Stress tolerance in bacterial populations is the ability to restore homeostasis after protracted exposure to lethal agents. The longer a bacterial population can withstand a lethal agent, the more tolerant it is considered to be. In the last two decades, antibiotic tolerance was given special attention because of a possible link between the tolerance level of bacterial pathogens and infection outcome in the clinic (1). Much like the evolution of antibiotic resistance, bacterial pathogens can evolve into a state of high-level tolerance (2). Unlike the limited number of mutations that provide antibiotic resistance, a wide range of mutations can increase the bacterial level of tolerance. This observation distinguishes a fundamental difference between the mechanisms of tolerance and resistance. Whereas de novo acquisition of resistance is based on specific changes at the antibiotic binding site, numerous mutations that slow the growth rate or extend the lag time increase the bacterial tolerance level. In both slow-growth and extended-lag mutants, the antibiotic target is less abundant and, therefore, primary damage accumulates more slowly (3). However, for most antibiotics, it is not the primary damage per se that kills the bacterial cell, but rather a downstream cascade of events that ends in secondary damage types (4–6). In theory, one would expect an additional evolutionary trajectory toward a high state of tolerance, driven by secondary damage attenuating mutations. However, the inherent difficulty of the study of secondary damage in isolation is the dearth of currently available research.
Measuring tolerance is more difficult than it may initially seem. The common practice of quantifying bacterial survival at time intervals by removal of antibiotic and cell plating on recovery plates is inherently flawed. While it accurately quantifies the amount of cell regrowth on the plates, the quantification of bacterial survival does not accurately reveal the time of cell death per se—that is, the standard laboratory tolerance assay does not take into account cell death on the recovery plate. As such, it either underestimates bacterial survival at a given time or overestimates cell death. Two very different scenarios might lead to bacterial cell death on the recovery plate. The first is somehow prosaic: Residual intracellular antibiotic present during recovery is responsible for cell killing on the plate. The second is far more intriguing: In the absence of antibiotic, the downstream damage cascade triggered by the primary damage is sufficient to complete the killing process without the necessity for new rounds of primary damage on the recovery plate.
For nonantibiotic lethal stress, there is at least one example of cell death on the recovery plate long after removal of the lethal agent: the tolerance to UV light irradiation. UV light damages chromosomal DNA due to the formation of cyclobutane pyrimidine dimer (CPD) cross-links between adjacent thymidine nucleotides. Even short-duration UV irradiation, typically a few minutes, is highly potent in killing bacteria, provided that the cells are kept in the dark after the irradiation (7, 8). Otherwise, light-dependent activity of photolyase decross-links CPD and prevents cell death on the recovery plate (9). This means that bacteria remain alive after the UV irradiation ends and death occurs at a later time. Of note, photoreactivation of CPD is effective only during a limited time window immediately after UV irradiation. Once this time window closes, photoreactivation is futile and cells die on the recovery plate. While UV tolerance inherently lends itself to the separation in time between the lethal agent and subsequent damage recovery, a similar system for the study of antibiotic tolerance has thus far been unavailable.
In PNAS, Hong et al. (10) describe an elegant experimental system for the separation of stress from recovery with respect to antibiotic tolerance in Escherichia coli. Using this system, they test in isolation the role of secondary damage in cell killing.
Two key features of this system are essential. The first is the use of nalidixic acid, a bactericidal antibiotic that binds reversibly to its target, the bacterial DNA gyrase (11), and that can be completely removed from bacterial cells. When the nalidixic acid concentration is above the minimum inhibitory concentration (MIC), DNA synthesis is blocked. However, promptly after the nalidixic acid is washed away, DNA synthesis resumes (12). Based on mass spectrometry quantification, Hong et al. (10) measure a decrease in intracellular nalidixic acid concentration to 0.002 MIC after washing and diluting treated cells. The second feature of the system is crucial for determining whether cells die when the antibiotic remains inside the cell or, alternatively, when cells are free of the antibiotic. If the former occurs, then no treatment on the recovery plate could possibly resuscitate the cells; however, if the latter occurs, then there might be a way for bacteria to avoid death and resuscitate on the recovery plate.
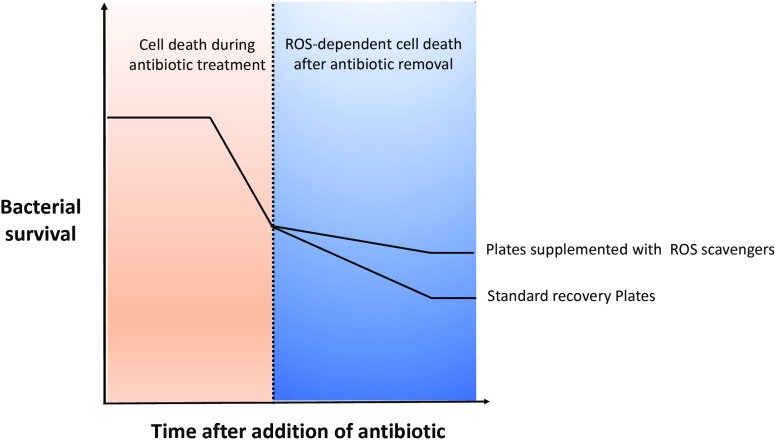
For UV irradiation, the repair of primary damage by photolyase proved instrumental for this important distinction. For nalidixic acid treatment, Hong et al. (10) discover that the sequestration of a secondary damaging agent, rather than repair of the primary damage, is essential. Specifically, a higher tolerance level is measured when compounds preventing the accumulation of reactive oxygen species (ROS) are added to the recovery plate (Fig. 1). Addition of the iron chelator bipyridyl to the recovery plate increased the number of colony-forming units by ∼30-fold. The generation of ROS and subsequent oxidative damage to many macromolecules is a known commonality shared among bactericidal antibiotics and other lethal stressors (13–19). Knowledge of the potentiating effect for ROS on cell killing, as well as the contrasting protective effect of ROS scavengers, is not new. However, this result indicates that ROS-dependent secondary damage itself can force cell death if it is preceded by a threshold amount of primary damage. In a complementing experiment, ROS scavengers had no effect on the formation of double-strand breaks, the primary damage induced by nalidixic acid. The development of tolerance, therefore, requires more than merely sustaining the primary damage. Full tolerance requires an additional step to overcome secondary damages.
Fig. 1.
Scavengers of ROS increase antibiotic tolerance after the antibiotic is no longer inside the bacterial cell. The continuous generation of ROS at this step promotes death of cells that survived the antibiotic exposure.
The protective effect of ROS scavengers on the recovery plate is counterintuitive because intracellular ROS are highly reactive and unstable (20). However, it suggests that ROS are continuously generated after antibiotic removal (Fig. 1). Indeed, when Hong et al. (10) use a fluorescent dye to monitor intracellular ROS on the recovery plate 1 h after plating, they could detect ROS in nalidixic acid treated cells, but not in control cells. Continuous generation of ROS is unlikely to stem from stable primary damage, at least in the case of nalidixic acid treatment. Instead, it is possible that the initial oxidative damage fuels new cycles of ROS generation, but this idea was not experimentally addressed by the authors in the current study.
Although both UV and nalidixic acid tolerance assays showcase cell killing on the recovery plate, it is worth emphasizing a principle difference between the two. When UV irradiation ends, the primary damage lingers. Therefore, the resuscitation time window for photoreactivation is open insofar as cells can sustain combined primary and secondary damages. In contrast, complete removal of nalidixic acid equates to the complete and instant removal of primary damage. In this case, the resuscitation time window occurs isolated from the primary damage and is mostly dependent on the sustaining of secondary damage.
In PNAS, Hong et al. describe an elegant experimental system for the separation of stress from recovery with respect to antibiotic tolerance in Escherichia coli. Using this system, they test in isolation the role of secondary damage in cell killing.
Both cases appear to shed light on a unique physiological condition of bacterial cells: injured but with a full, yet transient, recovery potential. What is the duration of this time window? According to Hong et al. (10), it can last many hours. The authors present intriguing data in a temperature-sensitive mutant of the replicative helicase DnaB. In this system, after lethal exposure at the restrictive temperature, tolerant cells are plated at the permissive temperature for recovery. Hence, there is a complete separation between primary damage and recovery in the DnaB experimental system as well. Furthermore, Hong et al. demonstrate higher tolerance on recovery plates supplemented with ROS scavengers or even purified catalase. This means that ROS contribute to cell killing on the recovery plate. Surprisingly, a clear increase in tolerance is seen, even when the authors wait 30 h before adding purified catalase to the recovery plate. However, because catalase is most probably cell impermeable, the mechanism for this extracellular prevention of ROS production, although intriguing, deserves more attention in future studies.
Studying secondary damage in isolation might prove challenging for additional classes of antibiotics such as aminoglycosides and β-lactams. Hong et al. (10) detect significant amounts of cell-bound kanamycin, even after washing treated cells. Although β-lactams are efficiently removed through washing and dilution, recovery plates supplemented with ROS scavengers do not increase tolerance. Nevertheless, the protective effect of coadministering ROS scavengers and β-lactams is dramatically enhanced when recovery plates are supplemented with ROS scavengers. This result suggests a much shorter resuscitation time window in the case of β-lactam–treated cells. It also illuminates an overlooked aspect in the relationship between the primary and secondary damages. Although some secondary damages of ROS are ubiquitous among bactericidal drugs, ROS-dependent secondary damage most probably has a drug-specific context as well.
The fictional world Thomas Mann created in the novel The Magic Mountain unfolds the lives of tuberculosis patients in a Swiss sanatorium. The patients spend hours each day lying down, taking their rest cure. Because of this, one rebellious patient refers to himself and all other patients as horizontal people. The present study by Hong et al. (10) uncovers the rest cure that tolerant bacteria take on their horizontal recovery plates. It is likely that future studies will provide exciting insights regarding this physiological state that bacteria pass through on the road to antibiotic tolerance.
Acknowledgments
This work was supported by the Department of Defense Grant PR171734 and by the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
See companion article on page 10064.
References
- 1.Meylan S, Andrews IW, Collins JJ (2018) Targeting antibiotic tolerance, pathogen by pathogen. Cell 172:1228–1238. [DOI] [PubMed] [Google Scholar]
- 2.Fridman O, Goldberg A, Ronin I, Shoresh N, Balaban NQ (2014) Optimization of lag time underlies antibiotic tolerance in evolved bacterial populations. Nature 513:418–421. [DOI] [PubMed] [Google Scholar]
- 3.Brauner A, Fridman O, Gefen O, Balaban NQ (2016) Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat Rev Microbiol 14:320–330. [DOI] [PubMed] [Google Scholar]
- 4.Davies BW, et al. (2009) Hydroxyurea induces hydroxyl radical-mediated cell death in Escherichia coli. Mol Cell 36:845–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis BB. (1988) The lethal action of aminoglycosides. J Antimicrob Chemother 22:1–3. [DOI] [PubMed] [Google Scholar]
- 6.Cho H, Uehara T, Bernhardt TG (2014) Beta-lactam antibiotics induce a lethal malfunctioning of the bacterial cell wall synthesis machinery. Cell 159:1300–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelner A. (2003) Effect of visible light on the recovery of Streptomyces griseus conidia from ultra-violet irradiation injury. DNA Repair (Amst) 2:630–636. [PubMed] [Google Scholar]
- 8.Dulbecco R. (1949) Reactivation of ultra-violet-inactivated bacteriophage by visible light. Nature 163:949. [DOI] [PubMed] [Google Scholar]
- 9.Sancar GB. (1990) DNA photolyases: Physical properties, action mechanism, and roles in dark repair. Mutat Res 236:147–160. [DOI] [PubMed] [Google Scholar]
- 10.Hong Y, Zeng J, Wang X, Drlica K, Zhao X (2019) Post-stress bacterial cell death mediated by reactive oxygen species. Proc Natl Acad Sci USA 116:10064–10071.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mustaev A, et al. (2014) Fluoroquinolone-gyrase-DNA complexes: Two modes of drug binding. J Biol Chem 289:12300–12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kantor GJ, Deering RA (1968) Effect of nalidixic acid and hydroxyurea on division ability of Escherichia coli fil+ and lon- strains. J Bacteriol 95:520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ (2007) A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130:797–810. [DOI] [PubMed] [Google Scholar]
- 14.Belenky P, et al. (2015) Bactericidal antibiotics induce toxic metabolic perturbations that lead to cellular damage. Cell Reports 13:968–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foti JJ, Devadoss B, Winkler JA, Collins JJ, Walker GC (2012) Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics. Science 336:315–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shatalin K, Shatalina E, Mironov A, Nudler E (2011) H2S: A universal defense against antibiotics in bacteria. Science 334:986–990. [DOI] [PubMed] [Google Scholar]
- 17.Gusarov I, Shatalin K, Starodubtseva M, Nudler E (2009) Endogenous nitric oxide protects bacteria against a wide spectrum of antibiotics. Science 325:1380–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mironov A, et al. (2017) Mechanism of H2S-mediated protection against oxidative stress in Escherichia coli. Proc Natl Acad Sci USA 114:6022–6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vilchèze C, et al. (2017) Enhanced respiration prevents drug tolerance and drug resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci USA 114:4495–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imlay JA. (2003) Pathways of oxidative damage. Annu Rev Microbiol 57:395–418. [DOI] [PubMed] [Google Scholar]