Significance
Cerebral malaria (CM) is a potentially fatal neurological complication caused by Plasmodium falciparum affecting mainly young children. In endemic areas, severe complications become less frequent as γδ-T cell responses decline. However, the role of γδ-T cells in CM pathogenesis remains unclear. Here, we show that mice deficient in γδ-T cells are resistant to experimental CM development when infected with Plasmodium berghei ANKA sporozoites. Importantly, we demonstrate that the presence of γδ-T cells enhanced the expression of Plasmodium immunogenic factors upon liver-stage infection and promoted subsequent blood-stage inflammatory responses. This study characterizes a critical pathogenic role of γδ-T cells in experimental CM that provides a strong rationale for their modulation toward achieving “clinical immunity” to malaria.
Keywords: cerebral malaria, gamma-delta T cells, Plasmodium, liver stage, interferon-gamma
Abstract
Cerebral malaria (CM) is a major cause of death due to Plasmodium infection. Both parasite and host factors contribute to the onset of CM, but the precise cellular and molecular mechanisms that contribute to its pathogenesis remain poorly characterized. Unlike conventional αβ-T cells, previous studies on murine γδ-T cells failed to identify a nonredundant role for this T cell subset in experimental cerebral malaria (ECM). Here we show that mice lacking γδ-T cells are resistant to ECM when infected with Plasmodium berghei ANKA sporozoites, the liver-infective form of the parasite and the natural route of infection, in contrast with their susceptible phenotype if challenged with P. berghei ANKA-infected red blood cells that bypass the liver stage of infection. Strikingly, the presence of γδ-T cells enhanced the expression of Plasmodium immunogenic factors and exacerbated subsequent systemic and brain-infiltrating inflammatory αβ-T cell responses. These phenomena were dependent on the proinflammatory cytokine IFN-γ, which was required during liver stage for modulation of the parasite transcriptome, as well as for downstream immune-mediated pathology. Our work reveals an unanticipated critical role of γδ-T cells in the development of ECM upon Plasmodium liver-stage infection.
Malaria remains a devastating global health problem, responsible for more than 200 million cases every year, leading to more than 400,000 annual deaths due to severe malaria, such as cerebral malaria (CM), caused by Plasmodium falciparum (1, 2). In endemic regions, adults and children older than 5 y acquired considerably rapid “clinical immunity” to malaria, characterized by a decrease in severe malaria episodes and an increase of asymptomatic P. falciparum infections (3). While the basic immunological mechanisms that contribute to clinical immunity to severe malaria remain unclear, it was recently shown to associate with decreased responsiveness of Vγ9Vδ2 T cells (4), the main subset of circulating human γδ-T lymphocytes. These increase dramatically, from 1% to 5% (in healthy donors) to 30–40% of all peripheral blood T cells, during primary P. falciparum infections (5–7). In fact, it was suggested almost 25 y ago that the acquisition of clinical immunity in endemic areas could be due to the down-regulation of Vγ9Vδ2 T cell functions (8).
In addition to being potentially deleterious (4, 9), expanded Vγ9Vδ2 T cells have also been suggested to play protective roles in controlling parasite density (10–13), namely by killing extracellular merozoites (10), by acting as antigen-presenting cells for αβ-T cells in response to Plasmodium intraerythrocytic-stage parasites (14), and more recently by preventing parasite recrudescence (13). Moreover, mouse γδ-T cells expressing CD40L enhanced dendritic cell activation, promoted Plasmodium berghei XAT (a nonlethal strain) clearance (15), and were required for induction of protective CD8 T cell responses upon sporozoites vaccination (16). Notably, a recent study revealed two distinct types of protective γδ-T cell responses to Plasmodium chabaudi in a murine model of blood-stage infection (13). This infection model is characterized by an early acute stage, with high parasitemia, and a postacute stage, with very low parasitemia, after which sterile immunity is achieved in wild-type (WT) mice (13, 17). The study by Mamedov et al. (13) showed that, after producing abundant IFN-γ in the acute phase, γδ-T cells shift to making high levels of macrophage-colony stimulating factor (M-CSF), in addition to CCL3 and CCL5 chemokines, in the late stage. Critically, both IFN-γ and M-CSF were essential for the prevention of parasitemic recurrence in this P. chabaudi infection model.
Going beyond the role of γδ-T cells in controlling parasite load, here we aimed to clarify the biological functions of γδ-T cells in malaria pathogenesis using a well-established rodent model of experimental cerebral malaria (ECM). Although the nature of ECM pathogenesis remains incompletely understood, there are two theories, the mechanical obstruction and the immunopathology hypotheses, that try to gather the complex interactions between several host and parasite factors to explain the process of cerebral malaria pathogenesis (18). ECM is considered an immune-mediated pathology that is also dependent on the accumulation/sequestration of parasites in the brain (19), features that are also seen in human cerebral malaria (20, 21). Importantly, two of its immunological hallmarks are the absolute dependence on αβ-T cells (22–26) and the proinflammatory cytokine IFN-γ, which constitutes a key pathogenic factor in severe malaria (23, 24, 27–29). In fact, IFN-γ–deficient and IFN-γR–deficient mice are completely resistant to ECM development after infection with P. berghei ANKA infected red blood cells (iRBCs) (23, 28–30). The paradox in the immune response to Plasmodium stems from proinflammatory cytokines like IFN-γ (and TNF-α) being associated both with protection against infection (parasite elimination) and with immunopathology (when the infection is not resolved) (23, 28, 29, 31). This further draws the attention to γδ-T cells, since they are major producers of IFN-γ (and TNF-α) in the immune response to Plasmodium in mice (32–34) as well as in humans (35, 36).
In a previous study, mice infected with P. berghei ANKA iRBCs and depleted of γδ-T cells by GL3 antibody treatment before infection (day 0) or at day 3 postinfection (p.i.) did not show any physical or histological evidence of ECM, whereas the disease developed fully if the γδ-T cell depletion was performed at day 5 p.i. With regard to the genetic deletion of γδ-T cells, i.e., TCRδ−/− mice, it was reported that >50% of animals developed ECM when infected with P. berghei ANKA iRBCs (37, 38). Notably, this route of infection bypasses the liver stage, which has been widely neglected with regard to its impact in ECM pathogenesis. In natural infections, malaria is transmitted through the bite of infected anopheline mosquitoes, where Plasmodium sporozoites are delivered into the skin, entering the bloodstream and homing to the liver (39, 40). Once inside the liver parenchyma, sporozoites invade a hepatocyte and develop into large exoerythrocytic forms that differentiate into thousands of merozoites. These infective forms egress from hepatocytes, invade RBCs, and initiate the blood-stage infection (41). The clinically “silent” liver stage is thus an essential step of the Plasmodium life cycle that always precedes the cyclic intraerythrocytic infection causing the characteristic clinical symptoms of malaria, such as CM. Despite being clinically silent, the liver stage has recently been shown to be immunologically active and elicit a robust innate type I IFN response against P. berghei sporozoites (42). Other studies on sporozoite infection have used Plasmodium yoelii, another rodent model that shows higher (lethal) susceptibility due to an altered immune response in the liver (43). Building on previous findings (44, 45), a recent report demonstrated, after a P. yoelli primary infection, that natural killer (NK) and natural killer T (NKT) cells increased in the liver and IFN-γ producers CD1d-restricted NKT cells were crucial to reduce parasite load upon secondary infection (46). γδ-T cells have also been shown to promote immunity against P. yoelli (47), with TCRδ−/− mice presenting higher parasite load in the liver and being more susceptible than wild type (WT) mice when challenged with very low doses of P. yoelii sporozoites (48). By contrast, the data on γδ-T cells in P. berghei ANKA infection are scarce, restricted to iRBC infection, and suggestive of a dispensable role in ECM pathogenesis (37).
In this study we hypothesized that the contribution of γδ-T cells to ECM pathogenesis might depend on liver involvement upon sporozoite infection, i.e., the natural course of infection. By comparing the outcome of P. berghei ANKA infection using sporozoites (Spz) versus iRBCs in TCRδ−/− or WT mice, we disclose an essential role for γδ-T cells in ECM upon liver-stage infection. We show that sporozoite infection induces a robust γδ-T cell activation that promotes IFN-γ-mediated responses, which impact on parasite virulence, blood-stage inflammation, and ECM pathogenesis.
Results
γδ-T Cells Are Essential for Liver Stage-Dependent ECM Pathogenesis.
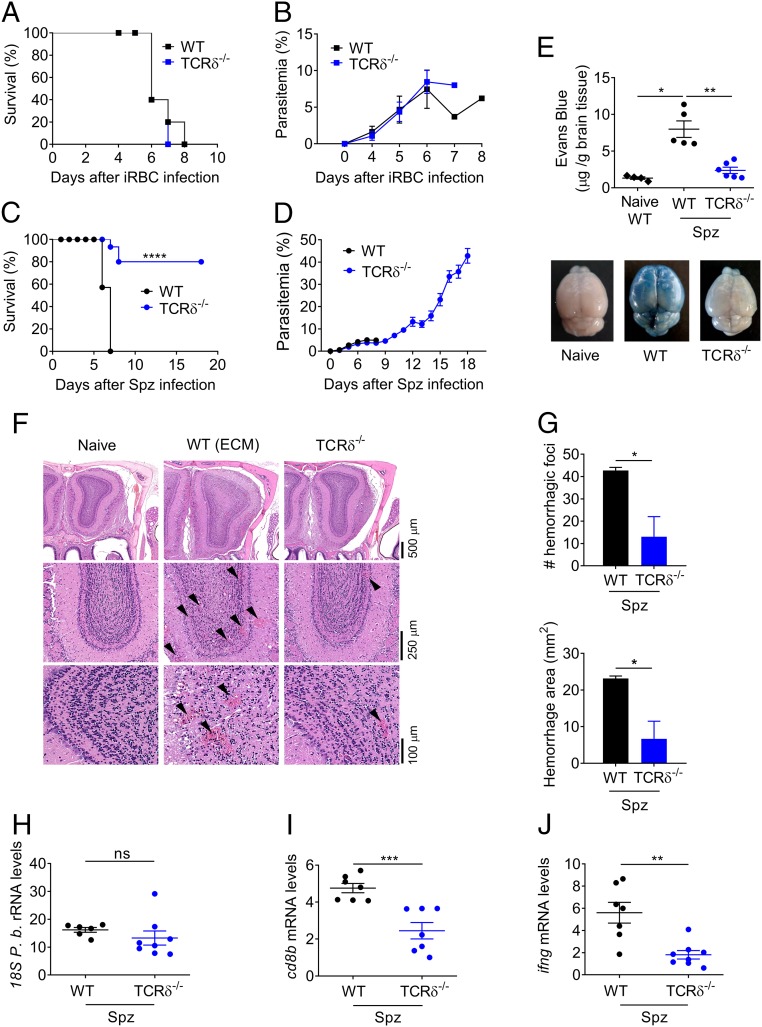
To evaluate the impact of γδ-T cells along the two developmental stages, intrahepatic and intraerythrocytic, of the Plasmodium parasite, we compared the infection outcome in TCRδ–deficient (TCRδ−/−) versus WT mice injected with either P. berghei ANKA Spz or iRBCs. As previously reported (37), TCRδ−/− mice infected with P. berghei ANKA iRBCs, i.e., bypassing the liver stage, developed parasitemia and succumbed to experimental cerebral malaria (100% of mortality between days 6 and 8, within the timeframe of ECM), similarly to WT animals (Fig. 1 A and B). In striking contrast, upon sporozoite and mosquito-bite infection, i.e., the natural route of infection, only 20–25% of the mice from the TCRδ−/− group developed ECM signs (P < 0.0001) (Fig. 1 C and D and SI Appendix, Fig. S1 A and B). Importantly, there were no significant differences in parasitemia between WT and TCRδ−/− mice during the first 7–9 d following sporozoite, iRBC, or mosquito-bite infections (Fig. 1 B and D and SI Appendix, Fig. S1B). Thus, the impact of γδ-T cells on ECM pathogenesis was not mediated through inhibition of parasite proliferation during the intraerythrocytic stage of infection. All TCRδ−/− animals that did not show ECM signs and survived beyond days 8–10, developed high parasitemia and anemia and were killed on day 18 after infection (Fig. 1D and SI Appendix, Fig. S1B). Of note, the differential ECM phenotype of TCRδ−/− mice upon Spz versus iRBC infection was always observed independently of the parasite inoculums (SI Appendix, Fig. S1 C and D).
Fig. 1.
γδ-T cells are essential for liver stage-dependent ECM pathogenesis. (A and C) Survival and (B and D) parasitemia curves for WT and TCRδ−/− mice. WT (n = 5–14) and TCRδ−/− (n = 5–15) mice were infected with 1 × 106 P. berghei ANKA GFP iRBCs by i.p. injection (A and B); or 2 × 104 P. berghei ANKA GFP Spz by i.v. injection (C and D). (C) Data combined from three independent experiments [****P < 0.0001 by Log-rank (Mantel–Cox) test]. (E) Assessment of BBB disruption by EB staining of noninfected (naïve, n = 5) versus Spz-infected WT (n = 5) or TCRδ−/− (n = 6) mice. (F) Representative microphotographs of coronal sections of mouse brain at the level of the olfactory bulb by H&E. Original magnification, 2.5×, 10×, and 20×. (Scale bars, 100–500 μm.) Images are representative of three mice. (G) Number and area of hemorrhage foci (n = 3) in the olfactory bulb (approximately at bregma 3.92). (H) Relative expression levels of parasite 18S P.b. rRNA, (I) cd8b, and (J) ifng in brain tissue of WT and TCRδ−/− (n = 6–8) Spz-infected mice (arbitrary units normalized to the housekeeping gene hprt). Data are represented as mean ± SEM. Each symbol in E and H–J represents an individual mouse. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, not significant; by Mann–Whitney U test.
These data reveal an unanticipated pathogenic role of γδ-T cells in experimental cerebral malaria that is strictly dependent on sporozoite infection.
γδ-T Cells Are Required for Neuroinflammation upon Sporozoite Infection.
To formally characterize the underlying pathology as ECM, we assessed blood–brain barrier (BBB) permeability, leukocyte infiltration, and histopathology following sporozoite infection. BBB disruption is a hallmark of ECM and has also been reported in human CM (49). While we observed a sevenfold increase in Evans blue (EB) accumulation in the brain parenchyma of infected versus naïve WT mice (P < 0.01), this did not occur in TCRδ−/− mice, which showed no evidence of BBB disruption (Fig. 1E). In addition, the brains of P. berghei ANKA-infected WT mice displayed typical ECM lesions, including multifocal hemorrhages and diffuse edema as shown by the increased volume of the organ compared with naïve mice (Fig. 1 F and G). The severity of such lesions was significantly decreased in TCRδ−/− mice, which show reduced brain enlargement/edema and decreased hemorrhage area (Fig. 1 F and G). These clear morphological differences were associated with the absence and presence of ECM signs in TCRδ−/− and WT mice, respectively. The ECM signs include hemi- or paraplegia, head deviation, tendency to roll over on stimulation, ataxia, and convulsions. Collectively, these data firmly ascribe the distinctive phenotypes of TCRδ−/− versus WT mice to ECM.
The concomitant presence of iRBCs and CD8+ αβ-T cells in the brain are necessary for ECM onset (19); and IFN-γ has been implicated as a critical immune mediator of ECM pathogenesis (28–30). While we did not observe any significant differences in parasite accumulation (as indicated by Plasmodium-specific 18S rRNA levels) in the brains of TCRδ−/− and WT mice (Fig. 1H), CD8β and IFN-γ mRNA expression levels were significantly lower in the brain of infected TCRδ−/− mice (Fig. 1 I and J). These results suggest that, upon sporozoite infection, γδ-T cells may support the migration or accumulation of inflammatory IFN-γ-producing and cytotoxic T cells in the brain.
γδ-T Cells Promote a Proinflammatory Microenvironment Without Affecting P. berghei Development in the Liver.
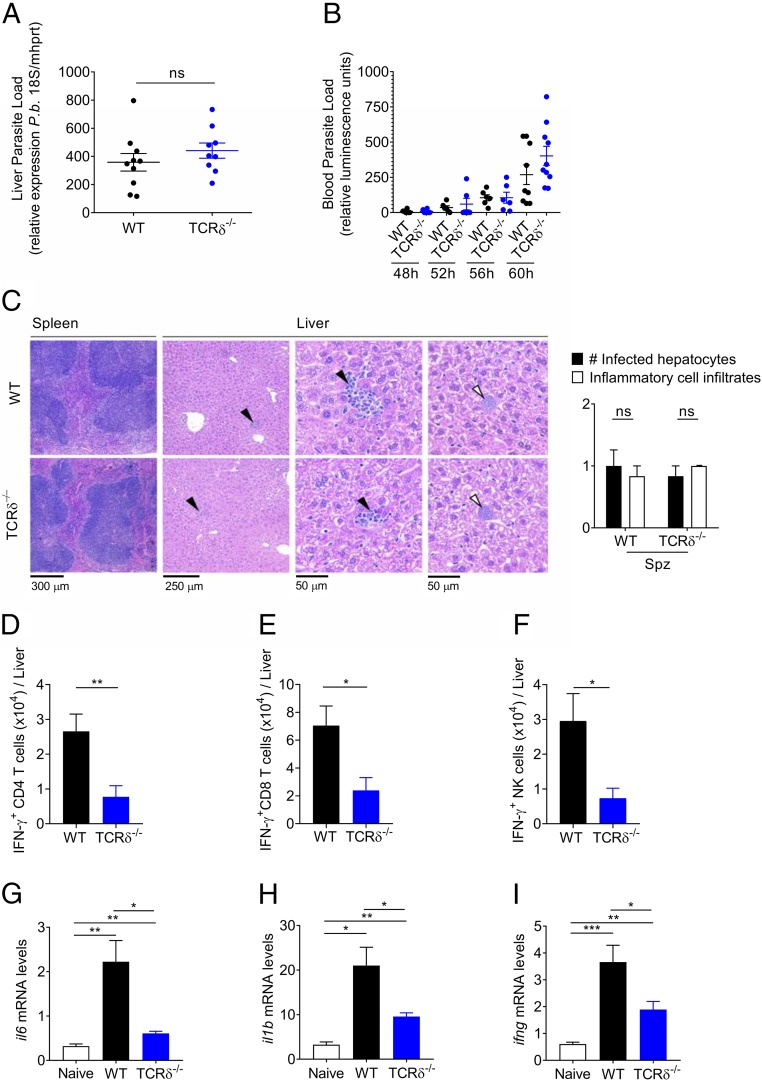
Since the pathogenic role of γδ-T cells in ECM pathogenesis seemed strictly dependent on sporozoite infection, we next assessed the impact of γδ-T cells on intrahepatic Plasmodium development, i.e., within 48 h of sporozoite infection. Interestingly, parasite levels were similar in livers of TCRδ−/− and WT mice (Fig. 2A). We next analyzed the prepatent period, i.e., the time between sporozoite inoculation and the appearance of parasites in the blood (patency), which correlates with the duration of the liver stage and the number of RBC-infective merozoites produced during this stage. Besides a nonsignificant trend at 60 h, we did not detect any significant differences in prepatency (Fig. 2B) nor in the first blood-stage replication cycles (SI Appendix, Fig. S2A) between TCRδ−/− and WT mice. Histological analyses of spleen and liver 48 h after sporozoite infection showed mild lymphoid hyperplasia of the spleen, for both TCRδ−/− and WT mice while, in the liver, the infected hepatocytes (white arrowhead) that can be identified are associated with minimal inflammatory cell infiltrates, granulocyte, and/or mononuclear cell rich (black arrowhead), with multifocal distribution (Fig. 2C). Thus, we could not find any significant morphological differences in the livers of TCRδ−/− and WT mice during Plasmodium liver-stage infection (Fig. 2C).
Fig. 2.
γδ-T cells promote a proinflammatory microenvironment in the liver without affecting P. berghei development. (A) Relative expression of parasite 18S P.b. rRNA in liver tissue of WT or TCRδ−/− mice measured by qRT-PCR at 48 h after Spz infection (n = 9–10). (B) Assessment of prepatent period at 48 h, 52 h, 56 h, and 60 h after infection with 5 × 104 luciferase P. berghei ANKA parasites using bioluminescence quantification (n = 9–10 mice per group). Each symbol in A and B represents an individual mouse. (C) Spleen and liver representative microphotographs of sections by H&E of WT or TCRδ−/− mice at 48 h after Spz infection (n = 6 mice per group). (Scale bars, 50–300 μm.) (D–F) Absolute number of IFN-γ producers among CD4+ and CD8+ T cells, and NK cells from livers of WT mice at 48 h after Spz infection (n = 6–7 mice per group). (G–I) Relative expression levels of il6, il1b, and ifng in liver tissue of WT or TCRδ−/− mice measured by qRT-PCR at 48 h after Spz infection (n = 6 mice per group). Data are represented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant; by Mann–Whitney U test.
We next assessed the underlying immune response in the liver of WT versus TCRδ−/− mice at day 2 post-Spz infection. Although there was no overt inflammatory pathology in either case (Fig. 2C), there was a clearly enhanced inflammatory microenvironment in the presence of γδ-T cells, both at the cellular (Fig. 2 D–F) and molecular (Fig. 2 G–I) levels. The percentages (SI Appendix, Fig. S2 B–D) and absolute numbers (Fig. 2 D–F) of IFN-γ producers among CD4+ and CD8+ T cells, and NK cells, were significantly reduced in TCRδ−/− compared with WT livers. Moreover, the mRNA expression levels of the inflammatory cytokines, IL-1β, IL-6, and IFN-γ, were also markedly lower in TCRδ−/− than in WT livers (Fig. 2 G–I).
Taken together, these results show that the ECM resistance of TCRδ−/− mice following Plasmodium sporozoite infection is not due to a direct impact on intrahepatic development of the parasite. Instead, our data pointed toward decreased proinflammatory responses in TCRδ−/− livers, which prompted us to assess the immune responses in the subsequent blood stage of infection.
Sporozoite Infection Induces Robust γδ-T Cell Activation That Promotes IFN-γ Responses.
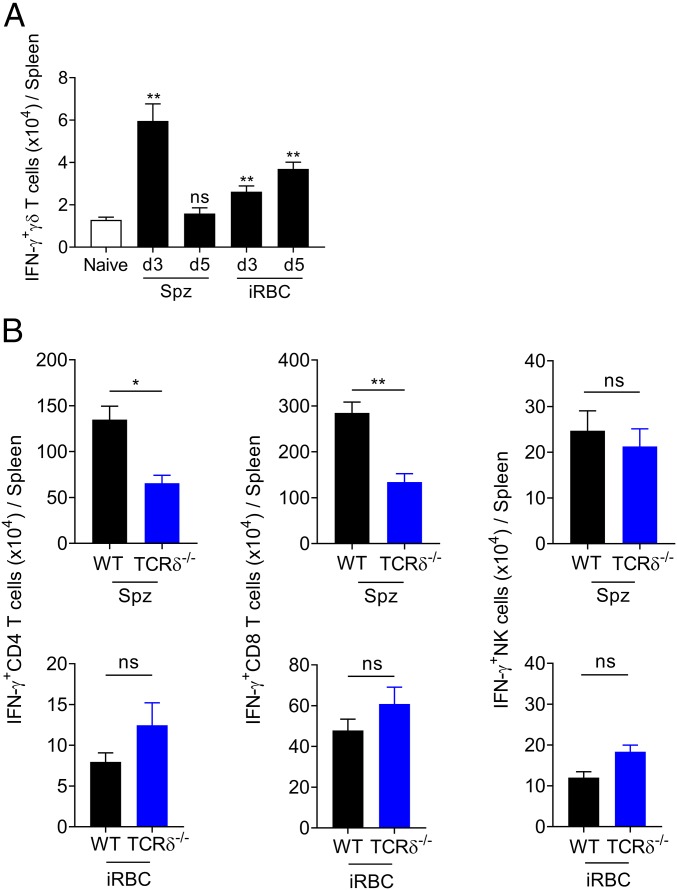
Previous studies have shown that ECM is an IFN-γ–dependent syndrome, with IFN-γ+ CD4+ T cells upstream of CD8+ T cell activation and recruitment to the brain (23, 30). To assess the impact of Spz and iRBC infection on IFN-γ responses induced in secondary lymphoid organs during the blood stage, we analyzed splenic lymphocyte subsets between days 3 and 5 after Spz or iRBC infections (Fig. 3). Besides the different kinetics of the γδ-T cell response to Spz and iRBC infection, we found maximum numbers of IFN-γ-producing γδ-T cells at day 3 post-Spz infection (Fig. 3A). These data suggest a more robust activation and effector function of γδ-T cells in response to Spz infection.
Fig. 3.
Sporozoite infection induces robust γδ-T cell activation that promotes IFN-γ responses. (A) Absolute number of splenic IFN-γ–expressing γδ-T cells at days 3 or 5 after Spz (n = 6–10 mice per group) or iRBC infection (n = 4–6 mice per group) **P < 0.01; ns, not significant; by Mann–Whitney U test compared with naïve. (B) Absolute number of splenic of IFN-γ–expressing CD4+ T cells, CD8+ T cells, and NK cells from WT and TCRδ−/− mice at day 5 after Spz and day 3 after iRBC infections (n = 5 mice per group). Data are represented as mean ± SEM. *P < 0.05; **P < 0.01; ns, not significant; by Mann–Whitney U test.
Building on the results obtained in the liver (Fig. 2 D–F), we next compared IFN-γ production by splenic αβ-T cells and NK cells following Spz or iRBC infection of WT or TCRδ−/− mice (Fig. 3B and SI Appendix, Fig. S3A). Given the 2-d delay between Spz and iRBC infection, we chose day 5 post-Spz and day 3 post-iRBC infection. We found reduced IFN-γ production by CD4+ and CD8+ T cells in TCRδ−/− mice exclusively upon Spz, but not iRBC infection (Fig. 3B). The global impact on cellular IFN-γ responses in Spz infection translated into reduced serum IFN-γ levels (SI Appendix, Fig. S3B). We further confirmed that, upon Spz infection, IFN-γ–deficient mice are completely resistant to ECM development (SI Appendix, Fig. S3 C and D), similarly to what has been observed with iRBC infection (22, 23, 30).
These results suggest that Spz infection induces a robust γδ-T cell response that promotes IFN-γ production by CD4+ and CD8+ T cells, which associates with ECM development.
γδ-T Cells and IFN-γ Modulate the Transcriptome and Pathogenicity of Liver-Derived Parasites.
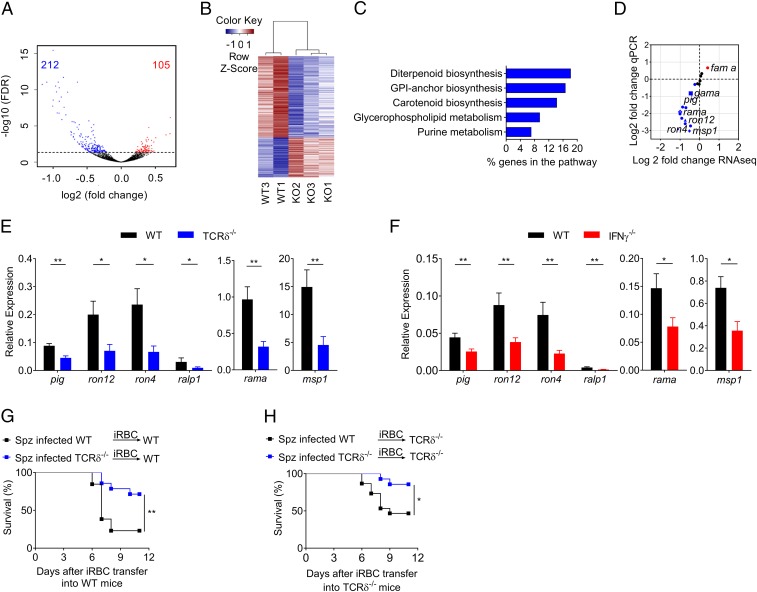
Since the relative quantity of parasites developing in the liver (Fig. 2A) and egressing to the blood (Figs. 1D and 2B and SI Appendix, Fig. S2A) was not significantly different in TCRδ−/− versus WT mice, we hypothesized that the distinctive inflammatory responses in the brain (Fig. 1 I and J) and spleen (Fig. 3) upon sporozoite infection could be due to qualitative differences between the parasites derived from the liver stage of both mouse strains. To assess this hypothesis, we performed genome-wide RNA sequencing (RNA-Seq) on liver stage-derived parasites from WT and TCRδ−/− mice, collected from the blood 74 h after sporozoite infection (Fig. 4 A and B) (50). Differential expression analysis performed using DESeq2 software revealed 317 genes differentially expressed according to statistical significance [false discovery rate (FDR) < 0.05] between WT and TCRδ−/− samples, which represents ∼6.3% of the parasite genome (Fig. 4A and SI Appendix, Table S1). A total of 212 genes were down-regulated while 105 genes were up-regulated in TCRδ−/− liver stage-derived parasites compared with WT liver stage-derived parasites (Fig. 4A and SI Appendix, Table S1). This suggests that the presence of γδ-T cells creates a liver microenvironment that modulates the expression of several P. berghei ANKA genes. A pathway enrichment analysis of the down-regulated genes in TCRδ−/− liver stage-derived parasites using the metabolic pathway enrichment analysis tool from the PlasmoDB website (Plasmodium Genomics Resource) revealed a significant enrichment for several parasite metabolic pathways. These include the diterpenoid biosynthetic pathway that makes dolichols, which are essential molecules for production of glycophosphatidylinositol (GPI) anchors (51), and the GPI-anchor biosynthetic pathway (Fig. 4C and SI Appendix, Table S2). Moreover, Gene ontology (GO) enrichment analysis of down-regulated genes, which identifies terms over- (or under)-represented using annotations from the gene set, showed that host cell surface receptor binding was one of the “molecular component” terms with the highest fold enrichment (SI Appendix, Table S3). Among the down-regulated genes in TCRδ−/− liver stage-derived parasites, we found four genes encoding for GPI-anchored proteins, rhoptry-associated membrane antigen (RAMA) [PBANKA_0804500], Apical Membrane Antigen 1 (AMA1) [PBANKA_091500], glycosylphosphatidylinositol-anchored micronemal antigen (GAMA) [PBANKA_070190], and merozoite surface protein 1, (MSP1) [PBANKA_0831000] (SI Appendix, Table S1). Furthermore, several genes encoding for rhoptry neck proteins (RONs), such as RON12 [PBANKA_0501400], RON4 [PBANKA_0932000], and Rhoptry-associated, leucine zipper-like protein 1 (RALP1) [PBANKA_0619700], were also significantly down-regulated (FDR < 0.05) (SI Appendix, Table S1). To validate this unanticipated set of RNA-Seq data, 19 differentially expressed genes were selected and quantified by qRT-PCR. For this set of genes, a positive correlation was observed between the RNA-Seq and qRT-PCR results [Pearson’s correlation index (r = 0.86)] (Fig. 4D). The mRNA expression levels of RON12, RON4, RALP1, RAMA, and MSP1, as well as phosphatidylinositol N-acetylglucosaminyltransferase, putative (PIG) [PBANKA_0812100], an enzyme involved in the GPI biosynthetic pathway, were significantly lower in TCRδ−/− liver stage-derived parasites (compared with WT), both determined by RNA-Seq and qRT-PCR (Fig. 4D). Additionally, the mRNA expression levels of these genes, PIG, RON12, RON4, RALP1, RAMA, and MSP1, encoding invasion-related merozoite proteins and GPI-anchored proteins were validated in independent samples (Fig. 4E). Given the reduced levels of IFN-γ in TCRδ−/− livers (compared with WT) (Fig. 2I) and the key role played by IFN-γ in ECM pathogenesis upon sporozoite infection (SI Appendix, Fig. S3 B–D), we assessed the mRNA expression levels of these Plasmodium genes in liver stage-derived parasites from IFN-γ−/− mice (Fig. 4F). Importantly, the transcriptional changes observed in liver stage-derived parasites from TCRδ−/− mice were fully reproduced in liver stage-derived parasites from IFN-γ−/− mice (Fig. 4 E and G), thus associating IFN-γ production with the transcriptional modulation of Plasmodium parasites by γδ-T cells.
Fig. 4.
γδ-T cells and IFN-γ modulate the transcriptome and pathogenicity of liver-derived parasites. (A) Volcano plot of log2 fold change of gene expression in parasites collected from Spz-infected TCRδ−/− versus WT mice against statistical significance of change (−log10 P value adjusted). Red and blue colors indicate transcripts up-regulated or down-regulated in parasites collected from Spz-infected TCRδ−/− mice (compared with WT mice), respectively. The number of genes differentially expressed (FDR < 0.05) is indicated on the plot. (B) Heatmap of relative gene expression for differentially expressed genes. Samples are clustered by Pearson’s correlation. (C) Metabolic pathway enrichment (Kyoto Encyclopedia of Genes and Genomes) analysis of the 212 down-regulated genes. The graph shows the percentage of the background genes altered in the RNA-Seq analysis for Benjamini–Hochberg (FDR < 0.05). (D) Scatterplot of fold changes of gene expression in parasites collected from Spz-infected TCRδ−/− versus WT mice. Shown is the correlation between the RNA-Seq versus qRT-PCR measurements, Pearson’s correlation index (r = 0.86). (E and F) Relative gene expression analysis of significantly down-regulated pig, ron12, ron4, ralp1, rama, and msp1 genes, (FDR < 0.05, fold change > 1.5) in parasites collected from (E) Spz-infected TCRδ−/− and from (F) Spz-infected IFNγ−/−, compared with WT mice (n = 5–9 mice per group). (G and H) Survival curves for WT and TCRδ−/− mice infected with 1 × 106 P. berghei ANKA GFP iRBC collected from WT and TCRδ−/− infected previously with 2 × 104 P. berghei ANKA GFP Spz (n = 13–16 mice per group). Data are represented as mean ± SEM. *P < 0.05; **P < 0.01; by Mann–Whitney U and Log-rank (Mantel–Cox) tests.
Finally, to functionally demonstrate the impact of such modulation in ECM pathogenesis, we performed adoptive transfer experiments of infected RBCs collected upon parasites’ egress from the liver. Thus, we infected naïve WT and TCRδ−/− mice with iRBCs taken from sporozoite-infected TCRδ−/− or WT mice and followed ECM development (Fig. 4 G and H). Consistent with our hypothesis, we observed that parasites obtained from TCRδ−/− donors were less pathogenic (as attested by higher survival rates) than those derived from WT donors (Fig. 4 G and H). Interestingly, TCRδ−/− recipient mice (Fig. 4H) showed higher survival rates than WT hosts (Fig. 4G), thus suggesting an additional contribution of host γδ-T cells to immunopathology.
Collectively, these results place γδ-T cells and IFN-γ at the core of liver stage-dependent modulation of parasite virulence, immunopathology, and ECM development.
Discussion
The impact of liver stage in the erythrocytic-stage of Plasmodium infection and in cerebral malaria pathogenesis has been widely neglected. Very few studies have addressed the potential of an early inflammatory response upon sporozoite infection in modulating downstream effector responses and consequently disease outcome during the erythrocytic stage. Previous studies have shown that preerythrocytic or early immune responses may modulate downstream host immune responses and reduce ECM development or clinical symptoms, respectively, in mice and in humans (52–55) or exacerbate symptoms in clinical malaria (9). Our study contributes to a better understanding of the key role of γδ-T cells in promoting the critical IFN-γ response upon sporozoite infection, which impacts in the immunogenicity of liver-derived parasites, blood-stage inflammation, and, ultimately, the development of ECM.
It has been described that IFN-γ can play different roles during P. berghei infection and depending on the time that is produced during infection it may be an important modulator of the developing immune responses and consequently disease outcome (56, 57). Previous studies have shown that the IFN-γ produced at 24 h after iRBC infection correlates with protection, whereas later production of IFN-γ during infection is essential for the development of ECM (56–58). Our study using sporozoite infection now demonstrates that the IFN-γ response is critical during the liver stage: IFN-γ modulates (likely through an effect on hepatocytes that remains to be dissected) the parasite transcriptome in the liver; and its immunogenic potential drives blood-stage inflammation, culminating in ECM development.
Our data further show that sporozoite infection induced a robust IFN-γ response by γδ-T cells that seemingly impacted on later αβ-T cell-mediated IFN-γ responses. In fact, previous studies have shown that IFN-γ is essential for effector (Th1) CD4+ T cell differentiation; and adoptively transferred CD4+ T cells, actively making IFN-γ, induced (likely via CXCL9 and CXCL10 induction) CD8+ T cell accumulation in the brain and drove ECM development (23).
The diverse innate-like and adaptive-like functions of γδ-T cells may suggest a role for these cells in the pathogenesis of ECM in a time-dependent manner (59). In fact in a previous study, mice depleted of γδ-T cells by monoclonal antibody at early time points, days 0 and 3 after infection with P. berghei iRBCs, were protected from ECM, whereas depletion at day 5 failed to protect from ECM (37). On the other hand, >50% of TCRδ−/− mice developed ECM upon iRBC infection. In our study, essentially all TCRδ−/− mice developed ECM following iRBC infection, in stark contrast with the high degree of protection observed after sporozoite infection.
We found that during the intrahepatic development of the parasite, the absence of γδ-T cells results in a less proinflammatory microenvironment that prevented ECM development, without any significant effect on parasite development or delay in prepatency. These findings suggest an increased parasite virulence and immunopathology independently of parasite growth rate or load.
It has been known for decades that mosquito transmission resets Plasmodium virulence and serial blood passage of Plasmodium through rodents, primates, or humans, increases parasite virulence and disease severity (60–62). Noteworthy, recent studies have shown that differences in gene expression between blood and mosquito-passaged parasites have an impact in parasite virulence and host immune responses (62–64).
Our transcriptional analyses of parasites developing in the presence or absence of γδ-T cells following sporozoite infection pointed toward several surface and microneme merozoite GPI-anchored proteins, such as MSP1 and RAMA, that have been considered potential targets of host immune cells during the invasion/rupture process of the infected red blood cells, inducing proinflammatory responses by immune cells that drive malaria pathogenesis and thus impact in the outcome of malaria infection (65–68). MSP1 is the most abundant merozoite surface protein and is thought to be involved in the initial attachment of the merozoite to the erythrocyte surface (69), while RAMA and GAMA, which are localized in the parasite secretory organelles (rhoptries and micronemes) during schizont development, then move to the merozoite surface before or during red blood cell invasion (70). Several of such surface proteins are cleaved and shed from the surface of the merozoite during the invasion process, that together with other parasite factors released during the rupture/invasion of host red blood cells may exert major immunomodulatory effects and influence the type of immune response and the severity of malaria infection (68). Moreover, several of these proteins have been proposed as potential candidates for inclusion in a multivalent subunit vaccine against malaria (71–73). Our data suggest that, upon sporozoite infection, γδ-T cells promote an IFN-γ–rich inflammatory microenvironment that modulates the expression levels of such immunogenic Plasmodium factors. Importantly, these observations seem to suggest that infection-induced immunopathology may impact in parasite gene expression and together have the potential to shape virulence (74, 75).
Our findings that the immune response induced by sporozoite infection is different from that elicited by iRBC infection highlight the importance of using the natural route of infection to study the pathogenesis of ECM (52, 54). However, our results may greatly contribute to our understanding of clinical immunity in humans. During primary P. falciparum or Plasmodium vivax infections, the major γδ-T cell subset in the peripheral blood, Vγ9Vδ2 T cells, can sense parasite-derived nonpeptidic phosphoantigens (76) and undergo striking polyclonal expansions (77). These cells produce high amounts of IFN-γ and TNF-α (36, 55), and can directly kill blood-stage merozoites (10). However, given the potent inflammatory effects of IFN-γ and TNF-α, Vγ9Vδ2 T cells may well contribute to severe malaria symptoms. In fact, it was suggested almost 25 y ago (8), and substantiated more recently, that Vγ9Vδ2 T cell loss/dysfunction underlies the acquisition of clinical immunity in malaria endemic areas (4).
Our murine data provide mechanistic insight into how γδ-T cells and IFN-γ drive ECM pathogenesis. Critically, the γδ-T cell contribution is strictly dependent on the liver stage of infection, which has been widely neglected in CM pathogenesis. This also highlights the importance of conducting research in animal models of severe malaria, since the dissection of liver-stage versus blood-stage phenomena would be particularly challenging in humans.
Overall, we believe our study constitutes an important step toward understanding the role of γδ-T cells in the pathogenesis of Plasmodium infection, which has major implications in disease control in endemic areas. While the attempt for a breakthrough in vaccination strategies is crucial since it may provide sterile immunity to Plasmodium, we should nurture the perspective of achieving clinical tolerance to malaria. In this context, clarifying the potential dual role of γδ-T cells in protection versus pathogenesis may provide new targets for intervention toward achieving host–parasite equilibrium without the complications of cerebral malaria.
Materials and Methods
Ethics Statements.
All animal care and experimental procedures were performed in accordance with European Union (EU) Directive 2010/63/EU, approved by the Animal Ethics Committee of Instituto de Medicina Molecular (IMM) (AEC_2011_10_AP_GDMalaria-IMM; AEC_2015_02_BSS_MicroRNA), following the Federation of European Laboratory Animal Science Associations (FELASA) guidelines and recommendations concerning laboratory animal welfare.
Mice.
C57BL/6J WT mice were obtained from Charles River Laboratories International Inc. and from specific pathogen-free facilities at the IMM. Tcr delta-deficient mice (Tcrdtm1Mom) (TCRδ−/−) (RRID:IMSR_JAX:002120), backcrossed more than 10 in C57BL/6 mice background, were originally obtained from The Jackson Laboratory and were bred in specific pathogen-free facilities at the IMM. Mice deficient for IFN-γ (B6.129S7-Ifngtm1Ts/J) (IFNγ−/−) (RRID:IMSR_JAX:002287) were obtained from The Jackson Laboratory. Six- to 12-wk old, male mice (sex matched in each experiment) were used throughout the experiments.
Plasmodium Infections and Disease Assessment.
For sporozoite and mosquito-bite infections, the complete life cycle of P. berghei ANKA was maintained by passage through naïve mice and Anopheles stephensi mosquitoes bred at the IMM insectarium. Mosquitoes infected with P. berghei ANKA expressing GFP (259cl2) (78) and luciferase (676m1cl1) (79) were generated and used throughout this study. For mosquito-bite infections, experimental mice were anesthetized and subjected to the bite of 7–10 P. berghei -GFP infected mosquitoes. For sporozoite infection, 2 × 104, 5 × 104, or 2 × 105 sporozoites obtained from the salivary glands of freshly dissected mosquitoes were injected i.v. For iRBC-initiated infection, cryopreserved (GFP)-expressing P. berghei parasites were passed once through C57BL/6 mice before being used to infect experimental animals. Experimental mice were infected by i.p. injection of 1 × 106 or 1 × 105 infected RBCs. In adoptive transfer experiments, donor mice were infected through i.v. injections of 2 × 104 (GFP)-expressing P. berghei sporozoites and 74 h after iRBCs were collected and used to infect recipient mice with 1 × 106 iRBCs through i.p. injection. Parasitemia was assessed by flow cytometry for mice infected with GFP-expressing P. berghei parasites, using tail blood, as previously described (78), and expressed as percentage of infected red blood cells. Survival is expressed as percentage. All mice were monitored daily for signs of ECM including hemi- or paraplegia, head deviation, tendency to roll over on stimulation, ataxia, and convulsions. To evaluate the signs of experimental cerebral malaria, we used a classification from 0 to 4 (80). Briefly, stage 0 indicates no detectable clinical symptoms; stage 1, ruffled fur; stage 2, ruffled fur and unbalancing; stage 3, motor impairment and respiratory distress; and stage 4, convulsions and/or coma (80).
BBB Permeability.
We assessed the BBB permeability using EB dye as described before (81). Briefly, mice were injected i.v. with 0.2 mL of PBS 1% EB dye (Sigma) when ECM signs were observed in control WT mice. Mice were killed 1 h after EB injection, perfused with 20 mL of PBS (1×), and brains were weighed and placed in 2 mL formamide (Merck) for 24 h at 37 °C to extract EB dye from the tissue. Absorbance was measured at λ = 620 nm (EB absorbance) and 740 nm (background) and compared with an EB standard curve in a multimode microplate reader (Infinite M200; Tecan). EB concentration was calculated using a standard curve of EB dye. The data are expressed as milligrams of EB per gram of brain tissue.
Hematoxylin and Eosin Staining.
Control WT and TCRδ−/− mice were killed with anesthetic overdose 48 h after sporozoites infection or when displaying overt signs of ECM. The head was harvested, formalin fixed, and decalcified with Calciclear. Grossly, coronal sections were performed at the level of the olfactory bulb (approximately at bregma 3.92) and caudal hippocampus (approximately at bregma −3.40) and processed for paraffin embedding, stained with hematoxylin and eosin (H&E), and examined by a pathologist blinded to experimental groups. Counting and measurement of the number and maximal diameter of hemorrhages in coronal sections of the brain, at the level of the olfactory bulb and posterior hippocampus, were performed using NDP.view2 software (Hamamatsu) in slides digitally scanned in the Hamamatsu NanoZoomerSQ.
Cell Preparation and Flow Cytometry.
Mice were killed and perfused with 20 mL PBS, and liver and spleen were removed from naïve and infected mice, on the indicated day after infection. Briefly, the liver and spleen were pressed through a nylon mesh cell strainer and suspended in RPMI medium. After one washing, the dissociated liver tissue was resuspended in 33% (vol/vol) Percoll and centrifuged at 800 × g for 25 min at room temperature. The pellet was resuspended in ammonium chloride/potassium (ACK) buffer to lyse erythrocytes and washed twice in 3% FCS-RPMI before counting. Collected mononuclear cells were counted using a standard hemocytometer and plated into a v-bottom 96-well plate for subsequent antibody staining for multicolor flow cytometry. The following antibodies (eBioscience) were used: Alexa Fluor 488-labeled anti–IL-17 (eBio17B7) (RRID: AB_763579); PE-labeled anti-TCRδ (eBioGL3) (RRID: AB_465934); PerCP-Cy5.5–labeled anti-CD3ε (145.2C11) (RRID: AB_1107000); PE-Cy7–labeled anti-NK1.1 (PK136) (RRID:AB_469664); Alexa Fluor 647-labeled anti–IFN-γ (XMG1.2) (RRID:AB_10393003); APC-eFluor 780-labeled anti-CD8 (SK1) (RRID: AB_1272185); and eFluor 450-labeled anti-CD4 (RM4-5) (RRID:AB_1272194). For intracellular, cells were stimulated with PMA (50 ng/mL) and ionomycin (1 μg/mL) (both from Sigma) for 4 h at 37 °C; 10 μg/mL Brefeldin A (Sigma) was added during the last 2 h. Cells were stained for the indicated cell surface markers, and intracellular staining was performed using fixation/permeabilization and permeabilization buffers (both from eBioscience), following the manufacturer’s instructions. Cells were analyzed on a FACS Fortessa (BD Biosciences) and using FlowJo (TreeStar) software (RRID:SCR_000410).
Plasmodium RNA Preparation.
C57BL/6J mice were infected with i.v. inoculation of 2 × 104 GFP-expressing P. berghei ANKA sporozoites purified from mosquito salivary glands. Parasites were isolated at exactly 74 h after sporozoites infection, at the late trophozoite stage of development. Briefly, whole blood was depleted of leukocytes by Plasmodipur filtration (EuroProxima); erythrocytes were centrifuged at 400 × g for 10 min and lysed with 0.15% (wt/vol) saponin (Sigma); samples were centrifuged at 1,000 × g for 5 min and washed with PBS; parasites were resuspended in TRIzol (Life Technologies) and snap-frozen on dry ice. We prepared three biological replicates of P. berghei ANKA sporozoites infection from five mice each. RNA was extracted as described (82), resuspended in water, and DNA removed with a TURBO DNA-Free kit (Applied Biosystems), according to the manufacturer’s instructions. RNA quantity/quality was determined on an Agilent 2100 Bioanalyzer RNA 6000 Nano chip.
cDNA Production and Real-Time PCR.
To assess gene expression in the liver and brain, mice were killed at 48 h after sporozoites infection and when infected control mice presented signs of ECM, respectively. The mice were perfused intracardially with PBS to remove circulating RBCs and leukocytes from the organs. RNA was isolated from brains and livers using TRIzol reagent (Invitrogen, Life Technologies), according to the manufacturer’s recommendation. The synthesis of the first-strand cDNA from the RNA templates was performed with random oligonucleotides (Invitrogen) using Moloney murine leukemia virus reverse transcriptase (Promega). qRT-PCR reactions were performed in the presence of SYBER Green (SYBER Green PCR master mix; Applied Biosystems) on a ViiA7 cycler (Applied Biosystems). Primers were either designed manually or by the Universal ProbeLibrary Assay Design Center (Roche). The transcripts of the genes of interest were amplified in parallel with housekeeping genes [hypoxanthine phosphoribosyltransferase (mhprt)] for mice transcripts and DNA/RNA-binding protein Alba 2, putative (PBANKA_13529200) and serine-tRNA ligase, putative (PBANKA_0615400) for Plasmodium transcripts in a final volume of 10 μL in 384-well plates (Applied Biosystems). Oligonucleotides used for the specific amplification of mouse genes were as follows:
hprt 5′-GTTGGATACAGGCCAGACTTTGTTG-3′ (forward) and 5′-GATTCAACCTTGCGCTCATCTTAGGC-3′ (reverse); Pb 18S 5′-AAGCATTAAATAAAGCGAATACATCCTTAC-3′ (forward) and 5′-GGAGATTGGTTTTGACGTTTATGTG-3′ (reverse); cd8b 5′TGCTCGAGATGTGATGAAGG-3′ (forward) and 5′-TCCCCTGTTGACTGGTCATT-3′ (reverse); ifng 5′-CACACTGCATCTTGGCTTTG-3′ (forward) and 5′-TCTGGCTCTGCAGGATTTTC-3′ (reverse); il-6 5′-TTCTTGGGACTGATGCTGGT-3′ (forward) and 5′-TCTGCAAGTGCATCATCGTT-3′ (reverse); il-1b 5′-CGGACCCCAAAAGATGAAGG-3′ (forward) and 5′-GCCACAGCTTCTCCACAGCCA-3′ (reverse); tnfa 5′-AATGGCCTCCCTCTCATCAGTT-3′ (forward) and 5′-CCACTTGGTGGTTTGCTACGA-3′ (reverse). Oligonucleotides used for the specific amplification of parasite genes: gama [PBANKA_070190] 5′-CAAATGGTGAGGAAACTGATGC-3′ (forward) and 5′-GAAAGCAAAGCGAATAAGGC-3′ (reverse); pig [PBANKA_081210] 5′-AACCCAAACTCACAGCGATGAC-3′ (forward) and 5′-GCAAAGCAGGAAGGAAAAAACC-3′; ron 12 [PBANKA_050140] 5′-AGGTAAGGAACAATCCCC-3′ (forward) and 5′-GAACTATCAAGAGTGCTTTCG-3′ (reverse); ron4 [PBANKA_093200] 5′-GAAGGGGACCAAAGCAACATAAAC-3′ (forward) and 5′-GCAGAAGCATTTCCAAAAGCG-3′ (reverse); rama [PBANKA_080450] 5′-TCAGTGAAAACATCGTTGGC-3′ (forward) and 5′-CATCATAATAATCCTTGTGGGC-3′ (reverse); ralp1 [PBANKA_061970] 5′-AATGAAAATGGAAATCCTGAAAG-3′ and 5′-TTGAAGTAACTCCCCTTGTCC-3′; msp1 [PBANKA_083100] 5′-CCCAAATCATACGGTAATGGTGG-3′ (forward) and 5′-TCCAGTAACTACAGGCACTTGTTCC-3′ (reverse); ama1 [PBANKA_091500]_ 5′-TAGACACCCCGCTGTTTATG-3′ (forward) and 5′-GGACAACTGGTTTCCCAATC-3′ (reverse); DNA/RNA-binding protein Alba 2, putative (alba2) [PBANKA_1359200] 5′-GGGTTGGTAAAGCCATAAGC-3′ (forward) and 5′-CATCTCCTGATTTTTTGTCGTC-3′ (reverse); serine–tRNA ligase, putative [PBANKA_061540] 5′-ATTGCTCAACCTTATCAAACTG (forward) and 5′-AGCCACATCTGAACAACCG-3′ (reverse). The relative changes in gene expression between experimental and control groups were calculated by the comparative CT method (ΔΔCT method) using hprt as internal control gene when analyzing the parasite Pb 18S and mouse genes and DNA/RNA-binding protein Alba 2, putative (alba2) and serine-tRNA ligase, putative as reference genes when normalizing parasite genes.
Bioluminescence Assay.
Plasmodium liver-stage infection was established by i.v. through retroorbital injection of luciferase-expressing P. berghei ANKA sporozoites. At the selected time points, 1 μL of blood was collected from the tail vein into 50 μL of lysis buffer. Luminescence was determined by adding 50 μL of d-luciferin dissolved in Firefly luciferase assay buffer (according to the manufacturer’s instructions) to 30 μL of lysate and immediately measured using a multiplate reader (Tecan) (83). Values of luciferase activity are expressed as relative luminescence units (RLUs).
Genome-Wide RNA Sequencing.
Gene expression analysis was determined by RNA sequencing of parasites collected from the blood at 74 h after infection of TCRδ−/− versus WT mice with 2 × 104 P. berghei ANKA sporozoites (samples were pooled from three mice per group). Barcoded stranded mRNA-seq libraries were prepared using the Illumina TruSeq kit implemented on the liquid handling robot Beckman FXP2. Obtained libraries were pooled in equimolar amounts and loaded on the sequencer HiSEq 2000 and sequenced bidirectionally, 100 bases from each end. The six samples were sequenced on one lane of HiSEq 2000, yielding ∼60 million reads per sample.
RNA-Seq Data Analysis.
Quality control of the raw sequence data from all of the samples was performed using FastQC High Throughput Sequence QC Report version 0.11.4. Raw reads were first mapped to the Mus musculus reference genome (GRCm38/mm10) using TopHat2 with default parameters. As expected, the majority of reads in these samples originated from mouse transcripts, although 8–14% of reads mapped uniquely to the P. berghei ANKA genome. Reads that failed to align to the mouse genome were then mapped to the P. berghei ANKA reference genome (October 2016, version 29) downloaded from PlasmoDB using TopHat version 2.0.13 with default parameters. Counts of mapped reads in exons were obtained from the resulting BAM files using feature Counts version 1.5.2 (84). Clustering analysis was done on read counts normalized for library size (DESeq2) using the R software environment. The clustering analysis of gene expression profiles revealed one of the samples to be a clear outlier displaying a skewed distribution of read counts and was discarded from further analysis (SI Appendix, Fig. S4). For the remaining samples, Pearson’s correlation coefficients were calculated using the counts of reads normalized by DESeq2 which showed a high level of similarity between the replicate samples collected from WT and TCRδ−/− (KO) mice (SI Appendix, Fig. S4). Differential expression analysis was performed using DESeq2 version 1.12.14 (85) using two replicates of WT and three replicates of KO. The heatmap (Fig. 4B) was done with the function heatmap.2 from the R package gplots (86). The genes up- and down-regulated with FDR <0.05 (Benjamini–Hochberg) were used for pathway and gene ontology (GO) enrichment analysis using the PlasmoDB enrichment tools.
Functional Enrichment Analysis.
GO terms and metabolic pathway enrichment analysis were performed with PlasmoDB (https://plasmodb.org/plasmo/) (RRID:SCR_013331) which sources GO information from Interpro (www.ebi.ac.uk/interpro/) (RRID:SCR_006695) and the annotation center. A FDR cutoff was set to 0.05 (SI Appendix, Tables S2 and S3).
Statistics.
Survival curves were compared by a Log-rank (Mantel–Cox) test. Statistical significance for remaining comparisons was analyzed by Mann–Whitney U tests (GraphPad Prism 5) (RRID:SCR_002798). Differences between experimental groups were considered significant at *P < 0.05, **P < 0.01, ***P < 0.001, or ****P < 0.0001. For the statistical analysis of RNA-Seq data, see RNA-Seq methods above. Data are represented as mean ± SE of mean (SEM).
Supplementary Material
Acknowledgments
We thank Daniel J. Pennington and Ana M. Vigário for important scientific discussions; Ana Parreira for P. berghei ANKA Anopheles stephensi mosquitoes and sporozoite production; Ana C. Pena, Ana R. Grosso, João Vieira, and Natacha Sousa (all from IMM) for technical and experimental advice; the staff of IMM rodent and flow cytometry facilities and the histology unit for technical assistance; and the European Molecular Biology Laboratory Genomics Core Facility (GeneCore) for performing the transcriptome sequencing (RNA-Seq). This work was supported by Fundação para a Ciência e a Tecnologia (FCT) (PTDC/SAU-OSM/099724/2008 to A.P.) and European Research Council (CoG_646701 to B.S.-S.). We also acknowledge UID/BIM/50005/2019, a project funded by Fundação para a Ciência e a Tecnologia (FCT)/Ministério da Ciência, Tecnologia e Ensino Superior (MCTES) through Fundos do Orçamento de Estado. A.P. was supported by a Ciência 2008 position of the Portuguese Ministry of Science and Technology, supported by a FCT fellowship (SFRH/BPD/110380/2015), and currently holds a research position supported by FCT (under decree-law no. 57/2016 of July 19th, as amended by law no. 57/2017). J.C.R., R.N., V.Z.-L., L.M.-S., and S.M. were supported by individual fellowships from FCT and European Molecular Biology Organization Long-Term Fellowships (IF/00013/2014, SFRH/BI/51054/2010, SFRH/BPD/81953/2011, ALTF 357-2009 and BPD-81953-2011, ALTF 960-2009, and PD/BD/114099/2015, respectively). L.M.-S. was also supported by the European Community’s Seventh Framework Programme (FP7/2007-2013) under Grant Agreement 242095 (EVIMalaR). Currently V.Z.-L. holds a research position supported by FCT (under decree-law no. 57/2016 of July 19th, as amended by law no. 57/2017), and J.C.R. holds a FCT investigator position (IF/00013/2014).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The RNA-seq data reported in this paper have been deposited in the Array Express database at European Molecular Biology Laboratory-European Bioinformatics Institute, https://www.ebi.ac.uk/arrayexpress (accession no. E-MTAB-6493).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1814440116/-/DCSupplemental.
References
- 1.World Health Organization . World Malaria Report 2018. World Health Organization; Geneva: 2018. [Google Scholar]
- 2.White NJ, et al. Malaria. Lancet. 2014;383:723–735. doi: 10.1016/S0140-6736(13)60024-0. [DOI] [PubMed] [Google Scholar]
- 3.Marsh K. Malaria–A neglected disease? Parasitology. 1992;104(Suppl 1):S53–S69. doi: 10.1017/s0031182000075247. [DOI] [PubMed] [Google Scholar]
- 4.Jagannathan P, et al. Loss and dysfunction of Vδ2+ γδ T cells are associated with clinical tolerance to malaria. Sci Transl Med. 2014;6:251ra117. doi: 10.1126/scitranslmed.3009793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho M, Webster HK, Tongtawe P, Pattanapanyasat K, Weidanz WP. Increased gamma delta T cells in acute Plasmodium falciparum malaria. Immunol Lett. 1990;25:139–141. doi: 10.1016/0165-2478(90)90105-y. [DOI] [PubMed] [Google Scholar]
- 6.Roussilhon C, Agrapart M, Ballet JJ, Bensussan A. T lymphocytes bearing the gamma delta T cell receptor in patients with acute Plasmodium falciparum malaria. J Infect Dis. 1990;162:283–285. doi: 10.1093/infdis/162.1.283-a. [DOI] [PubMed] [Google Scholar]
- 7.Roussilhon C, et al. Human TcR gamma delta+ lymphocyte response on primary exposure to Plasmodium falciparum. Clin Exp Immunol. 1994;95:91–97. doi: 10.1111/j.1365-2249.1994.tb06020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodier M, et al. Gamma delta T cells in the peripheral blood of individuals from an area of holoendemic Plasmodium falciparum transmission. Trans R Soc Trop Med Hyg. 1993;87:692–696. doi: 10.1016/0035-9203(93)90299-6. [DOI] [PubMed] [Google Scholar]
- 9.Stanisic DI, et al. γδ T cells and CD14+ monocytes are predominant cellular sources of cytokines and chemokines associated with severe malaria. J Infect Dis. 2014;210:295–305. doi: 10.1093/infdis/jiu083. [DOI] [PubMed] [Google Scholar]
- 10.Costa G, et al. Control of Plasmodium falciparum erythrocytic cycle: γδ T cells target the red blood cell-invasive merozoites. Blood. 2011;118:6952–6962. doi: 10.1182/blood-2011-08-376111. [DOI] [PubMed] [Google Scholar]
- 11.Farouk SE, Mincheva-Nilsson L, Krensky AM, Dieli F, Troye-Blomberg M. Gamma delta T cells inhibit in vitro growth of the asexual blood stages of Plasmodium falciparum by a granule exocytosis-dependent cytotoxic pathway that requires granulysin. Eur J Immunol. 2004;34:2248–2256. doi: 10.1002/eji.200424861. [DOI] [PubMed] [Google Scholar]
- 12.Elloso MM, van der Heyde HC, vande Waa JA, Manning DD, Weidanz WP. Inhibition of Plasmodium falciparum in vitro by human gamma delta T cells. J Immunol. 1994;153:1187–1194. [PubMed] [Google Scholar]
- 13.Mamedov MR, et al. A macrophage colony-stimulating-factor-producing γδ T cell subset prevents malarial parasitemic recurrence. Immunity. 2018;48:350–363.e7. doi: 10.1016/j.immuni.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howard J, et al. The antigen-presenting potential of Vγ9Vδ2 T-cells during Plasmodium falciparum blood-stage infection. J Infect Dis. 2017;215:1569–1579. doi: 10.1093/infdis/jix149. [DOI] [PubMed] [Google Scholar]
- 15.Inoue S, et al. Enhancement of dendritic cell activation via CD40 ligand-expressing γδ T cells is responsible for protective immunity to Plasmodium parasites. Proc Natl Acad Sci USA. 2012;109:12129–12134. doi: 10.1073/pnas.1204480109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaidi I, et al. γδ T cells are required for the induction of sterile immunity during irradiated sporozoite vaccinations. J Immunol. 2017;199:3781–3788. doi: 10.4049/jimmunol.1700314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stephens R, Culleton RL, Lamb TJ. The contribution of Plasmodium chabaudi to our understanding of malaria. Trends Parasitol. 2012;28:73–82. doi: 10.1016/j.pt.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunt NH, et al. Cerebral malaria: Gamma-interferon redux. Front Cell Infect Microbiol. 2014;4:113. doi: 10.3389/fcimb.2014.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baptista FG, et al. Accumulation of Plasmodium berghei-infected red blood cells in the brain is crucial for the development of cerebral malaria in mice. Infect Immun. 2010;78:4033–4039. doi: 10.1128/IAI.00079-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacPherson GG, Warrell MJ, White NJ, Looareesuwan S, Warrell DA. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 21.Ponsford MJ, et al. Sequestration and microvascular congestion are associated with coma in human cerebral malaria. J Infect Dis. 2012;205:663–671, and erratum (2012) 206:1483. doi: 10.1093/infdis/jir812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yañez DM, Manning DD, Cooley AJ, Weidanz WP, van der Heyde HC. Participation of lymphocyte subpopulations in the pathogenesis of experimental murine cerebral malaria. J Immunol. 1996;157:1620–1624. [PubMed] [Google Scholar]
- 23.Villegas-Mendez A, et al. IFN-γ-producing CD4+ T cells promote experimental cerebral malaria by modulating CD8+ T cell accumulation within the brain. J Immunol. 2012;189:968–979. doi: 10.4049/jimmunol.1200688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belnoue E, et al. On the pathogenic role of brain-sequestered alphabeta CD8+ T cells in experimental cerebral malaria. J Immunol. 2002;169:6369–6375. doi: 10.4049/jimmunol.169.11.6369. [DOI] [PubMed] [Google Scholar]
- 25.Claser C, et al. CD8+ T cells and IFN-γ mediate the time-dependent accumulation of infected red blood cells in deep organs during experimental cerebral malaria. PLoS One. 2011;6:e18720. doi: 10.1371/journal.pone.0018720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rénia L, et al. Pathogenic T cells in cerebral malaria. Int J Parasitol. 2006;36:547–554. doi: 10.1016/j.ijpara.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Waki S, et al. The role of T cells in pathogenesis and protective immunity to murine malaria. Immunology. 1992;75:646–651. [PMC free article] [PubMed] [Google Scholar]
- 28.Amani V, et al. Involvement of IFN-gamma receptor-medicated signaling in pathology and anti-malarial immunity induced by Plasmodium berghei infection. Eur J Immunol. 2000;30:1646–1655. doi: 10.1002/1521-4141(200006)30:6<1646::AID-IMMU1646>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 29.Rudin W, Favre N, Bordmann G, Ryffel B. Interferon-gamma is essential for the development of cerebral malaria. Eur J Immunol. 1997;27:810–815. doi: 10.1002/eji.1830270403. [DOI] [PubMed] [Google Scholar]
- 30.Villegas-Mendez A, et al. Gamma interferon mediates experimental cerebral malaria by signaling within both the hematopoietic and nonhematopoietic compartments. Infect Immun. 2017;85:e01035-16. doi: 10.1128/IAI.01035-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirunpetcharat C, Finkelman F, Clark IA, Good MF. Malaria parasite-specific Th1-like T cells simultaneously reduce parasitemia and promote disease. Parasite Immunol. 1999;21:319–329. doi: 10.1046/j.1365-3024.1999.00234.x. [DOI] [PubMed] [Google Scholar]
- 32.Seixas EM, Langhorne J. Gammadelta T cells contribute to control of chronic parasitemia in Plasmodium chabaudi infections in mice. J Immunol. 1999;162:2837–2841. [PubMed] [Google Scholar]
- 33.Ribot JC, et al. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ribot JC, et al. Cutting edge: Adaptive versus innate receptor signals selectively control the pool sizes of murine IFN-γ- or IL-17-producing γδ T cells upon infection. J Immunol. 2010;185:6421–6425. doi: 10.4049/jimmunol.1002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Ombrain MC, Robinson LJ, Mueller I, Schofield L. Mechanisms underlying early interferon-gamma production in human Plasmodium falciparum malaria. Clin Infect Dis. 2009;48:1482–1483. doi: 10.1086/598509. [DOI] [PubMed] [Google Scholar]
- 36.Robinson LJ, et al. Cellular tumor necrosis factor, gamma interferon, and interleukin-6 responses as correlates of immunity and risk of clinical Plasmodium falciparum malaria in children from Papua New Guinea. Infect Immun. 2009;77:3033–3043. doi: 10.1128/IAI.00211-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yañez DM, Batchelder J, van der Heyde HC, Manning DD, Weidanz WP. Gamma delta T-cell function in pathogenesis of cerebral malaria in mice infected with Plasmodium berghei ANKA. Infect Immun. 1999;67:446–448. doi: 10.1128/iai.67.1.446-448.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boubou MI, et al. T cell response in malaria pathogenesis: Selective increase in T cells carrying the TCR V(beta)8 during experimental cerebral malaria. Int Immunol. 1999;11:1553–1562. doi: 10.1093/intimm/11.9.1553. [DOI] [PubMed] [Google Scholar]
- 39.Amino R, Thiberge S, Shorte S, Frischknecht F, Ménard R. Quantitative imaging of Plasmodium sporozoites in the mammalian host. C R Biol. 2006;329:858–862. doi: 10.1016/j.crvi.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Vanderberg JP, Frevert U. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int J Parasitol. 2004;34:991–996. doi: 10.1016/j.ijpara.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Sturm A, et al. Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science. 2006;313:1287–1290. doi: 10.1126/science.1129720. [DOI] [PubMed] [Google Scholar]
- 42.Liehl P, et al. Host-cell sensors for Plasmodium activate innate immunity against liver-stage infection. Nat Med. 2014;20:47–53. doi: 10.1038/nm.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liehl P, Mota MM. Innate recognition of malarial parasites by mammalian hosts. Int J Parasitol. 2012;42:557–566. doi: 10.1016/j.ijpara.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Pied S, et al. Liver CD4-CD8- NK1.1+ TCR alpha beta intermediate cells increase during experimental malaria infection and are able to exhibit inhibitory activity against the parasite liver stage in vitro. J Immunol. 2000;164:1463–1469. doi: 10.4049/jimmunol.164.3.1463. [DOI] [PubMed] [Google Scholar]
- 45.Roland J, et al. NK cell responses to Plasmodium infection and control of intrahepatic parasite development. J Immunol. 2006;177:1229–1239. doi: 10.4049/jimmunol.177.2.1229. [DOI] [PubMed] [Google Scholar]
- 46.Miller JL, Sack BK, Baldwin M, Vaughan AM, Kappe SHI. Interferon-mediated innate immune responses against malaria parasite liver stages. Cell Rep. 2014;7:436–447. doi: 10.1016/j.celrep.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 47.Tsuji M, et al. Gamma delta T cells contribute to immunity against the liver stages of malaria in alpha beta T-cell-deficient mice. Proc Natl Acad Sci USA. 1994;91:345–349. doi: 10.1073/pnas.91.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McKenna KC, et al. Gammadelta T cells are a component of early immunity against preerythrocytic malaria parasites. Infect Immun. 2000;68:2224–2230. doi: 10.1128/iai.68.4.2224-2230.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rénia L, et al. Cerebral malaria: Mysteries at the blood-brain barrier. Virulence. 2012;3:193–201. doi: 10.4161/viru.19013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ribot JC, et al. 2019 RNA-seq of liver stage-derived parasites from wild-type and TCRδ−/− mice. ArrayExpress. Available at https://www.ebi.ac.uk/arrayexpress/experiments/E-MTAB-6493/. Deposited March 12, 2019.
- 51.Guggisberg AM, Amthor RE, Odom AR. Isoprenoid biosynthesis in Plasmodium falciparum. Eukaryot Cell. 2014;13:1348–1359. doi: 10.1128/EC.00160-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fernandes P, et al. A Plasmodium cross-stage antigen contributes to the development of experimental cerebral malaria. Front Immunol. 2018;9:1875. doi: 10.3389/fimmu.2018.01875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haussig JM, Matuschewski K, Kooij TW. Inactivation of a Plasmodium apicoplast protein attenuates formation of liver merozoites. Mol Microbiol. 2011;81:1511–1525. doi: 10.1111/j.1365-2958.2011.07787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lewis MD, et al. Chemical attenuation of Plasmodium in the liver modulates severe malaria disease progression. J Immunol. 2015;194:4860–4870. doi: 10.4049/jimmunol.1400863. [DOI] [PubMed] [Google Scholar]
- 55.D’Ombrain MC, et al. Association of early interferon-gamma production with immunity to clinical malaria: A longitudinal study among Papua New Guinean children. Clin Infect Dis. 2008;47:1380–1387. doi: 10.1086/592971. [DOI] [PubMed] [Google Scholar]
- 56.Mitchell AJ, et al. Early cytokine production is associated with protection from murine cerebral malaria. Infect Immun. 2005;73:5645–5653. doi: 10.1128/IAI.73.9.5645-5653.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grau GE, et al. Monoclonal antibody against interferon gamma can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc Natl Acad Sci USA. 1989;86:5572–5574. doi: 10.1073/pnas.86.14.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Souza JB, Williamson KH, Otani T, Playfair JH. Early gamma interferon responses in lethal and nonlethal murine blood-stage malaria. Infect Immun. 1997;65:1593–1598. doi: 10.1128/iai.65.5.1593-1598.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dantzler KW, Jagannathan P. γδ T cells in antimalarial immunity: New insights into their diverse functions in protection and tolerance. Front Immunol. 2018;9:2445. doi: 10.3389/fimmu.2018.02445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ebert D. Infectivity, multiple infections, and the genetic correlation between within-host growth and parasite virulence: A reply to Hochberg. Evolution. 1998;52:1869–1871. doi: 10.1111/j.1558-5646.1998.tb02267.x. [DOI] [PubMed] [Google Scholar]
- 61.Spence PJ, Jarra W, Lévy P, Nahrendorf W, Langhorne J. Mosquito transmission of the rodent malaria parasite Plasmodium chabaudi. Malar J. 2012;11:407. doi: 10.1186/1475-2875-11-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spence PJ, et al. Vector transmission regulates immune control of Plasmodium virulence. Nature. 2013;498:228–231. doi: 10.1038/nature12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Preiser PR, et al. Stage-specific transcription of distinct repertoires of a multigene family during Plasmodium life cycle. Science. 2002;295:342–345. doi: 10.1126/science.1064938. [DOI] [PubMed] [Google Scholar]
- 64.Bachmann A, et al. Mosquito passage dramatically changes var gene expression in controlled human Plasmodium falciparum infections. PLoS Pathog. 2016;12:e1005538. doi: 10.1371/journal.ppat.1005538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanders PR, et al. Distinct protein classes including novel merozoite surface antigens in Raft-like membranes of Plasmodium falciparum. J Biol Chem. 2005;280:40169–40176. doi: 10.1074/jbc.M509631200. [DOI] [PubMed] [Google Scholar]
- 66.Gilson PR, et al. Identification and stoichiometry of glycosylphosphatidylinositol-anchored membrane proteins of the human malaria parasite Plasmodium falciparum. Mol Cell Proteomics. 2006;5:1286–1299. doi: 10.1074/mcp.M600035-MCP200. [DOI] [PubMed] [Google Scholar]
- 67.Sanders PR, et al. A set of glycosylphosphatidyl inositol-anchored membrane proteins of Plasmodium falciparum is refractory to genetic deletion. Infect Immun. 2006;74:4330–4338. doi: 10.1128/IAI.00054-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deroost K, Pham TT, Opdenakker G, Van den Steen PE. The immunological balance between host and parasite in malaria. FEMS Microbiol Rev. 2016;40:208–257. doi: 10.1093/femsre/fuv046. [DOI] [PubMed] [Google Scholar]
- 69.Cowman AF, Healer J, Marapana D, Marsh K. Malaria: Biology and disease. Cell. 2016;167:610–624. doi: 10.1016/j.cell.2016.07.055. [DOI] [PubMed] [Google Scholar]
- 70.Topolska AE, Lidgett A, Truman D, Fujioka H, Coppel RL. Characterization of a membrane-associated rhoptry protein of Plasmodium falciparum. J Biol Chem. 2004;279:4648–4656. doi: 10.1074/jbc.M307859200. [DOI] [PubMed] [Google Scholar]
- 71.Topolska AE, Richie TL, Nhan DH, Coppel RL. Associations between responses to the rhoptry-associated membrane antigen of Plasmodium falciparum and immunity to malaria infection. Infect Immun. 2004;72:3325–3330. doi: 10.1128/IAI.72.6.3325-3330.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Naik RS, et al. Glycosylphosphatidylinositol anchors of Plasmodium falciparum: Molecular characterization and naturally elicited antibody response that may provide immunity to malaria pathogenesis. J Exp Med. 2000;192:1563–1576. doi: 10.1084/jem.192.11.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beeson JG, et al. Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. FEMS Microbiol Rev. 2016;40:343–372. doi: 10.1093/femsre/fuw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Long GH, Boots M. How can immunopathology shape the evolution of parasite virulence? Trends Parasitol. 2011;27:300–305. doi: 10.1016/j.pt.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 75.Day T, Graham AL, Read AF. Evolution of parasite virulence when host responses cause disease. Proc Biol Sci. 2007;274:2685–2692. doi: 10.1098/rspb.2007.0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guenot M, et al. Phosphoantigen burst upon Plasmodium falciparum schizont rupture can distantly activate Vγ9Vδ2 T cells. Infect Immun. 2015;83:3816–3824. doi: 10.1128/IAI.00446-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kurup SP, Harty JT. γδ T cells and immunity to human malaria in endemic regions. Ann Transl Med. 2015;3(Suppl 1):S22. doi: 10.3978/j.issn.2305-5839.2015.02.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Franke-Fayard B, et al. A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol Biochem Parasitol. 2004;137:23–33. doi: 10.1016/j.molbiopara.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 79.Franke-Fayard B, Waters AP, Janse CJ. Real-time in vivo imaging of transgenic bioluminescent blood stages of rodent malaria parasites in mice. Nat Protoc. 2006;1:476–485. doi: 10.1038/nprot.2006.69. [DOI] [PubMed] [Google Scholar]
- 80.Bienvenu AL, Ferrandiz J, Kaiser K, Latour C, Picot S. Artesunate-erythropoietin combination for murine cerebral malaria treatment. Acta Trop. 2008;106:104–108. doi: 10.1016/j.actatropica.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 81.van der Heyde HC, et al. Assessing vascular permeability during experimental cerebral malaria by a radiolabeled monoclonal antibody technique. Infect Immun. 2001;69:3460–3465. doi: 10.1128/IAI.69.5.3460-3465.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kyes S, Pinches R, Newbold C. A simple RNA analysis method shows var and rif multigene family expression patterns in Plasmodium falciparum. Mol Biochem Parasitol. 2000;105:311–315. doi: 10.1016/s0166-6851(99)00193-0. [DOI] [PubMed] [Google Scholar]
- 83.Zuzarte-Luis V, Sales-Dias J, Mota MM. Simple, sensitive and quantitative bioluminescence assay for determination of malaria pre-patent period. Malar J. 2014;13:15. doi: 10.1186/1475-2875-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liao Y, Smyth GK, Shi W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 85.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Warnes GR, et al. 2016 gplots: Various R Programming Tools for Plotting Data. R Package Version 3.0.1. Available at https://cran.r-project.org/. Accessed March, 11, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.