Significance
As agricultural lands rapidly expand in the tropics, they become critical for the future of tropical biodiversity, but little is known about their long-term conservation value. Over 12 years in Costa Rica, we measured the abundance and diversity of birds in agricultural areas and embedded forest remnants. We recorded 185 bird species in coffee plantations and 230 species at forest sites, but 69 out of 112 populations showed declines—mostly among more specialized, sedentary, and/or insectivorous species. Nevertheless, coffee plantations with modestly higher tree cover had higher bird diversity and capture rates. With limited opportunities to expand protected areas worldwide, even small improvements in farming practices can increase the long-term sustainability of tropical wildlife and its benefits to people.
Keywords: avian ecology, ecosystem services, global change, ornithology, tropical biology
Abstract
Tropical agriculture is a major driver of biodiversity loss, yet it can provide conservation opportunities, especially where protected areas are inadequate. To investigate the long-term biodiversity capacity of agricultural countryside, we quantified bird population trends in Costa Rica by mist netting 57,255 birds of 265 species between 1999 and 2010 in sun coffee plantations, riparian corridors, secondary forests, forest fragments, and primary forest reserves. More bird populations (69) were declining than were stable (39) or increasing (4). Declines were common in resident, insectivorous, and more specialized species. There was no relationship between the species richness of a habitat and its conservation value. High-value forest bird communities were characterized by their distinct species composition and habitat and dietary functional signatures. While 49% of bird species preferred forest to coffee, 39% preferred coffee to forest and 12% used both habitats, indicating that coffee plantations have some conservation value. Coffee plantations, although lacking most of the forest specialists, hosted 185 bird species, had the highest capture rates, and supported increasing numbers of some forest species. Coffee plantations with higher tree cover (7% vs. 13%) had more species with increasing capture rates, twice as many forest specialists, and half as many nonforest species. Costa Rican countryside habitats, especially those with greater tree cover, host many bird species and are critical for connecting bird populations in forest remnants. Diversified agricultural landscapes can enhance the biodiversity capacity of tropical countryside, but, for the long-term persistence of all forest bird species, large (>1,000 ha) protected areas are essential.
Worldwide conversion of native habitats into agriculture and other land use is the leading driver of the ongoing sixth mass extinction (1). Tropical forests are the world’s most species-rich terrestrial ecosystem, supporting up to 70% of plant and animal species worldwide—many of them threatened with extinction. This ranks tropical deforestation as one of the greatest threats to global biodiversity (2–6), with enormous scientific and societal implications.
Protected areas cover only 15% of the world’s land area (7), and, on their own, are thought to be insufficient in many dimensions to safeguard biodiversity into the future, especially in the face of climate change (8, 9). Agriculture, on the other hand, accounts for nearly 40% of global land cover and exceeds 80% in some tropical countries where forests were once the dominant land cover type (10). Despite their contribution to biodiversity loss, agricultural areas can retain considerable biodiversity (11). For example, a third of the world’s bird species have been recorded in human-dominated, mostly agricultural habitats (12). The retention of small forest remnants, such as riparian strips and individual trees, within tropical agricultural countryside can improve landscape connectivity and increase the effective area of forest reserves (13).
There is an urgent need for livelihoods and policies that sustain agricultural profits, biodiversity, and ecosystem services concurrently (14, 15). To advance this vision, we need long-term studies that yield insight into the drivers and impacts of biodiversity dynamics in human-dominated landscapes. Land-use intensification is rapidly increasing in many tropical areas, especially in the form of soy bean and oil palm monocultures where very few native species can survive (16). Nevertheless, in much of the tropics, agriculture still exists in a diverse patchwork of small farms, remnant trees, forest fragments, and riparian corridors, often bordering larger forest reserves in some places. The biodiversity of tropical countryside landscapes is further augmented in agroforests, such as shade coffee and shade cacao plantations, where the mixture of trees, shrubs, and crops is particularly valuable for biodiversity conservation, especially when native tree species are present (17–21).
In this study, we focus on birds because they occupy a wide range of ecological niches, have key ecological functions, are often highly specialized, are variably susceptible to disturbance, and their extinction risk increases with ecological specialization (22). They are also the best-known taxon, and there are established methods for studying changes in tropical bird biodiversity over many years (23). Assessing the responses of birds with different ecological specializations, foraging guild structures, and conservation statuses in tropical agricultural landscapes is important not only for conserving bird species, but also for maintaining the ecological functions and ecosystem services, such as pest control, pollination, and seed dispersal, that birds provide to farming communities (24, 25).
We examined the long-term effects of land use on the bird communities sampled in coffee plantations, riparian corridors, secondary forests, small forest fragments, and primary forest reserves in the human-dominated tropical countryside of southern Costa Rica. We focus on coffee because it is one of the most valuable export commodities for many tropical countries and can support high avian diversity (21).
Here, we summarize a 12-y ecological assessment of how land use shapes the region’s avian community. Specifically, we ask (i) how birds affiliate with a range of countryside habitat types, from coffee monoculture to extensive primary forest; (ii) what impact a modest increase in tree cover (from 7 to 13%) could have on the biodiversity conservation value of coffee; (iii) how the populations of different species in different habitats are changing through time; and (iv) which ecological traits make bird species differentially vulnerable to the loss of forest cover.
Results
Between 1999 and 2010, we had 57,255 captures of 265 species during 105,744 mist-net-hours (Fig. 1 and SI Appendix, Table S1). Below, we refer to coffee plantations with less than 10% tree cover as “open coffee” and coffee plantations with 10 to 20% tree cover as “shaded coffee” sites. Riparian, secondary, fragment, Las Cruces Forest Reserve, and Amistad International Park primary forest sites are sometimes collectively referred to as the “forest” sites.
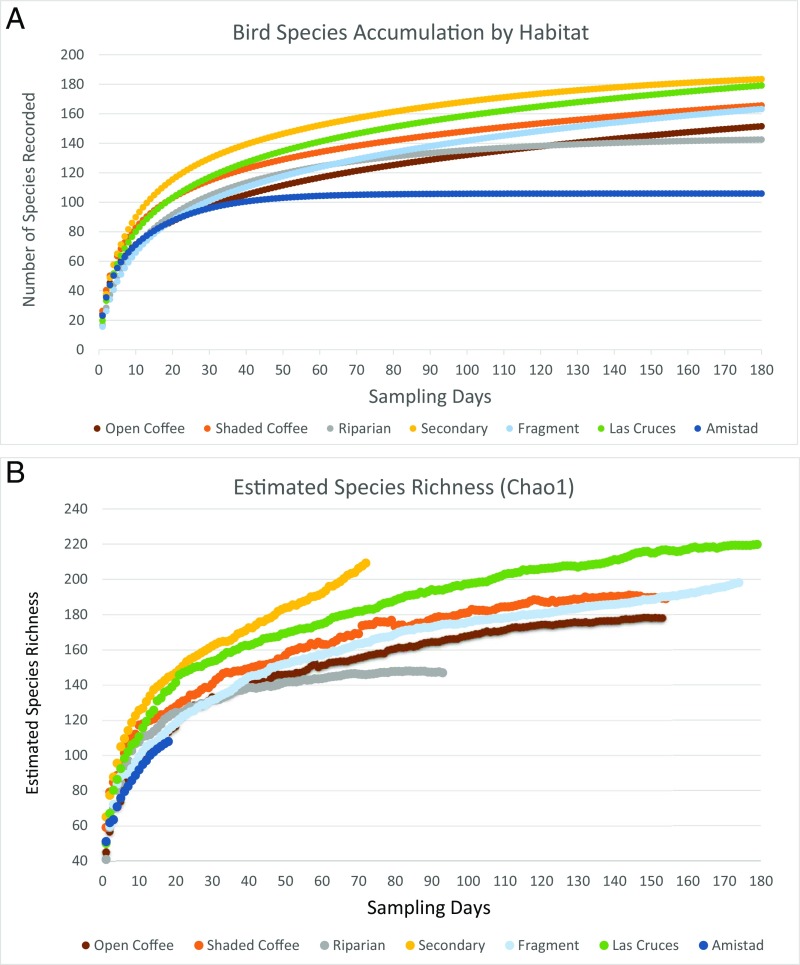
Fig. 1.
Species accumulation curves indicate the increase in bird species richness (∼20 more species) at shaded coffee sites with higher tree cover than open coffee sites (13% vs. 7%). (A) Species accumulation curves were calculated with Estimates 9.1, using sampling with replacement (62). All samples were extrapolated to 179 d to compare them with the full reference sample for Las Cruces, the largest sample in the dataset, with 179 sampling days (64). Note that for some of the habitats, extrapolated species numbers are higher than the numbers actually recorded. (B) Chao 1 estimates of species richness. Note the differences between actual species accumulation versus expected species richness with Chao 1. Estimated (Chao 1) species richness values for the Las Cruces forest reserve, forest fragments, and secondary forest are the highest, and Amistad is the lowest, with coffee and riparian sites having intermediate values.
Habitat Affiliation.
Species accumulation, richness, and similarity.
We had 29,740 captures of 185 species at the coffee sites and 27,515 captures of 230 species at the forest sites. The greatest number of species (179) was recorded at Las Cruces forest sites, followed by forest fragments (162), shaded coffee (161), secondary forest (158), open coffee (146), riparian (134), and Amistad primary forest (85). The 95% confidence intervals of species richness values for Las Cruces, fragments, shaded coffee, and secondary forest did not overlap with the 95% confidence intervals of open coffee, riparian, and Amistad species richness values (Fig. 1A). Chao 1 estimates of species richness (Fig. 1B) were highest for Las Cruces (220), followed by secondary forest (209), fragments (198), shaded coffee (189), open coffee (178), riparian (147), and Amistad (108). The 95% confidence intervals of Chao 1 species richness estimates for Las Cruces, fragments, and secondary forest did not overlap with the 95% confidence intervals of riparian and Amistad Chao 1 species richness estimates. Shaded coffee and open coffee Chao 1 estimates overlapped with those of riparian but not of Amistad. Nevertheless, as shown below, in-depth analyses of community composition are more informative on the conservation value of different habitats than species richness estimates alone. The bird community of the Amistad International Park, in particular, has a distinct species composition and habitat and diet functional signatures (Methods) characteristic of extensive primary forest bird communities.
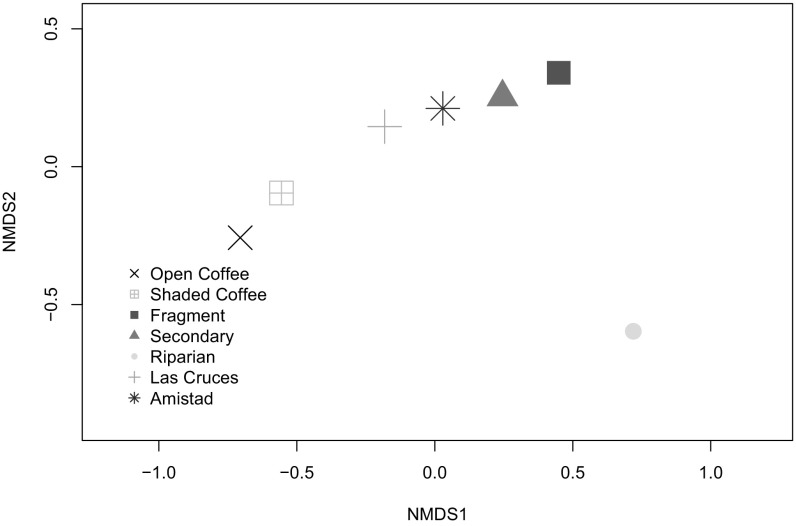
Morisita–Horn sample similarity indices (MHSIs) (Table 1 and SI Appendix, Table S2) and nonmetric multidimensional scaling (NMDS) analysis (Fig. 2 and SI Appendix, Fig. S2) indicated highly similar bird communities in open and shaded coffee habitats, with the forest communities becoming more differentiated along an increasing primary forest axis from riparian to Amistad and Las Cruces. Despite overlapping estimated species richness values for coffee (178 to 189) and forest (108 to 220) habitats, species composition differed substantially between them (MHSI ≤ 0.58), and there was little overlap between coffee and primary forest sites (MHSI = 0.06 to 0.20). Although Amistad primary forest’s bird community had higher similarity to that of secondary forest (0.63) than to that of Las Cruces (0.55), also reflected in the NMDS plot (SI Appendix, Fig. S2 and Table S2), this was a result of the hundreds of Swainson’s thrushes (Catharus ustulatus) migrating through Amistad, making up 41% of Amistad’s bird captures. When Swainson’s thrushes were excluded from the NMDS analysis, this difference disappeared (Fig. 2 and Table 1), and most of the results below also indicate that the Amistad International Park’s bird community metrics are most similar to those of the Las Cruces Forest Reserve. Swainson’s thrushes were present in all sampled habitats every dry season (boreal winter) sampling year (2003 to 2010), except in 2010, when they were unusually absent from open coffee, fragment, and riparian habitats, despite mist netting starting at the usual time. Even during this year, Swainson’s thrush capture rate was the highest at Amistad (2.62 birds per 100 mist-net-hours), an order of magnitude higher than the second highest (secondary forest, 0.23 birds per 100 mist-net-hours) and third highest (Las Cruces forest, 0.19 birds per 100 mist-net-hours) capture rates.
Table 1.
Morisita–Horn sample similarity indices
| Habitat | Shaded | Riparian | Secondary | Fragment | Las Cruces | Amistad |
| Open coffee | 0.93 | 0.51 | 0.54 | 0.32 | 0.16 | 0.05 |
| Shaded coffee | 0.58 | 0.60 | 0.36 | 0.17 | 0.07 | |
| Riparian | 0.80 | 0.64 | 0.55 | 0.37 | ||
| Secondary | 0.80 | 0.62 | 0.57 | |||
| Fragment | 0.86 | 0.80 | ||||
| Las Cruces | 0.82 |
Morisita–Horn sample similarity comparisons between habitats indicate highly similar bird communities of open and shaded coffee habitats, with the forest communities becoming more differentiated along the increasing primary forest axis from riparian sites to the Amistad International Park. Here, Swainson’s thrushes are excluded because large numbers migrated through the study region without wintering, and their preference for forest skews the similarity values, especially for Amistad, where they comprised 41% of all birds caught in the mist nets. See SI Appendix, Table S2 for the comparison that includes Swainson’s thrushes.
Fig. 2.
Nonmetric multidimensional scaling (NMDS) plot of habitats based on bird captures reveal similarity in community composition between the coffee sites and between the forest sites. Riparian sites are distinct. NMDS analyses group communities based on rank order of species abundance rather than absolute abundance, thus reducing the variability stemming from differential sampling of sites or capture rates. Here, Swainson’s thrushes are excluded because large numbers migrated through the study region without wintering, and their preference for forest skews the analysis, especially at Amistad where they comprised 41% of all birds caught in the mist nets. For the NMDS plot that includes Swainson’s thrushes, see SI Appendix, Fig. S2.
Habitat choice and forest dependence.
Most of the individuals sampled during the course of the study belonged to species whose primary habitat preference is closed-canopy forest across their global ranges (26), including approximately half of the individuals in coffee plantations (Fig. 3A). Compared with the forest sites, the coffee sites had higher proportions of nonforest birds and those with low forest dependence, and lower proportions of birds with high and medium forest dependence (Fig. 3B). Capture rates were higher (t = 7.06, P < 0.0001) at the coffee sites (81.7 birds per 100 net-hours) than at the forest sites (39.6 birds per 100 net-hours), which may partially be a result of most of the vegetation in coffee plantations being concentrated within three meters of the ground, making mist nets a more efficient sampling method in coffee.
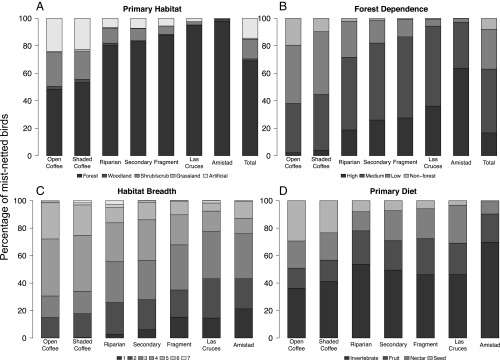
Fig. 3.
Changes in community composition and ecological profiles across habitats. (A) Primary habitat profiles of mist-netted individuals show the proportion of forest species increasing from open coffee to Amistad. Habitat types are described in detail in ref. 66. Primary habitat profiles were significantly different (χ2 > 11.6, P < 0.02), with the exception of open coffee versus shaded coffee (χ2 = 1.87, P = 0.76), secondary forest versus riparian forest (χ2 = 3.2, P = 0.53), and secondary forest versus fragment birds (χ2 = 3.5, P = 0.48). “Artificial” refers to human-created habitats, such as agricultural areas, pastures, orchards, and settlements. (B) Forest-dependence profiles of mist-netted individuals show an increase in forest dependence (26) from open coffee to Amistad. Forest-dependence profiles mostly differed between habitats (χ2 > 23.1, P < 0.01), with the exception of secondary forest versus riparian forest (χ2 = 15.4, P = 0.081), secondary forest versus fragments (χ2 = 6.4, P = 0.704), open coffee versus shaded coffee (χ2 = 16.2, P = 0.062), and fragment versus Las Cruces forest birds (χ2 = 16.3, P = 0.061). (C) Habitat specialists increase with increasing tree cover. Habitat breadth profiles of mist-netted individuals were significantly different (χ2 > 16.7, P < 0.01) or marginally so (fragments versus Las Cruces forest; χ2 = 12.1, P = 0.06), with the exception of open coffee versus shaded coffee (χ2 = 2.6, P = 0.86), shaded coffee versus riparian (χ2 = 11.1, P = 0.08), and secondary forest versus fragments (χ2 = 5.8, P = 0.45). Here, Swainson’s thrushes are excluded because large numbers migrated through the study region without wintering, and their preference for forest skews the proportions, especially at Amistad where they comprised 41% of all birds caught in the mist nets. (D) Bird community functional signatures of habitats based on the primary diet choice of bird species show a decrease in granivorous birds and an increase in insectivorous birds from open coffee plantations to Amistad primary forest.
Despite the twofold higher capture rates in coffee plantations, of the 265 species recorded in the study, 81 were recorded exclusively at the forest sites while only 35 were recorded exclusively at the coffee sites. Of the 185 species with more than six captures, 39% of bird species preferred coffee to forest, 49% preferred forest to coffee (t > 2, P < 0.05), and 12% were generalists that used both coffee and forest habitats (t < 2, P > 0.05). The 72 coffee species were evenly divided between the open and shaded coffee sites in their habitat preference. Of the 90 forest-preferring species, 46 preferred the primary forest sites (Las Cruces and/or Amistad) over riparian forest, forest fragments, or secondary forests, and the rest were forest generalists. Six species were only recorded in the riparian sites whereas no species were exclusive to the secondary forests or fragments.
On average, compared with birds in forest habitats, birds in coffee plantations have greater habitat breadth: i.e., they use more habitats across their global ranges (Fig. 3C). Average habitat breadth declined with increasing forest cover and quality, with the exception of Amistad, because of the hundreds of migrating Swainson’s thrushes making up 41% of Amistad’s captures. Without them, >70% of birds captured at Amistad belong to forest specialist species, with habitat breadth of three major habitats or fewer (Fig. 3C).
Community Structure.
All but nine of the bird species in our study partition into four primary diet guilds: frugivore, granivore, insectivore, and nectarivore (Fig. 3D). Insectivores had the lowest representation in open coffee plantations (36% of individuals) and highest in Amistad primary forest (70%) whereas granivores had the opposite pattern (29% and 1%, respectively). Frugivores had greater relative abundance in forest than in the coffee sites whereas proportions of nectarivores did not follow a pattern. Only one granivorous species was never recorded in coffee plantations (5% of 19 granivorous species) whereas three nectarivorous (11%), five carnivorous (71%), 16 frugivorous (34%), and 54 insectivorous (34%) species were never recorded at the coffee sites.
Population Change and Capture Trends.
Analysis of the capture-mark-recapture data using Cormack–Jolly–Seber population models showed that 60.5% more populations (69) are declining (λ < 1) than those that are stable (39) or increasing (4) (Fig. 4A and SI Appendix, Fig. S3). There were more population declines in the Las Cruces reserve than in the forest fragments that are nearly two orders of magnitude smaller, suggesting that Las Cruces’ ∼250-ha forest area (and an additional 115 ha of old pastures with regenerating forest) is not sufficient to sustain the populations of some forest birds. On the other hand, 13 of the 23 species with stable or increasing populations in coffee plantations were species of medium forest dependence.
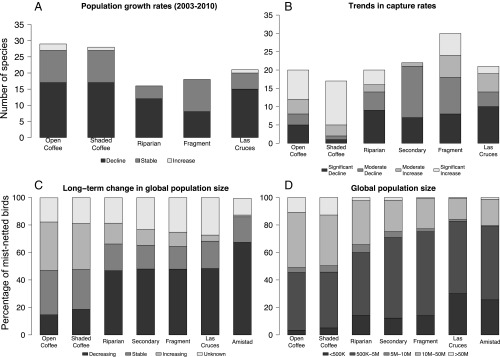
Fig. 4.
Population trends of bird species at the local and global level. (A) Population growth rates (λ), based on the analysis of the capture-mark-recapture data with Cormack–Jolly–Seber population models, indicate that more populations are declining than are increasing. Amistad International Park and secondary forest were not sampled for enough years to calculate comparable population growth rates. (B) Significant (P < 0.05) and moderate (0.05 < P < 0.10) changes in capture rates from 2003 to 2010 (from 2007 to 2010 for secondary forest). Forest habitats have more bird species with declining than increasing capture rates, but most of the forest specialists found at the forest sites no longer occur at the coffee sites and thus cannot decline further. Amistad International Park was not sampled for enough years to make it possible to calculate capture rate trends. (C) Global population trends of the mist-netted species (26). Bird species with globally declining populations increase with habitat tree cover. (D) Global population profiles of the mist-netted species (26). Bird species with globally small populations increase with habitat tree cover.
Overall, the ratio of declining to stable or increasing species was 1.7 for sedentary residents and 1.2 for the wintering populations of long-distance migrants. Sixty-two percent of declining populations (λ significantly <1) were primarily insectivores, 25% frugivores, 12% granivores, and 1% nectarivores. Not enough hummingbirds were banded to calculate λ values, however, and this excluded most of the common nectarivores.
Similar to λ values, significant (P < 0.05) and moderate (0.05 < P < 0.10) declines (77 populations) in capture rates (from 2003 to 2010) exceeded increases (53 populations). Shaded coffee species had the highest ratio of increases to declines (Fig. 4B). Eight of the 15 increases in shaded coffee plantations were species of medium and high forest dependence. When coffee plantations were analyzed at the site level, higher proportions of species were declining in individual open coffee plantations than in shaded coffee plantations (χ2 = 62.4, P < 0.0001) (SI Appendix, Fig. S4). Based on capture rates, the ratio of declining to increasing species was 1.8 for residents and 0.75 for long-distance migrants. Forty-nine percent of species with declining capture rates were primarily insectivores, 18% frugivores, 17% nectarivores, 13% granivores, and 3% were piscivores.
Species Traits and Vulnerability.
Species of conservation priority.
We captured 82 individuals from eight globally threatened or near threatened species (SI Appendix, Table S3). Most of these (87%) belonged to three migratory species. During the course of the study, we recorded 723 individuals of 11 species with globally restricted ranges [50,000 km2 or less (SI Appendix, Table S4), smaller than the area of Costa Rica (51,100 km2)].
When individuals (excluding recaptures) were analyzed with respect to the species’ global population trends, coffee plantations had the lowest proportion of birds with globally declining populations and the highest proportion of birds with globally increasing populations (Fig. 4C). Amistad primary forest had the opposite pattern, and the remaining forest habitats were in between. The differences between habitats were significant (all P < 0.03), except between coffee habitats (P = 0.71) and between fragment, riparian, and secondary forest habitats (all P > 0.21).
Primary forest sites had the most species with globally small populations. Two-thirds of the individual birds captured belonged to species with enough information to have global population estimates (26). Species with global populations under 500,000 birds had the highest representation at the primary forest sites of Las Cruces and Amistad whereas species with global populations of over 50 million birds had the highest representation at coffee plantations (Fig. 4D).
Individuals of human commensal bird species (known to benefit from people) favored heavily modified habitats, making up 25.0% of shaded coffee birds, followed by open coffee (22.9%), secondary forest (13.6%), fragment (12.1%), riparian (12.0%), Las Cruces (3.7%), and Amistad (1.3%) sites.
Mobility.
Modified habitats were heavily used by migratory species. Long-distance migrant species made up 21.6% of captured individuals (SI Appendix, Table S5). More than a third of the migrants consisted of Swainson’s thrushes, which migrated through the region in large numbers but did not overwinter, whereas the remaining species mostly wintered in the region. Excluding Swainson’s thrushes, wintering migratory bird species had higher representation in open coffee, shaded coffee, riparian, and secondary habitats, none of which differed from each other, and all differed from Las Cruces forest and Amistad primary forest, which did not differ from each other.
Reproductive status and productivity.
Riparian habitats had the highest proportion of nonmigratory (resident) birds in breeding condition (SI Appendix, Fig. S5), both for birds with brood patches (BPs) and with cloacal protuberances (CPs), whereas Amistad had the lowest proportions. The proportion of birds with CPs did not differ between habitats (all χ2 > 5.7, all P < 0.86), with the exception of Amistad. The proportion of riparian birds with BPs was significantly higher than those observed in other habitats (all χ2 > 13.6, all P < 0.034). The proportion of birds with BPs was significantly lower in Amistad than in other habitats (all χ2 > 12.8, all P < 0.047), with the exception of open coffee (χ2 = 2.02, P = 0.15). The ratio of the number of breeding birds with high/medium forest dependence to the number of breeding birds with low/no forest dependence increased from open coffee (0.66) to shaded coffee (0.83), forest fragments (0.79), riparian corridors (1.2), secondary forest (1.8), Las Cruces (2.7), and Amistad primary forest (7.1).
For nonmigratory species, the coffee sites had the highest ratios of hatch year (immature or juvenile) birds to adult birds of all categories [after hatch year (AHY), second year (SY), after second year (ASY), and older], followed by fragments, secondary forest, Las Cruces forest, riparian strips and Amistad, both when AHY birds were included in and excluded from the analyses (SI Appendix, Fig. S6), suggesting that coffee plantations are being used by young birds that cannot find territories in the forest habitats.
Discussion
Habitat Use.
Ninety of the 185 species (49%) with sufficient sample sizes (more than six captures) used forest habitats significantly more than coffee plantations. Seventy-two species (39%) had the opposite pattern while 23 species (12%) were habitat generalists that used both coffee and forest habitats. A quarter of these 185 species (46) were primary forest specialists, preferring the Las Cruces forest or the Amistad International Park to other habitats. Although 32 of these primary forest species had significantly higher capture rates in the 250-ha fragment of the Las Cruces forest than in the extensive Amistad International Park, some of this is a result of Amistad being situated at a higher elevation (1,500 m vs. 1,335 m). Four of these 32 species have elevational limits that only reach to 1,400 m, including gray-chested dove (Leptotila cassinii), rufous-tailed jacamar (Galbula ruficauda), white-whiskered puffbird (Malacoptila panamensis), and blue-crowned manakin (Lepidothrix coronata). Species that prefer coffee plantations showed no net preference for coffee plantations with more trees, with open coffee and shaded coffee sites significantly preferred by 17 species each, but there are some community differences discussed below. Of the 80 remaining species whose captures were not large enough for statistical comparisons, 38 were recorded only at the forest sites, and 24 were recorded only at coffee plantations, a distribution similar to that of the species with larger sample sizes.
Overall, the majority of the bird species recorded during our study are unlikely to persist in this human-dominated countryside landscape without the Las Cruces Forest Reserve (∼200 ha primary forest, 50 ha secondary forest, and 115 ha of old pastures with regenerating forest). However, the reserve itself may lose bird species in the future due to the ongoing bird population declines we observed, likely caused by a combination of an “extinction debt” (27, 28) and continuing forest degradation, plant species turnover, and loss of plant biomass (29). Most of the forest and primary forest species were recorded at significantly higher capture rates at Las Cruces than at the extensive Amistad International Park. However, Las Cruces is at a slightly lower elevation than Amistad. Additionally, the greater number of species at Las Cruces may also partially result from some of these species having remnant and inviable “living dead” populations in this isolated forest fragment that is the largest in the region. Furthermore, the regeneration of the second growth matrix surrounding Neotropical forest fragments can promote avian recolonization (30), and there has been active forest restoration bordering Las Cruces since 1998 (31). In that respect, the ongoing Las Cruces–Guaymí biological corridor project (31), which seeks to link the Las Cruces forest with the much larger Guaymí (Ngäbe) indigenous reserve (7,500 ha), is timely and essential to reduce the isolation of the Las Cruces Forest Reserve and to prevent the realization of its extinction debt.
Nearly half of the bird species recorded during our study either prefer coffee plantations to forest habitats, or they are habitat generalists that use a combination of coffee plantations and edge/secondary/open forest habitats. The coffee plantations in the region are sun coffee plantations with minimal tree cover, unlike shade coffee plantations that are better at conserving forest bird diversity (21, 32). Nevertheless, a small landscape-level difference in tree cover between our sites in open coffee plantations (averaging 7% cover) and in those shaded with more trees (13% cover) resulted in positive changes in the bird community, with increases in some bird species that are range-restricted, forest-dependent, and of global conservation concern (see below) (Fig. 3B and SI Appendix, Tables S3 and S4). However, the global collapse of the coffee market in the 1990s has resulted in a switch to other forms of agriculture and cattle production by some of the local coffee farmers, imperiling the quantity and quality of remaining forest habitat (27). Our results indicate that even seemingly minor losses of trees from existing coffee plantations (from 13 to 7% tree cover) will negatively impact the local bird community (Figs. 1, 3, and 4), especially forest species of conservation concern with limited distributions and declining populations. The conversion of coffee plantations to cattle pastures and other types of land use with lower woody plant cover is likely to result in net population losses in the majority of the bird species that use coffee habitats.
Forest Dependence.
There was no relationship between the species richness of a habitat and its conservation value, as indicated by the distinct habitat and dietary functional signatures of the forest bird communities. Nearly all of the birds captured in the Las Cruces (>94% of individual birds) and Amistad (>97%) forests are species with medium or high forest dependence (Fig. 3B). Understory bird communities of riparian strips (72%), secondary forests (82%), and small forest fragments (86%) are also predominantly comprised of bird species with medium and high forest dependence. This parallels these birds’ primary habitat choice of forest (Fig. 3A), indicating the crucial value of these forest remnants for maintaining forest biodiversity in this human-dominated landscape with only 28% forest cover—and declining (27). The proportions of these medium or high forest-dependence species were much lower in open (38%) and shaded (43%) coffee plantations. However, our data also show that half of the birds found in coffee plantations prefer forest habitat, suggesting that these areas still provide habitat for many forest-dependent bird species (Fig. 3A) and large numbers of migratory bird species. Underlining the conservation value of even modest increases in tree cover, the capture rate of high forest-dependence species nearly doubled (2.1% vs. 3.9% of individuals) and that of nonforest species halved (19.5% vs. 9.7% of individuals) in shaded coffee versus open coffee plantations (Fig. 3B).
Ecological Guilds and Ecosystem Services.
As is typically the case in the tropics (33), the proportion of insectivores was highest in the least disturbed and most extensive primary forest of the Amistad International Park and lowest in the open, agricultural habitat of coffee plantations (Fig. 3D). The opposite was the case for granivores, also in agreement with the distinct dietary functional signature of tropical forest bird communities (33). Nectarivores showed no consistent pattern although they did occur at the highest frequency at Las Cruces, possibly owing to the nearby presence of a botanical garden and other nectar-rich habitat types. Most forest species absent from coffee plantations are specialized forest understory insectivores, which are highly sensitive to forest fragmentation, degradation, and conversion (34–36). In addition to their ecological importance in tropical forests, insectivorous birds can provide valuable pest control services in coffee plantations (37, 38), and the declines of insectivorous birds in coffee plantations may have negative economic consequences for the coffee farmers. Frugivorous birds can also provide ecological benefits by providing seed dispersal services, and they have been shown to improve seed rain during ecological restoration efforts around Las Cruces (39). In contrast, granivorous bird species have the potential to be agricultural pests (24). Consequently, it is encouraging that even a slight increase in the tree cover of shaded coffee versus open coffee sites is reflected in increases in the proportions of insectivores and frugivores and a decrease in the proportion of granivores (Fig. 3D).
Limitations of Mist Netting.
This study was based on extensive mist netting and bird banding because of our focus on the region’s highly sensitive forest understory bird community (34) and our main goal to measure long-term population changes using capture-mark-recapture analyses (23, 40). However, mist netting cannot detect all of the species in a region’s avifauna, especially in diverse tropical forest bird communities with extensive stratification where point counts should also be used if possible (41). Therefore, our results cannot be generalized to the entire avifauna of this region with more than 400 bird species. Although mist nets and point counts have been shown to sample similar proportions of Neotropical forest species in groupings based on families, abundance, migratory status, habitat use, and foraging substrate guilds, mist nets sample a greater proportion of understory birds and small species than do point counts (41). In addition, canopy species, usually more tolerant of forest degradation and fragmentation, cannot be sampled effectively with mist nets (41). Consequently, our results primarily apply to forest understory birds, which also comprise the indicator group most sensitive to forest fragmentation and degradation in tropical forests in general and at Las Cruces in particular (34).
In addition, in shaded coffee (13% tree cover) and sun coffee (7% tree cover) plantations, most of the vegetation consists of 3-m-tall coffee plants. This scarcity of midstory and canopy forest vegetation results in a “compression” of the bird community, with some midstory and canopy bird species being forced to come down to 3 m or lower to stay in vegetation cover while moving through these plantations. These species of higher forest layers are rarely caught in mist nets at our forest sites where forest height can exceed 40 m. Consequently, coffee plantation bird communities sampled with mist nets are likely to have an inflated species richness compared with forested sites where upper story and canopy birds are rarely caught in mist nets. Therefore, compared with forest bird communities, the coffee plantation bird communities in our study are likely to be more impoverished than mist netting alone would indicate. For similar future studies in the region and in other comparable areas, we recommend a combination of mist netting and point counts, especially for studying the avifauna of higher vegetation layers.
Population Change over Time.
Population growth (λ) values and long-term trends in capture rates indicate that 1.5 to 1.6 more species are declining than are stable or increasing (Fig. 4 A and B and SI Appendix, Fig. S3). More species are declining in the 250-ha Las Cruces forest than in the 3- to 5-ha forest fragments that are 50 to 80 times smaller in area than the Las Cruces forest. Las Cruces forest hosts more bird species (Fig. 1), and some of these species have already disappeared from the small fragments (42). Furthermore, Las Cruces reserve itself is a 250-ha forest fragment, and even 1,000-ha tropical forest fragments are predicted to lose bird species 50 y after fragmentation (43). Populations of insectivores tend to be declining even in relatively large forest reserves on the Caribbean coasts of both Costa Rica and Panama, near where the present study was conducted (35, 36). Similar declines at the Las Cruces forest suggest that it may still be paying its extinction debt 60 y after regional deforestation. This underlines the urgency of the current land campaign to connect the Las Cruces forest to the 7,500-ha Guaymí (Ngäbe) reserve (31), especially in light of the ongoing net forest loss in the region (27).
Birds in shaded coffee had the highest ratio of increases to declines in species capture rates (Fig. 4B). It is encouraging to see that the small increase in tree cover (from 7 to 13%) in open vs. shaded coffee plantations influences bird species richness (Fig. 1) and bird communities (Fig. 3) positively and leads to long-term increases in some of the forest birds recorded there, indicating the importance of even modest efforts to keep and restore native trees in this agricultural countryside. These findings agree with our previous research showing the importance of forest remnants, riparian corridors, and individual trees for connecting this human-dominated landscape and for supporting some of the native forest bird diversity by providing breeding habitat, stepping stones for movement, habitat for surplus nonbreeders, and mosaics of different habitat types with varying phenologies of fruit, nectar, and insect production (12, 13, 44). Finally, populations of long-distance migratory species did better than those of the sedentary residents, in line with global assessments showing that migratory birds’ mobility helps reduce their extinction likelihood (45).
Species Traits.
Species of conservation priority.
In 12 y of mist netting, we captured only 82 near threatened birds and a single individual of a globally threatened species (SI Appendix, Table S3), a turquoise cotinga (Cotinga ridgwayi). This is not surprising as most of the threatened and near threatened species in Costa Rica are rare or uncommon, large species that seldom get caught in mist nets, such as cracids and macaws (26). All but 12 of these birds belonged to three Neotropical migrant species, which tend to prefer coffee plantations and more open forest habitats. Also of conservation significance are 723 individuals of 11 range-restricted species we recorded (SI Appendix, Table S4). Ninety-one percent of the range-restricted birds were captured at the forest sites, particularly in the Las Cruces forest where their capture rate was 14 to 31 times higher than in the coffee habitats. Species with globally declining populations and smaller global populations were also better represented in the forest habitats, particularly at Las Cruces and Amistad (Fig. 4 C and D). Approximately half of all individuals recorded in the forest habitats belonged to species with globally declining populations whereas only one-sixth of coffee individuals were in this category. The region’s coffee plantations with 7 to 13% tree cover are important for some species, but not for the species of greatest conservation concern, the large majority of which preferred forest habitats.
Migratory species.
Coffee plantations provide important habitat for some declining Neotropical migratory birds (32). Although some of our forest sites having a higher proportion of migratory birds seems contrary to the general pattern in the tropics (46), this was due to the large numbers of Swainson’s thrushes migrating through the study region without wintering. As they comprised 36% of all migratory individuals recorded, their preference for forest skewed the proportions, especially at Amistad, where they comprised 84% of all migratory birds caught in the mist nets. Excluding Swainson’s thrushes that only migrate through, wintering migratory birds did prefer coffee plantations and the more open riparian and secondary forest sites over the primary forest sites (SI Appendix, Table S5). Wintering migratory birds had the highest representation in shaded coffee plantations, indicating the importance of even small increases in tree cover for Neotropical migrants, which comprise the avian group that is the biggest focus of international conservation funding in the Neotropics.
Reproductive status and productivity.
Riparian sites had the highest proportion of nonmigratory birds in breeding condition (SI Appendix, Fig. S5), based on the presence of a brood patch (BP) (24%) or cloacal protuberance (CP) (14%). This is possibly due to the year-around presence of running water and associated plant and insect productivity, indicating the vital conservation value of these narrow riparian corridors in an agricultural matrix. Amistad birds had the lowest proportion of nonmigratory birds in breeding condition and the lowest ratio of young birds to adult birds (SI Appendix, Fig. S6). However, Amistad had the highest ratio (7.1) of the number of breeding birds with high/medium forest dependence to the number of breeding birds with low/no forest dependence, 9 to 11 times higher than the same ratios in forest fragments (0.79), shaded coffee (0.83), and open coffee (0.66). After Amistad, riparian sites had the lowest young:adult ratio whereas the coffee sites had the highest, the opposite of the pattern observed in birds in breeding condition. With the exception of Amistad, habitats with the highest proportions of breeding adults had the lowest proportions of young birds and vice versa. The low proportions of young birds in forest and riparian habitats are likely a result of these habitats being saturated with the territories of breeding adults, and coffee plantations may provide valuable “holding” habitat for young birds while they wait for territories to open up in the forest. However, in the long term, coffee plantations may be “sink” rather than “source” habitats, sensu Pulliam (47). Riparian strips and the Las Cruces forest may be acting as population sources and coffee plantations acting as population sinks for the young of some species, as was observed for fledgling white-throated robins (Turdus assimilis) in the coffee plantations bordering Amistad International Park (48).
Conclusions
We documented over 57,000 captures of 265 bird species during more than 105,000 mist-net hours of intensive sampling in the agricultural countryside of southern Costa Rica, to assess the decade-long changes in the bird populations. Despite the fact that less than a third of this landscape retains tree cover and much of the forest is degraded and fragmented, almost all of the native avifauna persist. Even though we recorded 235 species (89%) outside the Las Cruces and Amistad reserves, including in the coffee plantations, narrow riparian corridors, small forest fragments, and secondary forests, species lists alone can be misleading. Many of these species actually avoid disturbed habitats but have to fly through them to reach primary forest, and most (120) of these species were recorded fewer than 10 times in any of these nonprimary forest habitats. About half (90 of 185) of the adequately sampled species prefer forests and avoid coffee plantations, and 46 of these forest species are primary forest specialists, preferring the Las Cruces Forest Reserve or the Amistad International Park to other sites. There was no relationship between the species richness of a habitat and its conservation value, as indicated by the distinct composition, and habitat and dietary functional signatures of the forest bird communities.
Habitat generalists did better in coffee plantations, and specialists had higher capture rates in primary forest. Granivorous birds increased in the coffee sites whereas insectivores increased in primary forest. Due to their increased mobility, migratory bird species have nearly two times lower risk of extinction than do sedentary species (49), and, in our study, the migratory species also did better than the sedentary species. As expected, the coffee sites had higher proportions of nonforest birds and those with low forest dependence than did the forest sites. The forest sites, and especially the Las Cruces Forest Reserve, had the highest capture rate of globally range-restricted species. Capture rates of bird species with globally small and/or declining populations increased with primary forest cover, indicating that the Las Cruces Reserve and Amistad International Park forests have critical value for global bird conservation. Coffee plantations had the highest proportion of young birds, but, because these sites did not have the highest proportions of birds in breeding condition, these young birds may be coming from the forest sites, and coffee plantations may be population sinks for some species (48).
The region’s forest bird community is not safe in the long term, including those in the Las Cruces forest reserve, which is a 250-ha fragment with ongoing forest degradation (29) and is likely to lose bird species in the coming decades. Our population models and capture trends indicate that nearly twice as many bird populations are declining as are stable or increasing, a ratio that also applies to the Las Cruces forest. Although the ongoing land conservation and restoration campaign to connect the Las Cruces reserve to the 7,500-ha Guaymí (Ngäbe) Indigenous Reserve is an excellent initiative and has the potential to reduce bird declines, it is unlikely to be sufficient by itself to reverse the ongoing net forest loss (27) and degradation (29). Substantially more conservation, education, and outreach work needs to be undertaken with hundreds of farmers, cattle owners, and other locals, especially because the collapse of the coffee market in the 1990s has triggered a trend to replace the coffee plantations with other crops and cattle pastures (27). Our data show that even modest increases in tree cover in sun coffee plantations result in increases in bird capture rates, more forest-dependent bird species, more species of global conservation concern, and potentially improved avian ecosystem services. Furthermore, narrow riparian strips, small forest fragments, and regenerating forests, despite covering a small area and receiving no active protection, are disproportionately important in improving landscape connectivity, increasing the effective area of Las Cruces and other forest remnants (13), providing refugia to many forest species, and supporting avian biodiversity in the region.
Deforestation in this region has mostly occurred in the past 50 y, and studies of tropical avian extinction debt suggest that only about half of bird extinctions take place during half a century (43). Consequently, increasing the tree cover and landscape connectivity in the human-dominated countryside of southern Costa Rica is essential to increase its biodiversity capacity and to reduce future extinctions. As most of this landscape is already agricultural, relying on the existing forest reserves is not sufficient, and agricultural areas need to be better integrated into conservation programs. The conservation value of many tropical agricultural landscapes can be augmented substantially with modest investment and limited conflict because remnant trees, riparian strips, forest fragments, and their resident avifauna supply people and domestic animals with fruits, shade, clean water, crop pollination, and other ecosystem services (38, 44, 50–53). Working with individual landowners to provide them with clear incentives to conserve forest remnants should be a top priority, as exemplified by Costa Rica’s successful “Payment for Environmental Services” program (54). Diversified agricultural landscapes can enhance the biodiversity capacity of tropical countryside, and the conservation community needs to focus more on enhancing the conservation value of tropical countryside, to increase the chances of survival for thousands of declining species. Nevertheless, for the long-term persistence of all forest bird species, large (>1,000 ha) protected areas of primary forest are essential.
Materials and Methods
Study Region.
Our study was centered at the Las Cruces Biological Station (8°47′ N, 82°57′ W; 1,200 m above sea level) of the Organization for Tropical Studies, part of the Las Cruces Forest Reserve (referred to as Las Cruces above) in the canton of Coto Brus in southern Costa Rica. Mean annual temperature is 22 °C, and yearly average rainfall is around 3,500 mm. This previously forested region of southern Costa Rica is now dominated by sparsely shaded coffee plantations and pasture and is representative of human-dominated tropical areas that retain a substantial portion of their original biodiversity (12). The forest fragments have been isolated since the mid-1950s, and the Las Cruces forest is the largest midelevation fragment in the region (∼200 ha of primary forest, 50 ha of secondary forest, and 115 ha of old pastures with regenerating forest) (29, 31).
Sampling Sites.
Birds were captured at 19 sites representing seven major habitat types (SI Appendix, Fig. S1 and Table S1): the extensive primary rainforest of Amistad, three sites (Fila, Gamboa, and Rio Java) in the Las Cruces reserve, three primary and secondary forest fragments of 3 to 5 ha (Copal, Gamboa, and Los Angeles), three narrow riparian corridors [10- to 30-m-wide strips of forest along rivers (Panama, Sabalito, and Santa Teresa)], three secondary forest sites (Cañas Gordas, Los Pinos, and Melissa), three sun coffee plantations with minimal (5 to 9%) tree cover [referred to as open coffee above (El Puente, San Francisco, and San Gabriel)], and three sun coffee plantations with higher (12 to 14%) tree cover [referred to as shaded coffee above (La Isla, San Bosco, and Santa Teresa)]. Detailed descriptions of the sites and the vegetation surveys used to characterize them are provided in refs. 13 and 44. During analysis, data from multiple stations of one habitat type were pooled to increase the sample sizes of bird species analyzed and to increase the power of the analyses, especially for population change and capture trends.
The study area consists of fragments of Pacific premontane humid forest surrounded by pastures, plantations of coffee, other crops, and human settlements. Coffee plants (Coffea arabica L.) in the region are 2 to 3 m tall, partially shaded by banana plants (Musa spp.), poró trees (Erythrina poeppigiana Walpers), and scattered remnant trees that do not form a continuous canopy (12, 13). Based on our vegetation surveys, 25.7% of the landscape consists of forest remnants, including the Las Cruces forest reserve (8.1%), small forest fragments (7.3%), and riparian corridors (10.4%). Another 15.7% is covered in secondary forest and remnant trees. All of the bird banding stations were situated in this landscape at elevations between 900 and 1,335 m asl, with the exception of the Amistad primary forest site (∼1,500 m asl) situated in the ∼400,000-ha Amistad International Park (8°57′ N, 82°50′ W).
Data were collected during the wet seasons (June to September) of 1999 and 2002 and the dry seasons (January to May) of 2003 to 2010. To control for the effects of season, we only used the data from the 2003 to 2010 seasons for the analyses of capture rates, population growth rates, age, and breeding condition.
Sampling Regime.
To capture and mark birds, we mist-netted using 20 12-m, 36-mm mesh nets, with the exception of 1999, when 12 nets were used, following published methodology (40). Research was approved by a Stanford University Administrative Panel on Laboratory Animal Care (APLAC) protocol for care and use of laboratory animals. At each site, we operated the mist nets for 6 h beginning approximately half an hour before sunrise. No site was sampled for 2 d in a row, and we rotated daily between sites to minimize the drop in capture rate as a result of birds learning and avoiding net locations. Sampling bird communities using mist nets introduces some bias as certain species are far less likely to be captured in nets (41, 55). Large-bodied species or aerial insectivores tend to be underrepresented in mist-netting studies (55). Our results, therefore, are more representative of the smaller bodied species that frequent lower vegetation layers.
Data Analyses.
With the exception of mark-recapture analyses, individuals were counted only when trapped first (recaptures were excluded from the analysis) to avoid estimation bias from individuals that were recaptured many times (56). Then, all sites in each habitat category were combined, so as to compare the seven major habitat types. Pooling the data for each habitat was necessary to increase the power of the analyses, especially for population change and capture trends.
We estimated species survival independently for each habitat type using the R package “marked” (57). “Marked” estimates species survival by fitting mark-recapture data to a Cormack–Jolly–Seber model and was specifically designed for datasets with large numbers of individuals (57). We defined a population as all of the individuals of a species captured in a habitat type. Population growth rates (λ) were estimated using the R package “Rcapture” (58), which used log-linear modeling to assess demographic information from open populations. Amistad International Park was not sampled for enough years to make it possible to calculate population growth rates. We used the R package “vegan” (59) to conduct nonmetric multidimensional scaling (NMDS) analyses on the species communities in different habitat types where banding occurred. Species richness and abundance for each habitat were used in examining interhabitat variation in two dimensions. Lastly we used the “vegetarian” package (60) to estimate Morisita–Horn dissimilarity indices. The Morisita–Horn index was used because it has minimal sample size biases and is useful for large species assemblages with many rarely recorded species, as was the case in our study (61).
We used the Chao1 estimator in EstimateS 9.1.0 (62) to calculate estimated species richness [S(est)] for species’ relative abundance data (63). In the rarefaction curves of S(est), we extrapolated the habitats with fewer sampling sessions to the number of sessions of Las Cruces (179 sessions), to directly compare observed and estimated species richness in both habitats (64). Using this method, statistically robust extrapolation of samples is possible to make direct comparison of sites with different sample sizes, as was the case in our study (64). Individuals were randomized without replacement.
The frequency of breeding birds was determined for all habitats, by dividing the number of captures of nonmigratory (resident) species in breeding condition, as evidenced by cloacal protuberance or brood patch, by the total number of captures of those species (40). The ratio of juvenile to adult birds was also calculated for only the nonmigratory (resident) species. Birds in their first year (hatching year or newly fledged local birds) were classified as juveniles, and all birds in their second year or after were classified as adults, with the analyses done both including and excluding after hatch year (AHY) birds of undetermined age. Relative abundance was determined from the capture rate (number of birds per 100 net-hours), an index which controls for differing effort between habitats (65).
Information on bird species’ ecology was obtained from a global database containing the ecological traits of all of the world’s bird species (66) and is regularly updated. The most recent data on species’ conservation status, population size, population trends, and forest dependence were obtained from BirdLife International (26). Each species’ forest dependence was rated as high, medium, low, or nonforest (26). We classified mobility in three categories: long-distance migrant (Nearctic-Neotropical), altitudinal migrant, or sedentary. Human commensal species were defined as species that significantly benefit from associating with people and have often expanded their ranges as a result. Species’ diets were classified based on seven major food categories (seeds, fruits, nectar, invertebrates, carrion, vertebrates, and nonreproductive plant material) and ordered by priority in each species’ diet on a 10-point scale to determine primary diet, which consisted of the categories carnivore, frugivore, granivore, insectivore, nectarivore, and piscivore for the species in our study.
We constructed habitat and diet functional signatures of the bird community of each main habitat category. A functional signature of a set of bird species is the distribution of relative frequencies over a number of functional groups (67). For habitat preference, for example, the functional signature of a set of observed bird species could be as follows: 65% of the individuals recorded belong to a species whose first (primary) choice of habitat is forest, 30% of the individuals have shrub/scrub as primary habitat, and 5%, have grassland as primary habitat. The habitat signatures were constructed using the relative frequencies of the preferred habitats forest, woodland, shrub/scrub, savanna/grassland, riparian, and artificial (human-dominated habitats such as agriculture and settlements). The diet signatures were constructed using the preferred diets invertebrate, fruit, nectar, and seed.
Two-tailed t tests were used to test the hypotheses that capture and recapture rates, corrected for sampling effort, differed between different habitats for each species. For all our comparisons, we used Welch’s unequal variances t test that is used when the two samples have unequal variances and unequal sample sizes. Across different habitats, forest-dependence profiles, community diet signatures, primary habitat choices, global population profiles, proportions of juveniles, birds in breeding condition, and long-distance migrants were compared with χ2 tests.
Data Sharing Statement.
Species population change data used in the study are provided in SI Appendix, Supporting Information.
Supplementary Material
Acknowledgments
We thank the Moore Family Foundation, the National Geographic Society, the Wildlife Conservation Society, and the Winslow Foundation for financial support for this project. We thank the Costa Rican government (Ministerio de Ambiente y Energía) and the Organization for Tropical Studies for allowing us to work at the Las Cruces Biological Research Station; and L. D. Gómez, R. Quirós, E. Ramírez, Z. Zahawi, and other Las Cruces staff for their support. We appreciated the assistance of D. Aygen, S. Bangen, P. M. Cabezas, S. Jiménez Carvajal, M. Paniagua Castro, F. Fleischman, D. Goehring, S. Loarie, A. Ilama Meza, B. Serrano Nuñez, V. Ruiz-Gutiérrez, E. Castro Sandí, J. Figuroa Sandí, R. Figuroa Sandí, and A. Tapia in conducting the field work. We thank the Aragón, Barrantes, Gamboa, Granados, Pérez, and Piñeda families for allowing us to conduct our research on their properties. We thank William Laurance, Montague Neate-Clegg, Scott Robinson, Thomas Sherry, Tanya Williams, and the anonymous reviewers for their critical reviews and excellent suggestions that improved this paper greatly.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1802732116/-/DCSupplemental.
References
- 1.Ceballos G, et al. Accelerated modern human-induced species losses: Entering the sixth mass extinction. Sci Adv. 2015;1:e1400253. doi: 10.1126/sciadv.1400253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sodhi NS, Koh LP, Brook BW, Ng PKL. Southeast Asian biodiversity: An impending disaster. Trends Ecol Evol. 2004;19:654–660. doi: 10.1016/j.tree.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Dirzo R, Raven PH. Global state of biodiversity and loss. Annu Rev Environ Resour. 2003;28:137–167. [Google Scholar]
- 4.Laurance WF, Bierregaard RO., Jr . Tropical Forest Remnants: Ecology, Management, and Conservation of Fragmented Communities. Univ Chicago Press; Chicago: 1997. [Google Scholar]
- 5.Gibson L, et al. Primary forests are irreplaceable for sustaining tropical biodiversity. Nature. 2011;478:378–381. doi: 10.1038/nature10425. [DOI] [PubMed] [Google Scholar]
- 6.Tobias J, Şekercioğlu ÇH, Vargas FH. Bird conservation in tropical ecosystems: Challenges and opportunites. In: MacDonald DW, Willis KJ, editors. Key Topics in Conservation Biology 2. John Wiley & Sons; New York: 2013. pp. 258–276. [Google Scholar]
- 7.Juffe-Bignoli D, et al. 2014. Protected Planet Report 2014 (UN Environment World Conservation Monitoring Centre, Cambridge, United Kingdom)
- 8.Harris JBC, et al. The tropical frontier in avian climate impact research. Ibis. 2011;153:877–882. [Google Scholar]
- 9.Wormworth J, Şekercioğlu ÇH. Winged Sentinels: Birds and Climate Change. Cambridge Univ Press; Cambridge: 2011. [Google Scholar]
- 10.World Bank 2013 Agricultural land (% of land area). Available at data.worldbank.org/indicator/AG.LND.AGRI.ZS. Accessed December 25, 2017.
- 11.Daily GC, Ehrlich PR, Sanchez-Azofeifa GA. Countryside biogeography: Use of human-dominated habitats by the avifauna of southern Costa Rica. Ecol Appl. 2001;11:1–13. [Google Scholar]
- 12.Şekercioğlu ÇH, Loarie SR, Oviedo Brenes F, Ehrlich PR, Daily GC. Persistence of forest birds in the Costa Rican agricultural countryside. Conserv Biol. 2007;21:482–494. doi: 10.1111/j.1523-1739.2007.00655.x. [DOI] [PubMed] [Google Scholar]
- 13.Mendenhall CD, Şekercioğlu ÇH, Brenes FO, Ehrlich PR, Daily GC. Predictive model for sustaining biodiversity in tropical countryside. Proc Natl Acad Sci USA. 2011;108:16313–16316. doi: 10.1073/pnas.1111687108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy CM, et al. Optimizing land use decision-making to sustain Brazilian agricultural profits, biodiversity and ecosystem services. Biol Conserv. 2016;204:221–230. [Google Scholar]
- 15.Janzen D. Gardenification of wildland nature and the human footprint. Science. 1998;279:1312–1313. [Google Scholar]
- 16.Laurance WF. Emerging threats to tropical forests. Ann Mo Bot Gard. 2015;100:159–169. [Google Scholar]
- 17.Fischer J, Lindenmayer D. Landscape modification and habitat fragmentation: A synthesis. Glob Ecol Biogeogr. 2007;16:265–280. [Google Scholar]
- 18.Pimentel D, et al. Conserving biological diversity in agricultural/forestry systems: Most biological diversity exists in human-managed ecosystems. BioScience. 1992;42:354–362. [Google Scholar]
- 19.Greenberg R, Perfecto I, Philpott SM. Agroforests as model systems for tropical ecology. Ecology. 2008;89:913–914. doi: 10.1890/07-1578.1. [DOI] [PubMed] [Google Scholar]
- 20.Perfecto I, Vandermeer J. Year in Ecology and Conservation Biology 2008. Vol 1134. Blackwell Publishing; Oxford: 2008. Biodiversity conservation in tropical agroecosystems–A new conservation paradigm; pp. 173–200. [DOI] [PubMed] [Google Scholar]
- 21.Buechley ER, et al. Importance of Ethiopian shade coffee farms for forest bird conservation. Biol Conserv. 2015;188:50–60. [Google Scholar]
- 22.Şekercioğlu ÇH. Functional extinctions of bird pollinators cause plant declines. Science. 2011;331:1019–1020. doi: 10.1126/science.1202389. [DOI] [PubMed] [Google Scholar]
- 23.Şekercioğlu ÇH. Promoting community-based bird monitoring in the tropics: Conservation, research, environmental education, capacity-building, and local incomes. Biol Conserv. 2012;151:69–73. [Google Scholar]
- 24.Şekercioğlu ÇH. Ecological significance of bird populations. In: del Hoyo J, Elliott A, Christie DA, editors. Handbook of the Birds of the World: Old World Flycatchers to Old World Warblers. Vol 11. Lynx Edicions; Barcelona: 2006. pp. 15–51. [Google Scholar]
- 25.Wenny DG, Şekercioğlu ÇH, Cordeiro N, Rogers HS, Kelly D. Seed dispersal by fruit-eating birds. In: Şekercioğlu ÇH, Wenny DG, Whelan CJ, editors. Why Birds Matter. Univ Chicago Press; Chicago: 2016. pp. 107–146. [Google Scholar]
- 26.BirdLife International 2018 Birdlife data zone. Available at http://datazone.birdlife.org/. Accessed January 5, 2018.
- 27.Zahawi RA, Duran G, Kormann U. Sixty-seven years of land-use change in southern Costa Rica. PLoS One. 2015;10:e0143554. doi: 10.1371/journal.pone.0143554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sodhi NS, et al. Deforestation and avian extinction on tropical landbridge islands. Conserv Biol. 2010;24:1290–1298. doi: 10.1111/j.1523-1739.2010.01495.x. [DOI] [PubMed] [Google Scholar]
- 29.Zahawi RA, Oviedo-Brenes F, Peterson CJ. A degradation debt? Large-scale shifts in community composition and loss of biomass in a tropical forest fragment after 40 years of isolation. PLoS One. 2017;12:e0183133. doi: 10.1371/journal.pone.0183133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stouffer PC, Strong C, Naka LN. Twenty years of understorey bird extinctions from Amazonian rain forest fragments: Consistent trends and landscape-mediated dynamics. Divers Distrib. 2009;15:88–97. [Google Scholar]
- 31.Zahawi RA. The Las Cruces–Guaymí Biological Corridor in Southern Costa Rica. Organization for Tropical Studies; Durham, NC: 2015. [Google Scholar]
- 32.Komar O. Ecology and conservation of birds in coffee plantations: A critical review. Bird Conserv Int. 2006;16:1–23. [Google Scholar]
- 33.Şekercioğlu ÇH. Bird functional diversity and ecosystem services in tropical forests, agroforests and agricultural areas. J Ornithol. 2012;153(Suppl 1):S153–S161. [Google Scholar]
- 34.Şekercioğlu ÇH. Forest fragmentation hits insectivorous birds hard. Dir Sci. 2002;1:62–64. [Google Scholar]
- 35.Sigel BJ, Sherry TW, Young BE. Avian community response to lowland tropical rainforest isolation: 40 years of change at La Selva Biological Station, Costa Rica. Conserv Biol. 2006;20:111–121. doi: 10.1111/j.1523-1739.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 36.Sigel BJ, Robinson WD, Sherry TW. Comparing bird community responses to forest fragmentation in two lowland Central American reserves. Biol Conserv. 2010;143:340–350. [Google Scholar]
- 37.Johnson MD, Kellermann JL, Stercho AM. Pest reduction services by birds in shade and sun coffee in Jamaica. Anim Conserv. 2010;13:140–147. [Google Scholar]
- 38.Karp DS, et al. Forest bolsters bird abundance, pest control and coffee yield. Ecol Lett. 2013;16:1339–1347. doi: 10.1111/ele.12173. [DOI] [PubMed] [Google Scholar]
- 39.Cole RJ, Holl KD, Zahawi RA. Seed rain under tree islands planted to restore degraded lands in a tropical agricultural landscape. Ecol Appl. 2010;20:1255–1269. doi: 10.1890/09-0714.1. [DOI] [PubMed] [Google Scholar]
- 40.Ralph C, Dunn EH. Monitoring Bird Populations Using Mist Nets. Cooper Ornithological Society; Camarillo, CA: 2004. [Google Scholar]
- 41.Whitman AA, Hagan JMI, Brokaw NVL. A comparison of two bird survey techniques used in a subtropical forest. Condor. 1997;99:955–965. [Google Scholar]
- 42.Şekercioğlu ÇH, et al. Disappearance of insectivorous birds from tropical forest fragments. Proc Natl Acad Sci USA. 2002;99:263–267. doi: 10.1073/pnas.012616199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brooks TM, Pimm SL, Oyugi JO. Time lag between deforestation and bird extinction in tropical forest fragments. Conserv Biol. 1999;13:1140–1150. [Google Scholar]
- 44.Şekercioğlu ÇH, et al. Tropical countryside riparian corridors provide critical habitat and connectivity for seed-dispersing forest birds in a fragmented landscape. J Ornithol. 2015;156(Suppl 1):S343–S353. [Google Scholar]
- 45.Şekercioğlu ÇH. Conservation ecology: Area trumps mobility in fragment bird extinctions. Curr Biol. 2007;17:R283–R286. doi: 10.1016/j.cub.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 46.Poulin B, Lefebvre G. Dietary relationships of migrant and resident birds from a humid forest in central Panama. Auk. 1996;113:277–287. [Google Scholar]
- 47.Pulliam HR. Sources and sinks: Empirical evidence and population consequences. In: Rhodes OE Jr, Chester RK, Smith MH, editors. Population Dynamics in Ecological Space and Time. Univ Chicago Press; Chicago: 1996. pp. 45–70. [Google Scholar]
- 48.Cohen EB, Lindell CA. Survival, habitat use, and movements of fledgling White-Throated Robins (Turdus assimilis) in a Costa Rican agricultural landscape. Auk. 2004;121:404–414. [Google Scholar]
- 49.Horns JJ, Şekercioğlu ÇH. Conservation of migratory species. Curr Biol. 2018;28:R980–R983. doi: 10.1016/j.cub.2018.06.032. [DOI] [PubMed] [Google Scholar]
- 50.Daily GC, editor. Nature’s Services: Societal Dependence on Natural Ecosystems. Island Press; Washington, DC: 1997. [Google Scholar]
- 51.Ricketts TH, Daily GC, Ehrlich PR, Michener CD. Economic value of tropical forest to coffee production. Proc Natl Acad Sci USA. 2004;101:12579–12582. doi: 10.1073/pnas.0405147101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harvey CA, et al. Contribution of live fences to the ecological integrity of agricultural landscapes. Agric Ecosyst Environ. 2005;111:200–230. [Google Scholar]
- 53.Şekercioğlu ÇH. Ecosystem functions and services. In: Sodhi NS, Ehrlich PR, editors. Conservation Biology for All. Vol 13. Oxford Univ Press; Oxford: 2010. pp. 45–72. [Google Scholar]
- 54.Bank W. Costa Rica Improves the Efficiency of Its Payment for Environmental Services Program. World Bank; Washington, DC: 2015. [Google Scholar]
- 55.Wang Y, Finch DM. Consistency of mist netting and point counts in assessing landbird species richness and relative abundance during migration. Condor. 2002;104:59–72. [Google Scholar]
- 56.Remsen JV, Jr, Good DA. Misuse of data from mist-net captures to assess relative abundance in bird populations. Auk. 1996;113:381–398. [Google Scholar]
- 57.Laake JL, Johnson DS, Conn PB. marked: An R package for maximum likelihood and Markov Chain Monte Carlo analysis of capture-recapture data. Methods Ecol Evol. 2013;4:885–890. [Google Scholar]
- 58.Baillargeon S, Rivest L-P. Rcapture: Loglinear models for capture-recapture in R. J Stat Softw. 2007;19:1–31. [Google Scholar]
- 59.Oksanen J. 2016 Vegan: Ecological Diversity R Package, Version 2.5-4. Available at https://cran.r-project.org/web/packages/vegan/index.html. Accessed January 9, 2018.
- 60.Charney N, Record R. 2012 Vegetarian: Jost Diversity Measures for Community Data, Version 1.2. Available at https://cran.r-project.org/web/packages/vegetarian/index.html. Accessed January 9, 2018.
- 61.Magurran AE. Ecological Diversity and Its Measurement. Croom Helm; London: 1988. [Google Scholar]
- 62.Colwell RK. 2013 EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples, Version 9.1. Available at http://purl.oclc.org/estimates. Accessed January 13, 2018.
- 63.Hortal J, Borges PAV, Gaspar C. Evaluating the performance of species richness estimators: Sensitivity to sample grain size. J Anim Ecol. 2006;75:274–287. doi: 10.1111/j.1365-2656.2006.01048.x. [DOI] [PubMed] [Google Scholar]
- 64.Colwell R, et al. Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J Plant Ecol. 2012;5:3–21. [Google Scholar]
- 65.Newmark WD. Tropical forest fragmentation and the local extinction of understory birds in the eastern Usambara Mountains, Tanzania. Conserv Biol. 1991;5:67–78. [Google Scholar]
- 66.Sekercioğlu ÇH, Daily GC, Ehrlich PR. Ecosystem consequences of bird declines. Proc Natl Acad Sci USA. 2004;101:18042–18047. doi: 10.1073/pnas.0408049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aerts R, Spranghers S, Şekercioğlu ÇH. Conservation of the bird functional signature in a shade coffee landscape undergoing intensification. Bird Conserv Int. 2016;27:71–82. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.