Abstract
Context
The ovaries and adrenals are sources of androgens in women. Although dehydroepiandrosterone (DHEA), DHEA sulfate (DHEAS), and testosterone (T) all decline with age, these C19 steroids correlate poorly with parameters of androgen action in postmenopausal women.
Objective
To comprehensively compare the androgen profiles of pre- and postmenopausal women.
Methods
We quantified 19 steroids—including DHEA; DHEAS; T; androstenedione (A4); and the following adrenal-specific 11-oxygenated C19 steroids (11oxyandrogens): 11β-hydroxytestosterone (11OHT), 11-ketotestosterone (11KT), 11β-hydroxyandrostenedione (11OHA4), and 11-ketoandrostenedione (11KA4)—using liquid chromatography–tandem mass spectrometry in morning serum obtained from 100 premenopausal (age 20 to 40 years) and 100 postmenopausal (age ≥ 60 years) women. Double immunofluorescence of 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2) with cytochrome b5 (CYB5A) or sulfotransferase 2A1 (SULT2A1) was performed in normal adrenal glands obtained from eight premenopausal and eight postmenopausal women.
Results
DHEA, DHEAS, A4, and T were significantly higher in pre- than in postmenopausal women (2.9, 2.8, 2.9, and 1.6-fold, respectively; P < 0.0001). In contrast, the 11-oxyandrogens did not decrease with aging, and the 11OHT/T and 11OHA4/A4 ratios showed strong positive correlations with age (r = 0.5 and 0.8, respectively; P < 0.0001). Double immunofluorescence analysis showed that with the involution of the zona reticularis in the old adrenals, the sharp zonal segregation of HSD3B2 and CYB5A becomes less distinct, and areas of HSD3B2 and CYB5A overlap are observed.
Conclusions
Unlike DHEA, DHEAS, A4, and T, the 11oxyandrogens do not decline in aging women. Structural changes within the adrenal cortex might explain the evolution of androgen profiles in aging women.
This study of 100 pre- and 100 postmenopausal women shows that unlike other androgens, the adrenal 11-oxygenated C19 steroids do not decline with aging.
Despite intensive investigations, the contributions of androgens to physiology and sexuality in women, particularly after menopause, remain controversial. The adrenals are a major source of sex-steroid precursors during fetal life and adrenarche (1–3). From puberty throughout adult life, the ovaries and adrenals contribute to the production of testosterone (T) and androstenedione (A4) (4, 5), both directly and via metabolism of androgen precursors, including the adrenal-derived dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). With menopause, estrogen declines abruptly, but the ovarian androgen synthesis continues to a variable degree. Nevertheless, A4 and to a lesser extent T also decline with aging (6, 7). In contrast to the abrupt ovarian changes, adrenal C19 steroid production gradually declines with aging, which parallels the involution of the zona reticularis (ZR) (8, 9). The production of DHEA and DHEAS from the ZR peaks in the mid-20s and then declines progressively, such that by age 80 years, their concentrations are only about 20% of those at age 25 years (10). Given these multiple changes with aging, the contributions of the adrenals and ovaries to androgen economy during menopause are complex.
Besides DHEA and DHEAS, the adrenal gland also produces a set of underrecognized 11-oxygenated C19 steroids (11oxyandrogens), derived from A4 and T: 11β-hydroxyandrostenedione (11OHA4) and 11β-hydroxytestosterone (11OHT), which are converted in periphery to 11-ketoandrostenedione (11KA4) and 11-ketotestosterone (11KT), respectively (11–15). In vitro studies have shown that of these, 11KT is a bioactive androgen, with potency similar to that of T (12, 13, 16); subsequently, 11KT can be reduced to 11-ketodihydrotestosterone, an androgen analogous to dihydrotestosterone (13). Notably, 11OHA4 and 11KT are more abundant in the circulation of girls and young women than their precursors, A4 and T, respectively (15, 17). The dynamics of these adrenal-derived androgens in postmenopausal women has not been studied. We hypothesized that the adrenal is the major source of 11oxyandrogens during menopause. Herein, we show that in contrast with the traditional adrenal androgen precursors, DHEA and DHEAS, the 11oxyandrogens do not decline in aging women.
Patients and Methods
Human sera
Serum was obtained from reproductive-age (20 to 40 years) and postmenopausal (≥60 years) women who had morning (6:00 to 10:00 am) blood draws for annual health exams or minor health conditions in an outpatient setting (Table 1). Exclusion criteria comprised irregular menstrual bleeding, local or systemic hormonal contraceptive methods or hormonal replacement therapy, amenorrhea (in the reproductive-age group), immunosuppressive therapy, and active malignancies. Clinical information collected from chart reviews included patient demographic characteristics, blood pressure, metabolic profile, glycemic data, medications, and bone density. Hypertension was diagnosed if the systolic blood pressure was ≥ 140 mm Hg and/or the diastolic blood pressure was ≥ 90 mm Hg, or if antihypertensive medications were prescribed. Dyslipidemia was defined by serum concentrations of low-density lipoprotein cholesterol ≥ 140 mg/dL, high-density lipoprotein cholesterol < 40 mg/dL, triglyceride level ≥ 150 mg/dL, or use of lipid-lowering pharmacologic therapy; a hemoglobin A1c value ≥ 5.7% or antidiabetic agents indicated hyperglycemia. Bone density was assessed with dual-energy x-ray absorptiometry. The study was conducted with University of Michigan Institutional Review Boards approval.
Table 1.
Participants Characteristics
| Characteristic | Premenopausal Women (n = 100) | Postmenopausal Women (n = 100) | P Value |
|---|---|---|---|
| Age, y | 33 ± 6 | 70 ± 8 | <0.0001 |
| BMI, kg/m2 | 25.7 ± 7.4 | 26.7 ± 4.9 | 0.3 |
| Systolic blood pressure, mm Hg | 109 ± 11 | 127 ± 17 | <0.0001 |
| Diastolic blood pressure, mm Hg | 67 ± 8 | 69 ± 9 | 0.02 |
| Prevalence, n (%) | |||
| Hypertension | 3/100 (3) | 56/100 (56) | <0.0001 |
| Hyperglycemia | 6/32 (19) | 16/43(37) | 0.01 |
| Dyslipidemia | 15/55 (27) | 61/86 (71) | <0.0001 |
| DEXA results, n (%) | |||
| Normal | — | 24/57 (42) | |
| Osteopenia | — | 23/57 (40) | |
| Osteoporosis | — | 10/57 (18) |
Data are expressed as mean ± SD or n (%). Hyperglycemia and dyslipidemia were assessed in 32 and 55 premenopausal women and 43 and 86 postmenopausal women, respectively. DEXA was performed in 57 postmenopausal women.
Abbreviations: BMI, body mass index; DEX, dual-energy x-ray absorptiometry.
Steroid quantitation by liquid chromatography–tandem mass spectrometry
Quantitation of 19 steroids in peripheral serum was performed by liquid chromatography–tandem mass spectrometry, including 13 Δ4 steroids (including A4, T, 11OHA4, 11KA4, 11OHT, and 11OHT), two Δ5 steroids [DHEA and androstenediol (Adiol)] and four sulfated steroids [pregnenolone sulfate (PregS), 17-hydroxypregnenolone sulfate (17OHPregS), DHEAS, and Adiol-3-sulfate (AdiolS)]. Unlabeled and deuterium-labeled steroid were obtained from Sigma-Aldrich (St. Louis, MO); Cerilliant (Round Rock, TX); C/D/N Isotopes (Pointe-Claire, QC); Steraloids (Newport, RI); and the National Heart, Lung, and Blood Institute RTI International Metabolite Standards Synthesis Center (Research Triangle Park, NC) (18).
For measurement of Δ4 steroids, a mixture of 100 µL serum, 200 µL deionized water (diH2O), and internal standards at known concentrations was loaded on supported liquid extraction (Isolute; Biotage, Charlotte, NC) columns. After 5 minutes, the columns were washed twice with 700 µL methyl-tert-butyl ether (MTBE), and pressure was subsequently applied for 30 seconds to complete elution. The eluates were concentrated under nitrogen. For measurement of Δ5 steroids, serum was deproteinized with 200 µL methanol containing internal standards. The supernatant was mixed with 400 µL diH2O and extracted twice with 800 µL MTBE. The extracts were dried under pressurized nitrogen, and the residue was incubated in 100 µL reagent containing 2-picolinic acid, 2-methyl-6-nitrobenzonic anhydride, and 4-(dimethylamino) pyridine (25 mg, 20 mg, and 10 mg per 1 mL of acetonitrile, respectively) at room temperature for 90 minutes. The reaction was quenched with 400 µL of diH2O and extracted with 1 mL of MTBE, and the organic phase was separated and concentrated. The dried extracts were reconstituted with 100 µL methanol/diH2O (40:60 Δ4 or 75:25 Δ5) and transferred to 0.25-mL glass inserts. The sulfated steroids extraction was done as previously described (19). Samples (10 µL) were injected via autosampler and resolved with a two-dimensional chromatography method via a C4, 10 × 2.1-mm column (ThermoFisher Scientific, Waltham, MA) on an Agilent 1260 binary pump HPLC device, and a Kinetex 50 × 2.1-mm, 2.6-μm particle-size biphenyl column (Phenomenex, Torrance, CA) on an Agilent 1290 binary pump, using gradient elution with 0.2 mmol/L ammonium fluoride and methanol (18). The column effluent was directed into the source of an Agilent triple quadrupole tandem mass spectrometer (6495 for Δ4 steroids or 6490 for Δ5 and sulfated steroids) using electrospray ionization (18).
Steroids were analyzed by multiple reaction monitoring mode, and quantitation was performed as previously described (19). Intra-assay and interassay coefficients of variation were assessed by measuring quality control pooled serum samples five times within a run and across five different runs, respectively, and they were <12% for all steroids. The lower limit of detection for each steroid was defined by the minimum concentration achieving an extrapolated signal-to-noise ratio of 3, and it ranged from 0.19 ng/dL for A4 to 12.79 ng/dL for AdiolS (18).
Double immunofluorescence in human adrenal glands
To understand the mechanisms underlying the changes in adrenal androgen production that occur with aging, we examined the expression of key enzymes involved in the synthesis of active androgens and precursors within the adrenal cortex across ages. Adrenal glands were obtained from 10 deceased renal transplant donors (via the “Gift of Life” program) and from 6 patients who underwent en bloc nephrectomy and adrenalectomy for renal cancer who had not been exposed to glucocorticoids or chemotherapy before surgery (18). We performed double immunofluorescence of 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2) (anti-human rabbit polyclonal antibody, provided by Dr. C. R. Parker, University of Alabama at Birmingham) and cytochrome b5 (CYB5A) (anti-human mouse monoclonal antibody; Acris, San Diego, CA), or sulfotransferase 2A1 (SULT2A1) (anti-human mouse monoclonal antibody; R&D Systems Inc., Minneapolis, MN), respectively. Sections (5 µm) of the adrenal tissue from young (n = 8; median age, 32 years; range, 24 to 37 years) and old (n = 8; median age, 65 years; range, 60 to 79 years) women were incubated overnight with a mixture of primary antibodies at 4°C (1:1000 dilution for HSD3B2, 1:5000 dilution for CYB5A, and 1:2000 dilution for SULT2A1). The slides were subsequently incubated with a mixture of species-specific secondary fluorescent antibodies (Alexa Fluor 488–conjugated AffiniPure goat anti-mouse and Alexa Fluor 647–onjugated AffiniPure goat anti-rabbit, both diluted 1:100; Jackson ImmunoResearch, West Grove, PA) for 1 hour. Prolong Gold mounting medium with DAPI (Life Technologies, Eugene, OR) was used to visualize the cell nuclei. Immunofluorescence was viewed and scanned with an Olympus Motorized inverted system microscope (IX81; Olympus, Center Valley, PA). A color threshold tool was used for analyzing the areas expressing HSD3B2 (red) and either CYB5A or SULT2A1 (green).
Statistical analysis
GraphPad Prism7 (La Jolla, CA) and SAS 9.4 (SAS Institute Inc., Cary, NC) were used for statistical analysis. A nonparametric Mann-Whitney U test was used for two-group comparisons. Kruskal-Wallis test was used for multiple group comparison. Correlations between steroids and metabolic factors were assessed with the Spearman correlation test. Logistic regression for binary outcomes and multiple linear regression for continuous outcomes were used to assess associations between steroids and clinical parameters. Significance was accepted at an α level of 0.05.
Results
Demographic characteristics of participants
In total, 200 women were included in this study: 100 premenopausal (median age, 35 years; range, 20 to 40 years) and 100 postmenopausal (median age, 69 years; range, 60 to 89 years) (Table 1). Although body mass index was similar between the two groups, the prevalence rates of hypertension, hyperglycemia, and dyslipidemia were significantly higher in postmenopausal than in premenopausal women, as expected (P < 0.01 for all) (Table 1). Dual-energy x-ray absorptiometry was performed in 57 postmenopausal women, and osteopenia and osteoporosis were diagnosed in 23 (40%) and 10 women (18%), respectively.
Steroid profiles across age groups
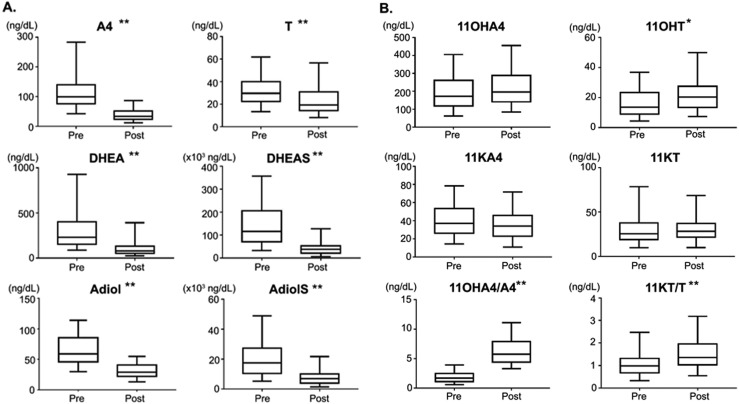
As previously observed (6, 7, 20–23), DHEA, DHEAS, A4, and T were significantly lower in post- vs premenopausal women (2.9, 2.8, 2.9, and 1.6-fold, respectively; P < 0.0001 for all) (Table 2). In contrast, the 11oxyandrogens were not lower in postmenopausal women (Table 1; Fig. 1). Notably, the relative contribution of 11oxyandrogens as compared with A4 and T was significantly higher in postmenopausal women than in their younger counterparts (P < 0.0001) (Table 2; Fig. 1).
Table 2.
Comparison of Circulating Steroid Concentrations Between Pre- and Postmenopausal Women
| Steroid | Premenopausal (n = 100) | Postmenopausal (n = 100) | Fold-Change Before/After | P Value |
|---|---|---|---|---|
| Steroid concentrations (ng/dL) | ||||
| Prog | 88 (21–1017) | 3.5 (2.8–5.0) | 25 | <0.0001 |
| 17OHProg | 81 (43–141) | 23 (15–34) | 3.5 | <0.0001 |
| Corticosterone | 253 (160–375) | 284 (150–538) | 0.9 | 0.2 |
| 11DOC | 5.0 (3.2–8.8) | 3.3 (2.0–5.3) | 1.5 | <0.0001 |
| 11dF | 26 (15–37) | 39 (20–61) | 0.7 | 0.0003 |
| Cortisol | 15,251 (11,322–21,432) | 16,991 (11,583–23,232) | 0.9 | 0.1 |
| Cortisone | 2623 (1897–3611) | 2147 (1631–2898) | 1.2 | 0.01 |
| DHEA | 232 (155–403) | 79 (52–134) | 2.9 | <0.0001 |
| Adiol | 59 (46–85) | 29 (22–41) | 2.0 | <0.0001 |
| PregS | 3040 (2007–4541) | 1088 (755–2266) | 2.8 | <0.0001 |
| 17OHPregS | 693 (423–951) | 329 (184–503) | 2.1 | <0.0001 |
| DHEAS | 116,268 (71,112–206,477) | 38,747 (22,258–53,873) | 3.0 | <0.0001 |
| AdiolS | 13,182 (7721–21,598) | 5091 (3014–7422) | 2.6 | <0.0001 |
| A4 | 99 (76–140) | 34 (23–51) | 2.9 | <0.0001 |
| T | 30 (22–40) | 19 (14–31) | 1.6 | <0.0001 |
| 11OHA4 | 172 (118–261) | 196 (141–289) | 0.9 | 0.06 |
| 11KA4 | 37 (26–55) | 34 (23–46) | 1.1 | 0.05 |
| 11OHT | 14 (9–23) | 20 (13–28) | 0.7 | 0.0002 |
| 11KT | 26 (19–38) | 28 (22–37) | 0.9 | 0.3 |
| Steroid ratios | ||||
| 11OHA4/A4 | 1.7 (1.1–2.5) | 5.8 (4.4–7.9) | <0.0001 | |
| 11KA4/A4 | 0.4 (0.2–0.6) | 0.9 (0.6–1.4) | <0.0001 | |
| 11OHT/T | 0.5 (0.3–0.7) | 0.9 (0.7–1.4) | <0.0001 | |
| 11KT/T | 1.0 (0.7–1.3) | 1.4 (1.0–2.0) | <0.0001 |
Data are expressed as median (interquartile range).
Abbreviations: 11DOC, 11-deoxycorticosterone; 11dF, 11-deoxycortisol; 17OHProg, 17α-hydroxyprogesterone; Prog, progesterone.
Figure 1.
Concentrations of traditional (A) and 11-oxygenated (B) C19 steroids in pre- and postmenopausal women. Whereas A4, T, DHEA, DHEAS, Adiol, and AdiolS all decline after menopause, 11OHA4, 11OHT, 11KA4, and 11KT are relatively similar between pre- and postmenopausal women. *P < 0.001; **P < 0.0001. The box contains the 25th to 75th percentiles, the whiskers mark the 5th and 95th percentiles, and the horizontal line within the box represents the median.
A negative correlation with age was observed for all sulfated steroids (r ranged from −0.44 for 17OHPregS to −0.7 for DHEAS) and for DHEA (r = −0.59), Adiol (r = −0.78), A4 (r= −0.72), and T (r = −0.39; P < 0.0001 for all). In contrast, 11OHT showed a modest positive correlation with age (r = 0.2; P = 0.004), and strong positive correlations were observed between age and ratios of 11oxyandrogens to A4 and T (r ranged from 0.35 for 11KT/T to 0.81 for 11OHA4/A4; P < 0.0001 for all). We did not find any meaningful associations between steroids and body mass index, hypertension, dyslipidemia, hyperglycemia, or bone density.
Double immunofluorescence
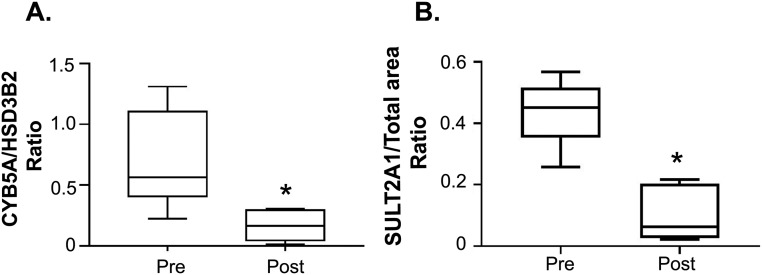
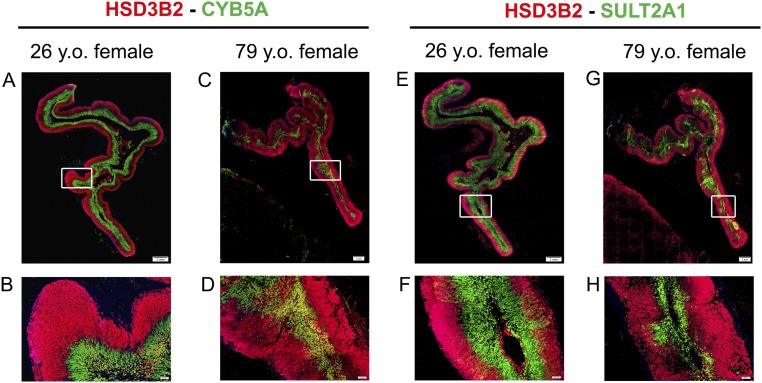
We performed double immunofluorescence of HSD3B2 with either CYB5A (both needed for the synthesis of Δ4 C19 steroids) or SULT2A1 in adrenal glands obtained from eight reproductive-age women (24 to 37 years old) and eight postmenopausal women (60 to 79 years old). Along with ZR involution, the old adrenal glands displayed smaller areas of both CYB5A and SULT2A1 expression compared with the young adrenals (P < 0.01) (Fig. 2). In the adrenal glands of reproductive-age women, HSD3B2 and CYB5A were sharply segregated to the zona fasciculata (ZF) and ZR, respectively (Fig. 3). SULT2A1 expression was present in the ZR and the inner ZF in both reproductive-age and postmenopausal women. In contrast, four of eight adrenals obtained from postmenopausal women (three of four women > age 70 years and one of four women in her 60s) revealed patchy areas composed of both HSD3B2 and CYB5A-expressing cells (Fig. 3).
Figure 2.
(A, B) Relative expression of androgenic enzymes (HSD3B2 and SULT2A1) and cofactor (CYB5A) within the adrenal cortex from eight reproductive-age (24 to 37 y) and eight postmenopausal (60 to 79 y) women, quantified by double immunofluorescence. Both the CYB5A/HSD3B2 and SULT2A1/total cortical area ratios decrease in postmenopausal women as a result of ZR involution with aging. Total cortical area was measured from the outer edge of HSD3B2 expression to the inner edge of SULT2A1 expression. The box contains the 25th to 75th percentiles, the whiskers mark the 5th and 95th percentiles, and the horizontal line within the box represents the median. *P < 0.01. Pre, premenopausal; Post, postmenopausal.
Figure 3.
Representative images for double immunofluorescence of HSD3B2 (red) and CYB5A (green) (A–D) or SULT2A1 (green) (E–H) in the adrenals of a pre- and a postmenopausal woman. In the young adrenal gland, HSD3B2 and CYB5A are segregated to the ZF and ZR, respectively (A, B), whereas the old adrenal demonstrates a thinner ZR and areas with intermingled expression of HSD3B2 and CYB5A (C, D). SULT2A1 is expressed in the ZR and lower ZF of both young and old adrenals (F, H), but the total SULT2A1 area is smaller in the old adrenal because of a thinner ZR.
Discussion
Consistent with previous data from both sexes (23), we found that the major adrenal androgen precursors—DHEA and DHEAS—decline in postmenopausal women. Similarly, A4 and T, steroids with dual gonadal and adrenal origin, are also lower in post- vs premenopausal women (6, 7, 20–22). In contrast, our major findings are that the adrenal-derived 11oxyandrogens do not decline with aging and that these steroids are more abundant than T and A4 in postmenopausal women.
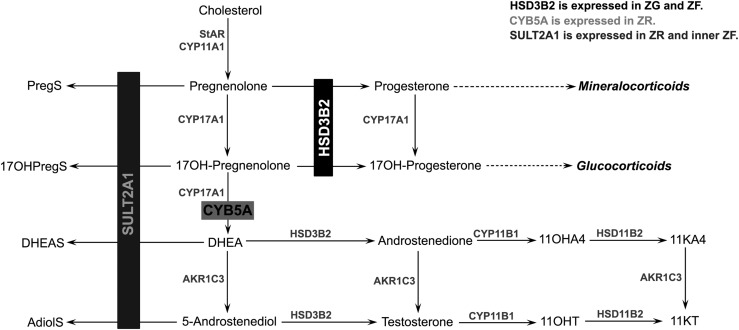
As previously recognized, we observed an involution of the ZR with aging (8, 9), which explains the age-related decline of both DHEAS and AdiolS. In addition, we found that PregS and 17OHPregS also decreased in older women, which suggests that these steroid sulfates are produced predominantly in the ZR and is consistent with the acute rise of PregS and 17OHPregS in response to cosyntropin (24). In parallel, we identified other structural transformations in the adrenal cortex of aging women that promote the production of active androgens. Normally, HSD3B2 and CYB5A, both necessary for the Δ4 androgen synthesis, are segregated to different adrenal zones, ZF and ZR, respectively (Figs. 3 and 4). As a result, the adrenal cortex produces abundant amounts of DHEA and DHEAS but much smaller amounts of active androgens. We found that this distinct zonal separation of HSD3B2 and CYB5A observed in the young adrenal glands appears to mingle in older women (Fig. 3). Such areas of close proximity of HSD3B2 and CYB5A found in the adrenal glands of postmenopausal women promote the conversion of nascent DHEA to A4, from which downstream 11oxyandrogens derive. In contrast, studies of ovarian tissue showed that LH/human chorionic gonadotropin receptors, aromatase (CYP19A1), and HSD3B2 expressions are greatly diminished after menopause, which reduces the ovarian production of androgens and estrogens (25).
Figure 4.
Adrenal C19 steroid pathway. The synthesis of C19 steroids requires both HSD3B2 and CYB5A), whereas the synthesis of sulfated steroids occurs via SULT2A1, which competes with HSD3B2 for the same substrates. Segregation of HDS3B2 and CYB5A to separate zones (ZF and ZR, respectively), along with abundant SULT2A1 expression, as observed in young adrenals, favors the synthesis of DHEAS. Conversely, lower SULT2A1 expression and proximity of HSD3B2- and CYB5A-expressing cells, as observed in old adrenals, favor the synthesis of active androgens. CYP11A1, cytochrome P450 cholesterol side-chain cleavage; CYP11B1, 11β-hydroxylase; CYP17A1, 17α-hydroxylase/17,20-lyase; HSD11B2, 11β-hydroxysteroid dehydrogenase, type 2; StAR, steroidogenic acute regulatory protein.
Menopause is associated with an array of highly variable clinical manifestations, including bone loss, increased cardiometabolic risk, sexual dysfunction, sleep disturbances, and skin and cognitive changes (26–28). In the face of abrupt estrogen loss, residual androgen synthesis is thought to mitigate some of these changes (29–32), yet the most relevant androgens have not been identified. For example, serum total, free, and bioavailable T measurements correlate weakly with sexual function in middle-aged women (33–35). The preservation of 11oxyandrogens as the dominant androgens in postmenopausal women suggest that these steroids might significantly contribute to menopausal physiology. Although we found no significant correlations of 11oxyandrogens with body mass index, hypertension, dyslipidemia, hyperglycemia, or bone density in postmenopausal women, this cross-sectional study with limited clinical information was not designed to test these associations. In a study of women with polycystic ovary syndrome (PCOS), O’Reilly et al. (17) found weak correlations of A4, 11OHA4, and 11KA4 with body mass index; however, these steroids did not differ between women with and without obesity and PCOS. In a subsequent study, the same group showed that the adipose tissue of women with PCOS expresses higher levels of the androgen-activating enzyme aldo-keto reductase type 1C3 than controls and that T and the T/A4 ratio are higher in adipose tissue than in serum from women with PCOS but not from controls (36).
This study comprehensively analyzed the serum androgen profiles of pre- and postmenopausal women, including the adrenal-derived 11oxyandrogens. We found that in stark contrast to DHEA, DHEAS, A4, and T, 11oxyandrogens do not decline in aging women. Structural changes within the adrenal cortex, including a regression of the ZR and disruption of the sharp separation of HSD3B2 and CYB5A, support the changes in serum androgens observed with aging in women. These findings, however, require confirmation in larger sets of normal adrenal glands, from carefully selected sources. The main limitation of our study resides in its cross-sectional design, which precludes causal inferences and longitudinal assessment. The relatively small number of patients and incomplete clinical data limit the analysis of associations between 11oxyandrogens and clinical outcomes. To what extent the preservation of 11oxyandrogens synthesis after menopause can compensate for the decline in T and androgen precursors remains to be determined in longitudinal studies of well-characterized cohorts, with careful assessment of sex steroid–related clinical outcomes.
Acknowledgments
We thank Dr. Scott Soleimanpour for use of the Olympus IX81 immunofluorescence microscope, Patrick O’Day for assistance with mass spectrometry assays, and Dr. Tom Giordano and Michelle Vinco for assistance with pathology blocks procurement.
Financial Support: This research was conducted while A.T.N. was a Glenn Foundation for Medical Research postdoctoral fellow (award N024780-00) and with support of grants from the National Institute of Diabetes and Digestive and Kidney Diseases (1K08DK109116), Claude D. Pepper Older Americans Independence Center (AG-024824), and the Michigan Institute for Clinical and Health Research (UL1TR000433), awarded to A.F.T.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 11KA4
11-ketoandrostenedione
- 11KT
11-ketotestosterone
- 11OHA4
11β-hydroxyandrostenedione
- 11OHT
11β-hydroxytestosterone
- 11oxyandrogens
11-oxygenated C19 steroids
- 17OHPregS
17-hydroxypregnenolone sulfate
- A4
androstenedione
- Adiol
androstenediol
- AdiolS
androstenediol-3 sulfate
- CYB5A
cytochrome b5
- DHEA
dehydroepiandrosterone
- DHEAS
dehydroepiandrosterone sulfate
- diH2O
deionized water
- HSD3B2
3β-hydroxysteroid dehydrogenase type 2
- MTBE
methyl-tert-butyl ether
- PCOS
polycystic ovary syndrome
- PregS
pregnenolone sulfate
- SULT2A1
sulfotransferase 2A1
- T
testosterone
- ZF
zona fasciculata
- ZR
zona reticularis
References
- 1. Mesiano S, Jaffe RB. Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev. 1997;18(3):378–403. [DOI] [PubMed] [Google Scholar]
- 2. Rege J, Turcu AF, Kasa-Vubu JZ, Lerario AM, Auchus GC, Auchus RJ, Smith JM, White PC, Rainey WE. 11-Ketotestosterone is the dominant circulating bioactive androgen during normal and premature adrenarche. J Clin Endocrinol Metab. 2018;103(12):4589–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Havelock JC, Auchus RJ, Rainey WE. The rise in adrenal androgen biosynthesis: adrenarche. Semin Reprod Med. 2004;22(4):337–347. [DOI] [PubMed] [Google Scholar]
- 4. Kirschner MA, Bardin CW. Androgen production and metabolism in normal and virilized women. Metabolism. 1972;21(7):667–688. [DOI] [PubMed] [Google Scholar]
- 5. Abraham GE. Ovarian and adrenal contribution to peripheral androgens during the menstrual cycle. J Clin Endocrinol Metab. 1974;39(2):340–346. [DOI] [PubMed] [Google Scholar]
- 6. Eisenhofer G, Peitzsch M, Kaden D, Langton K, Pamporaki C, Masjkur J, Tsatsaronis G, Mangelis A, Williams TA, Reincke M, Lenders JWM, Bornstein SR. Reference intervals for plasma concentrations of adrenal steroids measured by LC-MS/MS: Impact of gender, age, oral contraceptives, body mass index and blood pressure status. Clin Chim Acta. 2017;470:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elmlinger MW, Kühnel W, Wormstall H, Döller PC. Reference intervals for testosterone, androstenedione and SHBG levels in healthy females and males from birth until old age. Clin Lab. 2005;51(11-12):625–632. [PubMed] [Google Scholar]
- 8. Parker CR., Jr Dehydroepiandrosterone and dehydroepiandrosterone sulfate production in the human adrenal during development and aging. Steroids. 1999;64(9):640–647. [DOI] [PubMed] [Google Scholar]
- 9. Parker CR Jr, Slayden SM, Azziz R, Crabbe SL, Hines GA, Boots LR, Bae S. Effects of aging on adrenal function in the human: responsiveness and sensitivity of adrenal androgens and cortisol to adrenocorticotropin in premenopausal and postmenopausal women. J Clin Endocrinol Metab. 2000;85(1):48–54. [DOI] [PubMed] [Google Scholar]
- 10. Kroboth PD, Salek FS, Pittenger AL, Fabian TJ, Frye RF. DHEA and DHEA-S: a review. J Clin Pharmacol. 1999;39(4):327–348. [DOI] [PubMed] [Google Scholar]
- 11. Storbeck KH, Bloem LM, Africander D, Schloms L, Swart P, Swart AC. 11β-Hydroxydihydrotestosterone and 11-ketodihydrotestosterone, novel C19 steroids with androgenic activity: a putative role in castration resistant prostate cancer? Mol Cell Endocrinol. 2013;377(1-2):135–146. [DOI] [PubMed] [Google Scholar]
- 12. Rege J, Nakamura Y, Satoh F, Morimoto R, Kennedy MR, Layman LC, Honma S, Sasano H, Rainey WE. Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. J Clin Endocrinol Metab. 2013;98(3):1182–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pretorius E, Africander DJ, Vlok M, Perkins MS, Quanson J, Storbeck KH. 11-Ketotestosterone and 11-ketodihydrotestosterone in castration resistant prostate cancer: potent androgens which can no longer be ignored. PLoS One. 2016;11(7):e0159867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Swart AC, Schloms L, Storbeck KH, Bloem LM, Toit T, Quanson JL, Rainey WE, Swart P. 11β-hydroxyandrostenedione, the product of androstenedione metabolism in the adrenal, is metabolized in LNCaP cells by 5α-reductase yielding 11β-hydroxy-5α-androstanedione. J Steroid Biochem Mol Biol. 2013;138:132–142. [DOI] [PubMed] [Google Scholar]
- 15. Turcu AF, Nanba AT, Chomic R, Upadhyay SK, Giordano TJ, Shields JJ, Merke DP, Rainey WE, Auchus RJ. Adrenal-derived 11-oxygenated 19-carbon steroids are the dominant androgens in classic 21-hydroxylase deficiency. Eur J Endocrinol. 2016;174(5):601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Campana C, Rege J, Turcu AF, Pezzi V, Gomez-Sanchez CE, Robins DM, Rainey WE. Development of a novel cell based androgen screening model. J Steroid Biochem Mol Biol. 2016;156:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O’Reilly MW, Kempegowda P, Jenkinson C, Taylor AE, Quanson JL, Storbeck KH, Arlt W. 11-Oxygenated C19 steroids are the predominant androgens in polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(3):840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nanba AT, Rege J, Ren J, Auchus RJ, Rainey WE, Turcu AF. 11-Oxygenated C19 steroids do not decline with age in women. Zenodo.org. Deposited 7 January 2019 10.5281/zenodo.2532830. [DOI] [PMC free article] [PubMed]
- 19. Turcu AF, Rege J, Chomic R, Liu J, Nishimoto HK, Else T, Moraitis AG, Palapattu GS, Rainey WE, Auchus RJ. Profiles of 21-carbon steroids in 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2015;100(6):2283–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rothman MS, Carlson NE, Xu M, Wang C, Swerdloff R, Lee P, Goh VH, Ridgway EC, Wierman ME. Reexamination of testosterone, dihydrotestosterone, estradiol and estrone levels across the menstrual cycle and in postmenopausal women measured by liquid chromatography-tandem mass spectrometry. Steroids. 2011;76(1-2):177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90(7):3847–3853. [DOI] [PubMed] [Google Scholar]
- 22. Haring R, Hannemann A, John U, Radke D, Nauck M, Wallaschofski H, Owen L, Adaway J, Keevil BG, Brabant G. Age-specific reference ranges for serum testosterone and androstenedione concentrations in women measured by liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 2012;97(2):408–415. [DOI] [PubMed] [Google Scholar]
- 23. Burger HG, Dudley EC, Cui J, Dennerstein L, Hopper JL. A prospective longitudinal study of serum testosterone, dehydroepiandrosterone sulfate, and sex hormone-binding globulin levels through the menopause transition. J Clin Endocrinol Metab. 2000;85(8):2832–2838. [DOI] [PubMed] [Google Scholar]
- 24. Rege J, Nanba AT, Auchus RJ, Ren J, Peng HM, Rainey WE, Turcu AF. Adrenocorticotropin acutely regulates pregnenolone sulfate production by the human adrenal in vivo and in vitro. J Clin Endocrinol Metab. 2018;103(1):320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Havelock JC, Rainey WE, Bradshaw KD, Carr BR. The post-menopausal ovary displays a unique pattern of steroidogenic enzyme expression. Hum Reprod. 2006;21(1):309–317. [DOI] [PubMed] [Google Scholar]
- 26. El Khoudary SR, Shields KJ, Janssen I, Budoff MJ, Everson-Rose SA, Powell LH, Matthews KA. Postmenopausal women with greater paracardial fat have more coronary artery calcification than premenopausal women: the Study of Women’s Health Across the Nation (SWAN) cardiovascular fat ancillary study. J Am Heart Assoc. 2017;6(2):e004545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Waetjen LE, Crawford SL, Chang PY, Reed BD, Hess R, Avis NE, Harlow SD, Greendale GA, Dugan SA, Gold EB; Study of Womenʼs Health Across the Nation (SWAN) . Factors associated with developing vaginal dryness symptoms in women transitioning through menopause: a longitudinal study. Menopause. 2018;25(10):1094–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kravitz HM, Janssen I, Bromberger JT, Matthews KA, Hall MH, Ruppert K, Joffe H. Sleep trajectories before and after the final menstrual period in the Study of Women’s Health Across the Nation (SWAN). Curr Sleep Med Rep. 2017;3(3):235–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Benn M, Voss SS, Holmegard HN, Jensen GB, Tybjærg-Hansen A, Nordestgaard BG. Extreme concentrations of endogenous sex hormones, ischemic heart disease, and death in women. Arterioscler Thromb Vasc Biol. 2015;35(2):471–477. [DOI] [PubMed] [Google Scholar]
- 30. Jaspers L, Dhana K, Muka T, Meun C, Kiefte-de Jong JC, Hofman A, Laven JS, Franco OH, Kavousi M. Sex steroids, sex hormone-binding globulin and cardiovascular health in men and postmenopausal women: the Rotterdam Study. J Clin Endocrinol Metab. 2016;101(7):2844–2852. [DOI] [PubMed] [Google Scholar]
- 31. Montalcini T, Gorgone G, Gazzaruso C, Sesti G, Perticone F, Pujia A. Endogenous testosterone and endothelial function in postmenopausal women. Coron Artery Dis. 2007;18(1):9–13. [DOI] [PubMed] [Google Scholar]
- 32. van Geel TA, Geusens PP, Winkens B, Sels JP, Dinant GJ. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle mass, muscle strength and bone mineral density in postmenopausal women: a cross-sectional study. Eur J Endocrinol. 2009;160(4):681–687. [DOI] [PubMed] [Google Scholar]
- 33. Davis SR, Davison SL, Donath S, Bell RJ. Circulating androgen levels and self-reported sexual function in women. JAMA. 2005;294(1):91–96. [DOI] [PubMed] [Google Scholar]
- 34. Santoro N, Torrens J, Crawford S, Allsworth JE, Finkelstein JS, Gold EB, Korenman S, Lasley WL, Luborsky JL, McConnell D, Sowers MF, Weiss G. Correlates of circulating androgens in mid-life women: the study of women’s health across the nation. J Clin Endocrinol Metab. 2005;90(8):4836–4845. [DOI] [PubMed] [Google Scholar]
- 35. Wierman ME, Arlt W, Basson R, Davis SR, Miller KK, Murad MH, Rosner W, Santoro N. Androgen therapy in women: a reappraisal: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(10):3489–3510. [DOI] [PubMed] [Google Scholar]
- 36. O’Reilly MW, Kempegowda P, Walsh M, Taylor AE, Manolopoulos KN, Allwood JW, Semple RK, Hebenstreit D, Dunn WB, Tomlinson JW, Arlt W. AKR1C3-mediated adipose androgen generation drives lipotoxicity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(9):3327–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]