Significance
MAGT1 is a controversial protein that has been described as an endoplasmic reticulum (ER) localized subunit of the oligosaccharyltransferase (OST) complex involved in the posttranslational transfer of glycans onto proteins, but also as a magnesium (Mg2+) transporter at the plasma membrane. So far, mutations in MAGT1 have been associated with Mg2+ defects causing an immunodeficiency. We demonstrate that MAGT1-deficient patients have a defect in glycosylation, and, in addition, we describe a different phenotype for the disorder. These results confirm the presumed role of MAGT1 as a subunit of the OST.
Keywords: congenital disorders of glycosylation, CDG, XMEN, oligosaccharyltransferase complex
Abstract
Congenital disorders of glycosylation (CDG) are a group of rare metabolic diseases, due to impaired protein and lipid glycosylation. We identified two patients with defective serum transferrin glycosylation and mutations in the MAGT1 gene. These patients present with a phenotype that is mainly characterized by intellectual and developmental disability. MAGT1 has been described to be a subunit of the oligosaccharyltransferase (OST) complex and more specifically of the STT3B complex. However, it was also claimed that MAGT1 is a magnesium (Mg2+) transporter. So far, patients with mutations in MAGT1 were linked to a primary immunodeficiency, characterized by chronic EBV infections attributed to a Mg2+ homeostasis defect (XMEN). We compared the clinical and cellular phenotype of our two patients to that of an XMEN patient that we recently identified. All three patients have an N-glycosylation defect, as was shown by the study of different substrates, such as GLUT1 and SHBG, demonstrating that the posttranslational glycosylation carried out by the STT3B complex is dysfunctional in all three patients. Moreover, MAGT1 deficiency is associated with an enhanced expression of TUSC3, the homolog protein of MAGT1, pointing toward a compensatory mechanism. Hence, we delineate MAGT1-CDG as a disorder associated with two different clinical phenotypes caused by defects in glycosylation.
Congenital disorders of glycosylation (CDG) are a rapidly growing group of genetic diseases caused by defects in glycan synthesis, processing, and/or attachment. Glycosylation is an important co- and posttranslational modification of proteins and lipids, mediating their function, stability, and dynamics (1, 2). In the N-glycosylation of proteins, the lipid-linked oligosaccharide (LLO) is first built in the endoplasmic reticulum (ER) and subsequently transferred en bloc by the oligosaccharyltransferase (OST) complex from a lipidic dolichol carrier to an N-X-S/T residue of a nascent protein. Next, remodeling of the glycan structure continues in the Golgi apparatus (3). Patients with CDG show an extremely variable phenotype, ranging from intellectual disability (ID) to severe multiorgan failure and death (1).
Indispensable in this meticulously orchestrated glycosylation machinery is the transfer of glycans by the OST, a multisubunit protein complex consisting of a catalytic subunit (STT3A or STT3B), six shared subunits, and complex specific accessory subunits (4). The two complexes have distinct roles: STT3A is associated with the protein translocation channel and acts in a cotranslational fashion, while sites that are missed by STT3A can be posttranslationally glycosylated by STT3B (5). This interplay ensures the full N-glycosylation of proteins in mammalian cells. Both have accessory proteins that are specific for each of the catalytic subunits: DC2 and KCP2 are indispensable for STT3A function (6), while STT3B requires either MAGT1 or TUSC3 (7, 8). These two mutually exclusive paralogues share 66% amino acid sequence identity and are orthologs to the yeast OST subunits Ost6 and Ost3 (9). Both proteins possess a thioredoxin fold in the luminal domain, which has been described to be necessary for the glycosylation of STT3B substrates that are bracketed by disulfides. Remarkably, MAGT1 is also required for full STT3B glycosylation in an oxidoreductase-independent manner (7, 9). MAGT1 and TUSC3 are physically associated with STT3B, as was shown by native coimmunoprecipitation (7).
Intriguingly, MAGT1 has been swayed back and forth over the past years between the role as a subunit of the ER-localized OST, or as a magnesium (Mg2+) transporter, located at the plasma membrane. Indeed, MAGT1 was described to be required for Mg2+ uptake by vertebrate cells (10, 11). Mutations in this gene have been described to cause XMEN (X-linked immunodeficiency with magnesium defect, Epstein–Barr virus infection and neoplasia) (12). T lymphocytes of these patients displayed altered kinetics of Mg2+ influx, although the cellular levels of Mg2+ remained normal. The link between MAGT1 and glycosylation has not been assessed in these patients.
Here we describe three patients with pathogenic mutations in MAGT1. We demonstrate that MAGT1 deficiency causes a glycosylation defect and that the consequences of the MAGT1 mutations can be very broad. We studied two patients with a different clinical phenotype that is mostly characterized by intellectual and developmental disability. The third reported patient in this study has a MAGT1 mutation and the clinical phenotype of XMEN.
Results
Clinical Data and Mutation Analysis.
We identified three male patients with mutations in the X-linked gene MAGT1. Patient 1 (P1) and patient 2 (P2) were referred for metabolic screening because of developmental disability. This work-up revealed an abnormal (“type 1”) serum capillary zone electrophoresis and serum transferrin isoelectric focusing (sTf IEF) in both patients. Patient 3 (P3) was diagnosed with a primary immunodeficiency disorder (PID). The clinical data of the patients are summarized in Table 1.
Table 1.
Clinical and molecular summary of patients
| Characteristics | Patient 1 | Patient 2 | Patient 3 |
| Age at evaluation (years) | 13 | 11 | 17 |
| cDNA change | c.1068A > C | c.991C > T | c.938T > G |
| Protein change | p.Lys356Asn | p.Arg331* | p.Leu313* |
| Inheritance | Maternal transmission | De novo | Maternal transmission |
| Skewed X inactivation | Yes (98%) | NA | Yes (100%) |
| Intellectual/developmental disability | Yes | Yes | No |
| Behavior abnormalities | Yes | NT | No |
| Facial dysmorphism | Mild | Mild | No |
| Hepatomegaly | No | Yes | No |
| Immunological phenotype | |||
| CA EBV infection | No | No | Yes |
| Other infections | No | No | Yes |
| CD4:CD8 ratio | NT | NT | Normal |
| CD4 counts | Normal | NT | Decreased |
| Serum transferrin IEF | Type 1: 2-sialo form: 5.0% (normal range: 0–2.6) | Type 1 | Type 1: 2-sialo form: 7.0% (normal range: 0–2.6) |
CA EBV, chronic active Epstein Barr virus; IEF, isoelectric focusing; NA, not applicable; NT, not tested.
P1 and P2 received a molecular diagnosis after the use of our in-house CDG gene panel. Both boys were diagnosed with a hemizygous mutation in MAGT1 (NM_032121.5). P1 has a c.1068A > C mutation encoding a p.Lys356Asn. The mutation is not present in gnomAD nor in any other population database such as dbSNP, 1000 Genomes, and the ESP database. The nucleotide change affects a very well-conserved amino acid (AA) (SI Appendix, Fig. S1B). In addition, different prediction programs estimate that this variant is damaging. Furthermore, skewing analysis revealed that the mothers’ X chromosome containing the mutation is almost fully skewed (Table 1). All these genetic data imply the causal nature of the missense mutation in P1.
P2 has a de novo hemizygous loss-of-function mutation in MAGT1: c.991C > T, p.Arg331*.
A molecular diagnosis was reached for P3, after the use of a targeted panel covering immune genes. A hemizygous nonsense mutation c.938T > G, pLeu313* was found in MAGT1, which classifies P3 as an XMEN patient. In addition, P3 NK cells have reduced NKG2D steady state levels (SI Appendix, Fig. S2), which has previously been linked to the pathophysiology of XMEN disorder (13). Both loss-of-function mutations are not present in the aforementioned population databases. Interestingly, P3 also has a sTf IEF type 1 pattern.
Analysis of MAGT1.
In humans, MAGT1 is localized on the long arm of the X chromosome, at position Xq21.1. For the functional analysis of the mutations we selected, as described in the Material and Methods, two transcripts, MAGT1-204 (RefSeq NM_032121.5) and MAGT1-205, that respectively encode a 367- and 335-AA protein (SI Appendix, Fig. S1A). In-silico prediction models (CBS Prediction Servers; www.cbs.dtu.dk/services/FeatureP/) suggest that the 335-AA isoform harbors four transmembrane (TM) domains, and the 367-AA isoform has one more. The sequence of the shorter transcript is identical to the MAGT1-204 form, as the translation initiation site is found further upstream. It is important to note that efficiency of initiation is strongly dependent on the consensus sequence surrounding the start site (14). The first AUG in MAGT1-204 has a very poor context for translation. It is more likely that the second AUG will be used as translation initiation site, hence resulting in the exact same 335-AA product as the MAGT1-205 transcript. In addition, there is also no evidence for MAGT1 doublets on Western blots. In summary, the 335 isoform is most likely the only functional MAGT1 isoform (SI Appendix, Fig. S1C). Analysis of gene expression in the GTEx database (www.gtexportal.org) shows that MAGT1 is expressed in all studied tissues and organs.
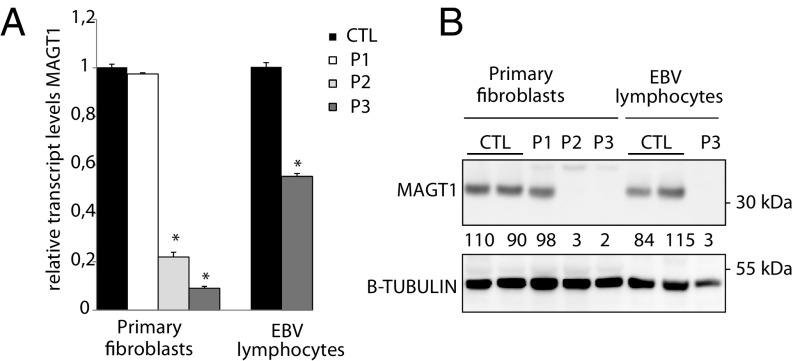
To look at the impact of the mutations, the relative expression levels of MAGT1 in patient-derived fibroblasts were assessed (Fig. 1A). In P1, relative MAGT1 transcript expression levels remained similar to the ones observed in controls. However, P2 fibroblasts showed a 75% reduction of MAGT1 transcript. That decrease was even more dramatic in P3, where only 6% residual MAGT1 transcript could be observed. An additional EBV-transformed lymphocyte cell line was available for P3, where about a 50% decrease of MAGT1 transcript could be seen compared with controls. This resource was unfortunately not available for P1 and P2. MAGT1 steady state levels were similar in P1 compared with control cell lines, hence suggesting that the p.Lys356Asn mutation does not affect MAGT1 stability (Fig. 1B). In P2 and P3, we observed an (almost) complete absence of the protein, confirming the loss-of-function nature of these mutations.
Fig. 1.
Expression levels of MAGT1. (A) Relative transcript levels of MAGT1 and (B) protein immunoblotting for MAGT1 in fibroblasts and EBV-transformed lymphocytes. β-Tubulin was used as a loading control. Values below the corresponding lane represent averaged normalized values (n = 3). Error bars represent SE of mean (SEM). *P < 0.05.
Mutations Alter the Expression Levels of TUSC3.
MAGT1 has been described as a subunit of the OST STT3B complex (7). Therefore, we studied the protein steady state levels of the catalytic subunits of the OST (STT3A and STT3B) and TUSC3, the homolog protein of MAGT1 (Fig. 2). These studies were performed in patient fibroblasts, in EBV-transformed lymphocytes, and in CRISPR/Cas9 knockout (KO) HEK293 cell lines. These cells were designed to not express either STT3A, STT3B, MAGT1, or TUSC3 (15).
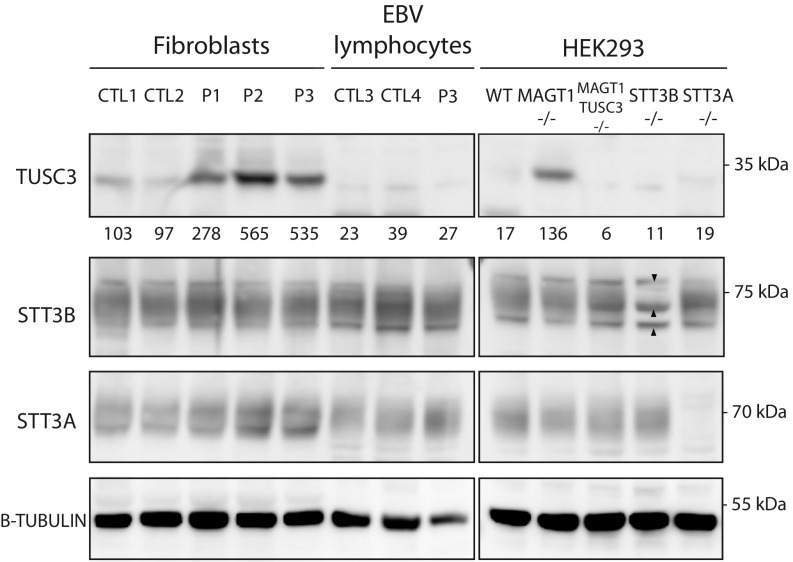
Fig. 2.
Stability of different OST subunits. Fibroblasts, EBV-transformed lymphocytes, and HEK293 cells were analyzed for protein steady state levels of TUSC3, STT3B, and STT3A. The arrowheads depict the nonspecific bands comigrating with STT3B. β-Tubulin was used as a loading control. Values below the TUSC3 blot represent averaged values normalized to the average of the control fibroblast cells (n = 3).
The expression levels of STT3B were variable in the different patient cell lines, but no significant differences were observed. Similar results were obtained for STT3A, except for P3 lymphocytes, in which a twofold increase in expression levels was observed. Remarkably, the assessment of steady state levels showed a marked increase of TUSC3 in all three patient fibroblast cells, which points toward a compensatory mechanism. Importantly, in both the control and patient lymphocytes there was no expression of TUSC3 (Fig. 2).
To frame the observations in patient-derived cells, steady state levels of the different subunits were assessed in KO HEK293 cells. The observed TUSC3 up-regulation in patient fibroblasts was also confirmed in the MAGT1−/− cell line. In summary, these results show that the mutations do not affect the stability of the catalytic subunits of the OST complex and that there is a strong enhancement of TUSC3 steady state levels in patient fibroblasts.
STT3B-Dependent Substrates Are Hypoglycosylated in Patient Cells.
The glycosylation of glycoproteins was studied directly in patient-derived fibroblasts to evaluate whether the MAGT1 mutations have an effect on the glycosylation of STT3B or STT3A substrates. Patients’ and control fibroblasts were transfected with either sex hormone binding globulin (SHBG), preprocathepsin C (pCatC), or preprosaposin (pSap) expression vectors for 24 h followed by pulse-chase labeling (Fig. 3A).
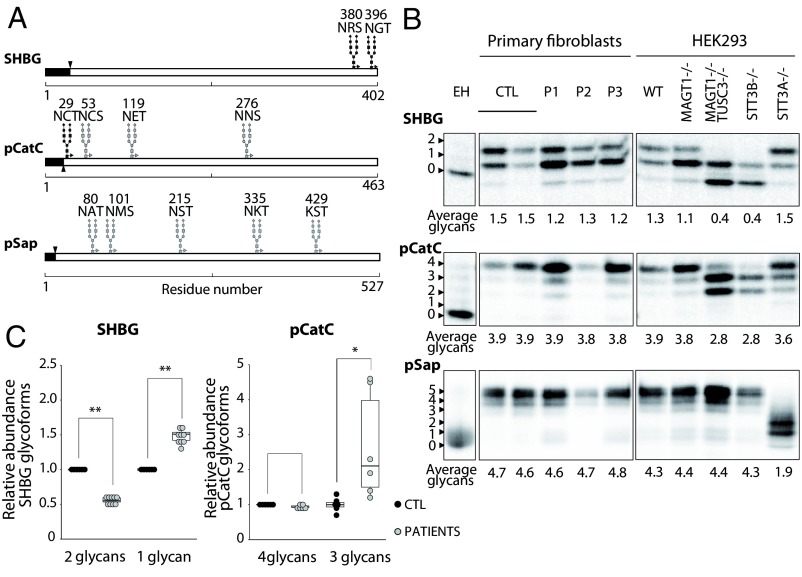
Fig. 3.
STT3B-dependent glycosylation is affected in patients’ fibroblasts. (A) Diagrams showing the glycosylation sites of SHBG, pCatC, and pSap. Black glycan structures indicate an STT3B-dependent site. Signal sequences are depicted in black. (B) HEK293 cells and fibroblasts were transfected with SHBG, pCatC, or pSap, followed by pulse-chase labeling. Quantified values are shown below gel lanes and represent the average number of glycans for the respective reporter (n = 3). (C) Quantification of the different glycoforms of SHBG and pCatC in fibroblasts, normalized to the averaged control samples. EH indicates endoglycosidase H treatment. *P < 0.5; **P < 0.005.
Pulse-chase labeling of transfected fibroblasts and HEK293 cells showed a mild, but significant (P < 0.05), hypoglycosylation pattern of SHBG in all patient cell lines (Fig. 3B). SHBG is a well-studied and characterized reporter of STT3B glycosylation and has two C-terminal glycosylation sites (N380RS and N396GT) (Fig. 3A). C-terminal sites are often skipped by the STT3A complex and are required to be glycosylated by STT3B (16). In addition, the reduction of the SHBG 2-glycan form by roughly 50% compared with controls and the 1.5-fold increase of the 1-glycan form confirm that the glycosylation of SHBG is affected in patient fibroblasts (Fig. 3C).
Next, we studied pCatC (Fig. 3A). This substrate has four N-glycosylation sites, of which one is a MAGT1 substrate (N29CT) (5). Pulse-chase labeling of pCatC revealed an increase in the glycoform carrying three glycans in P1 and P3 cells (Fig. 3C), but no difference in average total glycan count was observed (Fig. 3B). The defect was much less obvious in the P2 cells, possibly due to a very low incorporation of 35S label in this cell line. Therefore, this patient was not included in the calculation of the different pCatC glycoforms.
Third, the glycosylation of pSap was assessed. pSap has five N-glycosylation sequons that are glycosylated exclusively by the STT3A complex (5) (Fig. 3A). We therefore used pSap as a negative control. Pulse-chase labeling showed no hypoglycosylation defects, except in the STT3A−/− cell line (Fig. 3B). This confirms that our patients do not have a general glycosylation defect, but are only deficient in the STT3B-dependent glycosylation.
The patient fibroblasts show a tendency for an STT3B glycosylation defect in both pCatC and SHBG, but the reduction in the average glycan number was not as strong as in STT3B−/− or MAGT1−/−TUSC3−/− cells. This is in line with reports showing that enhanced expression of TUSC3 mitigates the MAGT1 defect (15). Indeed, our results suggest that the marked increase in TUSC3 steady state levels observed in patient cell lines (Fig. 2) compensates for the MAGT1 defect in patients.
MAGT1 Mutations Are Pathogenic in the Absence of TUSC3.
To assess whether the patients’ variants are pathogenic, the wild-type (WT) MAGT1-205 cDNA sequence (SI Appendix, Fig. S1) was mutagenized to harbor the corresponding point mutations. HEK293 MAGT1−/−TUSC3−/− cells were cotransfected with SHBG and the different MAGT1 expression vectors. The double-KO cell line was chosen to circumvent the issue of the increased expression of TUSC3 in the MAGT1−/− cells. Pulse-chase labeling showed a reduction of 70% in the average glycan number of the SHBG reporter protein in MAGT1−/−TUSC3−/− cells compared with WT cells. Complemented with the WT construct, the average number of glycans doubled (Fig. 4A). None of the MAGT1 cDNA sequences carrying the patients’ mutations was able to improve the glycosylation of SHBG, thereby indicating that these mutations are impairing the STT3B-mediated glycosylation. The same results were observed for complementation with the MAGT1-204 WT and mutagenized cDNA (SI Appendix, Fig. S3).
Fig. 4.
Glycosylation assessment of different substrates. (A) Assessment of SHBG glycosylation. MAGT1−/− TUSC3−/− cells were cotransfected with SHBG and the indicated MAGT1 transcripts. (B) Metabolic labeling of the endogenous pCatC and GLUT1 in HEK293 cells and EBV-transformed lymphocytes. (C) HEK293 cells, primary fibroblasts, and EBV-transformed lymphocytes were analyzed for protein steady state levels of pCatC and CatC. β-Tubulin was used as a loading control. (D) Diagram showing the STT3B-dependent glycosylation site of GLUT1. The signal sequence is depicted in black. (E) Relative abundance of the 4- and 3-glycans form for pCatC. (F) Relative steady state levels of pCatC and CatC in primary fibroblasts and EBV-transformed lymphoctyes. CatC was not quantifiable in HEK293 cells. Quantified values are shown below gel lanes and represent the average number of glycans for the respective reporter (n = 3). EH indicates endoglycosidase H treatment. Error bars represent SEM. *P < 0.05.
Next, the glycosylation status of two endogenous substrates, pCatC and glucose transporter 1 (GLUT1), was studied in lymphocytes as these cells do not express TUSC3 (Fig. 2). Quantitative glycoproteomics data and pulse-labeling experiments indicated that the N45QT site in the GLUT1 glucose transporter is an STT3B substrate (Fig. 4D). Metabolic pulse-chase labeling showed a marked hypoglycosylation pattern in P3 lymphocytes, with a decrease in the abundance of the fully glycosylated form of pCatC and an increase in the abundance of the band form carrying three and two glycans. Interestingly, GLUT1 is completely hypoglycosylated in P3 lymphocytes, with no visible protein carrying one glycan (Fig. 4B). Both results were similar to the glycosylation pattern observed in HEK293 MAGT1−/−TUSC3−/− and STT3B−/− cell lines. Taken together with the data obtained in fibroblasts, these results suggest that STT3B-dependent glycosylation is impaired in the XMEN patient (P3).
Moreover, pCatC is known to be first synthesized as a proenzyme and then processed into the mature cathepsin C (CatC). As a clear hypoglycosylation defect was demonstrated for pCatC in P3 lymphocytes, the steady state levels of endogenous pCatC were assessed to determine the effect of the glycosylation deficiency on the stable expression and the maturation of CatC. A variable glycosylation defect was observed in the three investigated patients (Fig. 4C). In P1, a strong hypoglycosylation defect could be observed, as the upper band (four glycans) is completely absent. In P2 and P3, an increase of the hypoglycosylated form (three glycans) is found (Fig. 4E). In P3 lymphocytes this defect becomes more pronounced, with barely any fully glycosylated pCatC. Also, the amount of pCatC is reduced in the patient cell lines. The steady state levels of the mature CatC were also severely reduced in P1 and P2 (Fig. 4F). In HEK293 cells, used as control, a hypoglycosylated band appeared for MAGT1−/−TUSC3−/− and STT3B−/− cell lines.
Discussion
We report an additional type of CDG caused by mutations in the X-linked gene MAGT1. Since numerous studies uncovered its role as a subunit of the OST complex, MAGT1 has been a candidate CDG gene (7, 15). It was proposed that MAGT1 or TUSC3, two mutually exclusive homologs, are specific accessory proteins of the STT3B subunit. They are crucial for the proper glycosylation of STT3B substrates due to their oxidoreductase activity (7, 8, 15). On the other hand, it was claimed that MAGT1 is a Mg2+ transporter, indispensable for Mg2+ uptake in vertebrate cells (10, 11). So far, mutations in MAGT1 have been associated with XMEN, an immunodeficiency characterized by chronic active EBV infection (12), but no glycosylation assays were performed in these patients. It was proposed that the defect in Mg2+ transport leads to a deficient activation of T lymphocytes and natural killer (NK) cells (13).
Here we describe two MAGT1-CDG patients with a phenotype that is mainly characterized by intellectual and developmental disability. In addition, we report a third patient with the clinical phenotype of XMEN. We showed that in P1 the missense mutation does not affect MAGT1 steady state levels. In sharp contrast is the complete absence of MAGT1 in P2 and P3, both harboring stop mutations. Moreover, we demonstrated that the expression levels of TUSC3 are elevated in the patients’ fibroblasts. This observation is restricted to fibroblasts, as lymphocytes do not express TUSC3. The molecular mechanism(s) by which TUSC3 is stabilized in response to a defect in MAGT1 are not elucidated yet. It was proposed that, in HEK293 cells, TUSC3 may be degraded because it does not compete efficiently with MAGT1 for incorporation in the STT3B complex (15). We hypothesize that a similar mechanism may occur in patients’ fibroblasts, explaining the increased levels of TUSC3.
Most importantly, we show that the glycosylation of specific STT3B substrates is altered in all three patient-derived cell lines. SHBG and pCatC were hypoglycosylated to a mild extent in patients’ fibroblasts, thereby confirming the previously described role of MAGT1 as an important subunit of the STT3B complex (7). This was reinforced by the fact that the glycosylation of pSap, an STT3A substrate, was not altered in MAGT1-deficient cells.
Due to the enhanced expression of TUSC3 that can mitigate the loss of the MAGT1 function, we confirmed the pathogenicity of the different mutations in MAGT1−/−TUSC3−/− cells. MAGT1 cDNA constructs carrying the various mutations reported in the patients were, contrarily to the WT sequence, not able to rescue the hypoglycosylation of the SHBG reporter. Moreover, the examination of EBV-transformed lymphocytes showed a glycosylation defect of two STT3B substrates (GLUT1, pCatC) in patient-derived cells. The lack of MAGT1 cannot be restored by TUSC3 in these cells, and the glycosylation defect associated with mutations in MAGT1 becomes much more pronounced than in fibroblasts and comparable to the STT3B−/− HEK293 cell line. As such, we were able to confirm the role of MAGT1 as a subunit of the OST in patient cells harboring mutations in MAGT1. Also, we demonstrate that EBV-transformed lymphocytes are the ideal patient-derived cellular model to assess MAGT1 mutations. In addition, by assessing steady state levels of pCatC it could be observed that the mutations lead to different levels of hypoglycosylation.
The missense mutation (p.Lys356Asn) in P1 does not affect the steady state protein expression. However, Lys356 and Glu268 form a salt bridge in MAGT1 that stabilizes a TM span, and we hypothesize that in P1 the AA substitution may destabilize that TM span (17). It seems that in general, MAGT1 is preferably incorporated over TUSC3 (15). Thus, the fact that MAGT1 is still stably expressed (but not functioning) might prevent TUSC3 to take over. On the other hand, P3 has a complete lack of MAGT1. In tissues that express TUSC3, the glycosylation deficiency can be bypassed by the latter. In other cells, such as lymphocytes, where TUSC3 is not expressed, the mutation becomes pathogenic. This may explain why XMEN patients in general only have an immunodeficiency. This hypothesis was confirmed by the study of the steady state levels of CatC, where P1 showed a strong hypoglycosylation effect of the pCatC and, in addition, decreased levels of matured CatC even though TUSC3 is expressed in fibroblasts. In P3 the defect was only partial in fibroblasts, but fully observable in the lymphocytes where the levels of the processed CatC were also lowered.
It is important to note the phenotypical difference between our MAGT1 patients and the previously reported ones. The two MAGT1-CDG patients we report here are mainly characterized by ID and developmental delay. On the other hand, patients with mutations in MAGT1 present with a PID that is characterized by CD4 lymphopenia and chronic active EBV infection (12). Neither one of our MAGT1-CDG patients has a history of infections. Remarkably, in a family with five affected males, patients with an intronic mutation in MAGT1 and a mutation in ATRX were diagnosed with ID and skin manifestations (18). The former was attributed to ATRX, a protein that has been linked to ID on multiple occasions, while the skin manifestations were linked to MAGT1. We wonder whether the ID could also be attributed to the mutations in MAGT1, as is the case in our patients. It was also claimed that MAGT1 deficiency causes ID in a family with a missense mutation (19). This variant (c.1028C > T; pVal311Gly) was later marked as “highly questionable” and retracted, as it was present in population databases (20). According to our analysis based on the American College of Medical Genetics and Genomics (ACMG) guidelines (21), we would also qualify this variant as likely benign. An assay to study the glycosylation would have to be performed to conclude on its pathogenicity.
The question arises whether MAGT1-CDG and XMEN represent two different phenotypes of a same clinical entity, or whether these are different disorders? Nonetheless, we were able to evidence glycosylation defects in cells derived from our XMEN patient (P3). This suggests that XMEN is a glycosylation disorder characterized mainly by an immunological phenotype. We favor the hypothesis describing that the defect in Mg2+ homeostasis occurs by an indirect mechanism involving the STT3B-dependent glycosylation of a protein involved in Mg2+ transport (22). We have attempted to assess Mg2+ fluxes, by following and adapting the protocol from Li et al. (12), but we have not been able to overcome the nonspecific reaction of MagFluo4 to Mg2+ and Ca2+ in CTL T cells (SI Appendix, Fig. S4). A question would then be why the defect in P1 and P2 shows no immunological involvement? We cannot exclude the possibility that the patients will not present with an immunodeficiency after infection with EBV, as the onset of disease also varies in the XMEN patient. Likewise, the interplay of TUSC3 and MAGT1 is not well understood, particularly in human tissues where TUSC3 and MAGT1 are expressed at different levels. A better understanding of the incorporation of these proteins into STT3B complexes in human tissues may be the key that will explain the different clinical presentation of the two ID patients compared with the XMEN patient. In addition, the previously reported STT3B-CDG patient presented with a severe congenital and developmental disorder, but not with immunological issues (23). Interestingly, this patient also had a mild type 1 sTf IEF pattern.
In summary, we identified three disease-causing mutations in MAGT1. Two of these mutations are responsible for a distinct phenotype, characterized by ID and developmental delay. All of these patients show anomalies in sTf IEF and the STT3B-dependent glycosylation, classifying these diseases caused by mutations in MAGT1 in the broad group of CDG. Further research still needs to be conducted to confirm whether other XMEN patients also present with aberrant glycosylation.
Materials and Methods
Ethics Statement.
Research on patients’ DNA and cells was approved by the Ethical Committee of the University Hospital of Leuven (approval nos. S59377, S58358, and S58466). All human subjects in this study provided informed consent.
Cell Lines.
CRISPR/Cas9-engineered HEK293 WT cells, depleted for either STT3A, STT3B, MAGT1, or TUSC3 were previously described (15). Primary fibroblasts from patients and controls were grown from a skin biopsy. EBV-transformed lymphocytes were derived from patient and control individuals. All cell lines were cultured in DMEM/F12 (Life Technologies) supplemented with 10% FBS (Clone III, HyClones) at 37 °C under 5% CO2.
Capillary Zone Electrophoresis and Isoelectric Focusing of Serum Transferrin.
Capillary zone electrophoresis and isoelectric focusing of serum transferrin were performed as previously described (24).
Gene Panel.
For P1 and P2, libraries were prepared from genomic DNA with the Illumina TruSeq DNA sample preparation kit and enriched for 79 glycosylation-related genes using a custom in-solution targeted assay (NimbleGen SeqCap EZ kit; Roche). For P3, the library was prepared with the KAPA High-Throughput Library Preparation Kit before enrichment for 290 immune-related genes using another custom in-solution targeted assay (NimbleGen SeqCap EZ kit; Roche). The enriched libraries were paired end sequenced on MiSeq (150 bp) or HiSeq2500 (100 or 125 bp, Illumina). The resulting reads were mapped to the reference genome hg19 using BWA, and variants were detected with GATK HaplotypeCaller after duplicate removal, realignment around indels, and base quality score recalibration.
X-Inactivation Assay.
Genomic DNA, derived from lymphocytes, was subjected to the androgen receptor (MIM# 313700) assay to determine X-inactivation ratios, as previously described in ref. 25.
RNA Extraction and Real-Time Quantitative PCR.
Total RNA was isolated from the different cell lines with RNeasy Mini kit (Qiagen). DNase treatment (Roche Diagnostics) was performed. Subsequently, 2 µg of purified total RNA was subjected to reverse transcription with the First-Strand cDNA synthesis kit (GE Healthcare).
PCRs were performed for MAGT1 (NM_032121), as well as HPRT (NM_000194) and GAPDH (NM_002046), which were used as an endogenous control for normalization. PCR primers were designed using the primer-BLAST software from NCBI and synthesized by Integrated DNA Technologies. PCR primer sequences will be provided on request. PCRs were performed using the 2× LightCycler 480 SYBR Green I Master. Data were analyzed using the LightCycler 480 Software (both Roche Applied Science). The comparative threshold cycle method described by Pfaffl was used to quantify the results (26).
Expression Vectors.
The different MAGT1 expression vectors were synthesized by e-Zyvec. Four protein-coding transcripts are described in Ensembl: MAGT1-201, -202, -203, and -204. Considering the lack of homology between MAGT1-203 and the yeast ortholog Ost3, we excluded this isoform.
Both MAGT1-201 and -204 encode the same 367-AA protein. For those two transcripts, we decided to choose the transcript MAGT1-204, in accordance with RefSeq. MAGT1-205 only differs from MAGT1-204 by shorter 3′ and 5′ UTRs. These two transcripts were used for the design of the WT MAGT1 expression vectors. Each one of the patient mutations were introduced in these vectors by site-directed mutagenesis. The expression vectors for SHBG (27), pCatC-HA (5), and pSAP-DDK-His (6) have all been described.
Protein Expression.
HEK293 cells were seeded in 60-mm dishes 24 h before transfection. Plasmid transfection was performed using 6 µg plasmid and Lipofectamine 2000 (Invitrogen) as transfection reagent in serum-free Opti-MEM medium (Gibco). Primary fibroblasts were transfected similarly, but with Lipofectamine LTX (Invitrogen). Cotransfections were performed with 4 µg of the MAGT1 vector and 4 µg of SHBG. All cells were assayed 24 h later.
Antibodies.
Anti-MAGT1 (17430-1-AP) and anti-TUSC3 (16039-1-AP) polyclonal antibodies were purchased from Proteintech, anti-BIP (3177S) from Bioké. Anti-STT3B and anti-STT3A antibodies were described previously (4, 5). Goat polyclonal antisera specific for CatC (AF1071) and SHBG (VFJ01) were purchased from R&D Systems and mouse monoclonal GLUT1 (ab40084) and β-tubulin (ab101019) from Abcam. The following antibodies against epitope tags were used: anti-HA (11867423001; Roche) and anti-DDK (F3165 anti-FLAG M2; Sigma).
Immunoblotting.
Ten micrograms of proteins were analyzed by SDS/PAGE and immunoblotted onto a nitrocellulose membrane (Thermo Fisher Scientific) with the indicated antibodies, as previously described (28). Signal detection was performed by autoradiography with ImageQuant LAS 4000, and quantification was performed with the Image Quant TL software (both GE Healthcare).
Pulse-Chase Radiolabeling and Immunoprecipitation.
Glycoprotein substrates in HEK293 and fibroblast cells were pulse-chase–labeled with Tran35S label (Perkin–Elmer), as previously described (7). Dry gels were exposed to a phosphor screen (Fujifilm), scanned in Typhoon FLA 9000, and quantified using Image Quant TL software (GE Healthcare).
Statistics.
Statistical analyses was performed in R. The Wilcoxon signed-rank test was used to compare control versus patient samples. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Dr. D. Sinasac, Dr. N. Wright, Dr. M. Alessandro, and K. Klassen, RN, for their valuable contribution; L. Coorevits and J. Cremers for technical assistance with flow cytometry; and I. Meyts for setup of the diagnostic gene panel for primary immune deficiencies. This research was supported by Research Foundation Flanders (FWO): under the frame of E-Rare-3, the ERA-Net for Research on Rare Diseases (ERA-NET Cofund action N°64578) (to G.M.), a research stay grant (FWO V417818N) (to E.B.), a Pegasus Marie Curie postdoctoral fellow (FWO 1207416N, 2012–2018) (to R.P.), a senior clinical investigator fellowship (to R. Schrijvers), and GLYCO4DIAG, an International Associated Laboratory grant from National Centre for Scientific Research (CNRS) and FWO (to F.F. and G.M.). The work was also supported by the National Institute of General Medical Sciences of the National Institutes of Health under award GM43768 (to R.G.), C1 KU Leuven fund (to R. Schrijvers), and by the Jaeken-Theunissen CDG Fund.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Pathogenic variant data related to this paper have been deposited in ClinVar (accession nos. SCV000898467.1, SCV000898468.1, and SCV000898469.1).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1817815116/-/DCSupplemental.
References
- 1.Jaeken J, Péanne R. What is new in CDG? J Inherit Metab Dis. 2017;40:569–586. doi: 10.1007/s10545-017-0050-6. [DOI] [PubMed] [Google Scholar]
- 2.Freeze HH, Chong JX, Bamshad MJ, Ng BG. Solving glycosylation disorders: Fundamental approaches reveal complicated pathways. Am J Hum Genet. 2014;94:161–175. doi: 10.1016/j.ajhg.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aebi M. N-linked protein glycosylation in the ER. Biochim Biophys Acta Mol Cell Res. 2013;1833:2430–2437. doi: 10.1016/j.bbamcr.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Kelleher DJ, Karaoglu D, Mandon EC, Gilmore R. Oligosaccharyltransferase isoforms that contain different catalytic STT3 subunits have distinct enzymatic properties. Mol Cell. 2003;12:101–111. doi: 10.1016/s1097-2765(03)00243-0. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz-Canada C, Kelleher DJ, Gilmore R. Cotranslational and posttranslational N-glycosylation of polypeptides by distinct mammalian OST isoforms. Cell. 2009;136:272–283. doi: 10.1016/j.cell.2008.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shrimal S, Cherepanova NA, Gilmore R. DC2 and KCP2 mediate the interaction between the oligosaccharyltransferase and the ER translocon. J Cell Biol. 2017;216:3625–3638. doi: 10.1083/jcb.201702159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cherepanova NA, Shrimal S, Gilmore R. Oxidoreductase activity is necessary for N-glycosylation of cysteine-proximal acceptor sites in glycoproteins. J Cell Biol. 2014;206:525–539. doi: 10.1083/jcb.201404083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohorko E, et al. Structural basis of substrate specificity of human oligosaccharyl transferase subunit N33/Tusc3 and its role in regulating protein N-glycosylation. Structure. 2014;22:590–601. doi: 10.1016/j.str.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Schulz BL, et al. Oxidoreductase activity of oligosaccharyltransferase subunits Ost3p and Ost6p defines site-specific glycosylation efficiency. Proc Natl Acad Sci USA. 2009;106:11061–11066. doi: 10.1073/pnas.0812515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goytain A, Quamme GA. Identification and characterization of a novel mammalian Mg2+ transporter with channel-like properties. BMC Genomics. 2005;6:48–66. doi: 10.1186/1471-2164-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou H, Clapham DE. Mammalian MagT1 and TUSC3 are required for cellular magnesium uptake and vertebrate embryonic development. Proc Natl Acad Sci USA. 2009;106:15750–15755. doi: 10.1073/pnas.0908332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li F-Y, et al. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature. 2011;475:471–476. doi: 10.1038/nature10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaigne-Delalande B, et al. Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science. 2013;341:186–191. doi: 10.1126/science.1240094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shabalina SA, Ogurtsov AY, Rogozin IB, Koonin EV, Lipman DJ. Comparative analysis of orthologous eukaryotic mRNAs: Potential hidden functional signals. Nucleic Acids Res. 2004;32:1774–1782. doi: 10.1093/nar/gkh313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cherepanova NA, Gilmore R. Mammalian cells lacking either the cotranslational or posttranslocational oligosaccharyltransferase complex display substrate-dependent defects in asparagine linked glycosylation. Sci Rep. 2016;6:20946. doi: 10.1038/srep20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shrimal S, Trueman SF, Gilmore R. Extreme C-terminal sites are posttranslocationally glycosylated by the STT3B isoform of the OST. J Cell Biol. 2013;201:81–95. doi: 10.1083/jcb.201301031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wild R, et al. Structure of the yeast oligosaccharyltransferase complex gives insight into eukaryotic N-glycosylation. Science. 2018;359:545–550. doi: 10.1126/science.aar5140. [DOI] [PubMed] [Google Scholar]
- 18.Qiao Y, et al. Variant ATRX syndrome with dysfunction of ATRX and MAGT1 genes. Hum Mutat. 2014;35:58–62. doi: 10.1002/humu.22465. [DOI] [PubMed] [Google Scholar]
- 19.Molinari F, et al. Oligosaccharyltransferase-subunit mutations in nonsyndromic mental retardation. Am J Hum Genet. 2008;82:1150–1157. doi: 10.1016/j.ajhg.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piton A, Redin C, Mandel JL. XLID-causing mutations and associated genes challenged in light of data from large-scale human exome sequencing. Am J Hum Genet. 2013;93:368–383. doi: 10.1016/j.ajhg.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richards S, et al. ACMG Laboratory Quality Assurance Committee Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cherepanova N, Shrimal S, Gilmore R. N-linked glycosylation and homeostasis of the endoplasmic reticulum. Curr Opin Cell Biol. 2016;41:57–65. doi: 10.1016/j.ceb.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shrimal S, Ng BG, Losfeld ME, Gilmore R, Freeze HH. Mutations in STT3A and STT3B cause two congenital disorders of glycosylation. Hum Mol Genet. 2013;22:4638–4645. doi: 10.1093/hmg/ddt312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carchon HA, Chevigné R, Falmagne JB, Jaeken J. Diagnosis of congenital disorders of glycosylation by capillary zone electrophoresis of serum transferrin. Clin Chem. 2004;50:101–111. doi: 10.1373/clinchem.2003.021568. [DOI] [PubMed] [Google Scholar]
- 25.Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet. 1992;51:1229–1239. [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bocchinfuso WP, Ma KL, Lee WM, Warmels-Rodenhiser S, Hammond GL. Selective removal of glycosylation sites from sex hormone-binding globulin by site-directed mutagenesis. Endocrinology. 1992;131:2331–2336. doi: 10.1210/endo.131.5.1425432. [DOI] [PubMed] [Google Scholar]
- 28.Rymen D, et al. MAN1B1 deficiency: An unexpected CDG-II. PLoS Genet. 2013;9:e1003989. doi: 10.1371/journal.pgen.1003989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.