Abstract
Background
The aim of this study was to investigate the role of deubiquitinase [ovarian tumor domain-containing protein 5 (OTUD5)] in regulating Akt ubiquitination and its effect on the radiosensitivity of cervical cancer.
Material/Methods
Cervical cancer C33A cells were cultured, and then 2 groups of cells (overexpressed cells and silenced cells) were established by overexpressing and silencing OTUD5 gene. Next, quantitative polymerase chain reaction (qPCR) was employed to detect the expression level of OTUD5 in cells in each group. Co-immunoprecipitation and Western blot (WB) analysis were applied to measure the expression level of phosphorylated protein kinase B (Akt) and the level of ubiquitination. The sensitivity of cells to radiotherapy in each group was detected via clone-forming efficiency assay. After that, Statistical Product and Service Solutions (SPSS) 17.0 software was employed for analyses. The t test, one-way analysis of variance (ANOVA), and p test were used. P<0.05 suggested that a difference was statistically significant.
Results
The levels of phosphorylated Akt and ubiquitination in OTUD5-overexpressed C33A cells were lower than those in the OTUD5-silenced group and control group. The sensitivity of OTUD5-overexpressed C33A cells to radiotherapy was higher than that of the OTUD5-silenced group and control group.
Conclusions
OTUD5 affects the radiosensitivity of cervical cancer through the regulation of Akt deubiquitination.
MeSH Keywords: Proto-Oncogene Proteins c-akt, Ubiquitination, Uterine Cervical Neoplasms
Background
Cervical cancer is the third most common cancer in women, with about 500 000 patients newly diagnosed each year, accounting for 5% of all new cancer patients. The main cause of cervical cancer is the persistently infectious human papilloma virus (HPV) [1] and 99.7% of cervical cancer patients have HPV infection. The treatment of cervical cancer is mostly successful in developed countries, but in developing countries most women are already in the middle or advanced stages at the time of diagnosis. Therefore, cervical cancer is still the second most common cause of cancer-related death in women in developing countries [2].
Radiotherapy is one of the most important methods for treatment of cervical cancer, and radiotherapy is becoming increasingly important with the wider application of conformal radiotherapy, intensity-modulated radiation therapy, image-protection radiation therapy, and the use of protons and heavy ions in clinical practice [3]. However, not all patients with cervical cancer have good responses to radiotherapy in clinical practice [4], and some patients only have good responses at the beginning of treatment. The radiosensitivity of patients is affected by different genetic phenotypes and changes in related genes [5,6]. For instance, among cervical cancer patients undergoing radiotherapy, those with KRAS mutation have worse prognoses than those without KRAS mutation [7]. Previous studies have shown the heterogeneity in primary tumors, i.e., there are different somatic mutations in tumor cells of the same tumor, which accumulate in different tumor subpopulations. Moreover, the treatment of tumors cannot be realized via radiotherapy as long as there are somatic mutations leading to radiotherapy resistance in patients. Therefore, heterogeneity and subpopulation diversity in tumor cells may result in good responses of patients only at the beginning of treatment [8]. In recent years, more and more studies have shown that the intrinsic radiosensitivity of tumor cells is affected by various factors, including injury repair, cell cycle arrest, apoptosis, and signal transduction [9,10].
Many studies have verified that the phosphatidylinositol 3-kinase (PI3K)/Akt pathway is one of the key factors involved in cancers [11]. Akt is a key signaling molecule that can phosphorylate many downstream molecules and link them to multiple interconnected signaling pathways. The PI3K/Akt signaling pathway participates in various biological behaviors such as cell cycle regulation, protein synthesis, angiogenesis, and cell migration [12,13]. As is well known, Akt is overactivated in many types of malignancies and is related to poor prognoses in some tumors [14]. However, the relationship between Akt and radiosensitivity of cervical cancer has not been previously studied or reported.
Ubiquitination is a protein translational modification process. Lysine residues of highly-conserved ubiquitin matrix proteins lead to the final degradation of proteins via cascade reactions of enzymes, including the connection of E1, E2, and E3 enzymes. On the contrary, deubiquitinases (DUBs)-mediated deubiquitination is able to hydrolyze Ub molecules on ubiquitin proteins, thereby influencing the stability or activity of proteins [15]. Recently, it has been demonstrated that DUBs are promising targets for cancer treatment, but it is difficult for their functions to be exerted in tumor cells. Cell lines were stably transfected with lentiviruses to construct cells with different expression levels of ovarian tumor domain-containing protein 5 (OTUD5), so as to investigate whether OTUD5 affects the radiosensitivity of cervical cancer cells by regulating the ubiquitination of Akt.
Material and Methods
Culture of cells
This study was entirely performed in China. The cervical cancer cell line C33A was purchased from the School of Life Sciences, Fudan University [medium: Roswell Park Memorial Institute (RPMI) 1640 medium (Corning, Corning, NY, USA), to which was added 10% fetal bovine serum (FBS, GIBCO, Thermo Fisher Scientific, Waltham, MA, USA), 1% benzylpenicillin sodium, and 1% streptomycin sulfate before use]. Cells were cultured in a 5% CO2 cell incubator at 37°C, cell growth was observed periodically, and follow-up experiments were carried out.
Human embryonic kidney 293T (HEK 293T) cells were human embryonic kidney cell lines purchased from the Cell Bank of Shanghai Institutes for Biological Sciences of Chinese Academy of Sciences (Shanghai, China), which were adherent-dependent epithelioid cells, with Dulbecco’s modified Eagle’s medium (DMEM) (containing 10% FBS) as the growth medium (Thermo Fisher Scientific, Waltham, MA, USA). Adherent cells were cultured into monolayer cells through growth and proliferation.
Transfection with lentiviruses
Overexpression lentivirus LV-OTUD5 and interference lentivirus LV-small interfering RNA (siRNA)-OTUD5 of OTUD5 gene (Human, GenBank serial number: NC_000023) were synthesized by Shanghai Genechem Co. (Shanghai, China), which also provided the empty vector control and negative control [they had been subjected to sequence comparison and verification using the Basic Local Alignment Search Tool (BLAST)].
Cells were plated in a 6-well plate the night before transfection. The density of bladder cancer cell line was 3×105 cells/well. On the next day, the overexpression lentivirus, interference lentivirus, and empty vector control were transferred into C33A cells using Lip2000 transfection kits (Invitrogen) when the degree of cell fusion reached 80%.
Quantitative real-time polymerase chain reaction (RT-PCR)
Total ribonucleic acid (RNA) was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Complementary deoxyribonucleic acid (cDNA) was synthesized using the TaKaRa PrimeScript™ Kit (Bio, Inc., Otsu, Japan). The gene sequences of target gene and internal reference β-actin were obtained from GenBank. Primers were designed using the primer design software Primer-Blast on the National Center for Biotechnology Information (NCBI) website. The sequences of the primers were synthesized by Sangon Biotech Co. (Shanghai, China). The reaction system was 20 μL. The reaction conditions were pre-denaturation at 95°C for 2 min and then 40 cycles of 95°C for 15 s and then at 60°C for 60 s. The calculation of relative messenger RNA (mRNA) expression was: 2−ΔΔCt [Δ cycle threshold (Ct)=Ct (target gene)−Ct (β-actin)]. The fold change among different treatments was calculated as 2−ΔΔCT, where ΔΔCT=experimental group ΔCt−control group ΔCt. The specific primer sequences are shown in Table 1.
Table 1.
Primer sequences of OTUD5 and β-actin mRNA for RT-PCR.
| Gene | Primer sequence |
|---|---|
| OTUD5 | Forward: 5′-TCCACAAGAGCCAAGGCAT-3′ |
| Reverse: 5′-GTGGCATAGAAGTGCAGCA-3′ | |
| β-actin | Forward: 5′-TGGTGAAGACGCCAGTGG A-3′ |
| Reverse: 5′-TTGTTACAGGAAGTCCCTTGCC-3′ |
Co-immunoprecipitation
Proteins were collected at 48 h after cell transfection. The cell culture dish was placed on ice and washed with pre-cooled phosphate-buffered saline (PBS) 3 times. Then, an appropriate amount of cell lysis buffer (containing protease inhibitors) was added, followed by lysis on ice for 10 min. Next, a cell scraper was utilized to gently scrape off cells, and cell lysis solution was transferred to a clean Eppendorf (Ep) tube using a pipette and gently pipetted on the ice to avoid air bubbles. Thereafter, centrifugation at 4°C and 13 000 rpm (the maximum speed) for 10 min was conducted, followed by collection of the supernatant. After that, a small amount of lysis buffer was transferred to a clean EP tube for Western blot (WB) analysis. To the remaining lysis buffer, we added 100 μL corresponding agarose beads containing Akt antibody (1: 200, #9272, Cell Signaling Technology, Beverly, MA, USA) and incubated the mixture overnight at 4°C with gentle shaking. After immunoprecipitation, agarose beads were sunk to the bottom of the tube through centrifugation at 3000 rpm and 4°C for 5 min. Then, the supernatant was carefully pipetted off, and the agarose beads at the bottom of the tube were rinsed with pre-cooled PBS 3 times. After that, 500 μL lysis buffer and 125 μL 5× sodium dodecyl sulfate (SDS) loading buffer were added and then boiled for 5 min to dissociate antigen, antibody, and beads. Lastly, the tube was centrifuged at 4°C for 10 min at the maximum speed, and the supernatant was taken for subsequent WB experiments.
WB
The concentration of total protein was detected using a bicinchoninic acid (BCA) protein assay kit (PIERCE). Samples were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) and transferred onto a polyvinylidene fluoride (PVDF) membrane (IPVH00010, Millipore, USA). Then, samples were incubated with primary antibody (Akt, 1: 1000, #9272; Phospho-Akt (Ser473), 1: 2000, #4060; OTUD, 1: 1000, #20087; β-Actin, 1: 1000, #4970 purchased from. Cell signaling technology, Beverly, MA, USA) at 4°C overnight and washed, followed by 1 h of incubation with horseradish peroxidase-conjugated secondary antibody (anti-rabbit IgG, 1: 2000, #7074 from Cell signaling technology, Beverly, MA, USA). Thereafter, enhanced chemiluminescence (ECL) mixture was added, and fluorescence imaging technique was applied to obtain images.
Clone-forming efficiency assay
Cells in the logarithmic growth phase were taken and subjected to trypsin digestion, so as to prepare into single cell suspensions. Then, a fully-automatic cell counter was used to measure the concentration of cells. Single-cell suspensions were diluted to the desired concentration and then inoculated in a 6-well plate at different densities based on different irradiation doses, with 3 mL complete medium in each well. The 6-well plate was gently shaken by making a cross, so that cells were evenly distributed. A parallel sample was set for each dose. Inoculated cells were put in the incubator at 37°C overnight to allow cells to adhere completely. Irradiation was performed using a Siemens linear accelerator. Irradiated cells were placed in the incubator for 14 d of culture, during which the movement of the 6-well plate was avoided as much as possible so as to facilitate clone formation. After that, the medium was discarded, cells were washed 3 times, and anhydrous ethanol solution containing crystal violet was added for fixation, then cells were stained and rinsed with slowly running water and the excess crystal violet solution was absorbed. Then, the plate was dried naturally at room temperature, and clones exceeding 106 cells were counted under a microscope. Clone-forming efficiency and cell survival score were calculated according to formulas. The experiment was repeated 3 times.
Statistical analyses
SPSS 17.0 software was employed for analyses. We performed the t test, ANOVA, and p test. P<0.05 suggested that a difference was statistically significant.
Results
Viral transfection experiments and validation
C33A cells transfected with overexpression virus LV-OTUD5 had green fluorescent protein (GFP) (Figure 1), of which the infection rate was over 90%. C33A cells transfected with downregulated virus LV-siRNA-OTUD5 and empty vector LV-siRNA-Control also showed GFP (Figure 2). Three knockout sequences – RNA interference 1 (RNAi1), RNAi2, and RNAi3 sequences – were constructed, and qPCR detection was conducted after transfection of C33A cells. In the comparison of mRNA expression level of OTUD5 among groups (Figure 3) we found that the mRNA expression level of OTUD5 in the RNAi1 group was the lowest, and the decrease rate of OTUD5 in RNAi1 sequence was the highest (74.67%). Therefore, RNAi3 was selected as the most effective target. After transfection, stable cell lines were constructed for subsequent experiments.
Figure 1.
Cellular morphology and fluorescence of C33A cells infected with the overexpression virus of OTUD5.
Figure 2.
Cellular morphology and fluorescence of C33A cells infected with the interference virus.
Figure 3.
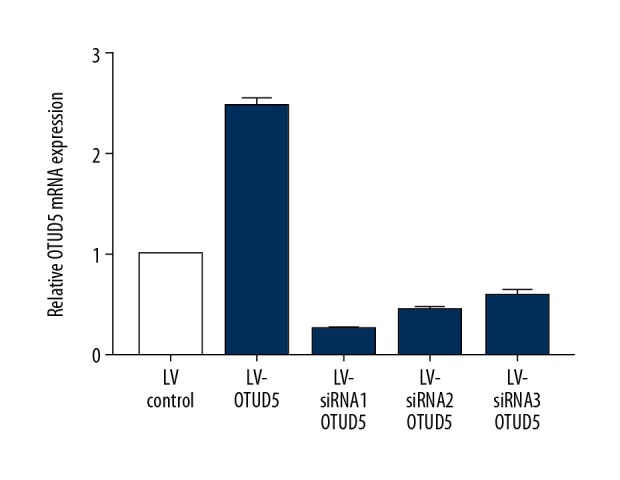
MRNA expression level of OTUD5 in each group detected via Qpcr.
Effects of OTUD5 expression on Akt phosphorylation and ubiquitination in interfered cell lines
Anti-Akt antibody was used to enrich protein complexes containing Akt through immunoprecipitation. Anti-Ub antibody was used to detect Ub molecules binding to Akt protein, reflecting the ubiquitination level of Akt. The results suggested that the levels of phosphorylated Akt and ubiquitination in OTUD5-overexpressed C33A cells were decreased compared with those in the OTUD5-silenced group and control group (Figure 4).
Figure 4.

Akt phosphorylation and ubiquitination levels measured by co-immunoprecipitation.
Clone-forming efficiency assay
Clone-forming efficiency assay was carried out to calculate the survival ratio of cells at different irradiation doses. The survival curves of cells were fitted using the one-hit multitarget model in GraphPad prism5 software (Figure 5), and the values of radiosensitive parameters, including survival fraction at 2 Gy (SF2), D0 (Gy), dose quasi-threshold (Dq, Gy), and N, were obtained (Table 2). The results indicated that the average survival ratio of cells in OTUD5-overexpressed group at each dose was lower than in the silenced group and control group, showing statistically significant differences. D0 is one of the indicators of the intrinsic radiosensitivity of tumor cells, which represents the average lethal dose for tumor cell survival. The value was 1.438 in the overexpressed group, which was lower than in the silenced group and control group (1.659 and 1.553, respectively, P<0.05).
Figure 5.

Influence of OTUD5 on radiosensitivity of cervical cancer cells. * p<0.05 compared to control. # p<0.05 compared to LV-OTUD5.
Table 2.
Effects of OTUD5 on parameters of radiosensitivity of cervical cancer cells.
| Group | SF2 | D0 (Gy) | Dq (Gy) | N |
|---|---|---|---|---|
| C33A/LV-OTUD5 | 0.391±0.061 | 1.438 | 0.976 | 1.688 |
| C33A/LV-siRNA-OTUD5 | 0.564±0.071 | 1.659 | 2.397 | 2.516 |
| C33A/LV-Control | 0.491±0.061 | 1.553 | 1.768 | 2.001 |
Discussion
Our study explored the role of OTUD5 by overexpression or knockdown strategies, showing that the increase in OTUD5 reduces the levels of phosphorylated Akt and ubiquitination in C33A cells. Furthermore, overexpression of OTUD5 elevated the sensitivity of C33A cells to radiotherapy. Akt, as a serine/threonine-specific protein kinase, is an important intracellular signal transducer of insulin and other growth factors, participating in the regulation of cell proliferation, metabolism, transcription, migration, and apoptosis [16]. As a result, it influences nearly all aspects of cancer biology. Furthermore, active Akt promotes the proliferation of cells, especially under stress, including genotoxic stress, so overactivated Akt in malignancies increases the resistance to chemotherapy and radiotherapy, thus affecting the prognosis of cancer treatment [17].
As an important post-translational modification, protein ubiquitination regulates various physiological functions. Ubiquitination leads to degradation of proteins in most cases, but certain types of ubiquitination are important pathways of signal activation and protein trafficking. The ubiquitin-specific protease 8 (USP8) removes ubiquitin from protein substrates, promotes tumor proliferation and invasion, and is correlated with poor overall survival [18]. Generally, ubiquitination via the ubiquitin chain of lysine 48 (K48) is involved in protein degradation, while ubiquitination via the K63 ubiquitin chain participates in important signal activation and protein trafficking pathways [19]. The ubiquitination of Akt has multiple effects on other aspects of the enzyme, and the activated Akt segment (Thr308) and hydrophobic group (Ser473) exert the functions of structure reorganization and enzyme promotion [20]. Ubiquitination regulates the stability of Akt protein. Previous studies have revealed that the degradation of ubiquitin-dependent Akt is regulated by mammalian target of rapamycin complex 2 (mTORC2)-mediated translationally coupled phosphorylation in a growth factor-independent manner, and the lack of Thr450 phosphorylation causes unstable Akt. Some researchers have proposed that after the K63 ubiquitination of the pleckstrin homology (PH) domain of Akt, a molecular platform is possibly formed, recruiting some effector molecules from the cytoplasm. Furthermore, with the collaboration of these effector molecules, Akt is localized on the cell membrane and activated by phosphorylation, suggesting that the regulation of Akt ubiquitination is capable of affecting the activation of Akt phosphorylation. We found that the expression level of phosphorylated Akt was decreased after the action of ubiquitinase, which is in agreement with the findings of previous studies.
Recently, 2 independent studies have found that cylindromatosis (CYLD), a tumor suppressor, is a deubiquitinase of Akt. Research indicated that CYLD is able to damage the activity of Akt by removing the K63-coupled ubiquitin chain on the Akt molecule upon the action of growth factors, including insulin-like growth factor 1 (IGF-1), interleukin-1 (IL-1), and epidermal growth factor (EGF), or stimulation of lung injury. Therefore, CYLD can suppress the occurrence and development of tumors and the formation of pulmonary fibrosis by lowering the activity of Akt [21]. Moreover, previous evidence indicated a relationship between ubiquitination in the regulation of Akt pathway and radiosensitivity. For instance, bortezomib enhances radiosensitivity in oral cancer through inhibition of IR-induced TRAF6 ubiquitination and TRAF6-mediated Akt activation [22]. These studies suggest that ubiquitination and deubiquitination play important roles in Akt cell membrane localization and phosphorylation activation. Recent findings show that the reduction of Glucose-Related Protein 78 (GRP78) increased chemo-sensitivity and has promise in the improvement of cisplatin-resistance in cervical cancer. Results of these previous studies and our present results offer a combination scaffold for chemoradiotherapy of cancer [23].
Conclusions
Our data demonstrate the influence of OTUD5 on the radiosensitivity of cervical cancer. This effect may be brought about by the regulation of Akt deubiquitination, which provides a new idea for further treatment of cervical cancer.
Footnotes
Source of support: The study was founded by Xuzhou Science and Technology Project (KC15SH023)
Conflict of interest
None.
References
- 1.Bosch FX, Lorincz A, Munoz N, et al. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–65. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Desantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. Cancer J Clin. 2014;64:252–71. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 4.Mccormack M, Kadalayil L, Hackshaw A, et al. A phase II study of weekly neoadjuvant chemotherapy followed by radical chemoradiation for locally advanced cervical cancer. Br J Cancer. 2013;108:2464–69. doi: 10.1038/bjc.2013.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabori U, Baskin B, Shago M, et al. Universal poor survival in children with medulloblastoma harboring somatic TP53 mutations. J Clin Oncol. 2010;28:1345–50. doi: 10.1200/JCO.2009.23.5952. [DOI] [PubMed] [Google Scholar]
- 6.Krakstad C, Chekenya M. Survival signalling and apoptosis resistance in glioblastomas: Opportunities for targeted therapeutics. Mol Cancer. 2010;9:135. doi: 10.1186/1476-4598-9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sreelekha TT, Nair MK, Jayaprakash PG, et al. Immunophenotype of mutant ras p21 and early response to radiotherapy in cancer of the uterine cervix. J Exp Clin Cancer Res. 1999;18:337–41. [PubMed] [Google Scholar]
- 8.Xia Q, Guo Y, Zhang Z, et al. Complete resequencing of 40 genomes reveals domestication events and genes in silkworm (Bombyx) Science. 2009;326:433–36. doi: 10.1126/science.1176620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeggo PA, Lobrich M. How cancer cells hijack DNA double-strand break repair pathways to gain genomic instability. Biochem J. 2015;471:1–11. doi: 10.1042/BJ20150582. [DOI] [PubMed] [Google Scholar]
- 10.Paull TT, Rogakou EP, Yamazaki V, et al. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10:886–95. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- 11.Robbins HL, Hague A. The PI3K/Akt pathway in tumors of endocrine tissues. Front Endocrinol (Lausanne) 2015;6:188. doi: 10.3389/fendo.2015.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saji M, Vasko V, Kada F, et al. Akt1 contains a functional leucine-rich nuclear export sequence. Biochem Biophys Res Commun. 2005;332:167–73. doi: 10.1016/j.bbrc.2005.04.109. [DOI] [PubMed] [Google Scholar]
- 13.Martelli AM, Tabellini G, Bressanin D, et al. The emerging multiple roles of nuclear Akt. Biochim Biophys Acta. 2012;1823:2168–78. doi: 10.1016/j.bbamcr.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Ocana A, Vera-Badillo F, Al-Mubarak M, et al. Activation of the PI3K/mTOR/AKT pathway and survival in solid tumors: Systematic review and meta-analysis. PLoS One. 2014;9:e95219. doi: 10.1371/journal.pone.0095219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suresh B, Lee J, Kim H, Ramakrishna S. Regulation of pluripotency and differentiation by deubiquitinating enzymes. Cell Death Differ. 2016;23:1257–64. doi: 10.1038/cdd.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martelli AM, Tabellini G, Bressanin D, et al. The emerging multiple roles of nuclear Akt. Biochim Biophys Acta. 2012;1823:2168–78. doi: 10.1016/j.bbamcr.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Pan S, Sun Y, Sui D, et al. Lobaplatin promotes radiosensitivity, induces apoptosis, attenuates cancer stemness and inhibits proliferation through PI3K/AKT pathway in esophageal squamous cell carcinoma. Biomed Pharmacother. 2018;102:567–74. doi: 10.1016/j.biopha.2018.03.109. [DOI] [PubMed] [Google Scholar]
- 18.Yan M, Zhao C, Wei N, et al. High expression of ubiquitin-specific protease 8 (USP8) is associated with poor prognosis in patients with cervical squamous cell carcinoma. Med Sci Monit. 2018;24:4934–43. doi: 10.12659/MSM.909235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–5. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- 20.Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11:9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- 21.Lim JH, Jono H, Komatsu K, et al. CYLD negatively regulates transforming growth factor-beta-signalling via deubiquitinating Akt. Nat Commun. 2012;3:771. doi: 10.1038/ncomms1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu YH, Wu WS, Lin LC, et al. Bortezomib enhances radiosensitivity in oral cancer through inducing autophagy-mediated TRAF6 oncoprotein degradation. J Exp Clin Cancer Res. 2018;37:91. doi: 10.1186/s13046-018-0760-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo C, Fan W, Jiang Y, et al. Glucose-related protein 78 expression and its effects on cisplatin-resistance in cervical cancer. Med Sci Monit. 2018;24:2197–209. doi: 10.12659/MSM.906413. [DOI] [PMC free article] [PubMed] [Google Scholar]




