Highlights
-
•
Low-dose and short-term exposure to methyl mercaptan had adverse effects on lung tissue.
-
•
Lipid peroxidation and alterations in blood antioxidant enzyme levels were observed following the exposure.
-
•
Oxidative damage is recognized as a potential mechanism for pulmonary stimulation and injury.
-
•
Malondialdehyde in lung tissue was significantly increased in both males and females exposure group.
Keywords: Pulmonary toxicity, Hematotoxicity, Short-term exposure, Odor compound, Methyl mercaptan
Abstract
The present study was carried out to evaluate the hematotoxicity and respiratory toxicity of methyl mercaptan in Sprague-Dawley rats. A dynamic exposure methodology was adopted in this study following 7 days of exposure by repeated inhalation. The concentration of methyl mercaptan used in the exposure was 0.5 ppm and the exposure time was 6 h/day for 7 days. After exposure, the rats were sacrificed to collect lung tissue and blood samples. Routine blood and serum biochemistry were conducted. Morphological injury of lung tissue was detected by hematoxylin and eosin staining. Decreased food consumption and body weight gain in both sexes were noted in the exposure group compared with the control group. Several significant changes in hematological parameters were observed. The results showed that the blood urea nitrogen (UREA) levels and superoxide dismutase (SOD) values were significantly decreased in exposed male rats. Malondialdehyde (MDA) in lung tissue was significantly increased in both males and females in the exposed group. In the histopathological examination of lung tissue, terminal bronchiole constriction, alveolar congestion, and erythrocyte exudation were observed, suggesting that the lungs may be target organs after inhaling methyl mercaptan and workers exposed to this concentration may cause some pulmonary stimulation and injury.
1. Introduction
Methyl mercaptan (CH3SH) exist widely in the waste treatment facilities, such as landill, composting plant and sewage plant, in addition, is often used in manufacturing and other industries. It is often added in natural gases because it has a strong smell, which can help finding leaks. It is also a waste product during papermaking. Sulfur compounds, including dimethyl sulfide (CH3)2S, dimethyl disulfide (CH3SSCH3), methyl mercaptan (CH3SH), and hydrogen sulfide (H2S), have been widely detected in the air of pulp mills [1,2]. The concentrations of these sulfur compounds ranged from 0.05–39 ppm. Hence, their toxicity, combined with high production, represents a threat to human health. Methyl mercaptan is also founded in anaerobic circumstances such as lake sediments and in intestinal microenvironment. In addition, methyl mercaptan occurred at nontoxic concentrations in some food and drink such as wine, cheese, and other foods, and has been detected in waste treatment facilities such as landfills [3]. The concentration of these compounds fluctuates greatly, ranging from several ppb to tens of ppm. Workers exposed to reduced sulfur compounds showed a decreased activity of the heme-synthesizing enzymes delta-amino-levulinic acid synthetase and heme synthase [4]. Some studies have indicated that workers exposed in high concentration of sulfur compounds have increased air flow obstruction, irritant-induced occupational asthma, and a suspected increase in mortality due to ischemic heart disease [5]. Prolonged exposure to odorous pollution can affect respiration and the central nervous system [6]. Among the odorous gases, sulfur compounds have lower odor threshold values and higher detection rates [7]. The main toxicity of sulfur compounds is to stimulate the respiratory tract [8,9]. Coughing and shortness of breath may occur with the inhalation of sulfur compounds. High concentrations of exposure may lead to severe shortness of breath and pulmonary effusion. Inhaling high concentrations of methyl mercaptan may also cause headache, vomiting, nausea, and unconsciousness [10]. The olfactory threshold of methyl mercaptan is very low. It is possible to smell methyl mercaptan at only 0.02 ppb in air [11]. However, higher smell thresholds of 1–2 ppb have also been reported, e.g., Wilby [12] and Leonardos et al. [13]. The literature contains very little information on the effect of methyl mercaptan on the living organisms, one effect is neurotoxicity of general intoxication with different levels represented paralysis of the locomotory muscles and respiration. A case of human death death has been reported after exposure to methyl mercaptan, at that time he was emptying the tanks containing methyl mercaptan [14]. A concentration of 20,000 mg/3 will kill a rat in 15 min [15]. The concentration of methyl mercaptan in the air, the respiratory rate of the individual, and the exposure time of this compound will determine the amount of toxicant absorbed by the respiratory system. The main mechanism of coma and death induced by methyl mercaptan is believed to be related to reversible inhibition of cytochrome c oxidase and inhibition of brain sodium (Na+) and potassium (K+) ATPase activities, which is similar to the effects of cyanide and hydrogen sulfide. The degree of inhibition is proportional to the amount of methyl mercaptan adsorbed on these membranes [16]. Methyl mercaptan is related to the pathogenesis of hepatic coma [17]. It was found that methyl mercaptan could stabilize erythrocyte membrane and inhibit sodium ion, potassium ion and ATPase [18]. Methyl mercaptan may play a role in inducing anesthetic-like effects. Occupational exposure to this compound through inhalation may cause adverse health effects in waste or wastewater treatment plants, pulp mills and oil refineries. Nearby residents fear that long-term exposure may have adverse health effects also [19]. Recently, studies have shown that continued exposure to odors can cause adverse effects ranging from physiological to psychology symptoms such as emotional stresses, states of anxiety and unease, etc [20]. The occupational safety and health administration (OSHA) permissible exposure limit (PEL) to this chemical is 10 ppm, while the National Institute for Occupational Safety and Health (NIOSH) recommended exposure limit (REL) and the American Conference of Governmental Industrial Hygienists (ACGIH) threshold limit value-time weighted average (TLV-TWA) is 0.5 ppm [21]. The level of methyl mercaptan in the air at waste landfill and community waste treatment facilities has been shown to be close to the NIOSH REL and ACGIH TLV-TWA values [22]. Opportunities for exposure have steadily increased due to the proliferation of the sources, which may affect the health of workers. Exposed workers seldom use safety masks to protect against odorous compounds. Severe metabolical responses to some reduced-S compounds, including methyl mercaptan, have been reported across a wide range of concentrations [23,24]. However, few studies have been reported at present on the pathophysiological results of long-term low-level inhalation of this compound. Likewise, there are few studies in the literature evaluating the effects of short term exposures to low-dose methyl mercaptan also. Epidemiological studies have shown that lung diseases are associated with inhalation drugs, including cigarette smoke, organic compounds and particulate matter. [25,26]. Moreover, Tansey et al. [27] in vivo examined acute and chronic toxicity of methyl mercaptan in Sprague-Dawley rats, but he mainly studied hepatotoxicity, lacking data on pulmonary toxicity and few parameters of blood biochemical components. The present experiment was arranged to observe the potential toxicity of methyl mercaptan in rats continuing for seven days of exposure. Our aim is to determine whether subacute exposure to methyl mercaptan vapor approaching to the recommended workplace concentration could lead to significant differences in the pulmonary tissue structure and metabolic performance parameters when compared with the data of control rats. These data will be of value in establishing occupational exposure standards or evaluating future occupational health risks.
2. Materials and methods
2.1. Animals
Male and female Sprague-Dawley (SD) rats were used in this experiment. These rats weighed 160 ± 20 g at the start of the experiment. SD rats were randomly divided into two groups: the control group (control males, n = 15; control females, n = 15) and the methyl mercaptan exposure group (exposed males, n = 15; exposed females, n = 15). The SD rats were housed, six per cage, in stainless-steel wire hanging rodent cages (170 mm [W] × 294 mm [D] × 176 mm [H]) in a breeding room. The indoor environment maintained at 21 ± 1 °C and at 50% humidity.
In all experiments, animals were continuously supplied tap water. Food supplies in the control group and the experimental group were stopped during exposure to aviod the difference of feeding behavior. Standard glass tubes matched a small menisci were used to supply water for both groups, in order to reduce the dissolution of methyl mercaptan vapor in the water. Throughout the experiment, animals were kept in a 12 -h light-dark cycle. All efforts are aimed at minimizing the number of animals used and their suffering. All experiments were approved by the local Ethics Committee for Animal Research and adhered to the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of China.
2.2. Methyl mercaptan inhalation exposure
Adaptive feeding of rats in laboratory conditions continuing for 7 days and the initial weights of these rats were recorded. After acclimation, the rats were treated with methyl mercaptan in a 0.6 m3 inhalation exposure chamber (Model 8050D, Hope-Med Co. Ltd., Tianjin, China) (Fig. 1). The concentration of the gas was monitored by a portable instrument in real time (Konor Model JA903, Konor Electronics, Shenzhen, China). A methyl mercaptan gas cylinder of 50 ppm was used (Haizhou Gas Co. Ltd., Shanghai, China). The gas was diluted with air after decompressing for animal delivery and fed to the chamber. The concentration of methyl mercaptan for the exposure was 0.5 ± 0.1 ppm. The exposure time was 6 h per day, continuing for 7 days. At the end of the exposure, the rats were taken out of the chamber and returned to their cages. After 7 days exposure, body weights were recorded and the rats were sacrificed to collect lung tissue and blood samples.
Fig. 1.
The inhalation exposure chamber system.
2.3. Sample preparation
Blood samples were collected through cardiac puncture under mild diethyl ether anesthesia, half samples with anticoagulant and half samples without. Blood samples with the anticoagulant were tested immediately for the determination of hematological parameters. The samples without anticoagulant were centrifuged at 4000 rpm for 10 min at 4 °C, and the serum samples were stored at −20 °C waiting for the subsequent biochemical analysis. The lung tissues were excised and one specimen was rinsed with cold normal saline and dried with filter paper. The tissue was quickly frozen in liquid nitrogen at −170 °C and stored at −80 °C waiting for the subsequent determination of malondialdehyde (MDA). Other lung tissue specimens were quickly fixed with paraformaldehyde solution for histopathological examination [[28], [29], [30]].
2.4. Hematological parameters
The nomenclature and abbreviations used for laboratory blood sample parameters are shown in Table 1.
Table 1.
Nomenclature and abbreviation of laboratory tests.
| Nomenclature | |||
|---|---|---|---|
| Hematological parameters | Serum biochemical parameters | ||
| WBC | White blood cell (109/ L) | Hepatic function | |
| RBC | Red blood cell (1012/ L) | STP | Serum total protein(g/dL) |
| HGB | Hemoglobin (g/L) | ALB | Albumin(g/dL) |
| MCV | Mean corpuscular volume (fL) | A/G | Albumin/Globin |
| MCH | Mean corpuscular hemoglobin(pg) | TBIL | Total bilirubin (mg/dL) |
| MCHC | Mean corpuscular hemoglobin concentration (g/L) | GGT | Gamma-glutamyl transpeptidase (IU/L) |
| PLT | Platelets (109/L) | DBIL | Direct bilirubin (mg/dL) |
| LYMPH | Lymphocytes (%) | ALT | Alanine aminotransferase (IU/L) |
| MONO | Monocytes (%) | ALP | Alkaline phosphatase(IU/L) |
| NEUT | Neutrophils (%) | ASTm | Aspartate aminotransferase isoenzyme (IU/L) |
| EOS | Eosinophils (%) | AST | Aspartate aminotransferase (IU/L) |
| BASO | Basophils (%) | LDH | Lactate dehydrogenase (IU/L) |
| RDW-C | Red cell distribution width-CV(%) | Renal function | |
| RDW-S | Red cell distribution width-SD(fL) | CREA | Creatinine (mg/dL) |
| PDW | Platelets distribution width(%) | UREA | Blood urea nitrogen (mmol/dL) |
| MPV | Mean platelets volume(fL) | GLU | Glucose (mg/dL) |
| PCT | Plateletcrit(%) | UA | Uric acid (μmol/dL) |
| HCT | Hematocrit(%) | RBP | Retinol binding protein (mg/L) |
| Hematological parameters | CysC | Cystatin C (mg/L) | |
| MDA | malondialdehyde (nmol/mg) | Cardiovascular function | |
| CK | Creatine kinase (IU/L) | ||
| CK-MB | Creatine kinase isoenzymes (IU/L) | ||
| ACE | Angiotensin converting enzyme (IU/L) | ||
| Thyroid function | |||
| TT3 | Total triiodothyronine (ng/dL) | ||
| TT4 | Total thyronine (ng/dL) | ||
| FT3 | Free triiodothyronine (ng/dL) | ||
| FT4 | Free thyronine (ng/dL) | ||
| Oxidative stress | |||
| SOD | Superoxide dismutase (IU/L) | ||
Blood samples were collected in tubes added the potassium salt ethylenediamine tetra-acetic acid (EDTA) for hematology test. These samples were examined immediately within 20 min using an automatic hematology analyzer (Advia 2120i, Siemens Diagnostics, New York, USA). In total, 17 hematological parameters were assessed, including red blood cell (RBC) count, white blood cell (WBC) count, hemoglobin (HGB), and mean cell volume (MCV) using a hematology auto-analyzer system.
2.5. Serum biochemical parameters
Blood biochemical parameters including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), glucose (GLU), total protein (TP), albumin (ALB), albumin/globulin ratio (A/G), blood urea nitrogen (BUN), and creatinine (CREA) were measured using an automated biochemistry analyzer (Toshiba 120FR, Tokyo, Japan). Total triiodothyronine (TT3), as a marker of thyroid function, was measured with an automatic analyzer (Hitachi 7070, Hitachi Ltd., Tokyo, Japan).
2.6. Lung tissue test and histopathological examination
Lung tissue MDA was determined colorimetrically by using Bio-Diagnostics Kits (Bio-Diagnostics Co. Giza, Egypt) [31]. Conventional techniques were used for the histopathological examination of the tissues [32]. After 24 h of fixation, samples were frozen with sucrose solution of 15%–30%, and then frozen in nitrogen. Each piece was cut at 15 μm thickness intervals by a cryostat (Leitz, Digital 1702, Germany) at −20 °C, then the slices were placed on glass slides and stained using hematoxylin and eosin (H&E) to evaluate tissue morphology. Histopathological parameters were obtained using an Olympus® Bx50 optical microscope (Olympus, Tokyo, Japan). A lung injury score was adopted to quantify changes in lung architecture visible under light microscopy [33,34]. The score of the degree of microscopic injury was calculated basing on the following condition: infiltrative around bronchioles and bronchi, exudation in bronchioles and bronchi, infiltration around blood vessels, edema, and atelectasis. The severity of injury was graded as follows for each of the five variables: no injury = 0, injury to 25% of the field = 1, injury to 50% of the field = 2, injury to 75% of the field = 3, and diffuse injury = 4. The highest possible score was 20 and the lowest was 0. The standards for the pathology scores of lung tissue are shown in Table 2.
Table 2.
Pathology score standard of lung tissue.
| score | A Infiltrative around bronchioles and bronchi | B Exudation in bronchioles and bronchi | C Infiltration around blood vessel | D Edema | E Atelectasis |
|---|---|---|---|---|---|
| 0 | None | None | None | None | None |
| 1 | <25% | <25% | <10% | Few | <25% |
| 2 | 25-50% | 25-50% | 10%-50% | Slightly | 25-50% |
| 3 | 50-75% | 50-75% | 50-75% | Severely | 50-75% |
| 4 | >75% | >75% | >75% | Completely | >75% |
Score = A+B + C+D + E.
2.7. Statistical analysis
Significant differences between data for the exposure and control groups were determined using a two-tailed t-test. Before the comparisons, the data were first examined for normal distribution. Body weight, hematological, and blood biochemical parameters were analyzed difference according to t-test [35]. Bartlett's test was used to determine whether the variance was homogeneous. The software used in the analysis was SPSS version 10 (SPSS Inc., Chicago, IL, USA). The value is expressed by mean value (± SEM). When P value is less than 0.05, the difference has statistical significance.
3. Results
3.1. Body weight of animals
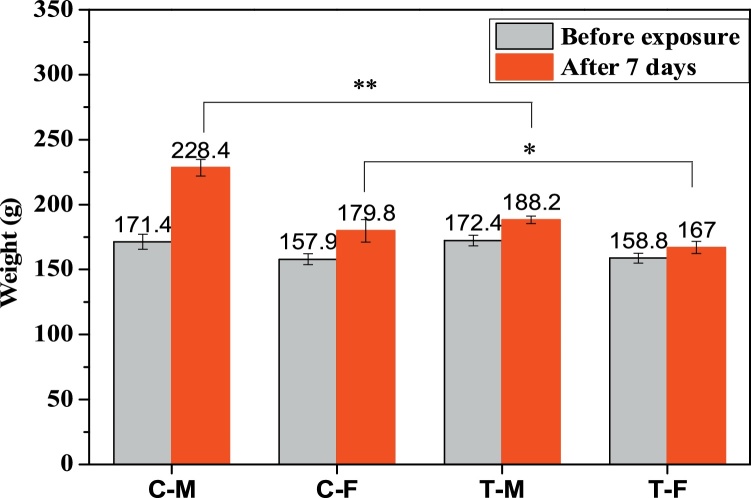
No mortalities were observed in any control of the exposed population during the 7- day period. However, during the exposure procedure, the rats tended to huddle together; this behavior was not observed in the control group. Before exposure, there were no significant differences in body weights among the four groups (male/female control groups and male/female exposure groups, p < 0.05). After the exposure period, The body weights of all rats increased obviously (Fig. 2). The rate of increase varied in each of the four groups. The terminal weights of the exposure groups were lower than those of the control groups. The weight of the male control group was found to be significantly higher than that of the male exposure group (p < 0.01), with the former weighing 40.2 g more than the latter on average. The weight of the female control group was found to be significantly higher than that of the female exposure group (p < 0.05), with the former weighing 12.8 g more than the latter on average. This result demonstrated that methyl mercaptan inhalation adversely affects body weight increases in male rats more significantly than in female rats.
Fig. 2.
Body weight changes of the rats exposed to methyl mercaptan.
3.2. Hematological values of the rats
In total, 17 hematological parameters were assessed in this study. The results of the hematology analyses for male and female rats are showed in Table 3. The only hematological change was a significant increase in PDW in exposed males (15 ± 0.1%) compared to control males (14.7 ± 0.1%) (p < 0.05). PDW in the female rats was not significantly different between the control and exposure groups (p < 0.05). There were no significant differences between the control and exposure groups for the other parameters measured.
Table 3.
Hematological parameters of the control and exposure groups.
| Parameter | Group |
|||
|---|---|---|---|---|
| Control-male | Control-female | Exposure-male | Exposure-female | |
| WBC (109/L) | 7.3 ± 2.6 | 6.6 ± 1.2 | 5.6 ± 4.8 | 5.3 ± 3.0 |
| RBC (1012/L) | 7.2 ± 0.1 | 6.5 ± 0.3 | 7.9 ± 1.0 | 6.3 ± 0.5 |
| HGB (g/dL) | 137 ± 4 | 137 ± 4 | 149 ± 18 | 123 ± 8 |
| HCT (%) | 42.8 ± 1.8 | 38.2 ± 1.9 | 46.7 ± 5.2 | 37.1 ± 1.8 |
| MCV (fL) | 59.3 ± 2.8 | 58.7 ± 1.7 | 59.3 ± 0.6 | 59.2 ± 1.8 |
| MCH (pg) | 19.0 ± 0.6 | 19.7 ± 0.6 | 18.9 ± 0.2 | 19.7 ± 0.3 |
| MCHC (g/dL) | 321.3 ± 4.9 | 335.0 ± 7.5 | 318.5 ± 3.5 | 333.5 ± 4.9 |
| PLT (109/L) | 992 ± 94 | 992 ± 62 | 1137 ± 203 | 962 ± 11 |
| NEUT (%) | 81.1 ± 1.4 | 83.6 ± 2.1 | 82.3 ± 1.3 | 82.3 ± 5.1 |
| LYMPH (%) | 3.9 ± 1.3 | 2.2 ± 0.8 | 3.5 ± 0.9 | 2.3 ± 0.1 |
| MONO (%) | 14.5 ± 1.3 | 13.9 ± 1.2 | 13.8 ± 1.4 | 14.8 ± 5.0 |
| EOS (%) | 0.3 ± 0.3 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 |
| BASO (%) | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.4 ± 0.1 |
| RDW-C(%) | 12.3 ± 0.7 | 11.4 ± 0.5 | 12.8 ± 01. | 12.8 ± 0.6 |
| RDW-S(fL) | 30.0 ± 0.6 | 27.7 ± 1.4 | 31.2±.2 | 31.4 ± 2.7 |
| PDW(%) | 14.7 ± 0.1 | 14.7 ± 0.1 | 15.0 ± 0.1* | 14.8 ± 0.1 |
| MPV(fL) | 5.7 ± 0.1 | 5.6 ± 0.1 | 5.9 ± 0.1 | 5.8 ± 0.1 |
| PCT(%) | 0.6 ± 0.1 | 0.5 ± 0.04 | 0.7 ± 0.1 | 0.6 ± 0.01 |
Values represent the mean ± SEM, n = 15. Control-male vs. Exposure-male, Control-female vs. Exposure-female, * P < 0.05. * *P < 0.01.
3.3. Serum and tissue biochemical values of the rats
Twenty-five serum biochemical parameters and one tissue biochemical parameter were analyzed in this study. The results of the serum biochemical tests for male and female rats are showed in Table 4. These parameters were related to hepatic, renal, cardiovascular, and thyroid functions and oxidative stress. In relation to hepatic function in the male exposure group, the values of STP and ALB were increased, while the values of ALT, ASTm, and LDH were decreased after exposure. Among the female rats, a significant difference in ALP was observed between the control and exposure groups (p < 0.05); ALP was decreased after exposure. The clinical findings of increases in STP and ALB were related to activation of the immune system, while increases in ALB, ALT, LDH, and ASTm values suggest liver damage or inflammation [36]. The results showed that methyl mercaptan did not have obvious toxicity to the liver at this concentration.
Table 4.
Serum and tissue biochemical values of the control and exposure groups.
| Sample | Parameter | Group |
|||
|---|---|---|---|---|---|
| Control -male | Control -female | Exposure -male | Exposure -female | ||
| Serum | Hepatic function | ||||
| STP(g/dL) | 49 ± 2 | 49 ± 4 | 55 ± 3 | 54 ± 3 | |
| ALB(g/dL) | 23.2 ± 0.8 | 24.3 ± 1.2 | 25.6 ± 1.5** | 26.5 ± 0.7 | |
| A/G | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.0 | 1.0 ± 0.1 | |
| TBIL(mg/dL) | 1.2 ± 0.4 | 0.6 ± 0.7 | 0.8 ± 0.6 | 1.0 ± 0.3 | |
| DBIL(mg/dL) | 0.4 ± 0.3 | 0.1 ± 0.6 | 0.4 ± 0.3 | 0.1 ± 0.1 | |
| ALT(IU/L) | 64 ± 8 | 65 ± 7 | 42 ± 9** | 53 ± 1 | |
| ALP(IU/L) | 282 ± 24 | 163 ± 5 | 225 ± 41 | 158 ± 7* | |
| GGT(IU/L) | 0.4 ± 1.1 | 1.3 ± 1.5 | 0.8 ± 0.4 | 1.5 ± 0.7 | |
| ASTm(IU/L) | 45 ± 8 | 29 ± 12 | 26 ± 14* | 42 ± 4 | |
| AST(IU/L) | 165 ± 36. | 139 ± 27 | 144 ± 41 | 171 ± 18 | |
| LDH(IU/L) | 1094 ± 276 | 497 ± 222 | 650 ± 228* | 786 ± 98 | |
| Renal function | |||||
| CREA (mg/dL) | 19 ± 5 | 32 ± 1 | 24 ± 2 | 29 ± 1* | |
| UREA(mmol/dL) | 6.6 ± 0.3 | 5.4 ± 0.2 | 6.1 ± 0.5 | 7.4 ± 0.1** | |
| GLU (mg/dL) | 17.4 ± 2.0 | 21.1 ± 8.8 | 14.8 ± 3.3 | 17.7 ± 1.3 | |
| UA (μmol/dL) | 225 ± 22 | 211 ± 144 | 149 ± 22* | 1 01 ± 9 | |
| RBP(mg/L) | 0.4 ± 1.9 | 1.7 ± 2.1 | 1.2 ± 2.0 | 3.0 ± 1.4 | |
| CysC(mg/L) | 0.19 ± 0.01 | 0.18 ± 0.01 | 0.18 ± 0.01 | 0.16 ± 0.07* | |
| Cardiovascular function | |||||
| CK (IU/L) | 4150 ± 2058 | 2456 ± 1803 | 3184 ± 1528 | ||
| CK-MB (IU/L) | 1030 ± 144 | 526 ± 164 | 968 ± 148 | ||
| ACE (IU/L) | 288 ± 18.0 | 271.7 ± 11.0 | 294.2 ± 15.1 | ||
| Thyroid function | |||||
| TT3 (ng/dL) | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.0 | 0.8 ± 0.0 | |
| TT4 (ng/dL) | 37.8 ± 5.2 | 40.5 ± 12.6 | 37.2 ± 1.8 | 36.7 ± 0.0 | |
| FT3 (ng/dL) | 3.1 ± 0.4 | 3.1 ± 0.6 | 3.2 ± 0.3 | 2.9 ± 0.0 | |
| FT4 (ng/dL) | 11.2 ± 1.9 | 11.8 ± 1.0 | 12.4 ± 0.9 | 11.6 ± 0.0 | |
| Oxidative stress | |||||
| Serum | SOD(IU/L) | 277 ± 36.7 | 149.7 ± 31.5 | 148.2 ± 51.5** | 179.5 ± 31.8 |
| Lung tissue |
MDA (nmol/mg) | 27.0 ± 2.8 | 24.8 ± 2.5 | 34.5 ± 4.3* | 31.8 ± 0.1* |
Values represent the mean ± SEM, n = 15. Control-male vs. Exposure-male, Control-female vs. Exposure-female, * P < 0.05. * *P < 0.01.
For parameters related to renal function, more differences were observed in the female groups than in the male groups. CREA and UREA are very important indicators of glomerular filtration function. CysC levels were determined by glomerular filtration rate also. Differences in CREA, UREA, and CysC were observed between the female control and exposure groups (CREA, p < 0.05; UREA, p < 0.01; CysC < 0.05). There was a significant increase in UREA levels, which changed from 5.4 ± 0.2 mmol/dL to 7.4 ± 0.1 mmol/dL, revealing that metabolism of methyl mercaptan may induce nephron damage in female rats. In the male groups, the only significant difference observed was for UA (p < 0.05); and this parameter is often correlated with hepatic function. However, as noted above, the levels of ALT, LDH, and ASTm were decreased in exposed male rats, which suggests that methyl mercaptan is not toxic in the liver. Parameters related to cardiovascular and thyroid function were not significantly different between the control and exposure groups, while SOD values, reflecting oxidative stress, were significantly decreased in the exposed male group (277 ± 36.7 vs 148.2 ± 51.5, p < 0.01). Lipid peroxidation content MDA in lung tissue demonstrated a significant increase in the exposure group compared with the control group (male: 27.0 ± 2.8 vs 34.5 ± 4.3, p < 0.05; female: 24.8 ± 2.5 vs 31.8 ± 0.1, p < 0.05).
3.4. Histopathology study
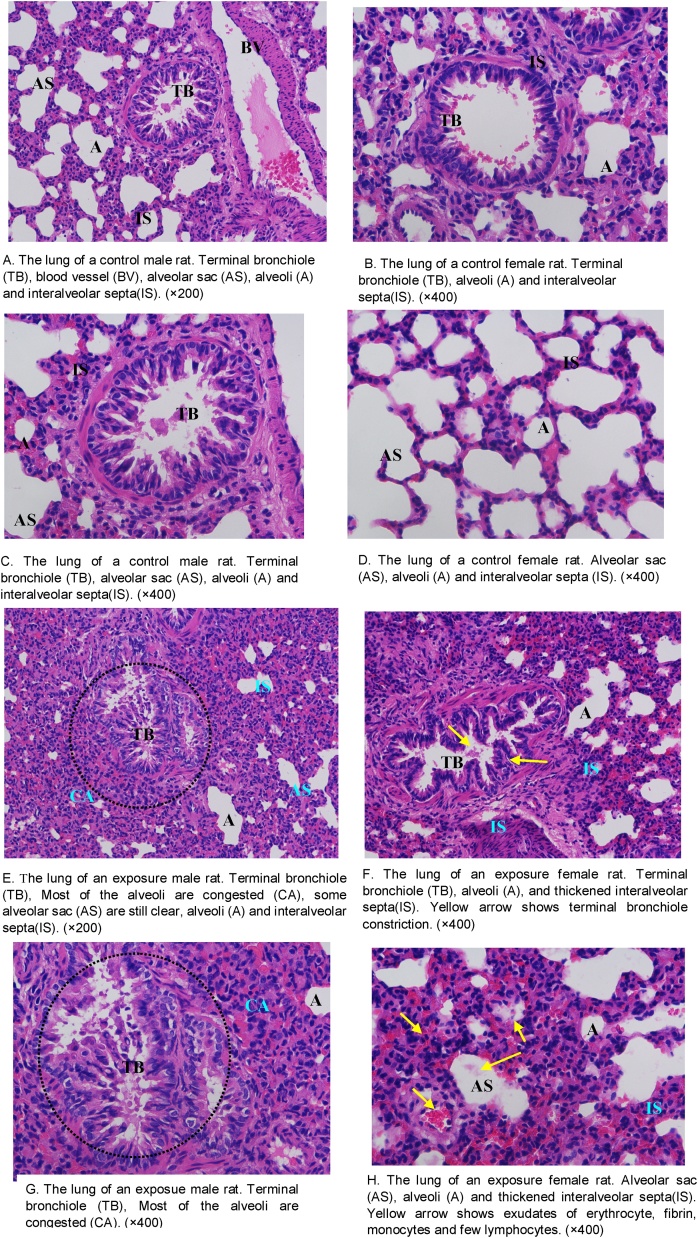
The alveolar sacs, alveoli, and bronchioles were found to be normal in the lung tissues of control animals. The alveolar sacs showed no signs of congestion or collapse (Fig. 3A–D). After 7 days exposure to methyl mercaptan, bronchioles were found to contain prominent goblet cells. Terminal bronchiole constriction (Fig. 3E & F) was observed in the exposure groups. The alveoli of exposed animals exhibited congestion, with exudation of erythrocytes, fibrin, monocytes, and few lymphocytes. Alveolar walls were observed to be thickened and exhibited erythrocyte diapedesis, especially in the female groups (Fig. 3G & H). The scores for lung tissue pathology for the four groups are shown in Table 5. The animals in the control groups had significantly lower scores than the animals in the exposure groups (control males, 0.8 ± 0.5 vs exposure males, 3.6 ± 2.4, p < 0.05; control females, 0.4 ± 0.1 vs exposure females, 4.9 ± 2.9, p < 0.05). The score of lung tissue pathology in the control groups was lower than that in the exposure groups. These findings suggest that exposure to inhaled methyl mercaptan is associated with bronchial and peri-bronchiolar inflammatory exudates that also involves the surrounding alveoli.
Fig. 3.
Histopathology of the lung tissue of control and exposure groups. Hematoxylin and eosin (H&E) stain.
Table 5.
Score of lung tissue pathology (mean ± SEM, n = 15).
| Control-male | Control-female | Exposure-male | Exposure-female | |
|---|---|---|---|---|
| A | 0 | 0 | 0.6 ± 0.5 | 1.2 ± 0.7 |
| B | 0 | 0 | 0.8 ± 0.5 | 1.1 ± 0.4 |
| C | 0.8 ± 0.5 | 0.4 ± 0.1 | 0.4 ± 0.4 | 0.8 ± 0.5 |
| D | 0 | 0 | 0.6 ± 0.4 | 0.6 ± 0.8 |
| E | 0 | 0 | 1.2 ± 0.6 | 1.2 ± 0.5 |
| Score | 0.8 ± 0.5 | 0.4 ± 0.1 | 3.6 ± 2.4* | 4.9 ± 2.9* |
The pathology score standard of lung tissue = A+B + C+D + E, Control-male vs. Exposure-male, Control-female vs. Exposure-female,* P < 0.05.
4. Discussion
Using an inhalation model of SD rats, we examined the toxicity effects of short term and low-dose methyl mercaptan exposure. Bodyweight of animals, hematology, clinical biochemistry, histopathology between control and exposure group were compared and significantly differences were observed.
After seven days of exposure at 0.5 ppm, the male group showed a 17.5% decrease in weight gain, while the female group showed a 6.7% decrease in weight gain compared with the control group. In an earlier study of the acute and subchronic toxicity of methyl mercaptan inhalation in rats, SD rats exposed to 2, 17, and 57 ppm of methyl mercaptan gas for three-month to observe subchronic toxicity and no mortality occurred in any group [27]. The most obvious effect was a decrease in body weight. The average body weight of each exposure group in end stage was lower than that of the control group. This difference was significant in the 57-ppm exposure group, which showed a 15% decrease in weight gain compared to the paired controls. These results were consistent with our research.
In the present study, we tested various hematological parameters of rats exposed to 0.5 ppm methyl mercaptan 6 h/day for 7 days. There were no significant differences between the control and exposure groups, except for a significant increase in PDW in exposed males.
Subsequently we investigated serum and tissue biochemical values of the rats. ALT, ASTm, and LDH were decreased after exposure. Significant increases in total protein were observed at all exposure levels in the study by Tansy et al., whereas a difference in this parameter was not observed among the groups in our experiment. The increased total protein in the Tansy et al. study may have been a result of dehydration, which is consistent with the observed decreases in water intake and the increase in fluid output. They also observed increases of conjugated and unconjugated serum bilirubin. The measurement of unconjugated bilirubin is estimated by the measurement of indirect bilirubin, and conjugated bilirubin is estimated by the measurement of DBIL. Indirect bilirubin was calculated using the values of TBIL and DBIL. In our study, TBIL and DBIL levels were not observed to be different among the groups. Tansy et al. suggested that the increases of serum cholesterol in the 2 and 17 ppm exposure groups observed in their study may have been associated with liver damage. The exposure time of our research was much shorter than that in the experiment by Tansy et al. and decreases of ALT, LDH, and ASTm values were observed, which may infer that the chemical will not cause a toxicity effect to the liver at such a low concentration of exposure and short exposure time. We consider the increase in PDW in the male group was considered to be an incidental finding, rather than representing a significant toxic hematological effect.
In recent years, with the development of free radical theory, it has been recognized that the occurrence of many lung diseases is related to an imbalance in the local oxidation-antioxidant system [37]. It is very important to keep a dynamic balance between oxygen free radicals and their eliminating agents for maintaining the normal structure and function of tissue cells. Oxidative stress can directly damage airway epithelium, aggravate airway inflammation, proinflammatory gene expression and eventually lead to airflow restriction [38]. Living organisms have different molecules that accelerate termination by neutralizing free radicals, thereby protecting cell membranes. One important antioxidant is superoxide dismutase (SOD). It has been suggested that a decrease of SOD activity in serum plays an important role in the pathological process of chronic lung injury and bronchitis. The decreased activity of SOD, one of the major oxygen free radical enzyme scavengers, might have resulted from enzyme consumption in an attempt by the host to limit the damage caused by reactive oxygen species. In this study, no statistically significant difference in SOD was observed between females in the control and exposed groups, while the male group showed a significant decrease after exposure. MDA produced by lipid peroxidation of polyunsaturated fatty acids, and the degree of lipid peroxidation can be estimated by the amount of MDA in tissues.
MDA in lung tissue in our study demonstrated a significant increase in the exposure group compared with the control group, suggesting lipid peroxidation as a possible mechanism of toxicity. Elevated oxidative stress and lipid peroxidation levels after methyl mercaptan exposure have been recognized as potential mechanisms that lead to observed terminal bronchiole constriction. These results revealed that inhalation of methyl mercaptan caused oxidative damage, with lipid peroxidation and alterations in blood antioxidant enzyme levels.
The lung is the first vital organ contacted with inhaled toxicants, and the most important target of these toxicants. In our study, bronchial and peri-bronchiolar inflammatory exudates in the lungs and surrounding alveoli were observed in the exposed groups. These findings suggest that some changes in the lung, kidney, and the immune system may be associated with subacute exposure to 0.5 ppm methyl mercaptan, which was recommended by ACGIH as the TLV-TWA. In the early stages of damage, a large number of inflammatory cells such as macrophages, neutrophils and lymphocytes infiltrate the interstitial tissue or alveoli. In the subsequent stages, substance related lesions occur, including epithelial damage with inflammation of tracheobronchial lesions. Continuous exposure may further cause to pulmonary fibrosis or edema, characterized by the loss of the original lung structure due to excessive expression and deposition of collagen and extracellular matrices [25,26]. A number of irritant gases have been related to bronchiolitis, causing inflammation or other adverse reactions [39]. Airway edema can occur after contact with these irritant gases, and other possible effects include headache, dizziness, tremors, seizure, nausea, and vomiting [40]. Among the survivors, these changes quickly disappeared. In patients progress to the second stage, physiological disorders include hypoxemia and associated restrictive or obstructive pulmonary dysfunction may develop [41]. In our research, bronchial and peri-bronchiolar inflammatory exudates were observed in lung tissues after exposure for seven days to 0.5 ppm methyl mercaptan, which present more severe effects than that in the study by Tansy et al. using a higher level of methyl mercaptan and animals with a lighter weight, about 90–100 g. This is proposed to be a compensatory change in the organism for protection at the beginning of exposure [42]. With prolonging of the exposure, lung tissue may be able to repair and restore the damage from the chemical exposure because of the developed immune system. Local wounds produced by toxic compound on the lung surface of rats may be recovered by a typical reparative process. Some research reported that it was cell migration and increased cell division in alveolar and bronchial tissues participated in these repairs [43].
Some studies have shown that the effects of pollutant inhalation on olfactory mucosa are dose- and time-dependent [44,45]. Animal models of chemical inhalation exhibited pathophysiological changes over a period of time, which was very similar to what was observed in patients with inhalation injury [46]. Atelectasis, emphysema and futher development of pulmonary edema decrease in arterial oxygen and progressive pulmonary deterioration, which caused to significant mortality [47]. After exposure to sulfur compounds, the lung pathology observed in different species was very similar. All other effects in this experiment are negligible and cannot be firmly established as compounds. From the results of our study, we think the value of ACGIH TLV of 0.5 ppm as an 8 h TWA may be too loose, and workers exposed to this concentration may cause some pulmonary stimulation and injury.
5. Conclusions
The exposure of rats to 0.5 ppm methyl mercaptan by inhalation over 7 days had adverse effects on clinical and histopathological parameters. Reduced food intake and body weight gain were noted in both sexes of the exposure group compared with the control group. Methyl mercaptan at 0.5 ppm may cause lung damage and activation of the immune system upon inhalation. The chemical had a greater adverse impact on renal function in female rats than in male rats. Lipid peroxidation and alterations in blood antioxidant enzyme levels were observed following methyl mercaptan exposure. Oxidative damage is recognized as a potential mechanism for terminal bronchiolar constriction, alveolar congestion, and erythrocyte exudation. Workers exposed to this concentration may cause some pulmonary stimulation and injury.
Conflict of interest
The authors declare that there are no conflicts of interests.
Transparency document
Acknowledgements
This work was financially supported by the Navy Foundation of China, National Natural Science Foundation of China (NO. 21507161), State Key Laboratory of Pollution Control and Resource Reuse Foundation, (NO. PCRRF14005), Research Fund of Tianjin Key Laboratory of Aquatic Science and Technology.
References
- 1.Kangas J., Jappinen P., Savolainen H. Exposure to hydrogen sulfide, mercaptans and sulfur dioxide in pulp industry. Aihaj. 1984;45(12):787–789. doi: 10.1080/15298668491400647. [DOI] [PubMed] [Google Scholar]
- 2.Leach J.M., Chung L.T.K. Gas concentration and occupational health in kraft mills. Tappi J. 1982;11:95–98. [Google Scholar]
- 3.Fang J.J., Zhang H., Yang N., Shao L.M., He P.J. Gaseous pollutants emitted from a mechanical biological treatment plant for municipal solid waste: Odor assessment and photochemical reactivity. J. Air Waste Manage. Assoc. 2013;63(11):1287–1297. doi: 10.1080/10962247.2013.822439. [DOI] [PubMed] [Google Scholar]
- 4.Tenhunen R., Savolainen H., Jäppinen P. Changes in haem synthesis associated with occupational exposure to organic and inorganic sulphides. Clin. Sci. 1983;64(2):187–191. doi: 10.1042/cs0640187. [DOI] [PubMed] [Google Scholar]
- 5.Torén K., Hagberg S., Westberg H. Health effects of working in pulp and paper mills: exposure, obstructive airways diseases, hypersensitivity reactions, and cardiovascular diseases. Am. J. Ind. Med. 1996;29(2):111–122. doi: 10.1002/(SICI)1097-0274(199602)29:2<111::AID-AJIM1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 6.Heaney C.D., Wing S., Campbell R.L., Caldwell D., Hopkins B., Richardson D., Yeatts K. Relation between malodor, ambient hydrogen sulfide, and health in a community bordering a landfill. Environ. Res. 2011;111(6):847–852. doi: 10.1016/j.envres.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jäppinen P., Kangas J., Silakoski L., Savolaine H. Volatile metabolites in occupational exposure to organic sulfur compounds. Arch. Toxicol. 1993;67(2):104–789. doi: 10.1007/BF01973679. [DOI] [PubMed] [Google Scholar]
- 8.Hanácek J., Korpás J. Respiratory reactions of nonanaesthetized cats following respiratory tract irritation with sulphur dioxide. Bratislavské Lekárske Listy. 1977;68(5):549–551. [PubMed] [Google Scholar]
- 9.Harbison R.D., Bourgeois M.M., Johnson G.T. Sulfur compounds hamilton & hardy’s industrial. Toxicology. 2015:391–400. [Google Scholar]
- 10.National Institute for Occupational Safety and Health (NIOSH) 2019. International Chemical Safety Cards (ICSC): Methyl Mercaptan.http://www.cdc.gov/niosh/ipcsneng/neng0299.html Available from: [accessed 21.01.15] [Google Scholar]
- 11.Nagata Y. 2003. Measurement of Odor Threshold by Triangle Odor Bag Method; pp. 118–127. [Google Scholar]
- 12.Wilby F.V. Variation in recognition odor threshold of a panel. J. Air Pollut. Control Assoc. 1969;19(2):96–100. [Google Scholar]
- 13.Leonardos G., Kendall D., Barnard N. Odor threshold determination of 53 odorant chemicals. J. Environ. Conserv. Eng. 1974;3(8):4–32. [Google Scholar]
- 14.Shults W.T., Fountain E.N., Lynch E.C. Methanethiol poisoning: irreversible coma and hemolytic anemia following inhalation. J. Am. Med. Assoc. 1970;211(13):2153–2154. doi: 10.1001/jama.211.13.2153. [DOI] [PubMed] [Google Scholar]
- 15.Ljunggren G., Norberg B. On the effect and toxicity of dimethyl sulfide, dimethyl disulfide and methyl mercaptan. Acta Physiol. 1943;4:248–255. [Google Scholar]
- 16.Luttrell W.E., Bobo M.E. Methyl mercaptan. J. Chem. Health Saf. 2015;22(5):37–39. [Google Scholar]
- 17.Zieve L., Doizaki W.M., Zieve J. Synergism between mercaptans and ammonia or fatty acids in the production of coma: a possible role for mercaptans in the pathogenesis of hepatic coma. J. Lab. Clin. Med. 1974;83(1):16–28. [PubMed] [Google Scholar]
- 18.Ahmed K., Zieve L., Quarfoth G. Effects of methanethiol on erthrocyte membrane stabilization and on Na+, K+- adenosine triphosphatase: relevance to hepatic coma. J. Pharmacol. Exp. Ther. 1984;228(1):103–108. [PubMed] [Google Scholar]
- 19.Palmiotto M., Fattore E., Paiano V., Celeste G., Colombo A., Davoli E. Influence of a municipal solid waste landfill in the surrounding environment: toxicological risk and odor nuisance effects. Environ. Int. 2014;68:16–24. doi: 10.1016/j.envint.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Aatamila M., Verkasalo P.K., Korhonen M.J., Suominen A.L., Hirvonen M., Viluksela M.K. Odour annoyance and physical symptoms among residents living near waste treatment centers. Environ. Res. 2011;111(1):164–170. doi: 10.1016/j.envres.2010.11.008. American Conference of Governmen- tal Industrial Hygienists (ACGIH) [DOI] [PubMed] [Google Scholar]
- 21.2014. TLVs and BEIs Based on the Documentation of the Threshold Limit Values for Chemical Substances and Physical Agents & Biological Exposure Indices ACGIH: Cincinnati, OH; p. 42. [Google Scholar]
- 22.Fang J.J., Zhang H., Yang N., Shao L.M., He P.J. Odor compounds from different sources of landfill: characterization and source identification. Waste Manage. 2012;32(7):1401–1410. doi: 10.1016/j.wasman.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Tenhunen R., Savolainen H., Jappinen P. Mortality from asthma and chronic obstructive pulmonary disease among workers in a soft paper mill: a case referent study. Br. J. Ind. Med. 1983;46:192–195. doi: 10.1136/oem.46.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato Y., Kuroda Y., Iitomi T., Kishi F., Fukuta Y., Abe T., Oshita S. Methylmercaptan poisoning usefulness of monitoring jugular venous oxygenation. Nihon Kyukyu Igakukai Zasshi. 2003;14(6):304–306. [Google Scholar]
- 25.Leala M.P., Brochettia R.A., Ignáciob A., Câmarab N.O.S., da Palmac R.K., de Oliveirac L.V.F., Silvaa D.F.T., Francoa A.L.S. Effects of formaldehyde exposure on the development of pulmonary fibrosis induced by bleomycin in mice. Toxicol. Rep. 2018;5:512–520. doi: 10.1016/j.toxrep.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H.Y., Cho E.S. 28-day inhalation toxicity of 3-methoxybutyl chloroformate in rats. Toxicol. Rep. 2018;5:213–219. doi: 10.1016/j.toxrep.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tansy M.F., Kendall F.M., Fantasia J., Landin W.E., Oberly R., Sherman W. Acute and subchronic toxicity studies of rats exposed to vapors of methyl mercaptan and other reduced- sulfur compounds. J. Toxicol. Environ. Health. 1981;8:71–88. doi: 10.1080/15287398109530051. [DOI] [PubMed] [Google Scholar]
- 28.Obici S., Otobone J.F., Da Silva Sela V.R., Ishida K., Da Silva J.C., Nakamura C.V., Cortez D.A.G., Audi E.A. Preliminary toxicity study of dichloromethane extract of kielmeyera coriaceastems in mice and rats. J. Ethnopharmacol. 2008;115(1):131–139. doi: 10.1016/j.jep.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Tan P.V., Mezui C., Enow-Orock G., Njikam N., Dimo T., Bitolog P. Teratogenic effects, acute and sub chronic toxicity of the leaf aqueous extract of ocimum suave wild (lamiaceae) in rats. J. Ethnopharmacol. 2008;115(2):232–237. doi: 10.1016/j.jep.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y.S., Kwon J.T., Shim I., Kim H.M., Kim P., Kim J.C., Lee K. Evaluation of toxicity to triclosan in rats following 28 days of exposure to aerosol inhalation. Regul. Toxicol. Pharmacol. 2015;71:259–268. doi: 10.1016/j.yrtph.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 32.Pothoven S.A., Nalepa T.F., Schneeberger P.J., Brandt S.B. Changes in diet and body condition of Lake Whitefish in Southern Lake Michigan associated with changes in benthos. N. Am. J. Fish. Manage. 2001;21(4):876–883. [Google Scholar]
- 33.Mrozek J.D., Smith K.M., Bing D.R., Meyers P.A., Simonton S.C., Connett J.E. Exogenous s Bx50 optical microscope (Olympus urfactant and partial liquid ventilation: physiologic and pathologic effects. Am. J. Respir. Crit. Care Med. 1997;156:1058–1065. doi: 10.1164/ajrccm.156.4.9610104. [DOI] [PubMed] [Google Scholar]
- 34.Rotta A.T., Gunnarsson B., Hernan L.J., Fuhrman B.P., Steinhorn D.M. Partial liquid ventilation influences pulmonary histopathology in an animal model of acute lung injury. J. Crit. Care. 1999;14(2):84–92. doi: 10.1016/s0883-9441(99)90019-9. [DOI] [PubMed] [Google Scholar]
- 35.Hamada C., Yoshino K., Matsumoto K., Nomura M., Yoshimura I. Tree-type algorithm for statistical analysis in chronic toxicity studies. J. Toxicol. Sci. 1998;23:173–181. doi: 10.2131/jts.23.3_173. [DOI] [PubMed] [Google Scholar]
- 36.Yin D., Xu X.Y., Li H.W., Corinne B., Pasan F., Terrence D.R. Acute and subacute toxicity studies of CMICE-013, a novel iodinated rotenone-based myocardial perfusion tracer, in Sprague Dawley rats and Gottingen minipigs. Regul. Toxicol. Pharmacol. 2016;80:195–209. doi: 10.1016/j.yrtph.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Wang F., Li C., Liu W., Jin Y. Oxidative stress levels of Kunming mice following short-term exposure to volatile organic compounds (VOCs) Mixture. J. Anim. Vet. Adv. 2012;11(18):3417–3426. [Google Scholar]
- 38.Young R.P., Hopkins R., Black P.N., Eddy C., Wu L., Gamble G.D., Mills G.D., Garrett J.E., Eaton T.E., Rees M.I. Functional variants of antioxidant genes in smokers with COPD and in those with normal lung function. Thorax. 2006;61(5):394–399. doi: 10.1136/thx.2005.048512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Camus P., Nemery B. A novel cause for bronchiolitis obliterans organizing pneumonia: exposure to paint aerosols in textile workshops. Eur. Respir. J. 1998;11(2):259–262. doi: 10.1183/09031936.98.11020259. [DOI] [PubMed] [Google Scholar]
- 40.Goyer N. Evaluation of occupational exposure to sulfur compounds in paper pulp kraft mills. Am. Ind. Hyg. Assoc. J. 1990;51(7):390–394. doi: 10.1080/15298669091369835. [DOI] [PubMed] [Google Scholar]
- 41.Fleming G.M., Chester E.H., Montenegro H.D. Dysfunction of small airwaysfollowing pulmonary injury due to nitrogen dioxide. Chest. 1979;75:720–721. doi: 10.1378/chest.75.6.720. [DOI] [PubMed] [Google Scholar]
- 42.Guven A., Lin W.Y., Leggett R.E., Kogan B.A., Levin R.M., Mannikarottu A. Effect of aging on the response of biochemical markers in the rabbit subjected to short-term partial bladder obstruction. Mol. Cell. Biochem. 2007;306(2):213–219. doi: 10.1007/s11010-007-9571-x. [DOI] [PubMed] [Google Scholar]
- 43.Simnett J.D., Fisher J.M. Cell division and tissue repair following localized damage to the mammalian lung. J. Morphol. 1976;48(2) doi: 10.1002/jmor.1051480204. 177-120. [DOI] [PubMed] [Google Scholar]
- 44.Newton P.E., Bolte H.F., Derelanko M.J., Hardisty J.F., Rinehart W.E. An evaluation of changes and recovery in the olfactory epithelium in mice after inhalation exposure to methylethylketoxime. Inhal. Toxicol. 2002;14:1249–1260. doi: 10.1080/08958370290084890. [DOI] [PubMed] [Google Scholar]
- 45.Katagiri T., Takeuchi T., Mine T., Noguchi T., Nishizawa T., Yamamoto S. Chronic inhalation toxicity and carcinogenicity studies of 3-chloro-2-methylpropene in BDF1 mice. Ind. Health. 2000;38:309–318. doi: 10.2486/indhealth.38.309. [DOI] [PubMed] [Google Scholar]
- 46.Herndon D.N., Traber L.D., Linares T.H. Etiology of the pulmonary pathophysiology associated with inhalation injury. Resuscitation. 1986;14:43–59. doi: 10.1016/0300-9572(86)90006-7. [DOI] [PubMed] [Google Scholar]
- 47.Lim Y.S., Chung M.H., Park S.H., Kim H.Y., Choi B.G., Lim H.W., Kim J.A., Yoo W.J. Acute and repeated inhalation lung injury by 3-methoxybutyl chloroformate in rats: CT-pathologic correlation. Eur. J. Radiol. 2007;62:227–234. doi: 10.1016/j.ejrad.2006.11.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.