Abstract
Background
This is the first published study assessing the parallelogram effect of degenerative structures around the apical vertebra. We evaluated the effect of degenerative structures around the apical vertebra and spinopelvic parameters on the severity of ADS.
Material/Methods
We retrospectively reviewed data on 144 patients with ADS. The coronal (coronal Cobb angle, CA) and sagittal (thoracic kyphosis, TK; sagittal vertical axis, SVA; pelvic incidence, PI; lumbar lordosis, LL; sacral slope, SS; pelvic tilt, PT) parameters, lumbar multifidus muscle atrophy (LMA), and facet joint osteoarthritis (FJOA) were evaluated. Multiple linear regression was used to assess the correlations.
Results
LL and PT were negatively correlated with CA (P<0.001), and the correlation between LL and SVA was positive (P<0.001), as was the correlation between PI and CA (P<0.001). The correlation between SS and SVA was negative (P<0.001). The correlation between CA and concave LMA at upper or lower intervertebral level of the apical vertebra was positive (P≤0.001). The convex LMA at upper and lower intervertebral levels was negatively correlated with CA (P<0.001). Convex LMA at the upper intervertebral level and concave LMA at the lower intervertebral level of the apical vertebra were negatively correlated with the SVA (P≤0.001). FJOA works similar to LMA (P<0.05).
Conclusions
Spinopelvic parameters are correlated with severity of ADS. The structures around the apical vertebra are very important to maintain global alignment of the spine via the parallelogram effect.
MeSH Keywords: Muscular Atrophy, Scoliosis, Statistics as Topic
Background
Adult degenerative lumbar scoliosis (ADS) can be diagnosed when a spinal deformity is identified with a coronal deviation of greater than 10º in a standing simple radiograph in a skeletally mature patient, especially in patients over 50 years of age, without a history of scoliosis [1]. The incidence of ADS is expected to increase as the patient’s age increases; it has been reported that ASD occurs in 1.4% to 32% of all adults, which makes it a major public health concern in the aging population [2].
Several studies reported correlations between low back pain or the degenerative flat-back, a deformity in the sagittal plane and the lumbar multifidus muscle atrophy (LMA) [1,3,4]. However, correlations between LMA, facet joint degeneration, spinopelvic parameters, and severity of ADS have not been investigated. Although Yagi et al. [5] analyzed the CSA of muscle in the L5–S1 level and stated that the cross-sectional area (CSA) of psoas (PS) and multifidus (MF) was significantly smaller in ADS, their methods could not reflect the characteristics of paravertebral muscles around the apex vertebra. Kalichman et al. [6] reported that there might be a correlation between facet tropism and facet degeneration. However, the association between facet degeneration and ADS was not fully discussed in their study. In addition, they used an axial CT (computed tomography)-based facet joint osteoarthritis (FJOA) grading system, which could not be used to evaluate the FJOA in the tilt motion segments in ADS, and their study has many limitations.
To discuss the correlation between LMA, FJOA in different intervertebral levels, spinopelvic parameters, and the severity of ADS, we simplified the approach to evaluating LMA and FJOA by using a magnetic resonance imaging (MRI)-based Goutallier classification system [7] and Pathria grading system [8]. These methods make the assessment of anatomical features on the tilted intervertebral plane much easier and more practical. Our study found the parallelogram effect of LMA by these methods, which could be used to predict progression in ADS, which has not been previously reported.
Material and Methods
Ethics statement
The Ethics Committee of the Xuanwu Hospital Capital Medical University approved this study (approval number [2018] 014). Written informed consent of each patient was obtained prior to the study.
Selection criteria
We reviewed patients with ADS that attended our inpatient clinic during the period from January 2016 to January 2018 for this study. Inclusion criteria included: patient’s age >50 years at the time of attendance; anteroposterior and lateral X-ray radiographs of total spine and magnetic resonance imaging (MRI) of the lumbar spine were available; and Cobb angle of lumbar deformity on the coronal plane was more than 10° on a standing posteroanterior film. Exclusion criteria included: history of scoliosis in the preceding time; history of severe spinal trauma, local infection, spinal inflammation, spinal tumor and spinal surgery; and presence of ankylosing spondylitis, Parkinson disease, and muscular dystrophy, which can predict other systemic diseases that can affect spinal alignment.
Radiographic measurement
Radiographic parameters were obtained via evaluating the standing whole-spine X-ray (Philips Digital Diagnost; Zhejiang Province, China) of the patients. The measurement methods were as follows: coronal Cobb angle (CA), the angle between inferior end plate of the caudal end vertebra and the superior end plate of the cephalad end vertebra on the coronal plane; sagittal vertical axis (SVA), the length on lateral radiographs from the C7 plumb line to a perpendicular line, which was drawn from the S1 superior end; thoracic kyphosis (TK), the angle between lower T12 end plate to T2 upper end plate; sacral slope (SS), the angle between the cranial sacral endplate and a horizontal line; lumbar lordosis (LL), the angle between T12 upper end plate and the S1 upper end plate; pelvic incidence (PI), the angle subtend by a perpendicular line from the S1 upper end plate and a line connecting the femoral head center to the S1 cephalad end plate center; and pelvic tilt (PT), the angle between the line through the midpoint of the sacral endplate to femoral heads axis and the vertical axis. Two researchers who were not involved in the patient encounters individually measured all parameters, and we used the averages of their measurement results.
MRIs evaluation
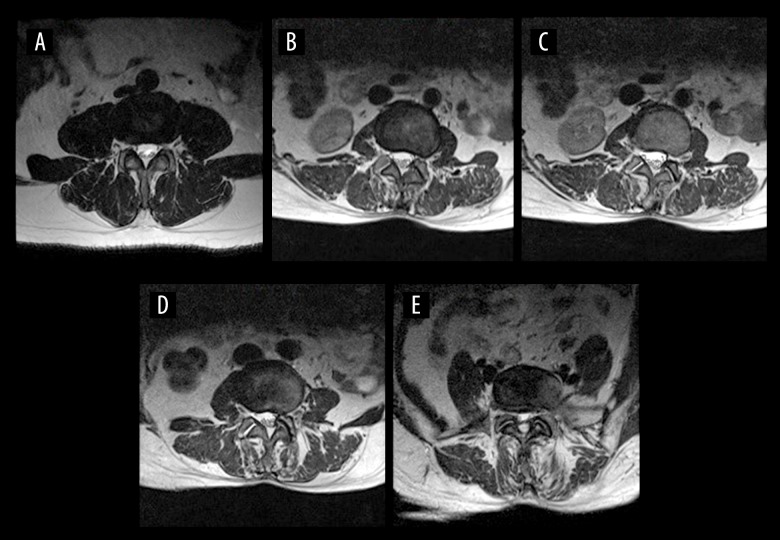
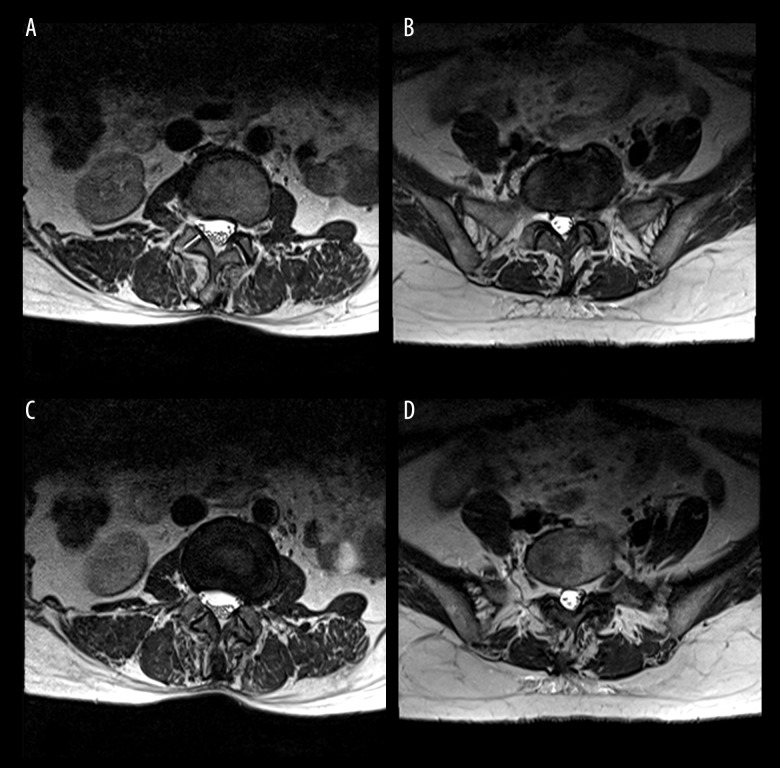
MRIs with 1.5-T Magnetom Symphony imaging systems (Siemens, Berlin, Germany) were used in this study. Three T2-weighted images (T2WI) in the axial position at intervertebral levels above and below the apical vertebras were obtained. The gap between slices was 0.1 mm. The thickness of slices was 4 mm. Multifidus muscles on the convex and concave side of the scoliotic curve were evaluated on the center slice of each of the 3 T2WIs. Muscle fatty degeneration in the lumbar multifidus muscle was quantified according to the Goutallier classification system [7] (Figure 1). Four grades of FJOA were defined using criteria similar to those reported by Pathria et al. [8] (Figure 2).
Figure 1.
Goutallier grade on axial T2W1 MRI showing: grade 0, muscle tissue is normal (A); grade 1, streaks of fat occur (B); grade 2, less fat than muscle (C); grade 3, amounts of muscle and fat tissue are equal (D); and grade 4, less muscle than fat (E).
Figure 2.
Pathria grade on axial T2W1 MRI showing: grade I, facet joint space is 2 mm or greater, no osteophytes or possible small osteophytes can be found (A); grade II, facet joint space is 1 mm to 2 mm, and/or definite small osteophytes can be found (B); grade III, facet joint space is less than 1 mm, and/or definite moderate osteophytes can be found (C); grade IV, facet joint space cannot be found (bone to bone), and/or large osteophytes can be found (D).
Statistical analysis
Statistical Package for the Social Sciences version 17.0 software (SPSS, Inc, Chicago, IL) was used for statistical analysis. Non-contiguous data were presented as numbers or ratios. In addition, continuous variables were reported as mean ± standard deviations (SD). Normality was evaluated by Shapiro-Wilk test. Wilcoxon rank sum test was used to evaluated the differences in LMA between the concave and convex sides at intervertebral levels above and below the apical vertebras, or between upper intervertebral levels and lower intervertebral levels of apical vertebras in concave or convex sides, as was the FJOA. Pearson’s correlation test was used to analyze the correlation between different parameters. The correlations between LMA, FJOA, spinopelvic parameters, and severity of scoliosis were analyzed by multiple linear regression. Kappa test was used to evaluate the agreement between researchers’ measurements. P value of statistical significance was less than 0.05.
Results
Patient demographics
There were144 ADS patients (52 males, 92 females); the mean age was 63.5±7.2 years (Table 1). Scoliotic curves typically had an apex at L3 (41.7%) and were convex to the left (58.3%). There was a wide range of severity of sagittal imbalance in these patients, with a mean value of 9.0±10.0 cm. However, coronal imbalance was relatively mild, with a mean value of 2.1±1.0.
Table 1.
Patient demographics.
| Number of cases | 144 |
|---|---|
| Gender (Male/Female) | 52/92 |
| Age (year) | 63.5±7.2 |
| Apex of ADS | |
| L1 | 12 (8.3%) |
| L2 | 48 (33.3%) |
| L3 | 60 (41.7%) |
| L4 | 24 (16.7%) |
| Side of convex | |
| Left | 84 (58.3%) |
| Right | 60 (41.7%) |
| CA (˚) | 25.4±10.0 |
| TK (˚) | 31.1±12.5 |
| LL (˚) | 24.9±21.6 |
| PI (˚) | 53.4±8.0 |
| PT (˚) | 29.2±7.3 |
| SS (˚) | 23.5±11.0 |
| CVA (cm) | 2.1±1.0 |
| SVA (cm) | 9.0±10.0 |
CA – coronal Cobb angle; TK – thoracic kyphosis; LL – lumbar lordosis; SS – sacral slope; PI – pelvic incidence; PT – pelvic tilt; SVA – sagittal vertical axis; ADS – adult degenerative scoliosis.
Comparison of LMA between different intervertebral levels or sides
Wilcoxon rank sum test showed that the upper LMA (U-LMA) significantly increased on the concave side compared with convex side at the upper intervertebral level (P<0.001). The lower LMA (L-LMA) similarly increased on the concave side compared with convex side at the lower intervertebral level (P=0.014). No significant difference was found between U-LMA and L-LMA on the concave side (P=0.988). U-LMA was significantly increased compared with L-LMA on the convex side (P<0.001; Table 2). Kappa test showed good agreement between researchers’ measurements (k=0.95).
Table 2.
Wilcoxon rank sum test comparing LMA between different intervertebral levels or sides of the scoliosis.
| Variables | Goutallier grade | Concave side | Convex side | Z value (convex–concave) | P value |
|---|---|---|---|---|---|
| Upper intervertebral level | 0 | 0 | 12 | −7.951 | <0.001 |
| 1 | 5 | 7 | |||
| 2 | 19 | 53 | |||
| 3 | 72 | 48 | |||
| 4 | 48 | 24 | |||
| Lower intervertebral level | 0 | 0 | 7 | −2.449 | 0.014 |
| 1 | 7 | 5 | |||
| 2 | 17 | 12 | |||
| 3 | 72 | 36 | |||
| 4 | 48 | 84 | |||
| Z value (lower–upper) | −0.015 | −7.341 | |||
| P value | 0.988 | <0.001 |
LMA – lumbar multifidus muscle atrophy.
Comparison of FJOA between different intervertebral levels or sides
The Pathria grade system showed that at the upper intervertebral level, FJOA (upper FJOA, U-FJOA) significantly increased on the concave side compared with convex side (P<0.001). At the lower intervertebral level, FJOA (lower FJOA, L-FJOA) similarly increased on the concave side compared with convex side (P<0.001). On the concave side, U-FJOA was more severe than L-FJOA (P<0.001). On the convex side, U-FJOA was significantly increased compared with L-FJOA (P<0.001; Table 3, Figure 3). Kappa test showed a good agreement between researchers’ measurements (k=0.98).
Table 3.
Wilcoxon rank sum test comparing FJOA between different intervertebral levels or sides of the scoliosis.
| Variables | Pathria grade | Concave side | Convex side | Z value (convex–concave) | P value |
|---|---|---|---|---|---|
| Upper intervertebral level | I | 0 | 0 | −7.941 | <0.001 |
| II | 12 | 108 | |||
| III | 60 | 24 | |||
| IV | 72 | 12 | |||
| Lower intervertebral level | I | 0 | 0 | −10.368 | <0.001 |
| II | 0 | 96 | |||
| III | 60 | 48 | |||
| IV | 84 | 0 | |||
| Z value (lower-upper) | −9.725 | −9.643 | |||
| P value | <0.001 | <0.001 |
FJOA – facet joint osteoarthritis.
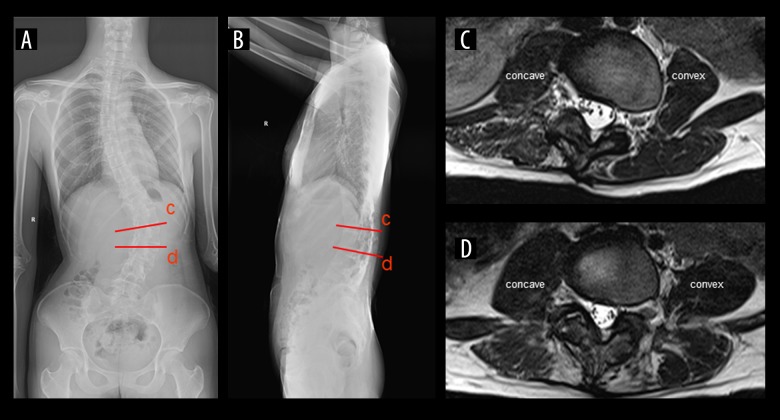
Figure 3.
A male patient (62 years old) with adult degenerative scoliosis (ADS). (A) Anteroposterior (AP) radiograph: coronal Cobb angle (CA), 48.0°; coronal vertical axis (CVA), −2.3 cm. (B) Standing lateral radiograph: thoracic kyphosis (TK), 11.5°; pelvic tilt (PT): 10.3°; lumbar lordosis (LL): 22.1°; pelvic incidence (PI): 31.5°; sacral slope (SS): 21.4°; sagittal vertical axis (SVA): −2.0 cm. (C) T2-weighted axial image at intervertebral levels above the apical vertebra (L1/L2): grade 1 LMA on the convex side; grade 2 LMA on the concave side. (D) T2-weighted axial images at intervertebral level below the apical vertebra (L2/3): grade 3 LMA on the convex side; grade 4 LMA on the concave side. The red lines show the corresponding positions of the axis images.
Correlation of LMA, spinopelvic parameters, and FJOA
Pearson correlation analysis (Table 4) showed positive correlations between SS and LL (P<0.001), PI and LL (P<0.001), PI and SS (P<0.001), LL and U-LMA on the concave side (P=0.009), U-LMA on the convex and concave side (P<0.001), PI and L-LMA (P<0.001), PT and L-LMA (P<0.001), U-LMA and L-LMA (P<0.001), PT and U-FJOA on the concave side (P=0.008), PI and U-FJOA on the convex side (P=0.003), PI and L-FJOA on the concave side (P=0.004), U-FJOA on the concave side and L-FJOA on the convex side (P<0.001), U-FJOA on the convex side and L-FJOA on the concave side (P < 0.001), and L-FJOA on the concave side and the convex side (P=0.004). Negative correlations were found between PT and LL (P<0.001), PT and SS (P<0.001), LL and U-FJOA on the concave side (P<0.001), SS and U-FJOA on the concave side (P<0.001), PI and U-FJOA on the concave side (P<0.001), U-LMA and U-FJOA both on the concave side (P<0.001), L-LMA on the convex side and U-FJOA (concave side, P=0.010; convex side, P<0.001), L-LMA on the convex side and L-FJOA (concave side, P=0.003; convex side, P<0.001); U-LMA and L-FJOA (P<0.001); L-LMA on the concave side and L-FJOA (concave side, P<0.001; convex side, P=0.005); U-FJOA on the concave side and convex side (P<0.001). To sum up, the results of Pearson correlation analysis were very complex; these indicated that, in multi-factor regression analysis, TK should be discussed as an independent factor, while other spinopelvic parameters should be analyzed as cofactors; factors in LMA and FJOA could be evaluated as cofactors in multi-factor regression analysis considering their significant correlations.
Table 4.
Pearson correlation analysis of spinopelvic parameters, concave or convex LMA and FJOA at upper or lower intervertebral level of the apical vertebra.
| Variables | TK | LL | SS | PI | PT | U-LMA | L-LMA | U-FJOA | L-FJOA | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concave side | Convex side | Concave side | Convex side | Concave side | Convex side | Concave side | Convex side | |||||||
| TK | Correlation | – | −0.012 | 0.02 | 0.012 | 0.020 | −0.032 | −0.003 | 0.049 | 0.035 | 0.046 | −0.031 | −0.016 | 0.006 |
| P value | – | 0.886 | 0.977 | 0.890 | 0.813 | 0.701 | 0.976 | 0.556 | 0.681 | 0.584 | 0.708 | 0.850 | 0.940 | |
| LL | Correlation | – | 0.900 | 0.621 | −0.561 | 0.217 | 0.007 | −0.142 | 0.045 | −0.583 | 0.104 | −0.014 | −0.671 | |
| P value | – | <0.001 | <0.001 | <0.001 | 0.009 | 0.933 | 0.090 | 0.594 | <0.001 | 0.214 | 0.866 | <0.001 | ||
| SS | Correlation | – | 0.758 | −0.575 | −0.021 | −0.123 | −0.083 | −0.076 | −0.388 | 0.163 | 0.126 | −0.449 | ||
| P value | – | <0.001 | <0.001 | 0.800 | 0.142 | 0.325 | 0.363 | <0.001 | 0.050 | 0.132 | <0.001 | |||
| PI | Correlation | – | 0.047 | −0.014 | −0.073 | 0.217 | 0.168 | −0.394 | 0.245 | 0.241 | −0.504 | |||
| P value | – | 0.572 | 0.870 | 0.386 | 0.009 | 0.045 | <0.001 | 0.003 | 0.004 | <0.001 | ||||
| PT | Correlation | – | −0.049 | 0.114 | 0.445 | 0.407 | 0.221 | 0.050 | 0.097 | 0.037 | ||||
| P value | – | 0.562 | 0.174 | <0.001 | <0.001 | 0.008 | 0.549 | 0.247 | 0.658 | |||||
| U-LMA | ||||||||||||||
| Concave side | Correlation | – | 0.713 | 0.385 | 0.501 | −0.514 | −0.523 | −0.737 | −0.680 | |||||
| P value | – | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| Convex side | Correlation | – | 0.630 | 0.501 | −0.099 | −0.581 | −0.739 | −0.294 | ||||||
| P value | – | <0.001 | <0.001 | 0.237 | <0.001 | <0.001 | <0.001 | |||||||
| L-LMA | ||||||||||||||
| Concave side | Correlation | – | 0.674 | 0.159 | −0.520 | −0.512 | −0.235 | |||||||
| P value | – | <0.001 | 0.057 | <0.001 | <0.001 | 0.005 | ||||||||
| Convex side | Correlation | – | −0.213 | −0.363 | −0.247 | −0.610 | ||||||||
| P value | – | 0.010 | <0.001 | 0.003 | <0.001 | |||||||||
| U-FJOA | ||||||||||||||
| Concave side | Correlation | – | −0.348 | −0.090 | 0.756 | |||||||||
| P value | – | <0.001 | 0.281 | <0.001 | ||||||||||
| Convex side | Correlation | – | 0.814 | 0.092 | ||||||||||
| P value | – | <0.001 | 0.272 | |||||||||||
| L-FJOA | ||||||||||||||
| Concave side | Correlation | – | 0.239 | |||||||||||
| P value | – | 0.004 | ||||||||||||
| Convex side | Correlation | – | ||||||||||||
| P value | – | |||||||||||||
LMA – lumbar multifidus muscle atrophy; FJOA – facet joint osteoarthritis; U-LMA – upper intervertebral level LMA; L-LMA – lower intervertebral level LMA; U-FJOA – upper intervertebral level FJOA; L-FJOA – lower intervertebral level FJOA; TK – thoracic kyphosis; LL – lumbar lordosis; SS – sacral slope; PI – pelvic incidence; PT – pelvic tilt.
Correlation of influencing factors and severity of ADS
There was a positive correlation between PI and CA (B=1.519, P<0.001). The correlations between PT (B=−0.558, P<0.001), LL (B=−0.335, P<0.001) and CA were negative. The R2 value in multiple linear regression of spinal pelvic parameters and CA was 0.705 (Table 5). The multiple linear regression (R2=0.887) of LMA, FJOA and CA showed that on the concave side, both U-LMA (B=3.367, P=0.025) and L-LMA (B=20.175, P<0.001) were positively correlated with CA. On the convex side, both U-LMA (B=−3.192, P<0.001) and L-LMA (B=−12.997, P<0.001) were negatively correlated with CA. On the convex side, U-FJOA (B=−11.241, P<0.001) and L-FJOA (B=−11.403, P<0.001) were negatively correlated with CA. On the concave side, U-FJOA (B=−5.185, P<0.001) was negatively correlated with CA, while L-FJOA (B=26.573, P<0.001) was positively correlated with CA (Table 5). Table 6 summarizes the correlations between spinal pelvic parameters, LMA and FJOA on concave or convex side, and SVA. Results of the correlation between spinal pelvic parameters and SVA showed a positively correlation between LL and SVA (B=0.342, P<0.001). A a negative correlation was found between SS and SVA (B=−0.751, P<0.001). Considering the R2 value was 0.129, this model had a low reliability. Multiple linear regression of LMA, FJOA, and SVA showed that on the concave side, U-LMA (B=−9.341, P<0.001), L-LMA (B=−9.413, P<0.001), and L-FJOA (B=−19.798, P<0.001) were negatively correlated with SVA. On the convex side, U-LMA (B=6.377, P<0.001), L-LMA (B=5.782, P<0.001), and U-FJOA (B=6.489, P<0.001) were negatively correlated with SVA. The R2 value of this model indicated a moderate predictive power (R2=0.446; Table 6).
Table 5.
Multiple linear regression analysis of influencing factors and CA.
| Influencing factors | Variables | B value | Standard error | t value | P value | R2 value |
|---|---|---|---|---|---|---|
| Spinopelvic parameters | LL | −0.335 | 0.041 | −8.104 | <0.001 | 0.705 |
| PI | 1.519 | 0.092 | 16.504 | <0.001 | ||
| PT | −0.558 | 0.096 | −5.824 | <0.001 | ||
| Constant | −31.079 | 3.589 | −8.660 | <0.001 | ||
| LMA | U-LMA | 0.887 | ||||
| Concave | 3.367 | 1.484 | 2.269 | 0.025 | – | |
| Convex | −3.192 | 0.550 | −5.805 | <0.001 | ||
| L-LMA | – | |||||
| Concave | 20.175 | 0.675 | 29.887 | <0.001 | – | |
| Convex | −12.997 | 0.704 | −1.406 | <0.001 | ||
| FJOA | U-FJOA | |||||
| Concave | −5.185 | 1.662 | −3.121 | 0.002 | ||
| Convex | −11.241 | 1.086 | −10.350 | <0.001 | ||
| L-FJOA | ||||||
| Concave | 26.573 | 2.252 | 11.798 | <0.001 | ||
| Convex | −11.403 | 1.724 | −6.614 | <0.001 | ||
| Constant | −15.631 | 13.266 | −1.178 | 0.241 |
CA – coronary Cobb angle; LL – lumbar lordosis; PI – pelvic incidence; PT – pelvic tilt; LMA – lumbar multifidus atrophy; FJOA, facet joint osteoarthritis; U-LMA – upper intervertebral level LMA; L-LMA – lower intervertebral level LMA; U-FJOA – upper intervertebral level FJOA; L-FJOA – lower intervertebral level FJOA.
Table 6.
Multiple linear regression analysis of influencing factors and SVA.
| Influencing factors | Variables | B value | Standard error | t value | P value | R2 value |
|---|---|---|---|---|---|---|
| Spinopelvic parameters | LL | 0.342 | 0.084 | 4.081 | <0.001 | 0.129 |
| SS | −0.751 | 0.165 | −4.560 | <0.001 | ||
| Constant | 18.093 | 2.328 | 7.771 | <0.001 | ||
| LMA | U-LMA | 0.446 | ||||
| Concave | −9.341 | 1.820 | −5.134 | <0.001 | ||
| Convex | 6.377 | 1.067 | 5.978 | <0.001 | ||
| L-LMA | ||||||
| Concave | −9.413 | 1.484 | −6.345 | <0.001 | ||
| Convex | 5.782 | 1.176 | 4.915 | <0.001 | ||
| FJOA | U-FJOA | |||||
| Convex | 6.489 | 2.011 | 3.226 | 0.002 | ||
| L-FJOA | ||||||
| Concave | −19.798 | 4.064 | −4.872 | <0.001 | ||
| Constant | 81.710 | 15.440 | 5.292 | <0.001 |
LL – lumbar lordosis; SS – sacral slope; SVA – sagittal vertical axis; LMA – lumbar multifidus atrophy; FJOA – facet joint osteoarthritis; U-LMA – upper intervertebral level LMA; L-LMA – lower intervertebral level LMA; U-FJOA – upper intervertebral level FJOA; L-FJOA – lower intervertebral level FJOA.
Discussion
The progression of adult spinal degenerative disease is usually combined with the degeneration of soft-tissue structures [9]. Considering MF is the most medially located back muscle and contributes to maintaining the spinal erector posture as well as rotating or abducting the spine, LMA may cause instability of the spine, which then results in the more severe degeneration of disc and facet in the lumbar spine [10]. In our study, the apical vertebras of ADS patients were more likely to occur in L3 or L4 segment. This may be explained by the important role that MF plays in maintaining the stability of the L3/L4 segment; LMA may play a larger role in L3 or L4 segments than in other segments [11].
The mechanism of LMA may be multifactorial. The immobilization and disuse of back muscles causes atrophy at different intervertebral levels [12–14]. In addition, nerve root compression or disc herniation always occurs in combination with paraspinal denervation and re-innervation; this is because the multifidus muscle is innervated by the medial branch of the lumbar nerve root’s dorsal ramus, as in the case above [15]. It has been reported that LMA can cause disc degeneration at the L3/L4 level, even though there is no denervation phenomenon at this level; furthermore, L4/L5 disc herniation can also cause LMA at the L5/S1 level [16]. All of these observations imply that the pathogenetic mechanisms of U-LMA and L-LMA are different; U-LMA may be caused by disuse, while L-LMA may result from denervation. Our study found that LMA on the concave side was more severe than on the convex side; in addition, there were differences in the causes of U-LMA and L-LMA on the convex side. These indicated that the previous study, which used the axial image obtained at the level of the apex of the curvature as a reference for comparison of the paraspinal muscle, might not be correct [17]. U-LMA may be the cause of ADS, while L-LMA may be the consequence of ADS; they should be discussed separately.
The results of bone mineral density in dual-energy X-ray absorptiometry were usually influenced by FJOA and intervertebral osteophyte formation, which made the discussion of osteoporosis very difficult in our research. Therefore, we focused on FJOA instead. It has been reported that the side of the spine that has more severe OA (osteoarthritis) has more sagittally oriented facets; articular process remodeling in the development of FJOA will result in facet orientation changes [6]. Similarly, our results showed that the change of FJOA had a certain lag compared with the progression of LMA, which indicated that FJOA might be a compensatory change.
In our research, Pearson correlation analysis showed there were correlations between LL, SS, PI, and PT, while TK was an independent factor. This is because spinopelvic parameters are geometrically related; however, thoracic segments are supported by ribs with relatively poor compensatory ability; therefore, the correlations between TK and other radiographic parameters are not significant [18]. The multifidus muscle can act like a bowstring and switch compression loading to stretch loading; some of the axial compression force can subsequently be transmitted from the disc to the anterior longitudinal ligament, and the spinopelvic parameters are ultimately maintained [19].
PI shows the compensatory ability of the lumbar spine and pelvis in maintaining global alignment of the spine. The occurrence of ADS may mean a more severe decompensated state in patients with high PI than in others. Therefore, PI was positively correlated with CA in our study. Lumbar hypolordosis was reported to be associated with lateral listhesis and vertebral rotation in ADS patients, which aggravates scoliosis [20]. In consequence, our results showed that LL and PT are both protective factors of CA, and LL was positively correlated with SVA. A previous study showed that an anterior shift in the C7 plumb line was correlated with a vertically oriented sacrum [20]. Therefore, our results showed that SS was negatively correlated with SVA.
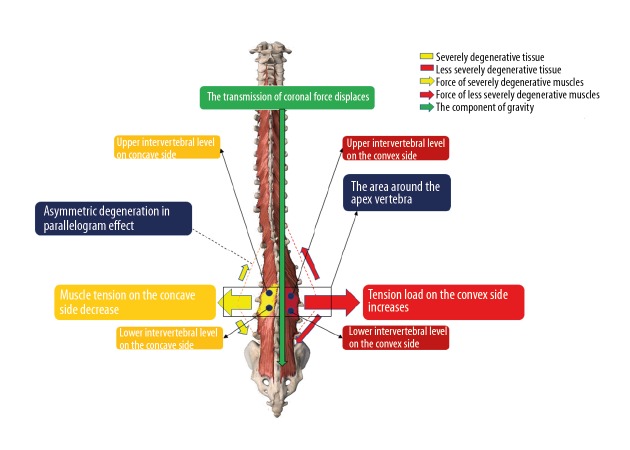
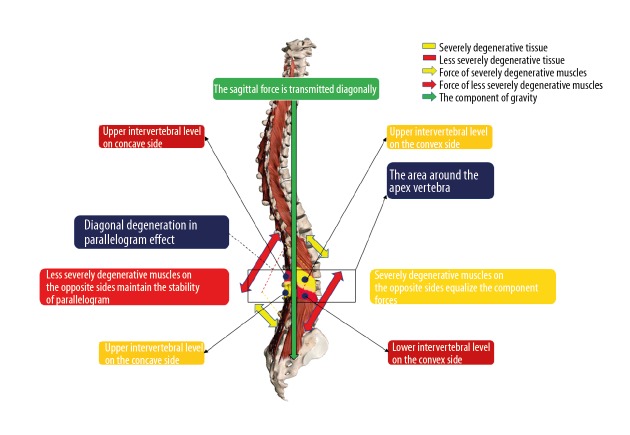
It has been reported that ADS patients prefer to suffer a localized myopathy rather than suffer from a progressive global muscle weakness related to age [5]. As a result, significant asymmetric LMA may be the primary cause of ADS and could be used to predict the progression of ADS. The correlations of LMA or FJOA at different intervertebral levels and severity of ADS were analyzed in our study. These correlations were concluded as “the parallelogram effect of degenerative structures around the apical vertebra”. The parallelogram effect includes 2 parts: the asymmetric effect in the coronal plane and the diagonal effect in the sagittal plane. Results in our study showed that U-LMA and L-LMA on the convex side were negatively correlated with CA, and U-LMA and L-LMA on the concave side were positively correlated with CA. These indicate that asymmetrical LMA can aggravate the coronal deformity via the asymmetric effect, which may influence the conduction of gravity in the coronal plane. This is like the shortening of 2 adjacent sides on the same diagonal side in a parallelogram, which can shift the diagonal line (Figure 4). In addition, convex U-LMA and concave L-LMA were negatively correlated with the SVA. This indicates that LMAs with similar severity on the diagonal through apical vertebra can balance the stress and secure the conduction of gravity in the sagittal plane via the diagonal effect, which is very important to maintaining sagittal balance (Figure 5). FJOA works similar to LMA. This parallelogram effect is first proposed in the present study. Patients with significant changes in parallelogram effect should receive treatment to prevent the progression of ADS.
Figure 4.
The asymmetric effect.
Figure 5.
The diagonal effect.
There are several limitations in our study. First, the retrospective, single-center design may have resulted in unavoidable selection bias. Second, a comparative analysis of different phases of ADS was not conducted, as the course ADS is difficult to follow. Third, all the conclusions were based on radiological data, not biomechanical evidence. Studies with a larger sample size that include patients at different stages of disease progression and biomechanical evidence are warranted to confirm the results of the present study.
Conclusions
In ADS patients, LL and PT are both protective factors against CA progression; high LL is a risk of sagittal imbalance. High SS protects against sagittal imbalance. LMA on the concave side is more severe than the convex side; on the convex side, there are differences in the causes of U-LMA and L-LMA. Asymmetric LMA may be positively correlated with CA, which can influence the coronal balance via the asymmetric effect. LMA on the diagonal through apical vertebra may be very important to maintain sagittal balance via the parallelogram effect. FJOA works similar to LMA. ADS patients with abnormal parallelogram effect should receive further therapy to prevent the progression of spinal deformity.
Acknowledgements
The authors would like to thank Yu-Xi Liu and Miao Ou-Yang who provided data support and corrected some mistakes.
Abbreviations
- LMA
lumbar multifidus muscle atrophy
- ADS
adult degenerative scoliosis
- FJOA
facet joint osteoarthritis
- PS
psoas
- MF
multifidus
- PI
pelvic incidence
- CSA
cross-sectional area
- CT
computed tomography
- MRI
magnetic resonance imaging
- LL
lumbar lordosis
- T2WI
T2-weighted images
- TK
thoracic kyphosis
- CA
coronal Cobb angle
- SVA
sagittal vertical axis
- SS
sacral slope
- PT
pelvic tilt
- SD
standard deviations
- U-LMA
upper intervertebral level LMA
- L-LMA
lower intervertebral level LMA
- U-FJOA
upper intervertebral level FJOA
- L-FJOA
lower intervertebral level FJOA
- OA
osteoarthritis
Footnotes
Source of support: This study was funded by the National Natural Science Foundation of China (No. 81672201 and No. 81871794) and the Beijing Municipal Commission of Health and Family Planning (No. PXM2017 026283 000002)
Conflict of interest
None.
References
- 1.Kim H, Lee CK, Yeom JS, et al. Asymmetry of the cross-sectional area of paravertebral and psoas muscle in patients with degenerative scoliosis. Eur Spine J. 2013;22:1332–38. doi: 10.1007/s00586-013-2740-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwab F, Dubey A, Gamez L, et al. Adult scoliosis: prevalence, SF-36, and nutritional parameters in an elderly volunteer population. Spine. 2005;30:1082–85. doi: 10.1097/01.brs.0000160842.43482.cd. [DOI] [PubMed] [Google Scholar]
- 3.Lee JC, Cha JG, Kim Y, et al. Quantitative analysis of back muscle degeneration in the patients with the degenerative lumbar flat back using a digital image analysis: Comparison with the normal controls. Spine. 2008;33:318–25. doi: 10.1097/BRS.0b013e318162458f. [DOI] [PubMed] [Google Scholar]
- 4.Yoshihara K, Shirai Y, Nakayama Y, Uesaka S. Histochemical changes in the multifidus muscle in patients with lumbar intervertebral disc herniation. Spine. 2001;26:622–26. doi: 10.1097/00007632-200103150-00012. [DOI] [PubMed] [Google Scholar]
- 5.Yagi M, Hosogane N, Watanabe K, et al. The paravertebral muscle and psoas for the maintenance of global spinal alignment in patient with degenerative lumbar scoliosis. Spine J. 2016;16:451–58. doi: 10.1016/j.spinee.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Kalichman L, Guermazi A, Li L, et al. Facet orientation and tropism: Associations with spondylolysis. J Spinal Disord Tech. 2010;23:101–5. doi: 10.1097/BSD.0b013e31819afb80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Battaglia PJ, Maeda Y, Welk A, et al. Reliability of the Goutallier classification in quantifying muscle fatty degeneration in the lumbar multifidus using magnetic resonance imaging. J Manipulative Physiol Ther. 2014;37:190–97. doi: 10.1016/j.jmpt.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Pathria M, Sartoris DJ, Resnick D. Osteoarthritis of the facet joints: Accuracy of oblique radiographic assessment. Radiology. 1987;164:227–30. doi: 10.1148/radiology.164.1.3588910. [DOI] [PubMed] [Google Scholar]
- 9.Wong E, Altaf F, Oh LJ, Gray RJ. Adult degenerative lumbar scoliosis. Orthopedics. 2017;40:e930–39. doi: 10.3928/01477447-20170606-02. [DOI] [PubMed] [Google Scholar]
- 10.Hodges P, Holm AK, Hansson T, Holm S. Rapid atrophy of the lumbar multifidus follows experimental disc or nerve root injury. Spine. 2006;31:2926–33. doi: 10.1097/01.brs.0000248453.51165.0b. [DOI] [PubMed] [Google Scholar]
- 11.Richardson CA, Jull GA. Muscle control-pain control. What exercises would you prescribe? Man Ther. 1995;1:2–10. doi: 10.1054/math.1995.0243. [DOI] [PubMed] [Google Scholar]
- 12.Bakou S, Cherel Y, Gabinaud B, et al. Type-specific changes in fibre size and satellite cell activation following muscle denervation in two strains of turkey (Meleagris gallopavo) J Anat. 1996;188:677–91. [PMC free article] [PubMed] [Google Scholar]
- 13.Bishop DL, Milton RL. The effects of denervation location on fiber type mix in self-reinnervated mouse soleus muscles. Exp Neurol. 1997;147:151–58. doi: 10.1006/exnr.1997.6605. [DOI] [PubMed] [Google Scholar]
- 14.Weber BR, Grob D, Dvorak J, Muntener M. Posterior surgical approach to the lumbar spine and its effect on the multifidus muscle. Spine. 1997;22:1765–72. doi: 10.1097/00007632-199708010-00017. [DOI] [PubMed] [Google Scholar]
- 15.Macintosh JE, Bogduk N. The biomechanics of the lumbar multifidus. Clin Biomech. 1986;1:205–13. doi: 10.1016/0268-0033(86)90147-6. [DOI] [PubMed] [Google Scholar]
- 16.Sun D, Liu P, Cheng J, et al. Correlation between intervertebral disc degeneration, paraspinal muscle atrophy, and lumbar facet joints degeneration in patients with lumbar disc herniation. BMC Musculoskelet Disord. 2017;18:167. doi: 10.1186/s12891-017-1522-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong JY, Suh SW, Modi HN, et al. The prevalence and radiological findings in 1347 elderly patients with scoliosis. J Bone Joint Surg Br. 2010;92:980–83. doi: 10.1302/0301-620X.92B7.23331. [DOI] [PubMed] [Google Scholar]
- 18.Fu X, Sun XL, Harris JA, et al. Long fusion correction of degenerative adult spinal deformity and the selection of the upper or lower thoracic region as the site of proximal instrumentation: A systematic review and meta-analysis. BMJ Open. 2016;6:e12103. doi: 10.1136/bmjopen-2016-012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kader DF, Wardlaw D, Smith FW. Correlation between the MRI changes in the lumbar multifidus muscles and leg pain. Clin Radiol. 2000;55:145–49. doi: 10.1053/crad.1999.0340. [DOI] [PubMed] [Google Scholar]
- 20.Kumar MN, Baklanov A, Chopin D. Correlation between sagittal plane changes and adjacent segment degeneration following lumbar spine fusion. Eur Spine J. 2001;10:314–19. doi: 10.1007/s005860000239. [DOI] [PMC free article] [PubMed] [Google Scholar]