Significance
Stroke is the leading cause of disability and fifth leading cause of mortality in the United States. Previous studies demonstrate a highly protective effect of inhibiting cyclooxygenase-2 (COX-2) after stroke, indicating that downstream PGE2 signaling pathways cause cerebral ischemic injury. Using conditional knockout strategies to study the role of PGE2 EP2 signaling in a model of cerebral ischemia, we determine that neuronal EP2 signaling, which is highly induced following cerebral ischemia, increases cerebral injury. We demonstrate that EP2 blockade in adult mice is highly cerebroprotective and myeloid EP2 signaling increases the poststroke innate immune response. Additionally, the present results differ from previous studies of congenitally null EP2 mice, reflecting a potential confounding effect of congenital deletion of EP2.
Keywords: PGE2, stroke, conditional knockout
Abstract
The inflammatory prostaglandin E2 (PGE2) EP2 receptor is a master suppressor of beneficial microglial function, and myeloid EP2 signaling ablation reduces pathology in models of inflammatory neurodegeneration. Here, we investigated the role of PGE2 EP2 signaling in a model of stroke in which the initial cerebral ischemic event is followed by an extended poststroke inflammatory response. Myeloid lineage cell-specific EP2 knockdown in Cd11bCre;EP2lox/lox mice attenuated brain infiltration of Cd11b+CD45hi macrophages and CD45+Ly6Ghi neutrophils, indicating that inflammatory EP2 signaling participates in the poststroke immune response. Inducible global deletion of the EP2 receptor in adult ROSA26-CreERT2 (ROSACreER);EP2lox/lox mice also reduced brain myeloid cell trafficking but additionally reduced stroke severity, suggesting that nonimmune EP2 receptor-expressing cell types contribute to cerebral injury. EP2 receptor expression was highly induced in neurons in the ischemic hemisphere, and postnatal deletion of the neuronal EP2 receptor in Thy1Cre;EP2lox/lox mice reduced cerebral ischemic injury. These findings diverge from previous studies of congenitally null EP2 receptor mice where a global deletion increases cerebral ischemic injury. Moreover, ROSACreER;EP2lox/lox mice, unlike EP2−/− mice, exhibited normal learning and memory, suggesting a confounding effect from congenital EP2 receptor deletion. Taken together with a precedent that inhibition of EP2 signaling is protective in inflammatory neurodegeneration, these data lend support to translational approaches targeting the EP2 receptor to reduce inflammation and neuronal injury that occur after stroke.
The COX-1 and inducible COX-2 catalyze the first committed step in PGE2 synthesis and function physiologically in the central nervous system to regulate synaptic plasticity, neurovascular coupling, and glial homeostasis. Of the five prostanoids downstream of COX—including PGE2, PGD2, PGF2α, prostacyclin, and thromboxane—PGE2 has emerged as a unique modulator of disease-promoting neuronal and inflammatory processes. In pathologic contexts, induction of COX-2 in neurons and glia leads to generation of PGE2 that signals through four G protein coupled receptors, EP1–EP4. In vivo studies of the EP receptor function using genetic knockout models have highlighted EP receptor-specific effects in a broad range of neurological disease models. For example, whereas the EP1 receptor elicits neurotoxic effects in models of cerebral ischemia (1), the EP4 receptor conversely mediates neuroprotective, vasodilatory, and antiinflammatory effects (2, 3). In models of familial Alzheimer’s disease (AD), ablation of EP2 or EP3 receptors blunts inflammatory responses, amyloid accumulation, and loss of synaptic proteins (4–7), whereas deletion of microglial EP4 elicits the opposite (8). Thus, genetic studies demonstrate beneficial as well as detrimental PGE2 EP signaling cascades that operate in receptor-specific ways.
The PGE2 EP2 receptor is a major regulator of maladaptive inflammatory responses in models of chronic neurodegenerative disease (9). In vivo, genetic ablation of the EP2 receptor suppresses adverse inflammatory responses in models of neurogenesis (10), innate immunity (11), AD (4, 5, 12, 13), Parkinson’s disease (PD) (14, 15), and amyotrophic lateral sclerosis (16). More recently, studies using myeloid cell conditional knockout strategies identified EP2 receptor-driven pathologic microglial responses in models of innate immunity, PD, and AD (4, 12, 15) where ablation of a microglial EP2 receptor increased microglial chemotaxis and phagocytosis and suppressed proinflammatory gene expression, synaptic injury, and memory deficits.
Stroke is the fifth leading cause of death and the leading cause of adult disability. Thrombolytic therapy is the pharmacologically approved therapy for stroke, however, it has a limited window of administration of 4.5 h; endovascular thrombectomy is a promising acute intervention with recent studies showing an extended treatment window in selected subsets of patients (17). Validation of neuroprotective strategies has been challenging, in part, because components of the neurotoxic cascade occur early and are short lived. However, stroke is a multiphasic process with the initial ischemic phase followed by secondary poststroke inflammatory responses that unfold over days to weeks (18–22). Following ischemia, an innate immune phase begins with infiltrating neutrophils and macrophages accumulating in the ischemic area with subsequent ingress of T and B cells. Experimental manipulations have suggested toxic as well as beneficial functions of the poststroke immune response (23–26). Given the role of myeloid EP2 signaling in suppressing beneficial immune responses, notably phagocytosis and termination of inflammation (4, 12, 15), we hypothesized that in the setting of early poststroke inflammation, inflammatory EP2 signaling may contribute to stroke severity. Using cell-specific and inducible conditional knockout strategies to study the role of EP2 signaling in the mouse middle cerebral artery occlusion-reperfusion (MCAo-RP) model of cerebral ischemia, we determine that both myeloid and neuronal cellular substrates mediate detrimental effects of EP2 signaling in this model. Moreover, the present results differ from previous studies of congenitally null EP2−/− mice that conversely suggest that EP2 signaling increases cerebral ischemic injury.
Results
Conditional Deletion of Myeloid EP2 Receptor Reduces Innate Immune Cell Trafficking in Stroked Brain.
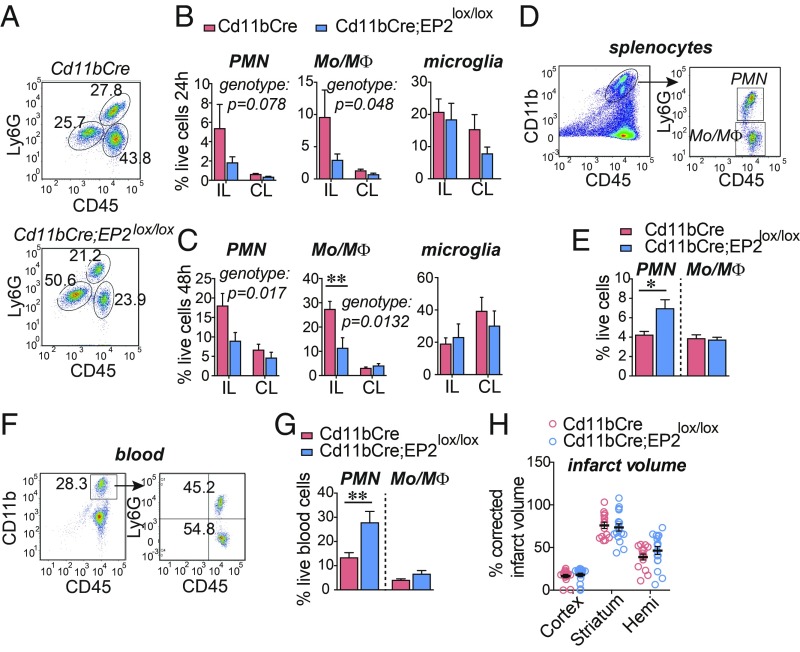
EP2 signaling in microglia suppresses critical immune functions, notably microglial termination of proinflammatory responses, phagocytosis, and generation of neurotrophic factors (4). Accordingly, we tested whether the loss of myeloid EP2 signaling would attenuate the poststroke inflammatory response. Previous quantification of genomic EP2 sequences in Cd11bCre;EP2lox/lox macrophages demonstrated that the Cd11bCre promoter elicits a ∼50% knockdown of genomic EP2 expression in myeloid lineage cells and does not alter numbers of brain microglia or subpopulations of macrophages, neutrophils, resident monocytes, or inflammatory monocytes in the spleen (15). Examination of Cd11bCre;EP2lox/lox and Cd11bCre mice demonstrated reduced levels of infiltrating CD11+CD45hiLy6Glo monocyte/macrophages (Mo/MΦ) and CD11+CD45+Ly6Ghi neutrophil polymorphonuclear leukocytes (PMNs) into the ischemic ipsilateral (IL) hemisphere (Fig. 1 A–C and SI Appendix, Fig. S1 A and B) at 24 and 48 h after MCAo-RP. Quantification of peripheral PMN and Mo/MΦ in the spleen and blood revealed a retention of neutrophils in the spleen (Fig. 1 D and E) and in blood (Fig. 1 F and G) in Cd11bCre;EP2lox/lox mice, suggesting that the loss of the myeloid EP2 receptor reduced neutrophil chemotaxis from the spleen to the brain. Knockdown of myeloid EP2 signaling also altered levels of immune factors (SI Appendix, Fig. S1 C and D). We conclude that reduction of myeloid EP2 signaling attenuated the poststroke innate immune response, but this was not sufficient to reduce overall cerebral injury (Fig. 1H), likely reflecting the 50% knockdown of the myeloid EP2 receptor (15).
Fig. 1.
Conditional deletion of the EP2 receptor in myeloid cells reduces immune cell infiltration after MCAo. Cd11bCre and Cd11bCre;EP2lox/lox C57B6/J 3 mo male mice underwent MCAo-RP and brain, spleen, and blood myeloid cells were examined. Data are presented as mean ± SEM. (A) Representative plots from Cd11bCre (Top) and Cd11bCre;EP2lox/lox (Bottom) ischemic hemispheres 48 h after MCAo. (B) At 24 h after MCAo, percent live cells in IL and contralateral (CL) hemispheres representing CD11b+CD45hiLy6Ghi neutrophils (PMN), CD11b+CD45hiLy6Glo Mo/MΦ and Cd11b+CD45int microglia are shown (n = 5–8 per group; two-way ANOVA, effects of genotype in italics). (C) At 48 h after MCAo, percentage of live cells representing PMN, Mo/MΦ, and microglia (n = 6 to 7 per group; two-way ANOVA, effects of genotype in italics; post hoc Bonferroni **P < 0.01). (D) Representative gating strategy to identify PMNs and Mo/MΦ in the spleen 48 h after MCAo. (E) At 48 h, percentage of live cells representing PMN and Mo/MΦ in the spleen (n = 8–11 per group; two-tailed Student’s t test, *P < 0.05). (F) Representative gating strategy to identify PMNs and Mo/MΦ in blood 48 h after MCAo. (G) At 48 h, percentage of live cells representing PMN and Mo/MΦ in blood (n = 8–11 per group; two-tailed Student’s t test, **P < 0.01). (H) Percentage of corrected infarct volume for the cortex, striatum, and hemisphere at 72 h after MCAo (n = 15–17 per group).
Inducible Global Deletion of EP2 Receptor Reduces Myeloid Trafficking to Brain and Cerebral Injury.
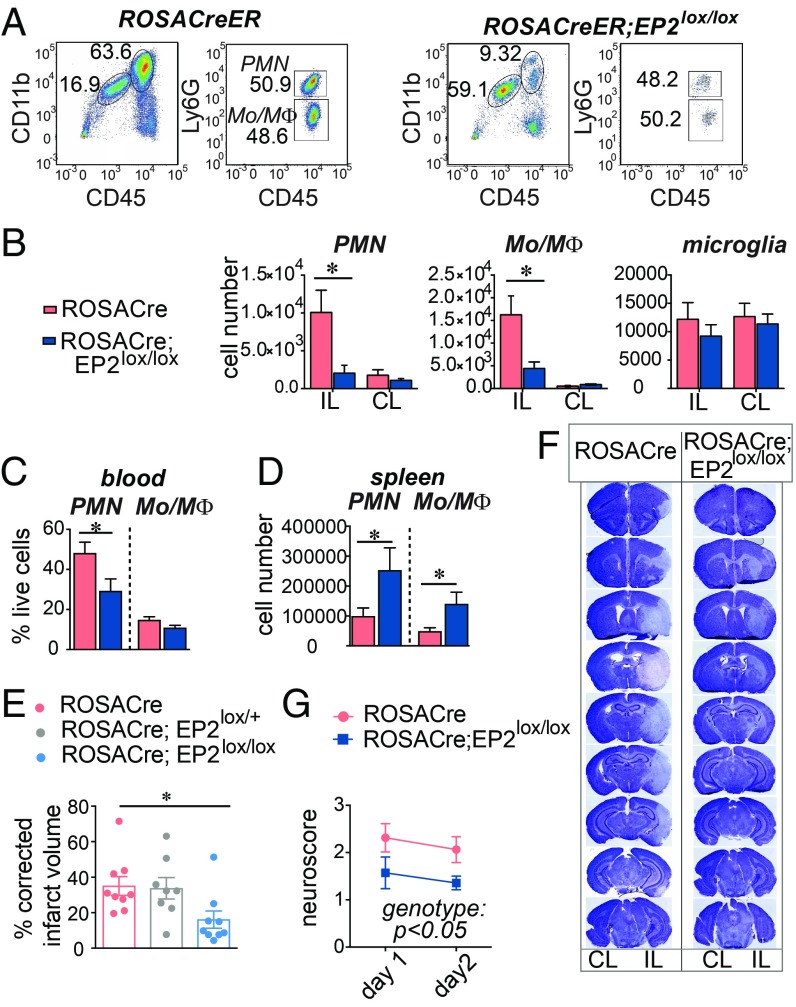
We then tested the effects of an inducible knockout generated using the ROSACreER line. Quantitative PCR demonstrated near total genomic excision of EP2 sequences in ROSACreER;EP2lox/lox mice (SI Appendix, Fig. S1E). Tamoxifen was administered at 6–8 wk of age to both ROSACreER and ROSACreER;EP2lox/lox mice, and MCAo-RP was carried out 4–6 wk later. At 48 h after MCAo-RP, levels of infiltrating (Mo/MΦ) and PMNs decreased in the ROSACreER;EP2lox/lox IL hemisphere with no effect on the numbers of microglia (Fig. 2 A and B). Spleen weight and splenocyte numbers were increased in ROSACreER;EP2lox/lox mice (SI Appendix, Fig. S1 F and G) as were numbers of splenic PMNs and Mo/MΦ (Fig. 2C), suggesting a reduced egress of splenocytes from the spleen with loss of the EP2 receptor. In blood, in line with the retention of myeloid cells in the spleen in ROSACreER;EP2lox/lox mice, lower levels of PMNs and Mo/MΦ populations were observed (Fig. 2D).
Fig. 2.
Inducible global deletion of the EP2 receptor reduces myeloid cell infiltration after MCAo and prevents cerebral injury. RosaCreER and RosaCreER;EP2lox/lox 3–4 mo C57B6/J male mice underwent MCAo followed by 48 h RP. Data are presented as mean ± SEM. (A) Representative plots of CD11b+CD45hiLy6Ghi PMN and CD11b+CD45hiLy6Glo Mo/MΦ from RosaCreER and RosaCreER;EP2lox/lox ischemic hemispheres 2 d after MCAo. (B) Cell numbers in IL and CL hemispheres for PMN, Mo/MΦ subsets, and microglia (n = 5–11 per group; two-way ANOVA; for PMN, effect of the genotype, P = 0.026, effect of the hemisphere, P = 0.02; for Mo/MΦ, effect of the genotype P = 0.017, effect of the hemisphere, P = 0.002; Tukey post hoc, *P < 0.05). (C) At 48 h, numbers of PMN and Mo/MΦ in the spleen (n = 5–9 per group; two-tailed Student’s t test, *P < 0.05). (D) At 48 h, percentage of live cells representing PMN and Mo/MΦ in blood 48 h after MCAo (n = 7–10 per group; two-tailed Student’s t test, *P < 0.05). (E) Neurological scores (n = 7 to 8 per group; repeated measure two-way ANOVA, effect of the genotype P = 0.0492; effect of time P < 0.0001). (F) Representative series of brain sections stained with Cresyl violet (CV) from RosaCreER and RosaCreER;EP2lox/lox mice 48 h after MCAo. Areas lacking CV staining were quantified for infarct volume. (G) Quantification of the percentage of corrected hemispheric infarct volume at 48 h after MCAo (n = 8 to 9 per group; one-way ANOVA P = 0.0328; Tukey’s post hoc *P < 0.05).
Stroke outcome measures, including measurements of neurological function (Fig. 2G) and volume of infarcted brain tissue (Fig. 2 E and F) were improved in ROSACreER;EP2lox/lox male mice. In female cohorts, overall stroke volumes did not differ between genotypes and were lower, a finding consistent with previous data indicating reduced injury in female vs. male genders. Body weights did not differ between genotypes and were 30.97 g ± 0.75 SE and 31.53 g ± SE 2.56 for male ROSACreER and ROSACreER;EP2lox/lox mice, respectively, and 23.15 g ± 0.61 and 23.78 g ± 0.49 for female ROSACreER and ROSACreER;EP2lox/lox mice, respectively.
EP2 Receptor Expression Is Induced in Neurons After MCAo-RP.
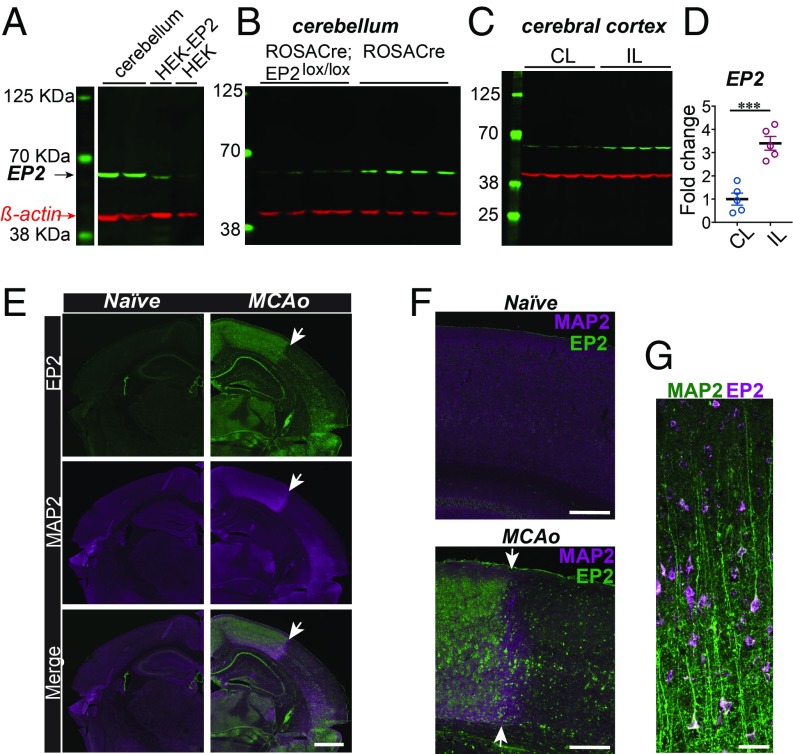
Our findings in ROSACreER;EP2lox/lox mice differed from previous findings using global EP2−/− mice (27, 28), raising the possibility that responses to cerebral ischemia may be confounded from compensatory or developmental processes brought about by congenital deletion of the EP2 receptor. In line with this, images from the Gene Expression Nervous System Brain Atlas (GENSAT; www.gensat.org/index.html) demonstrate high levels of EP2 receptor expression by postnatal day 7 in neurons of the developing forebrain, hippocampus, and cerebellum (SI Appendix, Fig. S2A). At adult stages, expression of the EP2 receptor largely disappears in the forebrain and hippocampus but persists in the cerebellum (SI Appendix, Fig. S2B). Immunoblot staining of EP2 protein using HEK cells overexpressing the EP2 receptor and cerebellar lysates derived from ROSACreER and ROSACreER;EP2lox/lox mice demonstrated specificity of an anti-EP2 antibody (Fig. 3 A and B). Immunoblot comparison of EP2 expression levels in the cerebral cortex in the ischemic vs. contralateral hemisphere revealed a 3.5-fold induction of an EP2 receptor 48 h after MCAo (Fig. 3 C and D). This induction was also observed by immunofluorescent staining (Fig. 3 E–G) where EP2 receptor expression was markedly induced in neurons in the ischemic penumbra and was mostly lost in the area of infarction as defined by the absence of MAP2 staining. Given the protection of inducibly ablating adult EP2 receptor expression using the ROSACreER line, we conclude that induction of the EP2 receptor in response to cerebral ischemia promotes cerebral injury.
Fig. 3.
EP2 receptor is highly induced in the brain in response to cerebral ischemia. (A) Validation of the anti-EP2 antibody using cerebellar lysates and HEK cells overexpressing the EP2 receptor (HEK-EP2; positive control) and HEK cell lysates (negative control). (B) EP2 receptor expression is decreased in the cerebellum in ROSACreER;EP2lox/lox 3 mo male mice. (C) The EP2 receptor is significantly induced in the IL cerebral cortex compared with the CL noninfarcted cortex 48 h after MCAo in 3 mo male mice. (D) Quantification of the EP2 protein in the IL and CL cortices 48 h after MCAo (n = 5 per group, two-tailed Student’s t test, ***P = 0.0003). Data are presented as mean ± SEM. (E) Immunofluorescent staining of naive and MCAo brains 48 h after MCAo, stained for the EP2 receptor and MAP2. (Scale bar, 1 mm.) The white arrow points to the border among infarcted tissue and penumbra. (F) Higher magnification of the border region between the infarcted and the penumbral parietal cortex and the corresponding area in the naive brain. (Scale bar, 250 μm.) (G) Layers V and VI of the ischemic hemisphere in the penumbra demonstrating colocalization of MAP2 (green) and the EP2 receptor (purple). (Scale bar, 50 μm.).
Effects of Neuronal EP2 Receptor Deletion in MCAo-RP.
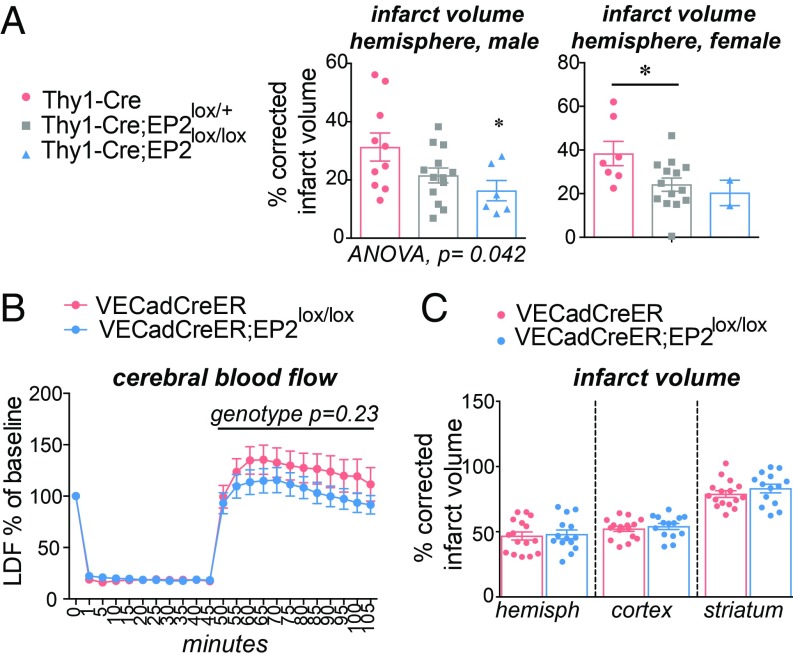
As the EP2 receptor is induced in neurons in the ischemic hemisphere, particularly in the penumbra, we next tested the contribution of neuronal EP2 signaling to cerebral ischemic injury using the Thy1-Cre line to selectively excise EP2 sequences in neurons after the second postnatal week (29), avoiding developmental effects from genetic deletion at earlier developmental stages (Fig. 4A). Measurement of infarct volume demonstrated a significant and gene-dose-dependent decrease in cerebral injury in male and female cohorts of Thy1-Cre, Thy1-Cre; EP2lox/+, and Thy1-Cre;EP2lox/lox mice. These findings indicate that induction of the neuronal EP2 receptor in the context of cerebral ischemia is highly detrimental and contrast with prior data in global EP2 receptor knockout mice using the same MCAo model where congenital deletion of the EP2 receptor was cerebroprotective (27, 28).
Fig. 4.
Effects of cell-specific deletion of the neuronal and endothelial EP2 receptors in MCAo-RP. Data are presented as mean ± SEM. (A) Deletion of the neuronal EP2 receptor in 3 to 4 mo Thy1-Cre;EP2lox/lox mice was protective in male and female cohorts (males: n = 6–10/group; P = 0.0417; females: n = 2–14/group; P = 0.0470; *P < 0.05 post hoc Tukey between male Thy1-Cre and Thy1-Cre;EP2lox/lox genotypes). (B) Measurement of relative CBF by LDF does not demonstrate any effect of genotype in VECadCreER;EP2lox/lox vs. control VECadCreER mice (n = 14–20 3 to 4 mo male mice per group; effect of genotype during RP from 45 min to 105 min, P = 0.2365). (C) Percentage of corrected infarct volume does not differ in 3 to 4 mo male VECadCreER;EP2lox/lox mice vs. control VECadCreER mice 24 h after MCAo (n = 14–16 per group).
Effects of Endothelial EP2 Receptor Deletion in MCAo-RP.
The EP2 receptor, along with the EP4 and prostacyclin (PGI2) receptors, mediate the vasodilatory effects of PGE2 (30, 31). Accordingly, we examined effects of inducibly deleting the EP2 receptor at adult stages in endothelial cells using the VECad-CreERT2 line (abbreviated VECadCreER; Fig. 4 B and C) wherein tamoxifen triggers excision of loxP sequences in both systemic and cerebral endothelia (32). This strategy has previously been utilized to identify cell-specific mechanisms of cerebroprotection of a related PGE2 receptor, the EP4 receptor (2). We measured relative cerebral blood flow (CBF) using laser Doppler flowmetry (LDF) in mice subjected to 60 min of ischemia followed by 60 min of RP. VECadCreER;EP2lox/lox mice did not show significant differences in CBF compared with VECadCreER mice, a finding consistent with previous LDF studies showing no effect of EP2 receptor deletion in global EP2 −/− mice (27, 28). Quantification of stroke volume by CV staining also showed no differences between genotypes, suggesting that the endothelial EP2 receptor does not contribute significantly to early cerebral ischemic injury.
Inducible Global Deletion of EP2 Receptor Does Not Disrupt Cognitive Function.
The divergent findings between stroke outcomes in congenital EP2−/− mice vs. ROSACreER;EP2lox/lox mice suggested that previously observed deficits in sensorimotor gating, anxiety, and spatial memory in EP2−/− mice (33, 34) might have been confounded by the early developmental expression of the EP2 receptor. Yang et al. (34) demonstrated a deficit in spatial memory in the Morris water maze, and Savonenko et al. (33) demonstrated impaired prepulse inhibition (PPI) and heightened anxiety in the open field and elevated plus maze tests in congenital EP2 −/− mice (35). We examined cohorts of 3 mo old male and female ROSACreER:EP2lox/lox and control ROSACreER mice (SI Appendix, Fig. S3) that had received tamoxifen at 8 wk of age and were tested 4 wk later. After ensuring that visual and motor skills were normal, mice were tested for deficits in PPI, a measure of sensorimotor gating where a brief and low-intensity acoustic stimulus is used to inhibit the startle reflex caused by a loud stimulus (SI Appendix, Fig. S3A). Previous examination of the PPI in EP2−/− mice demonstrated significant differences compared with wild-type control mice (33). Here, the PPI in ROSACreER:EP2lox/lox mice did not differ from control ROSACreER ER mice in either gender.
Additional behavioral testing included the activity chamber, a measure of motor activity, where both male and female ROSACreER:EP2lox/lox mice behaved similarly to ROSACreER control mice, and total distance and time traveled did not differ between genotypes (SI Appendix, Fig. S3B). In the open field test, a measure of novelty-induced exploratory activity and anxiety, there were no differences in distance traveled or time between genotypes in both genders (SI Appendix, Fig. S3C). In a second test of anxiety, the elevated plus maze, there were again no differences in the time in open arms between genotypes in both genders (SI Appendix, Fig. S3D). Finally, in the Morris water maze, a measure of spatial learning and long-term memory of a fixed location, there were no differences in escape latency (SI Appendix, Fig. S3E) or time in quadrants (SI Appendix, Fig. S3F) between genotypes of either gender. Taken together, adult-stage ablation of the EP2 receptor did not phenocopy the cognitive deficits observed in global EP2−/− mice, supporting a confounding effect of congenital deletion of the EP2 receptor.
Pharmacologic Inhibition of EP2 Prevents Cerebral Ischemic Injury.
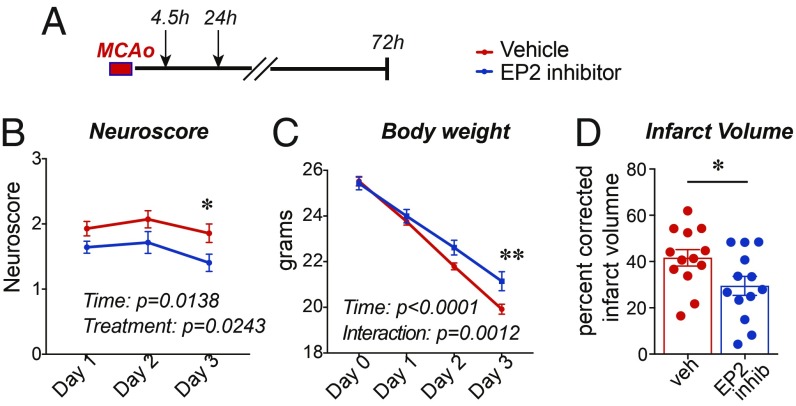
We then tested whether pharmacologic inhibition of the EP2 receptor would attenuate MCAo injury (Fig. 5). Recent studies have identified a series of EP2 receptor antagonists (36) that are brain penetrant and increase phagocytic activity of the microglia. We selected compound 52, a small molecule with low nanomolar binding affinity for the EP2 receptor (IC50 = 8 nM). C57BL/6J male mice underwent 45 min of MCAo as before with the examination of neuroscores over 72 h and quantification of infarct volume. Administration of compound 52 at 4.5 h and again at 24 h after MCAo improved neurological scores and reduced infarct volume, consistent with results observed with ROSACreER;EP2lox/lox mice.
Fig. 5.
Inhibition of the EP2 receptor is cerebroprotective. Data are presented as mean ± SEM. (A) The EP2 receptor inhibitor (compound 52 at 10 mg/kg) or vehicle were administered at 4.5 h and again at 24 h after MCAo to 3 mo C57BL/6J male mice (n = 21 per group). (B) Neurological scores over 72 h after MCAo (repeated measures, two-way ANOVA, effect of time P = 0.0138; effect of treatment, P = 0.0243; Bonferroni post hoc *P < 0.05 at day 3). (C) Body weight over 72 h after MCAo (repeated measures, two-way ANOVA, effect of time P < 0.0001, and effect of treatment, P = 0.0052; Tukey post hoc **P < 0.01 at day 3). (D) Percentage of infarct volume in mice 72 h after MCAo ± EP2 receptor inhibitor (n = 13 per group; Student’s two-tailed t test, P = 0.035).
Discussion
The PGE2 EP2 receptor has been studied using genetic approaches in models of inflammatory neurodegeneration, including models of innate immunity, AD, PD, and amyotrophic lateral sclerosis (4, 5, 10–16). In these models, progression of pathology occurs in the context of a prominent innate immune response and ablation of the EP2 receptor is highly beneficial. In this study, myeloid-specific effects of PGE2 EP2 signaling were examined in a model of cerebral ischemia where precedent has demonstrated that peripheral macrophages and neutrophils infiltrate the ischemic hemisphere over the first several days after stroke (18–22). Myeloid-specific knockdown of EP2 signaling in Cd11bCre;EP2lox/lox mice reduced brain infiltration of Cd11b+CD45hi macrophages and CD45+Ly6Ghi neutrophils, suggesting that the myeloid EP2 receptor enhances the poststroke innate immune response. Loss of the myeloid EP2 receptor did not alter infarct severity, potentially reflecting the 50% knockdown of EP2 genomic sequences in the Cd11bCre;EP2lox/lox line, a limitation of this approach; given this conditional knockout approach, we cannot rule out that myeloid EP2 ablation alone is sufficient to reverse cerebral ischemic injury, however, it was associated with marked decreases in myeloid cell infiltration. Significant reduction of both myeloid cell infiltration and cerebral injury was, however, observed following inducible global deletion of the EP2 receptor in adult RosaCreER;EP2lox/lox mice; here EP2 genomic sequences were reduced by ∼95% and suggested that nonmyeloid EP2 receptor-expressing cell types contribute to cerebral injury.
Unexpectedly, the reduction of stroke severity in ROSACreER;EP2lox/lox mice did not phenocopy previous findings in stroked global EP2−/− mice where EP2 receptor deletion instead increased cerebral injury (27, 28). As this suggested a potential confound of congenital EP2 receptor deletion, we investigated expression levels of the EP2 receptor basally and after MCAo. Basal expression of the EP2 receptor in naive mice was very low in the forebrain, but upon ischemic insult, EP2 receptor expression was markedly induced in penumbral cortical areas, particularly in neurons. Previous investigations (GENSAT) also confirmed low to absent EP2 receptor expression in the adult forebrain but high levels in the developing brain, suggesting an early role of neuronal EP2 signaling in brain development. We also examined cognitive function in ROSACreER;EP2lox/lox mice and found a normal behavioral phenotype that contrasted sharply with the previously described abnormal behavioral phenotypes of EP2−/− mice (33, 34). In retrospect, the lack of an effect of adult EP2 receptor ablation on behavior is not surprising as the basal expression level of the EP2 receptor is nearly undetectable in the forebrain in the adult.
Following cerebral ischemia, a marked induction of EP2 receptor expression was observed in the ischemic hemisphere, particularly in neurons of the penumbra, suggesting that neuronal EP2 receptor induction worsens cerebral injury. In support of that, ablation of the neuronal EP2 receptor postnatally in Thy1-Cre;EP2lox/lox mice was highly protective and phenocopied the rescue of the infarct volume seen in ROSACreER;EP2lox/lox mice. Subsequent examination of endothelial EP2 signaling in stroked VECadCreER;EP2lox/lox mice did not show changes in CBF or infarct volume. This observation is consistent with prior studies in EP2−/− mice where no changes in CBF were observed with loss of the EP2 receptor (27, 28, 37). The lack of an effect of conditional deletion of the endothelial EP2 receptor as well as the absence of a vascular phenotype following global EP2 receptor deletion may reflect redundancy in the regulation of CBF where, at least, two additional prostanoid signaling pathways can regulate vascular tone, including the vasodilatory PGI2 and EP4 signaling pathways.
In vivo, a marked cerebroprotective effect was found in Thy1-Cre;EP2lox/loxlox/lox and Thy1-Cre;EP2lox/+ mice compared with Thy1-Cre control mice, indicating a detrimental effect of neuronal EP2 receptor induction acutely after cerebral ischemia. Previous in vitro studies modeling effects of EP2 signaling employed primary neuronal cultures derived from embryonic cortical or hippocampal neurons or, alternatively, hippocampal slice cultures derived from the early postnatal brain (27, 38). These in vitro studies convincingly supported a neuroprotective effect of EP2 receptor signaling following pharmacologic application of EP2 receptor agonists in the setting of glutamate excitotoxicity and oxygen-glucose deprivation. However, given our current assessment of EP2 receptor expression in the adult brain, these observations might be explained by the fact that in vitro cultures utilized embryonic neurons that express high levels of the EP2 receptor and would therefore not be representative of adult neurons that are basally devoid of the EP2 receptor. Taken together with the cerebroprotective effects of a brain permeable EP2 inhibitor, our findings provide strong evidence that inhibition of EP2 signaling is highly protective in stroke through neuronal and immune mechanisms.
Nonsteroidal antiinflammatory drugs, which inhibit both COX-1 and COX-2, as well as COX-2 selective inhibitors are protective in multiple stroke models when administered acutely within the neuroprotective and vascular time windows (39–43). COX-2 inhibitors have not been considered for stroke therapy because of concern over vascular side effects from inhibition of beneficial PGE2 signaling pathways downstream of COX-2 (44). PGI2 is a vasodilatory PGE, but with sustained COX-2 inhibition, its synthesis is decreased, leading to a prothrombotic state from unopposed thromboxane A2 signaling in platelets (reviewed in ref. 44). It is interesting to note that inhibition of COX-2 as far out as 18 h after MCAo is also effective in limiting stroke damage in models of stroke (45), a time window that coincides with the poststroke inflammatory response. Of the four PGE2 receptors, maladaptive innate immune responses have been established for the EP2 receptor and include a persistence of proinflammatory responses from induction of COX-2 expression and PGE2 generation, a suppression of phagocytosis and lysosomal function, and an inhibition of neurotrophic support (4, 15, 46). Conversely, the EP4 receptor is highly anti-inflammatory (3) in addition to being neuroprotective and vasodilatory in stroke models (2). EP2 signaling therefore appears to be an effector of COX-2–mediated injury via myeloid and neuronal cell types. Selectively targeting the EP2 receptor pharmacologically also reduces stroke severity, providing proof of concept that toxic PGE2 signaling can be suppressed without the potential side effects from broader COX-2 inhibition.
In conclusion, we demonstrate that, in a rodent model of cerebral ischemia, ablation of myeloid EP2 signaling attenuates the poststroke inflammatory response. We also demonstrate that the EP2 receptor blockade using either an inducible knockout approach or a brain penetrant inhibitor in adult mice is highly protective via neuronal and inflammatory mechanisms. Finally, we uncover important differences in stroke and behavioral phenotypes between the original congenital global EP2−/− mouse model and the inducible adult ablation of the EP2 receptor in the ROSACreER;EP2lox/lox line, highlighting the importance of recognizing potential confounding effects from congenital gene deletion.
Materials and Methods
Animals.
This study was conducted in accordance with the National Institutes of Health guidelines for the use of experimental animals (47) and protocols were approved by the Institutional Animal Care and Use Committee. C57BL/6 EP2lox/+ mice were generated as previously described (15). Additional details are provided in the SI Appendix, Supplementary Methods.
Transient Focal Ischemia Model.
All MCAo experiments were performed by an experimenter blinded to genotype as described previously (2, 27). Some 12–16 wk old mice were subjected to 45 min of MCAo followed by 24 or 48 h of RP.
Quantification of Infarct Volume.
Infarct quantification was carried out by a second examiner who was blinded to genotype. After 23 or 47 h of RP, mice were lethally anesthetized, and brain tissue was harvested for infarct quantification using CV as previously described (2). For each brain, 20 µm sections at 800 μm intervals were stained with CV, imaged (Keyence microscope, BZ-9000 Series), and hemispheric stained regions were quantified using ImageJ (imagej.nih.gov/ij). Volumes across the intervals from the last to first sections were summed to calculate the total infarct volume; infarct volume was then corrected for edema by comparing the volume of contralateral noninfarcted hemisphere to minimize the contributions of cytotoxic and vasogenic edema, the infarct volume was determined using the indirect method, using the equation: % corrected infarct- 100 × [(Vc − Vl)/Vc], where Vc is the volume of a normal brain in the nonischemic hemisphere and Vl is the volume of normal gray matter in the lesioned ischemic hemisphere (48).
Measurement of Relative CBF.
LDF was performed in a blinded fashion 2 mm posterior and 3 mm lateral to the bregma over the parietal skull surface as previously described (2, 27). Here, mice underwent 60 min of MCA occlusion followed by 60 min of RP.
Statistical Analysis.
Data are expressed as mean ± SEM. Statistical comparisons were made in Prism software (GraphPad) using the Student’s t test (for two groups meeting normal distribution criteria by the Shapiro–Wilk normality test), the Mann–Whitney U test (for two groups not meeting normal distribution criteria), or ANOVA with the Bonferroni or Tukey multiple comparison tests (for groups across variables with multiple comparisons between groups). Data were subjected to the Grubbs’ test to identify the presence or absence of outlier data points for exclusion from analysis. For all tests, P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant R21NS087639 (to K.I.A.), NIH Grant R01NS045727 (to K.I.A.), Dean’s Fellowship (E.N.W.), and NIH NRSA Award NS086397 (to P.B.L.). GENSAT images are courtesy of the GENSAT Project, National Institute of Neurological Disorders and Stroke (NINDS) Contracts N01NS02331 and HHSN271200723701C to The Rockefeller University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1818544116/-/DCSupplemental.
References
- 1.Kawano T, et al. (2006) Prostaglandin E2 EP1 receptors: Downstream effectors of COX-2 neurotoxicity. Nat Med 12:225–229. [DOI] [PubMed] [Google Scholar]
- 2.Liang X, et al. (2011) Signaling via the prostaglandin E₂ receptor EP4 exerts neuronal and vascular protection in a mouse model of cerebral ischemia. J Clin Invest 121:4362–4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi J, et al. (2010) The prostaglandin E2 E-prostanoid 4 receptor exerts anti-inflammatory effects in brain innate immunity. J Immunol 184:7207–7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansson JU, et al. (2015) Prostaglandin signaling suppresses beneficial microglial function in Alzheimer’s disease models. J Clin Invest 125:350–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang X, et al. (2005) Deletion of the prostaglandin E2 EP2 receptor reduces oxidative damage and amyloid burden in a model of Alzheimer’s disease. J Neurosci 25:10180–10187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi J, et al. (2012) Inflammatory prostaglandin E2 signaling in a mouse model of Alzheimer disease. Ann Neurol 72:788–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoshino T, et al. (2007) Involvement of prostaglandin E2 in production of amyloid-beta peptides both in vitro and in vivo. J Biol Chem 282:32676–32688. [DOI] [PubMed] [Google Scholar]
- 8.Woodling NS, et al. (2014) Suppression of Alzheimer-associated inflammation by microglial prostaglandin-E2 EP4 receptor signaling. J Neurosci 34:5882–5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cimino PJ, Keene CD, Breyer RM, Montine KS, Montine TJ (2008) Therapeutic targets in prostaglandin E2 signaling for neurologic disease. Curr Med Chem 15:1863–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keene CD, et al. (2009) Protection of hippocampal neurogenesis from toll-like receptor 4-dependent innate immune activation by ablation of prostaglandin E2 receptor subtype EP1 or EP2. Am J Pathol 174:2300–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montine TJ, et al. (2002) Neuronal oxidative damage from activated innate immunity is EP2 receptor-dependent. J Neurochem 83:463–470. [DOI] [PubMed] [Google Scholar]
- 12.Johansson JU, Woodling NS, Brown HD, Wang Q, Andreasson KI (2015) Microarray analysis of the in vivo response of microglia to Aβ peptides in mice with conditional deletion of the prostaglandin EP2 receptor. Genom Data 5:268–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keene CD, et al. (2010) Suppressed accumulation of cerebral amyloid beta peptides in aged transgenic Alzheimer’s disease mice by transplantation with wild-type or prostaglandin E2 receptor subtype 2-null bone marrow. Am J Pathol 177:346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin J, et al. (2007) Prostaglandin E2 receptor subtype 2 (EP2) regulates microglial activation and associated neurotoxicity induced by aggregated alpha-synuclein. J Neuroinflammation 4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansson JU, et al. (2013) Suppression of inflammation with conditional deletion of the prostaglandin E2 EP2 receptor in macrophages and brain microglia. J Neurosci 33:16016–16032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang X, et al. (2008) The prostaglandin E2 EP2 receptor accelerates disease progression and inflammation in a model of amyotrophic lateral sclerosis. Ann Neurol 64:304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albers GW, et al. ; DEFUSE 3 Investigators (2018) Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 378:708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens SL, et al. (2002) The use of flow cytometry to evaluate temporal changes in inflammatory cells following focal cerebral ischemia in mice. Brain Res 932:110–119. [DOI] [PubMed] [Google Scholar]
- 19.Frangogiannis NG. (2007) Chemokines in ischemia and reperfusion. Thromb Haemost 97:738–747. [PubMed] [Google Scholar]
- 20.Wang Q, Tang XN, Yenari MA (2007) The inflammatory response in stroke. J Neuroimmunol 184:53–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gelderblom M, et al. (2009) Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 40:1849–1857. [DOI] [PubMed] [Google Scholar]
- 22.Iadecola C, Anrather J (2011) The immunology of stroke: From mechanisms to translation. Nat Med 17:796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seifert HA, et al. (2012) The spleen contributes to stroke induced neurodegeneration through interferon gamma signaling. Metab Brain Dis 27:131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ajmo CT Jr, et al. (2008) The spleen contributes to stroke-induced neurodegeneration. J Neurosci Res 86:2227–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostrowski RP, et al. (2012) Acute splenic irradiation reduces brain injury in the rat focal ischemic stroke model. Transl Stroke Res 3:473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dotson AL, Wang J, Saugstad J, Murphy SJ, Offner H (2015) Splenectomy reduces infarct volume and neuroinflammation in male but not female mice in experimental stroke. J Neuroimmunol 278:289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCullough L, et al. (2004) Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J Neurosci 24:257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmad M, et al. (2010) The PGE2 EP2 receptor and its selective activation are beneficial against ischemic stroke. Exp Transl Stroke Med 2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caroni P. (1997) Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J Neurosci Methods 71:3–9. [DOI] [PubMed] [Google Scholar]
- 30.Audoly LP, et al. (1999) Identification of specific EP receptors responsible for the hemodynamic effects of PGE2. Am J Physiol 277:H924–H930. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, et al. (2000) Characterization of murine vasopressor and vasodepressor prostaglandin E(2) receptors. Hypertension 35:1129–1134. [DOI] [PubMed] [Google Scholar]
- 32.Monvoisin A, et al. (2006) VE-cadherin-CreERT2 transgenic mouse: A model for inducible recombination in the endothelium. Dev Dyn 235:3413–3422. [DOI] [PubMed] [Google Scholar]
- 33.Savonenko A, et al. (2009) Impaired cognition, sensorimotor gating, and hippocampal long-term depression in mice lacking the prostaglandin E2 EP2 receptor. Exp Neurol 217:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang H, Zhang J, Breyer RM, Chen C (2009) Altered hippocampal long-term synaptic plasticity in mice deficient in the PGE2 EP2 receptor. J Neurochem 108:295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breyer RM, Kennedy CR, Zhang Y, Guan Y, Breyer MD (2002) Targeted gene disruption of the prostaglandin E2 EP2 receptor. Adv Exp Med Biol 507:321–326. [DOI] [PubMed] [Google Scholar]
- 36.Fox BM, et al. (2015) A selective prostaglandin E2 receptor subtype 2 (EP2) antagonist increases the macrophage-mediated clearance of amyloid-beta plaques. J Med Chem 58:5256–5273. [DOI] [PubMed] [Google Scholar]
- 37.Liu D, Wu L, Breyer R, Mattson MP, Andreasson K (2005) Neuroprotection by the PGE2 EP2 receptor in permanent focal cerebral ischemia. Ann Neurol 57:758–761. [DOI] [PubMed] [Google Scholar]
- 38.Echeverria V, Clerman A, Doré S (2005) Stimulation of PGE receptors EP2 and EP4 protects cultured neurons against oxidative stress and cell death following beta-amyloid exposure. Eur J Neurosci 22:2199–2206. [DOI] [PubMed] [Google Scholar]
- 39.Nakayama M, et al. (1998) Cyclooxygenase-2 inhibition prevents delayed death of CA1 hippocampal neurons following global ischemia. Proc Natl Acad Sci USA 95:10954–10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagayama M, Niwa K, Nagayama T, Ross ME, Iadecola C (1999) The cyclooxygenase-2 inhibitor NS-398 ameliorates ischemic brain injury in wild-type mice but not in mice with deletion of the inducible nitric oxide synthase gene. J Cereb Blood Flow Metab 19:1213–1219. [DOI] [PubMed] [Google Scholar]
- 41.Iadecola C, et al. (2001) Reduced susceptibility to ischemic brain injury and N-methyl-D-aspartate-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proc Natl Acad Sci USA 98:1294–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doré S, et al. (2003) Neuronal overexpression of cyclooxygenase-2 increases cerebral infarction. Ann Neurol 54:155–162. [DOI] [PubMed] [Google Scholar]
- 43.Ahmad M, Zhang Y, Liu H, Rose ME, Graham SH (2009) Prolonged opportunity for neuroprotection in experimental stroke with selective blockade of cyclooxygenase-2 activity. Brain Res 1279:168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Funk CD, FitzGerald GA (2007) COX-2 inhibitors and cardiovascular risk. J Cardiovasc Pharmacol 50:470–479. [DOI] [PubMed] [Google Scholar]
- 45.Sugimoto K, Iadecola C (2003) Delayed effect of administration of COX-2 inhibitor in mice with acute cerebral ischemia. Brain Res 960:273–276. [DOI] [PubMed] [Google Scholar]
- 46.Woodling NS, Andreasson KI (2016) Untangling the web: Toxic and protective effects of neuroinflammation and PGE2 signaling in Alzheimer’s disease. ACS Chem Neurosci 7:454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National Research Council (2011) Guide for the Care and Use of Laboratory Animals(National Academies Press, Washington, DC: ), 8th Ed. [Google Scholar]
- 48.Swanson RA, et al. (1990) A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab 10:290–293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.