Significance
Physical exercise is well known for its positive effects on general health (specifically, on brain function and health), and some mediating mechanisms are also known. A few reports have addressed intergenerational inheritance of some of these positive effects from exercised mothers or fathers to the progeny, but with scarce results in cognition. We report here the inheritance of moderate exercise-induced paternal traits in offspring’s cognition, neurogenesis, and enhanced mitochondrial activity. These changes were accompanied by specific gene expression changes, including gene sets regulated by microRNAs, as potential mediating mechanisms. We have also demonstrated a direct transmission of the exercise-induced effects through the fathers’ sperm, thus showing that paternal physical activity is a direct factor driving offspring’s brain physiology and cognitive behavior.
Keywords: intergenerational inheritance, cognition traits, moderate physical exercise, adult hippocampal neurogenesis, mitochondria
Abstract
Physical exercise has positive effects on cognition, but very little is known about the inheritance of these effects to sedentary offspring and the mechanisms involved. Here, we use a patrilineal design in mice to test the transmission of effects from the same father (before or after training) and from different fathers to compare sedentary- and runner-father progenies. Behavioral, stereological, and whole-genome sequence analyses reveal that paternal cognition improvement is inherited by the offspring, along with increased adult neurogenesis, greater mitochondrial citrate synthase activity, and modulation of the adult hippocampal gene expression profile. These results demonstrate the inheritance of exercise-induced cognition enhancement through the germline, pointing to paternal physical activity as a direct factor driving offspring’s brain physiology and cognitive behavior.
The beneficial effects of exercise for health are largely well known (1, 2), as well as its basic action profile—a hormetic, biphasic curve (3). Specifically, its anxiolytic, antidepressant, and procognitive effects have been described in detail in past decades (for a recent review, both in laboratory rodents and humans, see ref. 4). Moreover, physical exercise has also been linked to brain function and to specific behavior-related phenomena such as adult hippocampal neurogenesis (5). However, very little data exist on whether these exercise-mediated effects are inheritable. Because many of the positive effects of exercise on the brain are induced through epigenetic changes (for a recent review, see ref. 6) and some epigenetic changes have also been reported in runners’ sperm (7–9), we investigate whether the procognitive effects of exercise training in mice might be inherited by the (otherwise sedentary) progeny of runner fathers.
Several reports have revealed either no effects or positive effects of physical activity on the sedentary progeny of exercised pregnant female rodents and humans, due to its direct actions on fetuses through the placenta (10–12). Intergenerational transference of exercise-induced effects on mood and conditioned fear has been reported (13–15), and a recent paper (16) demonstrated the inheritance of an enriched environment-induced improvement in hippocampal long-term potentiation (LTP) through male sperm microRNAs. Despite the importance of knowing the basic biological mechanisms by which an activity-dependent improvement in cognition might be inherited by a nontrained progeny, to our knowledge, there are no reports addressing either the specific neuronal populations related to these improved functions or the cellular mechanisms mediating these effects. Furthermore, the inheritance of the effects on both nonspatial and spatial tasks and on cognitive processes, like object recognition memory and pattern separation (crucial to information processing), remain to be described. Taking into account that adult neurogenesis is a key player in hippocampal information processing and pattern separation (17) and the relevant role that mitochondrial function has for exercise-induced improvements in cognition (reviewed in ref. 18), we have designed hypothesis-driven patrilineal intergenerational inheritance experiments focusing on pattern separation, adult hippocampal neurogenesis, and nuclear-encoded mitochondrial protein effects. Considering this background, our working hypotheses are (i) the effects of exercise on some aspects of fathers’ cognition are inherited by the adult male offspring; (ii) the main features of the adult hippocampal neurogenesis subpopulations (cell proliferation, short- and long-term cell survival, and maturation) affected by the paternal exercise are inherited by the progeny; (iii) the changes in the pattern of gene expression in the hippocampus of exercised fathers might also be inherited by the offspring; and (iv) the main characteristics of mitochondrial functioning (either number or organelle activation or both) are transmitted intergenerationally.
To achieve these goals, we performed a triple approach to patrilineal intergenerational inheritance to test the strength of the biological process at hand. First, we compared litters from sedentary males with litters from the same males after training (running). This approach was used to minimize interfather variability (the progenitor effect). Second, we compared litters from sedentary males with litters from different, exercised males to study intergeneration effects of exercise in nonrelated offspring. This approach was used to compare animals from experimental groups processed at the same time and without the potentially confounding factor of the order of the litter. Third, to study whether these intergenerational exercise-driven effects were germline dependent, we designed an experiment in which interactions between male and female progenitors were eliminated by generating the progeny through in vitro fertilization (IVF) and embryo transfer. Our experimental design followed main gold-standard guidelines (19). Because we were interested in the cognitive effects of physical activity, we tested the pure effects of physical exercise, separating these effects from the cognitive influence of an environmental enrichment. To minimize intersubject variability, we employed moderately forced activity on a treadmill. Specific behavioral tests were used to analyze a possible enhancement of object recognition memory and spatial pattern separation. The novel object recognition (NOR) test is a common method used to assess the rodents’ ability to recognize a novel object in an environment without external cues and reinforcements. It is based on the rodent’s natural preference for novelty. When the animals are exposed to a familiar and a novel object, they spend more time exploring the novel object (20). There are several underlying neural circuits and brain structures involved in the NOR test (in which the hippocampal formation plays a key role) that support learning and memory processes, such as encoding, consolidation, and memory retrieval (21). On the other hand, pattern separation is a cognitive process that allows the formation of distinct representations out of similar inputs. A pattern separation task based on a novel object location test can be used to study spatial pattern separation, which is greatly supported by the dentate gyrus and adult hippocampal neurogenesis (22). Both the NOR test and the pattern separation task can be evaluated by a discrimination index (DI), which expresses the difference in the exploration times of the novel and familiar objects (the moving and fixed objects in the pattern separation task), divided by the total exploration time.
To relate the exercise effects with the heritability of the changes in specific hippocampal neuronal populations (including neurogenic populations), we used ad hoc-designed stereological protocols. Exercise-induced changes in gene expression in the brains of fathers and offspring were also analyzed, as well as the changes in induced methylation in the parents’ sperm. Finally, exercise-induced changes in mitochondrial physiology and cellular energetics in the liver, cerebellum, and hippocampus of fathers and offspring were also analyzed.
Results
Experimental Design and Inheritance of Behavioral Effects of Exercise.
Before exercise, we analyzed whether fathers had any cognitive differences (tested in a difficult NOR test), finding no significant differences between groups (Fig. 1 E and H). Next, one group underwent a moderate forced training protocol on a treadmill for 6 wk and the other group remained sedentary. After the application of the exercise protocol, all fathers were tested in a behavioral test battery based on Crawley (23), including the elevated plus maze (anxiety test) and a standard protocol of water maze in experiment A. These two tests revealed no significant differences between groups (SI Appendix, Fig. S14). Differences found in the rest of the tests are described below. In experiment A (SI Appendix, Fig. S1A), litters of sedentary fathers were compared with litters born from the same fathers after exercising. In experiment B (SI Appendix, Fig. S1B), litters from sedentary males were compared with litters from different, exercised males. Finally, in experiment C (SI Appendix, Fig. S1C), to eliminate interactions between males and their mates, IVF and embryo transfer were used to produce litters from different exercised and sedentary males. In adulthood, they underwent the same behavioral protocols as litters in experiments A and B. In every experiment, all litters were sedentary—only groups of fathers underwent physical exercise during the experiments.
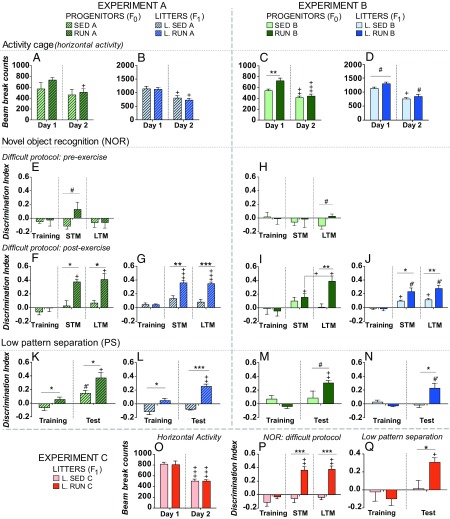
Fig. 1.
Intergenerational inheritance of memory enhancement and pattern separation improvement through physical exercise. (A–D) Horizontal locomotor activity in a novel and a known environment. The locomotor activity was assessed using an activity cage in a novel and a known environment (day 1 and day 2, respectively). Charts represent the horizontal locomotor activity of fathers (A) and litters (B) in experiment A and of fathers (C) and litters (D) in experiment B. (E–J) NOR memory enhancement in exercised fathers and their offspring. Results represent a DI. A DI score of >0.20 was set ad hoc to determine proper novel-object discrimination; statistically significant within-group differences were also considered between the training phase and the test phases. Before exercising, all groups of fathers were unable to discriminate the novel object in a difficult protocol (E and H). After 6 wk of physical exercise, only exercised fathers and their sedentary offspring showed memory enhancement in experiment A (F and G) and experiment B (I and J). (K–N) Improved pattern separation performance of exercised fathers and their offspring in a low separation test. Results represent a DI, which allows the discrimination between the exploration of the objects in different positions. A DI score of >0.20 was set ad hoc to determine proper discrimination; statistically significant within-group differences were also considered between the training phase and the test phases. After 6 wk of physical exercise, only runner fathers and their sedentary offspring showed an improved pattern separation performance in experiment A (K and L) and B (M and N). (O–Q) Results were replicated in experiment C. Locomotor activity (O) of the offspring in the IVF experiment in a novel and a known environment (day 1 and day 2, respectively). The offspring of exercised fathers also showed enhanced object recognition memory (P) and an improved pattern separation performance in a low pattern separation test (Q). All data are shown as mean ± SEM. For comparisons between independent groups, *P < 0.05, **P < 0.01, ***P < 0.001; tendencies 0.05 > #P < 0.09. For comparisons between dependent groups, +P < 0.05, ++P < 0.01, +++P < 0.00; tendencies 0.05 > #′P < 0.09. Extreme values were removed from the analysis. SED A, n = 4; RUN A, n = 5; L. SED A, n = 8; L. RUN A, n = 8; SED B, n = 10; RUN B, n = 5; L. SED B, n = 8; L. RUN B, n = 4; L. SED C, n = 10; L. RUN C, n = 13.
Litters in experiments A and B underwent a neurodevelopmental battery of tests (24), from postnatal day (P)2 to P15 (SI Appendix, Table S2). No relevant significant differences were found between groups (SI Appendix, Figs. S15 and S16). All animals in experiments A and B were tested at adult stages (3.5-mo-old litters, and 5.5-mo-old fathers).
First, the locomotor activity was analyzed using an activity cage in a novel and a known environment (day 1 and day 2, respectively). In experiment A (Fig. 1 A and B), a Wilcoxon signed-rank test indicated that exercised fathers (Z = −2.02, P = 0.043, r2 = 0.40) and both groups of litters (Z = −2.52, P = 0.012, r2 = 0.40) significantly reduced their activity on day 2. In experiment B (Fig. 1 C and D), exercised fathers showed significantly more activity than sedentary fathers on day 1 [t test, t (13) = −3.43, P = 0.004, g = 1.9, r2 = 0.50]. Both sedentary [t (9) = 4.25, P = 0.002, dz = 1.34, r2 = 0.67] and exercised fathers [t (4) = 14.23, P < 0.001, dz = 6.3, r2 = 0.98] significantly reduced their activity on day 2. On the other hand, litters from exercised fathers tended to show more activity than litters from sedentary fathers (Mann–Whitney U test, U = 6, P = 0.089, r2 = 0.24) on day 1. A Wilcoxon signed-ranked test revealed that both groups of litters significantly reduced their activity on day 2, but only litters from sedentary fathers showed a significant reduction (Z = −2.52, P = 0.012, r2 = 0.40 and Z = −1.83, P = 0.068, r2 = 0.41, respectively). In experiment C (Fig. 1O), a paired-sample t test indicated that litters from sedentary fathers [t (9) = 15.68, P < 0.001, dz = 4.96, r2 = 0.96] and litters from exercised fathers [t (12) = 6.03, P < 0.001, dz = 1.67, r2 = 0.75] also reduced their activity on day 2. Overall, in experiment A, exercised fathers and both groups of litters reduced their activity in a known environment. In experiment B, exercised fathers showed more activity than sedentary fathers in a novel environment, and both groups of fathers reduced their activity in a known environment. Finally, litters from sedentary fathers reduced their activity in a known environment. For additional activity-cage parameters, see SI Appendix, Figs. S2 and S6A.
Second, NOR memory was assessed. To assess memory enhancement, difficult and easy protocols were designed by modifying the time spent during the training phase (SI Appendix, Fig. S11 A and B). The easy protocol had a longer training phase and, although animals showed low DI scores, no significant differences were found between groups in experiments A and B (SI Appendix, Fig. S3 A–D). In contrast, the difficult protocol allowed the animals limited time to explore the objects in the training phase. Before exercising, all groups of fathers were unable to discriminate the novel object in the difficult protocol (Fig. 1 E and H), but after 6 wk of physical exercise, exercised fathers and their offspring showed memory enhancement (Fig. 1 F–J). In experiment A (Fig. 1F), a Mann–Whitney U test indicated that exercised fathers showed significantly higher DI scores than sedentary males in the short-term memory (STM) phase (U = 0, P = 0.021, r2 = 0.67) and in the long-term memory (LTM) phase (U = 0, P = 0.02, r2 = 0.67). A Friedman test revealed significant differences in the performance of exercised fathers throughout the test (X2 = 6.5, P = 0.039); post hoc analysis with Wilcoxon signed-rank tests showed significantly higher DI scores in both test phases (Z = −2.023, P = 0.043, r2 = 0.41). To analyze the offspring’s performance (Fig. 1G), a mixed ANOVA was run, with the phase of the test (training, STM, or LTM) as a within-subjects factor and the fathers’ exercise protocol (sedentary or runner) as a between-subjects factor. There was a significant main effect of the phase of the test [F(2.26) = 24.877, P < 0.001, ηp2 = 0.657], as well as a significant interaction between the phase of the test and the exercise protocol [F(2.26) = 10.779, P < 0.001, ηp2 = 0.453]. Pairwise comparisons corrected by Bonferroni showed that only litters from exercised fathers obtained significantly higher DI scores in both test phases (STM and LTM) compared with the training phase (P < 0.001). There was also a significant main effect of the fathers’ exercise protocol [F(1.13) = 45.021, P < 0.001, ηp2 = 0.776]. Pairwise comparisons corrected by Bonferroni revealed that litters from exercised fathers obtained higher DI scores than litters from the same fathers before exercising in the STM phase (P = 0.001) and in the LTM phase (P < 0.001). In experiment B (Fig. 1I), a Mann–Whitney U test indicated that exercised fathers showed significantly higher DI scores than sedentary fathers in the LTM phase of the test (U = 2.5, P = 0.006, r2 = 0.51). A Friedman test revealed significant differences in the performance of exercised fathers throughout the test (X2 = 10, P = 0.007). Post hoc analysis with Wilcoxon signed-rank tests indicated that exercised fathers showed significantly higher DI scores in both test phases (Z = −2.023, P = 0.043, r2 = 0.41) compared with the training phase. Furthermore, litters from exercised fathers (Fig. 1J) showed significantly higher DI scores than litters from different sedentary fathers in the STM phase (U = 2, P = 0.017, r2 = 0.47) and the LTM phase (U = 0, P = 0.007, r2 = 0.61). Although the average DI scores were low, a Friedman test revealed significant differences in the performance of litters from sedentary fathers throughout the test (X2 = 13, P = 0.002). Post hoc analysis revealed significantly higher DI scores in both test phases (Wilcoxon signed-rank test, Z = −2.52, P = 0.012, r2 = 0.40). On the other hand, litters from different exercised fathers tended to vary their performance throughout the test (Friedman test, X2 = 6, P = 0.05), and post hoc analysis with Wilcoxon signed-rank test indicated a tendency to show higher DI scores in both test phases (Z = −1.83, P = 0.068, r2 = 0.40). Nevertheless, only litters from exercised males reached DI scores >0.20. Finally, in experiment C (Fig. 1P), a Mann–Whitney U test indicated that litters from exercised fathers showed significantly higher DI scores compared with litters from sedentary fathers in the STM phase (U = 7, P = 0.001, r2 = 0.53) and in the LTM phase (U = 2, P < 0.001, r2 = 0.65). A Friedman test revealed that only litters from exercised fathers significantly varied their performance throughout the test (X2 = 12, P < 0.001), and post hoc analysis with a Wilcoxon signed-rank test showed significantly higher DI scores in both the STM phase (Z = −3.06, P = 0.002, r2 = 0.39) and the LTM phase (Z = −2.94, P = 0.003, r2 = 0.36) compared with the training phase. The total exploration times of all NOR tests are shown in SI Appendix, Figs. S3 E–N and S6B.
Finally, to determine whether these differences were test dependent, a pattern separation test with customizable difficulty thresholds was designed (SI Appendix, Fig. S11 C and D). While the easy protocol of the test (high pattern separation) was achieved by all litters in experiments A and B (SI Appendix, Fig. S4 A and C), only the exercised fathers and their offspring performed outstandingly in the difficult protocol (low pattern separation). In experiment A (Fig. 1 K and L), a Mann–Whitney U test revealed that exercised fathers showed significantly higher DI scores than sedentary males in the training phase (U = 2, P = 0.048, r2 = 0.43) and in the test phase (U = 2, P = 0.049, r2 = 0.43). A Wilcoxon signed-rank test indicated that both groups showed higher DI scores in the test phase compared with the training phase, although this difference was only statistically significant for exercised fathers (Z = −1.83, P = 0.068, r2 = 0.42 and Z = −2.23, P = 0.043, r2 = 0.41, respectively). Furthermore, litters from exercised fathers showed significantly higher DI scores than litters from the same fathers before exercising in the training phase [t (13) = −3, P = 0.010, g = 1.5, r2 = 0.41] and in the test phase [t (13) = −9.17, P < 0.001, g = 4.46, r2 = 0.87]. A paired-sample t test revealed that only litters from exercised fathers showed higher DI scores in the test phase compared with the training phase [t (7) = −6.07, P = 0.001, dz = 2.15, r2 = 0.84]. In experiment B (Fig. 1 M and N), exercised fathers tended to show higher DI scores than sedentary fathers in the test phase [t test, t (13) = −1.47, P = 0.067, g = 0.81, r2 = 0.24] and showed significantly higher DI scores in the test phase compared with the training phase [t (4) = −6.93, P = 0.002, dz = 3.10, r2 = 0.92]. Moreover, litters from exercised fathers showed significantly higher DI scores than litters from different sedentary fathers in the test phase (Mann–Whitney U test, U = 2, P = 0.017, r2 = 0.47) and tended to show higher DI scores in the test phase compared with the training phase (Wilcoxon signed-rank test, Z = −1.83, P = 0.068, r2 = 0.42). Finally, in experiment C (Fig. 1Q), only litters from exercised fathers showed significant differences between the training phase and the test phase [t (8) = −5.37, P = 0.001, dz = 1.79, r2 = 0.78] and significantly higher DI scores than litters from sedentary fathers in the test phase [t test, t (17) = −2.56, P = 0.020, g = 1.18, r2 = 0.28]. Because the pattern separation test is based on an object location test (OLT), a standard OLT was used as a control to ensure correct object location performance. No significant differences were found between groups in the test phase of OLT (SI Appendix, Fig. S4 D–G). The total exploration times of all pattern separation tests and OLT in experiments A and B are shown in SI Appendix, Fig. S4 H–R and in SI Appendix, Fig. S6C for experiment C.
Because the NOR and pattern separation tests were part of a behavioral phenotyping battery, we previously studied whether continuous exposure to complex testing had an effect on the animals’ DI scores in the easy protocols. To verify this, a control experiment was designed with naïve animals performing only the easy protocols of these tests. All animals—sedentary and runner—learned to discriminate normally in the control experiment without intergroup differences, thereby validating the easy and difficult experimental designs and explaining the low DI scores found in the data of the behavioral test battery (SI Appendix, Fig. S17).
All of the mentioned effects in fathers and litters were observed, both when analyzing data considering all siblings from a given litter as one sample to avoid litter effects and when analyzing data considering all animals from all litters inside each experimental group as independent samples (SI Appendix, Fig. S5). In experiment C, the litter effect was minimized by applying a mixed weaning strategy on P21 (SI Appendix, Fig. S1C).
Exercise-Induced Increase of Hippocampal Cell Proliferation and Immature Neuron Number Is Mimicked by Sedentary Litters Raised from Exercised Parents.
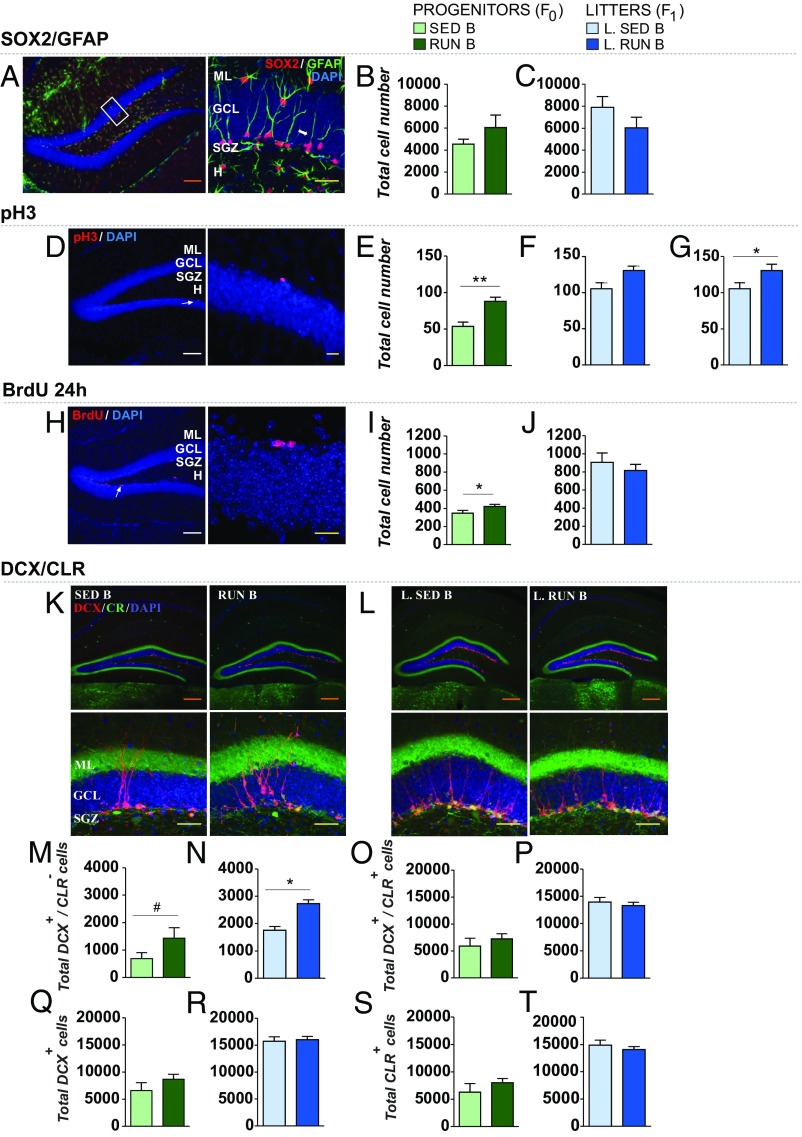
Nonspatial NOR and spatial pattern separation have been closely related to the hippocampal formation and impact on adult hippocampal neurogenesis (17, 25–27). For this reason, we analyzed adult hippocampal neural stem cell (NSC) number, cell proliferation, and differentiation. We measured cell proliferation and neurogenesis by immunohistochemistry of double staining of SOX2/GFAP, phosphohistone 3 (pH3), BrdU, and double staining of doublecortin/calretinin (DCX/CLR) (Fig. 2) in neural progenitors and their adult progeny within the hippocampal dentate gyrus. Fathers and litters of both experiments were killed after 24 h of survival time after BrdU injection. No significant variation was found in SOX2/GFAP cells, suggesting that the NSC number was not affected (Fig. 2 A–C). A t test revealed that exercised fathers (Fig. 2E), showed a significant increase in pH3+ cell number compared with sedentary fathers [t (13) = −3.65, P = 0.003, g = 2, r2 = 0.51], while no significant variation was found in their offspring (Fig. 2F). Because the variability in the litters was higher than the group mean variability for this variable (no litter effect), we also show the significant data from the independent sample analysis of the offspring (Fig. 2G; Mann–Whitney U test, U = 115, P = 0.032, r2 = 0.10). The increase in BrdU+ cells in runner fathers’ hippocampus (Mann–Whitney U test, U = 4, P = 0.027, r2 = 0.41) was not observed in the litters (Fig. 2 I and J). A Mann–Whitney U test revealed that the litters from exercised fathers had a significantly increased number of DCX+/CLR− immature neuroblasts (U = 1, P = 0.011, r2 = 0.54; Fig. 2N). In DCX+/CLR−, the fathers show a trend: t test, t (13) = −1.84, P = 0.089, g = 1, r2 = 0.21 (Fig. 2M). No significant differences were found between groups in the total number of DCX+/CLR+ cells (Fig. 2 O and P), in the total number of DCX+ cells (Fig. 2 Q and R), or in the total number of CLR+ cells (Fig. 2 S and T). A battery of histological parameters, including volume of the hippocampus and dentate gyrus, the volume occupied by the granule cell layer (GCL) and the area of the subgranular cell zone (SI Appendix, Fig. S7 A–I), the density of GFAP+ signal (SI Appendix, Fig. S7 J–L), and the total granule cell number (SI Appendix, Fig. S7 M–O), were also analyzed. This analysis revealed no changes between groups, except for an increase in the total GCL neuron number of runner parents compared with sedentary (SI Appendix, Fig. S7N), an effect not found in the litters (SI Appendix, Fig. S7O).
Fig. 2.
Increased neurogenesis in the offspring of exercised males. (A–C) Assessment of neural stem cells (NSCs) in the hippocampus. Representative image (A) of SOX2+/GFAP+ cells in the dentate gyrus (DG) (Left) and a magnification image showing the morphology of these cells (Right). Only cells that had their cell bodies in the subgranular zone (SGZ) and displayed a radial glia-like morphology with a long process across the granular cell layer (GCL) were taken into account. No significant differences were found in the total SOX2+/GFAP+ cells (B and C) in any of the experimental groups. (D–G) Cell proliferation. Representative image (D) of pH3+ cells in the GCL and SGZ of the hippocampus (Left) and a magnification image showing the morphology of these cells (Right). Only exercised males showed a significantly increased total number of pH3+ cells (E), while no significant differences were found in the offspring (F); as the variability in the litters was higher than the group mean variability (no litter effect), we also show the independent sample analysis where litters from runner fathers showed an increased number of pH3+ compared to controls (G). (H–J) Cell proliferation and 24-h cell survival. Representative image (H) of BrdU+ cells in the GCL and SGZ of the hippocampus (Left) and a magnification image showing the morphology of these cells (Right). Exercised fathers showed a significant increase in the total number of BrdU+ cells (I) compared to controls; no significant differences were found in the offspring (J). (K–T) Assessment of subpopulations of immature cells. Representative image of the total number of DCX+/CLR+ cells in the hippocampus of fathers (K) and litters (L) (Top) and magnification images showing the morphology of these cells (Bottom). Exercised fathers tended to show an increased total number of DCX+/CLR− cells (M), while litters showed a significantly increased number of these cells compared to controls (N); no significant differences were found between groups in the total number of DCX+/CLR+ cells (O and P), in the total number of DCX+ cells (Q and R), and in the total number of CLR+ cells (S and T). Orange scale bars, 200 μm; white scale bars, 100 μm; yellow scale bars, 20 μm. H, Hilus; ML, molecular layer. For intergroup differences between two independent groups, the t test was applied (if the variable was not normally distributed, the Mann–Whitney U test was used), *P < 0.05, **P < 0.01, ***P < 0.001; tendencies 0.05 > #P < 0.09 in Student’s t test or Mann–Whitney U test. For each test, extreme values were removed from the analysis. Data are shown as mean ± SEM. Group SED B, n = 10 and RUN B, n = 5; group L. SED B, n = 8, L. Group RUN B, n = 4 (G, L. SED B, n = 36 and L. RUN B, n = 14).
Descriptive Analysis of Gene Expression Shows the Biological Processes Affected Differentially in Each Generation and Suggests a Possible Mechanism of Epigenetic Inheritance.
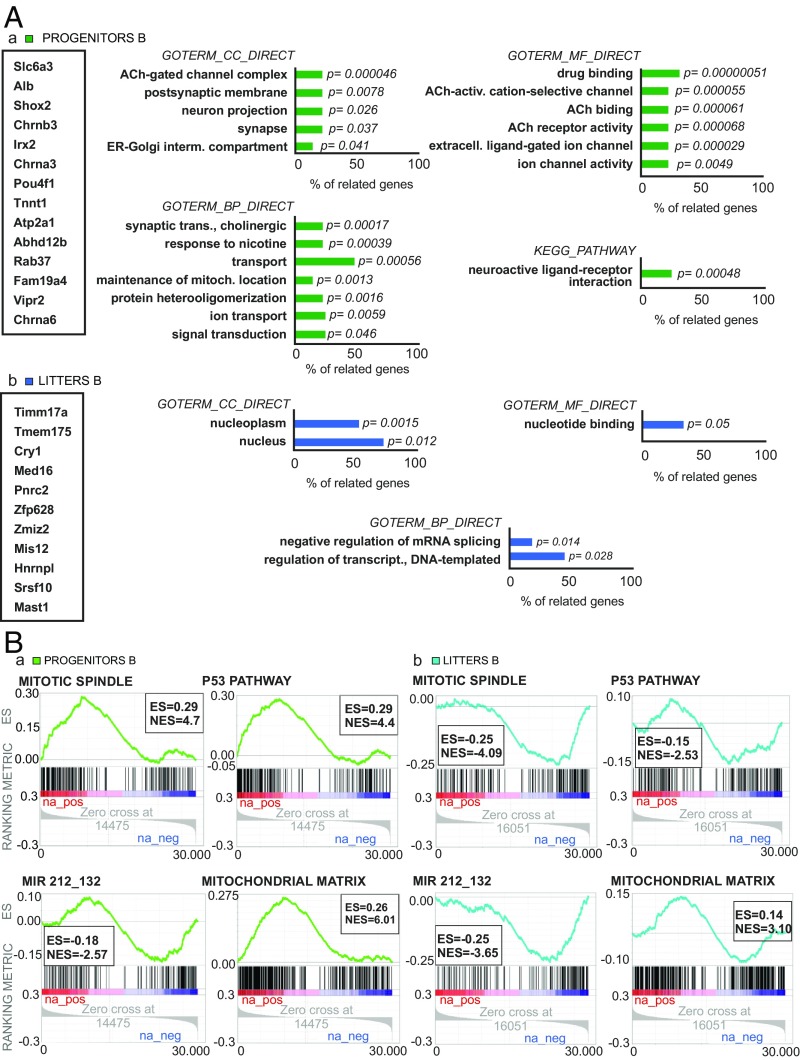
We performed RNA sequencing (RNA-seq) analysis to compare (i) the hippocampal gene expression levels of exercised fathers with respect to sedentary ones and (ii) the hippocampal gene expression levels of litters from exercised fathers with respect to the litters of the sedentary ones. All samples used in this analysis were from animals from experiment B. The Database for Annotation, Visualization, and Integrated Discovery (DAVID) software (v6.8) was used as a first approach to functionally describe the gene expression changes in our groups. We took into account only significantly differentially expressed genes (sDEGs) for this analysis [adjusted P value (adj-P) < 0.05]. sDEGs of each comparison are listed in Fig. 3A. We generated the annotation chart for each list of sDEGs (the databases used were GOTERM_CC_DIRECT, GOTERM_BP_DIRECT, GOTERM_MF_DIRECT, and KEGG_PATHWAY). Nineteen annotation terms relevant for neural tissue were enriched on the fathers’ list and five were enriched on the litters’ list (Fig. 3A). On the fathers’ list, the terms were mostly related to synaptic transmission, whereas they were mostly related to transcription processes on the litters’ list of sDEGs. We also generated an annotation table for each list to know all annotation terms related to our sDEGs (SI Appendix, Table S6).
Fig. 3.
Descriptive analysis of gene expression data. (A) Functional analysis for fathers’ comparison (a) and litters’ comparison (b). Boxes (Far Left) show the lists of sDEGs of each group (adj-P < 0.05). Graphs show the annotation terms enriched from each database consulted and the percentage of sDEGs associated with each term. P values indicate the significance of enrichment (EASE Score). (B) GSEA for fathers’ comparison (a) and litters’ comparison (b). Several gene sets related to metabolic processes, cell proliferation, cellular components, and microRNAs show enrichment in both groups. Graphs for Mitotic Spindle, P53 Pathway, MIR212_132, and Mitochondrial Matrix sets and their respective enrichment score (ES) and normalized enrichment score (NES) are shown to illustrate the most interesting findings. A positive enrichment score here indicates that genes related to a specific function show a trend to be overexpressed in exercised individuals; a negative enrichment score indicates that genes related to a specific function show a trend to be underexpressed in exercised individuals. Only expression data from experiment B was used in this analysis (exercised fathers, n = 4; sedentary fathers, n = 5; animals from exercised fathers, n = 7; animals from sedentary fathers, n = 8). ER, endoplasmic reticulum.
To further expand the qualitative description of gene expression, we performed a preranked gene set enrichment analysis (GSEA) of the RNA-seq data (Fig. 3B). Because GSEA is a threshold-free method of analysis, we generated the ranked list using all genes and their log2FoldChange-associated value. Several gene sets from three Molecular Signatures Database collections (H: hallmark gene sets, MIR: microRNA targets, and CC: gene ontology cellular component) showed enrichment in both comparisons (exercised fathers B vs. sedentary fathers B, and litters from exercised fathers B vs. litters from sedentary fathers B). We found 10 gene sets related to cell cycle and cell proliferation positively enriched in the fathers’ comparisons (SI Appendix, Table S1). A positive enrichment in this analysis indicates that overrepresented genes related to a specific function are found overexpressed in exercised fathers compared with the levels of expression in sedentary ones. Remarkably, all gene sets related to cell cycle and cell proliferation were negatively enriched in the litters’ comparison (SI Appendix, Table S1). A negative enrichment in this analysis indicates that overrepresented genes related to a specific function are found underexpressed in litters from exercised fathers compared with the levels of expression in the litters of sedentary fathers.
Moreover, we found sets of genes associated with microRNA activity enriched in both comparisons (197 microRNA sets enriched in fathers’ comparison and 200 in litters’ comparison). Interestingly, one of the negatively enriched sets in both comparisons was MIR212_132 (Fig. 3B and SI Appendix, Table S1). The negative enrichment in this case indicates that overrepresented genes that share putative target sites (seed matches) of this specific microRNA in their 3′ UTRs are underexpressed in exercised fathers compared with sedentary fathers, and in litters from exercised fathers compared with litters of the sedentary ones. This is interesting, as Benito et al. (16) implicated these RNAs in the intergenerational inheritance of environmental enrichment effects.
Parallel to other findings in this work, mitochondrial-related gene sets were found positively enriched in both comparisons (SI Appendix, Table S1). The positive enrichment in this case indicates that overrepresented genes that are related to these mitochondrial components are overexpressed in exercised fathers compared with sedentary fathers and in litters from exercised fathers compared with litters of the sedentary ones. For additional GSEA plots of enriched gene sets, see SI Appendix, Fig. S8. Heatmaps of sDEGs are shown in SI Appendix, Figs. S9 and S10.
The complete descriptive analysis shows that the replication of the cognitive advantage in the second generation is not due to a replication of the gene expression profile of the first one. Annotation terms enriched in sDEGs from fathers point to exercise affecting mostly synaptic transmission, whereas annotation terms enriched in sDEGs from litters point to changes in the way hippocampal cells regulate transcription. GSEA analysis shows that multitudes of biological processes are commonly affected in both generations, including processes related to other results in this paper (i.e., cell cycle, cell proliferation, and mitochondria), but these affects are not a perfect replication from the first generation to the second one. The change of expression in genes related to microRNAs, as GSEA shows, suggests them as a potential mechanism for epigenetic inheritance. These data gain interest, since we found no exercise-induced changes in methylation of male sperm DNA in a methylated DNA immunoprecipitation sequencing analysis (SI Appendix, Fig. S13 and Table S4), suggesting that intergenerational effects were not mediated by altered DNA methylation in spermatozoa. Further investigation is needed to clarify this hypothesis.
The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://ncbi.nlm.nih.gov/geo (accession no. GSE123582) (28).
The Progeny of Exercised Mice Exhibit Augmented Markers of Mitochondrial Function in the Hippocampus.
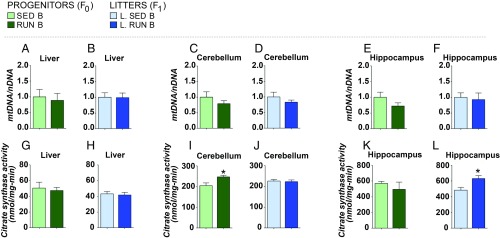
We next examined exercise-induced effects in mitochondrial physiology and cellular energetics in the liver, cerebellum, and hippocampus of the fathers and their respective offspring. The liver was selected because it is directly related to the production of insulin-like growth factor 1 after exercise. The cerebellum was selected because the increased motion associated with physical exercise may well change the activity of the cerebellum, and the hippocampus was selected because variations in physical activity are directly associated with changes in hippocampal neurogenesis rate. The ratio of mitochondrial DNA (mtDNA) to nuclear DNA (nDNA) copy number was not affected in the different experimental groups (Fig. 4 A–F), suggesting that mitochondrial number per cell is not affected. To determine possible modulations in mitochondrial performance, we analyzed the activity of citrate synthase, a marker of mitochondrial functionality (29) encoded in the nDNA, which participates in the tricarboxylic acid cycle in the matrix of the mitochondria. Citrate synthase activity was significantly greater in the cerebellum of exercised parents [Fig. 4I; t test, t (8) = −2.88, P = 0.022, g = 1.79, r2 = 0.50], while no modulations were detected in the hippocampus and the liver of these mice (Fig. 4 G and K). Interestingly, citrate synthase activity was significantly increased in the hippocampus of the offspring from exercised parents [Fig. 4L; t (11) = −2.86, P = 0.016, g = 1.6, r2 = 0.41], while no effects were found in liver and cerebellum lysates from the same individuals (Fig. 4 H and J).
Fig. 4.
Paternal exercise enhances markers of mitochondrial function in the hippocampus of the offspring. (A–F) mtDNA/nDNA ratio was determined by real-time PCR in the liver of fathers (A) and offspring (B), in the cerebellum of fathers (C) and litters (D), and in the hippocampus of fathers (E) and litters (F). (G–L) Citrate synthase activity measured in the liver of fathers (G) and litters (H), in the cerebellum of fathers (I) and litters (J), and in the hippocampus of fathers (K) and litters (L). All data are shown as mean ± SEM. For comparisons, *P < 0.05 for SED vs. RUN and L. SED vs. L. RUN in unpaired Student’s t test. SED B and RUN B, n = 5 per group; L. SED B, n = 7 to 8; L. RUN B, n = 6 to 8.
Discussion
Previous studies have addressed inter- or transgenerational inheritance of activity-induced effects on behavior (13–16). In these studies, limited results were found. No changes were observed in anxiety or depression-like behaviors of the filial (F)1 generation after using environmental enrichment (14). Exercise alone only suppressed reinstatement of juvenile fear memory in the study by Short et al. (13), and isolated animals were tested only at juvenile stages after weaning (15). Benito et al. (16) reported an enhancement of synaptic plasticity after environmental enrichment, with limited results in cognition. The present study shows a significant, large effect of fathers’ pure physical activity on both the NOR memory and the spatial pattern separation of their adult offspring as compared with the offspring of sedentary fathers or the offspring of the same fathers before exercising.
We have also found that offspring significantly replicated the exercise effects on the immature neuron subpopulation found in the fathers’ hippocampus. However, differences in cell proliferation were not replicated. This is not surprising, as pH3+ and 24-h survival BrdU+ cells are different subpopulations usually under compensating regulation. Our results indicate that specific subpopulations of cycling progenitors and immature, differentiating neurons in the dentate gyrus GCL are changed in sedentary brains because of the exercise program performed by their fathers.
Despite this replication of cognitive advantage and effects in the immature neuron subpopulation, we did not find a similar gene expression profile in both generations. There were no matches between sDEGs from the fathers’ comparison and from the litters’ comparison. DAVID analysis of RNA-seq data showed that different annotation terms were enriched for each sDEG list, whereas GSEA analysis showed that some relevant biological processes were affected in the same direction in both generations (i.e., mitochondrial processes), but others in opposite directions (i.e., cell cycle, cell proliferation). These results indicate that different gene expression profiles are mediating the same cognitive, cellular, and molecular outcomes in fathers and their offspring. Moreover, as for the neurogenesis-related sets, an exercise-induced increase in the proliferation of neural progenitors can be the final outcome of the intervention both in fathers and offspring, even though the gene expression patterns are different, due to different compensatory mechanisms in parents and offspring. Our work provides an extensive and detailed functional analysis of the gene expression changes induced by physical exercise done in two generations: the exercised one and their offspring. Extensive descriptive analyses were previously made by other groups (30), but they were restricted to the exercised animals and not their offspring.
The findings on mitochondrial proteins suggest that paternal exercise produces a specific reprogramming of hippocampal mitochondria in the offspring. In particular, we found that citrate synthase activity was enhanced, while mtDNA copy number per cell was unaffected. These data are suggestive of increased mitochondrial function and/or content in this specific area of the brain, which may have beneficial effects for the offspring. This finding is reinforced by our GSEA analysis, in which we found several gene sets related to mitochondria, enriched both in the fathers’ comparison and the litters’ comparison, including mitochondrial matrix set (where citrate synthase is located). The enhanced mitochondrial activity in the offspring might contribute to the cellular and behavioral changes observed in the present study. This is supported by recent works reporting that mitochondrial integrity is crucial for cell differentiation and dendritogenesis of newborn neurons (31) and for efficient lineage progression of adult NSCs in adult and aged hippocampus (32), as well as the finding that acute activation or deactivation of certain mitochondrial receptors is sufficient to modify memory abilities in adult mice (33). We believe that the fact that the mtDNA/nDNA ratio is not altered in the hippocampus of the offspring but that differences are found in the same samples in citrate synthase activity is remarkable and might reflect differences in mitochondrial functionality rather than differences in the number of mitochondria.
Therefore, our data demonstrate that the specific brain effects of a physical activity program can be intergenerationally inherited. These transmitted effects include (i) enhancing the performance of nonspatial and spatial cognitive tasks; (ii) increasing the number of specific cell populations of adult hippocampal neurogenesis, inducing changes in hippocampal gene expression; and lastly (iii) increasing hippocampal mitochondrial citrate synthase activity. We found no exercise-induced changes in methylation of male sperm DNA, suggesting that intergenerational effects were not mediated by altered DNA methylation in spermatozoa. Our GSEA suggests a possible mechanism of epigenetic inheritance, since we found a huge number of enriched sets related to microRNA activity. This indicates that many of the genes that are microRNA targets show a tendency to be found overexpressed or underexpressed in the hippocampus of exercised fathers and their offspring compared with sedentary groups. It has been reported that paternal sperm microRNAs drive the changes in the progeny of stressed fathers (34, 35). Some of the gene sets that were enriched in our study are the target of microRNAs that have been proved as key in the epigenetic inheritance of an LTP improvement [e.g., the key role of microRNA 212/132 reported by Benito et al. (16)]. Therefore, the paternal sperm microRNAs of exercised fathers could well be originating the changes we observed here in mitochondria, neurogenesis, and behavior. We cannot discard other epigenetic marks such as histone methylation (36) or H3 retention sites (37) that may have mediated phenotype transmission.
Our data suggest that the intergenerational transmission of these exercise effects is pleiotropic. Multiple mechanisms involved at different levels of the hippocampus mediate these effects. First, we found that specific gene sets were modified in exercised fathers and their sedentary offspring; second, at an organelle level, an increased mitochondrial function in the hippocampus of sedentary offspring of runner fathers was found; finally, at a tissue level, we found increased proliferation of hippocampal cells in both generations. Our gene expression analysis suggests mitochondrial and cell cycle-related genes as potential mechanisms mediating these effects in the hippocampus, whereas some of the microRNAs that were differentially regulated in the hippocampus of fathers and offspring are involved in the germline transmission of these changes (16). Further experiments would be worth carrying out to demonstrate whether transgenerational effects are also inherited (by examining the F2 generation).
Most importantly, we have shown that the cognitive effects are germline dependent, because the main behavioral results were robust after IVF and embryo transfer in a patrilineal design. These findings demonstrate a patrilineal intergenerational inheritance of improved cognitive abilities in adult progeny, pointing to the physical activity levels of fathers as an unexpected, relevant factor in the brain physiology and cognitive performance of their descendants.
Materials and Methods
Subjects.
C57/BL6J mice (Harlan Laboratories) were housed under standard laboratory conditions, with ad libitum access to food and water, in accordance with European Union Directive 2010/63/EU. All experiments were performed according to the European Community Guidelines (Directive 2010/05/2016) and Spanish Guidelines (Real Decreto 53/2013), and have been approved by the Committee of Ethics and Animal Experimentation of the Cajal Institute (20/05/2016), Ethics Committee (Subcommittee of Ethics) of the Spanish Research Council (07/27/2016) and the Animal Protection Area of the Ministry of Environment of the Community of Madrid (10/26/2016).
Male progenitors (F0).
In both experiments (A and B), animals were randomly assigned to the experimental conditions. Ten subjects were used in experiment A [referred to as group sedentary (SED) A and runner (RUN) A] and 15 subjects in experiment B (referred to as group SED B and RUN B). They shared a home cage with two dams during mating periods and were housed individually immediately afterward. Animals were 3.5 mo old at the start of the preexercise behavioral assessment and 5.5 mo old at the beginning of the behavioral battery, and they were killed at 7 mo of age. All comparisons were made on subjects of the same age for each comparison.
Male offspring (F1).
Only male offspring were used. To prevent litter effects, each dam was considered the experimental unit, and all siblings from a given litter were considered as one sample. In experiment A, litters from sedentary fathers (L. SED A) and litters from the same fathers after exercising (L. RUN A) resulted in a sample size of eight subjects in each group. In experiment B, litters from sedentary fathers (L. SED B) resulted in a sample size of eight, while litters from different exercised males (L. RUN B) resulted in a sample size of four. After weaning, subjects were housed with their respective siblings. Animals were 3 mo old at the start of the behavioral assessment and were killed at 5.5 mo of age. All comparisons were made on subjects of the same age for each comparison.
Experiment Design (Experiments A and B).
A polygamous trio was selected as a breeding strategy, always housing one male with two females per cage during a whole week (different females were selected for the following trios). Dams were separated when visibly pregnant to prevent overcrowding and to keep a correct record of which pups belong to which female. A cross-fostering strategy was implemented to minimize the impact of the mother on the offspring. Siblings from a given litter remained together and were culled to generate as balanced numbers as possible within and between experimental groups.
Experiment C Design.
Experiment C was implemented to test the transmission of the positive effects of exercise in cognition through the germline, eliminating interactions between male and female progenitors by IVF and embryo transfer. In adulthood, litters underwent the same behavioral protocols as litters in experiments A and B.
IVF and embryo transfer were conducted at the Mouse Embryo Cryopreservation Facility of the National Centre for Biotechnology, Spanish National Research Council and using described methods (38–40). C57BL/6JOlaHsd females were superovulated (41). The IVF protocols used are available through the Center for Animal Resources and Development web page (card.medic.kumamoto-u.ac.jp/card/english/sigen/index.html). The process produced 10 males from sedentary fathers (referred to as L. SED C) and 13 males from runner fathers (referred to as L. RUN C). Animals were 3 mo old at the start of the behavioral assessment and were killed at 4.5 mo of age.
Control Experiment Design.
A control experiment was carried out to test whether continuous exposure to complex testing had an effect on the animals’ performance in easy behavioral protocols. Two groups of six adult males (sedentary and runner) exclusively underwent easy protocols of NOR and pattern separation.
Exercise Protocol (All Experiments).
The exercise protocol that was used was modified from Trejo et al. (42). Mice ran at 1,200 cm/min for 40 min, 5 d a week. Sedentary mice remained in the same room without running throughout the duration of the protocol.
Behavioral Assessment.
Activity assessment.
To study the spontaneous locomotor activity in an open field arena, a VersaMax Legacy Open Field activity box (Omnitech Electronics) was used. Animals underwent a two-day protocol (5 min in the activity cage per day).
NOR protocols.
To assess memory enhancement, difficult and easy protocols were designed by modifying [from the original description (20) and recent modifications of the test (21, 43)] the time spent during the training phase (SI Appendix, Figs. S11 A and B and S12).
Pattern separation.
A modified version of the pattern separation test was used to study pattern separation performance enhancement. To do this, animals underwent two different protocols (SI Appendix, Fig. S11 C and D), referred to as low separation and high separation.
BrdU Injections.
All male experimental animals in experiments A and B received one i.p. injection of BrdU (50 mg/kg body weight; Sigma-Aldrich) 24 h before being killed.
Tissue Collection.
All male experimental animals were deeply anesthetized with pentobarbital (Euta-Lender). Each animal was transcardially perfused with 0.9% saline. The right hemisphere was used to store frozen tissue. The left hemisphere was fixed by immersion in 4% paraformaldehyde for histology.
Histology.
Coronal sections (50-μm width) were obtained on a Leica VT1000S vibratome. One random series was chosen for each immunohistochemistry as described previously (44). Slices were incubated for single or double staining (SI Appendix, Table S3). The Cavalieri method was used as described previously (45).
Stereology.
BrdU- and pH3-labeled cells were counted by the optical fractionator method. The physical-dissector method, adapted to confocal microscopy as previously described (46), was used to estimate the total number of SOX2+/GFAP+ cells, DCX+, and CLR+ cells. GFAP expression was analyzed in the dentate gyrus.
RNA-seq.
Total RNA extraction from hippocampal tissue.
The QuickGene RNA Tissue Kit SII (RT-S2) and the QG-Mini80 (Kurabo) was used to extract total RNA from hippocampal tissue of a random selection of fathers (n = 10) and of eight animals representing each litter per group (n = 16) in experiment B. The final number of useful samples for analysis was n = 9 fathers (sedentary fathers, n = 5; exercised fathers, n = 4) and n = 15 offspring (animals from sedentary fathers, n = 8; animals from exercised fathers, n = 7).
Stranded mRNA library preparation and sequencing.
RNA-seq libraries were made with the TruSeq Stranded mRNA LT Sample Prep Kit (cat. no. 15031047 Rev. E, October 2013; Illumina). The libraries were sequenced on HiSeq2000 (Illumina) using TruSeq SBS Kit v4. Image analysis, base calling, and quality scoring of the run were processed using the manufacturer’s software Real Time Analysis (RTA 1.18.66.3) and followed by generation of FASTQ sequence files by CASAVA.
RNA-seq data processing and analysis.
RNA-seq reads were mapped with STAR version 2.5.2a (ENCODE parameters for long RNA), and genes were quantified with RSEM version 1.2.28 (with default parameters). Normalization and differential expression were performed with DESeq2 version 1.10. We considered significant genes with a false discovery rate (FDR) of <5%.
Bioinformatic analysis of RNA-seq results of hippocampal tissue.
Only animals of experiment B were used in this analysis. DAVID v6.8 was used for the functional description of the sDEGs of each comparison (exercised fathers vs. sedentary fathers and litters from exercised fathers vs. litters from sedentary ones). sDEGs have adjusted P values associated with their log2FoldChange of <0.05. Four databases were chosen for the extraction of terms: GOTERM_BP_DIRECT, GOTERM_CC_DIRECT, KEGG_PATHWAY, and GOTERM_MF_DIRECT, and an EASE Score of 0.05 was set as a threshold. GSEA of RNA-seq data was performed with GSEA (Broad Institute, v3.0). A preranked analysis was performed using log2FoldChange as a ranking metric. Only gene sets with an FDR of <25% were considered for descriptive analysis following the guidelines set by the Broad Institute (https://software.broadinstitute.org/gsea/doc/GSEAUserGuideFrame.html).
Mitochondrial Assessment in Liver, Cerebellum, and Hippocampus.
mtDNA/nDNA ratio analysis.
Total DNA was extracted with the DNeasy Blood and Tissue Kit (QIAGEN). mtDNA was amplified using primers specific for the mitochondrial NADH dehydrogenase (ND1) gene. Primer sequences can be found in SI Appendix, Table S5. The RT-PCR was performed on individual DNAs by using iTAQ universal SYBR Green (Bio-Rad Laboratories). The relative DNA content was calculated by the 2−ΔΔCT method.
Citrate synthase activity.
Citrate synthase activity was determined in ∼50 µg of protein lysates following the method described by Spinazzi et al. (29). Citrate synthase was determined by spectrophotometric methods.
Statistical Analysis.
Depending on the type of comparison and the parameter analyzed, we used either the t test, the Mann–Whitney U test, the paired-sample t test, the Wilcoxon signed-ranked test, a repeated-measures ANOVA, a mixed ANOVA, a Friedman test followed by a post hoc Wilcoxon signed-ranked test, or the Chi-square test (detailed description of the statistical analysis can be found in the SI Appendix, Supplementary Materials and Methods). All data were analyzed using SPSS Statistics (IBM, v.24.0.0). For the dependent variables measured on a continuous scale, data are shown as mean ± SEM. To test normality, the Shapiro–Wilk test was applied. For each test, extreme values were removed from the analysis. For comparisons between independent groups (intergroup differences), *P < 0.05, **P < 0.01, ***P < 0.001; trends 0.05 ≥ #P < 0.09. For comparisons between dependent groups (intragroup differences), +P < 0.05, ++P < 0.01, +++P < 0.001; trends 0.05 ≥ #′P < 0.09. All graphs were created in GraphPad Prism 5. Effect size estimates are described as g (Hedges’ g), r2, and partial eta-squared (ηp2).
Supplementary Material
Acknowledgments
We thank Cesar Cobaleda [Centre of Molecular Biology Severo Ochoa (CBMSO), Spanish National Research Council/Autonomous University of Madrid (CSIC/UAM), Madrid, Spain] and Alberto González-de la Vega (MegaLab, Madrid, Spain) for expert assistance and advice of the RNA-seq, DAVID, and GSEA analysis; María Llorens-Martín (CBMSO, CSIC/UAM, Madrid, Spain) for useful discussions; Silvia Fernández (Cellular and Molecular Biology Unit, Cajal Institute, Madrid, Spain) and Laude Garmendia (Animal House, Cajal Institute, Madrid, Spain) for volunteer help and advice; the Image Analysis Unit of the Cajal Institute; Carmen Sandi (Brain Mind Institute, Lausanne, Switzerland) for helpful and useful advice and assistance; and all members of the National Centre for Biotechnology Mouse Embryo Cryopreservation Facility—María Jesús del Hierro, Marta Castrillo, and Lluís Montoliu—for their huge efforts and impressive involvement in the IVF experiments. This work was supported by the Spanish Ministry of Economy and Competitiveness Project Grants BFU2013-48907-R and BFU2016-77162-R (to J.L.T.), SAF2016-78845-R (to S.R.F.), RYC-2012-10193 and AGL2014-85739-R (to P.B.Á.), CP14/00105 and PI15/00134 (to A.M.-M.); by the Instituto de Salud Carlos III of the Spanish Ministry of Economy and Competitiveness; and by the European Regional Development Fund Grant PT17/0009/0019 (to A.E.-C). Á.F.-L. was funded by a CSIC JAE-Doc Programme grant and VPlan Propio US-Acceso Grant, I.L.-T. was funded by a predoctoral fellowship (FPI) grant, and K.R.M. was funded by a contract associated with the above-mentioned project grants awarded to J.L.T.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://ncbi.nlm.nih.gov/geo (accession no. GSE123582).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1816781116/-/DCSupplemental.
References
- 1.Martin A, et al. Physical activity, diet and other behavioural interventions for improving cognition and school achievement in children and adolescents with obesity or overweight. Cochrane Database Syst Rev. 2018;3:CD009728. doi: 10.1002/14651858.CD009728.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song D, Yu DSF, Li PWC, Lei Y. The effectiveness of physical exercise on cognitive and psychological outcomes in individuals with mild cognitive impairment: A systematic review and meta-analysis. Int J Nurs Stud. 2018;79:155–164. doi: 10.1016/j.ijnurstu.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Gradari S, Pallé A, McGreevy KR, Fontán-Lozano Á, Trejo JL. Can exercise make you smarter, happier, and have more neurons? A hormetic perspective. Front Neurosci. 2016;10:93. doi: 10.3389/fnins.2016.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rendeiro C, Rhodes JS. A new perspective of the hippocampus in the origin of exercise-brain interactions. Brain Struct Funct. 2018;223:2527–2545. doi: 10.1007/s00429-018-1665-6. [DOI] [PubMed] [Google Scholar]
- 5.Cooper C, Moon HY, van Praag H. On the run for hippocampal plasticity. Cold Spring Harb Perspect Med. 2018;8:a029736. doi: 10.1101/cshperspect.a029736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandes J, Arida RM, Gomez-Pinilla F. Physical exercise as an epigenetic modulator of brain plasticity and cognition. Neurosci Biobehav Rev. 2017;80:443–456. doi: 10.1016/j.neubiorev.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denham J, O’Brien BJ, Harvey JT, Charchar FJ. Genome-wide sperm DNA methylation changes after 3 months of exercise training in humans. Epigenomics. 2015;7:717–731. doi: 10.2217/epi.15.29. [DOI] [PubMed] [Google Scholar]
- 8.Donkin I, et al. Obesity and bariatric surgery drive epigenetic variation of spermatozoa in humans. Cell Metab. 2016;23:369–378. doi: 10.1016/j.cmet.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Ingerslev LR, et al. Endurance training remodels sperm-borne small RNA expression and methylation at neurological gene hotspots. Clin Epigenetics. 2018;10:12. doi: 10.1186/s13148-018-0446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gobinath AR, et al. Maternal exercise increases but concurrent maternal fluoxetine prevents the increase in hippocampal neurogenesis of adult offspring. Psychoneuroendocrinology. 2018;91:186–197. doi: 10.1016/j.psyneuen.2018.02.027. [DOI] [PubMed] [Google Scholar]
- 11.Gomes da Silva S, et al. Maternal exercise during pregnancy increases BDNF levels and cell numbers in the hippocampal formation but not in the cerebral cortex of adult rat offspring. PLoS One. 2016;11:e0147200. doi: 10.1371/journal.pone.0147200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim TW, Park HS. Physical exercise improves cognitive function by enhancing hippocampal neurogenesis and inhibiting apoptosis in male offspring born to obese mother. Behav Brain Res. 2018;347:360–367. doi: 10.1016/j.bbr.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Short AK, et al. Exercise alters mouse sperm small noncoding RNAs and induces a transgenerational modification of male offspring conditioned fear and anxiety. Transl Psychiatry. 2017;7:e1114. doi: 10.1038/tp.2017.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeshurun S, Short AK, Bredy TW, Pang TY, Hannan AJ. Paternal environmental enrichment transgenerationally alters affective behavioral and neuroendocrine phenotypes. Psychoneuroendocrinology. 2017;77:225–235. doi: 10.1016/j.psyneuen.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Yin MM, et al. Paternal treadmill exercise enhances spatial learning and memory related to hippocampus among male offspring. Behav Brain Res. 2013;253:297–304. doi: 10.1016/j.bbr.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 16.Benito E, et al. RNA-dependent intergenerational inheritance of enhanced synaptic plasticity after environmental enrichment. Cell Rep. 2018;23:546–554. doi: 10.1016/j.celrep.2018.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toda T, Gage FH. Review: Adult neurogenesis contributes to hippocampal plasticity. Cell Tissue Res. 2018;373:693–709. doi: 10.1007/s00441-017-2735-4. [DOI] [PubMed] [Google Scholar]
- 18.Bernardo TC, et al. Physical exercise and brain mitochondrial fitness: The possible role against Alzheimer’s disease. Brain Pathol. 2016;26:648–663. doi: 10.1111/bpa.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bohacek J, Mansuy IM. A guide to designing germline-dependent epigenetic inheritance experiments in mammals. Nat Methods. 2017;14:243–249. doi: 10.1038/nmeth.4181. [DOI] [PubMed] [Google Scholar]
- 20.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 21.Antunes M, Biala G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn Process. 2012;13:93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clelland CD, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crawley JN. What’s Wrong with My Mouse? Behavioral Phenotyping of Transgenic and Knockout Mice. John Wiley & Sons; Hoboken, NJ: 2017. [Google Scholar]
- 24.Fox WM. Reflex-ontogeny and behavioural development of the mouse. Anim Behav. 1965;13:234–241. doi: 10.1016/0003-3472(65)90041-2. [DOI] [PubMed] [Google Scholar]
- 25.Cohen SJ, Stackman RW., Jr Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav Brain Res. 2015;285:105–117. doi: 10.1016/j.bbr.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leal SL, Yassa MA. Integrating new findings and examining clinical applications of pattern separation. Nat Neurosci. 2018;21:163–173. doi: 10.1038/s41593-017-0065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pérez-García G, Guzmán-Quevedo O, Da Silva Aragão R, Bolaños-Jiménez F. Early malnutrition results in long-lasting impairments in pattern-separation for overlapping novel object and novel location memories and reduced hippocampal neurogenesis. Sci Rep. 2016;6:21275. doi: 10.1038/srep21275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGreevy KR, et al. Intergenerational transmission of the positive effects of physical exercise on brain and cognition. Gene Expression Omnibus. 2019 doi: 10.1073/pnas.1816781116. Available at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE123582. Deposited December 10, 2018. [DOI] [PMC free article] [PubMed]
- 29.Spinazzi M, Casarin A, Pertegato V, Salviati L, Angelini C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat Protoc. 2012;7:1235–1246. doi: 10.1038/nprot.2012.058. [DOI] [PubMed] [Google Scholar]
- 30.Grégoire CA, et al. RNA-sequencing reveals unique transcriptional signatures of running and running-independent environmental enrichment in the adult mouse dentate gyrus. Front Mol Neurosci. 2018;11:126. doi: 10.3389/fnmol.2018.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agnihotri SK, Shen R, Li J, Gao X, Büeler H. Loss of PINK1 leads to metabolic deficits in adult neural stem cells and impedes differentiation of newborn neurons in the mouse hippocampus. FASEB J. 2017;31:2839–2853. doi: 10.1096/fj.201600960RR. [DOI] [PubMed] [Google Scholar]
- 32.Beckervordersandforth R, et al. Role of mitochondrial metabolism in the control of early lineage progression and aging phenotypes in adult hippocampal neurogenesis. Neuron. 2017;93:560–573.e6. doi: 10.1016/j.neuron.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hebert-Chatelain E, et al. A cannabinoid link between mitochondria and memory. Nature. 2016;539:555–559. doi: 10.1038/nature20127. [DOI] [PubMed] [Google Scholar]
- 34.Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci. 2013;33:9003–9012. doi: 10.1523/JNEUROSCI.0914-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodgers AB, Morgan CP, Leu NA, Bale TL. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci USA. 2015;112:13699–13704. doi: 10.1073/pnas.1508347112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siklenka K, et al. Disruption of histone methylation in developing sperm impairs offspring health transgenerationally. Science. 2015;350:aab2006. doi: 10.1126/science.aab2006. [DOI] [PubMed] [Google Scholar]
- 37.Ben Maamar M, Sadler-Riggleman I, Beck D, Skinner MK. Epigenetic transgenerational inheritance of altered sperm histone retention sites. Sci Rep. 2018;8:5308. doi: 10.1038/s41598-018-23612-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takeo T, et al. Methyl-beta-cyclodextrin improves fertilizing ability of C57BL/6 mouse sperm after freezing and thawing by facilitating cholesterol efflux from the cells. Biol Reprod. 2008;78:546–551. doi: 10.1095/biolreprod.107.065359. [DOI] [PubMed] [Google Scholar]
- 39.Takeo T, Nakagata N. Combination medium of cryoprotective agents containing L-glutamine and methyl-beta-cyclodextrin in a preincubation medium yields a high fertilization rate for cryopreserved C57BL/6J mouse sperm. Lab Anim. 2010;44:132–137. doi: 10.1258/la.2009.009074. [DOI] [PubMed] [Google Scholar]
- 40.Takeo T, Nakagata N. Reduced glutathione enhances fertility of frozen/thawed C57BL/6 mouse sperm after exposure to methyl-beta-cyclodextrin. Biol Reprod. 2011;85:1066–1072. doi: 10.1095/biolreprod.111.092536. [DOI] [PubMed] [Google Scholar]
- 41.Takeo T, Nakagata N. Superovulation using the combined administration of inhibin antiserum and equine chorionic gonadotropin increases the number of ovulated oocytes in C57BL/6 female mice. PLoS One. 2015;10:e0128330. doi: 10.1371/journal.pone.0128330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trejo JL, Llorens-Martín MV, Torres-Alemán I. The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol Cell Neurosci. 2008;37:402–411. doi: 10.1016/j.mcn.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 43.Fontán-Lozano A, et al. Caloric restriction increases learning consolidation and facilitates synaptic plasticity through mechanisms dependent on NR2B subunits of the NMDA receptor. J Neurosci. 2007;27:10185–10195. doi: 10.1523/JNEUROSCI.2757-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trejo JL, Pons S. Phosphatidylinositol-3-OH kinase regulatory subunits are differentially expressed during development of the rat cerebellum. J Neurobiol. 2001;47:39–50. doi: 10.1002/neu.1014. [DOI] [PubMed] [Google Scholar]
- 45.Llorens-Martín M, Torres-Alemán I, Trejo JL. Pronounced individual variation in the response to the stimulatory action of exercise on immature hippocampal neurons. Hippocampus. 2006;16:480–490. doi: 10.1002/hipo.20175. [DOI] [PubMed] [Google Scholar]
- 46.Suh H, et al. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1:515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.