Significance
Amazing fossil discoveries over the last 30 years have led to the paleontological consensus that some Mesozoic mammaliaforms underwent ecomorphological diversification in the midst of dinosaurs. However, the ecological structure of Mesozoic mammaliaform communities remains unclear. Here, we quantify the ecological structure of extinct and extant small-bodied mammaliaform communities aiming to identify evolutionary and ecological drivers that have influenced those communities through time. We used body size, diet, and locomotion of constituent species to plot ecospace occupation and calculate ecological richness and disparity of those communities. We propose that the interplay of Late Cretaceous dental evolution, the rise of angiosperms, and competition with other vertebrates were critical in shaping the ecological structure of small-bodied mammaliaform communities through time.
Keywords: Mesozoic mammaliaform, mammal community, ecological structure, tribosphenic molar, angiosperm diversification
Abstract
The long-standing view that Mesozoic mammaliaforms living in dinosaur-dominated ecosystems were ecologically constrained to small size and insectivory has been challenged by astonishing fossil discoveries over the last three decades. By studying these well-preserved early mammaliaform specimens, paleontologists now agree that mammaliaforms underwent ecomorphological diversification during the Mesozoic Era. This implies that Mesozoic mammaliaform communities had ecological structure and breadth that were comparable to today’s small-bodied mammalian communities. However, this hypothesis remains untested in part because the primary focus of most studies is on individual taxa. Here, we present a study quantifying the ecological structure of Mesozoic mammaliaform communities with the aim of identifying evolutionary and ecological drivers that influenced the deep-time assembly of small-bodied mammaliaform communities. We used body size, dietary preference, and locomotor mode to establish the ecospace occupation of 98 extant, small-bodied mammalian communities from diverse biomes around the world. We calculated ecological disparity and ecological richness to measure the magnitude of ecological differences among species in a community and the number of different eco-cells occupied by species of a community, respectively. This modern dataset served as a reference for analyzing five exceptionally preserved, extinct mammaliaform communities (two Jurassic, two Cretaceous, one Eocene) from Konservat-Lagerstätten. Our results indicate that the interplay of at least three factors, namely the evolution of the tribosphenic molar, the ecological rise of angiosperms, and potential competition with other vertebrates, may have been critical in shaping the ecological structure of small-bodied mammaliaform communities through time.
Astounding fossil discoveries accumulated over the last 30 years have challenged the long-standing view that most Mesozoic mammaliaforms (the last common ancestor of Sinoconodon and Mammalia and all of its descendants; ref. 1) were constrained to a small part of the ecospace—generalized, small-bodied, nocturnal insectivores—and that they were able to diversify ecologically only after the extinction of nonavian dinosaurs at the Cretaceous–Paleogene (K/Pg) boundary (2–5). Near-complete skulls and skeletons of Mesozoic mammaliaforms have revealed unexpectedly specialized forms, including colonial-insect feeding diggers, semiaquatic carnivores, and even herbivorous gliders, some of which arose independently, multiple times in distinct lineages (e.g., refs. 6–10). Analyses of large-scale morphological datasets have similarly indicated that some mammaliaform clades underwent ecological radiation beginning in the Cretaceous, perhaps in-step with the rise to dominance of angiosperms (11–13), and others may have done so even earlier in the Jurassic (14, 15). These findings imply that Mesozoic mammaliaform communities possibly attained ecological breadth comparable to today’s small-bodied mammalian communities. This hypothesis is untested, however, because comprehensive analysis of mammaliaform fossil communities, rather than individual taxa or clades, has never been done; thus, how the ecological structure of small-bodied mammaliaform communities evolved through time—as well as the factors that shaped it—remain unknown.
Today various biotic and abiotic characteristics of the environment are thought to structure mammalian communities (e.g., refs. 16–19). These factors operate over ecological hierarchies via physiological and ecological processes, such as metabolism, predation, competition, climatic seasonality, primary productivity, and habitat tiering (20, 21). Given the fundamental nature of these processes, they likely operated in the past to shape Mesozoic mammaliaform communities as well. However, in light of major differences in the biotic and abiotic context (e.g., species composition) and the stage of mammaliaform evolution in the Mesozoic, we might expect significant differences in how those processes affected ecological structure.
Here, we aim to reconstruct how the ecology of mammaliaform communities has changed since the Mesozoic, and use the resulting patterns to elucidate intrinsic and extrinsic influences on community assembly through time. We use a “taxon-free” approach (i.e., an approach not relying on ecological inferences from modern relatives) to first quantify the ecological structure of 98 extant, small-bodied mammalian communities from diverse biomes around the world, an approach that has not been attempted at this scale (but see refs. 20, 22, and 23). We use this reference dataset to analyze five exceptionally preserved mammaliaform paleocommunities (two Jurassic, two Cretaceous, one Eocene). Comprehensive inference of the paleoecology of mammaliaform paleocommunities is typically limited by the incompleteness of the fossil record; most mammaliaform fossil localities, particularly those from Mesozoic terrestrial deposits, yield only isolated elements, predominantly teeth and fragmentary jaws. We restrict our study to paleocommunities from Konservat-Lagerstätten from the Mid-Upper Jurassic and Lower Cretaceous of northeastern China and the middle Eocene of Germany (24, 25) (SI Appendix, Table S1); consequently, many of the taxa are represented by nearly complete skulls and associated skeletons (24–27), which have been studied individually (autecology) but not together as a paleocommunity (synecology). The completeness of these fossil assemblages enables us to robustly categorize each species in each community, extant and extinct, according to three ecological parameters: body size, dietary preference, and locomotor mode (SI Appendix, Table S1 and Dataset S1). These parameters reflect important aspects of a species autecology, including physiology, food resource use, habitat use, and survival strategies (28). From the five body-size ranks, six dietary-preference ranks, and eight locomotor-mode ranks, we constructed a three-dimensional ecospace (sensu ref. 29) of 240 ecological parameter value combinations, hereafter referred to as eco-cells, that approximate functional niches or ecological roles (sensu refs. 30–32). We plotted the eco-cells occupied by each mammalian community in our study, and quantitatively characterized the filled ecospace using two taxon-free indices: ecological disparity (EDisp) and ecological richness (ERich). EDisp measures the magnitude of ecological differences among species of a community in a given ecospace. ERich tallies the number of eco-cells occupied by a community in a given ecospace. Our results indicate that both intrinsic (predominance of the tribosphenic molar) and extrinsic (ecological rise of angiosperms, potentially competition) factors have vitally shaped the ecological structure of mammaliaform communities through time.
Results and Discussion
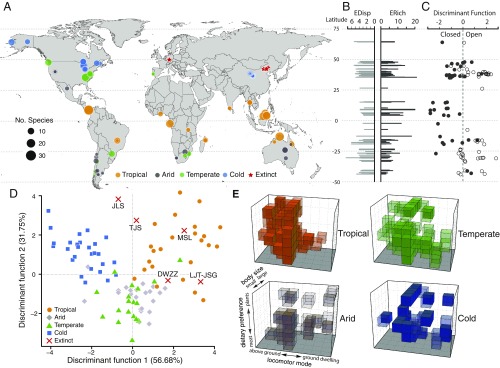
The 98 extant, small-bodied mammalian communities together occupy 41.25% of the theoretically possible modes of life in our ecospace (i.e., 99 of the 240 eco-cells; SI Appendix, Table S12). The most frequently occupied eco-cell in this realized ecospace (sensu ref. 29) is that for very small (<32 g), terrestrial insectivores (67 unique occurrences), followed closely by small (32–128 g), terrestrial herbivores (61 unique occurrences; SI Appendix, Table S13). Large regions of the ecospace are unoccupied, perhaps due to the low viability of some ecological combinations (28). For example, we do not find any saltatorial, fossorial, semifossorial, or semiaquatic frugivores (SI Appendix, Table S13), likely because fleshy fruits have limited availability in the habitats where these locomotor modes are most advantageous (open environments and aquatic environments, respectively; ref. 33). A conspicuous, sparsely filled region of the ecospace is that for mammals in our medium- to large body-size categories (128–2,048 g) with locomotor modes associated with open habitats (i.e., saltatorial, fossorial, and semifossorial; SI Appendix, Table S13; see also discussion below). It does not seem to be a case of ecological inviability because those eco-cells are filled by some fossil taxa in our study (SI Appendix, Tables S1 and S13).
Ecological Structure by Habitat Openness.
Our analysis shows that, within the realized ecospace of extant, small-bodied mammalian communities (filling 99 eco-cells), structure varies with habitat openness (SI Appendix, Figs. S5A and S6A and Tables S6 and S8). Closed-habitat communities differ from open-habitat communities in densely populating the region of ecospace that corresponds to tree-dwelling locomotor modes and in occupying the full range of diet types, including frugivory (SI Appendix, Figs. S5A and S6A). On average, closed-habitat communities also fill more eco-cells than open-habitat communities do (ERichclosed = 8.40, ERichopen = 4.75; Student’s t test, P < 0.001; SI Appendix, Fig. S2 and Tables S6 and S8); however, EDisp does not significantly differ with habitat openness (SI Appendix, Fig. S1 and Table S7). Although some ecologies are common in both habitat types (scansorial and terrestrial locomotion, omnivorous and insectivorous diets), our discriminant function analysis (DFA) indicates that open- and closed-habitat communities have distinct ecological compositions (Fig. 1D and SI Appendix, Figs. S5A and S6A). The distribution of body sizes is more even in closed-habitat communities, whereas in open-habitat communities smaller-bodied species (<128 g) predominate (SI Appendix, Fig. S5A and Tables S5 and S15). Open-habitat communities also have more granivores (they lack frugivores entirely) and more hopping and burrowing mammals; whereas closed-habitat communities have more mammals that climb and live within trees and, in addition to frugivores, have more carnivores and omnivores (SI Appendix, Fig. S5A and Tables S5 and S15).
Fig. 1.
Ecological structure of 98 extant, small-bodied mammalian communities sampled around the world. (A) The locations of the 98 extant, small-bodied mammalian communities and five extinct mammaliaform communities sampled in this study. (B) EDisp and ERich of the extant, small-bodied mammalian communities plotted against latitude. (C) DF1 scores from the habitat-openness DFA plotted against latitude. (D) Plot of DF1 vs. DF2 scores from the climate-type DFA and scores of the extinct communities projected onto the plot. (E) Composite plots of ecospace occupation of the extant, small-bodied mammalian communities from the four climate types, showing all occupied eco-cells for the communities from that climate type. See Fig. 2D for more detailed key to ecospace axes. MSL, Messel.
Ecological Structure by Climate Type.
The ecological structure of extant, small-bodied mammalian communities also varies with climate type. A caveat is that some climate categories are a composite of multiple vegetation types with divergent ecological signals; thus, differential sampling of vegetation types might misrepresent ecological structure in those climate types. Tropical climate communities, for example, are predominantly drawn from tropical forests (Dataset S1), overwhelming the markedly different ecological signal from savannas (see below, Ecological Structure by Vegetation Type). Therefore, like closed-habitat communities, tropical communities are shifted toward the region of the ecospace that corresponds to tree-dwelling locomotor modes (Fig. 1E, Left side of ecospace). Arid and cold communities—and to a lesser extent temperate communities—more densely populate the regions of the ecospace that correspond to ground-dwelling and burrowing locomotion (Fig. 1E, Right side), and are generally more spread across the ecospace than are tropical communities. Tropical mammalian communities have, on average, high ecological richness (ERichtropical = 8.63) and low ecological disparity (EDisptropical = 3.49; SI Appendix, Figs. S1 and S2 and Table S6); whereas mammalian communities in arid climates have lower ecological richness (ERicharid = 4.31) and occupy more disparate regions of the ecospace (EDisparid = 3.75; SI Appendix, Figs. S1 and S2 and Table S6). Temperate and cold climate communities have intermediate ecological richness (ERichtemperate = 7.10, ERichcold = 6.04) and significantly higher ecological disparity than communities in both tropical and arid climates (EDisptemperate = 4.12, EDispcold = 4.54; pairwise Student’s t test, P < 0.015; SI Appendix, Figs. S1 and S2 and Tables S6–S8). Our DFA discriminates well among climate types (Fig. 1D and SI Appendix, Fig. S11 A, C, and E). Tropical communities tend to have mammals in our medium- to very large body-size categories (>128 g), frugivorous and omnivorous diets, and arboreal locomotion (SI Appendix, Figs. S5B and S11 B and D and Tables S5 and S15). In contrast, cold communities strongly segregate by herbivorous, gliding, semiaquatic, semifossorial, and saltatorial mammals (SI Appendix, Figs. S11 A and B and S5B and Tables S5 and S15). Although temperate and arid communities both have many mammals of the smallest body-size category (<32 g), temperate communities tend to have more carnivorous, insectivorous, and scansorial mammals, whereas arid communities have more granivorous and fossorial mammals (SI Appendix, Fig. S11 E and F and Tables S5 and S15).
Ecological Structure by Vegetation Type.
Our results at the level of vegetation type show the clearest pattern of variation in ecological structure (Fig. 2B and SI Appendix, Fig. S6C). Tropical forest communities form a tight and densely packed cluster in the region of the ecospace corresponding to tree-dwelling locomotor modes, with tropical seasonal forest communities showing slightly greater variation in locomotor type. Savanna communities more sparsely populate the ecospace around the central region. All other vegetation types have communities that better span the ecospace and are less densely packed than tropical forest communities. Although both types of tropical forest communities have high ecological richness (ERichtropicalrainforest = 9.33, ERichtropicalseasonalforest = 10.13), they differ from each other in that tropical rainforests have markedly lower ecological disparity than tropical seasonal forests (EDisptropicalrainforest = 3.20, EDisptropicalseasonalforest = 4.07; SI Appendix, Figs. S1 and S2 and Tables S6–S8). Conversely, communities in more open vegetation types (i.e., savanna, grassland, shrubland, and desert) tend to have low ecological richness {ERich[range] = 3.78–5.65}, and, with the exception of savanna communities, have relatively high ecological disparity {EDisp[range] = 3.54–4.22; SI Appendix, Tables S6 and S7}. Communities in temperate and boreal forests are intermediate in ecological richness (ERichtemperateforest = 8.19, ERichborealforest = 6.00), but have high ecological disparity (EDisptemperateforest = 4.27, EDispborealforest = 4.26; SI Appendix, Tables S6–S8). The results of the DFA clearly separate tropical forests from all other vegetation types, primarily due to the emphasis on the medium- to very large body-size categories (>128 g), frugivory, and arboreality among their mammals (SI Appendix, Fig. S12 A, C, and E and Tables S5 and S15). Savanna communities also form a relatively distinct grouping, driven by the prominence of omnivory and terrestrial locomotion (SI Appendix, Fig. S5C and Tables S5 and S15). Boreal and temperate forest communities segregate from most open-habitat communities (savannas, grasslands, shrublands, and deserts) and are characterized by mammals of very small body size (<32 g), herbivorous and carnivorous diets, and gliding, terrestrial, and semiaquatic locomotion; communities from more open-habitat vegetation types emphasize granivory and semifossorial, fossorial, and saltatorial locomotion (SI Appendix, Fig. S5C and Tables S5 and S15).
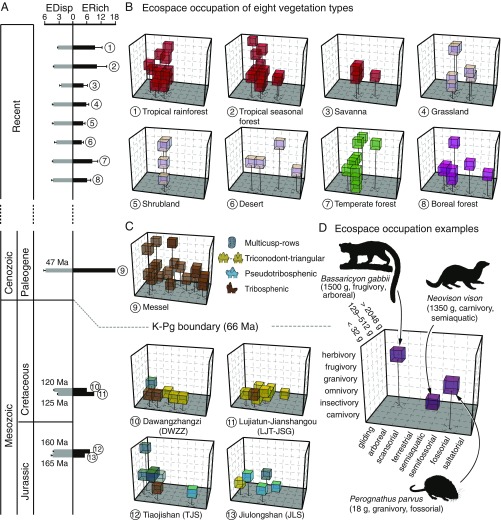
Fig. 2.
Ecospace occupation of small-bodied mammaliaform communities through time. (A) EDisp and ERich of small-bodied mammaliaform communities from the Mesozoic, Eocene, and Recent. (B) Exemplar plots of ecospace occupation of the extant, small-bodied mammalian communities from the eight vegetation types. (C) Plots of ecospace occupation of two Jurassic, two Cretaceous, and one Eocene small-bodied mammaliaform communities with colors corresponding to tooth types. (D) An example of ecospace occupation of three extant, small-bodied mammalian species. Each exemplar plot was generated by bootstrapping the eco-cells in each environmental type. See SI Appendix, Materials and Methods for details.
Factors Shaping Ecological Structure in Extant Communities.
Overall, our results imply that the ecological structure of extant, small-bodied mammalian communities is strongly influenced by vegetation type, which in turn depends on climatic factors and to a lesser degree, soil type and disturbance regime (e.g., refs. 34–37). Indeed, the first discriminant function (DF1) in each DFA strongly correlates with both mean annual temperature and mean annual precipitation (SI Appendix, Figs. S13 and S14). Vegetation type likely shapes the ecological structure of extant, small-bodied mammalian communities by controlling the availability and spatial distribution of food types and habitat throughout the year. Rainforests form one end of the vegetation spectrum, where a combination of year-round warm temperatures and rainfall promotes dense, vertically stratified, complex habitats with rich food resources located above ground (38). It follows that extant, small-bodied mammalian communities in these habitats are characterized by arboreal and frugivorous taxa (refs. 20, 22, and 39 and SI Appendix, Fig. S5C and Tables S5 and S15). The high net primary production (NPP) of rainforests (e.g., refs. 40 and 41) supports a greater number of individuals apportioned across more species than can most other habitats (e.g., refs. 42 and 43), as implied by the high ecological richness of those communities. In addition, high temperatures are thought to be correlated with elevated metabolic rates and associated shorter generation times and higher mutation rates (43, 44). Through intensified biotic interactions and faster selection, warm climates may therefore promote accelerated speciation and ultimately higher species richness (45, 46). In addition, the more complex, vertical stratification of rainforests promotes closer packing of mammalian species according to their ecologies (i.e., niche partitioning; refs. 47–51), resulting in low ecological disparity in these communities.
Extant, small-bodied mammalian communities in tropical seasonal and temperate forests share the elevated ecological richness with rainforest communities, but have significantly higher ecological disparity (SI Appendix, Figs. S1 and S2 and Table S6). This difference might reflect the (seasonally) drier, deeper, and/or cooler soils in those vegetation types, easing physiological and substrate constraints to allow a wider range of locomotor modes (e.g., fossoriality; refs. 52 and 53). Boreal forests experience even greater seasonality in temperature and water availability and have overall much lower NPP than other forests (54). These differences, along with the impact of lower temperatures on metabolic and evolutionary rates (43, 44), might explain why ecological richness is lower in these forests, despite high ecological disparity, possibly reflecting a “thinning” of niches and ecospace occupation. Seasonal access to food or protection in trees might also explain the relative scarcity of obligate arboreal and/or frugivorous small mammals in both temperate and boreal forests.
On the other end of the vegetation spectrum, open, arid vegetation types (e.g., deserts, shrublands, and many grasslands) support few or no trees, such that resources for small-bodied mammals are concentrated at or below ground level, rather than in the canopy (54). This shift away from trees as a source of food and shelter in these open, arid vegetation types translates to greater prevalence of semifossorial, fossorial, and saltatorial locomotion and granivory and to some degree herbivory among extant, small-bodied mammalian communities. Also, the associated, lower aboveground NPP (54) supports fewer small-bodied mammalian species, which in turn occupy relatively fewer niches (i.e., low ERich; SI Appendix, Table S13). Desert communities are an extreme case, with several ecological categories entirely missing (SI Appendix, Table S13). Because the occupied niches tend to be more specialized, hence distinct in our ecospace, ecological disparity is relatively high for desert communities. The paucity of mammals in our large- to very large body-size categories (>512 g) in some of these open-habitat (desert and grassland) communities is consistent with the observed “medium body-size gap” (∼500–8,000 g) in body-size-by-rank diagrams (cenograms) for mammal communities from open habitats (refs. 18, 55, and 56, see also, ref. 57). This body-size gap might be a byproduct of the limited size range of both leaves and insects in open habitats (58) or the evolution of increasing upper body-size limit, combined with an invariant minimum size, among mammals during the Cenozoic (59).
Small-bodied mammalian communities in savannas have a unique ecological structure, a pattern that is consistent across the three continents sampled herein. They are species poor and do not contain many highly specialized taxa. Moreover, although we do not find the medium body-size gap typical of open habitat communities, the range of locomotor and diet types is restricted in a way that is more similar to open habitats (SI Appendix, Fig. S5A and Table S5). The scarcity of small mammals adapted to, and living exclusively in savannas may relate to the strong seasonality of this vegetation type, the frequent fires, and disturbance from large-bodied herbivores (34, 60). These unpredictable environments may be what forces many species in savanna regions to instead rely on nearby gallery forests, which provide more reliable and diverse sources of food and shelter (60, 61). The lack of both vertical complexity and horizontal heterogeneity in savannas may also adversely affect the number of available niches (61).
Ecological Structure of Paleocommunities.
The five small-bodied mammaliaform paleocommunities in our dataset together fill only 38 of the 240 eco-cells (∼16%). The realized ecospace of the paleocommunities strongly overlaps that of the 98 extant communities, but differs in that (i) the paleocommunities entirely lack unambiguous granivores and frugivores, and (ii) some regions of the ecospace that are relatively unfilled in the extant dataset (semifossorial carnivores, larger-bodied saltatorial and fossorial forms) are more densely filled in the paleocommunities (Figs. 1E and 2 B and C and SI Appendix, Fig. S6 and Tables S1 and S13).
The ecological structure of the individual, small-bodied mammaliaform paleocommunities differ from one another and from the extant communities. The differences are not driven by variation in sample size (SI Appendix, Fig. S3 and Table S9) and, judging by available paleoenvironmental proxy and sedimentological data, are not caused by variation in local environmental conditions or by systematic taphonomic bias (SI Appendix, Table S19). Indeed, all of our fossil localities have been reconstructed as mainly closed habitats (forests) in temperate-subtropical climates (SI Appendix, Table S19). Thus, the differences among the paleocommunities and extant communities do not chiefly relate to differences in climate or vegetation type, but to other evolutionary or ecological factors that influenced the ecological structure of small-bodied mammaliaform communities through time.
To explore how turnover in taxonomic composition and functional evolution played into these differences in ecological structure, we categorized higher-level mammaliaform taxa from these paleocommunities into four dental architectural types: (i) the triconodont-triangular type for eutriconodontans and “symmetrodontans” having cheek teeth with three primary cusps, in a row or in a triangle, that function primarily in shearing; (ii) the multicusp-rows type for haramiyidans and multituberculates having cheek teeth with multiple cusps in multiple rows that function in crushing and grinding; (iii) the tribosphenic type for therians (eutherians and metatherians) having a true tribosphenic molar pattern (62, 63) with shearing and crushing function; and (iv) the pseudotribosphenic type for docodontans and shuotherids having cheek teeth approximating a tribosphenic pattern (63, 64), also with shearing and crushing function.
Our analysis shows that the Mid-Late Jurassic communities [Jiulongshan (JLS) and Tiaojishan (TJS)], which consist of mostly nontribosphenic mammaliaforms, have low dietary diversity. They consist of mostly insectivores and omnivores (Fig. 2C); the only herbivore is a haramiyidan (multicusp-rows), and the only carnivore is the docodontan Castorocauda lutrasimilis (pseudotribosphenic). C. lutrasimilis is also the only taxon with body mass over 512 g; body size in these communities is otherwise centered on our very-small- to small body-size categories (<128 g) (SI Appendix, Tables S1 and S13). There is, on the other hand, spread along the locomotor axis, with all but the saltatorial and semifossorial modes occupied and an emphasis on tree-related locomotion, especially in TJS and mostly among haramiyidans (multicusp-rows) and eutriconodontans (triconodont-triangular); the semiaquatic and fossorial modes are occupied by docodontans (pseudotribosphenic). The eutherian (tribosphenic) was very small (<32 g), insectivorous, and scansorial, traits considered plesiomorphic within this group. The ecological disparity of these communities is relatively high {EDisp[range] = 3.40–4.40}, akin to values in open habitats and nontropical forests, whereas the ecological richness is modest {ERich[range] = 5.00–7.00}, corresponding to the lower range of forests and grasslands (SI Appendix, Table S6). Paleoenvironmental classifications from DFA and Whittaker plots conducted at different levels (habitat openness, climate type, vegetation type) contradict each other and paleoproxy data (SI Appendix, Table S19). JLS, for example, is classified as cold climate and tropical rainforest by DFA and shrubland/woodland by Whittaker biome plots. The conflicting signals from the EDisp/ERich values, paleoenvironmental classification results, and paleoproxy data imply that these Mid-Late Jurassic mammaliaform communities were nonanalog.
The Early Cretaceous mammaliaform communities [Dawangzhangzi (DWZZ) and Lujiatun-Jianshangou (LJT-JSG)] include many eutriconodontans and symmetrodontans (both triconodont-triangular), a multituberculate (multicusp-rows), and three eutherians (tribosphenic); docodontans and shuotherids (both psuedotribosphenic) are absent (SI Appendix, Table S1). These communities are even more constrained to the lower part of the ecospace than those of the Jurassic (Fig. 2C). There is a single omnivorous multituberculate (multicusp-rows), and in the LJT-JSG there are several carnivores, all eutriconodontans (triconodont-triangular). Body-size diversity varies greatly between the two communities: LJT-JSG has the full spectrum, including larger-bodied eutriconodontans (>2,048 g), whereas DWZZ is restricted to the very small to small categories (<128 g) (SI Appendix, Tables S1 and S13); this difference does not seem to be a product of taphonomic size bias in light of the larger-bodied vertebrate taxa preserved in DWZZ (e.g., the ornithischian dinosaur Jinzhousaurus yangi) (65). As in the Jurassic communities, the range of locomotor modes is broad, containing all but gliding, saltatorial, and fossorial modes. Eutriconodontans account for much of this diversity, whereas eutherians (tribosphenic) retain relatively plesiomorphic ecologies [very small (<32 g), arboreal and scansorial insectivores]. The ecological disparity of these communities is very low {EDisp[range] = 2.87–3.27}, similar to tropical rainforest and savanna communities, and the ecological richness is medium to high {ERich[range] = 6.00–9.00}, in the range of boreal forest to tropical rainforest communities (SI Appendix, Table S6). The paleoenvironmental classifications from DFA and Whittaker plots are again contradictory; for example, the Whittaker scheme variously classifies LJT-JSG as woodland/shrubland, temperate seasonal forest, and temperate/tropical seasonal forest, and DWZZ as more open and arid (SI Appendix, Table S19). This discrepancy among interpretations from the EDisp/ERich values, paleoenvironmental classification results, and paleoproxy data implies that the Early Cretaceous mammaliaform communities were also nonanalog.
The middle Eocene small-bodied mammalian community from Messel consists entirely of eutherians and metatherians (tribosphenic) and is very rich (21 species) compared with the Mesozoic communities and most extant communities in our dataset (SI Appendix, Table S1). It also has a much greater spread across the ecospace than the Mesozoic communities, a pattern that is not the byproduct of greater sample size (SI Appendix, Tables S6 and S9). The dietary preferences are more diverse and redundant than in the Mesozoic communities; unambiguous frugivores and granivores are missing, but preserved gut contents indicate that all four herbivores and some omnivores from Messel incorporated fruit and seeds into their diet (66, 67). In terms of locomotion, there is a mix of tree-related (arboreal), terrestrial, and open, dry ground-related modes, including two fossorial and four saltatorial taxa. Gliding and scansorial forms are not recorded, but note that we omitted taxa with powered flight from this study, including eight Messel bats (all insectivores) (67–69). Whereas all body-size categories are represented in this mammalian paleocommunity, there is an emphasis on our medium- to very-large body-size categories (>128 g), consistent with forest habitats (SI Appendix, Tables S1 and S13). Both the ecological disparity and ecological richness of this extinct community are higher than for any extant, small-bodied mammalian community {EDisp[Messel] = 5.73, ERich[Messel] = 19.0; SI Appendix, Table S6}, and the relatively minor spatial or temporal averaging of the Messel fossil deposits suggest that these differences are not taphonomic artifacts. On the basis of paleobotanical and sedimentological data, Messel has been reconstructed as a multistratal canopy forest with abundant lianas, but with herb-covered wetland microhabitats and drier vegetation in more distal uplands (67, 70–72). The classifications from the DFA and Whittaker plots are mostly consistent with the paleoproxy data (tropical, tropical rainforest, or tropical seasonal rainforest/savanna), except for the classifications as open (DFA) and shrubland/woodland (Whittaker biome) (SI Appendix, Table S19). These discrepancies are likely due to the large number of saltatorial forms in this paleocommunity, potentially reflecting drier and/or more heterogeneous local habitats in Messel than previously recognized.
Together, this sequence from the Mid-Late Jurassic to the middle Eocene shows that ecospace occupation of small-bodied mammaliaform paleocommunities expanded through time toward the present, but it did so differentially along ecological axes. Locomotor diversification occurred earlier during the Mesozoic and across most groups of mammaliaforms (15), perhaps facilitated by the diversity of substrates available in local environments and by similar potential to evolve postcranial adaptations among higher-level taxa. In contrast, diets were constrained to primarily carnivory, insectivory, and omnivory until the Eocene; omnivory in our Mesozoic paleocommunities is found only in mammaliaforms with pseudotribosphenic and multicusp-row cheek teeth, and the sole herbivore is a medium-sized (128–512 g), gliding haramyidan (multicusp-rows) from the Jurassic. Body-size diversification was also constrained; only one mammaliaform (a docodontan) falls in our large body-size category (512–2,048 g) in the Jurassic communities, and the three that fall in our large- to very-large categories (>512 g) in one of the Cretaceous communities (LJT-JSG) are all carnivores from the same group of eutriconodontans (Gobiconodontidae; SI Appendix, Table S1). It is not until the Eocene Messel community, consisting solely of tribosphenic eutherians and metatherians, that we see mammals above our medium body-size (128–512 g) category take on diverse diets (SI Appendix, Table S1). Given the temporal gaps in our sampling, it remains for future studies to detail how dietary, locomotor, and body-size diversity changed in mammaliaform communities during the Late Cretaceous and Paleocene, but global-scale analyses do show increases in larger, herbivorous forms (e.g., multituberculates and metatherians) before the K/Pg boundary (11, 12, 73).
Factors Shaping Ecological Structure Through Time.
Despite the Mesozoic-to-Eocene ecospace expansion, none of the studied communities closely match extant, small-bodied mammalian communities. Particularly the Mesozoic ones are nonanalog in their ecospace occupation as well as in their ecological disparity and richness values (SI Appendix, Table S6). Although taphonomy can distort the paleoecological signal in the fossil record (e.g., incomplete sampling might artificially deflate the numbers of taxa), the resampled ecological structure of the extant communities (SI Appendix, Tables S9 and S10) and the unique ecospace occupation in the extinct communities (Fig. 2C and SI Appendix, Table S13) indicate that preservation is not the only cause for the differences in the ecological structure of the extinct and extant, small-bodied mammaliaform communities.
We propose that several factors played a role in shaping these ecological patterns. First, a key extrinsic factor is that the Mesozoic communities studied herein were part of ecosystems with no or few angiosperms. The major, early taxonomic diversification of crown-group angiosperms occurred by the late Early Cretaceous—nearly coeval with our Early Cretaceous communities but after those from the Mid-Late Jurassic (74–76). Perhaps more importantly for this study, angiosperms did not become abundant components in forests until the Late Cretaceous or early Paleocene (77–79). By the Eocene, angiosperms were both diverse and dominant in the forest canopy and understory, as structural elements and as significant sources of food for mammals and their prey (e.g., insects). Indeed, mammalian reliance on plants, and in particular angiosperms, as habitat, food, or both is not just evident from tooth morphology and preserved gut contents in Messel mammalian specimens, but plant–animal interactions are also supported by the high proportion of Messel fruits and seeds with traits typically associated with protection against (hard walls) or promotion of (fleshy walls) consumption by animals (67).
The lack of angiosperms notwithstanding, Mesozoic communities contained mammaliaforms with tree-related life modes, including arboreal, scansorial, and gliding forms and, rarely, even herbivores (a Jurassic haramyidan; Fig. 2C and SI Appendix, Table S1). These mammaliaforms likely relied on conifers or other seed plants for habitat, and their leaves and possibly seeds (e.g., of Ginkgoales) for food. However, by creating more complex habitats (such as in multistratal forests) and offering more rapidly proliferating, nutrient-rich leaves, fleshy fruits, seeds, and tubers, angiosperms fundamentally changed terrestrial ecosystems and opened up substantial regions of the ecospace for colonization by a diversity of mammals and other forest-dwelling animals. The Mid-Cenozoic rise to ecological dominance of open-habitat grasses and other associated, arid-adapted angiosperms later established the granivore guild, spurring another wave of changes in mammalian community structure (80).
A second extrinsic factor that potentially limited ecospace filling by mammaliaforms in Mesozoic ecosystems is selective pressure from other vertebrates. Predation by dinosaurs and other larger-bodied vertebrates is often invoked to explain the narrow range of body sizes among Mesozoic mammaliaforms (mostly ≤5 kg; ref. 81). By limiting our comparative extant dataset to small-bodied mammalian communities (≤5 kg), we acknowledge this apparent body-size upper limit in the Mesozoic but cannot robustly test the factors that shaped it using the fossil record. The small sizes of Mesozoic mammaliaforms would have imposed physiological constraints to dietary specialization, but these would apply equally to many extant, small-bodied mammals (82–84). Other potential causes for differential ecospace filling of small-bodied mammaliaform communities through time include niche incumbency (85) and competition for food resources and habitat (81, 86). In the Early Cretaceous Jehol biota, for example, a number of vertebrates overlapped with resident mammaliaforms in body size, diet, and habitat: small-bodied (∼0.5 kg), insectivorous squamates (e.g., Dalinghosaurus longidigitus) (87), carnivorous dinosaurs that ranged between 1 and 3 kg in body mass (e.g., Jinfengopteryx elegans and Sinovenator changii) (88, 89), a small-bodied (∼1 kg), insectivorous pterosaur (Jeholopterus ningchengensis) (90), as well as juveniles of large-bodied herbivorous dinosaurs (e.g., Liaoningosaurus paradoxus) (91). We hypothesize that the competitive dynamics among mammaliaforms, birds, and squamates in Mesozoic communities were similar to what they experience today, and thus, would not have differentially affected mammaliaform ecospace filling in the Mesozoic. In contrast, small- and medium-sized nonavian dinosaurs and juveniles of large-bodied dinosaurs represent a substantial departure from modern ecosystems. Whereas most extant birds and squamates are diurnal, some Mesozoic dinosaurs (herbivores and small theropods) and pterosaurs are thought to have been nocturnal, cathemeral, and/or crepuscular (92; but see ref. 93) and would therefore have temporally overlapped with nocturnal Mesozoic mammaliaforms. Inclusion of nonmammaliaform vertebrates, particularly dinosaurs, into ecospace analyses or food web studies may shed more light on whether dietary niche incumbency or some other unknown competitive advantage of these taxa influenced the ecological structure of Mesozoic mammaliaform communities.
Mammaliaform dental evolution represents a third major factor—but intrinsic rather than extrinsic—that potentially influenced the ecological structure of mammaliaform communities through time. Mammals with tribosphenic molars arose by the Mid-Late Jurassic in the northern hemisphere [94; but see earlier appearance of Australosphenida in the southern hemisphere (95)], but they were not taxonomically or numerically abundant until the Late Cretaceous (96); nontribosphenic forms predominated in earlier mammaliaform communities. We hypothesize that the dental architectural types varied in their capacity for heritable phenotypic variation (i.e., evolvability; ref. 97) in a way that affected functional diversity and, in turn, the expansion of dietary diversity through time. The high evolvability of the tribosphenic molar is demonstrated by studies showing that minor genetic or developmental modifications can produce major phenotypic changes in mammal teeth (98–103); it is corroborated by the extensive diversity of tooth shapes (104) and the repeated, independent evolution of adaptive dental traits (e.g., hypocone; ref. 105), both of which are documented in extant and extinct therians.
Although the genetic and developmental basis of tooth-shape formation in extinct mammaliaform lineages is inherently less knowable (but see refs. 106 and 107), our results support the idea that nontribosphenic dentitions were more morphologically constrained, and therefore restricted to certain dietary categories. The triconodont-triangular type, mainly capable of puncturing and shearing, was limited to insectivory and carnivory (108). The multicusp-rows type, especially when paired with a posteriorly directed chewing motion, could achieve crushing and grinding necessary for omnivory and herbivory (109), but were nevertheless constrained in how these dietary adaptations were achieved (as evidenced by the extreme enlargement of the multicusped molars and associated reduction of the blade-like premolars in the Paleogene taeniolabidids; ref. 11). In contrast, the tribosphenic and pseudotribosphenic types had more internal cutting ridges and a basin and could shear and crush insects in a more efficient way than the triconodont-triangular type (63, 110 and references therein). Some pseudotribosphenics (docodontans) had enhanced crushing for omnivory, and occlusal simulations suggest that others may have even evolved a transverse, grinding mode of chewing that made herbivory possible. Shuotheriids (pseudotribosphenics) did not develop a grinding function, but their fossil record is still sparse (111). Therians (tribosphenics) were insectivorous for most of the Mesozoic, as shown in our paleocommunities (Fig. 2), but by the Late Cretaceous some increased their crushing capacity by broadening both the upper molar protocone and the occluding lower molar talonid basin. Additionally, enhancement of the lingual phase of chewing (i.e., phase II chewing; ref. 112) brought about grinding function, which led to the omnivory and herbivory evident in the Eocene Messel and extant mammalian communities. Phase II chewing evolved perhaps as early as the mid-Cretaceous (Turonian, ca. 90 Ma) in zhelestid eutherians of Middle Asia (113, 114), and it was taxonomically and geographically widespread by the latest Cretaceous–earliest Paleogene (ca. 66 Ma) in archaic ungulates, plesiadapiform primates, and possibly the metatherian Glasbius (110, 115–118). Taken together, these three factors as well as others less detectable in the fossil record (e.g., digestive physiology) may have worked jointly or in sequence to shape mammaliaform ecospace filling through time. In a separate but revealing case, pretribosphenic mesungulatids from the Late Cretaceous of South America independently evolved the grinding function necessary for omnivory and herbivory (114), supporting the idea that the interplay between ecological opportunity (rise to dominance of angiosperms) and evolutionary potential (dental evolvability) has been critical in shaping the evolution of mammaliaform ecological structure.
Conclusion
In this paper, we quantified ecological structure in a large sample of extant, small-bodied mammalian communities across diverse biomes. Our results show that these communities occupy a limited volume of the theoretical ecospace. Within the realized ecospace, variation in the ecological structure of communities is linked to vegetation, which in turn depends on climate and other abiotic/biotic factors. Accordingly, geographically distant communities from similar vegetation/climate types have similar ecological structure, despite differences in taxonomic composition resulting from distinct biogeographic histories (22). We also show the converse, that climate/vegetation type is predictable from the ecological structure of extant, small-bodied mammalian communities, corroborating previous studies (22, 23). These results caution that studies of diversity through time with uneven paleoenvironmental distribution of sampling efforts could yield biased results (see refs. 119 and 120). As an example, a late Maastrichtian mammalian community (e.g., Hell Creek Formation, United States) might have higher species richness and ecological richness relative to a late Campanian community (e.g., Djadochta Formation, Mongolia) simply due to environmental differences (wet subtropical environment vs. semiarid desert).
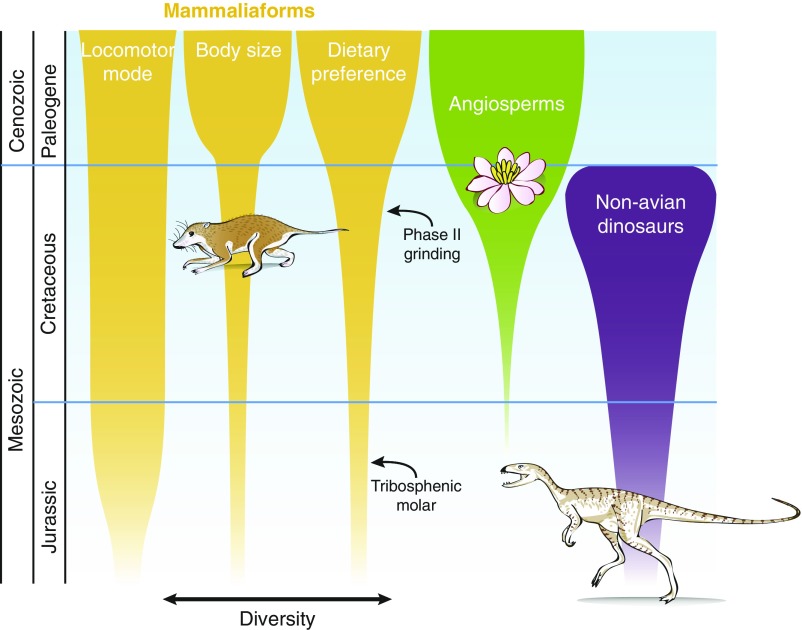
Our analysis of five exceptionally preserved small-bodied mammaliaform paleocommunities shows that ecospace occupation of the Mesozoic communities was constrained and markedly nonanalog relative to both that of the Eocene and extant communities. Despite the deterministic relationship between vegetation/climate and ecological structure of modern communities, our data indicate that major changes in evolutionary and ecological boundary conditions impacted the expansion of ecospace occupation of mammaliaform communities through time (117, 121). In the words of Hutchinson (122) modified by Bambach and colleagues (29: p19), “the evolutionary play is not only staged in the ecological theatre [...]; the ecological theatre itself evolves.” Specifically, we hypothesize that at least three factors affected the ecological expansion of mammaliaform communities: the rise to ecological dominance of angiosperms, the emergence of grinding function (i.e., phase II chewing) in tribosphenic mammals, both in the Late Cretaceous–early Paleocene, and the extinction of nonavian dinosaurs at the K/Pg boundary (Fig. 3). Furthermore, because the synergy of these intrinsic and extrinsic influences (123) might have been crucial, the resulting ecological assembly of mammalian communities came only after a substantial macroevolutionary lag (124) relative to the origin of tribosphenic mammals in the Jurassic. We conclude that the ecological structure of small-bodied mammaliaform communities is a product of current and historical intrinsic and extrinsic factors (123). A broader understanding of such mammaliaform community dynamics through time is critical to our understanding of the processes that govern ecosystem assembly and maintenance—topics that are still hotly debated (125, 126), but which directly bear on efforts to forecast and mitigate the evolutionary and ecological consequences of our current biodiversity crisis (127–129).
Fig. 3.
Schematic diagram showing the relative timing of the expansion of mammaliaform communities along three ecological axes (this study), the ecological rise to dominance of angiosperms (11, 75), and taxonomic diversification of nonavian dinosaurs (130). Note that expansion of mammaliaform communities along these ecological axes resulted from evolution within multiple clades.
Materials and Methods
Full details of materials and methods are provided in SI Appendix. In brief, we compiled taxonomic and ecological data of 98 extant, nonvolant, small-bodied mammalian communities from the primary literature. These communities provide broad coverage of habitat, climate, and vegetation types (Dataset S1). We selected five extinct mammaliaform communities (two Jurassic, two Cretaceous, one Eocene) from Konservat-Lagerstätten (SI Appendix, Table S1) and compiled their ecological data based on inferences from well-preserved fossil remains. We categorized each species in each community according to three ecological parameters: body size, dietary preference, and locomotor mode (SI Appendix, Table S2). We used five body-mass categories for body size, six dietary-preference categories (carnivory, insectivory, omnivory, granivory, frugivory, and herbivory), and eight locomotor-mode categories (gliding, arboreal, scansorial, terrestrial, semiaquatic, semifossorial, fossorial, and saltatorial) to define the ecospace (Dataset S1). These categorical data were transformed to numerical values before analyses (see SI Appendix for details). We quantified the magnitude of the filled ecospace of each community using two indices: EDisp, which measures the magnitude of ecological differences among species in the ecospace, and ERich, which measures the number of eco-cells occupied by a community in the ecospace. We then compared ecospace occupation and ecological structure indices of extant communities grouped according to environmental types at three different levels: habitat openness, climate type, and vegetation type. We conducted DFA and null model tests to assess how well ecological structure discriminates among environmental types and whether the patterns of ecospace occupation are significant across environmental types, respectively. We incorporated the extinct mammaliaform communities into the DFA and compared ecological structure indices to infer past environmental type(s).
Supplementary Material
Acknowledgments
We thank Zhe-Xi Luo, Pei-Ji Chen, Gang Li, Xiang-Ning Yang, Yu-Kun Shi, Jin Meng, Fang-Yuan Mao, Yuang-Qing Wang, and Qing-Jin Meng for providing access to the Mesozoic fossil specimens; Christian Sidor, Patricia Kramer, Janneke Hille Ris Lambers, Lauren Buckley, Zhe-Xi Luo, David Grossnickle, Danielle Fraser, Matthew Carrano, S. Kathleen Lyons, Anna K. Behrensmeyer, and David Polly for valuable suggestions and discussion; and members of the G.P.W. laboratory (Jonathan Calede, Lauren DeBey, Dave DeMar, Jr., Stephanie Smith, Luke Weaver, Alexandria Brannick, Paige Wilson, and Jordan Claytor) for constructive comments on earlier drafts of this manuscript. Funding was provided by the University of Washington’s Department of Biology and Burke Museum of Natural History and Culture, and we acknowledge the WRF-Hall Fellowship, Smithsonian Postdoctoral Fellowship, State Key Laboratory of Palaeobiology and Stratigraphy (Nanjing Institute of Geology and Palaeontology, CAS) (Grant 183106), the National Natural Science Foundation of China (Grant 41688103) (to M.C.), and the US National Science Foundation Grants EAR-1325674 (to G.P.W.) and EAR-1253713 (to C.A.E.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1820863116/-/DCSupplemental.
References
- 1.Rowe T. Definition, diagnosis, and origin of Mammalia. J Vertebr Paleontol. 1988;8:241–264. [Google Scholar]
- 2.Simpson GG. The beginning of the age of mammals. Biol Rev Camb Philos Soc. 1937;12:1–46. [Google Scholar]
- 3.Archibald JD. Structure of the KT mammal radiation in North America: Speculations on turnover rates and trophic structure. Acta Palaeontol Pol. 1983;28:7–17. [Google Scholar]
- 4.Alroy J. The fossil record of North American mammals: Evidence for a Paleocene evolutionary radiation. Syst Biol. 1999;48:107–118. doi: 10.1080/106351599260472. [DOI] [PubMed] [Google Scholar]
- 5.Smith FA, et al. The evolution of maximum body size of terrestrial mammals. Science. 2010;330:1216–1219. doi: 10.1126/science.1194830. [DOI] [PubMed] [Google Scholar]
- 6.Hu Y, Meng J, Wang Y, Li C. Large Mesozoic mammals fed on young dinosaurs. Nature. 2005;433:149–152. doi: 10.1038/nature03102. [DOI] [PubMed] [Google Scholar]
- 7.Luo Z-X, Wible JR. A Late Jurassic digging mammal and early mammalian diversification. Science. 2005;308:103–107. doi: 10.1126/science.1108875. [DOI] [PubMed] [Google Scholar]
- 8.Ji Q, Luo Z-X, Yuan C-X, Tabrum AR. A swimming mammaliaform from the Middle Jurassic and ecomorphological diversification of early mammals. Science. 2006;311:1123–1127. doi: 10.1126/science.1123026. [DOI] [PubMed] [Google Scholar]
- 9.Zheng X, Bi S, Wang X, Meng J. A new arboreal haramiyid shows the diversity of crown mammals in the Jurassic period. Nature. 2013;500:199–202. doi: 10.1038/nature12353. [DOI] [PubMed] [Google Scholar]
- 10.Luo ZX, et al. Mammalian evolution. Evolutionary development in basal mammaliaforms as revealed by a docodontan. Science. 2015;347:760–764. doi: 10.1126/science.1260880. [DOI] [PubMed] [Google Scholar]
- 11.Wilson GP, et al. Adaptive radiation of multituberculate mammals before the extinction of dinosaurs. Nature. 2012;483:457–460. doi: 10.1038/nature10880. [DOI] [PubMed] [Google Scholar]
- 12.Grossnickle DM, Newham E. Therian mammals experience an ecomorphological radiation during the Late Cretaceous and selective extinction at the K-Pg boundary. Proc Biol Sci. 2016;283:20160256. [Google Scholar]
- 13.Meredith RW, et al. Impacts of the cretaceous terrestrial revolution and KPg extinction on mammal diversification. Science. 2011;334:521–524. doi: 10.1126/science.1211028. [DOI] [PubMed] [Google Scholar]
- 14.Close RA, Friedman M, Lloyd GT, Benson RBJ. Evidence for a Mid-Jurassic adaptive radiation in mammals. Curr Biol. 2015;25:2137–2142. doi: 10.1016/j.cub.2015.06.047. [DOI] [PubMed] [Google Scholar]
- 15.Chen M, Wilson GP. A multivariate approach to infer locomotor modes in Mesozoic mammals. Paleobiology. 2015;41:280–312. [Google Scholar]
- 16.Barnosky AD, Hadly EA, Bell CJ. Mammalian response to global warming on varied temporal scales. J Mammal. 2003;84:354–368. [Google Scholar]
- 17.Beever EA, Brussard PF, Berger J. Patterns of apparent extirpation among isolated populations of pikas (Ochotona princeps) in the Great Basin. J Mammal. 2003;84:37–54. [Google Scholar]
- 18.Travouillon KJ, Legendre S. Using cenograms to investigate gaps in mammalian body mass distributions in Australian mammals. Palaeogeogr Palaeoclimatol Palaeoecol. 2009;272:69–84. [Google Scholar]
- 19.Lyons SK, et al. Holocene shifts in the assembly of plant and animal communities implicate human impacts. Nature. 2016;529:80–83. doi: 10.1038/nature16447. [DOI] [PubMed] [Google Scholar]
- 20.Fleming TH. Numbers of mammal species in North and Central American forest communities. Ecology. 1973;54:555–563. [Google Scholar]
- 21.Olff H, Ritchie ME, Prins HH. Global environmental controls of diversity in large herbivores. Nature. 2002;415:901–904. doi: 10.1038/415901a. [DOI] [PubMed] [Google Scholar]
- 22.Andrews P, Lord JM, Evans EMN. Patterns of ecological diversity in fossil and modern mammalian faunas. Biol J Linn Soc Lond. 1979;11:177–205. [Google Scholar]
- 23.Hernández Fernández M, et al. Identification problems of arid environments in the Neogene–Quaternary mammal record of Spain. J Arid Environ. 2006;66:585–608. [Google Scholar]
- 24.Schaal S, Ziegler W. Messel: An Insight into the History of Life and of the Earth. Oxford Univ Press; Oxford: 1993. [Google Scholar]
- 25.Meng J. Mesozoic mammals of China: Implications for phylogeny and early evolution of mammals. Natl Sci Rev. 2014;1:521–542. [Google Scholar]
- 26.Meng J, Hu Y-M, Li C-K, Wang Y-Q. The mammal fauna in the Early Cretaceous Jehol Biota: Implications for diversity and biology of Mesozoic mammals. Geol J. 2006;41:439–463. [Google Scholar]
- 27.Zhou Z. The Jehol Biota, an Early Cretaceous terrestrial Lagerstatte: New discoveries and implications. Natl Sci Rev. 2014;1:543–559. [Google Scholar]
- 28.Eisenberg JF. The Mammalian Radiations: Analysis of Trends in Evolution, Adaptation, and Behavior. University of Chicago Press; Chicago: 1981. [Google Scholar]
- 29.Bambach RK, Bush AM, Erwin DH. Autecology and the filling of ecospace: Key metazoan radiations. Palaeontology. 2007;50:1–22. [Google Scholar]
- 30.Elton C. Animal Ecology. Sidgwick and Jackson; London: 1927. [Google Scholar]
- 31.Odum EP. Fundamentals of Ecology. 3rd Ed Saunders; Philadelphia: 1971. [Google Scholar]
- 32.Polechová J, Storch D. Ecological niche. In: Jørgensen SK, Fath BD, editors. Encyclopedia of Ecology. Elsevier; Oxford: 2008. pp. 1088–1097. [Google Scholar]
- 33.Herrera CM. Seed dispersal by vertebrates. In: Herrera CM, Pellmyr O, editors. Plant-Animal Interactions. Blackwell; Oxford: 2002. pp. 185–208. [Google Scholar]
- 34.Sankaran M, et al. Determinants of woody cover in African savannas. Nature. 2005;438:846–849. doi: 10.1038/nature04070. [DOI] [PubMed] [Google Scholar]
- 35.Sankaran M, Ratnam J, Hanan N. Woody cover in African savannas: The role of resources, fire and herbivory. Glob Ecol Biogeogr. 2008;17:236–245. [Google Scholar]
- 36.Lehmann J, et al. Biochar effects on soil biota–A review. Soil Biol Biochem. 2011;43:1812–1836. [Google Scholar]
- 37.Iio A, Hikosaka K, Anten NPR, Nakagawa Y, Ito A. Global dependence of field-observed leaf area index in woody species on climate: A systematic review. Glob Ecol Biogeogr. 2014;23:274–285. [Google Scholar]
- 38.Ricklefs RE. The Economy of Nature. W. H. Freeman and Company; New York: 2008. [Google Scholar]
- 39.van Dam JA, Weltje GJ. Reconstruction of the late Miocene climate of Spain using rodent paleocommunity successions: An application of end-member modelling. Palaeogeogr Palaeoclimatol Palaeoecol. 1999;151:267–305. [Google Scholar]
- 40.Cramer W, et al. Comparing global models of terrestrial net primary productivity (NPP): Overview and key results. Glob Change Biol. 1999;5:1–15. [Google Scholar]
- 41.Gillman LN, et al. Latitude, productivity and species richness. Glob Ecol Biogeogr. 2015;24:107–117. [Google Scholar]
- 42.Francis AP, Currie DJ. Global patterns of tree species richness in moist forests: Another look. Oikos. 1998;81:598–602. [Google Scholar]
- 43.Rohde K. Latitudinal gradients in species diversity: The search for the primary cause. Oikos. 1992;65:514–527. [Google Scholar]
- 44.Brown JH. Why are there so many species in the tropics? J Biogeogr. 2014;41:8–22. doi: 10.1111/jbi.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gillooly JF, Allen AP, West GB, Brown JH. The rate of DNA evolution: Effects of body size and temperature on the molecular clock. Proc Natl Acad Sci USA. 2005;102:140–145. doi: 10.1073/pnas.0407735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allen AP, Gillooly JF, Savage VM, Brown JH. Kinetic effects of temperature on rates of genetic divergence and speciation. Proc Natl Acad Sci USA. 2006;103:9130–9135. doi: 10.1073/pnas.0603587103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hutchinson GE. Homage to Santa Rosalia or why are there so many kinds of animals? Am Nat. 1959;93:145–159. [Google Scholar]
- 48.MacArthur RH, Levins R. The limiting similarity, convergence, and divergence of coexisting species. Am Nat. 1967;101:377–385. [Google Scholar]
- 49.MacArthur R. Species packing and competitive equilibrium for many species. Theor Popul Biol. 1970;1:1–11. doi: 10.1016/0040-5809(70)90039-0. [DOI] [PubMed] [Google Scholar]
- 50.Schoener TW. Resource partitioning in ecological communities. Science. 1974;185:27–39. doi: 10.1126/science.185.4145.27. [DOI] [PubMed] [Google Scholar]
- 51.Novack-Gottshall PM. General models of ecological diversification. I. Conceptual synthesis. Paleobiology. 2016;42:185–208. [Google Scholar]
- 52.McNab BK. The metabolism of fossorial rodents: A study of convergence. Ecology. 1966;47:712–733. [Google Scholar]
- 53.Quesada M, et al. Succession and management of tropical dry forests in the Americas: Review and new perspectives. For Ecol Manage. 2009;258:1014–1024. [Google Scholar]
- 54.Leuschner C. Vegetation and ecosystems. In: Van der Maarel E, Franklin J, editors. Vegetation Ecology. Wiley-Blackwell; Oxford: 2013. pp. 285–307. [Google Scholar]
- 55.Legendre S. Analysis of mammalian communities from the late Eocene and Oligocene of Southern France. Palaeovertebrata. 1986;16:191–212. [Google Scholar]
- 56.Legendre S. Les communautés de mammifères du Paléogène (Eocène supérieur et Oligocène) d’Europe occidentale: Structures, milieux et évolution. Münchner Geowissenschaftliche Abhandlungen. 1989;16:1–110. [Google Scholar]
- 57.Menéndez I, et al. Body-size structure of Central Iberian mammal fauna reveals semidesertic conditions during the middle Miocene Global Cooling Event. PLoS One. 2017;12:e0186762. doi: 10.1371/journal.pone.0186762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gingerich PD. 1989. New Earliest Wasatchian Mammalian Fauna from the Eocene of Northwestern Wyoming: Composition and Diversity in a Rarely Sampled High-Floodplain Assemblage (Museum of Paleontology, University of Michigan, Ann Arbor)
- 59.Alroy J. Cope’s rule and the dynamics of body mass evolution in North American fossil mammals. Science. 1998;280:731–734. doi: 10.1126/science.280.5364.731. [DOI] [PubMed] [Google Scholar]
- 60.Redford KH, da Fonseca GAJB. The role of gallery forests in the zoogeography of the Cerrado’s non-volant mammalian fauna. Biotropica. 1986;18:126–135. [Google Scholar]
- 61.August PV. The role of habitat complexity and heterogeneity in structuring tropical mammal communities. Ecology. 1983;64:1495–1507. [Google Scholar]
- 62.Crompton AW. The origin of the tribosphenic molar. In: Kermack DM, Kermack KA, editors. Early Mammals. Academic; London: 1971. pp. 65–87. [Google Scholar]
- 63.Davis BM. Evolution of the tribosphenic molar pattern in early mammals, with comments on the “dual-origin” hypothesis. J Mamm Evol. 2011;18:227–244. [Google Scholar]
- 64.Butler PM. Docodont molars as tribosphenic analogues. Mem Mus Natl Hist Nat Ser C. 1988;53:329–340. [Google Scholar]
- 65.Wang X-L, Xu X. A new iguanodontid (Jinzhousaurus yangi gen. et sp. nov.) from the Yixian Formation of western Liaoning, China. Chin Sci Bull. 2001;46:1669–1672. [Google Scholar]
- 66.Collinson ME, et al. The value of X-ray approaches in the study of the Messel fruit and seed flora. Palaeobiodivers Palaeoenviron. 2012;92:403–416. [Google Scholar]
- 67.Storch G. Paleobiological implications of the Messel mammalian assemblage. In: Gunnell GF, editor. Eocene Biodiversity: Unusual Occurrences and Rarely Sampled Habitats. Springer Science+Business Media; New York: 2012. pp. 215–235. [Google Scholar]
- 68.Habersetzer J, Richter G, Storch G. Paleoecology of early middle Eocene bats from Messel, FRG. Aspects of flight, feeding and echolocation. Hist Biol. 1994;8:235–260. [Google Scholar]
- 69.Morlo M, Schaal S, Mayr G, Seiffert C. An annotated taxonomic list of the middle Eocene (MP 11) Vertebrata of Messel. Cour Forschungsinst Senckenberg. 2004;252:95–108. [Google Scholar]
- 70.Wilde V. Untersuchungen zur Systematik der Blattreste aus dem Mitteleozan der Grube Messel bei Darmstadt (Hessen, Bundesrepublik Deutschland) Cour Forschungsinst Senckenberg. 1989;115:1–213. [Google Scholar]
- 71.Wilde V, Micklich N. The lake and its immediate surroundings. In: Gruber G, Micklich N, editors. Messel Treasures of the Eocene. Hessisches Landesmuseum Darmstadt; Darmstadt: 2007. pp. 52–55. [Google Scholar]
- 72.Lenz OK, Wilde V, Riegel W. Short-term fluctuations in vegetation and phytoplankton during the middle Eocene greenhouse climate: A 640-kyr record from the Messel oil shale (Germany) Int J Earth Sci. 2011;100:1851–1874. [Google Scholar]
- 73.Wilson GP. Mammals across the K/Pg boundary in northeastern Montana, U.S.A.: Dental morphology and body-size patterns reveal extinction selectivity and immigrant-fueled ecospace filling. Paleobiology. 2013;39:429–469. [Google Scholar]
- 74.Anderson CL, Bremer K, Friis EM. Dating phylogenetically basal eudicots using rbcL sequences and multiple fossil reference points. Am J Bot. 2005;92:1737–1748. doi: 10.3732/ajb.92.10.1737. [DOI] [PubMed] [Google Scholar]
- 75.Magallón S, Hilu KW, Quandt D. Land plant evolutionary timeline: Gene effects are secondary to fossil constraints in relaxed clock estimation of age and substitution rates. Am J Bot. 2013;100:556–573. doi: 10.3732/ajb.1200416. [DOI] [PubMed] [Google Scholar]
- 76.Magallón S, Gómez-Acevedo S, Sánchez-Reyes LL, Hernández-Hernández T. A metacalibrated time-tree documents the early rise of flowering plant phylogenetic diversity. New Phytol. 2015;207:437–453. doi: 10.1111/nph.13264. [DOI] [PubMed] [Google Scholar]
- 77.Wing SL, Boucher LD. Ecological aspects of the Cretaceous flowering plant radiation. Annu Rev Earth Planet Sci. 1998;26:379–421. [Google Scholar]
- 78.Crifò C, Currano ED, Baresch A, Jaramillo C. Variations in angiosperm leaf vein density have implications for interpreting life form in the fossil record. Geology. 2014;42:919–922. [Google Scholar]
- 79.Boyce CK, Lee J-E, Feild TS, Brodribb TJ, Zwieniecki MA. Angiosperms helped put the rain in the rainforests: The impact of plant physiological evolution on tropical biodiversity. Ann Mo Bot Gard. 2010;97:527–540. [Google Scholar]
- 80.Jardine PE, Janis CM, Sahney S, Benton MJ. Grit not grass: Concordant patterns of early origin of hypsodonty in Great Plains ungulates and Glires. Palaeogeogr Palaeoclimatol Palaeoecol. 2012;365–366:1–10. [Google Scholar]
- 81.Lillegraven JA. Introduction. In: Lillegraven JA, Kielan-Jaworowska Z, Clemens WA, editors. Mesozoic Mammals: The First Two-Thirds of Mammalian History. University of California Press; Berkeley, CA: 1979. pp. 1–6. [Google Scholar]
- 82.Clauss M, et al. The maximum attainable body size of herbivorous mammals: Morphophysiological constraints on foregut, and adaptations of hindgut fermenters. Oecologia. 2003;136:14–27. doi: 10.1007/s00442-003-1254-z. [DOI] [PubMed] [Google Scholar]
- 83.Price SA, Hopkins SSB. The macroevolutionary relationship between diet and body mass across mammals. Biol J Linn Soc Lond. 2015;115:173–184. [Google Scholar]
- 84.Pineda-Munoz S, Lazagabaster IA, Alroy J, Evans AR. Inferring diet from dental morphology in terrestrial mammals. Methods Ecol Evol. 2016;8:481–491. [Google Scholar]
- 85.Jablonski D, Sepkoski JJ., Jr Paleobiology, community ecology, and scales of ecological pattern. Ecology. 1996;77:1367–1378. [PubMed] [Google Scholar]
- 86.Van Valen L, Sloan RE. Contemporaneity of late Cretaceous extinctions. Nature. 1977;270:193. [Google Scholar]
- 87.Evans SE, Wang Y. The Early Cretaceous lizard Dalinghosaurus from China. Acta Palaeontol Pol. 2005;50:725–742. [Google Scholar]
- 88.Xu X, Norell MA, Wang X-L, Makovicky PJ, Wu X-C. A basal troodontid from the Early Cretaceous of China. Nature. 2002;415:780–784. doi: 10.1038/415780a. [DOI] [PubMed] [Google Scholar]
- 89.Ji Q, et al. First avialian bird from China (Jinfengopteryx elegans gen. et sp. nov.) Geol Bull China. 2005;24:197–210. [Google Scholar]
- 90.Wang X-L, Zhou Z-H, Zhang F-C, Xu X. A nearly completely articulated rhamphorhynchoid pterosaur with exceptionally well-preserved wing membranes and “hairs” from Inner Mongolia, northeast China. Chin Sci Bull. 2002;47:226–230. [Google Scholar]
- 91.Xu X, Wang X-L, You H-L. A juvenile ankylosaur from China. Naturwissenschaften. 2001;88:297–300. doi: 10.1007/s001140100233. [DOI] [PubMed] [Google Scholar]
- 92.Schmitz L, Motani R. Nocturnality in dinosaurs inferred from scleral ring and orbit morphology. Science. 2011;332:705–708. doi: 10.1126/science.1200043. [DOI] [PubMed] [Google Scholar]
- 93.Hall MI, Kirk EC, Kamilar JM, Carrano MT. Comment on “Nocturnality in dinosaurs inferred from scleral ring and orbit morphology”. Science. 2011;334:1641, author reply 1641. doi: 10.1126/science.1208489. [DOI] [PubMed] [Google Scholar]
- 94.Luo Z-X, Yuan C-X, Meng Q-J, Ji Q. A Jurassic eutherian mammal and divergence of marsupials and placentals. Nature. 2011;476:442–445. doi: 10.1038/nature10291. [DOI] [PubMed] [Google Scholar]
- 95.Martin T, Rauhut OWM. Mandible and dentition of Asfaltomylos patagonicus (Australosphenida, Mammalia) and the evolution of tribosphenic teeth. J Vertebr Paleontol. 2005;25:414–425. [Google Scholar]
- 96.Luo Z-X. Transformation and diversification in early mammal evolution. Nature. 2007;450:1011–1019. doi: 10.1038/nature06277. [DOI] [PubMed] [Google Scholar]
- 97.Kirschner M, Gerhart J. Evolvability. Proc Natl Acad Sci USA. 1998;95:8420–8427. doi: 10.1073/pnas.95.15.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jernvall J, Keränen SVS, Thesleff I. Evolutionary modification of development in mammalian teeth: Quantifying gene expression patterns and topography. Proc Natl Acad Sci USA. 2000;97:14444–14448. doi: 10.1073/pnas.97.26.14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kangas AT, Evans AR, Thesleff I, Jernvall J. Nonindependence of mammalian dental characters. Nature. 2004;432:211–214. doi: 10.1038/nature02927. [DOI] [PubMed] [Google Scholar]
- 100.Kavanagh KD, Evans AR, Jernvall J. Predicting evolutionary patterns of mammalian teeth from development. Nature. 2007;449:427–432. doi: 10.1038/nature06153. [DOI] [PubMed] [Google Scholar]
- 101.Salazar-Ciudad I, Jernvall J. A computational model of teeth and the developmental origins of morphological variation. Nature. 2010;464:583–586. doi: 10.1038/nature08838. [DOI] [PubMed] [Google Scholar]
- 102.Moustakas JE, Smith KK, Hlusko LJ. Evolution and development of the mammalian dentition: Insights from the marsupial Monodelphis domestica. Dev Dyn. 2011;240:232–239. doi: 10.1002/dvdy.22502. [DOI] [PubMed] [Google Scholar]
- 103.Harjunmaa E, et al. Replaying evolutionary transitions from the dental fossil record. Nature. 2014;512:44–48. doi: 10.1038/nature13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ungar PS. Mammal Teeth: Origin, Evolution, and Diversity. 1st Ed The Johns Hopkins University Press; Baltimore: 2010. [Google Scholar]
- 105.Hunter JP, Jernvall J. The hypocone as a key innovation in mammalian evolution. Proc Natl Acad Sci USA. 1995;92:10718–10722. doi: 10.1073/pnas.92.23.10718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sánchez-Villagra M. Embryos in Deep Time. University of California Press; Berkeley: 2012. [Google Scholar]
- 107.Halliday TJD, Goswami A. Testing the inhibitory cascade model in Mesozoic and Cenozoic mammaliaforms. BMC Evol Biol. 2013;13:79. doi: 10.1186/1471-2148-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kielan-Jaworowska Z, Cifelli R, Luo Z-X. Mammals from the Age of Dinosaurs. Columbia Univ Press; New York: 2004. [Google Scholar]
- 109.Wall CE, Krause DW. A biomechanical analysis of the masticatory apparatus of Ptilodus (Multituberculata) J Vertebr Paleontol. 1992;12:172–187. [Google Scholar]
- 110.Schultz JA, Martin T. Function of pretribosphenic and tribosphenic mammalian molars inferred from 3D animation. Naturwissenschaften. 2014;101:771–781. doi: 10.1007/s00114-014-1214-y. [DOI] [PubMed] [Google Scholar]
- 111.Luo Z-X, Ji Q, Yuan C-X. Convergent dental adaptations in pseudo-tribosphenic and tribosphenic mammals. Nature. 2007;450:93–97. doi: 10.1038/nature06221. [DOI] [PubMed] [Google Scholar]
- 112.Butler PM. The milk-molars of Perissodactyla, with remarks on molar occlusion. J Zool. 1952;121:777–817. [Google Scholar]
- 113.Harper AM. 2011. Three dimensional analysis of large scale dental wear in Late Cretaceous eutherians, Dzharakuduk region Uzbekistan. Master thesis (San Diego State University, San Diego)
- 114.Harper T, Parras A, Rougier GW. Reigitherium (Meridiolestida, Mesungulatoidea) an enigmatic Late Cretaceous mammal from Patagonia, Argentina: Morphology, affinities, and dental evolution. J Mamm Evol. 2018;8:1–32. [Google Scholar]
- 115.Butler PM. Some functional aspects of molar evolution. Evolution. 1972;26:474–483. doi: 10.1111/j.1558-5646.1972.tb01951.x. [DOI] [PubMed] [Google Scholar]
- 116.Kay RF, Hiiemae KM. Jaw movement and tooth use in recent and fossil primates. Am J Phys Anthropol. 1974;40:227–256. doi: 10.1002/ajpa.1330400210. [DOI] [PubMed] [Google Scholar]
- 117.Crompton AW, Kielan-Jaworowska Z. Molar structure and occlusion in Cretaceous therian mammals. In: Butler PM, Joysey KA, editors. Development, Function, and Evolution of Teeth. Academic; New York: 1978. pp. 249–287. [Google Scholar]
- 118.Dewar EW. Functional diversity within the Littleton fauna (early Paleocene), Colorado: Evidence from body mass, tooth structure, and tooth wear. PaleoBios. 2003;23:1–19. [Google Scholar]
- 119.Wing SL, Dimichele WA. Conflict between local and global changes in plant diversity through geological time. Palaios. 1995;10:551–564. [Google Scholar]
- 120.Vilhena DA, Smith AB. Spatial bias in the marine fossil record. PLoS One. 2013;8:e74470. doi: 10.1371/journal.pone.0074470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Olson EC. Community evolution and the origin of mammals. Ecology. 1966;47:291–302. [Google Scholar]
- 122.Hutchinson GE. The Ecological Theater and the Evolutionary Play. Yale Univ Press; New Haven: 1965. [Google Scholar]
- 123.Jablonski D. Approaches to macroevolution: 2. sorting of variation, some overarching issues, and general conclusions. Evol Biol. 2017;44:451–475. doi: 10.1007/s11692-017-9434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jablonski D, Bottjer DJ. The origin and diversification of major groups: Environmental patterns and macroevolutionary lags. In: Taylor PD, Larwood GP, editors. Major Evolutionary Radiations. Clarendon Press; Oxford: 1990. pp. 17–57. [Google Scholar]
- 125.Hubbell SP. The Unified Neutral Theory of Biodiversity and Biogeography. Princeton Univ Press; Princeton: 2011. [DOI] [PubMed] [Google Scholar]
- 126.Odling-Smee FJ, Laland KN, Feldman MW. Niche Construction. Princeton Univ Press; Princeton: 2013. [Google Scholar]
- 127.Pereira HM, et al. Scenarios for global biodiversity in the 21st century. Science. 2010;330:1496–1501. doi: 10.1126/science.1196624. [DOI] [PubMed] [Google Scholar]
- 128.Parmesan C, Duarte C, Poloczanska E, Richardson AJ, Singer MC. Overstretching attribution. Nat Clim Chang. 2011;1:2–4. [Google Scholar]
- 129.Stegner MA, Holmes M. Using palaeontological data to assess mammalian community structure: Potential aid in conservation planning. Palaeogeogr Palaeoclimatol Palaeoecol. 2013;372:138–146. [Google Scholar]
- 130.Fastovsky DE, et al. Shape of Mesozoic dinosaur richness. Geology. 2004;32:877–880. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.