Significance
The knowledge of nonliterate societies may vanish in silence jeopardizing indigenous peoples’ livelihoods. Yet, this cultural component is missed by studies on ecosystem services that have historically emphasized the biological dimension. Here we fill this gap by introducing indigenous knowledge networks representing the wisdom of indigenous people on plant species and the services they provide. This approach allows us to assess how knowledge held by 57 Neotropical indigenous communities is structured locally and regionally, how it is influenced by turnover in biological and cultural heritage, and how the progressive loss of biocultural heritage may undermine the resilience of these communities.
Keywords: ecosystem services, network science, tropical ecosystems, biocultural diversity, indigenous societies
Abstract
Indigenous communities rely extensively on plants for food, shelter, and medicine. It is still unknown, however, to what degree their survival is jeopardized by the loss of either plant species or knowledge about their services. To fill this gap, here we introduce indigenous knowledge networks describing the wisdom of indigenous people on plant species and the services they provide. Our results across 57 Neotropical communities show that cultural heritage is as important as plants for preserving indigenous knowledge both locally and regionally. Indeed, knowledge networks collapse as fast when plant species are driven extinct as when cultural diffusion, either within or among communities, is lost. But it is the joint loss of plant species and knowledge that erodes these networks at a much higher rate. Our findings pave the road toward integrative policies that recognize more explicitly the inseparable links between cultural and biological heritage.
Indigenous communities of tropical regions have assembled sophisticated knowledge about plants and their services (1–3), which has significantly enhanced local livelihoods (4) and global economies (5, 6) (Fig. 1). Unlike the burning of the Library of Alexandria, however, the knowledge acquired by nonliterate societies may vanish in silence (7–10). Recently, platforms such as the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services have recognized the need to incorporate into science assessments and policy the underexplored role that culture plays in enhancing the beneficial contributions of nature to people (11–13). So far, however, studies of indigenous knowledge of plant services are typically affected by two sets of limitations. First, these studies are based on aggregate indicators such as the number of uses, useful species, or uses per species known within a community (14), leaving out essential information on the identity of species and uses and their relationships. Second, previous studies have generally documented knowledge at small scales or within few ethnic groups. As a result, we miss the macroscopic insights needed to assess the regional turnover of knowledge and its resilience to localized loss of plant species and cultural heritage. Here, we fill these gaps by addressing how knowledge about plant services held by 57 Neotropical indigenous communities (Fig. 2A) is structured locally, how it changes regionally, and how it is eroded by the progressive loss of biological and cultural heritage. We do so by depicting indigenous knowledge networks as bipartite graphs, in which nodes on one set represent plant species, nodes on the other set represent plant services, and a link connecting a plant species to a service indicates that the indigenous community knows that the plant provides them that service (Fig. 2B). We refer to links as indigenous knowledge because they convey people’s discovery of a particular service provided by a given plant. Our macroecological approach, in turn, allows us to link these local knowledge networks to the regional pool of knowledge.
Fig. 1.
Indigenous knowledge of plant services. Daily life in indigenous communities where palm products play a central role: (1) Macuna using Astrocaryum jauari fruits as fish bait. (2) Tikuna selling a souvenir bow made from Iriartea deltoidea stem. (3) Carijona using Iriartella setigera blowgun to hunt. (4) Edible beetle grubs from the felled and decaying stems of Bactris gasipaes. (5) Yucuna using mortar made from the stem of Bactris gasipaes to pound coca leaves that are ritually chewed. (6) Split Socratea exorrhiza stems as house walls and floors. (7) Embera house thatched with Welfia regia. (8) Cofán extracting edible oil from Oenocarpus bataua fruits. (9) Achuar eating Mauritia flexuosa fruits. (10) Tsa’chila playing the marimba instrument made from Iriartea deltoidea stems. Image courtesy of Susana Cámara-Leret (artist).
Fig. 2.
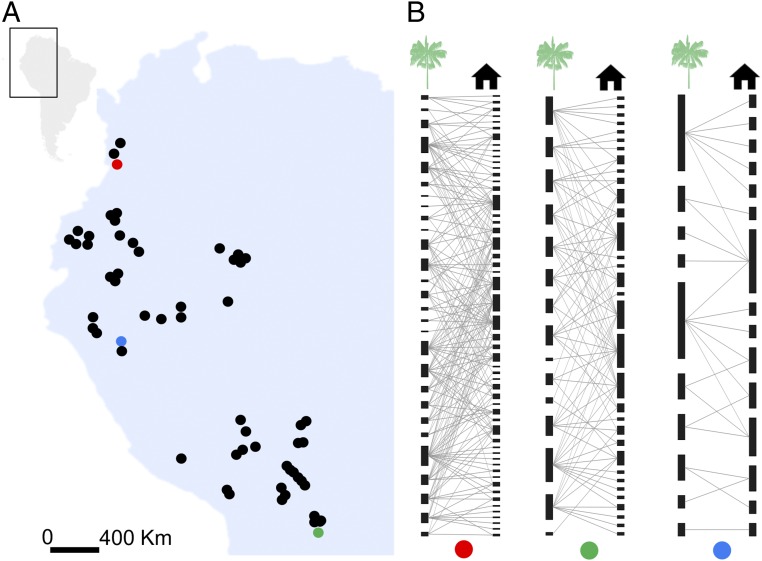
Geographic distribution of the communities studied and the architecture of indigenous knowledge networks. (A) Map of northwestern South America showing the geographic location of the 57 communities. (B) Example of three local knowledge networks (indicated by the colored dots in the map). Nodes under the green palm and black house symbols represent plant species and plant services, respectively. A link between two nodes indicates the knowledge the indigenous community has on the plant service provided by that plant species. For names of communities, see SI Appendix, Fig. S1.
Our study area in northwestern South America is exceptionally rich in biocultural diversity: it is home to c. 110 indigenous ethnic groups, the Amazon wilderness area, and the Andes and Chocó biodiversity hotspots (Fig. 2A). We focus on palms (Arecaceae), one of the most economically important plant families in the tropics (15), which provide essential ecosystem services to inhabitants in our study area (14). During 18 mo of fieldwork, interviews were conducted with inhabitants from 57 communities about the services forest palms provide, following a standard protocol (16). Communities knew a range of 7–41 palm species (mean ± SD, 17.8 ± 8.4 species) and 12–94 palm services (mean ± SD, 36.4 ± 18.5 services; SI Appendix, Table S1). These services span the hierarchy of human needs from human nutrition and medicine to ritual and spiritual needs (Fig. 1) (17).
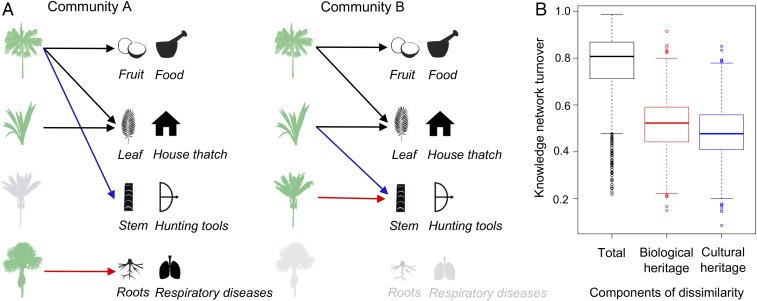
We start by addressing how biological and cultural heritage jointly influence the structure of indigenous knowledge networks across our study area. We do so by decomposing the total dissimilarity in knowledge networks for every pair of indigenous communities (β) into the components of plants and services (node turnover; βbio) and indigenous knowledge turnover (link turnover; βcul), respectively (18) (Fig. 3A). In addition, we calculated the relative importance of each component, dividing each component of turnover by the overall turnover, to indicate to what extent knowledge networks are mainly shaped by the biological or cultural heritage of indigenous communities. We found that total dissimilarity in knowledge increases linearly with the turnover in plants and services (nodes). Indigenous knowledge (link) turnover was weakly related to turnover in species composition, highlighting that turnover in cultural preferences adds complementary information to understanding total network dissimilarity. Our results show that communities share only moderate amounts of knowledge, even about shared species. Notably, we found that the relative contribution of biological and cultural heritage was similar, at ∼50% (range, 0.15–0.91 and 0.09–0.85, respectively; Fig. 3B). In other words, variation in the realization of ecosystem services in the study area is as strongly constrained by turnover in the biological and the cultural dimensions.
Fig. 3.
Plant and knowledge turnover among local indigenous knowledge networks. (A) The presence of different species in two given communities can lead to different knowledge about nature’s services (red arrows). Knowledge about shared species between communities may be either shared (black arrows), or not (blue arrows). (B) Boxplots showing the total knowledge network turnover across the 57 communities studied and the components resulting from turnover in biological heritage (nodes) and cultural heritage (links), respectively.
Next, we quantify to what extent the total knowledge of the services provided by plants is distributed across the different indigenous communities. This serves to identify whether communities are equivalent in terms of their local knowledge, or whether there are a few keystone communities. To do so, we first aggregate the knowledge networks of all local communities to build what we refer to as the indigenous knowledge metaweb (i.e., the global knowledge that communities have of the services provided by all plants occurring in the study area). Second, we calculate the dissimilarity (β′) of each local community to the indigenous knowledge metaweb. We found a twofold range of variability in this amount across communities (range, 0.27–0.57; mean, 0.41 ± 0.07), indicating strong variation between communities in their dissimilarity to the metaweb. Eight communities, for example, have a significantly larger amount of knowledge (Methods), and can therefore be considered culturally keystone communities. Differences in the dissimilarity of local communities to the metaweb were unrelated to the fraction of species each community contained, or to community population size, but were significantly and negatively related to the number of informants and to the fraction of services in relation to the metaweb (SI Appendix, Fig. S2). Thus, keystone communities are not as defined by their species pool but, rather, by their people’s knowledge. We hypothesize this may be analogous to the species–area relationship of biogeography (19), whereby as human communities grow in size, their ability to explore nature’s services may increase, fostering more innovations. Interestingly, communities with a similar use of shared plants do not account for a similar proportion of the metaweb (SI Appendix, Fig. S3). Such an absence of redundancy means that in theory, both communities would have to be conserved to represent the total pool of knowledge.
To better understand what drives the turnover in knowledge across communities, we explored the role of horizontal and vertical information diffusion (20). Under a horizontal diffusion scenario, communities would share more knowledge with their geographical neighbors. Alternatively, under a vertical diffusion scenario, communities would share more knowledge with closely related kin. To test for cultural proximity, we used two measures of linguistic distance: a binary index that quantifies the presence/absence of languages in each community and a quantitative index of the fraction of societal members speaking each language. We ran Mantel tests to explore whether differences between knowledge networks were related to geographic or linguistic distance between communities. We found that correlations between components of knowledge networks and geographic distance were in most cases statistically significant (SI Appendix, Table S2), supporting the notion that differences in knowledge increase as communities are farther apart. Interestingly, we found that communities that share knowledge about shared plant species were not necessarily geographically closer. Linguistic distance was also statistically significantly correlated with knowledge turnover. However, this was no longer the case, or correlations were very low, when we partialled out the effect of geographic distance (SI Appendix, Table S2). This indicates that knowledge about nature’s services has a strong horizontal diffusion component (21), underpinned by floristic similarity (22).
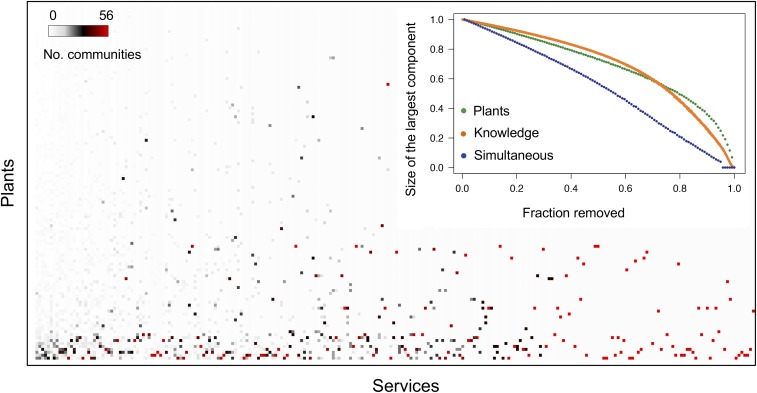
The loss of knowledge about nature’s services and its effect on the metaweb cannot be understood by studying local communities in isolation. Accordingly, we examined how the simulated loss of species (plant nodes) or knowledge (links) across communities affects the robustness of the metaweb. Because species extinction is mainly driven by habitat loss and species with small geographic ranges tend to have a higher extinction threat (23), we removed plant species following the inverse order of their geographic range. Also, because knowledge extinction will happen faster for links cited in one versus several communities, we first removed the least-cited links from the metaweb. We then measured how the fraction of removed plant nodes or links affects the mean size of the largest remaining cluster, a measure of network robustness (24), after 1,000 simulations (Methods and Fig. 4). We found that the size of the largest cluster decreases at least as fast when knowledge is lost as when plant species are driven extinct. This indicates that, although plant loss threatens human well-being (25), loss of cultural heritage is equally important in constraining our realization of nature’s services. More important, Fig. 4 shows that it is the simultaneous loss of biological and cultural heritage that leads to a much faster erosion of the indigenous knowledge metaweb.
Fig. 4.
Regional indigenous knowledge metaweb and its robustness to the extinction of plants and knowledge. Matrix rows and columns indicate plant species and services, respectively, across the entire area of study, whereas dots depict that a particular plant species is employed for that specific service. The color of dots denotes the number of communities that cite a given plant–service link. The metaweb is made up of 120 plant species, 250 services, and 1,743 links. (Inset) Robustness of the metaweb, measured as the size of the largest component, to the selective removal of an increasing fraction of plant nodes, links (indicating knowledge), and both plant nodes and links. For names of plant species and services, see SI Appendix, Fig. S4.
Although the emphasis has been traditionally placed on the ever-increasing effect of human activities on land and oceans, there is a greater need to understand both the biological and the cultural dimensions that underpin the realization of ecosystem services. Variation in knowledge can influence which benefits people obtain from nature, whereas variation in plants can constrain knowledge pools. Scaling this interplay between knowledge and plant species at a regional scale is also essential to draw generalizations that transcend the idiosyncrasies of individual communities and obtain a mechanistic understanding of the drivers that shape human–plant interactions. Given the joint effects of plants and cultural heritage on the robustness of the indigenous knowledge metaweb, further studies linking both factors are important to maximize the discovery of nature’s contributions to people. This will be especially crucial in biodiversity hotspots and wilderness areas, where most of Earth’s cultural and biological diversity co-occur (26) and where ongoing linguistic extinction (27) could substantially diminish the ability of future generations to identify and benefit from natural resources.
Materials and Methods
Study Area.
Our study area encompasses three biomes (Amazonia, Andes, and Chocó) within four countries in northwestern South America (Colombia, Ecuador, Peru, and Bolivia). The 57 communities in which ethnobotanical data were collected can be classified ethnically into indigenous (n = 46), mestizo (n = 10), and Afro-American (n = 1), although in this study we generically refer to them as indigenous. Study communities were located in the southern Amazon (n = 23), the northern Amazon (n = 15), the Andes (n = 13), and Chocó (n = 6). We analyzed data from communities that had at least 10 participants.
Palm Services.
Approval for this study was granted by the Committee for Ethical Research of the Autonomous University of Madrid (no. 48–922). Before initiating data collection, we obtained oral informed consent at the community level and then from individuals before each interview. Participants were informed of their right to discontinue the interviews at any time, and that all of the information provided would be anonymized. Ethnobotanical data about palms was collected making 2,137 interviews from March 2010 to December 2011, using a standard protocol (16, 28). Communities were selected on the basis of having a uniform ethnic composition, different accessibility to markets, and access to primary forests to harvest palm resources. Two types of participants were interviewed in each community: experts and general informants. Experts were selected by consensus during a meeting of societal members, and subsequently interviewed using a walk-in-the woods approach, by which all palm species present in forests were recorded and their vernacular names and services registered. Before interviewing experts, a list of palm species occurring in the region was compiled using refs. 29–31 to ensure that all palm species were taken into account. Once experts were interviewed, we used the list of vernacular names reported by experts to perform household interviews with general informants. General informants were selected in a stratified manner to have a representative sample of sex (women, n = 1,076; men, n = 1,071) and age (18–30 y, 28%; 31–40 y, 23%; 41–50 y, 20%; 51–60 y, 13%; >60 y, 16%) classes. The number of informants were correlated with community size (r = 0.428; P < 0.001) so that there was no bias in the sampling effort. Interviews were made in Spanish or with a local interpreter when needed. Field identification of palm specimens was performed using refs. 29–31, and specimens were collected whenever our field identification needed confirmation. Palm collecting permits were obtained from the Instituto Amazónico de Investigaciones Científicas Sinchi (Colombia), the Ministry of Environment (Ecuador), the Instituto Nacional de Recursos Naturales (Peru), and the Dirección General de Biodiversidad y Áreas Protegidas (Bolivia), and field studies did not involve endangered or protected species. Voucher specimens (n = 203) are deposited in the herbaria AAU, AMAZ, CHOCO, COL, LPB, and QCA, acronyms according to Thiers (32). We followed the World Checklist of Palms to unify nomenclature (33). Palm services cited by informants were classified into one of 10 categories (and their respective subcategories) following the Economic Botany Data Collection Standard (34), with the modifications proposed by Macía et al. (14). Categories included Animal food, Construction, Cultural, Environmental, Fuel, Human food, Medicinal and veterinary, Toxic, Utensils and tools, and Other uses. We defined a “service” as the concatenation of palm part, category, and subcategory.
Palm Species Distributions.
We made 1° grid square resolution distribution maps for the 120 useful native palm species occurring in the study area (SI Appendix, Table S3), using ArcGIS 10.1 (ESRI Inc; see ref. 17 for details). We quantified the geographic range size of each of the palm species in our study area by counting the number of 1° grid squares in which it is present. Species distributions were intersected with coordinates of each indigenous community to derive local species pools. In some cases, informants reported species from a different ecoregion (e.g., Amazonian species reported in the Chocó). Knowledge about these nonnative species was removed from each community to ensure uniformity across communities and equal exposure to palm resources among participants within each community.
Turnover in Plant–Service Interactions.
We decomposed the total dissimilarity between two given indigenous knowledge networks (β) into two components: plants and services (node) turnover (βbio), where differences in the presence/absence of links between plants and the services they provide are the result of a plant being present in one community but not in the other, and indigenous knowledge (link) turnover (βcul), where differences in the presence/absence of links between plants that co-occur in both communities and the services they provide are a consequence of the cultural knowledge that one community, but not the other, has on the service that plant provides. The relative importance of each component indicates to what extent knowledge networks are shaped by biological heritage (βbio/β close to one) or by cultural heritage (βcul/β close to one). Next, we calculated the cultural distance between community i and the equivalent network (same nodes) in the metaweb (βi′), and classified communities into keystone (βi′< one SD from the mean β′ value and a human population size <500), source (βi′< one SD from the mean β′ value and a human population size >1,000), sink (βi′> one SD from the mean β′ value), and standard (within one SD from the mean β′ value; SI Appendix, Fig. S5). Thus, our classification of keystone communities accounts for their disproportionate contribution to the indigenous knowledge metaweb in proportion to their human population size. Human population size within communities ranged from 30 to 13,000 (mean = 717; SD = 1,872). We measured turnover in plant–service interactions between communities with the betalink function, and the distance of each community from the metaweb with the beta_os_prime function, both in the R package BETALINK (35). To explore whether similarity between communities in their use of shared plants [1−(βcul/β)] relates to how much a given community resembles the metaweb (1−β′), the list of pairwise values of shared knowledge 1−(βcul/β) was converted to a distance matrix, using the list2dist function of the R package SPAA (36). This distance matrix was used to perform a hierarchical clustering using the hclust function of the R package STATS (37). We then plotted the cluster diagram using the as.phylo function of the R package APE (38), coloring branches by their β′ values, using the function contMap in the R package PHYTOOLS (39).
Language Data.
In each community, we conducted interviews to understand the socioeconomic profile of all participants, including the languages spoken by them. Twenty-two languages were spoken in the study communities. We used this linguistic information to build two indices that were then used in our Mantel tests (see Mantel Test). The first index was binary, containing presence/absence information on the languages spoken within each community. The second index contained quantitative information on the fraction of community members speaking a given language.
Mantel Test.
Mantel tests were run to find out whether turnover of knowledge networks (i.e., β, βbio, βcul, βbio/β and βcul/β) correlated with differences in geographic or linguistic distance. Geographic distances between communities were calculated using latitudinal and longitudinal coordinates and were log-transformed before computing Euclidean distances. Linguistic distances between communities were calculated using a presence/absence matrix of languages spoken in each community or using an abundance matrix with the fraction of individuals that speak a given language in each community. To control for spatial autocorrelation and to avoid inflating the apparent importance of the linguistic variables included in the analyses, we also ran partial Mantel tests, in which the correlation with logarithmically transformed geographical distances was partialled out before calculating the correlation between the different knowledge components and linguistic dissimilarities. The standardized form of the Mantel statistic (rM) was used, which is equivalent to the Pearson correlation coefficient between two dissimilarity matrices. The statistical significance of each correlation was established at the P < 0.001 level with a Monte Carlo permutation test, using 999 random permutations. All analyses were made using the vegdist and mantel functions in the R package VEGAN (40).
Simulations.
We assessed the effect of species extinction or knowledge loss on the metaweb by simulating the loss of plant species nodes and links stochastically. Nodes were removed with a probability proportionally inverse to their geographic range. Similarly, links were removed with a probability inverse to their frequency of citation. In addition, we simultaneously removed plant nodes and links to test for the joint loss of plants and knowledge. Because the ratio between the number of links (n = 1,743) and nodes (n = 120) was ∼15:1, we removed as many links as indicated by this ratio for every node removed. In all analyses, we ran 1,000 different simulations of node/link removals, using a different random seed to initiate the stochastic process. Each simulation was run until 100% of the nodes/links were removed. After 1,000 runs, we measured how the fraction of nodes fp or links fl removed affects the mean size of the largest remaining cluster S, until its collapse (24, 41). The size of the largest cluster is an indicator of the robustness of the networks, because as a critical fraction of nodes (links) are removed, the network gets fragmented (i.e., extant nodes belong to several disjointed subnetworks). All simulations were made in MATLAB (42). To plot the metaweb, we sorted the metaweb by row and column totals, using the sortmatr function (43), and plotted it using the heatmap function of the R package STATS (37).
Data and Materials Availability.
The data that support the findings of this study are available in the SI Appendix, Table S4.
Supplementary Material
Acknowledgments
We thank the 57 communities and participants of fieldwork interviews. We are also grateful to the research teams at Universidad Autónoma de Madrid, Universidad Nacional de Colombia, Pontificia Universidad Católica del Ecuador, and Universidad Mayor de San Andrés for facilitating our research. We extend our gratitude to H. Balslev for encouragement, to J. C. Copete, M. Soto, N. Paniagua, L. Camelo, R. Bussmann, and M. Jaimes for assistance in fieldwork, and to S. Cámara-Leret for providing the illustration in Fig. 1. Ethnobotanical data originate from the PhD work of R.C.-L., supervised by M. J. Macía and funded by the European Union, 7th Framework Programme [FP7-PALMS-Contract 212631 to H. Balslev (main Principal Investigator)]. J.B. and M.A.F. were supported by the Swiss National Science Foundation (Grant 31003A_169671 to J.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1821843116/-/DCSupplemental.
References
- 1.Schultes RE, Raffauf RF (1990) The Healing Forest: Medicinal and Toxic Plants of the Northwest Amazonia (Dioscorides Press, Portland, OR: ). [Google Scholar]
- 2.Gadgil M, Berkes F, Folke C (1993) Indigenous knowledge for biodiversity conservation. Ambio 22:151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultes RE, von Reis S, eds (1995) Ethnobotany: Evolution of a Discipline (Chapman and Hall Ltd, Portland, OR: ). [Google Scholar]
- 4.McDade TW, et al. (2007) Ethnobotanical knowledge is associated with indices of child health in the Bolivian Amazon. Proc Natl Acad Sci USA 104:6134–6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balick MJ, Mendelsohn R (1992) Assessing the economic value of traditional medicines from tropical rain forests. Conserv Biol 6:128–130. [Google Scholar]
- 6.Willis KJ, ed (2017) The State of the World’s Plants 2017 (Royal Botanic Gardens, Kew, UK: ). [PubMed] [Google Scholar]
- 7.Schultes RE. (1994) Burning the library of Amazonia. Sciences (New York) 34:24–30. [Google Scholar]
- 8.Martínez-Ballesté A, Martorell C, Caballero J (2006) Cultural or ecological sustainability? The effect of cultural change on Sabal palm management among the lowland Maya. Ecol Soc 11:27. [Google Scholar]
- 9.Reyes-García V, et al. (2014) Cultural change and traditional ecological knowledge. An empirical analysis from the Tsimane’ in the Bolivian Amazon. Hum Organ 73:162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ladio AH. (2011) Traditional knowledge of edible wild native and exotic plants in the context of cultural change in human populations of arid Patagonia. Biorem Biodiv Bioavail 5:60–64. [Google Scholar]
- 11.Díaz S, et al. (2018) Assessing nature’s contributions to people. Science 359:270–272. [DOI] [PubMed] [Google Scholar]
- 12.Isbell F, et al. (2017) Linking the influence and dependence of people on biodiversity across scales. Nature 546:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thaman R, et al. (2013) The contribution of Indigenous and local knowledge systems to IPBES: Building synergies with science. (IPBES Expert Meeting Report) [United Nations Educational, Scientific and Cultural Organization (UNESCO), Paris].
- 14.Macía MJ, et al. (2011) Palm uses in northwestern South America: A quantitative review. Bot Rev 77:462–570. [Google Scholar]
- 15.Johnson D. (2011) Tropical Palms (FAO, Rome: ). [Google Scholar]
- 16.Cámara-Leret R, et al. (2012) A standard protocol for gathering palm ethnobotanical data and socioeconomic variables across the tropics. Medicinal Plants and the Legacy of R.E. Schultes, eds Ponman BE, Bussmann RW (Missouri Botanical Garden Press, St. Louis: ), pp 41–71. [Google Scholar]
- 17.Cámara-Leret R, et al. (2017) Fundamental species traits explain provisioning services of tropical American palms. Nat Plants 3:16220. [DOI] [PubMed] [Google Scholar]
- 18.Poisot T, Canard E, Mouillot D, Mouquet N, Gravel D (2012) The dissimilarity of species interaction networks. Ecol Lett 15:1353–1361. [DOI] [PubMed] [Google Scholar]
- 19.Rosenzweig ML. (1995) Species Diversity in Space and Time (Cambridge Univ Press, Cambridge, UK: ). [Google Scholar]
- 20.Pagel M, Mace R (2004) The cultural wealth of nations. Nature 428:275–278. [DOI] [PubMed] [Google Scholar]
- 21.Cámara-Leret R, Paniagua-Zambrana N, Svenning JC, Balslev H, Macía MJ (2014) Geospatial patterns in traditional knowledge serve in assessing intellectual property rights and benefit-sharing in northwest South America. J Ethnopharmacol 158:58–65. [DOI] [PubMed] [Google Scholar]
- 22.Saslis-Lagoudakis CH, et al. (2014) The evolution of traditional knowledge: Environment shapes medicinal plant use in Nepal. Proc Biol Sci 281:20132768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawton JH, May RM (1995) Extinction Rates (Oxford Univ Press, Oxford: ). [Google Scholar]
- 24.Albert R, Jeong H, Barabási A-L (2000) Error and attack tolerance of complex networks. Nature 406:378–382. [DOI] [PubMed] [Google Scholar]
- 25.Díaz S, Fargione J, Chapin FS 3rd, Tilman D (2006) Biodiversity loss threatens human well-being. PLoS Biol 4:e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorenflo LJ, Romaine S, Mittermeier RA, Walker-Painemilla K (2012) Co-occurrence of linguistic and biological diversity in biodiversity hotspots and high biodiversity wilderness areas. Proc Natl Acad Sci USA 109:8032–8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krauss M. (1992) The world’s languages in crisis. Language 68:4–10. [Google Scholar]
- 28.Paniagua-Zambrana N, Macía MJ, Cámara-Leret R (2010) Toma de datos etnobotánicos de palmeras y variables socioeconómicas en comunidades rurales. Ecol Boliv 45:44–68. [Google Scholar]
- 29.Galeano G, Bernal R (2010) Palmas de Colombia: Guía de Campo (Universidad Nacional de Colombia, Bogotá, Colombia: ). [Google Scholar]
- 30.Borchsenius F, Pedersen HB, Balslev H (1998) Manual to the Palms of Ecuador (Aarhus Univ Press, Aarhus, Denmark: ). [Google Scholar]
- 31.Moraes M. (2004) Flora de Palmeras de Bolivia (Universidad Mayor de San Andxs, La Paz, Bolivia: ). [Google Scholar]
- 32.Thiers B. (2019) Index Herbariorum: A global directory of public herbaria and associated staff. New York botanical garden’s virtual herbarium. Available at sweetgum.nybg.org/science/ih/. Accessed January 1, 2019.
- 33.Govaerts R, et al. (2019) World checklist of Arecaceae. Available at wcsp.science.kew.org/home.do. Accessed January 1, 2019.
- 34.Cook FE. (1995) Economic Botany Data Collection Standard (Royal Botanic Gardens, Kew, UK: ). [Google Scholar]
- 35.Poisot T. (2016) betalink: Beta-Diversity of Species Interactions. R Package Version 2.2.1. Available at https://CRAN.R-project.org/package=betalink. Accessed January 1, 2019.
- 36.Zhang J. (2016) spaa: SPecies Association Analysis. R Package Version 0.2.2. Available at https://CRAN.R-project.org/package=spaa. Accessed January 1, 2019.
- 37.R Core Team (2018) R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing) Version 3.5.2. Available at https://www.r-project.org/. Accessed January 1, 2019.
- 38.Paradis E, Schliep K (2019) Ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35:526–528. [DOI] [PubMed] [Google Scholar]
- 39.Revell LJ. (2012) phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3:217–223. [Google Scholar]
- 40.Oksanen J, et al. (2018) Vegan: Community Ecology Package. R Package Version 2.5-3. Available at https://CRAN.R-project.org/package=vegan. Accessed January 1, 2019.
- 41.Solé RV, Montoya JM (2001) Complexity and fragility in ecological networks. Proc Biol Sci 268:2039–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.The MathWorks Inc (2014) MATLAB and Statistics Toolbox Release (The MathWorks, Inc., Natick, MA: ). [Google Scholar]
- 43.Vázquez DP, Chacoff NP, Cagnolo L (2009) Evaluating multiple determinants of the structure of plant-animal mutualistic networks. Ecology 90:2039–2046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.