Abstract
The first synthetic carbohydrate based potential anti-Salmonella Enteritidis vaccine has been developed by conjugating a synthetic tetrasaccharide antigen with bacteriophage Qβ. High levels of specific and long lasting anti-glycan IgG antibodies were induced by the conjugate, which completely protected mice from lethal bacterial challenge in a passive transfer model.
Graphical Abstract
The conjugate of synthetic Salmonella Enteritidis tetrasaccharide with bacteriophage Qβ induced powerful anti-glycan IgG responses for complete protection from lethal challenges of bacteria.
Salmonella, a genus of Gram negative facultative anaerobe enteric pathogens, causes a range of human diseases from self-limiting gastroenteritis to enteric fevers.1 Salmonella Enteritidis (S. Enteritidis) is one of the most common strains of non-typhoidal Salmonella (NTS) worldwide capable of lethal invasive and systemic infections.2 The Centers for Disease Control and Prevention (CDC) Foodborne Diseases Active Surveillance Network have shown that salmonellosis accounts for estimated 1.2 million illnesses in USA, and 54% of hospitalizations and 43% of deaths reported due to food poisoning.3 While the incidence of food borne diseases such as Shiga toxin-producing E. coli infections has decreased by more than 50% over the past two decades, little progress has been made in controlling Salmonella with a 10% increase in human salmonellosis cases during the same period despite multiple concerted interventions to reduce transmission through the food chain.4
Conventionally, invasive Salmonella infections are treated with antimicrobial agents. However, the overuse of antibiotics in medicine as well as in livestock rearing has led to the emergence of multidrug resistant strains, leading the CDC to designate Salmonella as a pathogen of serious concern in their report on antimicrobial threats in the USA.5 Despite the frequency and severity of NTS disease, no licensed human NTS vaccines are available yet. There is an urgent need to develop an NTS vaccine that could complement other control and prevention strategies.
Bacterial cell surface polysaccharides can be potential antigenic targets due to their abundance on the cell surface.6 The surface polysaccharide in most NTS serovars is the O polysaccharide (OPS) of lipopolysaccharide, for which structural differences are used to define Salmonella serogroups. The OPS of S. Enteritidis, a serogroup D Salmonella serovar, is characterized by a main repeating tetrasaccharide of α-D-mannose (Man)-(1→4)-α-L-rhamnose (Rha)-(1→3)-α-D-galactose (Gal)-with the 3-O position of Man decorated with a rare sugar, tyvelose (3,6-dideoxy-D-arabinohexose Tyv).7 Some of the Gal units can also be functionalized with a glucose at the 6-O position. To date, carbohydrate based anti-S. Enteritidis vaccine studies have utilized polysaccharides from the corresponding bacterial serovars,8 which require a series of purification to remove endotoxins and other host impurities. Furthermore, isolated polysaccharides are inhomogeneous, rendering it difficult to pinpoint the epitope structures needed for protection.
Herein, through a concise [2+2] glycosylation strategy, we have prepared structurally well-defined tyvelose containing tetrasaccharide 1, which represents one repeat unit of Salmonella serogroup D OPS (Figure 1). The synthetic tetrasaccharide 1 was conjugated to a powerful carrier, bacteriophage Qβ with over 300 copies of glycan immobilized per Qβ particle. The Qβ-glycan 1 conjugate was found to elicit potent IgG antibody responses in both mice and rabbits. Excitingly, passive transfer of antisera from rabbits immunized with Qβ-glycan 1 provided complete protection against fatal S. Enteritidis challenge in mice.
Figures 1.
Schematic demonstration of synthetic vaccine strategy.
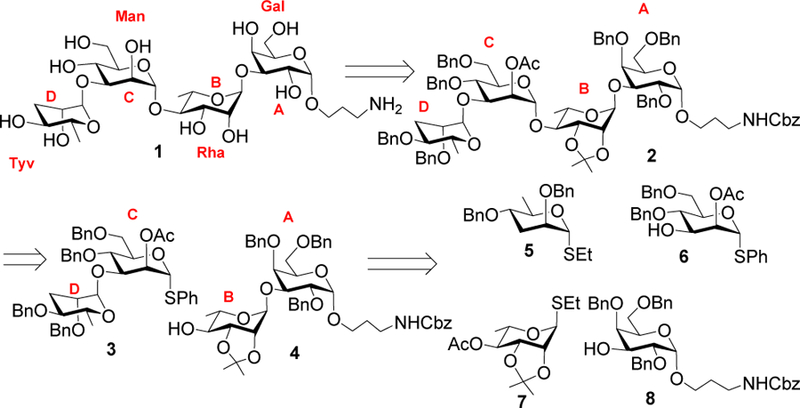
We envisioned that protected tetrasaccharide derivative 2 would be a suitable precursor to tetrasaccharide 1 (Scheme 1). Benzyl (Bn) and acetonide groups were primarily used as protective groups in 2 as these electron donating groups can enhance building block reactivities in glycosylations.9 The 2-O position of the mannosyl unit of 2 is protected as an O-acetate, which can be deprotected selectively for future chain elongation to synthesize longer oligosaccharides. Tetrasaccharide 2 could be prepared from a disaccharide thioglycoside donor 3 and a disaccharide acceptor 4 using a [2+2] glycosylation strategy, which in turn could be obtained from monosaccharide building blocks 5-8.
Scheme 1.
Retrosynthetic analysis of tetrasaccharide 1.
The synthesis of the rare tyvelose donor 5 started from ethyl 1-thio-β-D-mannopyranoside 910 by regioselective tert-butyldimethylsilyl (TBS) protection of the C-3 and C-6 hydroxyl groups11 to afford compound 10 in a 65% yield (Scheme 2a). Benzylation of compound 10 followed by removal of the TBS groups furnished compound 11 in 85% yield over two steps. Barton-McCombie deoxygenation12, 13 was applied to compound 11 by first converting the two free hydroxyl groups of 11 to xanthate esters 12 with subsequent radical mediated reduction14 to furnish tyvelose donor 5. The mannosyl building block 6 was prepared from 4,6-di-O-benzyl-1-thio-α-D-mannopyranoside 13,15 which was selectively acetylated in its 2-OH in 90% yield (Scheme 2b). Stereoselective 1,2-cis glycosylation of thioglycoside derivative 1416 with 3-N-benzyloxycarbonyl (Cbz)-amino-1-propanol 15 promoted by N-iodosuccinimide (NIS) and triflic acid (TfOH) followed by in situ removal of the p-methoxybenzyl (PMB) group afforded compound 8 in 65% yield together with the β-anomer in minor quantity (~10%), which was removed by column chromatography (Scheme 2c). The α-configuration of the newly formed glycosidic linkage in 8 was confirmed by the coupling constant (J1,2) between the H-1 and H-2 of the glycoside product [δ 4.71 (d, J = 3.0 Hz, 1 H, H-1)].
Scheme 2.
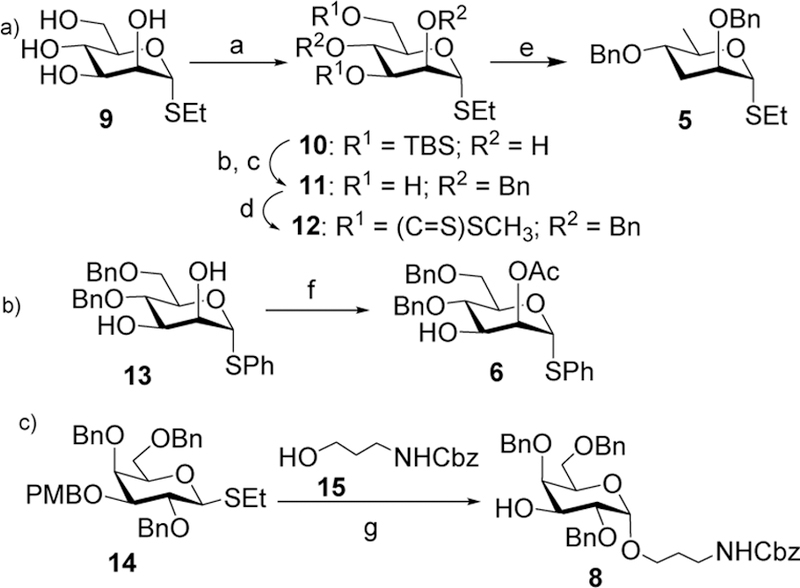
Reagents and conditions: (a) TBSCl, imidazole, DMF, room temperature, 12 h, 65%; (b) NaH, BnBr, DMF, room temperature, 1 h, 94%; (c) cat. p-TSA, CH3OH, room temperature, 91%; (d) NaH, imidazole, CS2, THF then CH3I, room temperature, 87%; (e) tri-n-butyl tin hydride, AIBN, toluene, reflux, 3 h, 45 %; (f) Triethylortho acetate, p-TSA, DMF, room temperature, 90%; (g) NIS, TfOH, CH2Cl2:Et2O (1:3), −20 oC, 0.5 h, 65%.
With all building blocks in hand, chemoselective glycosylation between 5 and 6 was performed with NIS and trimethylsilyl trifluoromethanesulfonate (TMSOTf) as the promoter (Scheme 3a).17 As deoxy sugars typically have higher anomeric reactivities than the fully oxygenated counterparts,9 selective activation of tyvelose 5 over the bifunctional mannoside 6 was achieved, furnishing αt-yvelosylated CD disaccharide 3 in 85% yield together with the minor β-anomer (~5%), which was separated by column chromatography. The formation of the α-glycosidic linkage in compound 3 was confirmed by the coupling constant values of 13C-1 and H-1 [δ 99.3 (JC1/H1 = 167.8 Hz, C-1D), 85.8 (JC1/H1 = 167.8 Hz, C-1C)].18 In order to prepare the AB disaccharide, stereoselective coupling of the L-rhamnosyl thioglycoside donor 719 with acceptor 8 produced disaccharide 15 in 82% yield, which was de-acetylated affording disaccharide acceptor 4 in quantitative yield (Scheme 3b).
Scheme 3.
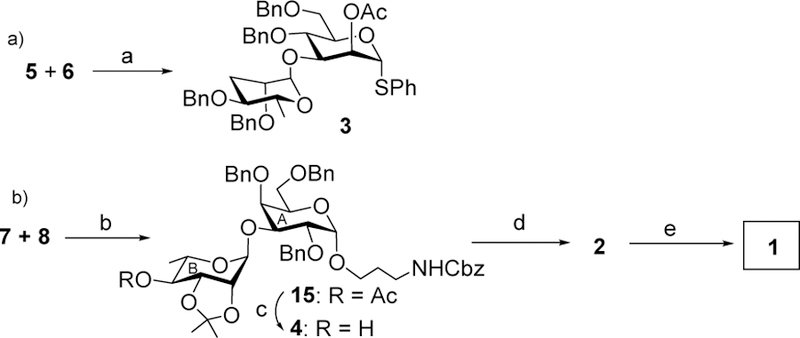
Reagents and conditions: (a) NIS, TMSOTf, CH2Cl2, −20 oC, 45 min, 85%; (b) NIS, TMSOTf, CH2Cl2, −30 oC, 25 min, 82%; (c) NaOCH3, CH3OH, room temperature, 1 h, 95%; (d) NIS, TMSOTf, 3, CH2Cl2, −15 °C, 40 min, 78%; (e) (i) NaOCH3, CH3OH, room temperature, 2 h (ii) 80% AcOH, 80 °C, 2 h; (iii) 20% Pd(OH)2-C, H2, CH3OH, room temperature, 16 h, 57% for three steps.
The union of AB and CD disaccharides was performed by stereoselective glycosylation of acceptor 4 with disaccharide donor 3 using NIS and TMSOTf as the promoter, generating α-anomer 2 exclusively in 78% yield (Scheme 3b). Deprotection of tetrasaccharide 2 was carried out by deacetylation, followed by acid hydrolysis and hydrogenolysis to produce tetrasaccharide 1 in 57% overall yield. The structure of compound 1 was confirmed by NMR analysis [δ 5.04 (br s, 1 H, H-1C), 5.00 (br s, 1 H, H-1A), 4.99 (br s, 1 H, H-1B), 4.88 (br s, 1 H, H-1D) in 1H NMR and δ 101.9 (JC1/H1 = 171.1 Hz, C-1C), 101.3 (JC1/H1 = 169.9 Hz, C-1B), 101.2 (JC1/H1 = 170.0 Hz, C-1D),
98.4 (JC1/H1 = 170.4 Hz, C-1A) in the 13C NMR spectrum].
Bioconjugation of 1 was investigated next for immunization studies. Carbohydrates are generally type 2 thymus independent antigens that do not engage helper T cells and thus do not robustly cause antibody class switch, affinity maturation and generation of immune memory. Conjugation to a protein carrier can engage T-cell help to polysaccharide-specific B-cells. We thus explored the use of the virus like particle bacteriophage Qβ as a carrier for the S. Enteritidis glycan antigen. Due to its ability to present haptens in an organized manner with high density, bacteriophage Qβ has been previously shown to impart effective carrier function for vaccines against cancer and inflammation.20, 21 Head to head comparisons between Qβ and a gold standard protein carrier, keyhole limpet hemocyanin (KLH) in glycopeptide based anti-cancer vaccine studies found that Qβ is superior to KLH for induction of high IgG antibodyiters and protection from tumor development.22 However, Qβ has not been explored to date as a carrier for carbohydrate-based anti-bacterium vaccines. Tetrasaccharide 1 derivatized with a bifunctional linker 16, was incubated with bacteriophage Qβ23,24 (Scheme 4). , Mass spectrometry analysis of the resulting Qβ-glycan 1 conjugate indicated that there were on average 334 copies of glycans per capsid (Figure S1). Glycan 1 was also conjugated with bovine serum albumin (BSA) for use as a capture antigen in enzyme linked immunosorbent assays (ELISA) to assess anti-glycan antibody responses (Figure S2).
Scheme 4.
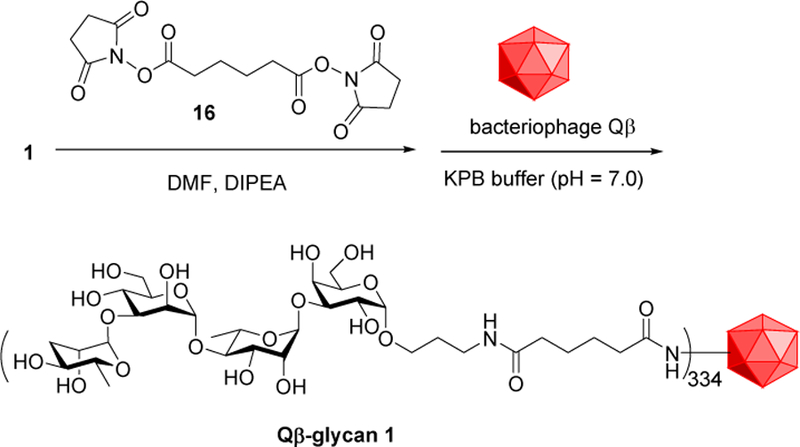
Conjugation of S. Enteritidis glycan 1 with Qβ.
To determine whether Qβ-glycan 1 conjugate could induce anti-glycan antibodies, groups of mice were administered subcutaneously with Qβ-glycan 1 (1 μg and 4 μg of carbohydrate per injection respectively) on days 0, 14 and 28. As controls, groups of mice received Qβ or glycan 1 alone following the same immunization protocol. Sera were collected on day 35 post immunization. ELISA analysis using BSA-glycan 1 as the coating antigen showed that high levels of anti-glycan 1 IgG antibodies were induced, with an average IgG titer of 487,000 and 980,000 ELISA units for groups receiving 1 μg and 4 μg of glycan respectively (Figure 2a). By comparison, controls immunized with Qβ or glycan 1 alone gave anti-glycan IgG titers below 2,000 ELISA units suggesting that linking glycan 1 with Qβ was critical for high anti-glycan antibody responses. Analysis of the subtypes of antibody generated by Qβ-glycan 1 showed that IgG2b, IgG2c and IgG3 were the major subtypes of antibodies while IgM antibody levels were insignificant (Figure S3).
Figure 2. Robust and long-lasting anti-glycan 1 antibody responseswere induced by Qβ-glycan 1 in mice.
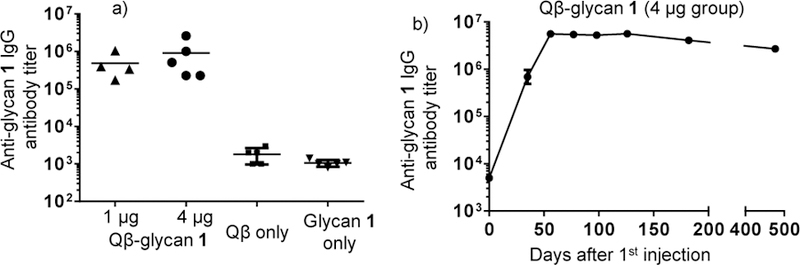
a) Anti-glycan 1 IgG titers on day 35 after first immunization from Qβ-glycan 1 (1 and 4 μg of glycan)immunized and control groups. Each symbol represents one mouse. Geometric mean titers (GMT) are indicated by solid bars. b) Average IgG titers induced by Qβ-glycan 1 (4 μg) monitored over 477 days.The persistence trend from 1 μg group was similar.
Induction of long-lasting immune responses is an important attribute of a successful vaccine. The longevity of antibody responses elicited by Qβ-glycan 1 were thus monitored over time (4 μg group data shown in Figure 2b). The levels of anti-glycan 1 IgG antibodies reached a plateau (6,000,000 ELISA units) 56 days after the first immunization, which was maintained for 120 days. At day 477, half of the peak IgG level still remained. These results suggest that durable anti-glycan 1 IgG responses were induced by Qβ-glycan 1.
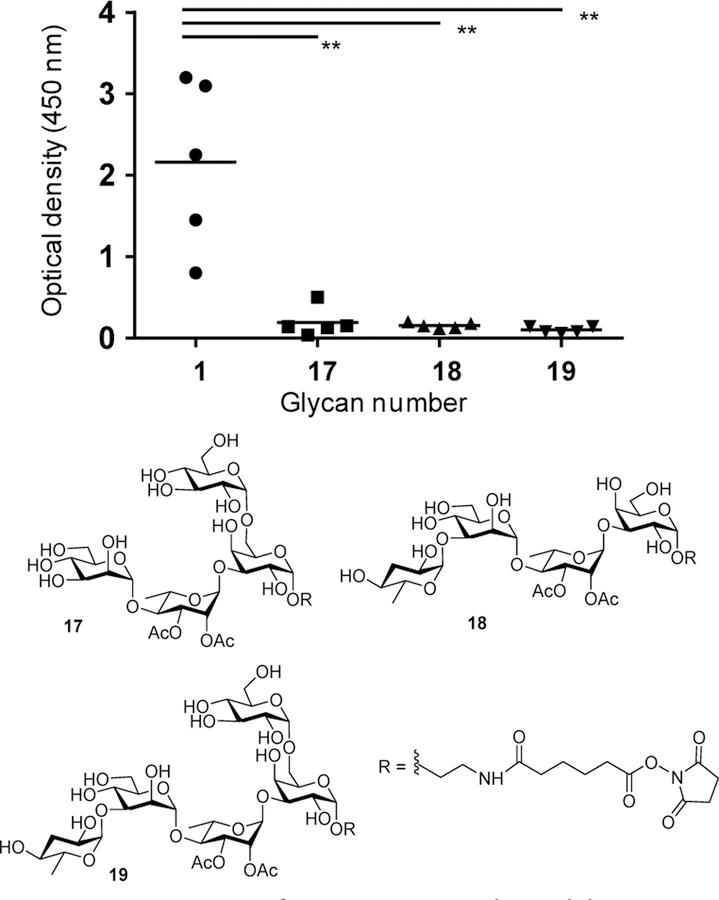
To establish the selectivity and specificity of antibody induced by Qβ-glycan 1, glucoside containing tetrasaccharide backbone 17, as well as Salmonella Paratyphi A OPS tetrasaccharide 18 and pentasaccharide 19 were synthesized25 and conjugated with BSA for use as ELISA antigens. Analyses with these BSA glycan conjugates demonstrated that post-immune sera maximally bound the BSA-glycan 1 (Figure 3), suggesting that the non-reducing end tyvelose may be the immunodominant epitope.
Figure 3. IgG antibodies in sera from mice immunized with Qβ-glycan 1 were highly selective towards glycan 1 when assessed against various synthetic Salmonella glycans by ELISA.
Symbols represent individual mouse, and solid bars indicate the group GMT. Differences between groups were assessed by Mann-Whitney rank-sum test, **p < 0.01. (Serum dilution at 51,200 fold).
In addition to mice, rabbits are commonly used as an animal model for immune response evaluation. Accordingly, rabbits were immunized subcutaneously with Qβ-glycan 1, and sera were collected prior to immunization and on days 35, 49 and 56 following the first immunization. ELISA analyses with BSA-glycan 1 found that robust IgG responses were induced in rabbits, with IgG titers reaching 83,579,000 and 150,175,000 ELISA units by day 56 (Table S1), which were more than 6,000-fold higher than those obtained with sera from Qβ immunized control rabbits.
We next wanted to confirm that antibodies induced could bind to native polysaccharides from the bacteria. For this, the homologous serotype native core-O-polysaccharide (COPS) was isolated from S. Enteritidis and immobilized in ELISA wells. Anti-sera from rabbits and mice were assessed. Three anti-Salmonella LPS monoclonal antibodies (mAbs) were added as negative (anti-S. Paratyphi OPS mAb 6347) and positive controls (mAbs 6391 and 6393 specific for the conserved core). As expected, mAbs 6391 and 6393 bound the COPS whereas mAb 6347 did not (Figure S4). Sera from immunized rabbits robustly recognized the purified S. Enteritidis COPS. However, unexpectedly, mouse sera did not bind to the native COPS antigen strongly. These disparities may possibly reflect differences in the paratope repertoire between these two species, or molecular differences in the binding pockets of rabbit compared to mouse antibodies.
As strong recognition of OPS was mediated by IgG antibodies induced by Qβ-glycan 1 in rabbits, we next assessed whether these antibodies could bind to the cognate antigen on intact bacteria. Rabbit sera were analyzed by flow cytometry for binding to S. Enteritidis R11, an invasive strain isolated from the blood of a patient in Mali.26 Whereas negligible binding was found for either serum taken pre-immunization or from rabbits immunized with the Qβ carrier alone, IgG antibodies in sera from Qβ-glycan 1 immunized rabbits potently bound to R11 (Figure 4), thus confirming that glycan 1-induced antibodies in rabbits can bind cell associated OPS, a key step for enabling anti-bacterial activity.
Figure 4. Qβ-glycan 1 induced rabbit antibodies bound S. Enteritidis bacteria.
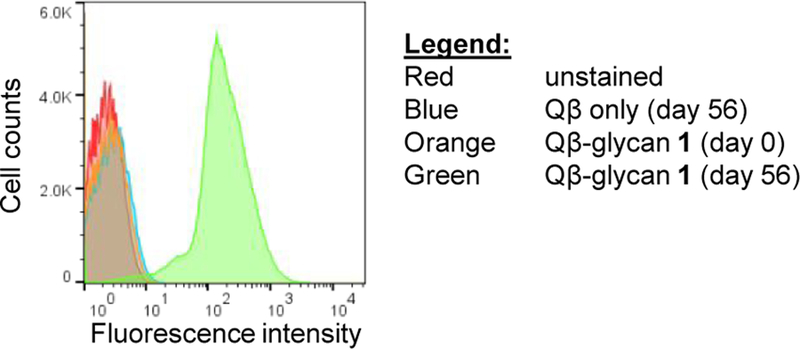
Flow cytometry analysis measuring binding to clinical S. Enteritidis strain R11 by anti-rabbit IgG secondary antibody alone (red), pre-immune rabbit sera (orange), Qβ induced rabbit sera (blue), or Qβ-glycan 1 post-immunization sera (green) (1:10,000 dilution).
Opsonophagocytic uptake (OPA) of antibody-bound bacteria into phagocytes with killing by oxidative burst is an important functional mechanism for antibody-mediated clearance of NTS.27 We assessed uptake of R11 cells into J774 mouse macrophages after incubation with pre-immune, Qβ-induced, or anti-Qβ-glycan 1 sera from rabbits. While the pre-immune and Qβ immunized rabbit sera did not cause significant bacterial uptake relative to media alone, antisera from Qβ-glycan 1 immunized rabbits markedly enhanced macrophage opsonisation of R11 bacteria (Figure S5).
The protective efficacy of Qβ-glycan 1 anti-sera against fatal R11 infection was evaluated next in mice as there were no suitable rabbit models for Salmonella infections. Mice were passively administered PBS (n=7), different dilutions of pooled pre-immune (n=12/group) or Qβ-glycan 1 vaccine-induced anti-sera (n=12/group) and then challenged intraperitoneally with an LD100 dose of R11. Whereas all mice in the PBS group succumbed to infection by day 7, and all but one out of the two groups of mice receiving pre-immune sera died by day 8, excitingly, 100% of the mice receiving post-immune sera (1:100 or 1:500 dilution, 24 total) survived the bacterial challenge (Figure 5).
Figure 5. Transfer of Qβ-glycan 1 induced rabbit sera to mice protected against fatal S. Enteritidis challenge.
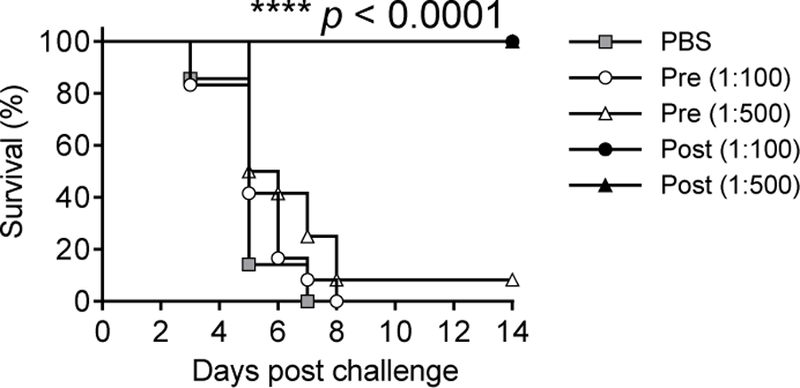
Mice were administered intraperitoneally PBS alone (n=7/group), pre-or post-immune sera (n=12/group) from Qβ-glycan 1 immunized rabbits (diluted as 1:100 or 1:500 in PBS) respectively, followed by intraperitoneal challenge with a lethal dose of S. Enteritidis R11 (n = 12 for each group). Statistical significance was determined by log-rank test. P values (****p<0.0001) were found between survival rates of groups receiving post-immune sera and those of groups receiving pre-immune sera or PBS.
In summary, we report the development of the first synthetic oligosaccharide based anti-S. Enteritidis vaccine. An efficient 2+2 glycosylation strategy was established for the synthesis of the tetrasaccharide repeating unit of the OPS of S. Enteritidis, bearing a rare tyvelose monosaccharide moiety at the non-reducing end. By linking to a bacteriophage Qβ virus like particle, we generated a construct that powerfully elicited IgG antibodies specific for S. Enteritidis OPS glycans. This suggests that a single tetrasaccharide repeat is sufficient to produce anti-OPS antibodies. Post-immune sera from rabbits recognized cell-associated LPS on an invasive S. Enteritidis clinical isolate and enhanced opsonophagocytic uptake into macrophages. Furthermore, the sera provided 100% protection against challenge by a lethal dose of S. Enteritidis bacteria in the mouse model of invasive NTS diseases. These results demonstrate that Qβ can be a powerful carrier for carbohydrate based anti-microbial vaccines.
Other NTS vaccines, based on mucosally administered live attenuated strains and antigens, have been tested in pre-clinical and clinical studies.6,8 One potential drawback of using live strains for vaccination is the concern of infection especially in infants and toddlers, the group at highest risk for invasive Salmonella diseases. Using the synthetic antigen as reported herein can complement vaccine strategies using antigens isolated directly from nature. Furthermore, synthetic antigen can help shine light on the identity of the protective epitope, enriching the knowledge on vaccine design against Salmonella serovars of human importance.
Supplementary Material
Acknowledgements
We thank the National Institutes of Health (Grant R01 CA225105 and R03 AI111054) for financial support.
Footnotes
Conflicts of interest The authors declare no conflicts of interest.
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/x0xx00000x
References
- 1.Graham SM, Curr. Opin. Infect Dis, 2002, 15, 507–512. [DOI] [PubMed] [Google Scholar]
- 2.Feasey NA, Dougan G, Kingsley RA, Heyderman RS and Gordon MA, Lancet, 2012, 379, 2489–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foodborne Diseases Active Surveillance Network (FoodNet), https://www.cdc.gov/foodnet/reports/prelim-data-intro-2017.html. [DOI] [PMC free article] [PubMed]
- 4.Adams DA, Thomas KR, Jajosky RA, Foster L, Baroi G, Sharp P, Onweh DH, Schley AW and Anderson WJ, Summary of notifiable diseases — United States, 2015, 2017. [DOI] [PubMed]
- 5. https://www.cdc.gov/drugresistance/biggest_threats.html.
- 6.Meiring JE, Patel P, Patel P and Gordon MA, Exp. Rev. Vaccines, 2018, 17, 673–651. [DOI] [PubMed] [Google Scholar]
- 7.Snyder DS, Gibson D, Heiss C, Kay W and Azadi P, Carbohydr. Res, 2006, 341, 2388–2397. [DOI] [PubMed] [Google Scholar]
- 8.Simon R, Tennant SM, Wang JY, Schmidlein PJ, Lees A, Ernst RK, Pasetti MF, Galen JE and Levine MM, Infect. Immun, 2011, 79, 4240–4249 and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z, Ollmann IR, Ye X-S, Wischnat R, Baasov T and Wong C-H, J. Am. Chem. Soc, 1999, 121, 734–753. [Google Scholar]
- 10.Fried J and Watz DE, J. Am. Chem. Soc, 1949, 71, 140–143. [DOI] [PubMed] [Google Scholar]
- 11.Du Y, Zhang MM and Kong FZ, Org. Lett, 2000, 2, 3797–3800. [DOI] [PubMed] [Google Scholar]
- 12.Barton DHR and McCombie SW, J. Chem. Soc., Perkin Trans. 1, 1975, 1574–1585.
- 13.Robins MJ, Wilson JS and Hansske F, J. Am. Chem. Soc, 1983, 105, 4059–4065. [Google Scholar]
- 14.Clive DLJ, Chittattu G and Wong CK, J. Chem. Soc., Chem. Commun, 1978, 41–42.
- 15.Iino K, Iwamoto S, Kasahara Y, Matsuda K, Tonozuka T, Nishikawa A, Ito Y and Matsuo I, Tetrahedron Lett, 2012, 53, 4452–4456. [Google Scholar]
- 16.Dhara D, Mandal PK and Misra AK, Tetrahedron: Asymm, 2014, 25, 263–267. [Google Scholar]
- 17.Veeneman GH, van Leeuwen SH and van Boom JH, Tetrahedron Lett, 1990, 31, 1331–1334. [Google Scholar]
- 18.Bock K and Pedersen C, J. Chem. Soc. Perkin Trans. 2, 1974, 293–297.
- 19.Lowary TL, Eichler E and Bundle DR, J. Org. Chem, 1995, 60, 7316–7327. [Google Scholar]
- 20.Yin Z, Wu X, Kaczanowska K, Sungsuwan S, Comellas-Aragones M, Pett C, Yu J, Baniel C, Westerlind U, Finn MG and Huang X, ACS Chem. Biol, 2018, 13, 1668–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohsen MO, Zha L, Cabral-Miranda G and Bachmann MF, Semin. Immunol, 2017, 34, 123–132. [DOI] [PubMed] [Google Scholar]
- 22.Wu X, Yin Z, McKay C, Pett C, Yu J, Schorlemer M, Gohl T, Sungsuwan S, Ramadan S, Baniel C, Allmon A, Das R, Westerlind U, Finn MG and Huang X, J. Am. Chem. Soc, 2018, 140, 16596–16609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiedler JD, Brown SD, Lau J and Finn MG, Angew. Chem. Int. Ed, 2010, 49, 9648–9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin Z, Wright WS, McKay C, Baniel C, Kaczanowska K, Bentley P, Gildersleeve JC, Finn MG, BenMohamed L and Huang X, ACS Chem. Biol, 2015, 10, 2364–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manuscript in preparation.
- 26.Tennant SM, Wang JY, Galen JE, Simon R, Pasetti MF, Gat O and Levine MM, Infect. Immun, 2011, 79, 4175–4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramachandran G, Boyd MA, MacSwords J, Higginson EE, Simon R, Galen JE, Pasetti MF, Levine MM and Tennant SM, Clin. Vaccine Immunol, 2016, 23, 520–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.