Significance
The shape (linear vs. nonlinear), direction (negative vs. positive), and strength of the relationship between invasive alien species (IAS) abundance and native species diversity determines which invaders present the greatest risk to ecosystems. Yet, the form of the relationship between abundance and impact was previously unknown. Our metaanalyses reveal a strongly negative, convex relationship between invader abundance and native populations or communities when invaders are at higher trophic levels. Thus, on average, invasive species’ impacts are strongest at low invader abundance, highlighting the need for proactive policies to prevent introduction and eradicate early infestations. When invaders are at the same trophic levels, their impacts tended to be negative and linear, suggesting that treatment could benefit native communities regardless of invasion stage.
Keywords: community ecology, density dependence, ecological impacts, invasive species, per capita effect
Abstract
To predict the threat of biological invasions to native species, it is critical that we understand how increasing abundance of invasive alien species (IAS) affects native populations and communities. The form of this relationship across taxa and ecosystems is unknown, but is expected to depend strongly on the trophic position of the IAS relative to the native species. Using a global metaanalysis based on 1,258 empirical studies presented in 201 scientific publications, we assessed the shape, direction, and strength of native responses to increasing invader abundance. We also tested how native responses varied with relative trophic position and for responses at the population vs. community levels. As IAS abundance increased, native populations declined nonlinearly by 20%, on average, and community metrics declined linearly by 25%. When at higher trophic levels, invaders tended to cause a strong, nonlinear decline in native populations and communities, with the greatest impacts occurring at low invader abundance. In contrast, invaders at the same trophic level tended to cause a linear decline in native populations and communities, while invaders at lower trophic levels had no consistent impacts. At the community level, increasing invader abundance had significantly larger effects on species evenness and diversity than on species richness. Our results show that native responses to invasion depend critically on invasive species’ abundance and trophic position. Further, these general abundance–impact relationships reveal how IAS impacts are likely to develop during the invasion process and when to best manage them.
Invasive alien species (IAS) have negative effects on native species populations (i.e., decreased population sizes) and communities (i.e., reduction in species diversity). These negative impacts have been observed for many invasive alien taxa and across ecosystems (1–5). However, previous syntheses have assessed the effect of invader presence/absence without considering how impact might change with increasing invader abundance. As a result, the general shape of the relationship between invader abundance and native population or community response remains unknown. Understanding how invader impacts change with abundance is critical for predicting the severity of the impacts across recipient habitats (3, 6, 7), assessing the costs and benefits of treatment (8, 9), and prioritizing management actions (10).
Frameworks for assessing IAS impacts typically rely on assumed relationships between invader abundance and impact. For example, Parker et al. (11) proposed that an invader’s impacts are a function of its total range, abundance, and per capita effect (I = R*A*E). This equation specifies that impacts increase linearly with abundance, with no density-dependent relationship between abundance and per capita effect. Later impact frameworks explicitly hypothesized density-dependent relationships, with impacts increasing or decreasing nonlinearly with invader abundance (12, 13). The variety of possible relationships between abundance and impact highlights the strong need for an empirical assessment of this fundamental question across taxa (8). Moreover, it is unknown whether relationships between abundance and impact depend on the trophic positions of invading and native species. One review of invasive impact studies concluded that there was no clear effect of trophic position on impacts (14), while another metaanalysis focused on marine ecosystems suggested that impacts on native species switched from positive to negative, if invaders were in lower vs. higher trophic levels, respectively (4).
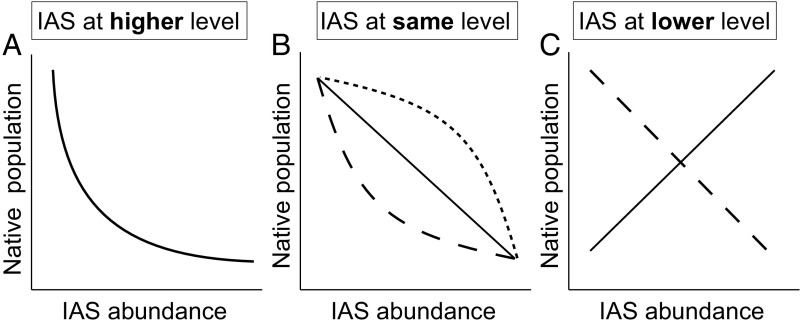
Classical ecological theory suggests that when an invasive alien species is at a higher trophic level than a native species, the invader is likely to cause a strong nonlinear decline in the native species population due to density dependence and a number of processes that alter the per capita effects of the invasive species (Fig. 1A and refs. 15 and 16). For example, the introduction of a novel alien predator or herbivore can lead to rapid decreases in native prey or plant population sizes (14, 17). Following this initial decline, native populations might later stabilize at lower sizes by persisting in refuges, through adaptation (evolution, phenotypic or behavioral plasticity), or by reaching a lower carrying capacity balanced by immigration of new individuals. These responses would result in a nonlinear relationship between invader abundance and native population size. For example, Benkwitt (18) observed a nonlinear decline in sizes of native fish populations following the introduction of the predatory invasive lionfish (Pterois volitans) in the Caribbean. Impacts at the community level are also hypothesized to be stronger when the IAS is at a higher trophic level than the invaded native species assemblage (19, 20), but the general shape of the relationship is unknown.
Fig. 1.
Hypothesized relationships between IAS abundance and native species’ population response. (A) IAS at higher trophic levels could prey upon natives, leading to a nonlinear decline of native species population sizes. (B) IAS at the same trophic level could compete with natives, leading to a linear decline (solid line), if competition is independent of density, or a nonlinear decline (dashed lines), if competition is density-dependent. (C) IAS at lower trophic levels could provide food or habitat resources, leading to a linear population increase (solid line), or could reduce resources for native species, leading to a linear decrease (dashed line).
When an invasive alien species is at the same trophic level as a native species, the invader could cause either a linear or nonlinear decline in the native species population size (Fig. 1B). Competition is the main mechanism for IAS impact when invasive and native species occupy the same trophic level (21). The impacts of competition could be linear, if per capita competitive effects are not density-dependent. However, field studies have also shown that competition can be density-dependent, leading to nonlinear declines in native species population sizes (22). Impacts at the community level for IAS at the same trophic level vary with the spatial scale of analysis (23), but the shape of the response relative to invader abundance is unknown.
Finally, when an invasive alien species is at a lower trophic level than a native species, the relationship between invader abundance and native species population size could be positive or negative (Fig. 1C). The direction of this relationship depends on whether the IAS acts as a novel resource for the native species or reduces resources upon which the native species depends. Previous metaanalyses of invader presence vs. absence suggest that negative impacts may be more likely. For example, the presence of invasive alien plants reduces the abundance of native animals (5), particularly native herbivorous insects (24), which are often specialists of native plants (25). Similarly, invasive primary producers in freshwater systems can have a negative effect on native fish (2), likely by disrupting access to resources. The direction of native community-level responses to IAS at lower trophic levels is even less clear. Previous metaanalyses in marine and freshwater ecosystems have found that invaders at lower trophic levels tended to increase (4) or have no significant overall effect on (2) the diversity of benthic invertebrates at higher trophic levels. Thus, impacts at the community level for IAS at lower trophic levels remain poorly understood.
Here, we present a global metaanalysis of responses of native species and communities to gradients of IAS abundance, quantifying the direction, strength, and shape of this relationship for different trophic interactions. We develop generalizations based on comprehensive empirical evidence of how the abundance–impact relationship varies between (i) native population and community responses (e.g., individual species abundance vs.. species diversity), (ii) invader taxon (plant, animal), and (iii) recipient habitat (freshwater, terrestrial, marine). This analysis of abundance–impact relationships across ecosystems provides a key test of ecological theory related to species and community-level responses to novel species interactions.
Results
We analyzed data from 1,258 unique case studies reported in 201 papers. Of the papers included in the dataset, 94 evaluated invasive plants, and 107 evaluated invasive animals (SI Appendix, Table S3.1). Almost all of the plant studies were terrestrial, whereas studies of invasive alien animals were well-distributed across habitat types. Spatially, most of the data were collected in North America, Europe, Australia, or New Zealand (SI Appendix, Fig. S3.1). This pattern is consistent with known biases in the invasion ecology literature (26), but the studies nonetheless encompass a broad range of alien taxa across habitat types.
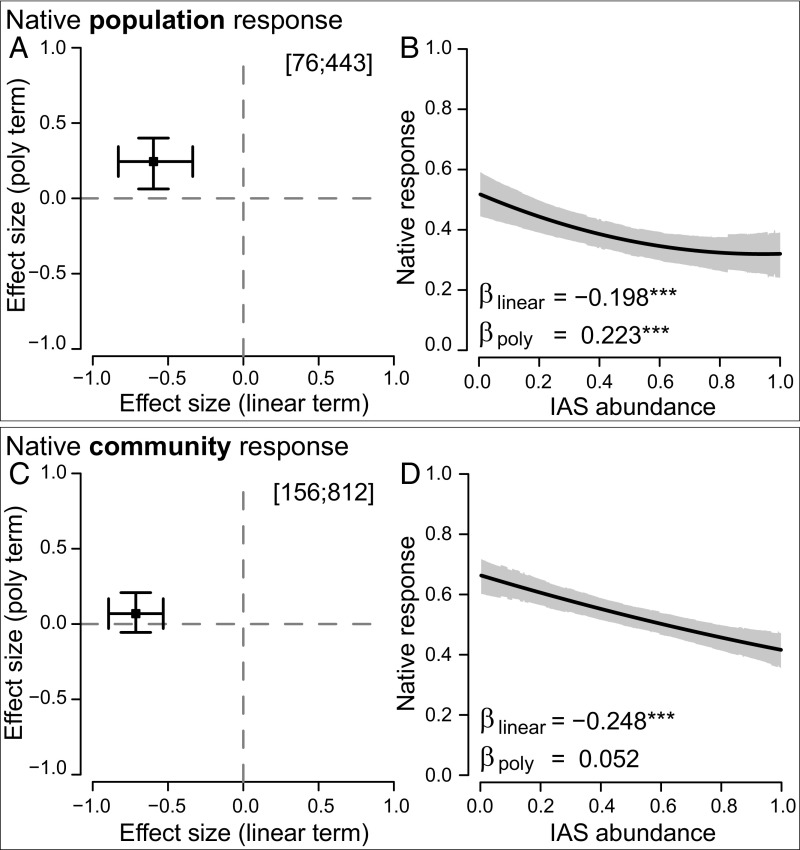
Native responses to IAS abundance at the population level had a significantly negative linear component but a significantly positive polynomial component, resulting in a nonlinear relationship with the most rapid rate of decline in native populations occurring at low invader abundance (Fig. 2 A and B; summary statistics for model contrasts are given in SI Appendix, Table S3.2). Native species populations declined by an average of 20% as IAS abundance increased (Fig. 2B). Native responses to IAS at the community level also had a significantly negative linear component, but no significant polynomial component, resulting in a negative linear shape (Fig. 2 C and D). Native community metrics (richness, diversity, evenness, or multispecies abundance) declined by an average of 25% as IAS abundance increased (Fig. 2D).
Fig. 2.
The shape of native species’ responses is nonlinear at the population level but linear at the community level. (A and C) Analyses based on partial-r. (B and D) The slope analyses. Numbers in brackets are total papers and studies analyzed. Effect size estimates in A and C are statistically supported when 95% credible interval bars do not cross the zero lines. Slope plots show model predictions (black line) with gray shading indicating the 95% credible zone. Significant linear (βlinear) or polynomial (βpoly) regression terms are indicated by asterisks (***P < 0.001).
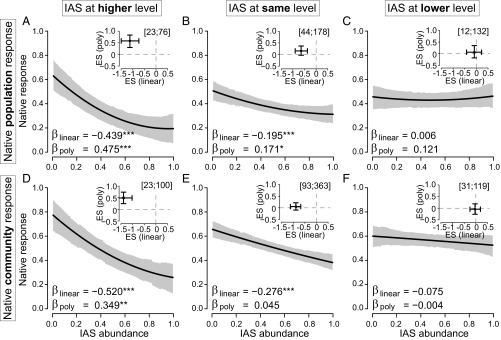
Abundance–impact relationships varied substantially and significantly depending on the relative trophic positions of the invasive and native species (Fig. 3). When IAS were at a higher trophic level, their impacts on native species populations and communities were strongly negative and nonlinear (Fig. 3 A and D). As IAS at higher trophic levels increased in abundance, native populations declined by an average of 44%, and native community metrics declined by an average of 52% (Fig. 3 A and D). However, IAS impacts weakened as their trophic position shifted from higher to lower (Fig. 3). For IAS at the same trophic level, native populations declined by an average of 20%, and native community metrics declined by an average of 28%. When IAS were at the same trophic level, their impacts on native species were significantly negative and nonlinear (Fig. 3B), while their impacts on communities were significantly negative and linear (Fig. 3E). When IAS were at a lower trophic level, they had no consistent impact on native species or communities (Fig. 3 C and F).
Fig. 3.
The shape and strength of IAS impacts on native species and communities depends strongly on relative trophic position. Results from the slope metaanalyses are shown in the main panels, and results from the partial-r metaanalyses are inset. (A–C) Native species’ population responses to invaders at higher, the same, and lower trophic levels, respectively. (D–F) Native community-level responses to invaders at higher, the same, and lower trophic levels, respectively. Significant linear (βlinear) or polynomial (βpoly) regression terms are indicated by asterisks (*P < 0.05; **P < 0.01; ***P < 0.001).
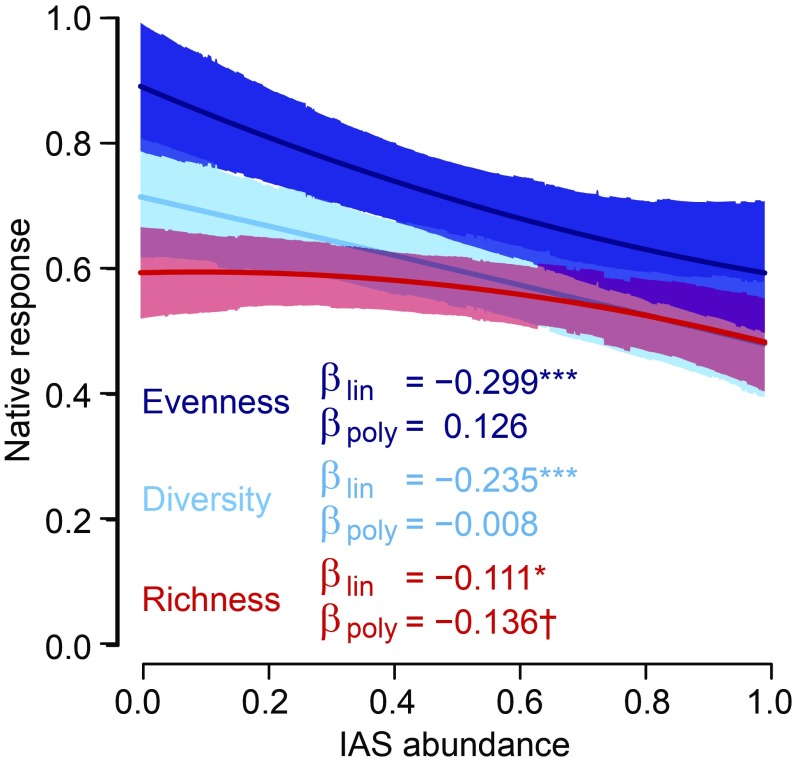
At the community level, increasing invader abundance had a significant negative effect on native species’ richness, Shannon diversity, and Pielou evenness (Fig. 4 and SI Appendix, Fig. S3.2). Although species richness was by far the most commonly reported diversity metric (85 papers, 218 studies), linear impacts were significantly more negative for native species evenness (P = 0.004) and diversity (P = 0.04; Fig. 4). On average (across all trophic categories) there were no significant nonlinearities between IAS abundance and community-level diversity. However, species richness showed a marginally nonsignificant negative polynomial term (P = 0.052; impacts on richness were more likely to be weakest at low invader abundance), and the polynomial term for richness was significantly lower than that for evenness (P = 0.01; Fig. 4).
Fig. 4.
IAS have significant negative linear effects on native community-level richness (red), diversity (cyan), and evenness (blue). There were significant differences between community-level responses for both linear and polynomial terms, which are reported in the results. Lines show model predictions, with shading indicating the 95% credible zone. Significant linear (βlin) or polynomial (βpoly) regression terms are indicated as follows:†P < 0.10; *P < 0.05; ***P < 0.001.
Compared with trophic position, recipient habitat (terrestrial, freshwater, or marine) explained little variation in the impacts of IAS on native species and communities (SI Appendix, Fig. S3.3). IAS at higher trophic levels generally had strongly negative, nonlinear effects on native species and communities regardless of habitat type, with freshwater habitat showing the strongest curvature. IAS at the same trophic level generally had negative linear effects across habitat types, although there was some curvature in freshwater habitat. IAS at lower trophic levels generally had no effect, although species and communities in terrestrial habitats were likely to show a weak negative linear response (SI Appendix, Fig. S3.3).
Responses of native species and communities to IAS abundance varied depending on invader taxon (animals vs. plants; SI Appendix, Fig. S3.4). At a higher trophic level, invasive animals had significant negative nonlinear effects on native species and communities (there were no plants at higher trophic levels). Invasive animals and plants at the same trophic level both drove negative impacts in native species, but responses to invasive animals were significantly nonlinear, while those to invasive plants were significantly linear. At lower trophic levels, invasive animals had no consistent impacts, while invasive plants had a small but significant negative linear effect (partial-r P = 0.002; SI Appendix, Fig. S3.4). Linear effect sizes did not vary significantly among study types (spatial, temporal, experimental studies; SI Appendix, Fig. S3.5).
Discussion
Our global metaanalysis quantifies general trends in the direction, shape, and strength of the relationship between IAS abundance and native response across trophic levels, invader taxon, and recipient habitat. Negative impacts of IAS clearly predominate across terrestrial, freshwater, and marine habitats and are caused by both animal and plant invaders. Negative impacts are common when IAS are at higher or the same trophic level as native species, and native population or community declines of 20–25% were typical. Across trophic interactions, invader taxon, and recipient habitat, there were no general trends of invader abundance having a positive effect on native populations or communities. Our results also show that native responses to IAS can be strongly nonlinear (convex), suggesting that impacts are strongest at low levels of IAS abundance during the earliest stages of invasion.
When IAS were at higher trophic levels, impacts were consistently nonlinear for both native populations and communities (Fig. 3 A and D). A nonlinear effect on native species populations is supported by ecological theory of predator–prey interactions (Fig. 1A). IAS at higher trophic levels are also thought to have stronger effects on native communities than those at other trophic levels (19). However, a general nonlinear effect on native communities has not been previously described. Low invader abundance is most likely to occur early in the invasion process. Thus, early detection and rapid response to new invasions (27, 28) will be most effective for reducing impacts of invasive animals because they are most likely to impose nonlinear effects on recipient habitats (SI Appendix, Fig. S3.4 A and B). Similarly, eradicating animal invaders, such as alien mammals on islands (29), is a much more effective means of supporting native species than reducing the populations of abundant animal invaders. If eradication is not possible, our results suggest that once IAS at higher trophic levels reach high abundance, management will be less effective for mitigating impacts.
When IAS were at the same trophic level as natives, our results highlight a consistent, negative impact on both populations and communities (Fig. 3 B and E). This negative impact tended to be linear for community-level metrics. However, our results also suggest that nonlinear responses to invaders at the same trophic level are likely when the native response is at the population level (Fig. 3B) and particularly when the IAS is an animal (SI Appendix, Fig. S3.4B). Density-dependent competition is common in animal species (30). Although density-dependent competition has also been observed for plant species (13, 22), it was not evident in our analysis (SI Appendix, Fig. S3.4D). Thus, nonlinear relationships between an invasive and native species at the same trophic level appear most likely to occur when the invader is an animal. Our results also suggest that IAS can precipitate negative, linear effects on native communities at the same trophic level (Fig. 3E). For IAS mainly interacting with native communities on the same trophic level (e.g., as competitors), management aimed at reducing IAS abundance could be effective for promoting community diversity at any stage of invasion.
We did not find consistent, significant relationships between IAS abundance and native population or community response when IAS were at a lower trophic level (Fig. 3 C and F). However, negative, linear effects were more likely to be observed when the recipient habitat was terrestrial (SI Appendix, Fig. S3.3C) and when the invader was a plant (SI Appendix, Fig. S3.4E). Previous metaanalyses have suggested that IAS impacts can cascade up to higher trophic levels (2, 5, 24), which could be due to a loss of native resources. For example, native insects tend to be specialists (25); thus, competitive suppression of native plants by invasive alien plants is likely to negatively affect native insects and potentially animals at higher trophic levels that feed on insects (24). In contrast to Thomsen et al. (4), on average we found no consistent impacts of IAS at lower trophic levels in marine habitats (SI Appendix, Fig. S3.3I). Some marine IAS are foundation species that create new habitat structure, which can increase space and physical resources for native species (31). Our results for marine habitat suggest that in these systems, natives may be experiencing both positive and negative effects from IAS (SI Appendix, Fig. S3.3I). Overall, the lack of significant positive effects and presence of several weak but significant negative effects suggests that IAS at lower trophic levels tend to remove resources for native consumers rather than add them. Thus, management of invasive abundance at any stage of invasion may provide some benefit for native species at higher trophic levels, particularly for terrestrial plant invasions.
Our analysis highlights a consistent, negative effect of IAS abundance across all three community-level metrics (Fig. 4). These results contrast with previous findings of increased community richness due to the addition of alien species (32). However, Sax and Gaines (32) focused on the establishment phase of invasion, before spread and impact (e.g., ref. 33). Our results show that as invaders become more abundant, community-level impacts are clearly negative. This negative effect was significantly stronger for evenness and diversity than for richness. Species richness is a conservative measure of community-level changes, requiring species extinctions or additions to register change. Metapopulation models of invasive alien plants suggest that they could take hundreds of years to cause extinctions [i.e., a decline in species richness (7)]. Our results also suggest that community evenness is likely to decline predominantly linearly, whereas richness is more likely to decline more slowly early in the invasion process and more rapidly later, at high invader abundance (negative polynomial; Fig. 4 and SI Appendix, Fig. S3.2). This pattern may be due to a tendency of invasive species to affect common native species early in the invasion process and rare native species only later (34). While extinctions leading to lower richness may not be apparent until later stages of invasion, changes in species abundance and therefore evenness may occur more quickly and appear to be more sensitive metrics of community change (Fig. 4).
In conclusion, regardless of trophic level, taxon, or recipient habitat, we found that increasing the abundance of IAS has pronounced negative impacts on native species’ populations and communities. In many cases, negative, strongly nonlinear relationships suggest that rapid declines in native species’ population sizes can occur at initial stages of the invasion process. The presence of nonlinear relationships highlights the increasing need for early detection and rapid response (EDRR) to new IAS (27). EDRR is cost-effective (35) and the only point at which eradication is feasible (36). Increasing trade (37), disturbance (38), and climate change (39) make it likely that IAS will continue to be introduced. Avoiding the ecological impacts of invasive species will require a much stronger commitment to proactive policies designed to prevent novel introductions (38) as well as increased management targeting the early stages of invasion.
Materials and Methods
Literature Search.
We searched the Web of Science Core Collection for all records through December 31, 2016. Our search terms (SI Appendix, part 1) were chosen to identify papers that focused on the impacts of IAS on native populations or communities and that contained information on the abundance or density of the IAS. We assessed the titles of the 7,557 returned papers for those reporting native impacts of an IAS across an abundance gradient. We reviewed the 490 resulting papers to identify those that fit the following criteria: (i) it was either explicit or likely that the native response was caused by the IAS, (ii) the paper presented at least four IAS abundance values and corresponding native response values such that shape could be measured, and (iii) the paper included empirical data.
The vast majority of relevant papers focused on single IAS, but we also included papers that involved multiple IAS. We only considered papers where the response variable(s) measured native species abundance (biomass, cover, density, or proportion) and/or measured native community response (multispecies abundance, Shannon diversity, species richness, or Pielou evenness). We included observational studies across space (spatial; measurements along an IAS abundance gradient) or over an invasion time series (temporal; IAS abundance changing over time) as well as experimental manipulations of IAS abundance.
Data Extraction.
Where empirical data were presented graphically, we used the Web Plot Digitizer application (https://github.com/ankitrohatgi/WebPlotDigitizer/releases) to extract values. If the data were transformed, we back-transformed them. When the raw empirical data were not presented in full, we emailed corresponding authors to request them. When possible, we calculated Shannon diversity and Pielou evenness from abundance and species richness data. Where papers presented multiple datasets or multiple combinations of IAS abundance and native responses, we extracted these as distinct datasets (hereafter, studies), such that single papers could contribute multiple studies to our analysis.
Data Categorization.
We extracted trophic relationships between the IAS and native species or community from the paper or sources cited within the paper. Trophic categories included “Same” when the native and IAS occupied the same trophic level; “Lower” when the IAS was at a lower trophic level than the native; and “Higher” when the IAS was at a higher trophic level than the native. When trophic information was not reported, we categorized some interactions based on kingdom (e.g., invasive plant vs. native plant was always “Same”; invasive plant vs. native animal was always “Lower”). For studies of invasive alien animal vs. native animal with no trophic information presented in the paper, we used a Google Scholar search for the IAS as well as “diet” or “feed” to identify the relative trophic position of the IAS. In cases where the invasive and native animals were fish, we also searched for trophic status in FishBase (www.fishbase.org). Species whose trophic position changed during their life cycle (e.g., fish can switch from competitors at juvenile stages to predators as adults) and species with unknown trophic positions were excluded from the trophic analyses.
In addition to trophic level, we analyzed the results by invader taxon (plant, animal), habitat (terrestrial, freshwater, marine), and study type (spatial, temporal, experimental). Marine algae were categorized as plants. Wetland plants were considered terrestrial, with only floating plants considered freshwater or marine. Experimental studies that took place over space or time were categorized as experimental. Observational studies over both space and time were categorized as multiple.
Metaanalysis.
We used two complementary metaanalyses to evaluate the relationship between IAS abundance and native species’ responses at the population and community level. Results from both metaanalyses were used to determine the direction and strength of linear and polynomial components to the invasive abundance–native response relationship. Results from the second metaanalysis were additionally used to reconstruct the average shape of this relationship. Both metaanalyses used a regression model to extract information on response direction, strength, and shape (curvature) from the raw IAS abundance–native response data:
| [1] |
where y was the native response, x was the IAS abundance, β0 was the intercept, βlinear was the linear regression term, and βpoly was the second-order polynomial regression term. The regression model was fit separately to raw data for each study.
The first metaanalysis derived effect sizes from Fisher-transformed partial correlation coefficients associated with each regression term from Eq. 1 (ref. 40; hereafter, partial-r metaanalysis):
| [2] |
| [3] |
where r is the partial correlation coefficient for one of the regression terms in Eq. 1 (βlinear or βpoly), t is the corresponding model t value, and df are the degrees of freedom associated with the same regression coefficient (40). Partial-r effect sizes were calculated separately for the linear and polynomial terms in Eq. 1 for each study. Effect size measurement error variance (mev) was calculated as 1/(n − 3), where n is the sample size for a study (41). We mean-centered the IAS abundance (x) for each study before fitting Eq. 1. Repositioning of the x axis to a mean of zero has no impact on invasive abundance–native response shape, but reduced dependence between linear and polynomial effect sizes within studies (42). Metaanalysis of the partial-r effect sizes allowed us to determine the strength and direction of linear and polynomial components of the regression fit.
The second metaanalysis derived effect sizes from the three regression terms (β0, βlinear, βpoly) in Eq. 1 (hereafter, slope metaanalysis). However, an analysis of regression terms requires that IAS abundance and native responses (x and y variables) be on a comparable scale (regression terms are scale-dependent; refs. 43 and 44). Thus, we rescaled the raw data (both invasive abundance, x, and native responses, y) by dividing by the maximum raw data value to create a scale of 0–1. We then mean-centered the rescaled IAS abundance values before analysis using Eq. 1 to generate three regression-term effect sizes (β0, βlinear, βpoly). We used the regression model-reported SE for each regression term as an estimate of effect size mev (44). Results from the slope metaanalysis were used to determine the shape of the relationship between IAS abundance and native responses and provided an additional test of the magnitude of linear and polynomial regression terms (SI Appendix, part 1).
Bayesian mixed-effects models (MCMCglmm in R version 3.5.1; refs. 45 and 46) were used for all metaanalyses of the IAS–native response relationship and to test for variation in invasive impacts among different trophic categories, between community- and population-level responses, in different habitats, and between invasive animals and plants. Full analytical details are presented in SI Appendix, part 1 and code is available at ref. 47.
Data Availability.
Citations of papers analyzed in this metaanalysis are presented in SI Appendix, part 2 and data are available at ref. 48.
Supplementary Material
Acknowledgments
We thank E. Beaury, D. Blumenthal, J. Dukes, J. Finn, J. Gill, D. Goldberg, and T. Morelli for valuable discussions. C. Karounos and T. Cross assisted with initial data extraction. This work was initiated at a working group led by C.J.B.S., B.A.B., A.E.B., and R.E. and was supported by the Albert and Elaine Borchard Foundation. B.A.B. gratefully acknowledges the hospitality of D. Richardson and the Centre for Invasion Biology at Stellenbosch University during an academic sabbatical. M.V. is grateful for funding from the projects IMPLANTIN (CGL2015-65346-R) of the Spanish Ministerio de Ciencia, Innovación y Universidades, and i-LINK-1198 from the Consejo Superior de Investigaciones Científicas.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Data used in the study are available on UMass Scholarworks at https://scholarworks.umass.edu/data/67/. R scripts used in the study are available on Zenodo at https://doi.org/10.5281/zenodo.2605254.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1818081116/-/DCSupplemental.
References
- 1.Cameron EK, Vilà M, Cabeza M. Global meta-analysis of the impacts of terrestrial invertebrate invaders on species, communities and ecosystems. Glob Ecol Biogeogr. 2016;25:596–606. [Google Scholar]
- 2.Gallardo B, Clavero M, Sánchez MI, Vilà M. Global ecological impacts of invasive species in aquatic ecosystems. Glob Change Biol. 2016;22:151–163. doi: 10.1111/gcb.13004. [DOI] [PubMed] [Google Scholar]
- 3.Thomsen MS, Olden JD, Wernberg T, Griffin JN, Silliman BR. A broad framework to organize and compare ecological invasion impacts. Environ Res. 2011;111:899–908. doi: 10.1016/j.envres.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 4.Thomsen MS, et al. Impacts of marine invaders on biodiversity depend on trophic position and functional similarity. Mar Ecol Prog Ser. 2014;495:39–47. [Google Scholar]
- 5.Vilà M, et al. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol Lett. 2011;14:702–708. doi: 10.1111/j.1461-0248.2011.01628.x. [DOI] [PubMed] [Google Scholar]
- 6.Cassey P, Blackburn TM, Lockwood JL, Sax DF. A stochastic model for integrating changes in species richness and community similarity across spatial scales. Oikos. 2006;115:207–218. [Google Scholar]
- 7.Gilbert B, Levine JM. Plant invasions and extinction debts. Proc Natl Acad Sci USA. 2013;110:1744–1749. doi: 10.1073/pnas.1212375110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sofaer HR, Jarnevich CS, Pearse IS. The relationship between invader abundance and impact. Ecosphere. 2018;9:e02415. [Google Scholar]
- 9.Yokomizo H, Possingham HP, Thomas MB, Buckley YM. Managing the impact of invasive species: The value of knowing the density-impact curve. Ecol Appl. 2009;19:376–386. doi: 10.1890/08-0442.1. [DOI] [PubMed] [Google Scholar]
- 10.Byers JE, et al. Directing research to reduce the impacts of nonindigenous species. Conserv Biol. 2002;16:630–640. [Google Scholar]
- 11.Parker IM, et al. Impact: Toward a framework for understanding the ecological effects of invaders. Biol Invasions. 1999;1:3–19. [Google Scholar]
- 12.Thiele J, Kollmann J, Markussen B, Otte A. Impact assessment revisited: Improving the theoretical basis for management of invasive alien species. Biol Invasions. 2010;12:2025–2035. [Google Scholar]
- 13.Barney JN, Tekiela DR, Dollete ES, Tomasek BJ. What is the “real” impact of invasive plant species? Front Ecol Environ. 2013;11:322–329. [Google Scholar]
- 14.Ricciardi A, Hoopes MF, Marchetti MP, Lockwood JL. Progress toward understanding the ecological impacts of nonnative species. Ecol Monogr. 2013;83:263–282. [Google Scholar]
- 15.Lotka AJ. Elements of Physical Biology. Williams & Wilkins; Baltimore: 1925. [Google Scholar]
- 16.Volterra V. Fluctuations in the abundance of a species considered mathematically. Nature. 1926;118:558–560. [Google Scholar]
- 17.Strayer DL. Alien species in fresh waters: Ecological effects, interactions with other stressors, and prospects for the future. Freshw Biol. 2010;55:152–174. [Google Scholar]
- 18.Benkwitt CE. Non-linear effects of invasive lionfish density on native coral-reef fish communities. Biol Invasions. 2015;17:1383–1395. [Google Scholar]
- 19.Moyle PB, Light T. Biological invasions of fresh water: Empirical rules and assembly theory. Biol Conserv. 1996;78:149–161. [Google Scholar]
- 20.Estes JA, et al. Trophic downgrading of planet Earth. Science. 2011;333:301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 21.Levine JM, et al. Mechanisms underlying the impacts of exotic plant invasions. Proc Biol Sci. 2003;270:775–781. doi: 10.1098/rspb.2003.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Law R, Watkinson AR. Response-surface analysis of two-species competition: An experiment on Phleum arenarium and Vulpia fasciculata. J Ecol. 1987;75:871–886. [Google Scholar]
- 23.Powell KI, Chase JM, Knight TM. A synthesis of plant invasion effects on biodiversity across spatial scales. Am J Bot. 2011;98:539–548. doi: 10.3732/ajb.1000402. [DOI] [PubMed] [Google Scholar]
- 24.Litt AR, Cord EE, Fulbright TE, Schuster GL. Effects of invasive plants on arthropods. Conserv Biol. 2014;28:1532–1549. doi: 10.1111/cobi.12350. [DOI] [PubMed] [Google Scholar]
- 25.Tallamy DW. Do alien plants reduce insect biomass? Conserv Biol. 2004;18:1689–1692. [Google Scholar]
- 26.Hulme PE, et al. Bias and error in understanding plant invasion impacts. Trends Ecol Evol. 2013;28:212–218. doi: 10.1016/j.tree.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Westbrooks RG. New approaches for early detection and rapid response to invasive plants in the United States. Weed Technol. 2004;18:1468–1471. [Google Scholar]
- 28.Crall AW, et al. Developing cost-effective early detection networks for regional invasions. Biol Invasions. 2012;14:2461–2469. [Google Scholar]
- 29.Courchamp F, Chapuis J-L, Pascal M. Mammal invaders on islands: Impact, control and control impact. Biol Rev Camb Philos Soc. 2003;78:347–383. doi: 10.1017/s1464793102006061. [DOI] [PubMed] [Google Scholar]
- 30.Hairston NG, Smith FE, Slobodkin LB. Community structure, population control, and competition. Am Nat. 1960;94:421–425. [Google Scholar]
- 31.Gutiérrez JL. 2017. Modification of habitat quality by non-native species. Impact of Biological Invasions on Ecosystem Services, Invading Nature–Springer Series in Invasion Ecology, eds Vilà M, Hulme PE (Springer Int Publ, Cham, Switzerland), pp 33–47.
- 32.Sax DF, Gaines SD. Species diversity: From global decreases to local increases. Trends Ecol Evol. 2003;18:561–566. [Google Scholar]
- 33.Theoharides KA, Dukes JS. Plant invasion across space and time: Factors affecting nonindigenous species success during four stages of invasion. New Phytol. 2007;176:256–273. doi: 10.1111/j.1469-8137.2007.02207.x. [DOI] [PubMed] [Google Scholar]
- 34.Powell KI, Chase JM, Knight TM. Invasive plants have scale-dependent effects on diversity by altering species-area relationships. Science. 2013;339:316–318. doi: 10.1126/science.1226817. [DOI] [PubMed] [Google Scholar]
- 35.Leung B, et al. An ounce of prevention or a pound of cure: Bioeconomic risk analysis of invasive species. Proc Biol Sci. 2002;269:2407–2413. doi: 10.1098/rspb.2002.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rejmanek M, Pitcairn MJ. When is eradication of exotic pest plants a realistic goal? In: Veitch CR, Clout MN, editors. Turning the Tide: The Eradication of Invasive Species : Proceedings of the International Conference on Eradication of Island Invasives. Int Union for the Conserv of Nature; Gland, Switzerland: 2002. [Google Scholar]
- 37.Bradley BA, et al. Global change, global trade, and the next wave of plant invasions. Front Ecol Environ. 2012;10:20–28. [Google Scholar]
- 38.Early R, et al. Global threats from invasive alien species in the twenty-first century and national response capacities. Nat Commun. 2016;7:12485. doi: 10.1038/ncomms12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allen JM, Bradley BA. Out of the weeds? Reduced plant invasion risk with climate change in the continental United States. Biol Conserv. 2016;203:306–312. [Google Scholar]
- 40.Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: A practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82:591–605. doi: 10.1111/j.1469-185X.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- 41.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. John Wiley and Sons; West Sussex, UK: 2011. [Google Scholar]
- 42.Schielzeth H. Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol. 2010;1:103–113. [Google Scholar]
- 43.Becker BJ, Wu M-J. The synthesis of regression slopes in meta-analysis. Stat Sci. 2007;22:414–429. [Google Scholar]
- 44.Koricheva J, Gurevitch J, Mengersen K. Handbook of Meta-Analysis in Ecology and Evolution. Princeton Univ Press; Princeton, NJ: 2013. [Google Scholar]
- 45.R Core Team 2018 R: A Language and Environment for Statistical Computing (R Found for Stat Comput, Vienna), Version 3.1.2. Available at https://www.R-project.org.
- 46.Hadfield JD. MCMC methods for multi-response generalized linear mixed models: The MCMCglmm R package. J Stat Softw. 2010;33:1–22. [Google Scholar]
- 47.Whitlock R. 2019 doi: 10.5281/zenodo.2605253. First release of invasive species abundance-native species response meta-analysis code. Available at . . Deposited on March 25, 2019. [DOI]
- 48.Bradley BA. 2019 doi: 10.7275/tjbv-qn87. Abundance vs. Impact (AvI) databases and code. Available at . . Deposited on March 25, 2019. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Citations of papers analyzed in this metaanalysis are presented in SI Appendix, part 2 and data are available at ref. 48.