Abstract
Laccases are multicopper oxidases containing four copper atoms per monomer distributed in three redox sites. Because of its tremendous applications in different areas, isolation of new laccases with wide range of industrial implementation. The present study focuses on the optimization of laccase production from Bacillus sp. MSK-01 under solid state fermentation conditions using fruit juice waste as the substrate. MSKLAC was produced extracellularly by the bacteria. This laccase was able to oxidize ABTS and syringaldazine. Various nutritional and environmental factors were utilized for increasing the enzyme yield. Plackett Burman was used to study the influence of input parameters on laccase yield. Tween-80, initial moisture ratio and magnesium sulphate were the major influencing factor affecting laccase yield. Central composite design of RSM was used for the modelling of experiment. Desirability approach was used to optimize laccase yield. Maximum laccase yield of 1645 IUg−1 was obtained when 0.55% of tween -80, 1:2.34 initial moisture ratio and 300μM magnesium sulphate was used. A 470 fold increase in the yield of laccase from unoptimized condition was obtained.
Keywords: Biotechnology, Microbiology
Abbreviations: RSM, response surface methodology; CCD, central composite design; PB, Plackett-Burman; SSF, solid state fermentation; 3D, three dimensional
1. Introduction
Laccases (benzenediol: oxygen oxidoreductases; EC 1.10.3.2) are multicopper oxidases (MCOs) which catalyze the oxidation of a wide variety of organic and inorganic compounds with concomitant four electron reduction of molecular oxygen to water. They catalyze the oxidation of both phenolic and non-phenolic substrates [1]. Laccases are very useful enzymes with respect to their applications in industry. They have found uses in biotechnological applications such as biobleaching, xenobiotics bioremediation, decolourization of textile dyes, biosensors, food industry, plastic degradation, degradation of lignin in lignocelluloses for the production of biofuels etc. [2, 3, 4].
Besides stability of an enzyme at industrial conditions, major obstacle in the path of the actual use of an enzyme at industrial level is its production cost. The development of procedures for the production of highly efficient, environment friendly and cost effective enzyme is a primary approach [5]. Solid state fermentation (SSF) is an cost effective method for increasing the enzyme production and is defined as any fermentation process occurring in the absence or near absence of free liquid, using an inert or a natural substrate as a solid support [6]. It is an economical process as it provides higher volumetric productivities [7].
Although most of the times SSF has been observed to be successful for filamentous organisms such as fungi, actinomycetes etc. [7, 8] but recently there are reports where SSF has been found to be useful for the production of enzymes from bacteria [9, 10]. Laccases are known to be secreted intracellularly by the bacteria. Therefore, SSF for laccase production was not reported in previous studies. Recently, it has been found that laccases are also secreted extracellularly by bacteria [11]. Sharma et al. [12] optimized the laccase production from Lysinibacillus sp. using SSF. They reported that 10 mg (w/w) of used TATA acti green tea leaves per 5 g of wheat bran, 1% pulp and paper industry effluent (agro based), and 1% wine made from Sygium cumini enhanced the laccase production 2.69, 2.61, and 2.09 fold, respectively. Muthukumarasamy et al. [13] optimized laccase production from B. subtilis MTCC 2414 using rice bran and wheat bran agroresidues. Maximum production was achieved at temperature 30 °C (270 ± 2.78 U/mL), pH 7.0 (345 ± 3.14 U/mL), and 96 h (267 ± 2.64 U/mL) of incubation. The carbon and nitrogen sources resulted in high enzyme yield at 3% sucrose (275 ± 3.11 U/mL) and 3% peptone (352.2 ± 4.32 U/mL) for rice bran and 3% sucrose (247.4 ± 3.51 U/mL) and 3% peptone (328 ± 3.33 U/mL) for wheat bran, respectively. Nevertheless, due to scarcity of extracellular laccase producing bacteria, utilization of agro-industrial residues for bacterial laccase production is a rare task. Furthermore, the researchers have employed one factor at a time approach (OFAT) for the optimization of laccase production through SSF [12, 13]. OFAT methods are good in assessing the effect of each variable on response independently but the interactive effect of variables on the response can never be assessed using OFAT. Statistical software's like response surface methodology is an ideal modeling approach for designing of experiments. In this regard, in the present study, a new Bacillus sp. MSK-01 producing extracelluar laccase was isolated and laccase production has been optimized through response surface methodology (RSM) based desirability approach.
Furthermore, the success of solid state fermentation (SSF) process is related to the characteristics of the support/substrate used. Porosity and particle size of the substrate affect the surface area accessible to the organism. The size of the substrate favors gas and nutrient diffusion, rate of colonization, CO2 removal and downstream extraction [14]. Small substrate particles provide a large surface area for microbial attachment while larger particles provide better aeration but a limited surface for microbial attachment. Therefore, selection of substrate is an important parameter in SSF. The fruit juice industry generates large amount of waste which is of no use. This waste is however is rich in phenolic compounds. Therefore, it has a potency to become good substrate for laccase production. But, till now none of the researchers has reported the utilization of fruit juice waste as substrate for laccase production. Many studies are based on the utilization of wheat bran as substrate [15, 16, 17, 18]. Other substrates like olive leaves [19], rice straw [20], coffee husk [21] etc. were also used for laccase production in the past few years.
By deeply analyzing the literature, it was observed that extracellular laccase producing bacteria are very few till date, RSM was not utilized for the production of laccase by bacteria in SSF and fruit juice waste was not used as substrate. Keeping all this in mind, the present study was designed to optimize laccase production using fruit juice waste as raw material in SSF by using RSM based desirability approach.
2. Materials and methods
2.1. Chemicals
Guaiacol used in the present study was purchased from Sigma (USA). Other chemicals were obtained from Hi-media (India) and were of analytical grade. Fruit juice waste was obtained from a local vendor of fruit juices.
2.2. Laccase assay
Laccase assay was performed at 75 °C for 10 min in 0.1 M Tris-HCl buffer (pH 8.0) using guaiacol (2mM) as substrate [22, 23]. The change in absorbance due to the oxidation of guaiacol was monitored at 465 nm (ε = 12,000 M-1 cm-1) in a UV–Visible spectrophotometer. The enzyme unit was expressed in IU g−1. One unit of laccase (IU g−1) was defined as the amount of the enzyme required to transform 1 μmol substrate per min per gram of substrate.
2.3. Isolation of extracellular laccase-producing bacteria
Extracellular laccase-producing bacteria were isolated on M162 medium supplemented with 0.2 % yeast extract, 0.2 % tryptone, 100 μM CuSO4, and 2mM guaiacol [24]. The detailed composition of M162 is given in Table 1. Soil samples were collected from different areas where lignocellulosic waste was decomposing. Samples were enriched in M162 medium containing 2mM guaiacol and appropriate dilutions were plated. The plates were incubated at 37 °C for 48 h. The colonies showing reddish brown color were selected.
Table 1.
Composition of M162.
| Micronutrient solution (10X) | (100 ml) |
| H2SO4 (conc.) | 5.0 ml |
| Manganese sulphate monohydrate (MnSO4.H2O) | 2.28 g |
| Zinc sulphate heptahydrate (ZnSO4.7H2O) | 5.0 g |
| Boric acid | 5.0 g |
| Copper sulphate pentahydrate (CuSO4.5H2O) | 250 mg |
| Sodium molybdate dihydrate | 250 mg |
| Cobalt chloride hexahydrate (CoCl2.6H2O) | 450 mg |
| M162 (10X) | (L−1) |
| Calcium sulphate dihydrate (CaSO4.2H2O) | 0.4 g |
| Magnesium chloride hexahydrate (MgCl2.6H2O) | 2.0 g |
| Nitriloacetic acid 1.0g Micronutrient solution (10X) | 1.0 ml |
| Ferric citrate solution (0.01M) | 5.0 ml |
2.4. Selection of bacterial strains producing extracellular laccase
Selected bacterial cultures were screened for the presence of extracellular laccase activity. For this, 0.1 % of overnight-grown bacterial culture was inoculated in M162 broth containing 0.2 % yeast extract, 0.2 % tryptone, and 100 μM CuSO4. After 48 h, the culture supernatant was obtained by centrifugation at 7826 x g for 15 min and was used as extracellular enzyme. The laccase activity was examined in extracellular enzyme preparations using guaiacol as substrate. The strains exhibiting extracellular laccase activities were selected. Furthermore, the oxidation of 2,2′-azino-bis[3-ethylbenzthiazoline-6-sulphonic acid] (ABTS) and syringaldazine (SGZ) and tyrosine was also assayed to confirm the presence of true laccase activity.
2.5. Identification of the isolate
The morphology of the isolate was studied by Gram staining. Physiological and biochemical characterization was done according to Bergey's Manual of Determinative Bacteriology [25]. Furthermore, the organism was identified based on 16S rDNA sequence, wherein the genomic DNA was isolated from the culture. Its quality was evaluated on 1.0% Agarose Gel, a single band of high-molecular weight DNA was observed. Fragment of 16S rDNA gene was amplified by PCR using 27F & 1492 R primers. A single discrete PCR amplicon band of 1500 bp was observed when resolved on Agarose. The PCR amplicon was purified to remove contaminants. Forward and reverse DNA sequencing reaction of PCR amplicon was carried out with forward and reverse primers using BDT v3.1 Cycle sequencing kit on ABI 3730xl Genetic Analyzer. Consensus sequence of 16S rDNA gene was generated from forward and reverse sequence data using aligner software (outsourcing done by Eurofins Genomics India Pvt. Ltd., Banglore, India). Sequences homologous to isolate MSK-01 were obtained using EzTaxon. Sequences with high query coverage and homology were selected for phylogenetic analysis [26]. Multiple sequence alignment was done using MultAlin (version 5) [27], and phylogenetic tree was constructed using MEGA version 4.0 [28].
2.6. Solid state fermentation of MSK-01 laccase
2.6.1. Inoculum preparation
The inoculum was prepared by inoculating a single bacterial colony from freshly grown plates into M162 medium containing 0.2% yeast extract, 0.2% tryptone and 100 μM CuSO4 in 250 ml Erlenmeyer flask. The culture was incubated at 37 °C, 150 rpm for 24 h and used as inoculum.
2.6.2. Extraction of laccase
The fermented fruit juice waste from each flask was suspended in 25 ml Tris-HCL buffer (100 mM, pH 8.0) and shaken at 150 rpm for 45 min. The extrudates were squeezed through muslin cloth for maximizing the enzyme extraction and centrifuged at 7826 x g for 20 min. The enzyme solution thus obtained was assayed for laccase activity.
2.6.3. Selection of significant parameters by Plackett-Burman (PB).
A set of 12 experiments was designed using the Plackett-Burman design of the Design expert (version 9.0.7) software (Stat-Ease Corporation, USA) for 11 variables (Table 2) that were analyzed as possible factors affecting production based on literature search. The parameters evaluated were as follows: A: temperature, B: pH, C: incubation time, D: CuSO4.5H2O, E: moisture ratio, F: magnesium sulphate, G: yeast extract, H: maganese sulphate, J: moistening solution, K: Tween 80, L: inoculum. In each experiment, dried fruit jiuce waste was taken as the basal solid media (5g) in 250 ml erlenmyer falsk. Concentration levels were decided on the basis of literature reports on laccase production. All the trials were carried out in set of three and laccase yield was calculated as mean of these three values. The effect of each parameter on laccase yield was obtained as per following equation:
| (1) |
where, Vi is the effect of each input variable i under study, Yi+ and Yi- are responses (laccase yield) of trials at which the parameter was at its high and low level, respectively, and N is the total number of trials. The significance of the model was calculated by ANOVA. From the pareto chart, the factors showing highest positive effects were selected for optimization using Central Composite Design of Response Surface Methodology.
Table 2.
Parameters, experimental runs and responses of Placket-Burman design used for the selection of significant parameters for laccase production in solid state fermentation using Bacillus sp. MSK-01.
| Run | Time (h) | Temp (°C) | pH | CuSO4 (μM) | Moisture ratio | MgSO4 (μM) | MnSO4 (μM) | yeast extract (%) | Moistening solution | Tween -80 (%) | Inoculum (%) | Activity (IU/g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 96 | 37 | 9 | 0 | 1:1 | 0 | 100 | 0 | 1 | 0.1 | 10 | 105.75 |
| 2 | 48 | 37 | 9 | 100 | 1:1 | 0 | 0 | 0 | 0 | 0.1 | 20 | 39.66 |
| 3 | 96 | 30 | 7 | 0 | 1:2 | 0 | 100 | 0 | 0 | 0.1 | 20 | 73.67 |
| 4 | 96 | 30 | 9 | 100 | 1:1 | 100 | 100 | 0 | 0 | 0.0 | 10 | 84.33 |
| 5 | 48 | 37 | 7 | 100 | 1:2 | 0 | 100 | 0 | 1 | 0.0 | 10 | 26.67 |
| 6 | 48 | 37 | 9 | 0 | 1:2 | 100 | 100 | 0 | 0 | 0.0 | 20 | 25.58 |
| 7 | 96 | 37 | 7 | 0 | 1:1 | 1000 | 0 | 0 | 1 | 0.0 | 20 | 91.42 |
| 8 | 96 | 30 | 9 | 100 | 1:2 | 0 | 0 | 0 | 1 | 0.0 | 20 | 76.16 |
| 9 | 48 | 30 | 7 | 0 | 1:1 | 0 | 0 | 0 | 0 | 0.0 | 10 | 0.17 |
| 10 | 48 | 30 | 7 | 100 | 1:1 | 100 | 100 | 0 | 1 | 0.1 | 20 | 0.12 |
| 11 | 96 | 37 | 7 | 100 | 1:2 | 100 | 0 | 0 | 0 | 0.1 | 10 | 160.91 |
| 12 | 48 | 30 | 9 | 0 | 1:2 | 100 | 0 | 0 | 1 | 0.1 | 10 | 93.34 |
2.6.4. Optimization of laccase production using response surface methodology (RSM)
Central composite design (CCD) of RSM was used from software Design expert (version 9.0.7, Statease Inc., Minneapolis, USA) at α value of ±2 to further optimize the levels of significant variables. Laccase activity was recorded as response. Response data were fed and analyzed by the software to generate 3D plots indicating the optimum conditions and interaction among these factors. Regression analysis was performed on the data obtained. A second-order polynomial equation was used to fit the data by multiple regression procedure. A quadratic model was obtained as per following equation:
| (2) |
where, Y is the predicted response [laccase activity (IU g−1)], ??0 is the constant term, ??i the linear coefficients, ??ii the squared coefficients and ??ij the interaction coefficients. The quality of fitting by the polynomial model equation was expressed using coefficient of determination R2. Eq. (2) was used to construct 3D plots.
2.6.5. Prediction of optimum values for maximum laccase production
After getting the model equation that explains the process, it was used for optimization of the laccase production using the numerical optimization option of the software. Criteria were set for each independent variable and the response (dependent variable). The independent variables were kept in the range used by the experimental set up and the response was set maximum. A solution was generated with predicted levels of the independent variables and predicted maximum production.
2.6.6. Validation experiments
To check the validity of the chosen quadratic model, experiments were designed using the predicted optimum values of the parameters from Eq. (2). The laccase activity was measured and compared with the predicted value. Each experiment was conducted in triplicates and the data presented as mean ± SD.
3. Results and discussion
In this present study, a search has been made to isolate novel bacteria that produce extracellular laccase. The productivity of laccase from bacteria depends largely on the medium constituents and their concentration. Concentration of phosphate was found to play a significant role in the isolation of laccase producing bacteria. Other media components such as yeast extract, tryptone and glucose also play significant role. It has been reported in literature that phosphate exerts negative effect on laccase synthesis. M162 media contains no phosphate and is therefore used for the isolation of laccase producing bacteria [29]. A total of 2 bacteria, Isolate MSk-01 and Isolate MSK-03 giving extracellular activity were isolated. To confirm the presence of true laccase activity, isolates MSK-01 and MSK-03 were further screened on the basis of 2,2′-azino-bis[3-ethylbenzthiazoline-6-sulphonic acid] (ABTS) and syringaldazine (SGZ) oxidizing ability of their crude enzymes. Laccase from MSK-01 was able to oxidize ABTS and SGZ but not tyrosine while MSK-03 was not (data not shown); therefore, Isolate MSK-01 was selected as a source of extracellular laccsae. ABTS and SGZ are true substrate of laccase. Other substrates like guaiacol and dimethoxypphenol can be oxidized by enzymes other than laccase as well. Enzymes showing oxidation of ABTS and SGZ but not tyrosine are considered to be laccase specifically [11].
3.1. Identification of isolate MSK-01
With a view to identify the isolate MSK-01, it's morphological and biochemical traits were extensively studied. The organism showed Gram positive sporulating rods. All characteristic features indicated that isolate MSK-01 belongs to Genus Bacillus. Sequencing of the 16S rRNA gene has served as an authenticated tool for determining phylogenetic relationships between bacteria [30]. Therefore, to further identify the Isolate MSK-01 upto species level, partial sequencing of the 16S rRNA gene was carried out. The results of 16S rRNA partial gene sequence analysis correlated with the results of phenotypic identification that the strain under study belonged to the Genus Bacillus. However, Isolate MSK-01 was not having more than 89% similarity from the known strain of Bacillus. Therefore, the Isolate MSK-01 might be a new species belonging to Genus Bacillus. Therefore, for this study, the isolate was names as Bacillus sp. MSK-01.
3.2. Selection of significant parameters for optimum MSK laccase production
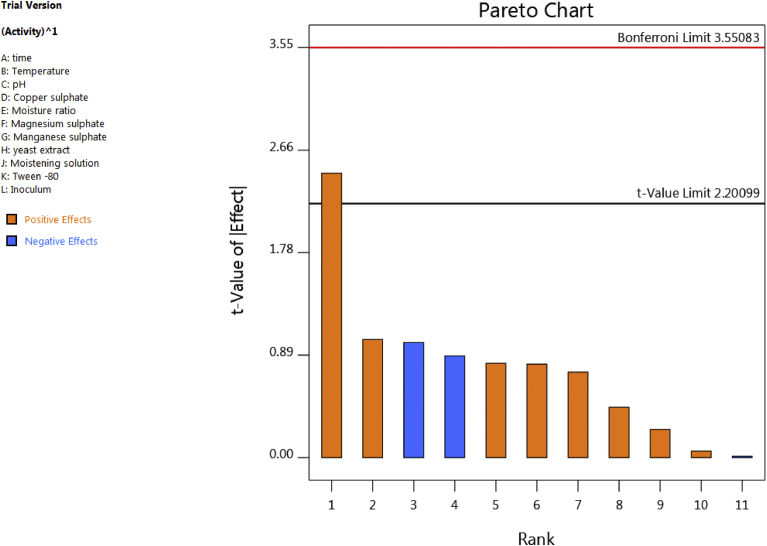
Plackett Burman analysis tells about the influence of each factor under study to the output responses. The factors which influence positively and negatively to enzyme production can be studied by PB analysis. A larger Placket Burman design is necessary for getting higher quality information on the significance of each real factor [31]. Therefore, 11 different parameters were selected on the basis of data available in literature and PB design was employed to choose significant parameters among them (Table 2). The influence of various parameters on MSK laccase production was estimated and graphically represented in the form of pareto chart (Fig. 1). Length of column represented the significance of the influence of studied parameters on enzyme yield. Among the 11 parameters, only inoculums concentration and manganese sulphate concentration negatively affected the production, rest all parameters have positive effect on laccase production. It can be due to fact that initial amount of inoculum to be added to the medium affects enzyme production as more number of cells in the inoculum would ensure rapid proliferation and biomass synthesis by shortening the lag phase. A very low inoculum level was inadequate for enzyme production while the inoculum level above optimum decreased the yield, probably due to competition for the nutrients [32]. Probably the initial inoculum was used at higher level resulted in negative effect on laccase production in the present study. In case of manganese, it is reported in literature that Mn2+ ions decrease the amount of laccase mRNA which resulted in decreased level of laccase in the presence of MnSO4 [33].
Fig. 1.
Pareto chart showing the effect of various factors on laccase production. Positive influencing factors were shown in orange while the negative one were shown in blue.
The results obtained from the pareto chart were analyzed by analysis of variance (ANOVA) test for their significance (Table 3). The results confirmed that the model was significant with p < 0.05. It was observed that the first three influential parameters were tween-80, initial moisture content and magnesium suplhate concentration. These three factors were selected for further increase in laccase production by RSM.
Table 3.
Analysis of variance for Placket-Burman response.
| Source | Sum of Squares | df | Mean Square | F-value | p-value | |||
|---|---|---|---|---|---|---|---|---|
| Model | 25010.64 | 10 | 2501.06 | 6937.12 | 0.0093 | significant | ||
| A-time | 13783.74 | 1 | 13783.74 | 38231.53 | 0.0033 | |||
| B-Temperature | 1244.40 | 1 | 1244.40 | 3451.56 | 0.0108 | |||
| C-pH | 430.32 | 1 | 430.32 | 1193.57 | 0.0184 | |||
| E-Moisture ratio | 1516.05 | 1 | 1516.05 | 4205.02 | 0.0098 | |||
| F-Magnesium sulphate | 1487.86 | 1 | 1487.86 | 4126.83 | 0.0099 | |||
| G-Manganese sulphate | 1765.16 | 1 | 1765.16 | 4895.96 | 0.0091 | |||
| H-yeast extract | 136.01 | 1 | 136.01 | 377.26 | 0.0327 | |||
| J-Moistening solution | 6.96 | 1 | 6.96 | 19.31 | 0.1425 | |||
| K-Tween -80 | 2383.46 | 1 | 2383.46 | 6610.94 | 0.0078 | |||
| L-Inoculum | 2256.67 | 1 | 2256.67 | 6259.24 | 0.0080 | |||
| Residual | 0.3605 | 1 | 0.3605 | |||||
| Cor Total | 25011.00 | 11 | ||||||
| Std. Dev. | 0.6004 | R2 | 1.0000 | |||||
| Mean | 64.81 | Adjusted R2 | 0.9998 | |||||
| C.V. % | 0.9264 | Predicted R2 | 0.9979 | |||||
| Adeq Precision | 280.2084 | |||||||
3.3. Response surface methodology
The levels of the three significant parameters which have positive influence on laccase production were used as central values to design experiments for central composite response surface design (Table 4).
Table 4.
Central composite rotary design matrix with experimental and predicted values of laccase production from Bacillus sp. MSK-01 in solid state fermentation.
| Run | A:Tween 80 |
B:Moisture ratio |
C:Magnesium sulphate |
Yield |
|---|---|---|---|---|
| % | ratio | micromolar | IU/g | |
| 1 | 1.00 | 2.00 | 100 | 383 |
| 2 | 0.55 | 1.50 | 300 | 1645.8 |
| 3 | 0.10 | 1.00 | 500 | 592 |
| 4 | 0.55 | 2.34 | 300 | 645.83 |
| 5 | 0.10 | 2.00 | 500 | 1327.5 |
| 6 | 0.10 | 2.00 | 100 | 1391.6 |
| 7 | 0.55 | 1.50 | 300 | 1650 |
| 8 | 1.00 | 2.00 | 500 | 300 |
| 9 | 0.55 | 1.50 | 36.36 | 450 |
| 10 | 1.00 | 1.00 | 100 | 975 |
| 11 | 0.55 | 1.50 | 300 | 1625 |
| 12 | 0.55 | 0.66 | 300 | 555.18 |
| 13 | 0.55 | 1.50 | 300 | 1620 |
| 14 | 0.10 | 1.00 | 100 | 340 |
| 15 | 0.55 | 1.50 | 300 | 1630 |
| 16 | 0.55 | 1.50 | 636.36 | 683.34 |
| 17 | 0.55 | 1.50 | 300 | 1664 |
| 18 | 1.00 | 1.00 | 500 | 1160.83 |
| 19 | 1.31 | 1.50 | 300 | 1237.5 |
| 20 | -0.21 | 1.50 | 300 | 1633.3 |
By applying multiple regression analysis on the experimental data, a predictive quadratic polynomial equation was constructed to describe the correlation between enzyme yield and three significant parameters as follows:
| Laccase yield (IU g−1) = +1639.52–109.68 * A +35.64 * B +50.02 * C -404.99 * A * B -10.63 * A * C -73.12 * B * C -74.56 * A2 -369.74 * B2 -381.70 * C2 -109.68 * A +35.64* B +50.02 * C -404.99 * A * B -10.63 * A * C -73.12 * B * C -74.56 * A2 -369.74 * B2 -381.70* C2 | (3) |
where, A, B and C were the coded values of tween 80, initial moisture level and concentration of magnesium sulphate respectively. The analysis of variance for the response surface quadratic model is summarized in Table 5. The p-values <0.05 indicated that the model terms are significant. In this case A, B, C, AB, BC, A2, B2, C2 are significant model terms. The p-value for lack of fit was 0.0777, indicating that this quadratic model adequately fit into the data. The determination coefficient, R2 (0.9974) indicated that the predicted and experimental values had perfect coherence with each other. The value of adjusted R2 (0.9913) suggested that the variation of 99.13% in the enzyme activity was attributed to the independent variables and only 0.87% of the total variation could not be explained by the model.
Table 5.
Analysis of variance for central composite design.
| Source | Sum of Squares | df | Mean Square | F-value | p-value | |||
|---|---|---|---|---|---|---|---|---|
| Model | 5.275E+06 | 9 | 5.861E+05 | 822.15 | <0.0001 | significant | ||
| A-Tween 80 | 1.643E+05 | 1 | 1.643E+05 | 230.48 | <0.0001 | |||
| B-Moisture ratio | 17346.66 | 1 | 17346.66 | 24.33 | 0.0006 | |||
| C-Magnesium sulphate | 34173.83 | 1 | 34173.83 | 47.94 | <0.0001 | |||
| AB | 1.312E+06 | 1 | 1.312E+06 | 1840.69 | <0.0001 | |||
| AC | 904.61 | 1 | 904.61 | 1.27 | 0.2863 | |||
| BC | 42767.89 | 1 | 42767.89 | 60.00 | <0.0001 | |||
| A2 | 80111.34 | 1 | 80111.34 | 112.38 | <0.0001 | |||
| B2 | 1.970E+06 | 1 | 1.970E+06 | 2763.69 | <0.0001 | |||
| C2 | 2.100E+06 | 1 | 2.100E+06 | 2945.42 | <0.0001 | |||
| Residual | 7128.55 | 10 | 712.86 | |||||
| Lack of Fit | 5698.42 | 5 | 1139.68 | 3.98 | 0.0777 | not significant | ||
| Pure Error | 1430.13 | 5 | 286.03 | |||||
| Cor Total | 5.282E+06 | 19 | ||||||
| Std. Dev. | 26.70 | R2 | 0.9987 | |||||
| Mean | 1075.49 | Adjusted R2 | 0.9974 | |||||
| C.V. % | 2.48 | Predicted R2 | 0.9913 | |||||
| Adeq Precision | 70.9115 | |||||||
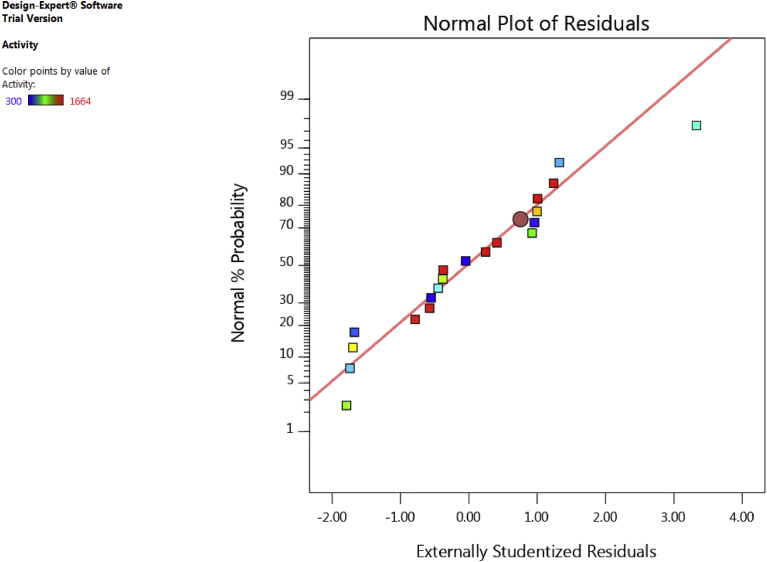
3.4. Diagnosis of the statistical properties of the model
Statistical properties of the model can be analyzed by the normal plot for residuals. Data points should be approximately linear. A non-linear pattern (such as an S-shaped curve) indicates non-normality in the error term, which may be corrected by a transformation. The residuals of laccase yield show normal distribution (Fig. 2). Their alignment on the drawn line indicates that there are no abnormalities in the data.
Fig. 2.
Normal probability plot of residuals of laccase yield showing their normal distribution.
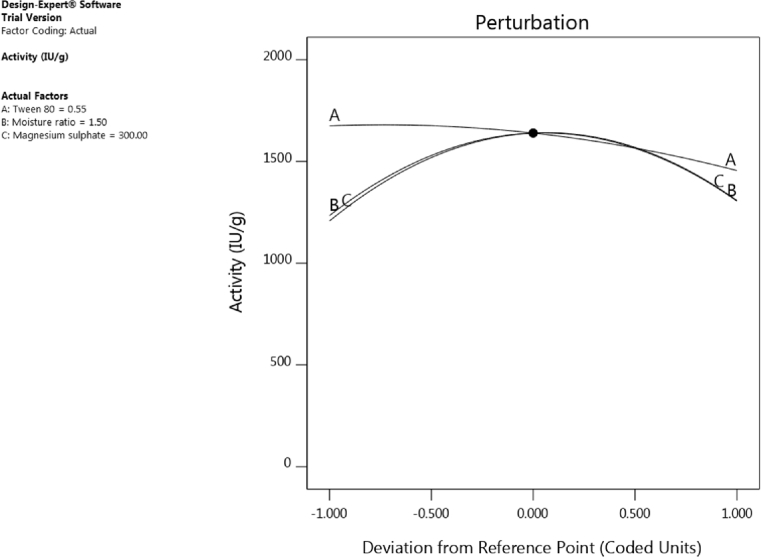
3.5. Perturbation plot
The perturbation plot shows how the response changes as each factor moves from the chosen reference point, with other factors were held constant at the reference value. Fig. 3 shows that laccase yield was affected more by tween-80 followed by initial moisture content and concentration of magnesium sulphate. Increasing the concentration of tween-80 resulted in increase in laccase yield while increasing the level of magnesium sulphate and initial moisture content upto the coded reference point of 0.25 increased laccase yield. But further increase beyond the coded point decreased laccase yield.
Fig. 3.
Perturbation graph for three factors affecting laccase yield.
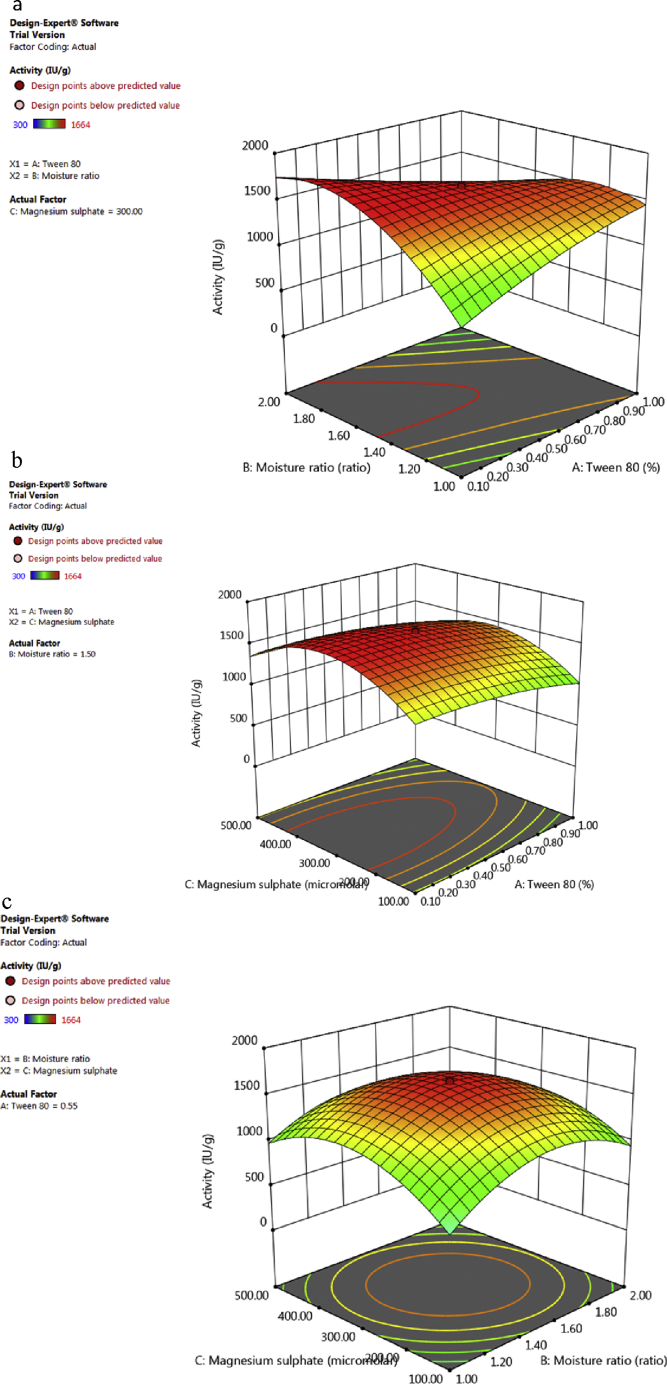
3.6. Interaction between variables
The interaction between two variables when the third is kept at its optimum value is presented in 3D graphs (Fig. 4). The 3D graphs showed how laccase production is affected by the variation of two factors at a time keeping the other at optimal condition. Laccase production increased with increase in initial moisture content and concentration of Tween-80 individually (Fig. 4a) but both factors resulted in decrease in laccase yields in combination. Initial moisture content also plays an important role in SSF [34]. The availability of initial water in lower or higher amounts affects microbial activity adversely. Initial moisture content of 1:1.5 (45%) was found to be optimum for MSK laccase production. At 1:0.5 moisture content, the organism was unable to grow significantly and at 1:2 moisture ratio, due to high moisture content, growth of bacteria ceases. This can be due to the reason that the porosity of the medium decreases with the increase in moisture content leading to the difficulties of oxygen transfer and heat release in the medium [35]. An initial moisture content of 65% was the optimum for laccase production by Streptomyces psammoticus [7]. Laccase from fungi Tremetes versicolor and Pleurotus sajorkaju was produced maximally at 80% (w/v) moisture level [19, 35].
Fig. 4.
Three dimensional response surface plots showing the effect of interaction of (a) Tween 80 and Moisture ratio (b) Moisture ratio and magnesium sulphate (c) Tween-80 and Magnesium sulphate on laccase production from Bacillus sp. MSK-01.
The use of surfactants for enzyme production in SSF has also been well-documented [18]. Surfactants increase the permeability of the membrane's lipid bilayer, which facilitates the secretion of enzymes. Maximum yield of extracellular MSK laccase was achieved when 0.5% tween-80 was supplemented to the medium. Tween 80 has been reported to enhance the secretion of laccases in Pycnoporus sanguineus [36], Ganoderma sp. KK-02 [37], Trametes trogii [38] etc.
Similarly, magneisum sulphate and initial moisture content increased the laccase yield upto central value, further increase resulted in decrease in laccase production (Fig. 4b). Tween-80 and magnesium sulphate resulted in maximum laccase production at central level (Fig. 4c).
3.7. Validation of predicted variables for maximum laccase production
As the maximum yield was obtained in central run, therefore the model was internally validated. At the end of RSM, 470 fold (from 3.50 IU/g – 1645 IU/g) increase in laccase yield could be achieved. A remarkable increase in the enzyme activity (470 fold increase) was achieved under optimized conditions. Thus, the results of optimization studies helped in substantial increase in yield of laccase from Bacillus sp. MSK-01 which shall help in its industrial application.
4. Conclusions
Bacterial enzyme production through fermentation of solid substrates is a possible solution to the cost related problems encountered in enzyme production. In this study, Bacillus sp. MSK-01 was cultured under SSF conditions using fruit juice waste as substrate. The fruit juice waste is of no value and thus adds no cost to the production. Laccase production in SSF was optimized. Maximum laccase yield of 1645 IUg−1 was obtained when 0.55% of tween -80, 1:2.34 initial moisture ratio and 300μM magnesium sulphate was used. A 470 fold increase in the yield of laccase from unoptimized condition was obtained. Thus, production of MSK-01 laccase under optimized conditions in SSF will be an economically feasible laccase production system.
Declarations
Author contribution statement
Sonica Sondhi: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Kiranjot Saini: Performed the experiments.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
I sincerely thank Chandigarh Group of Colleges, Landran, Mohali for providing me the necessary facilities for carrying out the research work.
References
- 1.Chandra R., Chowdhary P. Properties of bacterial laccases and their application in bioremediation of industrial wastes. Environ. Sci. Process. Impacts. 2015;17:326–342. doi: 10.1039/c4em00627e. [DOI] [PubMed] [Google Scholar]
- 2.Buddolla V., Bandi R., Avilala J., Arthala P.K., Golla N. Fungal laccases and their applications in bioremediation. Enzym. Res. 2014;2014:163242. doi: 10.1155/2014/163242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sondhi S., Kaur R., Kaur S., Kaur P.S. Immobilization of laccase-ABTS system for the development of a continuous flow packed bed bioreactor for decolorization of textile effluent. Int. J. Biol. Macromol. 2018;117:1093–1100. doi: 10.1016/j.ijbiomac.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Kudanga T., Le Roes-Hill M. Laccase applications in biofuels production: current status and future prospects. Appl. Microbiol. Biotechnol. 2014;98:6525–6542. doi: 10.1007/s00253-014-5810-8. [DOI] [PubMed] [Google Scholar]
- 5.Osma J.F., Toca Herrera J.L., Couto S.R. Cost analysis in laccase production. J. Environ. Manag. 2011;92:2907–2912. doi: 10.1016/j.jenvman.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 6.Chhaya R., Modi H.A. Statistical optimization of laccase producing Streptomyces chartreusis by solid state fermentation. CIBTech J. Microbiol. 2013;3(1):2319–3867. [Google Scholar]
- 7.Niladevi K.N., Sukumaran R.N., Prema P. Utilization of rice straw for laccase production by Streptomyces psammoticus in solid-state fermentation. J. Ind. Microbiol. Biotechnol. 2007 doi: 10.1007/s10295-007-0239-z. [DOI] [PubMed] [Google Scholar]
- 8.Sun J., Guo N., Niu L.L., Wang Q.F., Zang Y.P., Zu Y.G., Fu Y.J. Production of laccase by a new Myrothecium verrucaria MD-R-16 Isolated from Pigeon Pea [Cajanus cajan (L.) Millsp. and its application on dye decolorization. Molecules. 2017;22:673–689. doi: 10.3390/molecules22040673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koyani R.D., Rajput K.S. Solid state fermentation: comprehensive tool for utilization of lignocellulosic through biotechnology. J. Bioprocess. Biotech. 2015;5:258. [Google Scholar]
- 10.Kaur P.S., Sondhi S., Kaur S., Kaur H., Bansal S. Statistical Optimization of the production of α-amylase from Bacillus licheniformis MTCC 1483 using paddy straw as substrate. J. Commer. Biotechnol. 2017;23:37–45. [Google Scholar]
- 11.Sondhi S., Sharma P., Saini S., Puri N., Gupta N. Purification and characterization of an extracellular, thermo-alkali-stable, metal tolerant laccase from Bacillus tequilensis SN4. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma A., Gupta V., Khan M., Balda S., Gupta N., Capalash N., Sharma P. Flavonoid-rich agro-industrial residues for enhanced bacterial laccase production by submerged and solid-state fermentation. 3 Biotech. 2017;7:201–208. doi: 10.1007/s13205-017-0836-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muthukumarasamy N.P., Jackson B., Raj A.J., Sevanan M. Production of extracellular laccase from Bacillus subtilis MTCC 2414 using agro residues as a potential substrate. Biochem. Res. Int. 2015;2015:1–9. doi: 10.1155/2015/765190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couto S.R., Sanroman M.A. Application of solid-state fermentation to ligninolytic enzyme production. Biochem. Eng. J. 2005;22:211–219. [Google Scholar]
- 15.Revankar M.S., Desai K.M., S.S. Solid-state fermentation for enhanced production of laccase using indigenously isolated Ganoderma sp. Appl. Biochem. Biotechnol. 2007;143:16–26. doi: 10.1007/s12010-007-0029-0. [DOI] [PubMed] [Google Scholar]
- 16.DeSouza C.G.M., Perlata R.M. Purification and characterization of the main laccase produced by the white rot fungus Pleurotus pulmonarius on wheat bran solid state medium. J. Basic Microbiol. 2003;43:278–286. doi: 10.1002/jobm.200390031. [DOI] [PubMed] [Google Scholar]
- 17.Elshafei A.M., Hassan M.M., Haroun B.M., Elsayed M.A., Othman A.M. Optimization of laccase production from Penicillium martensii NRC 345. Adv. Life Sci. 2012;2(1):31–37. [Google Scholar]
- 18.Nandal P., Ravella S.R., Kuhad R.C. Laccase production by Coriolopsis caperata RCK2011: optimization under solid state fermentation by Taguchi DOE methodology. Sci. Rep. 2013;3:1–7. doi: 10.1038/srep01386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aydinoglu T., Sargin S. Production of laccase from Trametes versicolor by solid-state fermentation using olive leaves as a phenolic substrate. Bioprocess Biocyst. Eng. 2012;36:215–222. doi: 10.1007/s00449-012-0777-2. [DOI] [PubMed] [Google Scholar]
- 20.Risdianto H., Sofianti E., Suhardim S.H., Setiadi T. Optimization of laccase production using white rot fungi and agriculture wastes in solid state fermentation. ITB J. Eng. Sci. 2012;44:93–105. [Google Scholar]
- 21.Gonzalez J.C., Medina S.C., Rodriguez A., Osma J.F., Almeciga Diaz C.J., Sanchez O.F. Production of Trametes pubescens laccase under submerged and semi-solid culture conditions on agro industrial wastes. PLoS One. 2013;8:73721. doi: 10.1371/journal.pone.0073721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh G., Ahuja N., Sharma P., Capalash N. Response surface methodology for the optimized production of an alkalophilic laccase from gamma-proteobacterium JB. BioResources. 2009;4(2):544–553. [Google Scholar]
- 23.Sheikhi F., Ardakani M.R., Enayatizamir N., Rodriguez-Couto S. The determination of assay for laccase of Bacillus subtilis WPI with two classes of chemical compounds as substrates. Indian J. Microbiol. 2012;52(4):701–707. doi: 10.1007/s12088-012-0298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sondhi S., Sharma P., George N., Chauhan P.S., Puri N., Gupta N. An extracellular thermo-alkali-stable laccase from Bacillus tequilensis SN4, with a potential to biobleach softwood pulp. 3 Biotech. 2015;5(2):175–185. doi: 10.1007/s13205-014-0207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holt J.G., Krieg N.R., Sneath P.H.A., Staley J.T., Williams S.T., Williams W. ninth ed. 1994. Bergey’s Manual of Determinative Bacteriology. Waverly Baltimore MD. [Google Scholar]
- 26.Chun J., Lee J.H., Jung Y., Kim M., Kim S., Kim B.K., Lim Y.W. EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int. J. Syst. Evol. Microbiol. 2007;57:2259–2261. doi: 10.1099/ijs.0.64915-0. [DOI] [PubMed] [Google Scholar]
- 27.Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988;16(22):10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamura K., Dudley J., Nei M., Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 29.Kaur K., Singh G., Gupta V., Capalash N., Sharma P. Impact of phosphate and other medium components on physiological regulation of bacterial laccase production. Biotechnol. Prog. 2017;33(2):541–548. doi: 10.1002/btpr.2408. [DOI] [PubMed] [Google Scholar]
- 30.Gatson J.W., Benz B.F., Chandrasekaran C., Satomi M., Venkateswaran K., Hart M.E. Bacillus tequilensis sp. nov isolated from a 2000 year old Mexican shaft tomb, is closely related to Bacillus subtilis. Int. J. Syst. Evol. Microbiol. 2006;56:1475–1484. doi: 10.1099/ijs.0.63946-0. [DOI] [PubMed] [Google Scholar]
- 31.Li J., Ma C., Ma Y., Li Y., Zhou W., Xu P. Medium optimization by combination of response surface methodology and desirability function: an application in glutamine production. Appl. Microbiol. Biotechnol. 2007;74:563–571. doi: 10.1007/s00253-006-0699-5. [DOI] [PubMed] [Google Scholar]
- 32.Patel H., Gupte A., Gupte S. Effect of different culture conditions and inducers on production of laccase by a basidiomycete fungal isolate Pleurotus ostreatus HP-1 under solid state fermentation. BioResources. 2009;4(1):268–284. [Google Scholar]
- 33.Manubens A., Canessa P., Folch C., Avila M., Salas L., Vicun R. Manganese a¡ects the production of laccase in the basidiomycete Ceriporiopsis subvermispora. FEMS Microbiol. Lett. 2007;275:139–145. doi: 10.1111/j.1574-6968.2007.00874.x. [DOI] [PubMed] [Google Scholar]
- 34.Soccol C.R., Ferreira da Costa E.S., Letti L.A.J., Karp S.G., Woiciechowski A.L., de Souza Vandenberghe L.P. Recent developments and innovations in solid state fermentation. Biotechnol. Res. Innov. 2017;2017 [Google Scholar]
- 35.Patrick F., Mtui G., Mshandete A.M., Kivaisi A. Optimization of laccase and manganese peroxidase production in submerged culture of Pleurotus sajorcaju. Afr. J. Biotechnol. 2011;10:10166–10177. [Google Scholar]
- 36.Pointing S.B., Jones E.B.G., Vrumed L.L.P. Optimization of laccase production by P. sanguineus in submerged liquid culture. Mycologia. 2000;92:139–144. [Google Scholar]
- 37.Sharma K.K., Shrivastava B., Sastry V.R.B., Sehgal N., Kuhad R.C. Middle-redox potential laccase from Ganoderma sp.: its application in improvement of feed for monogastric animals. Sci. Rep. 2013;3:1299. doi: 10.1038/srep01299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levin L., Forchiassin F., Viale A. Ligninolytic enzyme production and dye decolorization by Trametes trogii: application of the Plackett-Burman experimental design to evaluate nutritional requirements. Process Biochem. 2005;40:1381–1387. [Google Scholar]