Significance
PD-1 blockade is a cancer immunotherapy effective in various types of cancer. However, we observed rapid cancer progression, called hyperprogressive disease (HPD), in ∼10% of advanced gastric cancer patients treated with anti–PD-1 monoclonal antibody. Tumors of HPD patients possessed highly proliferating FoxP3+ Treg cells after treatment, contrasting with their reduction in non-HPD tumors. In vitro PD-1 blockade augmented proliferation and suppressive activity of human Treg cells. Likewise, murine Treg cells that were deficient in PD-1 signaling were more proliferative and immunosuppressive. Thus, HPD may occur when PD-1 blockade activates and expands tumor-infiltrating PD-1+ Treg cells to overwhelm tumor-reactive PD-1+ effector T cells. Depletion of the former may therefore help treat and prevent HPD.
Keywords: regulatory T cells, PD-1, hyperprogressive disease, immune-checkpoint blockade
Abstract
PD-1 blockade is a cancer immunotherapy effective in various types of cancer. In a fraction of treated patients, however, it causes rapid cancer progression called hyperprogressive disease (HPD). With our observation of HPD in ∼10% of anti–PD-1 monoclonal antibody (mAb)-treated advanced gastric cancer (GC) patients, we explored how anti–PD-1 mAb caused HPD in these patients and how HPD could be treated and prevented. In the majority of GC patients, tumor-infiltrating FoxP3highCD45RA−CD4+ T cells [effector Treg (eTreg) cells], which were abundant and highly suppressive in tumors, expressed PD-1 at equivalent levels as tumor-infiltrating CD4+ or CD8+ effector/memory T cells and at much higher levels than circulating eTreg cells. Comparison of GC tissue samples before and after anti–PD-1 mAb therapy revealed that the treatment markedly increased tumor-infiltrating proliferative (Ki67+) eTreg cells in HPD patients, contrasting with their reduction in non-HPD patients. Functionally, circulating and tumor-infiltrating PD-1+ eTreg cells were highly activated, showing higher expression of CTLA-4 than PD-1− eTreg cells. PD-1 blockade significantly enhanced in vitro Treg cell suppressive activity. Similarly, in mice, genetic ablation or antibody-mediated blockade of PD-1 in Treg cells increased their proliferation and suppression of antitumor immune responses. Taken together, PD-1 blockade may facilitate the proliferation of highly suppressive PD-1+ eTreg cells in HPDs, resulting in inhibition of antitumor immunity. The presence of actively proliferating PD-1+ eTreg cells in tumors is therefore a reliable marker for HPD. Depletion of eTreg cells in tumor tissues would be effective in treating and preventing HPD in PD-1 blockade cancer immunotherapy.
Cancer immunotherapy represented by immune checkpoint blockade (ICB) such as anti–CTLA-4 and anti–PD-1 monoclonal antibodies (mAbs) instigates cytotoxic CD8+ T lymphocytes (CTLs) to kill cancer cells (1, 2). ICB has shown clinical efficacy in multiple cancer types and even in patients with advanced stages of cancer (3–5). However, the therapeutic efficacy of ICB is currently limited to 15–30% of treated cancer patients. More importantly, rapid disease progression [known as hyperprogressive disease (HPD)], rather than cancer regression, has been reported recently in certain cancer patients treated with anti–PD-1 mAb (6–8). To make ICB safer and more effective for cancer immunotherapy, the mechanism of HPD needs to be elucidated.
Regulatory T (Treg) cells are an immunosuppressive subset of CD4+ T cells, characterized by specific expression of the transcription factor forkhead box protein P3 (FoxP3) (9–14). They are abundant in tumor tissues, and there is accumulating evidence that FoxP3+ Treg cells, among various types of immunosuppressive cells in tumor tissues, play key roles in hindering effective antitumor immunity in cancer patients (14–19). In addition, there are observations that, among tumor-infiltrating lymphocytes (TILs), not only activated and exhausted CD4+ and CD8+ T cells, but also a fraction of Treg cells express PD-1 (20–25). Whereas the clinical efficacy of PD-1 blockade has been attributed to its augmentation of effector functions of tumor-infiltrating T cells, especially CTLs, possible effects of PD-1 blockade on PD-1–expressing Treg cells in tumor tissues remain unknown (1, 2).
FoxP3+ T cells in humans are heterogeneous in function (14). They comprise suppressive Treg cells and nonsuppressive conventional T (Tconv) cells, as human naive CD4+ T cells transiently up-regulate FoxP3 expression upon T cell receptor (TCR) stimulation (26). Accordingly, human FoxP3+CD4+ T cell population can be fractionated into the following three subsets based on the expression levels of the naive T cell marker CD45RA and FoxP3 or CD25 (14, 18, 19, 27): Fraction (Fr) I naive Treg cells (CD45RA+CD25lowFoxP3lowCD4+); Fr. II effector Treg (eTreg) cells (CD45RA–CD25highFoxP3highCD4+); and Fr. III non-Treg cells (CD45RA–CD25lowFoxP3lowCD4+). Fr. II eTreg cells, which highly express CTLA-4, are the predominant tumor-infiltrating FoxP3+CD4+ T cells in the majority of cancers (14, 18, 19, 28).
In this study, we have determined the Treg cell fraction in the tumor and the peripheral blood that expresses PD-1 and examined the effects of PD-1 blockade on FoxP3+ Treg cells in vitro and in vivo, in humans and mice. In particular, we have examined HPD in gastric cancer (GC) patients during anti–PD-1 mAb therapy by analyzing tumor samples obtained by biopsy before and after therapy. We have found that PD-1 blockade or deficiency enhances proliferation and immunosuppressive activity of PD-1+ Treg cells in humans and mice. Our results indicate a key role of PD-1+ eTreg cells in HPD development and suggest that depletion of Treg cells in tumor tissues could be effective in treating HPD during anti–PD-1 mAb therapy.
Results
HPDs Are Observed in ∼10% of Advanced GC Patients Treated with Anti–PD-1 mAb.
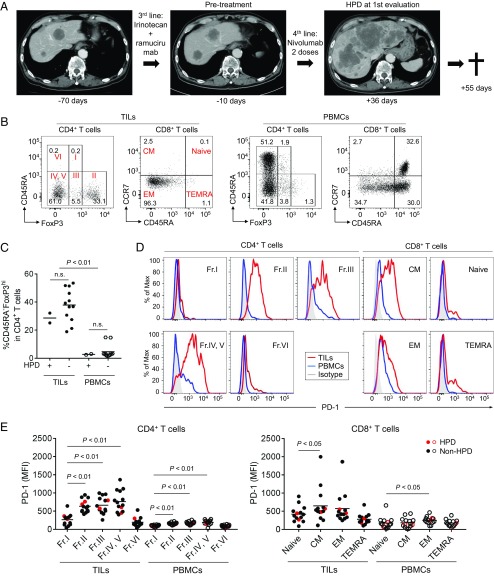
Thirty-six patients with advanced GC who received anti–PD-1 mAb (nivolumab) from October to December 2017 were enrolled in this study (SI Appendix, Table S1). Nine and 10 patients achieved partial responses (PR) and stable diseases (SD), respectively, by anti–PD-1 mAb treatment at the first evaluation 4–6 wk after starting the treatment. Among 17 patients with progressive diseases (PD), four patients (11.1% of 36 patients) were diagnosed as HPD during anti–PD-1 mAb treatment (Fig. 1A and SI Appendix, Table S1). HPD was defined as previously reported (6): time-to-treatment failure <2 mo, >50% increase in tumor burden compared with pretreatment imaging, and >twofold increase in progression speed. Despite good performance status (PS) before the treatment, three HPD patients died of tumor progression within a very short period (20–65 d) after the initial administration of anti–PD-1 mAb (SI Appendix, Table S2). Most HPD patients (three among four cases) suffered from multiple metastatic lesions especially in the liver (SI Appendix, Table S1).
Fig. 1.
Clinical course of an HPD patient and PD-1 expression by various T cell fractions in tumors and the periphery. (A) Clinical course of an HPD patient. A 73-y-old male with an MDM2 amplification (case 3 in SI Appendix, Table S2) received anti–PD-1 mAb (nivolumab) as fourth-line treatment. After two doses of anti–PD-1 mAb, his performance status became poor, and the computed tomography showed rapid disease progression diagnosed as HPD. Fifty-five days after the initial administration of anti–PD-1 mAb, he died of tumor progression. (B) TILs and PBMCs collected from 14 GC patients before anti–PD-1 mAb treatment were subjected to flow cytometry. Representative flow cytometry plots of CD4+ T cells (CD45RA and FoxP3) and CD8+ T cells (CCR7 and CD45RA) are shown. (C) Frequency of CD45RA−FoxP3highCD4+ eTreg cells in 14 GC patients. (D and E) PD-1 expression by each CD4+ and CD8+ T cell fraction. TILs and PBMCs collected from 14 GC patients before anti–PD-1 mAb treatment were subjected to flow cytometry. Representative flow cytometry staining for PD-1 by each CD4+ and CD8+ T cell fraction. Red, TILs; blue, PBMCs; gray, isotype control (D). Summary of PD-1 expression by each CD4+ and CD8+ T cell fraction in 14 GC patients (E). Red circle, HPD patients; black circle, non-HPD patients; naive (CCR7+CD45RA+); CM, central memory (CCR7+CD45RA−); EM, effector memory (CCR7−CD45RA−); TEMRA, terminally differentiated effector memory (CCR7−CD45RA+).
In 21 patients among 36 GC patients, formalin-fixed paraffin-embedded (FFPE) samples at pretreatment were available for genome analyses and subjected to next-generation sequencing (SI Appendix, Table S3). As summarized for genomic features of the patients in SI Appendix, Fig. S1 and Tables S2 and S4, one HPD patient possessed MDM2 gene amplification as reported in other types of cancer (case 3 in SI Appendix, Table S2) (7), while no patients without HPD had the MDM2 gene family alteration (SI Appendix, Fig. S1). Other genetic changes found in HPD patients (such as ERBB2 amplification, KRAS amplification, TP53 mutation, and PIK3CA mutation) were also detected in non-HPD patients (SI Appendix, Fig. S1), suggesting that these mutations were unlikely to be specifically involved in HPD.
eTreg Cells in TILs Highly Express PD-1.
The lack of common genetic alterations in HPD prompted us to examine immune responses in HPD patients. Paired (pre- and posttreatment) fresh tumor samples were obtained from 14 (2 HPD and 12 non-HPD) patients among 36 GC patients for phenotypic and functional analyses of Treg cells in TILs. While Fr. II eTreg cells were more abundant in TILs compared with peripheral blood mononuclear cells (PBMCs), there was no significant difference between HPD and non-HPD patients in eTreg cell frequency in TILs and PBMCs (Fig. 1 B and C). Notably, Fr. II eTreg cells and also Fr. III cells in HPD and non-HPD patients highly expressed PD-1 at a comparable level as effector/memory CD45RA−FoxP3−CD4+ TILs, which could be further dissected into the CD25+ (Fr. IV) and the CD25− (Fr. V) populations (Fig. 1 D and E) (27). The PD-1 expression levels of Fr. II TILs were also comparable with those of central memory (CM) or effector memory (EM) type CD8+ TILs. Thus, eTreg cells, which expressed PD-1 as highly as effector CD8+ and CD4+ T cells, would likely be targeted by anti–PD-1 mAb, particularly in tumor tissues.
Frequency of Proliferating (Ki67+) eTreg Cells Is Increased in TILs of HPD Patients and Decreased in Non-HPD Patients After Anti–PD-1 mAb Treatment.
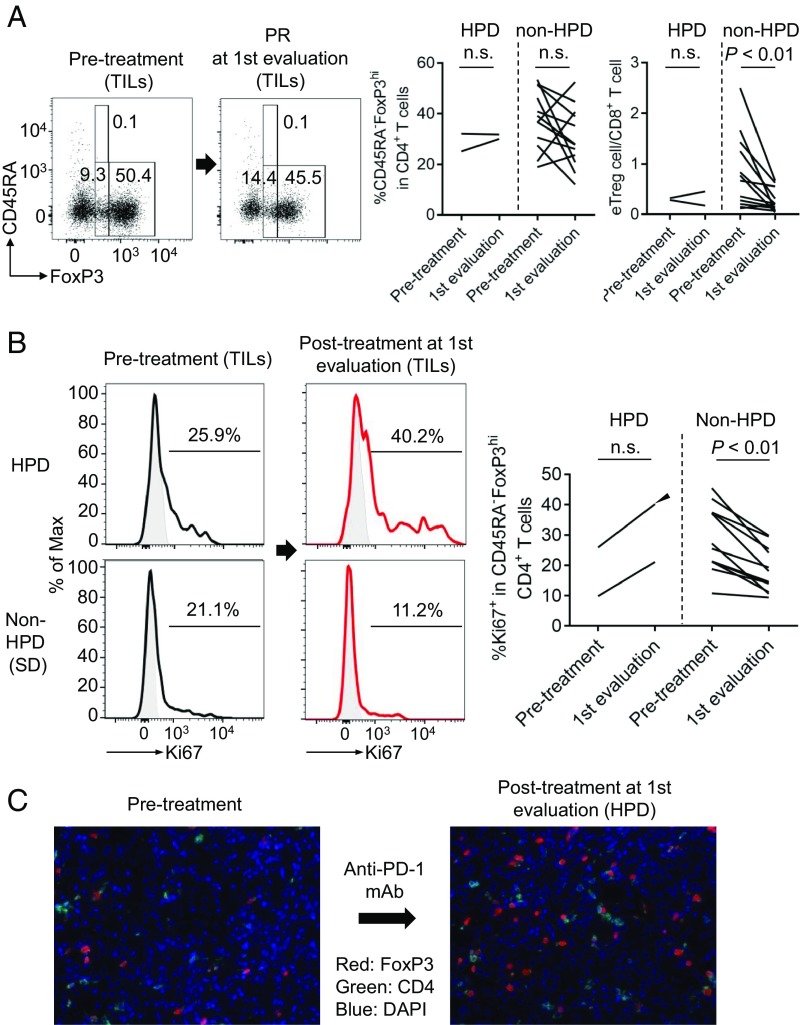
To address in vivo effect of PD-1 blockade on Treg cells, we examined the changes in the ratio of immune cells, particularly eTreg cells, in TILs from HPD patients before anti–PD-1 mAb treatment and at first evaluation 4–6 wk after starting the treatment (Fig. 2 and SI Appendix, Fig. S2). The kinetics of eTreg cell frequency in TILs was variable in patients with or without HPD (Fig. 2A). While HPD patients did not show significant changes in the ratio of eTreg cells to CD8+ T cells in TILs after anti–PD-1 treatment, non-HPD patients exhibited significant decrease in the ratio (Fig. 2A). The frequency of PD-1+, CTLA-4+, or Ki67+ eTreg cells in HPD patients’ TILs before treatment was not significantly different from non-HPD patients’ TILs (SI Appendix, Fig. S2). Notably, however, the frequency of Ki67+ eTreg cells in TILs was increased, albeit not significantly, during anti–PD-1 mAb treatment in HPD patients, contrasting with the significant decrease in non-HPD patients (Fig. 2B). We also measured the ratio of Ki67+ eTreg cells to Ki67+CD8+ T cells in TILs and found that it was reduced in the non-HPD group after treatment while remaining unchanged in the HPD group (SI Appendix, Fig. S3). In one HPD patient without MDM2 gene alteration (case 1 in SI Appendix, Table S2), the frequency of Ki67+ eTreg cells in the tumor was markedly higher after treatment. Immunohistochemistry confirmed the presence of a larger number of Foxp3+CD4+ T cells in posttreatment tumor compared with pretreatment tumor (Fig. 2 B and C). These results collectively indicate that HPD would likely occur when CD8+ T cells are not dominant over eTreg cells in tumor tissues and that the dominance is dependent on the proliferative response of eTreg cells rather than CD8+ T cells to anti–PD-1 therapy.
Fig. 2.
Immunological features of HPD patients. (A and B) TILs and PBMCs collected from 14 GC patients before and after anti–PD-1 mAb treatment were subjected to flow cytometry. (A, Left) Representative flow cytometry plots for eTreg cells of kinetic changes of eTreg cells in TILs from pretreatment to first evaluation. (A, Right) Summaries of kinetic changes of eTreg cells in two HPD patients and 12 non-HPD patients. (B, Left) Representative staining of Ki67 by eTreg cells in TILs of kinetic changes from pretreatment to first evaluation. Black, anti–PD-1 mAb (−); red, anti–PD-1 mAb (+); gray, isotype control. (B, Right) Summary of kinetic changes of Ki67+ eTreg cells in two HPD patients and 12 non-HPD patients. One patient who experienced HPD without any MDM2 gene alterations had very high Ki67+ eTreg cell infiltration at HPD state (an arrowhead; case 1 in SI Appendix, Table S2). n.s., not significant. (C) FFPE slides of case 1 before and after treatment were subjected to immunohistochemical staining of tumor-infiltrating Treg cells.
PD-1+ eTreg Cells Are Higher in CTLA-4 Expression and More Proliferative than PD-1− eTreg Cells.
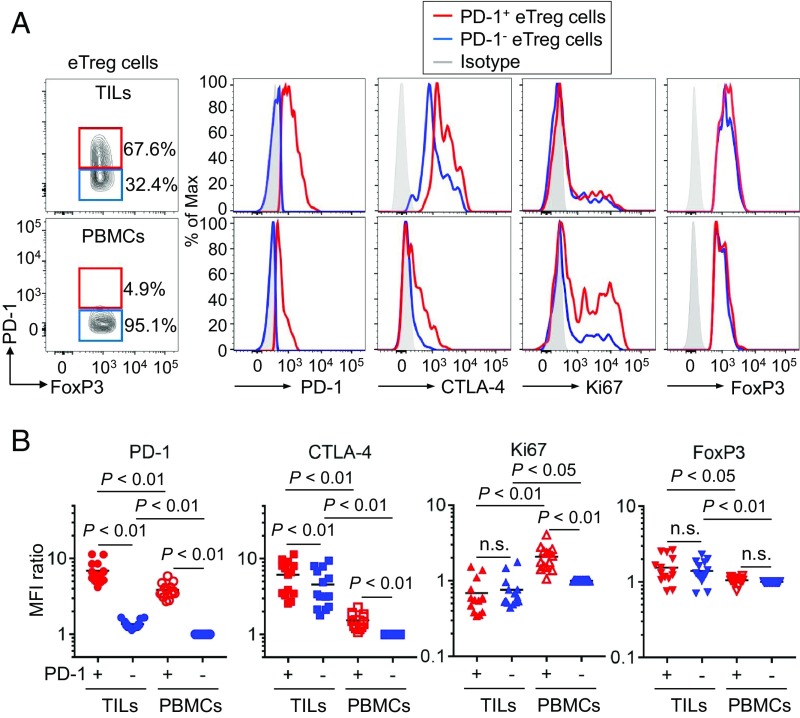
We then investigated the activation status of PD-1+ and PD-1− eTreg cells in TILs. CTLA-4, a key molecule for Treg-mediated suppression (29, 30), was expressed at higher levels by eTreg cells in TILs compared with PBMCs, and the expression was higher in PD-1+ eTreg cells than PD-1− ones (Fig. 3 A and B). In addition, Ki67 expression was much higher in PD-1+ eTreg cells compared with PD-1− eTreg cells in PBMCs (Fig. 3 A and B). Collectively, PD-1+ eTreg cells in TILs were actively proliferating and appeared to be potently immunosuppressive as indicated by high CTLA-4 expression.
Fig. 3.
Phenotypic and functional differences between PD-1+and PD-1− eTreg cells. (A and B) TILs and PBMCs collected from 14 GC patients before anti–PD-1 mAb treatment as in Fig. 1 were subjected to flow cytometry. Representative staining for PD-1, CTLA-4, Ki-67, and FoxP3 of PD-1+ and PD-1− eTreg cells from TILs or PBMCs. Naive Treg cells were used to demarcate PD-1+ and PD-1− eTreg cell fractions. Red, PD-1+ eTreg cells; blue, PD-1− eTreg cells; gray, isotype control (A). Summary for expression level detected by MFI (Mean Fluorescence Intensity) of PD-1, CTLA-4, Ki-67, and FoxP3 in PD-1+ and PD-1− eTreg cells in 14 GC patients (B). MFI for each molecule relative to MFI of PD-1− eTreg cells in PBMCs were summarized. n.s., not significant.
Anti–PD-1 mAb Augments Treg Cell-Mediated Immunosuppressive Activity in Vitro.
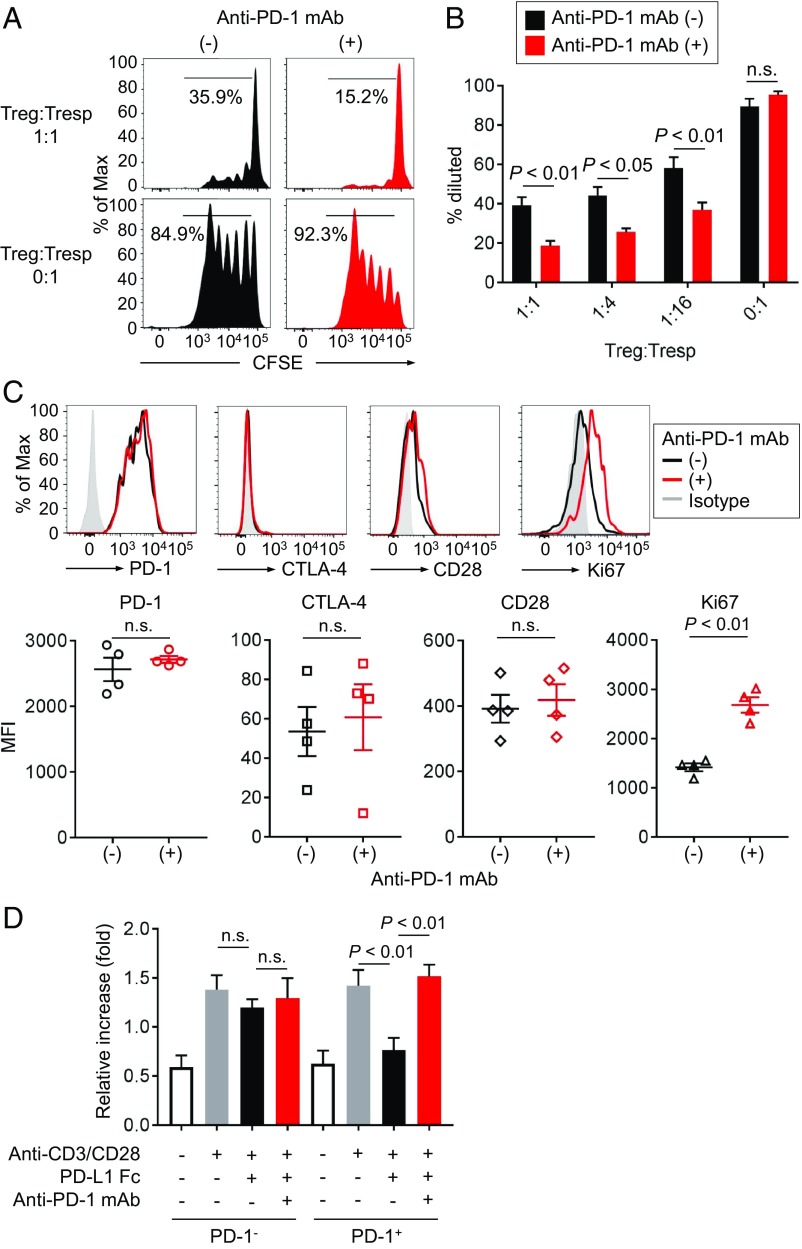
Since both TCR and CD28 signals, which are inhibited by PD-1, play crucial roles in Treg cell maintenance and immunosuppressive function (31–33), we examined whether PD-1 blockade could enhance Treg cell-mediated immunosuppressive function. We first analyzed proliferative capacity of carboxyfluorescein diacetate succinimidyl ester (CFSE) labeled CD8+ T cells cultured with or without PD-1+CD45RA−CD25highCD4+ T cells (eTreg cells) in the presence of anti-CD3 mAb and antigen-presenting cells (APCs) (SI Appendix, Fig. S4). While responder CD8+ T cells (Tresp cells) vigorously proliferated in the absence of eTreg cells, the proliferation of Tresp cells was significantly suppressed by eTreg cells. Addition of anti–PD-1 mAb to the cell culture further augmented the suppression mediated by eTreg cells (Fig. 4 A and B). In addition, anti–PD-1 mAb treatment significantly increased Ki67 expression by PD-1+ eTreg cells (Fig. 4C). This was validated by PD-L1 Fc Ig, which significantly decreased the proliferation of PD-1+ eTreg cells, but not PD-1− eTreg cells. The proliferation was restored with further addition of anti–PD-1 mAb (Fig. 4D). These findings collectively indicate that PD-1 expressed by eTreg cells can be a negative regulator of Treg cell-mediated immunosuppressive function and Treg cell proliferation and that PD-1 blockade augments immunosuppressive activity and proliferation of eTreg cells.
Fig. 4.
Role of PD-1 in Treg cell-mediated immune suppression. (A and B) PD-1+CD45RA−CD25highCD4+ T cells (eTreg cells) were sorted from PBMCs, and CFSE-labeled CD8+ T cells (Tresp cells) from PBMCs were cocultured with the indicated ratio of the sorted PD-1+ eTreg cells for 5 d with anti-CD3 mAb and irradiated APCs. Proliferation of Tresp cells was determined by CFSE dilution. Representative CFSE staining (A) and percent of proliferating Tresp cells in the cultures with the indicated ratio of Treg cells and Tresp cells (B). (C) eTreg cells in the cultures were subjected to flow cytometry to examine activation and proliferative status. (Top) Phenotypic changes (PD-1, CTLA-4, and CD28 expression) and proliferative capacity (Ki-67 expression) of eTreg cells with/without anti–PD-1 mAb (nivolumab). Black, anti–PD-1 mAb (−); red, anti–PD-1 mAb (+); gray, isotype control. (Bottom) Summary for expression levels detected by MFI of PD-1, CTLA-4, CD28, and Ki-67 of eTreg cells with/without anti–PD-1 mAb in four healthy individuals. (D) Proliferation of Treg cells. PD-1− or PD-1+ eTreg cells were sorted from PBMCs of healthy individuals and cultured with/without PD-L1 Fc Ig and/or anti–PD-1 mAb in the presence of anti-CD3 mAb and anti-CD28 mAb. Forty-eight hours after incubation, the proliferation of PD-1− or PD-1+ eTreg cells was evaluated by WST-1 assay. Ratio of the absorbance at 48 to 0 h is shown.
PD-1–Deficient Treg Cells Are Highly Proliferative and Immunosuppressive in Mice.
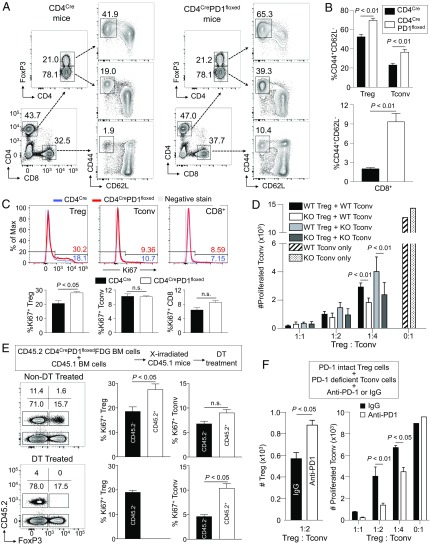
To confirm the above findings in humans, we investigated the role of PD-1 in Treg cells by generating CD4CrePD1floxedFoxP3IRES-DTR-GFP (FDG) mice with T cell-specific PD-1 deficiency (SI Appendix, Fig. S5). While CD4CrePD1floxedFDG and control CD4CreFDG mice had comparable frequencies of Treg cells, FoxP3−CD4+ (Tconv), and CD8+ T cells, the former possessed higher proportions of activated CD44+CD62L− cells in the various T cell populations, although they did not manifest discernable autoimmunity (Fig. 5 A and B). Interestingly, only Ki67+ Treg cells, but not Ki67+ Tconv cells and CD8+ T cells, were significantly increased in CD4CrePD1floxedFDG mice compared with CD4CreFDG mice (Fig. 5C). Given that Treg cells are highly dependent on basal TCR signaling to proliferate (33, 34) and possess a higher frequency of Ki67+ self-renewing cells than CD4+ Tconv or CD8+ T cells at steady state (27), it is likely that stronger TCR signaling due to PD-1 deficiency readily augments Treg cell proliferation.
Fig. 5.
Strong proliferation and immunosuppressive function of murine Treg cells with PD-1 deficiency. (A–C) Spleen cells from 9- to 10-wk-old CD4CreFDG and CD4CrePD1floxedFDG mice were subjected to flow cytometry. Representative flow cytometry plots of activated CD44+CD62L− cells in FoxP3+CD4+ Treg cells, FoxP3−CD4+ Tconv cells, and CD8+ T cells in the indicated mice are shown (A). Summary for the percentage of activated (CD44+CD62L−) cells in each cell population (B). Representative staining (Top) and summary (Bottom) of proliferating (Ki67+) Treg cells, Tconv cells, and CD8+ T cells (C). n = 4 per each group. Blue, CD4CreFDG mouse; red, CD4CrePD1floxedFDG mouse; gray, negative stain control. (D) PD-1–intact (wild-type; WT) and PD-1–deficient (knockout; KO) Treg and Tconv cells were collected from CD4CreFDG and CD4CrePD1floxedFDG mice, respectively. PD-1–intact and PD-1–deficient Tconv cells were stained with CellTrace violet (CTV) and cocultured with either PD-1–intact or PD-1–deficient Treg cells at the indicated ratios of Treg cells and Tresp cells. After 3 d culture, the number of proliferating cells was measured by CTV dilution. (E) Bone marrow chimeric (BMC) mice were generated by transferring bone marrow (BM) cells comprising 70% CD45.1 and 30% CD45.2 CD4CrePD1floxed FDG into lethally irradiated recipient CD45.1 mice. DT was administered in BMC mice to deplete PD-1–deficient CD45.2+ Treg cells. Five days after DT treatment, spleen cells were collected and subjected to flow cytometry. Representative staining of CD4+ T cells in spleens (Left) and percentages of proliferating (Ki67+) Treg cells and Tconv cells (Right). n = 4–5 in each group. (F) PD-1–intact Treg cells were cocultured with PD-1–deficient Tconv cells labeled with CTV in the presence of either anti–PD-1 or isotype-matched IgG mAb. The number of FoxP3+CD4+ Treg cells recovered (Left) and the number of proliferating Tconv cells (Right) are shown. Numbers on flow cytometry plots indicate percentages of gated populations. Data are representative of at least two independent experiments.
We next addressed whether the lack of increase in Ki67+ Tconv cells in CD4CrePD1floxedFDG mice was due to enhanced immunosuppressive function of PD-1–deficient Treg cells. To assess this possibility, we compared immunosuppressive function of PD-1–intact and PD-1–deficient Treg cells by in vitro suppression assay. The results showed that PD-1–deficient Treg cells were more suppressive against the proliferation of Tconv cells from either CD4CreFDG or CD4CrePD1floxedFDG mice (Fig. 5D). It is thus possible that in CD4CrePD1floxedFDG mice, enhanced immunosuppression by PD-1–deficient Treg cells suffices to prevent proliferation of PD-1–deficient Tconv cells.
We further sought to exclude any extrinsic effects of PD-1 deficiency on the proliferation of Treg cells because PD-1–deficient mice are known to be prone to autoimmunity. To this end, we transferred bone marrow (BM) cells composed of 70% CD45.1 wild-type (WT) mice and 30% CD45.2 CD4CrePD1floxedFDG mice into lethally irradiated CD45.1 host mice. Consistent with our observations above, CD45.2+ PD-1–deficient Treg cells had increased Ki67 expression compared with CD45.2− PD-1–intact Treg cells (Fig. 5E, Top). No significant difference in Ki67 expression was observed between CD45.2+ PD-1–deficient Tconv cells and CD45.2− PD-1–intact Tconv cells. To determine the importance of PD-1–deficient Treg cells in suppressing PD-1–deficient Tconv cells, we administered diphtheria toxin (DT) in the bone marrow chimeric (BMC) mice to deplete CD45.2+ PD-1–deficient Treg cells. This gave rise to an increase in Ki67+ PD-1–deficient CD45.2+ Tconv cells compared with PD-1–intact CD45.2− Tconv cells (Fig. 5E, Bottom). The latter was likely to be still suppressed by CD45.2− PD-1–intact Treg cells, which remained constant in frequency (15.7% in non–DT-treated and 17.5% in DT-treated) but were less efficient in suppressing PD-1–deficient Tconv cells, as shown in the in vitro suppression assay in Fig. 5D.
In addition, to confirm the role of PD-1 in Treg cells, we examined whether blocking PD-1 signaling with anti–PD-1 mAb would increase Treg cell immunosuppressive function in vitro. In the in vitro suppression assay containing PD-1–deficient Tconv cells, PD-1–intact Treg cells, and anti–PD-1 mAb, PD-1 blockade on Treg cells not only increased their numbers but also resulted in greater suppression of PD-1–deficient Tconv cell proliferation (Fig. 5F).
Collectively, these results indicate that PD-1 deficiency or blockade in Treg cells augments their proliferation and immunosuppressive activity in vivo and in vitro and renders them a memory/effector phenotype in vivo.
PD-1–Deficient Treg Cells Potently Suppress Antitumor Response by PD–1–Deficient Effector T Cells and Promote Tumor Growth in Mice.
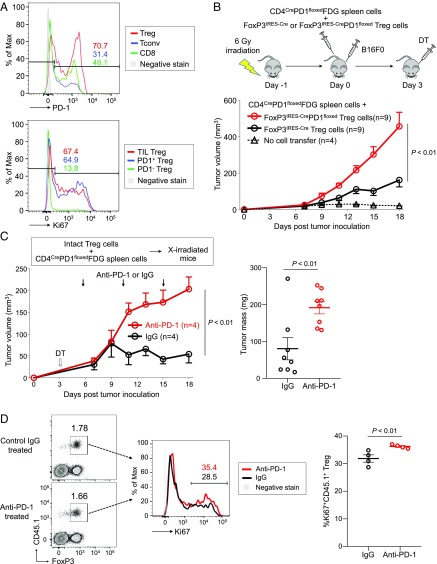
We next assessed the effects of Treg-specific PD-1 deficiency or blockade on antitumor immune responses in mice. With B16F0 murine melanoma model, we found that the majority of tumor-infiltrating Treg cells expressed PD-1 as high as Tconv cells and CD8+ T cells. Along with the high PD-1 expression, tumor-infiltrating Treg cells were also highly Ki67-positive (Fig. 6A).
Fig. 6.
Increased tumor growth by PD-1–deficient Treg cells. (A) C57BL/6 mice were inoculated with B16F0 melanoma cells in the right rear flank. Fifteen days after inoculation, T cells were prepared from tumors and draining inguinal lymph nodes and subjected to flow cytometry. Representative flow cytometry staining for PD-1 expressed by Treg cells (red), Tconv cells (blue), and CD8+ T cells (green) in TILs (Top) and Ki67 expressed by TIL Treg cells (red) from tumor and PD-1+ Treg cells (blue) and PD-1− Treg cells (green) from draining lymph nodes (Bottom). (B) C57BL/6 mice were lympho-depleted by 6-Gy irradiation and then were transferred with spleen cells from CD4CrePD1floxedFDG mice and Treg cells from either FoxP3IRES-Cre or FoxP3IRES-CrePD1floxed mice. After cell transfer, mice were injected s.c. with B16F0 cells. DT was administered intraperitoneally 3 d after cell transfer to deplete Treg cells from the CD4CrePD1floxedFDG transferred fraction. Tumor growth of B16 tumors was measured over 18 d. (C) Irradiated (6 Gy) CD45.2 B6 mice were transferred with CD45.2 CD4CrePD1floxedFDG spleen cells and PD-1–intact CD45.1 Treg cells. Mice were injected with B16 tumor cells and DT as in B, and anti–PD-1 or isotype-matched IgG mAb was administered on days 5, 10, and 15. Tumor growth of B16 tumors was measured over 18 d (Left). Tumor masses measured on day 18 are shown (Right). (D) Tumor-draining lymph nodes in anti–PD-1 mAb-treated or control mice were collected on day 18 posttransfer to assess transferred CD45.1+ Treg cells. Representative flow cytometry staining (Left) and percentage (Right) of proliferating (Ki67+) transferred CD45.1+ Treg cells from both groups. Data are representative of at least two independent experiments.
We then examined antitumor activity of PD-1–deficient effector T cells by transferring spleen cells from either CD4CreFDG or CD4CrePD1floxedFDG mice into lympho-depleted mice, which were injected s.c. with B16F0 melanoma cells. Three days later, transferred FDG Treg cells were depleted by administering DT. Whereas tumor development did not differ significantly between the two groups without DT treatment, tumors were markedly reduced in the DT-treated CD4CrePD1floxedFDG group, indicating that PD-1–deficient effector T cells possess stronger antitumor activity than PD-1–intact ones upon Treg cell depletion (SI Appendix, Fig. S6).
We next assessed whether PD-1 deficiency in Treg cells would enhance their ability to suppress in vivo antitumor responses in lympho-depleted mice transferred with PD-1–deficient or PD-1–intact Treg cells. Since PD-1–deficient and PD-1–intact Treg cells differ in their proportions of activated cells, we collected CD44−CD62L+ naive Treg cells from either FoxP3IRES-Cre or FoxP3IRES-CrePD1floxed mice to ensure that any effect would result from only the intrinsic deficiency of PD-1 in Treg cells. FoxP3IRES-Cre or FoxP3IRES-CrePD1floxed Treg cells were cotransferred with CD4CrePD1floxedFDG spleen cells into lympho-depleted (6-Gy–irradiated) mice (Fig. 6B). All recipient mice received subcutaneous injection of B16F0 melanoma cells immediately following cell transfer and were treated with DT 3 d later to deplete Treg cells from the CD4CrePD1floxed FDG inoculum. At the end of 18 d, we found mice transferred with PD-1–intact Treg cells had considerably smaller tumors, whereas those with PD-1–deficient Treg cells bore much larger tumors (Fig. 6B). The result suggests that PD-1–deficient Treg cells strongly favor tumor development.
To further determine the effect of Treg cell-specific PD-1 blockade on B16F0 tumor development, we cotransferred PD-1–intact Treg cells with CD4CrePD1floxedFDG spleen cells into lympho-depleted mice, which received DT 3 d after cell transfer and then anti–PD-1 or control mAb treatment on days 5, 10, and 15 post cell transfer. Mice treated with anti–PD-1 mAb developed significantly larger tumors than those treated with control mAb (Fig. 6C). Additionally, anti–PD-1 mAb induced higher Ki67 expression in Treg cells compared with control (Fig. 6D).
Taken together, PD-1 blockade in Treg cells results in their expansion and enhances their immunosuppressive activity, promoting tumor growth even in the presence of potent antitumor immune responses mediated by PD-1–deficient Tconv cells.
Proliferative eTreg Cells Can Be Targeted by Anti–CTLA-4, Anti–OX-40, or Anti-CCR4 mAb.
Last, to evaluate eTreg-specific markers in humans as potential therapeutic targets especially in HPD, 22 TIL samples from GC patients who underwent surgical resection were subjected to flow cytometric analysis (SI Appendix, Table S5). In these samples, eTreg cells expressed CTLA-4, OX-40, and CCR4 at significantly higher levels compared with CD4+ or CD8+ Tconv cells, with a comparable level of LAG-3 (SI Appendix, Fig. S7). These data suggest that antibodies, especially cell-depleting ones, targeting CTLA-4, OX-40, and CCR4 can be used for the treatment and prevention of HPD by specifically depleting eTreg cells (28).
Discussion
Immune checkpoint blockade, particularly by anti–PD-1 mAb, is now widely used as a cancer immunotherapy, with occasional development of HPD with poor clinical outcome. A previous study reported that 12 of 131 (9%) patients with various types of cancer succumbed to HPD during anti–PD-1 mAb treatment and that no GC patients (0/2) experienced HPD (6). In our present study, the occurrence rate of HPD in anti–PD-1 mAb-treated advanced GC patients was ∼10% (4 among 36). Our recent independent study with advanced GC patients also showed the development of HPD with poor prognosis in 21% (13 of 62) of anti–PD-1–treated patients (35), further underlining a need to determine the cause of HPD and design treatment strategies against it.
Our results in humans and mice have demonstrated that PD-1 deficiency or blockade enhanced the activation of both Treg and Tconv cells, the former suppressing and the latter augmenting antitumor immunity. PD-1 attenuates TCR signal and also CD28 costimulatory signal; PD-1 blockade or deficiency in T cells therefore enhances TCR and CD28 signal intensity, which activates Treg cells as well as Tconv cells (31–33). A previous animal study showed that PD-1–deficient Treg cells had increased immunosuppressive activity and better protected against autoimmune diseases compared with PD-1–intact Treg cells, indicating that lack of PD-1 signaling enhances the immunosuppressive function of Treg cells (20). On the other hand, it was recently reported that PD-L1 binding to PD-1 during peripheral Treg cell generation from Tconv cells was critical for the maintenance of long-term FoxP3 expression and in vivo regulation of immune responses against murine colitis and graft versus host disease (21). Furthermore, Treg cells have been shown to exhibit immunosuppressive activity via direct interaction between PD-1 on Treg cells and PD-L1 on CD8+ T cells, an activity significantly decreased by PD-1 blockade in an animal model of chronic infection (22). It is also worth noting that some tumor-infiltrating Treg cells express PD-L1 in addition to PD-1 (36, 37), raising the likelihood of reciprocal PD-1 and PD-L1 signaling that allows for a delicate control of immune cell homeostasis within tumor tissues. These results suggest that PD-1 signaling contributes to the function and maintenance of Treg cells depending on various factors ranging from the Treg cell subtype in question to the mechanism-of-action by which Treg cells control diseases (e.g., chronic viral infection and cancer) through their target cells of choice such as APCs or others (13, 14, 30).
The success of anti–PD-1 mAb therapy hinges on its ability to unleash effector T cells from PD-1–dependent inhibition to enable them to kill tumor cells. This strategy, however, can be supplanted by various means, one of which is the proliferation of Treg cells and augmentation of their immunosuppressive activity as shown in the present study. In tumor tissues, activated PD-1+ Treg cells, which highly express CTLA-4, may result in more Treg: APC aggregates coupled with CTLA-4–dependent down-regulation of the costimulatory molecules CD80 and CD86 on APCs, hence restricting APC access for Tconv cells and their activation (29, 30, 38). Additionally, proliferating PD-1+ Treg cells may rapidly absorb IL-2 and deprive it from tumor-reactive effector T cells (30). With such Treg cell-mediated immune suppression in tumor tissues, the antitumor efficacy of PD-1 blockade may rest on the balance between reinvigoration of effector T cells and augmentation of PD-1+ Treg cell proliferation and suppression. In the event that the latter effect is more dominant, tumor cells could escape effector T cell killing and grow uncontrollably (SI Appendix, Fig. S8). This is supported by the finding that Treg cells were increased in nonresponders to anti–PD-1 mAb treatment for malignant melanoma and decreased in responders (39), consistent with our observation in the HPD cases. The underlying reason for preferential expansion of Treg cells upon PD-1 blockade remains unknown. Although the tumors of HPD and non-HPD patients harbor high PD-1+ Treg cells, which serve as a major predisposition, it is reasonable to speculate that the former may inherently be enriched in factors (e.g., adenosine and indoleamine 2,3-dioxygenase) that support eTreg cell expansion and induction upon PD-1 blockade. Future work ought to assess the metabolite content of HPD tumors to dissect unique features that favor and sustain Treg cell proliferation. At present, it would be worthwhile to evaluate the proliferative response of Treg cells in TILs during anti–PD-1 mAb treatment to identify potential HPD patients. According to recent reports using mass cytometry, the relative frequencies of certain T cell subsets and expression of activation/migration markers (e.g., CD45RO and CD11a) may be used to predict clinical outcome following PD-1 blockade (40, 41). ICOS is highly correlated with Ki-67 expression in Treg cells (42) and, indeed, expressed by proliferating Treg cells in gastric cancer TILs (43). It was also shown that despite constant cell cycling of the majority of tumor-infiltrating T cells, only a few specific clusters increased in size in response to anti–PD-1 mAb therapy (40). Such findings are useful for future studies to assess relevant cellular and molecular defects that would induce and expand PD-1+ Treg cells in the tumor tissue, causing HPD.
Our study strongly indicates that inhibiting Treg cell proliferation could be an important strategy to treat and prevent HPD in high-risk patients under PD-1 blockade therapy. This is underscored by our mouse studies, which clearly suggest that by leaving the PD-1 pathway intact in Treg cells and not inducing their proliferation, PD-1 blockade on non-Treg effector T cells may be more effective in eliminating tumor cells. Notably, combination of nivolumab and ipilimumab (anti–CTLA-4 mAb), which reportedly target Treg cells for depletion (44, 45), can decrease HPD in malignant melanoma patients (46). OX-40–targeting therapy, which reportedly suppresses Treg cells, is presently under clinical trial with several solid tumors, and phase I trials have shown promising antitumor activity (47). Furthermore, we have previously shown that CCR4 was specifically and predominantly expressed by highly suppressive eTreg cells in malignant melanoma; in vitro treatment of melanoma TILs with anti-CCR4 mAb (mogamulizumab) indeed depleted melanoma-infiltrating CCR4+ eTreg cells and efficiently expanded and activated both CD4+ and CD8+ T cells specific for a cancer-testis antigen expressed by the melanoma (48). Several early-phase clinical trials with mogamulizumab, used as an eTreg cell depletion reagent, either as monotherapy or in combination with ICBs, are being conducted in advanced solid tumors. The combination of nivolumab and mogamulizumab is also being investigated in a Japanese phase I trial for solid tumors (49). The occurrence rate of HPD in this combination therapy appears to be lower than nivolumab monotherapy, warranting further studies with large cohorts. Besides antibody-mediated therapy, cytokine therapy could be a viable option as well. For example, antitumor effects of anti–PD-1 mAb were greatly enhanced by increasing systemic IL-27 levels. This was attributed to reduced IL-2 production and reduced IL-2Rα expression in Tconv cells and Treg cells, respectively, which limited Treg cell number in anti–PD-1 mAb-treated mice (50). Further development of strategies for Treg cell depletion in cancer tissues is required.
In conclusion, the development of HPD in advanced GC patients during anti–PD-1 mAb therapy is associated with proliferation of Treg cells in tumor tissues. PD-1 blockade promotes cell cycling of Treg cells and augments Treg cell-mediated immune suppression, likely through stronger TCR signaling. Currently, monitoring of Treg cells in the tumor during anti–PD-1 mAb treatment is not routinely practiced. Our study calls for a need to do so. Further confirmation with large cohort studies is required, especially for designing novel strategies to better detect and treat HPD. Depletion of tumor-infiltrating eTreg cells or attenuation of their immunosuppressive function could be instrumental in treating and preventing HPD, as our study suggests.
Materials and Methods
All participants provided written informed consent. This study was approved by the institutional review boards of National Cancer Center and was conducted in accordance with ethics guidelines including the Declaration of Helsinki. Mice were used according to protocols approved by Osaka University animal care and use committee. Details about materials and methods are provided in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank Ms. Tomoka Takaku, Miyuki Nakai, Konomi Onagawa, Megumi Takemura, Chie Haijima, Megumi Hoshino, Kumiko Yoshida, and Miho Ozawa for their technical assistance. This study was supported by Grants-in-Aid for Scientific Research [S Grant 17H06162 (to H.N.), Challenging Exploratory Research Grant 16K15551 (to H.N.), Young Scientists Grant 17J09900 (to Y. Togashi)], Japan Society for the Promotion of Science Research Fellow [Grants 17K18388 (to Y. Togashi) and 18J21161 (to T. Kamada)] and Grant-in-Aid for Specially Promoted Research [Grant 16H06295 (to S.S.)] from the Ministry of Education, Culture, Sports, Science and Technology of Japan, by the Project for Cancer Research, by Therapeutic Evolution [P-CREATE, Grants 16cm0106301h0002 (to H.N.), 18cm0106340h0001 (to Y. Togashi), and 18cm0106303h0003 (to S.S.)] Core Research for Evolutional Science and Technology [CREST, Grant 17gm0410016h0006 (to S.S.)], and Leading Advanced Projects for medical innovation [LEAP, Grant 18gm0010005h0001 (to S.S.)] from Japan Agency for Medical Research and Development (AMED), by the National Cancer Center Research and Development Fund [no. 28-A-7 (to H.N.)], by the Naito Foundation (to Y. Togashi and H.N.), by the Takeda Foundation (to Y. Togashi), by the Kobayashi Foundation for Cancer Research (to Y. Togashi), by the Novartis Research Grant (to Y. Togashi), by the Bristol-Myers Squibb Research Grant (to Y. Togashi), and by the Sagawa Holdings Foundation (to Y. Togashi). The analysis of immune status was executed in part as a research program supported by Ono Pharmaceutical Co., Ltd.
Footnotes
Conflict of interest statement: The Sponsor declares a conflict of interest. Y. Togashi has received honoraria and grants from Ono Pharmaceutical as to this work, honoraria and grants from Bristol-Myers Squibb and AstraZeneca, and honoraria from Chugai Pharmaceutical and Merck Sharp & Dohme (MSD) outside of this study. K.S. received honoraria and grants from Ono Pharmaceutical and Bristol-Myers Squibb and grants from MSD outside of this study. H.N. received honoraria and grants from Ono Pharmaceutical as to this work, honoraria and grants from Bristol-Myers Squibb and Chugai Pharmaceutical, and grants from Taiho Pharmaceutical, Daiichi-Sankyo, Kyowa-Hakko Kirin, Zenyaku Kogyo, Astellas Pharmaceutical, Sysmex, and BD Japan outside of this study. Other authors declare no competing financial interests.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1822001116/-/DCSupplemental.
References
- 1.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Champiat S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. 2017;23:1920–1928. doi: 10.1158/1078-0432.CCR-16-1741. [DOI] [PubMed] [Google Scholar]
- 7.Kato S, et al. Hyperprogressors after immunotherapy: Analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res. 2017;23:4242–4250. doi: 10.1158/1078-0432.CCR-16-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Champiat S, et al. Hyperprogressive disease: Recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol. 2018;15:748–762. doi: 10.1038/s41571-018-0111-2. [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 10.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 11.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 12.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 14.Wing JB, Tanaka A, Sakaguchi S. Human FOXP3+ regulatory T cell heterogeneity and function in autoimmunity and cancer. Immunity. 2019;50:302–316. doi: 10.1016/j.immuni.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Onizuka S, et al. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor α) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 16.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: A common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–5218. [PubMed] [Google Scholar]
- 17.Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 18.Saito T, et al. Two FOXP3+CD4+ T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. 2016;22:679–684. doi: 10.1038/nm.4086. [DOI] [PubMed] [Google Scholar]
- 19.Togashi Y, Nishikawa H. Regulatory T cells: Molecular and cellular basis for immunoregulation. Curr Top Microbiol Immunol. 2017;410:3–27. doi: 10.1007/82_2017_58. [DOI] [PubMed] [Google Scholar]
- 20.Zhang B, Chikuma S, Hori S, Fagarasan S, Honjo T. Nonoverlapping roles of PD-1 and FoxP3 in maintaining immune tolerance in a novel autoimmune pancreatitis mouse model. Proc Natl Acad Sci USA. 2016;113:8490–8495. doi: 10.1073/pnas.1608873113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stathopoulou C, et al. PD-1 inhibitory receptor downregulates asparaginyl endopeptidase and maintains Foxp3 transcription factor stability in induced regulatory T cells. Immunity. 2018;49:247–263.e7. doi: 10.1016/j.immuni.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park HJ, et al. PD-1 upregulated on regulatory T cells during chronic virus infection enhances the suppression of CD8+ T cell immune response via the interaction with PD-L1 expressed on CD8+ T cells. J Immunol. 2015;194:5801–5811. doi: 10.4049/jimmunol.1401936. [DOI] [PubMed] [Google Scholar]
- 23.Lowther DE, et al. PD-1 marks dysfunctional regulatory T cells in malignant gliomas. JCI Insight. 2016;1:e85935. doi: 10.1172/jci.insight.85935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montler R, et al. OX40, PD-1 and CTLA-4 are selectively expressed on tumor-infiltrating T cells in head and neck cancer. Clin Transl Immunology. 2016;5:e70. doi: 10.1038/cti.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asano T, et al. PD-1 modulates regulatory T-cell homeostasis during low-dose interleukin-2 therapy. Blood. 2017;129:2186–2197. doi: 10.1182/blood-2016-09-741629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyara M, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 28.Ha D, et al. Differential control of human Treg and effector T cells in tumor immunity by Fc-engineered anti-CTLA-4 antibody. Proc Natl Acad Sci USA. 2019;116:609–618. doi: 10.1073/pnas.1812186116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wing K, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 30.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi T, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: Induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 32.Zhang R, et al. An obligate cell-intrinsic function for CD28 in Tregs. J Clin Invest. 2013;123:580–593. doi: 10.1172/JCI65013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levine AG, Arvey A, Jin W, Rudensky AY. Continuous requirement for the TCR in regulatory T cell function. Nat Immunol. 2014;15:1070–1078. doi: 10.1038/ni.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vahl JC, et al. Continuous T cell receptor signals maintain a functional regulatory T cell pool. Immunity. 2014;41:722–736. doi: 10.1016/j.immuni.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki A, et al. Predictive factors for hyperprogressive disease during nivolumab as anti-PD1 treatment in patients with advanced gastric cancer. Gastric Cancer. January 9, 2019 doi: 10.1007/s10120-018-00922-8. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, et al. FoxA1 directs the lineage and immunosuppressive properties of a novel regulatory T cell population in EAE and MS. Nat Med. 2014;20:272–282. doi: 10.1038/nm.3485. [DOI] [PubMed] [Google Scholar]
- 37.Chevalier MF, et al. Conventional and PD-L1-expressing regulatory T cells are enriched during BCG therapy and may limit its efficacy. Eur Urol. 2018;74:540–544. doi: 10.1016/j.eururo.2018.06.045. [DOI] [PubMed] [Google Scholar]
- 38.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci USA. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weber JS, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31:4311–4318. doi: 10.1200/JCO.2013.51.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei SC, et al. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell. 2017;170:1120–1133.e17. doi: 10.1016/j.cell.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krieg C, et al. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat Med. 2018;24:144–153. doi: 10.1038/nm.4466. [DOI] [PubMed] [Google Scholar]
- 42.Miyara M, et al. Sialyl Lewis x (CD15s) identifies highly differentiated and most suppressive FOXP3high regulatory T cells in humans. Proc Natl Acad Sci USA. 2015;112:7225–7230. doi: 10.1073/pnas.1508224112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagase H, et al. ICOS+ Foxp3+ TILs in gastric cancer are prognostic markers and effector regulatory T cells associated with Helicobacter pylori. Int J Cancer. 2017;140:686–695. doi: 10.1002/ijc.30475. [DOI] [PubMed] [Google Scholar]
- 44.Romano E, et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci USA. 2015;112:6140–6145. doi: 10.1073/pnas.1417320112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arce Vargas F, et al. TRACERx Melanoma; TRACERx Renal; TRACERx Lung Consortia Fc effector function contributes to the activity of human anti-CTLA-4 antibodies. Cancer Cell. 2018;33:649–663.e4. doi: 10.1016/j.ccell.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larkin J, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:1270–1271. doi: 10.1056/NEJMc1509660. [DOI] [PubMed] [Google Scholar]
- 47.Hamid O, et al. First in human (FIH) study of an OX40 agonist monoclonal antibody (mAb) PF-04518600 (PF-8600) in adult patients (pts) with select advanced solid tumors: Preliminary safety and pharmacokinetic (PK)/pharmacodynamic results. J Clin Oncol. 2016;34(Suppl 15):3079. [Google Scholar]
- 48.Sugiyama D, et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci USA. 2013;110:17945–17950. doi: 10.1073/pnas.1316796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto N, et al. Anti-CC-chemokine receptor 4 (CCR4) antibody mogamulizumab (Moga) and nivolumab (Nivo) combination phase I study in patents with advanced or metastatic solid tumor. Ann Oncol. 2017;28(Suppl 5):v605–v649. [Google Scholar]
- 50.Zhu J, et al. IL-27 gene therapy induces depletion of Tregs and enhances the efficacy of cancer immunotherapy. JCI Insight. 2018;3:e98745. doi: 10.1172/jci.insight.98745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.