); these metrics were also evaluated as continuous measures. For individuals with multiple OSA diagnoses recorded over the study period, the first such documentation was selected as their index diagnosis; if no PSG could be retrieved from the time of this initial diagnosis, subsequent records were reviewed for relevant PSG reports.
Table 1.
OSA severity measures defined based on clinical guidelines and prior studies.
| Measure | Definition | Categories |
|---|---|---|
| AHI | # apnea or hypopnea events per hour of sleep | Mild = 5–14.9 |
| Moderate = 15–29.9 | ||
| Severe > 30 | ||
| Hypoxemic burden, Tsat90 | % of sleep time spent with oxygen saturation levels <90% | Mild ≤ 1.2% |
| Moderate = 1.2–12% | ||
| Severe ≥ 12% | ||
| Oxygen desaturation index, ODI3 | # times per hour sleep that blood oxygen levels drop by 3% | Mild/moderate/severe (defined by tertiles) |
| Oxygen saturation nadir (lowest_satO2) | Lowest level of oxygen saturation observed | Mild = 86–90% |
| Moderate = 80–85% | ||
| Severe ≤ 80% | ||
| Arousal index | # arousals per hour of sleep | Mild ≤ 20 |
| Moderate = 21–40 | ||
| Severe ≥ 40 |
In addition to OSA severity indicators, we abstracted additional information regarding patient characteristics from medical records and administrative databases including: age at sleep apnea diagnosis, sex, race (White, Black and others), Hispanic ethnicity (Hispanic, non-Hispanic), healthcare utilization, smoking history (never smoker, former smoker, current smoker), and body mass index (BMI). Healthcare utilization was categorized according to the total number of healthcare visits to UWM facilities and providers in the five years subsequent to OSA diagnosis, including outpatient clinic visits and inpatient visits (categorized into <5 or ≥5).
2.3. Statistical methods
In descriptive analyses, we examined the distribution of patient characteristics according to participant type (i.e., subcohort member, case). We also examined OSA severity indicators by subcohort/case status with a Chi square test. We used weighted Cox proportional hazards regression to calculate hazard ratios (HR) and 95% confidence intervals (CI) for the association of OSA severity measures with cancer incidence; weighting was based on methods by Borgan et al, which accounts for the oversampled subcohort members from the UWM sleep center by assigning a weight of >1 to subcohort members based on sampling population size and 1 to cases (Borgan and Samuelsen, 2003). Cancer cases not included in the randomly sampled subcohort (n = 304 out of 328), contributed to the analysis only from the time immediately preceding their cancer diagnosis (i.e., left truncation). Analyses were adjusted for age at OSA diagnosis, sex, race, smoking and BMI. We also conducted age (<60 vs ≥60 years at OSA diagnosis) and sex stratified analyses.
We performed several sensitivity analyses: excluding participants in the bottom 25% or alternatively the bottom 10% of total sleep time on PSG (given that these patients may not have slept long enough to yield an informative PSG); restricting to the OSA patients diagnosed with OSA at the UWM Sleep Center; and restricting to only those with a receipt of CPAP order from the medical record, at any time subsequent to the initial OSA diagnosis date, was recorded as an indicator for CPAP treatment. All analyses were conducted in Stata 14.0 (College Station, Texas) (StataCorp, 2015) with significance level considered at 0.05 alpha level.
3. Results
The subcohort had a median follow-up of 1851 days after OSA diagnosis (IQR: 1002-2,835 days). Baseline characteristics of the study population are provided in Table 2, by subcohort status. Among those in the randomly sampled subcohort, the mean age at OSA diagnosis was 50 years, 43% were female, 75% were white, 44% were obese, 13% were smokers, and 90% had >5 clinical visits in the last 5 years. The case group was slightly older at OSA diagnosis (mean age = 57 years) and with higher AHI (mean 47 events/h sleep vs. 41 events/h sleep in the subcohort), but was otherwise similar in the distribution of sex, race, BMI, smoking prevalence, and hospital and clinic visits. Furthermore, the distributions of the OSA severity indicators were similar across the two groups, including similarities in the proportion of participants with missing values.
Table 2.
Sub-cohort SEER linked Study population attributes.
| Comparison (n = 304) | Subcohort (n = 1162) | |
|---|---|---|
| Mean age (SD) | 57.2 (10.8) | 50.4 (12.9) |
| Sex | ||
| Females | 43.1 | 42.8 |
| Males | 56.9 | 57.2 |
| Race | ||
| White | 75.3 | 71.1 |
| Black | 10.9 | 12.5 |
| Other | 13.8 | 16.4 |
| BMI, % | ||
| Healthy Weight (18.5–24.9), kg/m2 | 7.24 | 10.8 |
| Overweight (25.0–29.9) | 22.0 | 19.3 |
| Obese (≥30) | 44.1 | 43.3 |
| Missing | 26.6 | 26.7 |
| Smoking, % | ||
| Current | 12.5 | 13.3 |
| Former | 28.0 | 18.9 |
| Never | 37.2 | 42.1 |
| Missing | 22.4 | 25.7 |
| Visit in 5 years | ||
| 5+ | 90.1 | 87.2 |
| <5 | 9.9 | 12.8 |
| Location of diagnosis | ||
| UW Medicine Sleep Center | 67.4 | 67.5 |
| Affiliated clinic | 32.6 | 32.5 |
| OSA severity indicators, mean (SD) | ||
| AHI % missing |
47.0 (31.1) | 41.3 (34.7) |
| 25.3 | 27.6 | |
| Tsat 90% % missing |
12.0 (20.4) | 11.9 (22.0) |
| 26.6 | 29.9 | |
| ODI_3% % missing |
26.3 (28.5) | 27.7 (32.4) |
| 30.3 | 42.9 | |
| Lowest_satO2 % missing |
83.3 (9.00) | 82.7 (11.0) |
| 25.7 | 27.3 | |
| Arousal index % missing |
45.8 (27.3) | 43.3 (27.8) |
| 29.3 | 32.4 |
Definition of abbreviations:
OSA: Obstructive sleep apnea.
AHI: Apnea or Hypopnea events per hour of sleep;
TSat90%: percent of nighttime spent with oxygen saturation, 90%.
ODI_3%: Oxygen desaturation index number times per hour sleep that blood oxygen levels drop by 3%.
Lowest_satO2: Oxygen saturation nadir Lowest level of oxygen saturation observed.
Arousal index: number of arousals per hour of sleep.
The risk of cancer across levels of OSA severity indices is shown in Table 3. Overall, compared with individuals in the lowest AHI category (5–14.9 events/h of sleep), indicating mild OSA, the adjusted HR (95% CI) for cancer incidence associated with moderate (15–29.9 events/h of sleep) and severe (>30 events/h of sleep) OSA were 0.72 (0.40, 1.29) and 0.87 (0.52, 1.45), respectively. Similarly, cancer risk among those subjects with moderate or severe hypoxemic burden (based on Tsat90) was not significantly different from the cancer risk among those with mild hypoxemic burden (HR = 1.16 (0.73, 1.83) and 1.13 (0.69, 1.86), respectively). Associations with oxygen desaturation index, oxygen desaturation nadir, and arousal index were also not statistically significant, albeit with HRs <1. The five indicators yielded consistently null results when modeled as continuous measures. Similar findings were also observed in age- and sex-specific analyses.
Table 3.
Overall and sex specific association of OSA severity metrics with cancer incidence in SEER linked sub-cohort.
| Overall (N = 1466) |
Males (n = 838) |
Females (n = 628) |
Age < 60 (n = 1053) |
Age 60 + (n = 413) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted HR (95% CI) | Pvalue | Adjusted HR (95% CI) | Pvalue | Adjusted HR (95% CI) | Pvalue | Adjusted HR (95% CI) | Pvalue | Adjusted HR (95% CI) | Pvalue | |
| AHI | ||||||||||
| Continuous | 0.98 (0.95, 1.01) | 0.301 | 0.98 (0.93, 1.02) | 0.316 | 1.00 (0.96, 1.04) | 0.957 | 1.01 (0.98, 1.05) | 0.548 | 0.97 (0.91, 1.03) | 0.343 |
| 5–14.9 | ||||||||||
| 15–29.9 | 0.72 (0.40, 1.29) | 0.272 | 0.39 (0.16, 0.96) | 0.04 | 0.86 (0.40, 1.88) | 0.71 | 0.76 (0.38, 1.50) | 0.430 | 0.44 (0.13, 1.51) | 0.191 |
| 30+ | 0.87 (0.52, 1.45) | 0.590 | 0.40 (0.18, 0.89) | 0.024 | 1.28 (0.67, 2.46) | 0.449 | 0.79 (0.43, 1.46) | 0.453 | 0.96 (0.32, 2.86) | 0.94 |
| Tsat90, % | ||||||||||
| Continuous | 1.02 (0.98, 1.07) | 0.291 | 1.05 (0.99, 1.11) | 0.108 | 1.01 (0.95, 1.08) | 0.784 | 1.02 (0.98, 1.07) | 0.321 | 1.04 (0.93, 1.16) | 0.472 |
| <1.2 | ||||||||||
| 1.2–12.9 | 1.16 (0.73, 1.83) | 0.526 | 1.25 (0.63, 2.48) | 0.522 | 1.13 (0.59, 2.19) | 0.709 | 1.11 (0.63, 1.95) | 0.725 | 1.19 (0.48, 2.91) | 0.707 |
| 13+ | 1.13 (0.69, 1.86) | 0.626 | 1.46 (0.72, 3.00) | 0.3 | 1.07 (0.52, 2.20) | 0.853 | 1.40 (0.81, 2.41) | 0.229 | 0.90 (0.32, 2.51) | 0.835 |
| ODI_3percent | ||||||||||
| Continuous | 0.99 (0.96, 1.02) | 0.557 | 1.00 (0.97, 1.03) | 0.975 | 0.98 (0.91, 1.05) | 0.564 | 1.00 (0.98, 1.03) | 0.743 | 0.96 (0.88, 1.05) | 0.394 |
| 1st tertile | ||||||||||
| 2nd tertile | 0.91 (0.57, 1.45) | 0.682 | 1.31 (0.62, 2.78) | 0.481 | 0.77 (0.41, 1.46) | 0.422 | 0.67 (0.38, 1.17) | 0.725 | 1.68 (0.86, 4.32) | 0.282 |
| 3rd tertile | 0.73 (0.43, 1.25) | 0.242 | 1.03 (0.46, 2.28) | 0.070 | 0.60 (0.27, 1.30) | 0.197 | 0.92 (0.50, 1.68) | 0.779 | 0.50 (0.16, 2.48) | 0.213 |
| Lowest_satO2 | ||||||||||
| Continuous | 0.96 (0.85, 1.08) | 0.468 | 0.93 (0.77, 1.11) | 0.427 | 1.00 (0.88, 1.13) | 0.942 | 0.89 (0.78, 1.02) | 0.105 | 1.23 (0.88, 1.72) | 0.225 |
| 86–90 | ||||||||||
| 80–85 | 0.93 (0.57, 1.53) | 0.786 | 0.89 (0.45, 1.77) | 0.736 | 1.26 (0.63, 2.51) | 0.521 | 1.07 (0.60, 1.93) | 0.814 | 0.54 (0.18, 1.61) | 0.274 |
| <80 | 0.89 (0.53, 1.50) | 0.666 | 0.87 (0.41, 1.82) | 0.704 | 1.01 (0.45, 2.25) | 0.977 | 0.81 (0.44, 1.51) | 0.515 | 1.01 (0.37, 2.78) | 0.985 |
| Arousal Index | ||||||||||
| Continuous | 1.01 (0.97, 1.04) | 0.744 | 1.00 (0.95, 1.05) | 0.987 | 1.02 (0.97, 1.08) | 0.463 | 1.03 (0.98, 1.07) | 0.236 | 1.00 (0.92, 1.07) | 0.902 |
| <20 | ||||||||||
| 21–40.9 | 0.80 (0.48, 1.35) | 0.413 | 0.41 (0.19, 0.88) | 0.022 | 1.28 (0.64, 2.60) | 0.386 | 0.75 (0.39, 1.46) | 0.402 | 0.73 (0.28, 1.93) | 0.527 |
| 41+ | 0.91 (0.54, 1.34) | 0.728 | 0.44 (0.20, 0.96) | 0.039 | 1.48 (0.74, 2.97) | 0.270 | 0.94 (0.49, 1.80) | 0.853 | 0.81 (0.30, 2.16) | 0.675 |
Definition of abbreviations: AHI: apnea-hypopnea index;
sleep apnea; TSat90 ¼ percent of nighttime spent with oxygen saturation, 90%.
ODI: Oxygen desaturation index # times per hour sleep that blood oxygen levels drop by 3%.
Lowest_satO2 Oxygen saturation nadir Lowest level of oxygen saturation observed.
Arousal index # arousals per hour of sleep.
*Adjusted for age, sex, body mass index, race, smoking status stratified on sleep center.
Continuous measures: estimates for 5 unit increase (exception: 5unit decrease for lowest_sat02).
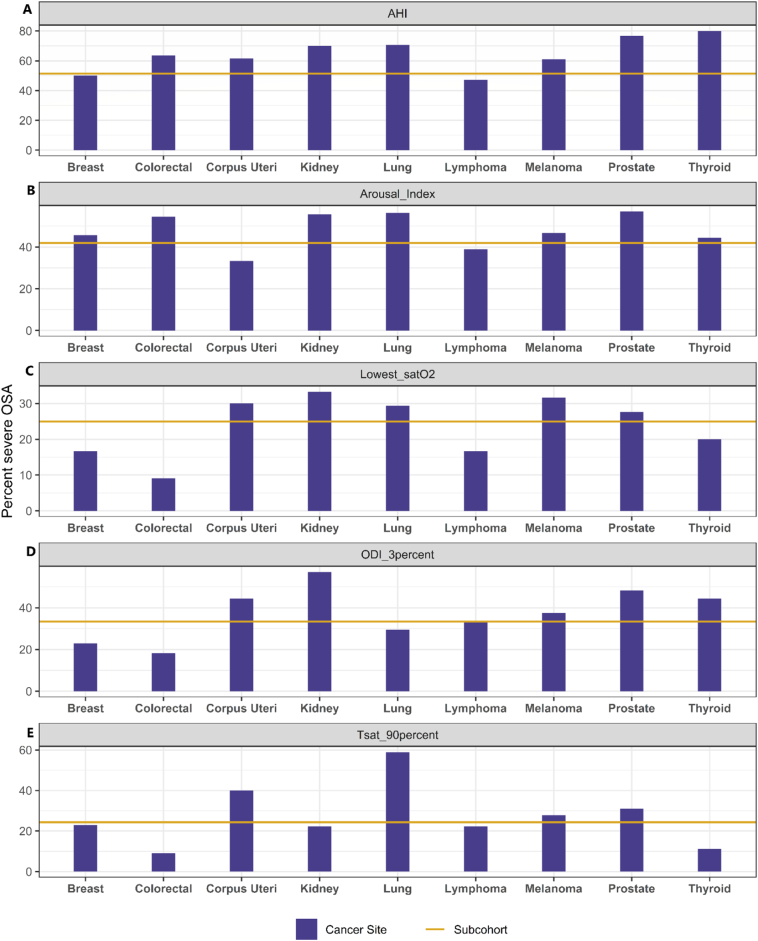
Fig. 2 shows the proportion of study participants with severe OSA according to different indices across common cancer sites. Among individuals with cancers of the prostate, corpus uteri, and lung, and in those with melanoma, the proportion of individuals with severe OSA was significantly higher relative to the subcohort for all OSA severity indices. Among those with kidney cancer, the proportion with severe OSA was elevated relative to the subcohort for four out of five severity metrics. Moreover, the proportion of cancer cases with severe OSA based on elevated AHI, was higher than that in the subcohort for all evaluated cancer sites, with the exception of breast cancer and lymphoma.
Fig. 2.
Proportion of participants with severe obstructive sleep apnea (OSA) by 5 indicators across cancer sites. Severe OSA cut-off by indicators with p-values comparing distribution across cancer sites and subcohort.
A) AHI: Apnea or Hypopnea events per hour of sleep: >30, p = 0.001.
B) Arousal Index: number of arousals per hour of sleep: >40, p = 0.298.
C) Lowest_satO2: lowest level of oxygen saturation n observed: <80%, p = 0.974.
D) ODI_3percent: number of times per hour sleep that blood oxygen levels drop by 3%: highest tertile, p = 0.974.
E) Tsat90percent: percent of sleep time spent with oxygen saturation levels <90%: 12%, p = 0.29.
The results from the sensitivity analysis was congruent and similar with the main analysis.
4. Discussion
In this study, the proportion of patients with severe OSA was higher among individuals with prostate, melanoma, corpus uteri, lung cancer, thyroid and kidney cancers than among those in the randomly sampled subcohort of individuals with OSA. However, in a multivariable-adjusted regression analyses, we found that overall cancer risk in individuals with moderate or severe OSA was not significantly different from individuals with mild OSA. Although limited by small numbers, we observed no statistically significant association between the prescription of a CPAP and subsequent cancer.
Our current findings do not fully corroborate prior animal studies and in vitro models which have clearly shown the oncogenic potential of intermittent hypoxia and fragmented sleep, two of the hallmark characteristics of OSA. Specifically, exposures to intermittent hypoxia in a mouse model of melanoma resulted in increased tumor growth and tumor metastasis (Almendros et al., 2012b). Other recent human studies have reported a 7% increased risk of cancer incidence per 10-unit increase in Tsat90 (Campos-Rodriguez et al., 2013), and have observed higher ODI 3% and AHI to be associated with greater aggressiveness of cutaneous melanoma (Martinez-Garcia et al., 2018).
We compared cancer incidence between moderate and severe sleep apnea to mild sleep apnea but found no evidence of a significant association. Such negative findings in the exploration of severity indicators may reflect the presence of carcinogenic potential for all levels of OSA severity, such that increments in such risk over the severity spectrum may require much larger sample sizes. Indeed, increased cardiovascular risk has been observed even with very mild levels of sleep apnea in previous studies (Peppard et al., 2000; Shahar et al., 2001).
Furthermore, due to scarcity of data on CPAP adherence, we were unable to account for treatment in our study; this may partially explain our null results. Indeed, 25% of the cohort had no information regarding CPAP prescription and, among those patients with a CPAP prescription, usage data were missing in 80% of cases. CPAP prescriptions do not necessarily translate into CPAP usage, with long-term adherence rates estimated to be only 60–70% regardless of disease severity, with adherence being defined as use >4 h/night 5 nights per week, clearly far from ideal adherence (Rotenberg et al., 2016). However, the results remained unchanged when we restricted the analysis to patients who had indicators of a CPAP order in their medical record. In addition, we have no information regarding other OSA treatments, such as surgery or oral appliances, which may also impact our results in unpredictable ways.
This study has some key limitations. First, 27%–40% of OSA patients were missing information across the 5 severity indicators (Supplemental Table 1). Those patients with missing severity metrics differed from those with complete data with respect to demographic factors, smoking and BMI. For example, the majority of those missing a severity indicator value were females, younger, and Black. The proportion of OSA patients missing smoking, BMI and hospital and clinic visits data was also higher among those missing severity indicators.
In addition, we did not have data on other potential confounding variables, such as alcohol consumption and occupation (e.g., shift work) which could both impact sleep apnea (Simou et al., 2018; Paciorek et al., 2011) and cancer risk (Praud et al., 2016; Purdue et al., 2015). However, we adjusted for other available key confounders, such as BMI and smoking, which are both associated with alcohol consumption. Also given the positive relationship of alcohol consumption and OSA and cancer risk respectively we expect that our observed null results would likely have been further attenuation had we been able to adjust for this particular confounder.
Furthermore, our study lacked statistical power for an analysis of OSA with site-specific cancer incidence. Therefore, given the heterogeneity of the relationship between OSA and the incidence of site-specific cancers (Gozal et al., 2016; Sillah et al., 2018; Silberfarb et al., 1993), it is plausible that the associations when all cancer types are combined will mask the effect of the OSA severity indicators on risk of specific anatomic sites. Our ascertainment of cancer diagnoses was also limited to the 13-county catchment area of the CSS cancer registry. As such, we could have missed other cancers diagnosed if or when individuals with sleep apnea moved outside the 13-county region. For instance, based on linkage with the National Death Index (NDI), 5 patients in the subcohort died of cancer, but were not captured as having experienced a cancer diagnosis via our cancer registry linkage due to having been diagnosed outside the registry catchment area. In addition, we had a relatively short follow-up period (mean = 5.3 years); it is plausible that the impact of OSA on cancer risk involves a longer induction period, such that longer follow-up duration might be necessary to observe true associations. Lastly, there is a potential for patient referral bias in our clinical cohort. Specifically, referred patients are more likely to be engaged with their healthcare and therefore more likely to receive a cancer diagnosis, which will reduce the heterogeneity of cancer diagnosis across the OSA severity groups. This bias could in part explain the null effect estimates observed in the study.
A strength of our study compared to prior work (Campos-Rodriguez et al., 2013; Martinez-Garcia et al., 2018; Martinez-Garcia et al., 2014b) is our comprehensive assessment of OSA severity indicators. We also had the ability to link sleep apnea cases with a population-based cancer registry, allowing for more comprehensive identification of cancer cases in our cohort.
In conclusion, no significant associations emerged between 5 key measures of OSA severity and cancer incidence; however, we consistently found a higher proportion of severe OSA among patients with kidney, prostate, melanoma, corpus uteri, and lung cancers. Larger studies with complete datasets on OSA severity indicators are needed to definitively establish whether dose-dependent relationships are indeed operationally detectable in OSA patients and site-specific cancer risk.
The following is the supplementary data related to this article.
OSA severity indicator data availability and distribution.
Funding
This work was supported by grant number NIH: P30CA015704, R03CA201806 and T32CA094880.
Conflict of interest
The authors declare there is no conflict of interest.
References
- Acker J., Richter K., Piehl A., Herold J., Ficker J., Niklewski G. Obstructive sleep apnea (OSA) and clinical depression—prevalence in a sleep center. Sleep and Breathing. 2016:1–8. doi: 10.1007/s11325-016-1411-3. [DOI] [PubMed] [Google Scholar]
- Akbarpour M., Khalyfa A., Qiao Z., Gileles-Hillel A., Almendros I., Farré R., Gozal D. Altered CD8+ T-cell lymphocyte function and TC1 cell stemness contribute to enhanced malignant tumor properties in murine models of sleep apnea. Sleep. 2017;40(2) doi: 10.1093/sleep/zsw040. [DOI] [PubMed] [Google Scholar]
- Almendros I., Montserrat J.M., Torres M. Obesity and intermittent hypoxia increase tumor growth in a mouse model of sleep apnea. Sleep Med. 2012;13(10):1254–1260. doi: 10.1016/j.sleep.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Almendros I., Montserrat J.M., Ramirez J. Intermittent hypoxia enhances cancer progression in a mouse model of sleep apnoea. Eur. Respir. J. 2012;39(1):215–217. doi: 10.1183/09031936.00185110. [DOI] [PubMed] [Google Scholar]
- Almendros I., Wang Y., Becker L. Intermittent hypoxia-induced changes in tumor-associated macrophages and tumor malignancy in a mouse model of sleep apnea. Am. J. Respir. Crit. Care Med. 2014;189(5):593–601. doi: 10.1164/rccm.201310-1830OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger L.K., Rajaratnam S., Wang W., OBrien C.S., Sullivan J.P., Quadri S. Common sleep disorders increase risk of motor vehicle crashes and adverse health outcomes in firefighters. J. Clin. Sleep Med. 2015;11(3):233–240. doi: 10.5664/jcsm.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgan Ø., Samuelsen S.O. A review of cohort sampling designs for cox's regression model: potentials in epidemiology. Norsk Epidemiologi. 2003;13(2) [Google Scholar]
- Campos-Rodriguez F., Martinez-Garcia M.A., Martinez M. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am. J. Respir. Crit. Care Med. 2013;187(1):99–105. doi: 10.1164/rccm.201209-1671OC. [DOI] [PubMed] [Google Scholar]
- Christensen A.S., Clark A., Salo P. Symptoms of sleep disordered breathing and risk of cancer: a prospective cohort study. Sleep. 2013;36(10):1429–1435. doi: 10.5665/sleep.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillos-Zapata C., Avendano-Ortiz J., Hernandez-Jimenez E. Hypoxia-induced PD-L1/PD-1 crosstalk impairs T-cell function in sleep apnoea. Eur. Respir. J. 2017;50(4) doi: 10.1183/13993003.00833-2017. (Print 2017 Oct. doi: 1700833 [pii]) [DOI] [PubMed] [Google Scholar]
- Ernster V.L. Nested case-control studies. Prev. Med. 1994;23(5):587–590. doi: 10.1006/pmed.1994.1093. [DOI] [PubMed] [Google Scholar]
- Fang H., Miao N., Chen C., Sithole T., Chung M. Risk of cancer in patients with insomnia, parasomnia, and obstructive sleep apnea: a nationwide nested case-control study. J. Cancer. 2015;6(11):1140. doi: 10.7150/jca.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D., Farré R., Nieto F.J. Putative links between sleep apnea and cancer: from hypotheses to evolving evidence. CHEST Journal. 2015;148(5):1140–1147. doi: 10.1378/chest.15-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D., Ham S.A., Mokhlesi B. Sleep apnea and cancer: analysis of a nationwide population sample. Sleep. 2016;39(8):1493–1500. doi: 10.5665/sleep.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao L., Lee H., Lin H., Tsai M., Chung S. Healthcare service utilization by patients with obstructive sleep apnea: a population-based study. PLoS One. 2015;10(9) doi: 10.1371/journal.pone.0137459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendzerska T., Gershon A.S., Hawker G., Tomlinson G., Leung R.S. Obstructive sleep apnea and incident diabetes. A historical cohort study. Am. J. Respir. Crit. Care Med. 2014;190(2):218–225. doi: 10.1164/rccm.201312-2209OC. [DOI] [PubMed] [Google Scholar]
- Kendzerska T., Leung R.S., Hawker G., Tomlinson G., Gershon A.S. Obstructive sleep apnea and the prevalence and incidence of cancer. CMAJ. 2014;186(13):985–992. doi: 10.1503/cmaj.140238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalyfa A., Almendros I., Gileles-Hillel A. Circulating exosomes potentiate tumor malignant properties in a mouse model of chronic sleep fragmentation. Oncotarget. 2016 doi: 10.18632/oncotarget.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konecny T., Kara T., Somers V.K. Obstructive sleep apnea and hypertension: an update. Hypertension. 2014;63(2):203–209. doi: 10.1161/HYPERTENSIONAHA.113.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langholz B. Wiley StatsRef: Statistics Reference Online; 2014. Case–control Study, Nested. [Google Scholar]
- Li L., Ren F., Cao J., Chen B. Relevant mechanism of intermittent hypoxia-induced melanoma lung metastases in a murine model of sleep apnea. Chest. 2016;149(4) [Google Scholar]
- Marshall N.S., Wong K., Cullen S., Knuiman M.W., Grunstein R.R. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study Cohort. J. Clin. Sleep Med. 2014;10(4):355–362. doi: 10.5664/jcsm.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Garcia M.A., Campos-Rodriguez F., Duran-Cantolla J. Obstructive sleep apnea is associated with cancer mortality in younger patients. Sleep Med. 2014;15(7):742–748. doi: 10.1016/j.sleep.2014.01.020. [DOI] [PubMed] [Google Scholar]
- Martinez-Garcia M.A., Martorell-Calatayud A., Nagore E. Association between sleep disordered breathing and aggressiveness markers of malignant cutaneous melanoma. Eur. Respir. J. 2014;43(6):1661–1668. doi: 10.1183/09031936.00115413. [DOI] [PubMed] [Google Scholar]
- Martinez-Garcia M.A., Campos-Rodriguez F., Nagore E., Martorell A., Rodriguez-Peralto J.L., Riveiro-Falkenbach E.…Cabriada V. Sleep-Disordered breathing is independently associated with increased aggressiveness of cutaneous melanoma: A multicenter observational study in 443 patients. Chest. 2018;154(6):1348–1358. doi: 10.1016/j.chest.2018.07.015. [DOI] [PubMed] [Google Scholar]
- Miao Z., Zhao T., Wang Z. Influence of different hypoxia models on metastatic potential of SGC-7901 gastric cancer cells. Tumor Biol. 2014;35(7):6801–6808. doi: 10.1007/s13277-014-1928-7. [DOI] [PubMed] [Google Scholar]
- Nanduri J., Prabhakar N.R. Impact of Sleep and Sleep Disturbances on Obesity and Cancer. Springer; 2014. Intermittent hypoxia: mechanistic pathways influencing cancer; pp. 103–119. [Google Scholar]
- Nieto F.J., Peppard P.E., Young T., Finn L., Hla K.M., Farré R. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am. J. Respir. Crit. Care Med. 2012;186(2):190–194. doi: 10.1164/rccm.201201-0130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciorek M., Korczyński P., Bielicki P., Byśkiniewicz K., Zieliński J., Chazan R. Obstructive sleep apnea in shift workers. Sleep Med. 2011;12(3):274–277. doi: 10.1016/j.sleep.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Peppard P.E., Young T., Palta M., Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N. Engl. J. Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- Peppard P.E., Young T., Barnet J.H., Palta M., Hagen E.W., Hla K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praud D., Rota M., Rehm J. Cancer incidence and mortality attributable to alcohol consumption. Int. J. Cancer. 2016;138(6):1380–1387. doi: 10.1002/ijc.29890. [DOI] [PubMed] [Google Scholar]
- Purdue M.P., Hutchings S.J., Rushton L., Silverman D.T. The proportion of cancer attributable to occupational exposures. Ann. Epidemiol. 2015;25(3):188–192. doi: 10.1016/j.annepidem.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotenberg B.W., Murariu D., Pang K.P. Trends in CPAP adherence over twenty years of data collection: a flattened curve. Journal of Otolaryngology-Head & Neck Surgery. 2016;45(1):43. doi: 10.1186/s40463-016-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria-Martos F., Benitez I., Giron C. Biomarkers of carcinogenesis and tumour growth in patients with cutaneous melanoma and obstructive sleep apnoea. Eur. Respir. J. 2018;51(3) doi: 10.1183/13993003.01885-2017. (Print 2018 Mar. doi: 1701885 [pii]) [DOI] [PubMed] [Google Scholar]
- Shahar E., Whitney C.W., Redline S. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the sleep heart health study. Am. J. Respir. Crit. Care Med. 2001;163(1):19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- Silberfarb P.M., Hauri P.J., Oxman T.E., Schnurr P. Assessment of sleep in patients with lung cancer and breast cancer. J. Clin. Oncol. 1993;11(5):997–1004. doi: 10.1200/JCO.1993.11.5.997. [DOI] [PubMed] [Google Scholar]
- Sillah A., Watson N.F., Schwartz S.M., Gozal D., Phipps A.I. Sleep apnea and subsequent cancer incidence. Cancer Causes Control. 2018:1–8. doi: 10.1007/s10552-018-1073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simou E., Britton J., Leonardi-Bee J. Alcohol and the risk of sleep apnoea: a systematic review and meta-analysis. Sleep Med. 2018;42:38–46. doi: 10.1016/j.sleep.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research The report of an American Academy of Sleep Medicine task force. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- StataCorp . StataCorp LP; College Station, TX: 2015. Stata Statistical Software: Release 14. [Google Scholar]
- Toffoli S., Michiels C. Intermittent hypoxia is a key regulator of cancer cell and endothelial cell interplay in tumours. FEBS J. 2008;275(12):2991–3002. doi: 10.1111/j.1742-4658.2008.06454.x. [DOI] [PubMed] [Google Scholar]
- Wan J., Chai H., Yu Z. HIF-1α effects on angiogenic potential in human small cell lung carcinoma. J. Exp. Clin. Cancer Res. 2011;30(1):1. doi: 10.1186/1756-9966-30-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
OSA severity indicator data availability and distribution.