Abstract
BACKGROUND:
While very low birth weight (VLBW) infants often require multiple red blood cell transfusions, efforts to minimize transfusion-associated risks have resulted in more restrictive neonatal transfusion practices. However, whether restrictive transfusion strategies limit transfusions without increasing morbidity and mortality in this population remains unclear. Recent epidemiologic studies suggest that severe anemia may be an important risk factor for the development of necrotizing enterocolitis (NEC). However, the mechanism whereby anemia may lead to NEC remains unknown.
STUDY DESIGN AND METHODS:
The potential impact of anemia on neonatal inflammation and intestinal barrier disruption, two well-characterized predisposing features of NEC, was defined by correlation of hemoglobin values to cytokine levels in premature infants and by direct evaluation of intestinal hypoxia, inflammation and gut barrier disruption using a pre-clinical neonatal murine model of phlebotomy-induced anemia (PIA).
RESULTS:
Increasing severity of anemia in the preterm infant correlated with the level of IFN-gamma, a key pro-inflammatory cytokine that may predispose an infant to NEC. Gradual induction of PIA in a pre-clinical model resulted in significant hypoxia throughout the intestinal mucosa, including areas where intestinal macrophages reside. PIA-induced hypoxia significantly increased macrophage pro-inflammatory cytokine levels, while reducing tight junction protein ZO-1 expression and increasing intestinal barrier permeability. Macrophage depletion reversed the impact of anemia on intestinal ZO-1 expression and barrier function.
CONCLUSIONS:
Taken together, these results suggest that anemia can increase intestinal inflammation and barrier disruption likely through altered macrophage function, leading to the type of predisposing intestinal injury that may increase the risk for NEC.
INTRODUCTION:
Preterm births comprise at least 1 of every 10 live births annually and are the leading contributor to neonatal mortality worldwide.1 One of the most common complications of preterm birth, particularly in very low birth weight (VLBW) infants weighing < 1500 g, is anemia.2,3 As a result, preterm infants frequently receive multiple transfusions within the first weeks of life, primarily owing to an immature hematopoietic system coupled with phlebotomy losses from frequent blood sampling.2–4 However, as red blood cell (RBC) transfusion is not without risk, many clinicians have opted for more restrictive neonatal RBC transfusion practices in recent years.5 Despite changes in practice, there is no clear consensus as to whether a restrictive RBC transfusion strategy is effective in limiting transfusions without increasing morbidity and mortality in this population.
Although allowing more permissive levels of anemia may be justified in certain patient populations,6 epidemiologic studies have raised concerns regarding the safety of increased tolerance of neonatal anemia in preterm infants. These studies have found severe anemia to be an independent risk factor for necrotizing enterocolitis (NEC), a devastating intestinal disease and major cause of preterm infant mortality.5,7–11 Indeed, retrospective studies and a meta-analysis of trials comparing restrictive vs. liberal transfusion strategies suggested a potentially higher risk of NEC among preterm infants managed by restrictive transfusion approaches (pooled relative risk 1.6; 95% CI 0.8–3.1).9,12 Moreover, a recent multicenter, prospective observational study evaluating time-varying exposure to anemia reported a higher risk of NEC among infants with severe anemia compared to those without severe anemia (adjusted hazard ratio 6.0; 95% CI 2.0–18.0)8. Importantly, while previous studies suggest that transfusion itself may cause transfusion-associated gut injury (TRAGI),13 this study failed to find an association between transfusion and NEC,8 suggesting the severity of anemia rather than exposure to RBC transfusion is a primary risk factor for NEC in this population.
While NEC accounts for 1 in 10 deaths in US neonatal intensive care units,14 the underlying factors that alter the intestinal environment and lead to an increased risk of developing NEC has remained inadequately understood for many years. While the etiology of NEC is incompletely defined, it is multifactorial, involving multiple insults including formula feeding, microbial imbalance, inadequate oxygen delivery, immature intestinal development and/or possible genetic factors that remain to be determined. Although NEC is a multifactorial disease, aberrant immune regulation and uncontrolled inflammation coupled with an impaired intestinal barrier has been a common pathogenic feature that can cause intestinal injury and increase the risk of progression to ischemic and inflamed bowel characteristic of NEC. However, the factors that drive this aberrant immune regulation and barrier dysfunction are incompletely understood.
Given the recent clinical association that suggests anemia may be a risk factor for NEC and previous data implicating immune dysregulation as a key priming event in NEC development,9,11,12,15–18 we sought to define the impact of anemia itself on neonatal immune function and intestinal injury, two key risk factors that may increase the susceptibility to developing NEC. Our results demonstrate that anemia in VLBW preterm infants is associated with a significant increase in IFNγPhlebotomy-induced anemia (PIA)19,20 in a pre-clinical model not only increases intestinal mucosa hypoxia, but likewise increases the pro-inflammatory activity of intestinal macrophages. PIA results in significant decreases in tight junction ZO-1 expression with concomitant loss in intestinal barrier function, while removal of macrophages reverses the impact of anemia on ZO-1 expression and intestinal barrier activity. Taken together, these results suggest that anemia can directly alter neonatal immune and intestinal barrier function, leading to the type of intestinal injury that may predispose a neonate to subsequent development of NEC.
MATERIALS AND METHODS:
Serum collection and cytokine analysis –
Residual serum samples were prospectively collected in a cohort of infants admitted to the University of Iowa neonatal intensive care unit (NICU) between February 2013 and May 2017, with institutional review board consent. Cytokine concentration was determined using a commercial electrochemiluminescence detection plate assay (Meso Scale Discovery) for IFNγ, IL10, IL12p70, IL1β, IP10 and IL18, according to the manufacturer’s recommendations, except that a dilution of 1:4 instead of 1:2 was employed to allow detection in the linear range. Neonates who developed NEC or sepsis were excluded from this analysis to reduce the probability of confounding changes in cytokine levels that could reflect other disease processes and not anemia alone.
Mice –
C57Bl/6 (B6) pups were acquired from timed pregnant dams (Charles River, Wilmington, MA). Mice were housed in Emory University Department of Animal Resources facilities. All procedures were performed according to approved Institutional Care and Use Committee (IACUC) protocols.
Phlebotomy Induced Anemia (PIA) –
Anemia was induced by twice-daily phlebotomy (5.25 μL/g), from day three after birth (postnatal day 3 (P3)) to obtain and maintain the desired hematocrit of <25% as outlined previously.19,20 Graduated glass microhematocrit tubes were used to measure blood draws and hematocrit. Control pups were pricked through the neck scruff without bleeding.
Hypoxyprobe and Immunofluorescence Staining –
On P8, Hypoxyprobe-1 (Pimonidazole HCl) (Hypoxyprobe, inc.) was administered by intraperitoneal (IP) injection (60 mg/kg) according to the manufacturer’s protocol. Intestines were harvested after 1 hour. Tissue sections were prepared as described previously.21 5 μm tissue sections were stained with anti-pimonidazole (clone 4.3.11.3) (1:500, overnight, 4°C), followed by goat anti-mouse IgG Alexa-555 (ThermoFisher) (1:500, 1 hour, room temperature). Alternatively, sections were incubated with rabbit anti-mouse HIF1α (1:500, 1 hour, room temperature) followed by goat anti-rabbit IgG Alexa-555 (ThermoFisher) (1:100 FITC rat anti-mouse CD11b, 1 hour, room temperature). Antibodies were diluted in PBS, 0.1% BSA, 0.1% Triton-x-100. Stained sections were mounted using Prolong Gold anti-fade mounting media with DAPI (ThermoFisher). Images (20x) were captured using the Zeiss Axio Imager followed by analysis using ImageJ software (NIH). Total fluorescence was calculated as corrected total fluorescence (CTF) = integrated density – (area selected x mean fluorescence of background). Each CTF value was calculated for the total area acquired for a 20X image.
Flow Cytometry Analysis –
Immune cells were isolated from intestinal lamina propria as outlined previously.22 Cells were stained with anti-mouse-CD45, anti-mouse-CD3, anti-mouse CD4, anti-mouse-CD8α, anti-mouse-Ly6c, anti-mouse-CD11b, anti-mouse-CD44, anti-mouse-CD49b (DX5), anti-mouse-IFNγ (BD bioscience), anti-mouse-CD45R/B220 (eBioscience), anti-mouse-TNFα, anti-mouse-Ly6G, anti-mouse-CD11c, anti-mouse-F4/80, anti-mouse-CD40, anti-mouse-CD69 (Biolegend) and live/dead stain (Life Technologies).
Intestinal permeability and macrophage depletion –
Pups were fasted 2 hours and gavaged with 750 mg/kg of fluorescein isothiocyanate (FITC) conjugated dextran (10kD, Sigma-Aldrich). After 1 hour, total serum FITC-dextran was determined as described previously.23 To deplete macrophages, pups were injected intraperitoneally with 30 μL of 5 mg/mL clodronate- or PBS-filled liposomes (Liposoma) on P5 and P7, with assessment of depletion 3 days after initial clodronate administration, similar to protocols used previously;24,25 alterations in the vascular barriers of pups may permit intestinal access to clodronate following IP injection.23,26 However, to confirm the impact of IP clondronate injection, intestinal macrophage depletion was assessed on P8 by flow cytometry.
Statistical analysis –
Analysis of cytokines as a function of hemoglobin values was accomplished by linear regression of log cytokine on hemoglobin, with a random effect added to account for within-infant correlation. For murine studies, statistical analysis was performed using one-way ANOVA with Tukey’s post-test for multiple comparisons or Student’s t-test for comparison of two groups. Significance was defined as p < 0.05.
RESULTS:
To determine whether anemia may contribute to immune dysregulation, we first examined serum for potential changes in cytokines with respect to hemoglobin levels in neonates during their NICU stay. To accomplish this, hemoglobin values and serial samples for cytokine measurements were obtained from 68 prospectively enrolled infants free of NEC or sepsis (41 females, 27 males) with a range of birth weights (279 to 1010 grams, mean 734 grams, median 771 grams) and gestational ages (range 22.3–28.6 weeks, mean 25.8 weeks). The average number of samples per infant was 12.1 (median 12, range 2–23), with a total of 461–826 samples per cytokine. Cytokines analyzed were chosen based on sample availability, compatibility on the Meso Scale platform, prior studies suggesting potential involvement in the development of NEC and overall capacity to detect inflammation.15–17,27–32 Among the cytokines analyzed (IFNγ, IL10, IL12p70, IL1β, IP10 and IL18), IFNγ, a potent pro-inflammatory cytokine implicated in intestinal inflammation and injury,27–29 significantly increased with anemia severity (Fig. 1). Thus, increasing severity of anemia in preterm infants correlates with pro-inflammatory IFNγ production.
Figure 1. Hemoglobin negatively correlates with serum cytokine levels in preterm infants.
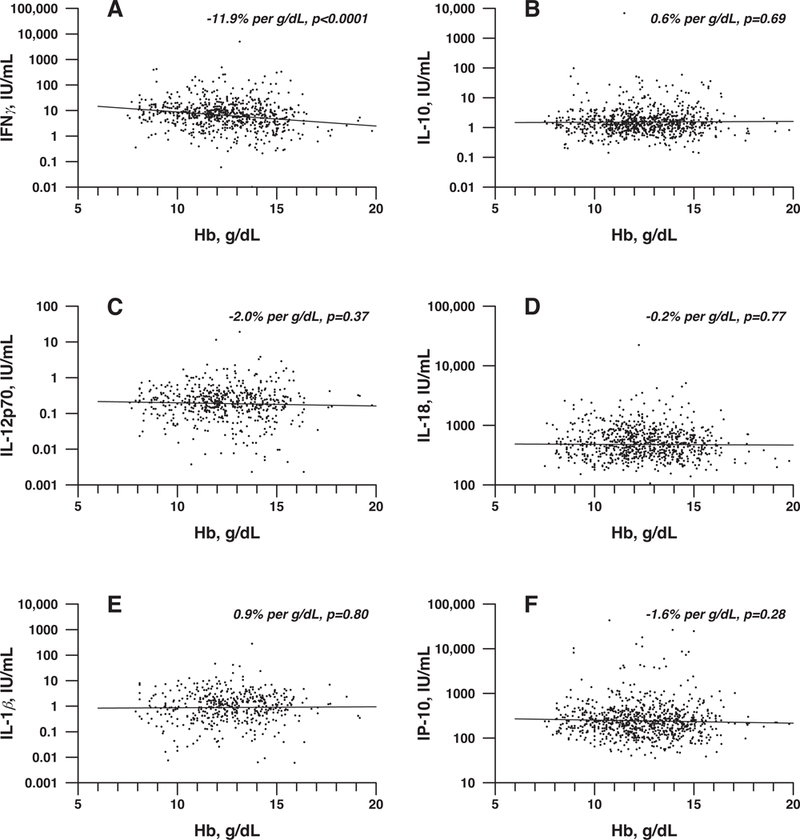
(A-F) Regression analysis was performed between hemoglobin level and IFNγ (A), IL-10 (B), IL-12p70 (C), IL-18 (D), IL-1β (E) or IP-10 (F). Hemoglobin showed significant negative correlation with IFNγ (P < 0.0001).
Given the association between anemia and IFNγ in preterm neonates, and the previous data suggesting that anemia may be a risk factor for later NEC development,8 we next employed a recently established pre-clinical murine model of phlebotomy-induced anemia (PIA),19,20 which mimics the rate and onset of anemia observed in preterm infants (Fig. 2A,B). As alterations in oxygen availability caused by anemia may impact overall intestine function,33,34 we first assessed whether anemia itself may result in significant alterations in tissue oxygenation within the intestine. To accomplish this, we directly examined the level of tissue hypoxia between anemic and control pups by injecting both anemic and littermate controls with pimonidazole HCl (Hypoxyprobe), a probe routinely used to evaluate tissue hypoxia,35–37 on P8. Detection of gut hypoxia using Hypoxyprobe demonstrated that while the epithelial lining of the gut exhibited some hypoxia, similar to previous reports,35,37 anemia induced significant increases in gut hypoxia that extended well beyond the epithelial layer and into the lamina propria (LP) (Fig. 2C,D). Taken together, these results suggest that anemia induces significant hypoxia within the gut mucosa, which may contribute to abnormal intestinal homeostasis and altered immune regulation.
Figure 2. Phlebotomy induced anemia (PIA) results in increased hypoxia within the intestinal mucosa.
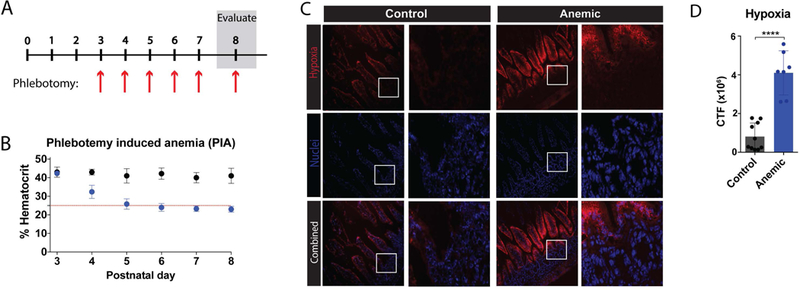
(A) Schematic of protocol for induction of PIA. (B) Percent hematocrit of neonates with (blue) or without (black) phlebotomy on days 3–8 after birth (P3-P8) as indicated. Red line indicates threshold for anemia (25%). (C) Fluorescence microscopy imaging of intestines isolated from control or anemic pups at P8, following administration of Hypoxyprobe (Pimonidazole HCl), which forms adducts in hypoxic tissue. Frozen tissue was stained for tissue hypoxia (pimonidazole) (red) and nuclei (DAPI) (blue). White boxes indicate enlarged regions shown to the right of each 20x field. (D) Quantification of fluorescent hypoxia in 2C. CTF = corrected total fluorescence. Data are presented as mean ± SEM, ****P < 0.0001.
As dysregulation of oxygen gradients has been described in various conditions such as inflammatory bowel disease (IBD) and NEC,33,34,38 we next sought to determine whether increased tissue hypoxia resulting from PIA might play a primary role in immune regulation within the gut in the absence of pre-existing inflammatory dysregulation. To accomplish this, we first examined the total cell number of immune populations isolated from the LP of either anemic pups or littermate controls on P8. No difference in the total number of macrophages, dendritic cells (DCs), CD4+ and CD8+ T cells, NK cells or B cells was observed when comparing anemic pups and littermate controls (Fig. 3). These results suggest that while PIA induces hypoxia within the intestinal mucosa (Figure 2C,D), PIA failed to significantly alter the number of key immune populations.
Figure 3. Anemia does not significantly alter immune population numbers in the small intestine.
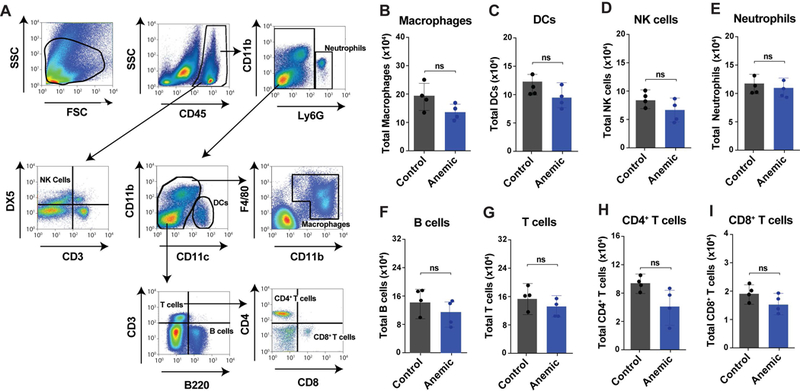
(A) Gating strategy for identification of key leukocyte populations as indicated. (B-I) Total cell counts per small intestine for macrophages (B), dendritic cells (DCs) (C), natural killer (NK) cells (D), neutrophils (E), B cells (F), CD3+ T cells (G), CD4+ T cells (H) and CD8+ T cells (I). Data are presented as mean ± SEM, ns = non significant.
While anemia did not induce significant changes in cell numbers (Fig. 3), we next sought to determine whether the activation state and cytokine expression of immune cells within the intestine is affected by severe anemia. To accomplish this, immune cells isolated from the LP were assessed for expression of cell surface activation markers and production of key inflammatory cytokines, IFNγ and TNFα, both cytokines previously shown to impact intestinal inflammation and barrier function.39–41 As T cells often represent a primary source of IFNγ,42 which was increased in serum of anemic premature infants (Fig. 1), we first assessed the activation of CD4+ and CD8+ T cells. However, no significant change in cytokine levels or cell surface activation markers could be detected in CD4+ or CD8+ T cells (Fig. 4A,B). While T cells are present in the LP, innate immune cells often represent the first responders to alterations in intestinal hemeostasis.43,44 Consistent with this, previous studies implicate intestinal macrophages in the development of NEC.15,45 To examine potential changes in intestinal macrophages, resident macrophages were examined for potential alterations in cell surface activation and cytokine production. Intestinal resident macrophages in anemic mice expressed increased CD40, a marker of pro-inflammatory macrophages,46,47 and produced significantly more IFNγ and TNFα when compared to controls (Fig. 4C). As NK cells and DCs can also play key roles in innate immune responses to alteration in the intestinal mucosa in other settings,43,44 we likewise examined NK cells and DCs for potential changes in activation and cytokine secretion following the development of anemia. In contrast to significant changes associated with macrophage activation in anemic pups, no changes in activation or cytokine secretion were observed in either NK cells nor DCs following the development of anemia (Fig. 4D,E). These results are consistent with previous results implicating macrophages in NEC pathology48 and strongly suggest that gut resident macrophages may play a key role in the development of anemia-induced increases in intestinal inflammation.
Figure 4. Anemia increases gut macrophage activation and pro-inflammatory cytokine production within the intestinal mucosa.
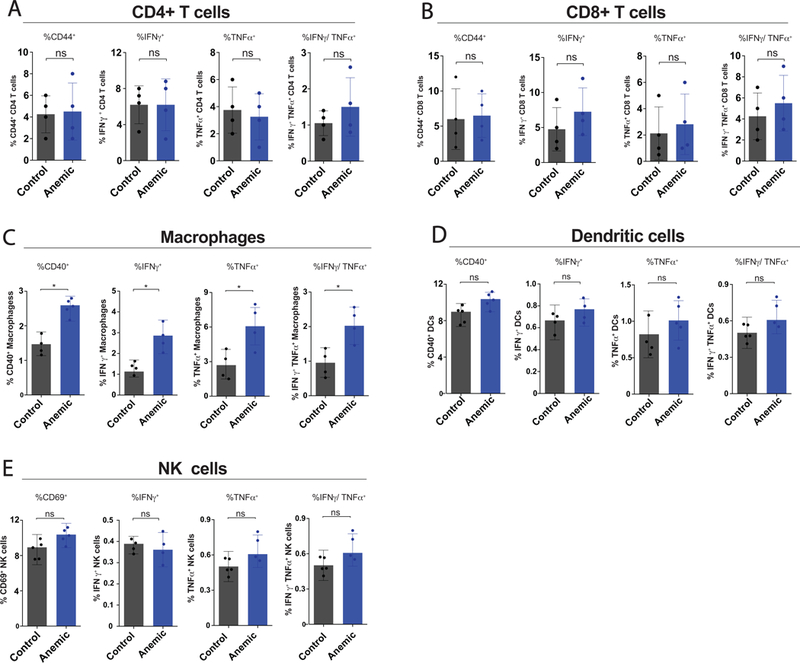
(A-B) Quantification of percent CD44+, IFNγ+, TNFα+ and IFNγ/TNFα double positive, CD4+ (A) or CD8+ (B) T cells isolated from the lamina propria of control or anemic pups. (C-D) Quantification of percent CD40+, IFNγ+, TNFα+ and IFNγ/TNFα double positive macrophages (C) or dendritic cells (DCs) (D) isolated from the lamina propria of control or anemic pups. (E) Quantification of percent CD69+, IFNγ+, TNFα+ and IFNγ/TNFα double positive natural killer (NK) cells isolated from the lamina propria of control or anemic pups. Data are presented as mean ± SEM, *P < .05.
Given the ability of anemia to induce tissue hypoxia in the LP and previous studies demonstrating that hypoxia can induce pro-inflammatory signaling in macrophages,49–51 we next sought to determine whether gut hypoxia might directly impact macrophage activation in anemic mice. To accomplish this, we first assessed whether hypoxia occurs specifically in areas where gut macrophages reside. To test this, we evaluated areas of gut hypoxia for gut resident macrophages. Anemic pups not only displayed significant increases in gut hypoxia, but this hypoxia began to permeate areas where gut resident macrophages reside (Fig. 5A,B), strongly suggesting that gut resident macrophages may be exposed to local changes in oxygen levels that could impact macrophage function. Previous studies suggest that exposure of macrophages to hypoxic conditions can induce expression of the transcription factor hypoxia-inducible factor (HIF), a key regulator of cellular responses to hypoxic conditions.50–54 HIF1α in particular has recently been shown to be responsible for IFNγ expression in macrophages following exposure to hypoxic conditions.55 As a result, we next defined the impact of neonatal anemia on HIF1α expression in intestinal macrophages. Consistent with previous results, HIF1α expression could be observed along the epithelial surface in both anemic and control pups, where physiological hypoxia is thought to regulate barrier function.33,56,57 In contrast, anemia-induced increases in the intestinal hypoxia following PIA resulted in increased and expanded HIF1α expression, which could be observed within intestinal macrophages (Fig. 5C,D,E). Taken together, these results suggest that not only does neonatal anemia induce intestinal hypoxia, but also that the ensuing intestinal hypoxia impacts resident macrophages by inducing the up-regulation of HIF1α, a known factor responsible for inducing macrophage IFNγ production.55
Figure 5. Intestinal macrophages exposed to hypoxia in anemic pups express HIF1α.
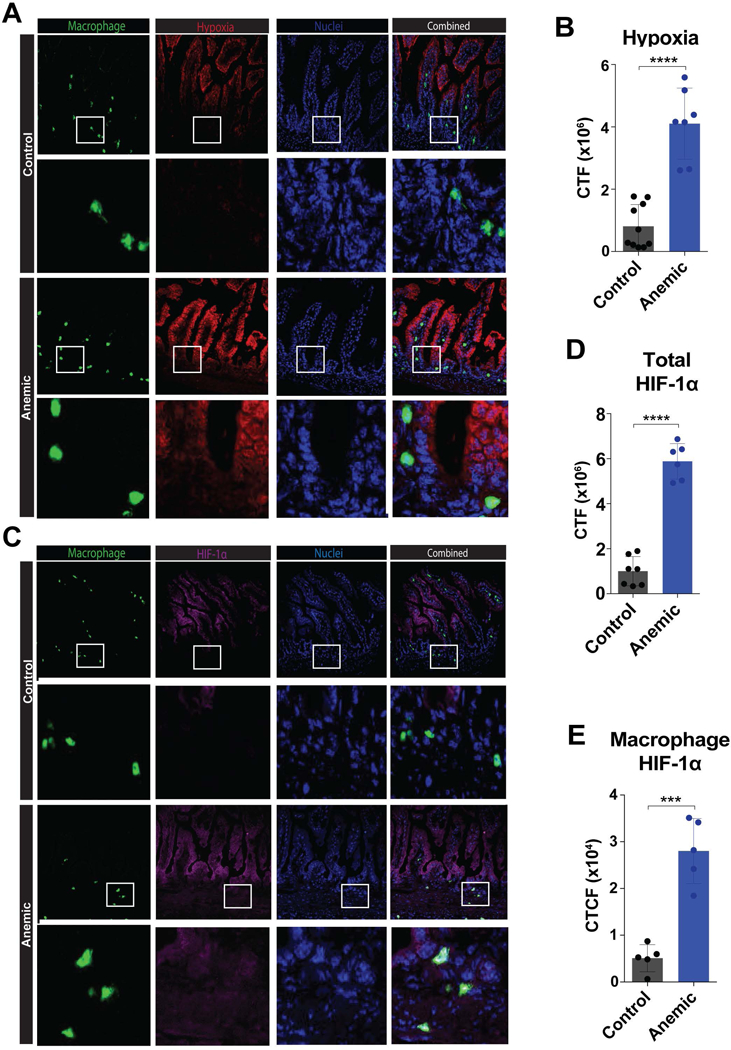
(A) Fluorescence microscopy imaging of intestines isolated from control or anemic pups at P8, following administration of Hypoxyprobe (Pimonidazole HCl) 1 hour prior to sacrifice and collection of intestinal tissue followed by staining of frozen sections for macrophages (CD11b) (green), hypoxia (pimonidazole) (red) and nuclei (DAPI) (blue). White boxes indicate enlarged regions directly below each corresponding 20x image. (B) Quantification of hypoxia staining in 5A. (C) Fluorescence microscopy imaging of intestines isolated from control or anemic pups at P8, followed by staining for macrophages (CD11b) (green), HIF1α (magenta) and nuclei (DAPI) (blue). White boxes indicate enlarged regions directly below each corresponding 20x image (D-E) Quantification of total HIF1α staining in 5C (D) and macrophage specific HIF1α staining in 5C (E). CTF = corrected total fluorescence. Data are presented as mean ± SEM, ***P < .001 ****P < .0001.
An additional critical component associated with the development of intestinal inflammatory conditions is increased gut permeability.58 Consistent with this, inflammation can directly impair gut barrier function.41,59 As a result, we next investigated the potential impact of neonatal anemia on the expression of epithelial tight junctions, key features of the intestinal epithelium responsible for normal barrier function.60–62 To accomplish this, we first examined the expression of ZO-1, a critical component of the epithelial tight junction,63,64 in anemic or control mice. Consistent with the possibility that anemia-induced inflammation may impact barrier function, ZO-1 expression was reduced in anemic mice when compared to controls (Fig. 6A,B). To determine whether alterations in ZO-1 were functionally relevant with respect to barrier function, we next directly assessed barrier permeability in anemic or control neonates. When fluorescein isothiocyanate (FITC)-dextran (10kDa) was administered by oral gavage to anemic and control mice, followed by quantification in the blood 1 hour later,23 there were significantly higher blood levels of FITC-dextran in anemic mice, demonstrating increased intestinal barrier permeability following PIA (Fig. 6C). Taken together, these data demonstrate decreased integrity of the gut in anemic neonates.
Figure 6. Anemia induces a decrease in epithelial barrier function through macrophages.
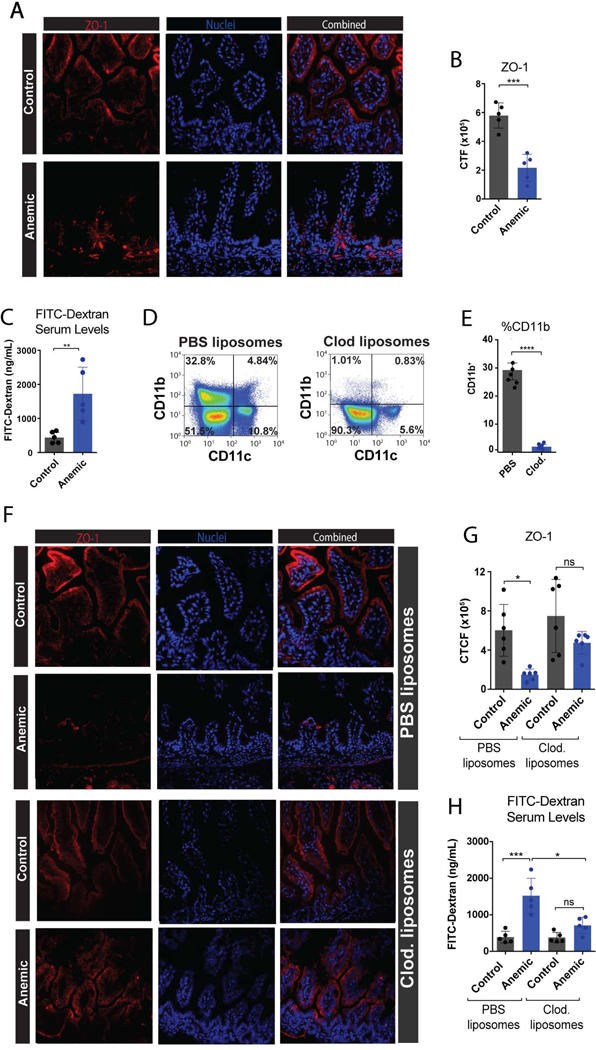
(A) Fluorescence microscopy imaging of intestines isolated from control or anemic pups at P8, followed by staining for ZO-1 (red) and nuclei (DAPI) (blue). (B) Quantification of ZO-1 staining in 6A. (C) Quantification of FITC-Dextran detectable in serum following oral gavage into control or anemic neonates. (D) Representative flow dot plots showing number of CD11b+ macrophages following treatment with PBS control liposomes or clodronate liposomes. (E) Quantification of CD11b+ macrophages isolated from neonatal intestines following treatment with PBS control liposomes or clodronate liposomes as indicated. (F) Fluorescence microscopy imaging of intestines isolated from control or anemic pups at P8 with or without clodronate treatment as indicated. (G) Quantification of ZO-1 staining in 6F. (H) Quantification of FITC-Dextran detectable in serum following oral gavage into control or anemic neonates with or without clodronate treatment as indicated. CTF = corrected total fluorescence. Data are presented as mean ± SEM, ns = non significant, *P < .05, **P < .01, ***P < .001, ****P < .0001.
Given the specific impact of anemia on intestinal macrophage activation and cytokine secretion, we next sought to more directly define the potential role of macrophages in anemia-induced alterations in gut barrier function. To accomplish this, we depleted macrophages using clodronate liposomes, a commonly used approach to assess the role of macrophages in various processes in vivo,25,65,66 in both anemic and control pups. Consistent with previous studies, clodronate-treated recipients displayed significant reductions in macrophage numbers (Fig. 6D,E). Equally important, clodronate treatment resulted in near normal ZO-1 expression following the development of neonatal anemia when compared to recipients that received empty liposomes as a control (Fig. 6F,G). Likewise, clodronate treated anemic mice exhibited near normal levels of gut barrier function when compared to controls (Fig. 6H). These results suggest that macrophages may represent key immunological sentinels that regulate the impact of anemia on intestinal injury and barrier function.
Discussion:
Anemia is one of the most common complications of preterm birth, especially in VLBW infants.2,3,14 As clinicians have shifted towards restrictive transfusion practices in the past decade,5 recent studies have raised concerns regarding the safety of increased tolerance of neonatal anemia.5,7–11 For example, retrospective studies and a recent multicenter prospective observational cohort study report that anemia is a significant risk factor for the development of NEC.8,67 However, the underlying mechanisms whereby anemia may increase later NEC development have remained unclear. As a result, in this study we sought to examine the impact of anemia itself on immune function and intestinal injury. Our data demonstrate that anemia alone appears to alter immune function and intestinal injury, both of which have previously been shown to potentially increase the risk for developing of NEC.15–17,27–32,58,68 In doing so, these data provide a novel mechanistic link between anemia and intestinal alterations that may increase an infant’s susceptibility to developing NEC.
Given prior clinical data suggesting that anemia is associated with a higher risk of NEC,8,9,12 coupled with previous results implicating alterations in immune function as a priming event for later NEC onset,9,11,12,15–18 we sought to define the impact of anemia on neonatal immune function. To accomplish this, we examined potential changes in inflammatory cytokine secretion with respect to neonatal anemia in premature infants. Among the cytokines analyzed, we found significant increases in IFNγ with the development of neonatal anemia, indicating that anemia may induce pro-inflammatory cytokine secretion in preterm infants. While IFNγ is not the only cytokine associated with the onset of NEC, and reports vary regarding the role of this cytokine and others in NEC pathology,18,27,29,69,70 previous studies have indicated that this cytokine is a key regulator in the early onset of intestinal inflammation.41,59 Differences in cytokine changes reported in prior studies may in part reflect variations in patient populations coupled with the frequency of sampling; some studies utilized a few predetermined time points and may have therefore not been able to similarly capture changes associated with anemia.18 Future studies will certainly be needed to determine whether similar alterations occur in other patient populations. However, as our study sought to determine the role of anemia as a priming event in intestinal inflammation in the absence of additional insults, the increased production of IFNγ by macrophages, which serve as sentinels and key regulators of innate immune responses in intestinal homeostasis, suggests that anemia-induced changes in macrophage function may serve as key priming events that create the type of pro-inflammatory environment that may sensitize neonates to additional injury or stress. Concurrent with this, our clinical data suggest the association of additional pro-inflammatory cytokines with increased anemia. However, as our clinical data are correlative in nature, these results certainly do not demonstrate that the increased INFγ observed in anemic infants is a direct consequence of enhanced intestinal macrophage production.
As anemia often occurs in the association with inflammatory conditions within the intestine, where compromises in gastrointestinal tract function can result in bleeding and impaired iron absorption, in addition to the well-documented negative impact of inflammatory cytokines on erythropoiesis,71 it has been challenging to determine if anemia is a causal risk factor or an outcome of intestinal injury, such as NEC, in clinical studies. However, while inflammation can certainly cause anemia, our pre-clinical model, where anemia is deliberately induced in the absence of any known baseline inflammation, demonstrated that anemia can independently promote pro-inflammatory cytokine secretion by macrophages within the intestine. These data complement our clinical findings and suggest that while inflammation can certainly impact erythropoiesis and therefore contribute to anemia, anemia itself may be an independent driver of intestinal inflammation and injury.
While HIF has been previously shown to regulate inflammation within the intestine,33,55 early studies actually suggested that induction of HIF1α results in up-regulation of protective pathways along the intestinal mucosa, often manifested by increased expression of tight junction proteins and enhanced intestinal barrier function.33,57 Indeed, physiological levels of hypoxia are critical to maintain gut homeostasis and barrier function. As such, hypoxia and HIF signaling are important for intestinal barrier regulation during both homeostasis and active inflammation.56 Consistent with this, physiological levels of both hypoxia and HIF expression are routinely observed along the intestinal epithelial barrier.33,56,57 However, while alterations in the magnitude and extent of physiological hypoxia within the intestinal wall may initially facilitate the ability of HIF1α to enhance barrier function in epithelial cells, our results demonstrate that anemia clearly drives marked, non-physiologic hypoxia beyond the intestinal epithelium and into the LP, where resident immune cells, such as macrophages, reside. These anemia-induced changes in the oxygen gradient increased HIF1α expression not only in intestinal epithelium, but also in macrophages, resulting in significant increases in pro-inflammatory cytokine expression in these cells. As pro-inflammatory cytokines, such as IFNγ and TNFα, can significantly impair gut barrier function and also attenuate the protective impact of HIF activity in epithelial cells,39–41,72 increased HIF1α in macrophages with accompanying pro-inflammatory cytokine secretion likely compromises gut barrier function. Consistent with this, recent results demonstrate that hypoxia not only enhances HIF1α expression in macrophages, but that HIF1α is required for macrophage pro-inflammatory cytokine secretion.55,73 Moreover, the ability of clodronate to not only deplete intestinal macrophages, but also reverse anemia-induced intestinal injury, suggest that in this model macrophages may serve as a key population responsible for detecting and subsequently responding to anemic insults; prolonged pro-inflammatory programming in macrophages may therefore tip the balance between tolerance and inflammation in additional immune populations within the gut upon secondary stress events. In this manner, macrophage cytokine secretion likely outweighs the potentially beneficial increase in epithelial HIF1α expression, leading to an overall decrease in tight junction protein expression and increased barrier permeability, both key factors that can predispose neonates to NEC.58
Given prior epidemiologic data demonstrating an increased risk of NEC in anemic neonates and our present findings implicating anemia as a causal factor in intestinal inflammation, severe anemia may increase the risk of intestinal injury that may predispose VLBW infants to subsequent development of NEC. As anemia is also associated with chronic inflammatory conditions within the intestines, such as IBD and graft versus host disease,33,38 the use of lower hemoglobin thresholds in these populations may likewise unintentionally contribute to intestinal inflammation and injury. Thus, while anemia in various clinical settings may reflect a variety of factors that directly contribute to blood loss and impaired erythropoiesis,71 anemia itself may provide a positive feedback loop of pro-inflammatory macrophage activation and subsequent intestinal injury that could exacerbate these underlying conditions. Clearly, whether the macrophage response to anemia observed in the present study is developmentally regulated and/or whether anemia drives similar inflammatory programs in other disease states remains to be tested. However, while these studies are clearly outside the scope of the present study, given the present findings, future studies may benefit from considering these possibilities.
There are clearly limitations with the current study that should be considered. For example, it would have been experimentally optimal to study intestinal macrophage function in VLBW infants in parallel with serum cytokine analysis. However, as many gut resident immune cells, including macrophages, do not circulate, sampling preterm infants for gut resident macrophage number and function, in addition to direct evaluation of intestine barrier activity during the development of anemia is not currently feasible. As a result, we decided to complement this study with a previously validated pre-clinical model of PIA to directly evaluate the impact of anemia on intestine immunity and barrier function.19,20 However, even in this model, significant barriers exist, as the size and overall cellular availability of individual mouse pups limit the scope of analysis that can be accomplished in this setting. Indeed, sample volume limitations in both clinical and animal studies of neonatal immune function have likely contributed to challenges associated with conducting studies that produce a level of understanding in neonates comparable to that obtained from similarly conducted studies in adult mice and patients clinically. Importantly, the rate of anemia employed in this study was designed to match the rate observed in many patients and does not induce detectable changes in heart rate or the development of acidosis that would be expected to occur in the setting of significant hemodynamic alterations/shock.19,20 While nearly all pups reached our threshold of severe anemia (25% Hct) on P7, followed by analysis of the potential impact of anemia the following day, future studies will be necessary to examine the effects of extended anemia exposure and threshold variations. Furthermore, it is not clear whether the lack of ability of anemia to induce NEC in this model accurately reflects what actually occurs clinically. However, as most VLBW anemic neonates do not develop NEC, these results appear to at least be consistent with what we have observed clinically and suggest that while anemia alone may not drive NEC, it may reflect a key predisposing factor, consistent with our recent results. Hence, other predisposing factors (e.g. genetic) and secondary insults (formula feeding, dysbiosis) may be necessary for NEC to develop in the setting of significant anemia.
In summary, as NEC is likely multifactorial, our data suggests that anemia may be a key factor that primes the neonatal intestine to a variety of potential secondary insults, such as dysbiosis, infection, immature immune development and other genetic factors that remain to be determined. While anemia appears to increase the risk of NEC in various prospective studies, as current NEC models employing external factors, such as cold-stress, hypoxic gas, vascular ligation, bacterial administration, or transfusion, to induce gut injury that can result in histologic injury are controversial,74 we elected instead to define the potential impact of anemia on the early events that may adversely affect intestinal function and therefore predispose an infant to NEC.68 However, not all VLBW infants that develop severe anemia develop NEC. As a result, while anemia may be a risk factor the development of NEC, the presence or absence of secondary insults, as well the other genetic and environmental factors, such as baseline variability in severity of and responses to anemia, may in part, dictate whether NEC develops in this setting. Thus, while anemia may be an important factor, future studies will be needed to further characterize its effect upon challenge with secondary insults. For example, as our study exclusively focused on the impact of anemia on neonatal immune function and intestinal injury, we did not define the impact of RBC transfusion in this setting. As our recent study demonstrated that anemia alone correlated with NEC irrespective of RBC transfusion,8 these results guided the focus of the current study. However, given the present findings and recent studies suggesting that transfusion may also impact neonatal immune function,75 future studies will be needed to decipher appropriate hemoglobin thresholds and optimal transfusion protocols to reduce or prevent anemia-induced intestinal inflammation and injury.
Acknowledgements:
This work was supported in part by the National Institutes of Health Early Independence grant DP5OD019892 and the National Heart Lung and Blood Institute grant R01HL13557501 to S.R.S. as well as by the National Heart Lung and Blood Institute Program Project Grant P01HL04692520 to J.A.W.
Footnotes
Conflict- of- interest disclosure: The authors declare no competing financial interests.
REFERENCES:
- 1.WHO. Preterm birth Fact sheet: World Health Organization; 2016.
- 2.Widness JA. Pathophysiology of Anemia During the Neonatal Period, Including Anemia of Prematurity. Neoreviews. 2008;9(11):e520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123(5):615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin JC, Strauss RG, Kulhavy JC, et al. Phlebotomy overdraw in the neonatal intensive care nursery. Pediatrics. 2000;106(2):E19. [DOI] [PubMed] [Google Scholar]
- 5.Keir AK, Yang J, Harrison A, Pelausa E, Shah PS, Canadian Neonatal N. Temporal changes in blood product usage in preterm neonates born at less than 30 weeks’ gestation in Canada. Transfusion. 2015;55(6):1340–1346. [DOI] [PubMed] [Google Scholar]
- 6.Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel RM, Kandefer S, Walsh MC, et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med. 2015;372(4):331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel RM, Knezevic A, Shenvi N, et al. Association of Red Blood Cell Transfusion, Anemia, and Necrotizing Enterocolitis in Very Low-Birth-Weight Infants. JAMA. 2016;315(9):889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh R, Visintainer PF, Frantz ID 3rd, , et al. Association of necrotizing enterocolitis with anemia and packed red blood cell transfusions in preterm infants. J Perinatol. 2011;31(3):176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maheshwari A, Patel RM, Christensen RD. Anemia, red blood cell transfusions, and necrotizing enterocolitis. Semin Pediatr Surg. 2018;27(1):47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh R, Shah BL, Frantz ID 3rd., Necrotizing enterocolitis and the role of anemia of prematurity. Semin Perinatol. 2012;36(4):277–282. [DOI] [PubMed] [Google Scholar]
- 12.Derienzo C, Smith PB, Tanaka D, et al. Feeding practices and other risk factors for developing transfusion-associated necrotizing enterocolitis. Early Hum Dev. 2014;90(5):237–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohamed A, Shah PS. Transfusion associated necrotizing enterocolitis: a meta-analysis of observational data. Pediatrics. 2012;129(3):529–540. [DOI] [PubMed] [Google Scholar]
- 14.Jacob J, Kamitsuka M, Clark RH, Kelleher AS, Spitzer AR. Etiologies of NICU deaths. Pediatrics. 2015;135(1):e59–65. [DOI] [PubMed] [Google Scholar]
- 15.Liu Z, Sun X, Tang J, et al. Intestinal inflammation and tissue injury in response to heat stress and cooling treatment in mice. Mol Med Rep. 2011;4(3):437–443. [DOI] [PubMed] [Google Scholar]
- 16.De Plaen IG. Inflammatory signaling in necrotizing enterocolitis. Clin Perinatol. 2013;40(1):109–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter CJ, De Plaen IG. Inflammatory signaling in NEC: Role of NF-kappaB, cytokines and other inflammatory mediators. Pathophysiology. 2014;21(1):55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maheshwari A, Schelonka RL, Dimmitt RA, et al. Cytokines associated with necrotizing enterocolitis in extremely-low-birth-weight infants. Pediatr Res. 2014;76(1):100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallin DJ, Zamora TG, Alexander M, Ennis KM, Tran PV, Georgieff MK. Neonatal mouse hippocampus: phlebotomy-induced anemia diminishes and treatment with erythropoietin partially rescues mammalian target of rapamycin signaling. Pediatr Res. 2017;82(3):501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallin DJ, Tkac I, Stucker S, et al. Phlebotomy-induced anemia alters hippocampal neurochemistry in neonatal mice. Pediatr Res. 2015;77(6):765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zerra PE, Cox C, Baldwin WH, et al. Marginal zone B cells are critical to factor VIII inhibitor formation in mice with hemophilia A. Blood. 2017;130(23):2559–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodyear AW, Kumar A, Dow S, Ryan EP. Optimization of murine small intestine leukocyte isolation for global immune phenotype analysis. J Immunol Methods. 2014;405:97–108. [DOI] [PubMed] [Google Scholar]
- 23.Patel RM, Myers LS, Kurundkar AR, Maheshwari A, Nusrat A, Lin PW. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am J Pathol. 2012;180(2):626–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aurora AB, Porrello ER, Tan W, et al. Macrophages are required for neonatal heart regeneration. J Clin Invest. 2014;124(3):1382–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weisser SB, van Rooijen N, Sly LM. Depletion and reconstitution of macrophages in mice. J Vis Exp. 2012(66):4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xing T, Camacho Salazar R, Chen YH. Animal models for studying epithelial barriers in neonatal necrotizing enterocolitis, inflammatory bowel disease and colorectal cancer. Tissue Barriers. 2017;5(4):e1356901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Zhu L, Fatheree NY, et al. Changes in intestinal Toll-like receptors and cytokines precede histological injury in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2009;297(3):G442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leaphart CL, Qureshi F, Cetin S, et al. Interferon-gamma inhibits intestinal restitution by preventing gap junction communication between enterocytes. Gastroenterology. 2007;132(7):2395–2411. [DOI] [PubMed] [Google Scholar]
- 29.Cho SX, Berger PJ, Nold-Petry CA, Nold MF. The immunological landscape in necrotising enterocolitis. Expert Rev Mol Med. 2016;18:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halpern MD, Holubec H, Dominguez JA, et al. Up-regulation of IL-18 and IL-12 in the ileum of neonatal rats with necrotizing enterocolitis. Pediatr Res. 2002;51(6):733–739. [DOI] [PubMed] [Google Scholar]
- 31.Emami CN, Chokshi N, Wang J, et al. Role of interleukin-10 in the pathogenesis of necrotizing enterocolitis. Am J Surg. 2012;203(4):428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng PC, Li K, Chui KM, et al. IP-10 is an early diagnostic marker for identification of late-onset bacterial infection in preterm infants. Pediatr Res. 2007;61(1):93–98. [DOI] [PubMed] [Google Scholar]
- 33.Shah YM. The role of hypoxia in intestinal inflammation. Mol Cell Pediatr. 2016;3(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horton KK. Pathophysiology and current management of necrotizing enterocolitis. Neonatal Netw. 2005;24(1):37–46. [DOI] [PubMed] [Google Scholar]
- 35.Aguilera KY, Brekken RA. Hypoxia Studies with Pimonidazole in vivo. Bio Protoc. 2014;4(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xing D, Liu L, Marti GP, et al. Hypoxia and hypoxia-inducible factor in the burn wound. Wound Repair Regen. 2011;19(2):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitney AK, Campbell EL. Imaging Inflammatory Hypoxia in the Murine Gut. Methods Mol Biol. 2016;1422:115–126. [DOI] [PubMed] [Google Scholar]
- 38.Nauta TD, van Hinsbergh VW, Koolwijk P. Hypoxic signaling during tissue repair and regenerative medicine. Int J Mol Sci. 2014;15(11):19791–19815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta. 2009;1788(4):864–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166(2):409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ito R, Shin-Ya M, Kishida T, et al. Interferon-gamma is causatively involved in experimental inflammatory bowel disease in mice. Clin Exp Immunol. 2006;146(2):330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. [DOI] [PubMed] [Google Scholar]
- 43.Newburg DS, Walker WA. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res. 2007;61(1):2–8. [DOI] [PubMed] [Google Scholar]
- 44.Kayama H, Takeda K. Functions of innate immune cells and commensal bacteria in gut homeostasis. J Biochem. 2016;159(2):141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MohanKumar K, Namachivayam K, Chapalamadugu KC, et al. Smad7 interrupts TGF-beta signaling in intestinal macrophages and promotes inflammatory activation of these cells during necrotizing enterocolitis. Pediatr Res. 2016;79(6):951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, Perlman H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am J Respir Cell Mol Biol. 2013;49(4):503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carlsen HS, Yamanaka T, Scott H, Rugtveit J, Brandtzaeg P. The proportion of CD40+ mucosal macrophages is increased in inflammatory bowel disease whereas CD40 ligand (CD154)+ T cells are relatively decreased, suggesting differential modulation of these costimulatory molecules in human gut lamina propria. Inflamm Bowel Dis. 2006;12(11):1013–1024. [DOI] [PubMed] [Google Scholar]
- 48.MohanKumar K, Kaza N, Jagadeeswaran R, et al. Gut mucosal injury in neonates is marked by macrophage infiltration in contrast to pleomorphic infiltrates in adult: evidence from an animal model. Am J Physiol Gastrointest Liver Physiol. 2012;303(1):G93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol. 2009;9(9):609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zinkernagel AS, Johnson RS, Nizet V. Hypoxia inducible factor (HIF) function in innate immunity and infection. J Mol Med (Berl). 2007;85(12):1339–1346. [DOI] [PubMed] [Google Scholar]
- 51.Palazon A, Goldrath AW, Nizet V, Johnson RS. HIF transcription factors, inflammation, and immunity. Immunity. 2014;41(4):518–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakayama T, Kurobe H, Sugasawa N, et al. Role of macrophage-derived hypoxia-inducible factor (HIF)-1alpha as a mediator of vascular remodelling. Cardiovasc Res. 2013;99(4):705–715. [DOI] [PubMed] [Google Scholar]
- 53.Cramer T, Yamanishi Y, Clausen BE, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112(5):645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walmsley SR, Cadwallader KA, Chilvers ER. The role of HIF-1alpha in myeloid cell inflammation. Trends Immunol. 2005;26(8):434–439. [DOI] [PubMed] [Google Scholar]
- 55.Acosta-Iborra B, Elorza A, Olazabal IM, et al. Macrophage oxygen sensing modulates antigen presentation and phagocytic functions involving IFN-gamma production through the HIF-1 alpha transcription factor. J Immunol. 2009;182(5):3155–3164. [DOI] [PubMed] [Google Scholar]
- 56.Glover LE, Lee JS, Colgan SP. Oxygen metabolism and barrier regulation in the intestinal mucosa. J Clin Invest. 2016;126(10):3680–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saeedi BJ, Kao DJ, Kitzenberg DA, et al. HIF-dependent regulation of claudin-1 is central to intestinal epithelial tight junction integrity. Mol Biol Cell. 2015;26(12):2252–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moore SA, Nighot P, Reyes C, et al. Intestinal barrier dysfunction in human necrotizing enterocolitis. J Pediatr Surg. 2016;51(12):1907–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Madara JL, Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989;83(2):724–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809. [DOI] [PubMed] [Google Scholar]
- 61.Fanning AS, Mitic LL, Anderson JM. Transmembrane proteins in the tight junction barrier. J Am Soc Nephrol. 1999;10(6):1337–1345. [DOI] [PubMed] [Google Scholar]
- 62.Harhaj NS, Antonetti DA. Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol. 2004;36(7):1206–1237. [DOI] [PubMed] [Google Scholar]
- 63.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273(45):29745–29753. [DOI] [PubMed] [Google Scholar]
- 64.Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103(3):755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Rooijen N, Hendrikx E. Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol Biol. 2010;605:189–203. [DOI] [PubMed] [Google Scholar]
- 66.Danenberg HD, Fishbein I, Gao J, et al. Macrophage depletion by clodronate-containing liposomes reduces neointimal formation after balloon injury in rats and rabbits. Circulation. 2002;106(5):599–605. [DOI] [PubMed] [Google Scholar]
- 67.Kirpalani H, Zupancic JA. Do transfusions cause necrotizing enterocolitis? The complementary role of randomized trials and observational studies. Semin Perinatol. 2012;36(4):269–276. [DOI] [PubMed] [Google Scholar]
- 68.Hackam DJ, Upperman JS, Grishin A, Ford HR. Disordered enterocyte signaling and intestinal barrier dysfunction in the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg. 2005;14(1):49–57. [DOI] [PubMed] [Google Scholar]
- 69.Benkoe T, Baumann S, Weninger M, et al. Comprehensive evaluation of 11 cytokines in premature infants with surgical necrotizing enterocolitis. PLoS One. 2013;8(3):e58720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abdelhamid AE, Chuang SL, Hayes P, Fell JM. In vitro cow’s milk protein-specific inflammatory and regulatory cytokine responses in preterm infants with necrotizing enterocolitis and sepsis. Pediatr Res. 2011;69(2):165–169. [DOI] [PubMed] [Google Scholar]
- 71.Weiss G, Gasche C. Pathogenesis and treatment of anemia in inflammatory bowel disease. Haematologica. 2010;95(2):175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Glover LE, Irizarry K, Scully M, et al. IFN-gamma attenuates hypoxia-inducible factor (HIF) activity in intestinal epithelial cells through transcriptional repression of HIF-1beta. J Immunol. 2011;186(3):1790–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu L, Lu Y, Martinez J, et al. Proinflammatory signal suppresses proliferation and shifts macrophage metabolism from Myc-dependent to HIF1alpha-dependent. Proc Natl Acad Sci U S A. 2016;113(6):1564–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.The Neu J. ‘myth’ of asphyxia and hypoxia-ischemia as primary causes of necrotizing enterocolitis. Biol Neonate. 2005;87(2):97–98. [DOI] [PubMed] [Google Scholar]
- 75.Dani C, Poggi C, Gozzini E, et al. Red blood cell transfusions can induce proinflammatory cytokines in preterm infants. Transfusion. 2017;57(5):1304–1310. [DOI] [PubMed] [Google Scholar]


