Abstract
Endocrine-disrupting chemicals are known to interfere with normal reproductive function and hormone signaling. Phthalates, bisphenol A, pesticides, and environmental contaminants such as polychlorinated biphenyls and dioxins are known endocrine-disrupting chemicals that have been shown to negatively affect both male and female reproduction. Exposure to these chemicals occurs on a daily basis owing to these compounds being found in plastics, personal care products, and pesticides. Recently, studies have shown that these chemicals may cause transgenerational effects on reproduction in both males and females. This is of concern because exposure to these chemicals prenatally or during adult life can negatively impact the reproductive health of future generations. This mini-review summarizes the endocrine-disrupting chemicals that humans are exposed to on a daily basis and what is known about the transgenerational effects that these chemicals may have on male and female reproduction.
The Endocrine Society defines an endocrine-disrupting chemical (EDC) as “an exogenous chemical, or mixture of chemicals, that can interfere with any aspect of hormone action” (1). EDCs are known to display nonmonotonic dose-response curves because hormones interact with and activate their receptors in a nonlinear fashion and this leads to a U-shaped or inverted U-shaped curve (2–4). EDCs are found in many different products, including plasticizers, personal care products, and pesticides. Furthermore, EDCs such as polychlorinated biphenyls (PCBs) and dioxins are found in environmental contaminants. Humans are constantly exposed to EDCs on a daily basis by ingestion, inhalation, and dermal contact, and with this constant exposure has come detrimental effects on reproductive health. Specifically, EDCs are known to decrease fertility in both male and female animal models and they are associated with subfertility/infertility in humans (2, 5). These chemicals can affect the production of steroids (steroidogenesis), ovarian follicle development and growth (folliculogenesis), and development and maturation of sperm (spermatogenesis), leading to complications with reproduction (6–10).
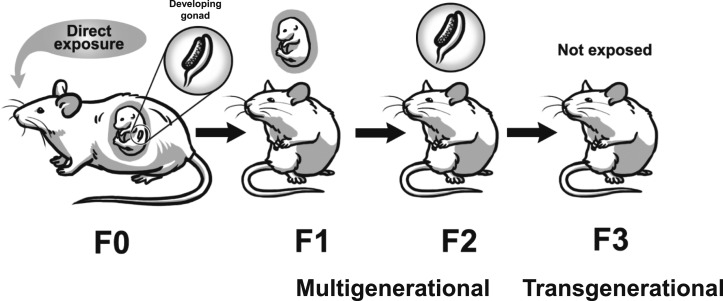
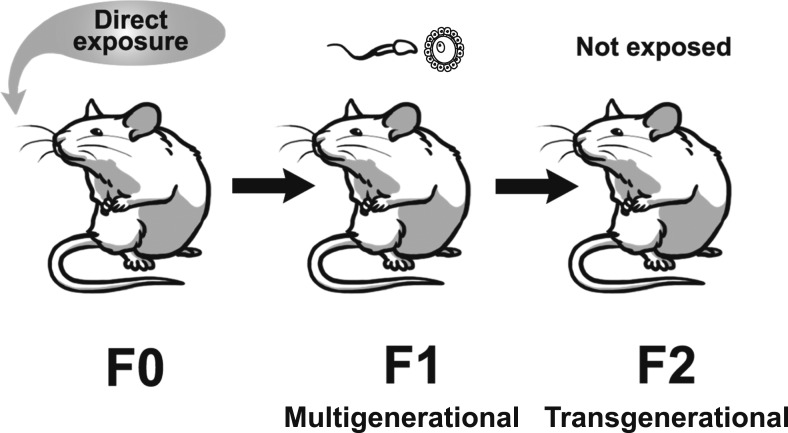
Recently, EDCs have been shown to cause multigenerational and transgenerational effects on reproduction in both male and female rodents (11–13). The exposure routes for multigenerational and transgenerational effects are different for males and females. If the filial generation (F0) female is pregnant and exposed to an EDC, her offspring, which are the F1 generation, are directly exposed to the EDC in utero (14–16). The F2 generation is exposed as germ cells within the F1 generation that is developing during pregnancy (14–16). Effects of EDCs observed in the F1 or F2 generations are considered to be multigenerational (14–16). The F3 generation is the first generation with no direct exposure to the EDC, so this exposure would be considered ancestral exposure and effects would be considered transgenerational (Fig. 1) (14–16). In contrast, when F0 males or nonpregnant F0 females are exposed to an EDC, effects in the produced F1 generation are considered multigenerational through exposure in the germline, and effects in the F2 generation, the first generation not directly exposed, are considered transgenerational in nature (Fig. 2) (14–16).
Figure 1.
Schematic representing multigenerational effects vs transgenerational effects from prenatal exposure to EDCs. Effects in the F1 and F2 generations are considered multigenerational and effects in the F3 generation are considered transgenerational.
Figure 2.
Schematic representing multigenerational effects vs transgenerational effects from adult exposure to EDCs. Effects in the F1 generation are considered multigenerational and effects in the F2 generation are considered transgenerational.
These transgenerational effects are thought to be mediated via epigenetic mechanisms (17). Epigenetic alterations of the germline can include DNA methylation, histone modifications, and noncoding RNAs (17, 18). Changes in epigenetics must be transmitted through the germline to the unexposed generation to cause effects on subsequent generations and cause transgenerational phenomena (17). During DNA methylation, DNA methyltransferases catalyze the addition of a methyl group to the cytosine base within a CpG dinucleotide context, and methylation is usually associated with the repression of transcription (19). Histone modification involves altering the chromatin structure by changing the interaction of DNA with other histones, therefore increasing or decreasing the possibility of transcription (16, 20). Lastly, noncoding RNAs, including small and long noncoding RNAs, are involved in chromatin function and can modulate gene expression via gene silencing and activation by influencing the mechanisms of DNA methylation and histone modifications (21, 22). Out of these alterations to the epigenome, DNA methylation is the most commonly studied and it is thought to be the primary epigenetic mechanism to mediate the effects of EDCs through the germline (23, 24).
Known EDCs that affect male and female reproduction include phthalates, bisphenol A (BPA), pesticides, and environmental contaminants, including PCBs and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). In this mini-review, we focused on these chemicals because they are known endocrine disruptors that humans are exposed to daily and they have been shown to cause transgenerational effects, specifically on reproduction [Tables 1 (11, 13, 25–44) and 2 (11, 32, 33, 35, 37–39, 42, 43, 45–49)].
Table 1.
The Transgenerational Effects of EDCs on Female Reproduction
| Effects | Chemical | Species | Dose | Reference |
|---|---|---|---|---|
| Anogenital distance/index | DEHP | Rat | 20 mg/kg | Meltzer et al. (25) |
| DEHP | Mouse | 750 mg/kg/d | Rattan et al. (26) | |
| Phthalate mixture | Mouse | 200 mg/kg/d | Zhou et al. (13) | |
| Mix of EDCs | Rat | Mix of EDCs: refer to paper | Manikkam et al. (11) | |
| PCBs | Rat | 1 mg/kg | Mennigen et al. (41) | |
| Epigenetic effects | MXC | Rat | 200 mg/kg/d | Manikkam et al. (34) |
| DDT | Rat | 50 mg/kg BW/d | Skinner et al. (35) | |
| DDT | Rat | 25 mg/kw BW/d | Nilsson et al. (36) | |
| Vinclozolin | Rat | 100 mg/kg BW/d | Nilsson et al. (36) | |
| Vinclozolin | Mouse | 100 mg/kg/d | Guerrero-Bosagna et al. (39) | |
| Vinclozolin | Rat | 100 mg/kg BW/d | Nilsson et al. (40) | |
| TCDD | Rat | 100 ng/kw BW/d | Manikkam et al. (42) | |
| Estrous cyclicity | DEHP | Mouse | 20 µg/kg/d | Rattan et al. (26) |
| DEHP | Mouse | 20 and 200 µg/kg/d, 500 and 750 mg/kg/d | Brehm et al. (29) | |
| BPA | Mouse | 50 µg/kg/d | Ziv-Gal et al. (31) | |
| Fertility | DEHP | Mouse | 20 µg/kg/d | Rattan et al. (26) |
| Phthalate mixture | Mouse | 200 mg/kg/d | Zhou et al. (13) | |
| BPA | Mouse | 0.5 µg/kg/d | Ziv-Gal et al. (31) | |
| TCDD | Mouse | 10 µg/kg | Bruner-Tran et al. (43) | |
| Follicle number | DEHP | Mouse | 0.05 and 5 mg/kg/d | Pocar et al. (27) |
| DEHP | Mouse | 20 and 200 µg/kg/d, 500 and 750 mg/kg/d | Rattan et al. (28) | |
| Phthalates and BPA | Rat | BPA (25 and 50 mg/kg BW/d), DEHP (375 and 750 mg/kg BW/d), and DBP (33 and 66 mg/kg BW/d) | Manikkam et al. (32) | |
| Mix of EDCs | Rat | Mix of EDCs: refer to paper | Nilsson et al. (40) | |
| Mix of EDCs | Rat | Mix of EDCs: refer to paper | Manikkam et al. (11) | |
| TCDD | Rat | 100 ng/kg BW/d | Manikkam et al. (42) | |
| Gene expression | BPA | Mouse | 0.5, 20, 50 µg/kg/d | Berger et al. (30) |
| Litter size | DEHP | Rat | 20 mg/kg | Meltzer et al. (25) |
| Ovarian disease (including cystic ovaries) | Phthalates and BPA | Rat | BPA (25 and 50 mg/kg BW/d), DEHP (375 and 750 mg/kg BW/d), and DBP (33 and 66 mg/kg BW/d) | Manikkam et al. (32) |
| MXC | Rat | 200 mg/kg/d | Manikkam et al. (34) | |
| DDT | Rat | 25 and 50 mg/kg BW/d | Skinner et al. (35) | |
| DDT | Rat | 25 mg/kg BW/d | Nilsson et al. (36) | |
| Vinclozolin | Rat | 100 mg/kg/d | Nilsson et al. (36) | |
| Vinclozolin | Mouse | 200 mg/kg/d | Guerrero-Bosagna et al. (39) | |
| Mix of EDCs | Rat | Mix of EDCs-refer to paper | Nilsson et al. (40) | |
| TCDD | Rat | 100 ng/kg BW/d | Manikkam et al. (42) | |
| Puberty | DEHP | Mouse | 20 µg/kg/d, 500 and 750 mg/kg/d | Rattan et al. (26) |
| BPA | Mouse | 0.5 and 50 µg/kg/d | Ziv-Gal et al. (31) | |
| Phthalates and BPA | Rat | BPA (25 and 50 mg/kg BW/d), DEHP (375 and 750 mg/kg BW/d), and DBP (33 and 66 mg/kg BW/d) | Manikkam et al. (32) | |
| Atrazine | Rat | 25 mg/kw BW/d | McBirney et al. (33) | |
| Mix of EDCs | Rat | Mix of EDCs: refer to paper | Manikkam et al. (11) | |
| TCDD | Rat | 100 ng/kg BW/d | Manikkam et al. (42) | |
| Reproductive aging | DEHP | Mouse | 20 µg/kg/d, 500 and 750 mg/kg/d | Brehm et al. (29) |
| Reproductive organ size | DEHP | Mouse | 20 and 200 µg/kg/d, 500 mg/kg/d | Brehm et al. (29) |
| Phthalate mixture | Mouse | 200 µg/kg/d, 200 mg/kg/d | Zhou et al. (13) | |
| Phthalates and BPA | Rat | BPA (25 and 50 mg/kg BW/d), DEHP (375 and 750 mg/kg BW/d), and DBP (33 and 66 mg/kg BW/d) | Manikkam et al. (32) | |
| Sex ratio | DEHP | Mouse | 20 µg/kg/d | Rattan et al. (26) |
| Vinclozolin | Rat | 100 mg/kg/d | Anway et al. (38) | |
| DDE | Rat | 100 mg/kg | Song and Yang (37) | |
| Sex steroid hormones | DEHP | Mouse | 20 µg/kg/d, 500 and 750 mg/kg/d | Brehm et al. (29) |
| PCBs | Rat | 1 mg/kg | Mennigen et al. (41) | |
| Uterine infections and adenomyosis | DDT | Rat | 50 mg/kg BW/d | Skinner et al. (35) |
| TCDD | Mouse | 10 µg/kg | Bruner-Tran et al. (44) |
Abbreviations: DDT, dichlorodiphenyltrichloroethane; DEHP, di(2-ethylhexyl) phthalate; MXC, methoxychlor.
Table 2.
The Transgenerational Effects of EDCs on Male Reproduction
| Effects | Chemical | Species | Dose | Reference |
|---|---|---|---|---|
| Anogenital distance/index | Mix of EDCs | Rat | Mix of EDCs: refer to paper | Manikkam et al. (11) |
| Epigenetic effects | DBP | Rat | 500 mg/kg | Yuan et al. (45) |
| Atrazine | Rat | 25 mg/kw BW/d | McBirney et al. (33) | |
| DDE | Rat | 100 mg/kg | Song et al. (47) | |
| DDE | Rat | 100 mg/kg | Song and Yang (37) | |
| Vinclozolin | Rat | 100 mg/kg/d | Anway et al. (48) | |
| Vinclozolin | Mouse | 100 mg/kg/d | Guerrero-Bosagna et al. (39) | |
| Fertility | DDE | Rat | 100 mg/kg | Song et al. (47) |
| DDE | Rat | 100 mg/kg | Song and Yang (37) | |
| Vinclozolin | Rat | 100 mg/kg/d | Anway et al. (48) | |
| TCDD | Mouse | 1 µg/kg | Bruner-Tran et al. (43) | |
| Gene expression | DDE | Rat | 100 mg/kg | Song et al. (47) |
| Puberty | DEHP | Mouse | 500 mg/kg | Doyle et al. (46) |
| Mix of EDCs | Rat | Mix of EDCs: refer to paper | Manikkam et al. (11) | |
| Reproductive organ size | DDE | Rat | 100 mg/kg | Song et al. (47) |
| Seminiferous tubules | DEHP | Mouse | 500 mg/kg | Doyle et al. (46) |
| DDE | Rat | 100 mg/kg | Song and Yang (37) | |
| PCBs | Mouse | 10 and 100 µg/kg/d | Pocar et al. (49) | |
| Sertoli cells | DBP | Rat | 500 mg/kg | Yuan et al. (45) |
| Sex steroid hormones | Mix of EDCs | Rat | Mix of EDCs: refer to paper | Manikkam et al. (11) |
| TCDD | Rat | 100 ng/kg BW/d | Manikkam et al. (42) | |
| Spermatogenetic capacity | Vinclozolin | Rat | 100 mg/kg/d | Anway et al. (38) |
| Sperm morphology | TCDD | Mouse | 10 µg/kg | Bruner-Tran et al. (43) |
| Sperm motility | DDE | Rat | 100 mg/kg | Song et al. (47) |
| Vinclozolin | Rat | 100 mg/kg/d | Anway et al. (48) | |
| Sperm number | DBP | Rat | 500 mg/kg | Yuan et al. (45) |
| DEHP | Mouse | 500 mg/kg | Doyle et al. (46) | |
| DDT | Rat | 50 mg/kg BW/d | Skinner et al. (35) | |
| DDE | Rat | 100 mg/kg | Song et al. (47) | |
| DDE | Rat | 100 mg/kg | Song and Yang (37) | |
| Vinclozolin | Rat | 100 mg/kg/d | Anway et al. (48) | |
| Vinclozolin | Mouse | 200 mg/kg/d | Guerrero-Bosagna et al. (39) | |
| Sperm viability | PCBs | Mouse | 1, 10, and 100 µg/kg/d | Pocar et al. (49) |
| Testis disease | Phthalates and BPA | Rat | BPA (25 mg/kg BW/d), DEHP (375 mg/kg BW/d), and DBP (33 mg/kg BW/d) | Manikkam et al. (32) |
| Atrazine | Rat | 25 mg/kw BW/d | McBirney et al. (33) | |
| DDT | Rat | 50 mg/kg BW/d | Skinner et al. (35) | |
| Vinclozolin | Mouse | 100 mg/kg/d | Guerrero-Bosagna et al. (39) |
Abbreviations: DBP, dibutyl phthalate; DDE, dichlorodiphenoxydichloroethylene; DDT, dichlorodiphenyltrichloroethane; DEHP, di(2-ethylhexyl) phthalate.
Phthalates
Phthalates are a group of chemicals widely used in many consumer products, including building materials, food storage containers, personal care products, and medical devices (50). With this widespread use, >18 billion pounds of phthalates are produced annually (51). Phthalates provide plasticity to materials such as polyvinyl chloride and they lubricate and act as solvents in other products (50). Low-molecular-weight phthalates include diethyl phthalate (DEP), dibutyl phthalate (DBP), and diisobutyl phthalate (DiBP). These low-molecular-weight phthalates are found in colognes, perfumes, nail polish, pharmaceuticals, and some adhesives (52, 53). High-molecular-weight phthalates include di(2-ethylhexyl) phthalate (DEHP), benzyl butyl phthalate (BzBP), and di-isononyl phthalate (DiNP). These high-molecular-weight phthalates are heavily used as plasticizers in polyvinyl chloride plastics, flooring, food packaging, medical tubing, plastic toys, and paints (52). In addition to being present in consumer products, phthalates are detected in indoor air and household dust (54).
Owing to the widespread use of phthalates in many different consumer products, humans are exposed daily to these chemicals. Based on studies in the United States and Germany, the estimated range of daily human exposure to DEHP is ∼3 to 30 µg/kg/d, DEP is 2.32 to 12 μg/kg/d, BzBP is 0.26 to 0.88 μg/kg/d, DBP is 0.84 to 5.22 μg/kg/d, and DIBP is 0.12 to 1.4 μg/kg/d (55, 56). Currently, daily human exposure for DiNP is not known, but occupational exposure levels reach up to 26 µg/kg/d, whereas exposure in infants can reach levels of up to 120 µg/kg/d (57, 58). Furthermore, occupational exposure to DEHP has been estimated to be between 143 and 286 µg/kg/d (55, 56).
Phthalates have been shown to negatively affect both male and female reproduction in humans and animal models. In women, increased exposure to monoethyl phthalate, a metabolite of DEP, increased time to pregnancy (59). Furthermore, a high sum of phthalate metabolites found in personal care products was associated with an increased risk of ever experiencing hot flashes (60). In men, DEHP metabolites were negatively associated with progressive sperm motility (61). In animal studies, phthalates have been shown to reduce ovarian follicle growth and decrease hormone production in female CD-1 mice (62). Furthermore, DEHP (20 and 200 µg/kg/d, 500 and 750 mg/kg/d) has been shown to cause early reproductive senescence in male CD-1 mice by impairing testosterone production, reducing sperm quality, and decreasing fertility (63).
In addition to directly affecting both male and female reproductive outcomes, phthalates have been shown to have transgenerational effects on male reproduction (Table 2). Yuan et al. (45) dosed timed pregnant Sprague-Dawley rats (F0) by oral gavage with DBP (500 mg/kg) from embryonic day 8 until 14 and found that DBP decreased the numbers of sperm and Sertoli cells in the F1, F2, and F3 generations of male Sprague-Dawley rats. This decrease in sperm and Sertoli cells may be due to a reduction in betaine-homocysteine S-methyltransferase, causing genome-wide hypomethylation, as well as a reduction of follistatin-like 3, which is a modulator of Sertoli cell number and spermatogenesis (45). In a similar study in which timed pregnant CD-1 mice (F0) were dosed with vehicle control or DEHP (500 mg/kg) by oral gavage, DEHP exposure delayed the onset of puberty in the F3 generation of male CD-1 mice as assessed by measuring the penis detachment from the prepuce in males starting at postnatal day (PND) 20 (46). Furthermore, DEHP exposure increased the number of abnormal seminiferous tubules and decreased sperm count in the F3 and F4 generations (46). Although the transgenerational effects discovered in these studies are very important, these effects were observed at doses higher than reported for human exposure. Thus, future studies should determine the transgenerational impact of phthalates on male reproduction using environmentally relevant doses and exploring different exposure periods.
In females, different phthalates or mixtures of phthalates have been shown to cause transgenerational effects on reproduction (Table 1). Ancestral exposure to DEHP via oral gavage from gestational day 14 until birth increased litter size (20 mg/kg), decreased pup weight at PND 3 (20 mg/kg), and decreased anogenital distance, which is a marker of in utero exposure to androgens or androgenic chemicals, at PND 15 (300 mg/kg) in the F3 generation of female Sprague-Dawley rats (25). This suggests that phthalates have antiandrogenic effects in the F3 generation. Furthermore, Rattan et al. (26) showed that ancestral exposure to DEHP at human relevant doses of 20 µg/kg/d and high doses of 500 and 750 mg/kg/d from gestational day 10.5 until birth caused several adverse effects on the fertility of CD-1 mice. Specifically, ancestral exposure to DEHP (20 µg/kg/d, 500 and 750 mg/kg/d) accelerated the onset of puberty in female CD-1 mice (26). DEHP exposure (20 µg/kg/d) also disrupted estrous cyclicity by increasing the time these mice spent in estrus and decreasing the time these mice spent in diestrus. Furthermore, DEHP exposure increased the number of female pups per litter (20 µg/kg/d) and decreased female pup anogenital index in the F3 generation of mice (750 mg/kg/d) (26). Although these transgenerational studies examining the effects of phthalates on female reproduction used a wide range of doses, future studies should examine different windows of exposure to determine windows of sensitivity to phthalates.
Phthalates have also been shown to cause transgenerational effects on the ovary (Table 1). For example, ancestral exposure to chow containing DEHP (0.05 and 5 mg/kg/d) from gestational day 0.5 until the end of lactation decreased primordial follicle numbers, but increased preantral follicles in the F3 generation of female CD-1 mice (27). These data suggest that ancestral DEHP exposure may accelerate folliculogenesis in the ovary in a transgenerational manner. Furthermore, ancestral exposure to DEHP (20 and 200 µg/kg/d, 500 and 750 mg/kg/d) from gestational day 10.5 until birth decreased germ cell number (500 mg/kg/d), increased folliculogenesis (500 and 750 mg/kg/d), and decreased total follicle numbers (500 mg/kg/d) at PND 1 in female CD-1 mice (28). DEHP exposure (200 μg/kg/d and 500 mg/kg/d) also increased primordial, preantral, and total follicle numbers at PND 8 and decreased primordial (20 µg/kg/d) and preantral follicles (750 mg/kg/d) at PND 21 in CD-1 mice (28). These results suggest that prenatal exposure to DEHP leads to adverse transgenerational effects on normal ovarian function beginning at PND 1 and continuing at PNDs 8 and 21. Although many of the transgenerational effects were observed at environmentally relevant doses (20 and 200 µg/kg/d), several effects were observed at doses that were greater than daily human exposure (500 and 750 mg/kg/d). This suggests that different doses have different effects on different endpoints and that future studies should continue to include a range of doses. Such studies have increased our understanding of the effects of EDCs on reproduction.
Besides causing transgenerational effects on the ovary and fertility, phthalates have been shown to affect reproductive aging in females (Table 1). Specifically, ancestral exposure to DEHP (20 and 200 µg/kg/d, 500 and 750 mg/kg/d) from gestational day 11 until birth decreased ovarian weight (20 and 200 µg/kg/d, 500 mg/kg/d) at 1 year of age in the F3 generation of female CD-1 mice (29). It also increased estradiol levels (20 µg/kg/d), decreased testosterone levels (20 µg/kg/d and 500 mg/kg/d), increased FSH levels (500 mg/kg/d), increased LH levels (20 µg/kg/d), and decreased inhibin B levels (750 mg/kg/d) in mice at 1 year of age in the F3 generation (29). These data suggest that ancestral DEHP exposure may accelerate reproductive aging by altering sex steroid hormone levels, increasing gonadotropin hormone levels, and decreasing inhibin B levels. Furthermore, DEHP (20 and 200 µg/kg/d, 500 and 750 mg/kg/d) altered estrous cyclicity, causing the F3 females to spend most of their time in metestrus/diestrus, with mice in the 500 mg/kg/d group spending 100% of their time in metestrus/diestrus, clearly demonstrating persistent diestrus (29). Because persistent diestrus indicates that the rodent is no longer cycling and has reached reproductive senescence, these data suggest that DEHP exposure may cause early reproductive aging. Few studies have examined how phthalates or other EDCs affect reproductive aging, especially in a transgenerational manner. Therefore, future studies should examine whether EDCs affect reproductive aging and whether the timing of exposure affects the outcomes.
Most transgenerational studies focus on exposure to a single phthalate; however, humans are exposed to mixtures of chemicals such as phthalates on a daily basis. Thus, it is important to consider the effects of mixtures on reproduction (Table 1). In one study, Zhou et al. (13) dosed pregnant CD-1 mice (F0) from gestational day 10 until birth with a mixture of phthalates consisting of 35% DEP, 21% DEHP, 15% DBP, 15% DiNP, 8% DIBP, and 5% BzBP. This mixture decreased anogenital distance at PND 21 (200 mg/kg/d), increased uterine weight at PND 60 (200 µg/kg/d and 200 mg/kg/d), and decreased ovary weight at PND 60 (200 mg/kg/d) in the F3 generation of CD-1 mice (13). The mixture also decreased the percentage of females that produced live pups (500 mg/kg/d), and it decreased some fertility indices throughout breeding trials in the F3 generation (13). Such fertility indices included how successful females were at becoming pregnant, maintaining a pregnancy, and/or delivering pups (13). Furthermore, the mixture (200 mg/kg/d) delayed time to pregnancy, but it did not affect puberty or cyclicity in the F3 generation (13). Lastly, the mixture (500 mg/kg/d) decreased pup numbers in the F4 generation (13). The results from this study suggest that ancestral exposure to mixtures of phthalates can negatively affect female fertility in a transgenerational manner. However, many of the negative effects on fertility were observed in treatment groups with levels of phthalates comparable to occupational and medical exposure, but not to daily human intake of these phthalates. Given that few studies have examined the transgenerational effects of a mixture of phthalates on female reproduction, future studies are needed to confirm and expand these findings. Such studies should include a range of doses that mimic daily exposure as well as occupational and medical exposure.
Bisphenol A
BPA is a synthetic compound used as a plasticizer in epoxy resins and polycarbonate plastics. It can be found in many products, including food containers, baby bottles, food and beverage can liners, and thermal receipt papers. Owing to the widespread use of BPA, >5 million tons are produced annually worldwide (64). BPA can leach from products due to microwaving, cleaning, and exposure to UV light. Humans are exposed to BPA via dermal and oral exposure along with inhalation, but most exposure is oral (65). Studies have estimated that human exposure ranges from <1 μg/kg/d to almost 5 μg/kg/d (65). BPA can be found in urine, breast milk, blood, and ovarian follicular fluid (66, 67).
BPA has been shown to adversely affect reproduction in both males and females in human and laboratory animal studies. For example, higher BPA serum levels have been found in women with polycystic ovarian syndrome compared with women without polycystic ovarian syndrome (65). Furthermore, in CD-1 mice, prenatal exposure to BPA decreased preantral follicle numbers (0.5 and 20 µg/kg/d) and estradiol levels (20 and 50 µg/kg/d) in the F1 generation (68). Moreover, BPA disrupted germ cell nest breakdown (0.5, 20, and 50 µg/kg/d) and severely reduced fertility (0.5 µg/kg/d) in female mice (69). In males, BPA exposure decreased testosterone levels (5 and 25 mg/kg/d), increased sperm defects (25 mg/kg/d), and decreased sperm counts (25 µg/kg/d) in Wistar rats (70, 71). These findings suggest that BPA exposure may negatively affect male and female reproduction, specifically by affecting ovarian and testicular function.
Studies examining the transgenerational effects of BPA have focused more on female reproductive outcomes than male reproductive outcomes (Table 1). In one study, ancestral oral exposure to BPA (0.5, 20, and 50 µg/kg/d) from gestational day 11 until birth altered expression of apoptotic genes (Bcl2, Casp8, and Bax), increased expression of antioxidant genes (Sod1 and Gpx), altered expression of genes in the Igf family (Igf1 and Igfbp2), decreased expression of sex hormone receptor Esr1, and increased expression of steroidgenic enzymes (Cyp17a1 and Fshr) at PND 21 in the ovaries of female CD-1 mice in the F3 generation (30). However, BPA exposure did not affect apoptotic factors, antioxidant genes, autophagy genes, germ cell nest breakdown, or follicle counts at PND 4 in the F3 generation of CD-1 mice (30). Furthermore, BPA exposure did not affect follicle numbers at PND 21 in the F3 generation (30). In a similar study design, ancestral exposure to BPA (0.5, 20, and 50 µg/kg/d) from gestational day 11 until birth delayed the age of vaginal opening (0.5 and 50 µg/kg/d), delayed the timing of first estrus (50 µg/kg/d), and it (0.5 µg/kg/d) decreased the fertility index in the F3 generation of female CD-1 mice (31). Collectively, these data suggest that ancestral BPA exposures, including doses relevant to human exposure, affect ovarian genes and fertility in a transgenerational manner. Future studies, however, are needed to address the mechanisms underlying the transgenerational effects of BPA exposure.
One study has examined the transgenerational effects of a mixture containing BPA (Tables 1 and 2). In a transgenerational study by Manikkam et al. (32), Sprague-Dawley rats were exposed to two doses of mixtures of different plasticizers via daily IP injections from gestational day 8 to 14. The group treated with the highest doses of plastics in the study were designated as the high-dose group and were exposed to a mixture containing BPA [50 mg/kg body weight (BW)/d], DEHP (750 mg/kg BW/d), and DBP (66 mg/kg BW/d) (32). The other group was designated as the low-dose plastics group and was exposed to a mixture containing BPA (25 mg/kg BW/d), DEHP (375 mg/kg BW/d), and DBP (33 mg/kg BW/d) (32). The results indicate that ancestral exposure to the low dose of plastics increased the incidence of testis disease at 1 year of age in the F3 generation (32). The high- and low-dose plastic groups also increased pubertal abnormalities, the occurrence of primordial follicle loss, polycystic ovary disease, and uterine weight in the F3 generation of 1-year-old Sprague-Dawley rats (32). The results from this study suggest that ancestral exposure to mixtures of plasticizers affect reproductive organs and pubertal outcomes in the F3 generation of male and female rats. However, note that the treatment group designated as “low-dose plastics” is not truly low dose when compared with human exposure of these toxicants, and it only reflects the low dose used in the study. Future studies are required to determine whether environmentally relevant levels of the plastic mixtures affect reproduction in a transgenerational manner. Furthermore, future studies should focus on relevant routes of exposure. The previous studies on plastic mixtures used IP injections as the route of exposure, whereas humans are exposed via oral, dermal, or respiratory exposure.
Pesticides
Pesticides are toxicants that are purposely produced to kill weeds (herbicides), insects (insecticides), fungi (fungicides), and rodents (rodenticides) (72). Pesticides are used heavily for agricultural purposes and to help control infectious diseases. In 2011 and 2012, world pesticide usage totaled almost 6 billion pounds annually, with herbicides accounting for approximately half of this use (73). Owing to the constant use of pesticides, humans are exposed daily via ingestion, inhalation, and dermal contact. Furthermore, widespread use has been shown to be hazardous to humans and other living organisms because it has been linked to cancer, asthma, diabetes, Parkinson disease, and cognitive effects (72). Several pesticides have been shown to impact reproduction, including atrazine, methoxychlor, dichlorodiphenyltrichloroethane (DDT), p,p′-dichlorodiphenoxydichloroethylene (DDE), and vinclozolin.
Atrazine is one of the most effective and frequently used herbicides in the world. Humans can be exposed through occupational exposure when agricultural workers spray the herbicide on crops (74). Furthermore, atrazine contaminates soil, leading to human exposure through contaminated agricultural products and our waterways (74). Epidemiological studies have reported water levels of atrazine ranging from 5.9 µg/L to >10 µg/L (75). Studies have indicated that atrazine exposure can affect reproduction in both males and females. In women, atrazine exposure has been associated with an increase in menstrual cycle irregularity (76). In animal studies, atrazine delayed vaginal opening in female Sprague-Dawley rats (100 mg/kg) (77) and decreased sperm motility in male Fischer rats (60 and 120 mg/kg) (78). Most transgenerational studies on pesticides have not focused on atrazine; however, in one study, McBirney et al. (33) exposed pregnant Sprague-Dawley rats via IP injections of atrazine (25 mg/kg BW/d) from gestational day 8 to 14 and found that atrazine increased testicular disease and caused an early onset of puberty in females in the F3 generation (Tables 1 and 2). Additionally, atrazine exposure caused sperm differential DNA methylation regions, or epimutations, which were associated with testis disease in the F3 generation (33). Testis disease was characterized by the presence of histopathologies, including azoospermia, atretic seminiferous tubules, presence of vacuoles in basal regions of seminiferous tubules, sloughed germ cells in the lumen of seminiferous tubules, and lack of seminiferous tubal lumen (33). Owing to lack of transgenerational studies on atrazine, future studies should focus on how this chemical affects male and female reproduction in a transgenerational manner. Furthermore, future studies should expose rodents via different exposure routes to compare with human exposure. The previous studies on atrazine used IP injections, which is not a relevant route of exposure in humans.
Methoxychlor (MXC) is an organochlorine insecticide used on agricultural crops and livestock (79). Although MXC is no longer used in the United States, humans are still exposed from agricultural products due to the ability of MXC to remain in soil for long periods of time (80). Furthermore, imported food to the United States is contaminated with this insecticide. MXC concentrations in water mains range from 0.0177 to 0.8053 µg/L (81). Additionally, humans in other countries are exposed to MXC by spraying and from agricultural products that are affected by the presence of MXC in soil. Studies have shown that MXC is a reproductive toxicant in both female and male rodents. Quignot et al. (82) found that MXC (200 mg/kg) reduced ovarian weights and increased corpora lutea number in female Sprague-Dawley rats. MXC (200 mg/kg) also reduced the weights of the testis, epididymis, seminal vesicles, and the prostate in male Sprague-Dawley rats (82). Furthermore, MXC (25, 96, and 200 mg/kg/d) decreased sperm number in male rodents (83).
Transgenerational studies examining the effects of MXC on reproduction are limited (Table 1). However, one study showed that prenatal exposure to MXC (200 mg/kg/d) via IP injections from gestational day 8 to 14 increased the incidence of ovarian disease and polycystic ovary disease in the F3 generation of Sprague-Dawley rats (34). In contrast, MXC exposure did not affect puberty in males or females, levels of estradiol in females, or levels of testosterone in the F3 generation of Sprague-Dawley rats (34). However, more studies are needed to examine the mechanisms underlying the transgenerational effects of MXC on male and female reproduction using relevant dose exposures that mimic daily human exposure with a more environmentally relevant exposure route.
DDT is a well-known insecticide. It is no longer used in the United States, but Asian and African countries still make use of it today to control for malaria (84). Residents of cities in China have estimated daily intakes of DDT ranging from 31.5 to 52.1 ng/kg BW/d (85). Similar to other pesticides, DDT biodegrades slowly, leading to constant exposure in the environment and accumulation in our food chains (86). DDT is a known endocrine disruptor that has been shown to negatively affect fertility. In male Wistar rats, DDT (50 and 100 mg/kg) decreased testis weight and sperm motility compared with control (87). In female rats and mice, DDT increased uterine weight compared with control (88). The main metabolite of DDT is p,p′-DDE. This metabolite is still found in both water and sediment, leading to daily exposure (89). Similar to the parent compound of DDT, p,p′-DDE has been shown to affect reproduction. DDE (20 and 60 mg/kg) decreased motile sperm in Sprague-Dawley rats (90). Furthermore, DDE (125 µg/g) decreased average litter size in female Wistar rats compared with control (91).
Only a few studies have examined the transgenerational effects of DDT on male and female reproduction, and the doses used in these studies are higher than those reported in humans (Tables 1 and 2). Ancestral exposure to DDT (25 or 50 mg/kg BW/d) via IP injection from gestational day 8 until 14 increased the incidence of testis disease (50 mg/kg BW/d) and decreased sperm count (50 mg/kg BW/d) in the F3 generation of Sprague-Dawley rats (35). Furthermore, ancestral DDT (25 and 50 mg/kg BW/d) exposure increased the incidence of polycystic ovaries (25 and 50 mg/kg BW/d) and the occurrence of uterine infections (50 mg/kg BW/d) in the F3 generation of Sprague-Dawley rats (35). Additionally, DDT (25 mg/kg BW/d) increased the incidence of ovarian disease in the F4 generation (35). The transmission of the polycystic ovaries may be from sperm epimutations that were induced by DDT and because many of the identified genes have been associated with polycystic ovary disease (35). In a similar study by Nilsson et al. (36), ancestral exposure to DDT (25 mg/kg BW/d) via IP injections increased the occurrence of cystic ovaries in the F3 generation of Sprague-Dawley rats. Furthermore, DDT promoted the epigenetic transgenerational inheritance of ovarian disease susceptibility by causing changes to DNA methylation and noncoding RNA expression, as well as mRNA expression in ovarian granulosa cells from young female rats in the F3 generation. These changes may contribute to the dysregulation of the ovary that can promote disease susceptibility later in life (36). Although these studies show that DDT causes many transgenerational effects on reproduction, future studies should focus on lower doses and environmentally relevant routes of exposure.
The metabolite of DDT, DDE, has also been shown to cause transgenerational effects on male reproduction (Table 2). In one study, ancestral exposure to DDE (100 mg/kg) via oral gavage from gestational days 8 to 15 increased the percentage of male offspring with small testis and infertility in the F3 generation of Sprague-Dawley rats (47). Ancestral DDE exposure also decreased the number of sperm and sperm motility and the expression of Igf2, but increased the expression of H19 in sperm in the F3 generation of rats (47). The altered expression of Igf2 and H19 was caused by Igf2 differentially methylated region 2 hypomethylation (47). These data suggest that ancestral exposure to DDE alters the paternally transcribed Igf2 gene and maternally transcribed H19 gene, while altering sperm function in a transgenerational manner. Furthermore, another study by Song and Yang (37) with the same study design found that ancestral DDE (100 mg/kg) exposure decreased elongated spermatids in seminiferous tubules and decreased the concentration of mobile sperm in the F3 generation of Sprague-Dawley rats (37). This impaired spermatogenesis may be due to the transgenerational sperm DNA hypomethylation of H19 and Gtl2 retained in the somatic cells (37). Additionally, ancestral exposure to DDE (100 mg/kg) decreased the male fertility index for males paired with females and increased the ratio of females to males in the F3 generation of Sprague-Dawley rats (37). This work should be expanded by examining doses that are more relevant to human exposure, administering doses in a way that mimics daily exposure, and exploring a wider range of exposure windows.
Vinclozolin is a fungicide that is used on fruits, vegetables, ornamental plants, and turfgrass (92). With vinclozolin being used on so many foodstuffs, humans are often exposed through a contaminated diet (93). This is of concern because dietary exposure to vinclozolin is estimated to be 40 ng/kg/d in the United States population (94), and vinclozolin is known to be antiandrogenic (95). Furthermore, animal studies have shown that vinclozolin can affect reproduction in male Sprague-Dawley rats by altering sperm function (82).
Studies have examined the transgenerational effects of high doses of vinclozolin on reproduction in male and female rodents (Tables 1 and 2). A study by Anway et al. (48) found that ancestral exposure to vinclozolin (100 mg/kg/d) via daily IP injections from embryonic days 8 until 15 decreased sperm number and forward motility in sperm, leading to male infertility in the F3 generation of Sprague-Dawley rats. Anway et al. (48) also suggested that this phenotype could be caused by the altered DNA methylation patterns discovered in the male germline. Additionally, Anway et al. (38) found that ancestral exposure to vinclozolin (100 mg/kg/d) via IP injections from embryonic days 8 until 14 decreased the ratio of males to females in the F3 generation of Sprague-Dawley rats. Vinclozolin (100 mg/kg/d) also reduced spermatogenetic capacity in the F3 and F4 generations of Sprague-Dawley rats (38). Furthermore, ancestral exposure to vinclozolin (100 mg/kg BW/d) from gestational day 8 until 14 via IP injections increased the occurrence of cystic ovaries in the F3 generation of Sprague-Dawley rats (36). Furthermore, studies indicate that vinclozolin promoted epigenetic transgenerational inheritance by causing changes to DNA methylation, noncoding RNA expression, and mRNA expression in ovarian granulosa cells from young female rats in the F3 generation, which may contribute to the formation of cystic ovaries (36). Additionally, ancestral exposure to vinclozolin (100 or 200 mg/kg/d) via IP injections from embryonic days 7 until 13 increased testis disease (100 mg/kg/d), which was associated with a loss of spermatogenic activity, reduction in germ cells per tubule cross-section, and increased number of tubules with no spermatogenic cells (azoospermia) in male CD-1 mice (39). Moreover, vinclozolin (200 mg/kg/d) increased the percentage of females with ovarian cysts, but it decreased the percentage of motile epididymal sperm (200 mg/kg/d) and number of caudal epididymal sperm (200 mg/kg/d) in CD-1 mice in the F3 generation (39). Analysis of the F3 generation sperm epigenome identified differential DNA methylation regions that may suggest a role of epigenetic modifications in the germline that could explain the transgenerational phenotypes observed in the study (39). Although many transgenerational effects on vinclozolin have been observed, some studies have not observed the same transgenerational effects (96, 97). Therefore, future studies are needed to resolve the discrepant findings between studies.
Studies have also examined the transgenerational effects of mixtures containing pesticides on reproduction in females and males (Tables 1 and 2). Nilsson et al. (40) showed that ancestral exposure to vinclozolin, pesticides (permethrin and DEET), plasticizers (DEHP, DBP, and BPA), plasticizers (half of doses for DEHP, DBP, and BPA), dioxin, or jet fuel affected ovarian morphology and histology in female Sprague-Dawley rats. These chemicals decreased follicle counts (all treatments), increased the number of small ovarian cysts (all treatments), increased the number of large cysts (vinclozolin, pesticide, plasticizers, and jet fuel treatments), and decreased large antral follicles (pesticides only) at 1 year of age in the F3 generation of Sprague-Dawley rats (40). After examining the effects of vinclozolin in greater detail, it was found that vinclozolin alters expression of genes involved in lipid metabolism and steroid precursor synthesis in the epigenome and transcriptome of granulosa cells from ovarian follicles in the F3 generation, and that these alterations in gene expression have been shown to potentially be involved in the pathology of polycystic ovarian disease (40). In a study with a similar study design except for the inclusion of vinclozolin, Manikkam et al. (11) found that the mixture caused an early onset of puberty (plastic, plasticizers, dioxin, and jet fuel treatments), increased anogenital index (plasticizers, plasticizers, dioxin, and jet fuel treatments), decreased the number of ovarian follicles per section (all treatments), decreased primordial follicle numbers (all treatments), and decreased preantral follicle numbers (dioxin and jet fuel) in the F3 generation of female Sprague-Dawley rats. When examining males in this same study, these chemicals increased anogenital index (plasticizers and dioxin), caused an early onset of puberty (plasticizers and dioxin), and decreased testosterone levels (plasticizers, dioxin, and jet fuel) in the F3 generation of Sprague-Dawley rats (11). These studies are very important for understanding how mixtures of chemicals affect reproduction, but, importantly, note that some mixtures included high doses that may not be relevant to daily human exposure. Thus, future studies should be conducted using environmentally relevant doses of the mixtures.
Persistent Environmental Contaminants
Some endocrine disruptors are known to persist in our environment for long periods of time due to their insolubility and lipophilicity, leading to their ability to remain in soil. This persistence in the environment leads to human exposure daily through the food chain (98, 99). Some environmental contaminants that are considered to be EDCs include PCBs and TCDD.
PCBs are a group of industrial chemicals that were mass produced from the 1920s until they were banned in 1979 by the Toxic Substances Control Act owing to their toxicity (98). They were used in many products such as plasticizers in rubber and resins, carbonless copy paper, wax extenders, inks, hydraulic fluids, and lubricants (98). Almost 1.5 million tons of these chemicals were produced, and although their production was banned, they still persist in our environment today (100). PCBs are chemically and thermally stable and lipophilic in nature, allowing them to be highly resistant to degradation, so they can be readily found in soils and bodies of water, leading to bioaccumulation in cells and the food chain (98, 99, 101). The mean dietary exposure to PCBs in a cohort of 36,759 Swedish men was determined to be 3.3 ng/kg BW/d (102).
PCBs have been shown to affect fertility in males and females. In women who were offspring of fish eaters, in utero PCB exposure was associated with decreased fecundability (103). In human sperm, PCBs have been shown to decrease sperm motility and affect the fertilization potential of sperm (104). With rodents, exposure to PCBs (1, 10, and 100 µg/kg/d) in chow decreased gonad weight in both male and female mice and it decreased the diameter of the seminiferous tubules in male CD-1 mice (49). Furthermore, lactational PCB (1, 2, and 5 mg/kg BW/d) exposure decreased testis weight, anogenital distance, testosterone levels, and Leydig cells in male Wistar rats (105). In utero exposure to PCBs (2.5 mg/kg/d) decreased numbers of preantral and antral follicles and increased atresia in female Long-Evans rats (106).
PCBs have been shown to cause transgenerational effects in male and female rodents (Tables 1 and 2). Ancestral exposure to PCBs (1 mg/kg) via IP injections on embryonic days 16 and 18 decreased the anogenital index of females in the F3 maternal line at PNDs 7 and 14 in Sprague-Dawley rats (41). Furthermore, at PND 60, PCBs (1 mg/kg) increased estradiol levels in the maternal and paternal lines and progesterone in the maternal line of rats in the F3 generation (41). Similarly, Pocar et al. (49) found that ancestral exposure to PCB chow (10 and 100 µg/kg/d) during pregnancy and lactation increased the seminiferous tubule distribution, and it (1, 10, 100 µg/kg/d) decreased sperm viability in ∼3-month-old male CD-1 mice in the F3 generation. In contrast, PCB ancestral exposure did not significantly change any reproductive outcomes in the F3 generation female CD-1 mice (49). Importantly, note that the doses chosen for these studies exceed normal dietary intake levels of PCBs, which are estimated to be 3.3 ng/kg BW/d. Thus, future studies should test whether doses that mimic human exposure generate similar effects.
Dioxins are a group of chlorinated organic chemicals that are unfortunate byproducts of manufacturing processes, including pesticide manufacturing, chlorine bleaching of paper pulp, combustion, and waste incineration (107). One of the most toxic dioxins is TCDD. Dioxins including TCDD are strongly lipophilic and insoluble, leading them to concentrate in sediments and attach to microscopic plants and animals, which are eaten by larger animals, and eventually they become a part of our food chain (107, 108). Estimated daily exposure to TCDD is ∼0.1 to 0.3 pg/kg/d (107). Humans were also exposed to TCDD as a result of a chemical explosion on 10 July 1976 in Seveso, Italy, leading to the highest levels of TCDD exposure in a residential population (109). In women from the Seveso Women’s Health Study, dose-related increases in serum TCDD levels were associated with increased risk of infertility (110), but TCDD exposure was not associated with quality of ovarian function (111). TCDD exposure in Seveso during infancy and TCDD exposure through breast milk reduced sperm count, motility, and concentration in male offspring (112, 113). TCDD has also been shown to affect reproduction in rodents. Specifically, TCDD impaired Sertoli cell function in male Wistar rats (114) and decreased estradiol levels in female Sprague-Dawley rats (115).
Many studies have focused on the transgenerational effects of TCDD on male reproduction (Table 2). In one study, ancestral exposure to TCDD (100 ng/kg BW/d) via IP injections from gestational days 8 to 14 increased testosterone levels in 1-year-old Sprague-Dawley rats in the F3 generation (42). In contrast, in a study by Sanabria et al. (116) in which rats were ancestrally exposed to TCDD (1 µg/kg) via oral gavage on gestational day 15 only, TCDD did not affect testosterone levels in the F3 generation of male Wistar rats. Results between these studies may differ due to different exposure routes and length of exposure. Another study on the ancestral effects of TCDD exposure (10 µg/kg) on embryonic day 15.5 showed that TCDD decreased normal sperm morphology and caused sperm tail defects in a nonsignificant manner in the F3 generation of C57BL/6 mice (43). Moreover, ancestral exposure to TCDD (1 µg/kg) reduced the ability of F3 males to get females pregnant and it decreased gestation length among females that were pregnant in mice (43). Although TCDD exposure causes transgenerational effects on reproduction, little is known about the underlying mechanisms. Thus, future studies should focus on elucidating the TCDD-induced epigenetic/transgenerational mechanisms of inheritance.
Studies also have focused on the transgenerational effects of TCDD on female reproduction (Table 1). In one study, ancestral exposure to TCDD (100 ng/kg BW/d) via IP injections from gestational day 8 until 14 increased pubertal abnormalities, the incidence of primordial follicle loss, and polycystic ovary disease at 1 year of age in Sprague-Dawley rats (42). Analysis of the F3 generation sperm epigenome discovered epimutations that affected genes in pathways, including ribosome pathway, chemokine signaling pathway, and natural killer cell–mediated toxicity, which may relate to the disease phenotype of polycystic ovary disease observed in this study (42). Furthermore, Bruner-Tran et al. (44) found that ancestral exposure to TCDD (10 µg/kg) via oral gavage on embryonic day 15.5 caused adenomyosis, a condition that has symptoms similar to endometriosis such as reduced fertility, pelvic pain, heavy bleeding, and dysmenorrhea in female C57BL/6 mice in the F3 generation. In addition, TCDD (10 µg/kg) increased infertility and the incidence of preterm birth in female C57BL/6 mice in the F3 generation (43). Although these studies found that TCDD caused negative outcomes on reproduction, the doses used in these studies surpass the estimated daily exposure to TCDD. Moreover, these studies do not use exposure routes that are comparable to daily human exposure. Thus, future studies are needed that use relevant doses and routes of exposure.
Conclusion
Men and women are exposed to many EDCs, including phthalates, BPA, pesticides, and persistent environmental contaminants such as PCBs and TCDD on a daily basis. This is concerning because EDCs are known to affect normal reproduction. This mini-review summarizes that these EDCs negatively affect male and female fertility in a transgenerational manner. Animal studies show that in females, ancestral EDC exposure can alter litter size, alter anogenital distance, cause early puberty, disrupt estrous cyclicity, alter follicle numbers, cause early reproductive aging, decrease fertility, increase cysts in ovaries, alter sex ratios in pups, alter sex steroid hormone levels, and cause adenomyosis (Table 1). Furthermore, in males, ancestral EDC exposure can alter anogenital distance, cause early puberty, decrease fertility, cause testes disease, decrease sperm count and motility, alter sperm morphology, and alter sex steroid hormone levels (Table 2).
The mechanisms for how these chemicals affect reproduction, especially in a transgenerational manner, are not fully understood. More studies are needed to understand how these chemicals affect reproduction in both males and females, and how these effects occur over multiple generations. Furthermore, studies are necessary to understand transgenerational exposure through the male line. Note that future studies should be reproduced with different rodent strains to observe whether effects vary among species and strain. Studies on transgenerational effects of EDCs on humans are needed to understand how these chemicals affect human reproduction in comparison with animal studies. Additionally, many studies in this review have wide dose ranges that are not always relevant to human exposure. Therefore, more studies are needed with relevant dose ranges and mixtures of chemicals to compare with daily human exposure because humans are exposed to mixtures of chemicals on a daily basis.
Acknowledgments
The authors thank Catheryne Chiang for artwork.
Financial Support: This work was supported by National Institutes of Health/National Institute of Environmental Health Sciences Grants P01 ES022848 and ES028661 (to J.A.F.) and by US Environmental Protection Agency Grant RD-83459301 (to J.A.F.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- BPA
bisphenol A
- BW
body weight
- BzBP
benzyl butyl phthalate
- DBP
dibutyl phthalate
- DDE
dichlorodiphenoxydichloroethylene
- DDT
dichlorodiphenyltrichloroethane
- DEHP
di(2-ethylhexyl) phthalate
- DEP
diethyl phthalate
- DiBP
diisobutyl phthalate
- DiNP
di-isononyl phthalate
- EDC
endocrine-disrupting chemical
- MXC
methoxychlor
- PCB
polychlorinated biphenyl
- PND
postnatal day
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
References and Notes
- 1. Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, Woodruff TJ, Vom Saal FS. Endocrine-disrupting chemicals and public health protection: a statement of principles from the Endocrine Society. Endocrinology. 2012;153(9):4097–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT. EDC-2: the Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36(6):E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33(3):378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vandenberg LN. Low-dose effects of hormones and endocrine disruptors. Vitam Horm. 2014;94:129–165. [DOI] [PubMed] [Google Scholar]
- 5. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hannon PR, Flaws JA. The effects of phthalates on the ovary. Front Endocrinol (Lausanne). 2015;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel S, Zhou C, Rattan S, Flaws JA. Effects of endocrine-disrupting chemicals on the ovary. Biol Reprod. 2015;93(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rattan S, Zhou C, Chiang C, Mahalingam S, Brehm E, Flaws JA. Exposure to endocrine disruptors during adulthood: consequences for female fertility. J Endocrinol. 2017;233(3):R109–R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sweeney MF, Hasan N, Soto AM, Sonnenschein C. Environmental endocrine disruptors: effects on the human male reproductive system. Rev Endocr Metab Disord. 2015;16(4):341–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sidorkiewicz I, Zaręba K, Wołczyński S, Czerniecki J. Endocrine-disrupting chemicals—mechanisms of action on male reproductive system. Toxicol Ind Health. 2017;33(7):601–609. [DOI] [PubMed] [Google Scholar]
- 11. Manikkam M, Guerrero-Bosagna C, Tracey R, Haque MM, Skinner MK. Transgenerational actions of environmental compounds on reproductive disease and identification of epigenetic biomarkers of ancestral exposures. PLoS One. 2012;7(2):e31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen J, Wu S, Wen S, Shen L, Peng J, Yan C, Cao X, Zhou Y, Long C, Lin T, He D, Hua Y, Wei G. The mechanism of environmental endocrine disruptors (DEHP) induces epigenetic transgenerational inheritance of cryptorchidism [published correction appears in PLoS One. 2015;10(7):e0132749]. PLoS One. 2015;10(6):e0126403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou C, Gao L, Flaws JA. Exposure to an environmentally relevant phthalate mixture causes transgenerational effects on female reproduction in mice. Endocrinology. 2017;158(6):1739–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Skinner MK. What is an epigenetic transgenerational phenotype? F3 or F2. Reprod Toxicol. 2008;25(1):2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Skinner MK. Environmental stress and epigenetic transgenerational inheritance. BMC Med. 2014;12(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xin F, Susiarjo M, Bartolomei MS. Multigenerational and transgenerational effects of endocrine disrupting chemicals: a role for altered epigenetic regulation? Semin Cell Dev Biol. 2015;43:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Skinner MK. Endocrine disruptors in 2015: epigenetic transgenerational inheritance. Nat Rev Endocrinol. 2016;12(2):68–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Skinner MK. Endocrine disruptor induction of epigenetic transgenerational inheritance of disease. Mol Cell Endocrinol. 2014;398(1–2):4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schübeler D. Function and information content of DNA methylation. Nature. 2015;517(7534):321–326. [DOI] [PubMed] [Google Scholar]
- 20. Walker DM, Gore AC. Epigenetic impacts of endocrine disruptors in the brain. Front Neuroendocrinol. 2017;44:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Campbell M, Kung HJ, Izumiya Y. Long non-coding RNA and epigenetic gene regulation of KSHV. Viruses. 2014;6(11):4165–4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peschansky VJ, Wahlestedt C. Non-coding RNAs as direct and indirect modulators of epigenetic regulation. Epigenetics. 2014;9(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab. 2010;21(4):214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCarthy MM, Rissman EF. Epigenetics of Reproduction. In: Plant TM, Zeleznik AJ, eds. Knobil and Neill’s Physiology of Reproduction. 4th ed.San Diego, CA: Academic Press; 2015:2439–2501. [Google Scholar]
- 25. Meltzer D, Martinez-Arguelles DB, Campioli E, Lee S, Papadopoulos V. In utero exposure to the endocrine disruptor di(2-ethylhexyl) phthalate targets ovarian theca cells and steroidogenesis in the adult female rat. Reprod Toxicol. 2015;51:47–56. [DOI] [PubMed] [Google Scholar]
- 26. Rattan S, Brehm E, Gao L, Flaws JA. Di(2-ethylhexyl) phthalate exposure during prenatal development causes adverse transgenerational effects on female fertility in mice. Toxicol Sci. 2018;163(2):420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pocar P, Fiandanese N, Berrini A, Secchi C, Borromeo V. Maternal exposure to di(2-ethylhexyl)phthalate (DEHP) promotes the transgenerational inheritance of adult-onset reproductive dysfunctions through the female germline in mice. Toxicol Appl Pharmacol. 2017;322:113–121. [DOI] [PubMed] [Google Scholar]
- 28. Rattan S, Brehm E, Gao L, Niermann S, Flaws JA. Prenatal exposure to di(2-ethylhexyl) phthalate disrupts ovarian function in a transgenerational manner in female mice. Biol Reprod. 2018;98(1):130–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brehm E, Rattan S, Gao L, Flaws JA. Prenatal exposure to di(2-ethylhexyl) phthalate causes long-term transgenerational effects on female reproduction in mice. Endocrinology. 2018;159(2):795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berger A, Ziv-Gal A, Cudiamat J, Wang W, Zhou C, Flaws JA. The effects of in utero bisphenol A exposure on the ovaries in multiple generations of mice. Reprod Toxicol. 2016;60:39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ziv-Gal A, Wang W, Zhou C, Flaws JA. The effects of in utero bisphenol A exposure on reproductive capacity in several generations of mice. Toxicol Appl Pharmacol. 2015;284(3):354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One. 2013;8(1):e55387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McBirney M, King SE, Pappalardo M, Houser E, Unkefer M, Nilsson E, Sadler-Riggleman I, Beck D, Winchester P, Skinner MK. Atrazine induced epigenetic transgenerational inheritance of disease, lean phenotype and sperm epimutation pathology biomarkers. PLoS One. 2017;12(9):e0184306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Manikkam M, Haque MM, Guerrero-Bosagna C, Nilsson EE, Skinner MK. Pesticide methoxychlor promotes the epigenetic transgenerational inheritance of adult-onset disease through the female germline. PLoS One. 2014;9(7):e102091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Skinner MK, Manikkam M, Tracey R, Guerrero-Bosagna C, Haque M, Nilsson EE. Ancestral dichlorodiphenyltrichloroethane (DDT) exposure promotes epigenetic transgenerational inheritance of obesity. BMC Med. 2013;11(1):228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nilsson E, Klukovich R, Sadler-Riggleman I, Beck D, Xie Y, Yan W, Skinner MK. Environmental toxicant induced epigenetic transgenerational inheritance of ovarian pathology and granulosa cell epigenome and transcriptome alterations: ancestral origins of polycystic ovarian syndrome and primary ovarian insufiency. Epigenetics. 2018;13(8):875–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Song Y, Yang L. Transgenerational impaired spermatogenesis with sperm H19 and Gtl2 hypomethylation induced by the endocrine disruptor p,p′-DDE. Toxicol Lett. 2018;297:34–41. [DOI] [PubMed] [Google Scholar]
- 38. Anway MD, Memon MA, Uzumcu M, Skinner MK. Transgenerational effect of the endocrine disruptor vinclozolin on male spermatogenesis. J Androl. 2006;27(6):868–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guerrero-Bosagna C, Covert TR, Haque MM, Settles M, Nilsson EE, Anway MD, Skinner MK. Epigenetic transgenerational inheritance of vinclozolin induced mouse adult onset disease and associated sperm epigenome biomarkers. Reprod Toxicol. 2012;34(4):694–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nilsson E, Larsen G, Manikkam M, Guerrero-Bosagna C, Savenkova MI, Skinner MK. Environmentally induced epigenetic transgenerational inheritance of ovarian disease. PLoS One. 2012;7(5):e36129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mennigen JA, Thompson LM, Bell M, Tellez Santos M, Gore AC. Transgenerational effects of polychlorinated biphenyls: 1. Development and physiology across 3 generations of rats. Environ Health. 2018;17(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Dioxin (TCDD) induces epigenetic transgenerational inheritance of adult onset disease and sperm epimutations. PLoS One. 2012;7(9):e46249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bruner-Tran KL, Ding T, Yeoman KB, Archibong A, Arosh JA, Osteen KG. Developmental exposure of mice to dioxin promotes transgenerational testicular inflammation and an increased risk of preterm birth in unexposed mating partners. PLoS One. 2014;9(8):e105084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bruner-Tran KL, Duleba AJ, Taylor HS, Osteen KG. Developmental toxicant exposure is associated with transgenerational adenomyosis in a murine model. Biol Reprod. 2016;95(4):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yuan B, Wu W, Chen M, Gu H, Tang Q, Guo D, Chen T, Chen Y, Lu C, Song L, Xia Y, Chen D, Rehan VK, Sha J, Wang X. From the cover: metabolomics reveals a role of betaine in prenatal DBP exposure-induced epigenetic transgenerational failure of spermatogenesis in rats. Toxicol Sci. 2017;158(2):356–366. [DOI] [PubMed] [Google Scholar]
- 46. Doyle TJ, Bowman JL, Windell VL, McLean DJ, Kim KH. Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice. Biol Reprod. 2013;88(5):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Song Y, Wu N, Wang S, Gao M, Song P, Lou J, Tan Y, Liu K. Transgenerational impaired male fertility with an Igf2 epigenetic defect in the rat are induced by the endocrine disruptor p,p′-DDE. Hum Reprod. 2014;29(11):2512–2521. [DOI] [PubMed] [Google Scholar]
- 48. Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308(5727):1466–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pocar P, Fiandanese N, Secchi C, Berrini A, Fischer B, Schmidt JS, Schaedlich K, Rhind SM, Zhang Z, Borromeo V. Effects of polychlorinated biphenyls in CD-1 mice: reproductive toxicity and intergenerational transmission. Toxicol Sci. 2012;126(1):213–226. [DOI] [PubMed] [Google Scholar]
- 50. Schettler T. Human exposure to phthalates via consumer products. Int J Androl. 2006;29(1):134–139. [DOI] [PubMed] [Google Scholar]
- 51. Blount BC, Milgram KE, Silva MJ, Malek NA, Reidy JA, Needham LL, Brock JW. Quantitative detection of eight phthalate metabolites in human urine using HPLC-APCI-MS/MS. Anal Chem. 2000;72(17):4127–4134. [DOI] [PubMed] [Google Scholar]
- 52. Braun JM, Sathyanarayana S, Hauser R. Phthalate exposure and children’s health. Curr Opin Pediatr. 2013;25(2):247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. National Research Council (US) Committee on the Health Risks of Phthalates. Phthalates and Cumulative Risk Assessment: The Tasks Ahead. Washington, DC: The National Academies Press; 2008. [PubMed] [Google Scholar]
- 54. Rudel RA, Camann DE, Spengler JD, Korn LR, Brody JG. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ Sci Technol. 2003;37(20):4543–4553. [DOI] [PubMed] [Google Scholar]
- 55. Kavlock R, Boekelheide K, Chapin R, Cunningham M, Faustman E, Foster P, Golub M, Henderson R, Hinberg I, Little R, Seed J, Shea K, Tabacova S, Tyl R, Williams P, Zacharewski T. NTP Center for the Evaluation of Risks to Human Reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod Toxicol. 2002;16(5):529–653. [DOI] [PubMed] [Google Scholar]
- 56. Koch HM, Calafat AM. Human body burdens of chemicals used in plastic manufacture. Philos Trans R Soc Lond B Biol Sci. 2009;364(1526):2063–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hines CJ, Hopf NB, Deddens JA, Silva MJ, Calafat AM. Occupational exposure to diisononyl phthalate (DiNP) in polyvinyl chloride processing operations. Int Arch Occup Environ Health. 2012;85(3):317–325. [DOI] [PubMed] [Google Scholar]
- 58. Babich MA, Chen SB, Greene MA, Kiss CT, Porter WK, Smith TP, Wind ML, Zamula WW. Risk assessment of oral exposure to diisononyl phthalate from children’s products. Regul Toxicol Pharmacol. 2004;40(2):151–167. [DOI] [PubMed] [Google Scholar]
- 59. Thomsen AM, Riis AH, Olsen J, Jönsson BA, Lindh CH, Hjollund NH, Jensen TK, Bonde JP, Toft G. Female exposure to phthalates and time to pregnancy: a first pregnancy planner study. Hum Reprod. 2017;32(1):232–238. [DOI] [PubMed] [Google Scholar]
- 60. Ziv-Gal A, Gallicchio L, Chiang C, Ther SN, Miller SR, Zacur HA, Dills RL, Flaws JA. Phthalate metabolite levels and menopausal hot flashes in midlife women. Reprod Toxicol. 2016;60:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Axelsson J, Rylander L, Rignell-Hydbom A, Jönsson BA, Lindh CH, Giwercman A. Phthalate exposure and reproductive parameters in young men from the general Swedish population. Environ Int. 2015;85:54–60. [DOI] [PubMed] [Google Scholar]
- 62. Zhou C, Flaws JA. Effects of an environmentally relevant phthalate mixture on cultured mouse antral follicles. Toxicol Sci. 2017;156(1):217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Barakat R, Lin PP, Rattan S, Brehm E, Canisso IF, Abosalum ME, Flaws JA, Hess R, Ko C. Prenatal exposure to DEHP induces premature reproductive senescence in male mice. Toxicol Sci. 2017;156(1):96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Prins GS, Patisaul HB, Belcher SM, Vandenberg LN. CLARITY-BPA academic laboratory studies identify consistent low-dose bisphenol A effects on multiple organ systems [published online ahead of print 12 September 2018]. Basic Clin Pharmacol Toxicol. doi: 10.1111/bcpt.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA). Reprod Toxicol. 2007;24(2):139–177. [DOI] [PubMed] [Google Scholar]
- 66. Mendonca K, Hauser R, Calafat AM, Arbuckle TE, Duty SM. Bisphenol A concentrations in maternal breast milk and infant urine. Int Arch Occup Environ Health. 2014;87(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17(11):2839–2841. [DOI] [PubMed] [Google Scholar]
- 68. Mahalingam S, Ther L, Gao L, Wang W, Ziv-Gal A, Flaws JA. The effects of in utero bisphenol A exposure on ovarian follicle numbers and steroidogenesis in the F1 and F2 generations of mice. Reprod Toxicol. 2017;74:150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang W, Hafner KS, Flaws JA. In utero bisphenol A exposure disrupts germ cell nest breakdown and reduces fertility with age in the mouse. Toxicol Appl Pharmacol. 2014;276(2):157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wisniewski P, Romano RM, Kizys MM, Oliveira KC, Kasamatsu T, Giannocco G, Chiamolera MI, Dias-da-Silva MR, Romano MA. Adult exposure to bisphenol A (BPA) in Wistar rats reduces sperm quality with disruption of the hypothalamic–pituitary–testicular axis. Toxicology. 2015;329:1–9. [DOI] [PubMed] [Google Scholar]
- 71. Hass U, Christiansen S, Boberg J, Rasmussen MG, Mandrup K, Axelstad M. Low-dose effect of developmental bisphenol A exposure on sperm count and behaviour in rats. Andrology. 2016;4(4):594–607. [DOI] [PubMed] [Google Scholar]
- 72. Kim KH, Kabir E, Jahan SA. Exposure to pesticides and the associated human health effects. Sci Total Environ. 2017;575:525–535. [DOI] [PubMed] [Google Scholar]
- 73. US Environmental Protection Agency. Pesticides industry sales and usage 2008–2012 market estimates. Available at: https://www.epa.gov/pesticides/pesticides-industry-sales-and-usage-2008-2012-market-estimates. Accessed 28 November 2018.
- 74. Agency for Toxic Substances and Disease Registry. Toxicological profile for atrazine. Available at: https://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=338&tid=59. Accessed 29 November 2018.
- 75. Wirbisky SE, Freeman JL. Atrazine exposure and reproductive dysfunction through the hypothalamus-pituitary-gonadal (HPG) axis. Toxics. 2015;3(4):414–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cragin LA, Kesner JS, Bachand AM, Barr DB, Meadows JW, Krieg EF, Reif JS. Menstrual cycle characteristics and reproductive hormone levels in women exposed to atrazine in drinking water. Environ Res. 2011;111(8):1293–1301. [DOI] [PubMed] [Google Scholar]
- 77. Davis LK, Murr AS, Best DS, Fraites MJ, Zorrilla LM, Narotsky MG, Stoker TE, Goldman JM, Cooper RL. The effects of prenatal exposure to atrazine on pubertal and postnatal reproductive indices in the female rat. Reprod Toxicol. 2011;32(1):43–51. [DOI] [PubMed] [Google Scholar]
- 78. Kniewald J, Jakominić M, Tomljenović A, Simić B, Romać P, Vranesić D, Kniewald Z. Disorders of male rat reproductive tract under the influence of atrazine. J Appl Toxicol. 2000;20(1):61–68. [PubMed] [Google Scholar]
- 79. US Department of Health and Human Services. Toxicological profile for methoxychlor. Available at: https://www.atsdr.cdc.gov/toxprofiles/tp47.pdf. Accessed 29 November 2018.
- 80. Golovleva LA, Polyakova AB, Pertsova RN, Finkelshtein ZI. The fate of methoxychlor in soils and transformation by soil microorganisms. J Environ Sci Health B. 1984;19(6):523–538. [DOI] [PubMed] [Google Scholar]
- 81. Badach H, Nazimek T, Kamińska IA. Pesticide content in drinking water samples collected from orchard areas in central Poland. Ann Agric Environ Med. 2007;14(1):109–114. [PubMed] [Google Scholar]
- 82. Quignot N, Arnaud M, Robidel F, Lecomte A, Tournier M, Cren-Olivé C, Barouki R, Lemazurier E. Characterization of endocrine-disrupting chemicals based on hormonal balance disruption in male and female adult rats. Reprod Toxicol. 2012;33(3):339–352. [DOI] [PubMed] [Google Scholar]
- 83. Aoyama H, Chapin RE. Reproductive toxicities of methoxychlor based on estrogenic properties of the compound and its estrogenic metabolite, hydroxyphenyltrichloroethane. Vitam Horm. 2014;94:193–210. [DOI] [PubMed] [Google Scholar]
- 84. Cohn BA, La Merrill M, Krigbaum NY, Yeh G, Park JS, Zimmermann L, Cirillo PM. DDT exposure in utero and breast cancer. J Clin Endocrinol Metab. 2015;100(8):2865–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Man YB, Chan JK, Wu SC, Wong CK, Wong MH. Dietary exposure to DDTs in two coastal cities and an inland city in China. Sci Total Environ. 2013;463-464:264–273. [DOI] [PubMed] [Google Scholar]
- 86. Turusov V, Rakitsky V, Tomatis L. Dichlorodiphenyltrichloroethane (DDT): ubiquity, persistence, and risks. Environ Health Perspect. 2002;110(2):125–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ben Rhouma K, Tébourbi O, Krichah R, Sakly M. Reproductive toxicity of DDT in adult male rats. Hum Exp Toxicol. 2001;20(8):393–397. [DOI] [PubMed] [Google Scholar]
- 88. Tiemann U. In vivo and in vitro effects of the organochlorine pesticides DDT, TCPM, methoxychlor, and lindane on the female reproductive tract of mammals: a review. Reprod Toxicol. 2008;25(3):316–326. [DOI] [PubMed] [Google Scholar]
- 89. Sevin S, Kuzukiran O, Yurdakok-Dikmen B, Tutun H, Aydin FG, Filazi A. Selected persistent organic pollutants levels in the Ankara River by months. Environ Monit Assess. 2018;190(12):705. [DOI] [PubMed] [Google Scholar]
- 90. Quan C, Shi Y, Wang C, Wang C, Yang K. p,p′-DDE damages spermatogenesis via phospholipid hydroperoxide glutathione peroxidase depletion and mitochondria apoptosis pathway. Environ Toxicol. 2016;31(5):593–600. [DOI] [PubMed] [Google Scholar]
- 91. Makita Y, Omura M, Ogata R. Effects of perinatal simultaneous exposure to tributyltin (TBT) and p,p′-DDE [1,1-dichloro-2,2-bis(p-chlorophenyl) ethylene) on male offspring of Wistar rats. J Toxicol Environ Health A. 2004;67(5):385–395. [DOI] [PubMed] [Google Scholar]
- 92. Gray LE Jr, Ostby J, Monosson E, Kelce WR. Environmental antiandrogens: low doses of the fungicide vinclozolin alter sexual differentiation of the male rat. Toxicol Ind Health. 1999;15(1–2):48–64. [DOI] [PubMed] [Google Scholar]
- 93. Eustache F, Mondon F, Canivenc-Lavier MC, Lesaffre C, Fulla Y, Berges R, Cravedi JP, Vaiman D, Auger J. Chronic dietary exposure to a low-dose mixture of genistein and vinclozolin modifies the reproductive axis, testis transcriptome, and fertility. Environ Health Perspect. 2009;117(8):1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. US Environmental Protection Agency. Reregistration eligibility decision (RED): vinclozolin. Available at: https://nepis.epa.gov/Exe/ZyPDF.cgi/2000083T.PDF?Dockey=2000083T.PDF. Accessed 28 January 2019.
- 95. Gray LE, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, Veeramachaneni DN, Wilson V, Price M, Hotchkiss A, Orlando E, Guillette L. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update. 2001;7(3):248–264. [DOI] [PubMed] [Google Scholar]
- 96. Schneider S, Kaufmann W, Buesen R, van Ravenzwaay B. Vinclozolin—the lack of a transgenerational effect after oral maternal exposure during organogenesis. Reprod Toxicol. 2008;25(3):352–360. [DOI] [PubMed] [Google Scholar]
- 97. Schneider S, Marxfeld H, Gröters S, Buesen R, van Ravenzwaay B. Vinclozolin—no transgenerational inheritance of anti-androgenic effects after maternal exposure during organogenesis via the intraperitoneal route. Reprod Toxicol. 2013;37:6–14. [DOI] [PubMed] [Google Scholar]
- 98. Grimm FA, Hu D, Kania-Korwel I, Lehmler HJ, Ludewig G, Hornbuckle KC, Duffel MW, Bergman Å, Robertson LW. Metabolism and metabolites of polychlorinated biphenyls. Crit Rev Toxicol. 2015;45(3):245–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Borja J, Taleon MD, Auresenia J, Gallardo S Polychlorinated biphenyls and their biodegradation. Process Biochem. 2005;40(6):1999–2013. [Google Scholar]
- 100. Abraham WR, Nogales B, Golyshin PN, Pieper DH, Timmis KN. Polychlorinated biphenyl-degrading microbial communities in soils and sediments. Curr Opin Microbiol. 2002;5(3):246–253. [DOI] [PubMed] [Google Scholar]
- 101. Kania-Korwel I, Lehmler HJ. Toxicokinetics of chiral polychlorinated biphenyls across different species—a review. Environ Sci Pollut Res Int. 2016;23(3):2058–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bergkvist C, Berglund M, Glynn A, Julin B, Wolk A, Åkesson A. Dietary exposure to polychlorinated biphenyls and risk of myocardial infarction in men—a population-based prospective cohort study. Environ Int. 2016;88:9–14. [DOI] [PubMed] [Google Scholar]
- 103. Han L, Hsu WW, Todem D, Osuch J, Hungerink A, Karmaus W. In utero exposure to polychlorinated biphenyls is associated with decreased fecundability in daughters of Michigan female fisheaters: a cohort study. Environ Health. 2016;15(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Jiang LG, Cheng LY, Kong SH, Yang Y, Shen YJ, Chen C, Deng XH, Liu SZ, Chao L. Toxic effects of polychlorinated biphenyls (Aroclor 1254) on human sperm motility. Asian J Androl. 2017;19(5):561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Thangavelu SK, Elaiyapillai SP, Ramachandran I, Bhaskaran RS, Jagadeesan A. Lactational exposure of polychlorinated biphenyls impair Leydig cellular steroidogenesis in F1 progeny rats. Reprod Toxicol. 2018;75:73–85. [DOI] [PubMed] [Google Scholar]
- 106. Baldridge MG, Stahl RL, Gerstenberger SL, Tripoli V, Hutz RJ. Modulation of ovarian follicle maturation in Long-Evans rats exposed to polychlorinated biphenyls (PCBs) in-utero and lactationally. Reprod Toxicol. 2003;17(5):567–573. [DOI] [PubMed] [Google Scholar]
- 107. Birnbaum LS. The mechanism of dioxin toxicity: relationship to risk assessment. Environ Health Perspect. 1994;102(Suppl 9):157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Marinković N, Pašalić D, Ferenčak G, Gršković B, Stavljenić Rukavina A. Dioxins and human toxicity. Arh Hig Rada Toksikol. 2010;61(4):445–453. [DOI] [PubMed] [Google Scholar]
- 109. Preliminary report: 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure to humans—Seveso, Italy. Arch Dermatol. 1989;125(3):329–330. [DOI] [PubMed] [Google Scholar]
- 110. Eskenazi B, Warner M, Marks AR, Samuels S, Needham L, Brambilla P, Mocarelli P. Serum dioxin concentrations and time to pregnancy. Epidemiology. 2010;21(2):224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Warner M, Eskenazi B, Olive DL, Samuels S, Quick-Miles S, Vercellini P, Gerthoux PM, Needham L, Patterson DG, Mocarelli P. Serum dioxin concentrations and quality of ovarian function in women of Seveso. Environ Health Perspect. 2007;115(3):336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mocarelli P, Gerthoux PM, Patterson DG Jr, Milani S, Limonta G, Bertona M, Signorini S, Tramacere P, Colombo L, Crespi C, Brambilla P, Sarto C, Carreri V, Sampson EJ, Turner WE, Needham LL. Dioxin exposure, from infancy through puberty, produces endocrine disruption and affects human semen quality. Environ Health Perspect. 2008;116(1):70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Mocarelli P, Gerthoux PM, Needham LL, Patterson DG Jr, Limonta G, Falbo R, Signorini S, Bertona M, Crespi C, Sarto C, Scott PK, Turner WE, Brambilla P. Perinatal exposure to low doses of dioxin can permanently impair human semen quality. Environ Health Perspect. 2011;119(5):713–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Aly HA, Khafagy RM. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD)-induced cytotoxicity accompanied by oxidative stress in rat Sertoli cells: possible role of mitochondrial fractions of Sertoli cells. Toxicol Appl Pharmacol. 2011;252(3):273–280. [DOI] [PubMed] [Google Scholar]
- 115. Valdez KE, Shi Z, Ting AY, Petroff BK. Effect of chronic exposure to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin in female rats on ovarian gene expression. Reprod Toxicol. 2009;28(1):32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sanabria M, Cucielo MS, Guerra MT, Dos Santos Borges C, Banzato TP, Perobelli JE, Leite GA, Anselmo-Franci JA, De Grava Kempinas W. Sperm quality and fertility in rats after prenatal exposure to low doses of TCDD: a three-generation study. Reprod Toxicol. 2016;65:29–38. [DOI] [PubMed] [Google Scholar]