Abstract
Objective
To perform a systematic literature review (SLR) informing the European Lmmendations for the management of antiphospholipid syndrome (APS) in adults.
Methods
A SLR through January 2018 was performed. Research questions were constructed using the Patient, Intervention, Comparator, Outcome (PICO) format. We included data from articles that reported on each relevant intervention. Summary effect estimates were calculated for direct comparison studies that matched the PICO question exactly, and for studies with the relevant intervention and comparator. When meta-analyses were available, we used these estimates.
Results
From 7534 retrieved articles (+15 from hand searches), 188 articles were included in the review. In individuals with high-risk antiphospholipid antibody (aPL) profile without prior thrombotic or obstetric APS, two meta-analyses showed a protective effect of low-dose aspirin (LDA) against thrombosis. Two randomised controlled trials (RCTs) and three cohort studies showed no additional benefit of treatment with vitamin K antagonists at target international normalised ratio (INR) 3–4 versus INR 2–3 in patients with venous thrombosis. In patients with arterial thrombosis, two RCTs and two cohort studies showed no difference in risk of recurrent thrombosis between the two target INR groups. One open-label trial showed higher rates of thrombosis recurrences in triple aPL-positive patients treated with rivaroxaban than those treated with warfarin. RCTs and cohort studies showed that combination treatment with LDA and heparin was more effective than LDA alone in several types of obstetric APS. SLR results were limited by the indirect evidence and the heterogeneity of patient groups for some treatments, and only a few high-quality RCTs.
Conclusion
Well-designed studies of homogeneous APS patient populations are needed.
Keywords: antiphospholipid syndrome, systemic lupus erythematosus, thrombosis, pregnancy morbidity, management, systematic literature review, recommendations
Key messages.
What is already known about this subject?
EULAR has issued the 2019 recommendations for the management of antiphospholipid syndrome in adults.
What does this study add?
This is a systematic literature review of the available published evidence on primary and secondary prevention of thrombotic antiphospholipid syndrome and the management of obstetric antiphospholipid syndrome.
How might this impact on clinical practice?
This systematic literature review informed the task force for the ‘EULAR recommendations for the management of antiphospholipid syndrome in adults’ that will help guide practice for physicians from several medical specialties involved in the management of the syndrome.
Introduction
Antiphospholipid syndrome (APS) is a rare rheumatic and musculoskeletal disease characterised by recurrent arterial or venous thrombosis or pregnancy morbidity in association with persistent antiphospholipid antibodies (aPLs).1 The major goal of the management of APS is the prevention of first or recurrent thrombotic or obstetric complications. Oral anticoagulation with vitamin K antagonists (VKAs) is the cornerstone of the treatment of thrombotic APS; however, several aspects of its use such as the intensity of treatment, and the efficacy and safety of alternative treatments, remain controversial. Additionally, although combination treatment with low-dose aspirin (LDA) and heparin has been commonly used in pregnant women with recurrent pregnancy losses and those with prior thrombotic APS, use of this combination in different clinical expressions of obstetric APS is still debated.
Here, we report the results of the systematic literature review (SLR) that informed the ‘EULAR recommendations for the management of antiphospholipid syndrome in adults’,2 focused on primary and secondary prevention of thrombotic APS and the management of obstetric APS. We do not include here studies of thrombotic risk stratification and modification, because these informed the overarching principles, and studies of catastrophic APS, because these focused on precipitating factors.2
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.3 PubMed, Embase and Cochrane Library were used as data sources for the SLR of English-language published articles until 31 January 2018. The search terms for risk factors, patient education, primary prevention, management of thrombotic and obstetric APS, and catastrophic APS are provided as supplementary material (online supplementary text S1). Research questions on the recommendation topics were structured according to the PICO format (Patients, Intervention, Comparator, Outcomes).4
rmdopen-2019-000924supp002.docx (36.1KB, docx)
The literature searches were performed by an informationist at the National Institutes of Health library, in consultation with the methodologist. All titles and abstracts were reviewed by two physicians in charge for literature review (LA, ML) and the full-text articles were independently reviewed by three persons: one literature reviewer, convenor (MGGT) and methodologist (MMW). Data abstraction was performed by the two literature reviewers and independently double-checked by the convenor and methodologist.
We included data from either clinical trials or observational studies that reported on each relevant intervention, regardless of whether a comparator was used. We categorised primary studies into four groups based on how closely they corresponded to the PICO question. Direct comparison studies compared the two treatment alternatives specified in the PICO in the relevant population (ie, the P, I, C and O were correct). Indirect comparison studies compared the treatment alternatives of the PICO but not specifically in the relevant population (ie, P with some modification, correct I, C and O). Mixed treatment studies included a comparison but not exactly the treatment alternatives specified in the PICO (ie, either the I or C was tested but with some modification). Single treatment arm studies did not include a comparison but reported on one of the treatment alternatives specified in the PICO (ie, either the I or C was tested). Mixed treatment and single treatment arm studies provided only background information for the comparison.
Data analysis
For thrombotic APS, the outcomes were first or recurrent thrombosis and major bleeding. For obstetric APS, the main outcome was live birth. High-risk aPL profile was defined as the presence of any of the following: lupus anticoagulant, double or triple aPL positivity, or persistently high aPL titres. Low-risk aPL profile includes isolated anticardiolipin or anti-beta2 glycoprotein I antibodies at low-medium titres, particularly if transiently positive.2
Summary effect estimates were calculated separately for direct comparison studies that matched the PICO question exactly (ie, correct P, I, C and O), and for direct and indirect comparison studies combined. The former provided the most relevant evidence for the recommendations, while the latter provided evidence that was considered supportive. Summary effects were based on frequency of events in each treatment group and calculated as risk ratios. When primary studies (including meta-analyses) provided summary effect estimates, we used these estimates. In some instances, these were ORs or HRs. I² is a measure of heterogeneity of effects among studies, with a range of 0% (no heterogeneity) to 100% (high heterogeneity). The statistical software RevMan V.5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) was used.
We assessed the risk of bias of randomised controlled trials (RCTs) using the Cochrane tool.5 Summary evaluation of risk of bias was based on an overall assessment, with priority given to blinding and allocation concealment. We used the Newcastle-Ottawa tool to assess the quality of cohort studies.6 This tool outlines eight features of study design and execution that impact validity of the study results. We used established ratings of 0–3 stars, 4–6 stars and 7–9 stars to classify studies as low, intermediate or high quality.
Results
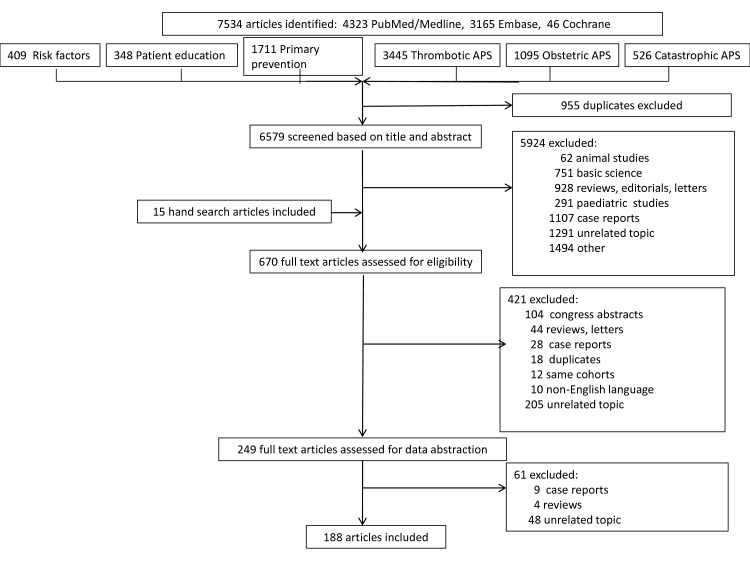
The number of retrieved articles at each step of the SLR is shown in figure 1. Articles on risk factors and patient education informing the overarching principles, and those on precipitating factors for catastrophic APS,2 are not included in this SLR. Detailed quality ratings of the included studies on primary prevention, and the management of thrombotic and obstetric APS are provided in online supplementary text S1.
Figure 1.
Flow chart of systematic literature review. APS, antiphospholipid syndrome.
Primary thromboprophylaxis with LDA in aPL-positive subjects
Asymptomatic aPL carriers (not fulfilling the classification criteria for thrombotic or obstetric APS) with a high-risk aPL profile with or without traditional risk factors
In a meta-analysis of studies of asymptomatic aPL carriers,7 most with high-risk aPL profiles, use of LDA was associated with a lower risk of thrombosis (table 1). Methodological quality of the primary studies was judged to be adequate overall, and there was no worrisome heterogeneity among study results as the directions were clear. In an individual-patient meta-analysis of five studies, LDA use was associated with a reduced risk of first thrombosis, although with a wide CI.8 Associations were stronger for protection from arterial events (HR 0.28; 95% CI 0.06 to 1.33) than venous events (HR 0.63; 95% CI 0.19 to 2.13). In an additional prospective cohort study of 119 asymptomatic aPL carriers followed for a mean of 9.1 years, LDA use was associated with lower odds of thrombosis, but the risk was not significantly different from no LDA use, likely due to limited power.9 Notably, 61% of patients had connective tissue disease. Methodological quality was intermediate.
Table 1.
Primary prevention of thrombosis with low-dose aspirin (LDA) in individuals with high-risk antiphospholipid antibody (aPL) profile, patients with systemic lupus erythematosus (SLE) and non-pregnant women with a history of obstetric antiphospholipid syndrome (APS) only
| Reference | Design | Comparison | Intervention | Control | Number of patients, intervention | Number of patients, control | Events, intervention | Events, control | Relative effect (95% CI) | Study quality |
| Asymptomatic individuals with high-risk aPL profile | ||||||||||
| Arnaud et al 20147 | Meta-analysis | Direct | LDA | No LDA treatment | 229 | 231 | 18 (7.8%) | 31 (13.4%) | OR 0.50 (0.25 to 0.99) | |
| Arnaud et al 20158 | Meta-analysis | Direct | LDA | No LDA treatment | 66 | 105 | NA | NA | HR 0.46 (0.17 to 1.20) | |
| Mustonen et al 20149 | Prospective cohort | Direct | LDA | No LDA treatment | 45 | 74 | 1 (2.2%) | 8 (10.8%) | OR 0.19 (0.02 to 1.56) | Intermediate quality |
| Patients with SLE and high-risk aPL profile | ||||||||||
| Arnaud et al 20147 | Meta-analysis | Direct | LDA | No LDA treatment | 261 | 179 | 24 (9.2%) | 30 (16.7%) | OR 0.55 (0.31 to 0.98) | |
| Arnaud et al 20158 | Meta-analysis | Direct | LDA | No LDA treatment | 109 | 83 | NA | NA | HR 0.47 (0.22 to 0.83) | |
| Patients with SLE and low-risk aPL profile | ||||||||||
| Tarr et al 200716 | Retrospective cohort | Direct | LDA | No LDA treatment | 52 | 29 | 1 (3.4%) | 2 (6.9%) | – | Intermediate quality |
| Tektonidou et al 200913 | Retrospective cohort | Direct | LDA | No LDA treatment | 87 | 57 | 11 (12.6%) | 18 (31.5%) | – | High quality |
| Total | Direct | 139 | 86 | 12 | 20 | RR 0.39 (0.20 to 0.74) I2=0% |
||||
| Non-pregnant women with a history of obstetric APS only (no prior thrombotic events), with or without SLE | ||||||||||
| Arnaud et al 20147 | Meta-analysis | Direct | LDA | No LDA treatment | 111 | 197 | 5 (4.5%) | 31 (15.7%) | OR 0.25 (0.10 to 0.62) | |
| Ruffatti et al 200921 | Retrospective cohort | Indirect | LDA | No LDA treatment | 130 | 231 | 8 (5.7%) | 22 (9.5%) | – | Intermediate quality |
Patients with systemic lupus erythematosus (SLE)
Patients with high-risk aPL profile
Pooled results of seven cohort studies10–16 and one RCT11 in a meta-analysis7 indicated lower risks of first thrombosis among patients treated with LDA compared with patients not treated with LDA. There was heterogeneity among studies (I2=47%) but the direction of effect was clear (table 1). Among three of these studies that reported on bleeding complications,11–13 no patients in either treatment group had major bleeding. Methodological quality of the primary studies was judged to be adequate overall. In five studies, all or a majority of patients had high-risk aPL profiles,10–12 14 15 while three primary studies included patients with low-risk and high-risk aPL profiles in unknown proportions.
A pooled analysis of individual-patient data from five studies (including four studies exclusively or predominately of patients with high risk profiles) showed a lower risk of first thrombosis in patients with SLE treated with LDA.8 Protection was similar for arterial events (HR 0.47) and venous events (HR 0.39), although the smaller number of events in these subgroups led to wide confidence limits. Data on bleeding complications were not reported in this meta-analysis.
A retrospective cohort study reported thrombotic events in 4% of patients with SLE treated with LDA alone, compared with 33% of those not treated with LDA or hydroxychloroquine (HCQ), after 8 years of follow-up.17 However, only 23% of patients had positive aPL.
Patients with low-risk aPL profile
Analysis of the two studies13 16 that included the smallest proportions of patients with high-risk profiles indicated a lower risk of first thrombosis among patients treated with LDA (risk ratio (RR) 0.39), with no heterogeneity (table 1). No bleeding events were reported in one study,13 while the other study did not comment on bleeding.16
Non-pregnant women with a history of obstetric APS only (no prior thrombotic events), with or without SLE
In five studies10 14 18–20 included in a meta-analysis7 that reported on primary prevention with LDA among women with a history of obstetric APS, use of LDA was associated with a lower risk of thrombosis (table 1). In two primary studies,10 14 patients with SLE comprised 24% and 27% of patients, and a third study18 reported 38% of women had secondary APS, so the results mainly pertain to women without SLE. Methodological quality of the primary studies was judged to be adequate overall. Indirect evidence was provided by a retrospective study of 370 patients with aPL, 57% of whom had obstetric APS.21 Long-term treatment with LDA in 130 patients (nine also with oral anticoagulation) was associated with a lower risk of first thrombosis (5.7% vs 9.5%), although the treatment results were not specific to the subset of women with past obstetric APS. Losses to follow-up were not reported in this study (table 1). Three studies22–24 reported outcomes of treatment with LDA without a comparison treatment arm (online supplementary table 1).
rmdopen-2019-000924supp001.docx (89.2KB, docx)
Secondary thromboprophylaxis in APS
Patients with definite APS and first venous thrombosis
Treatment with VKA at conventional versus high-intensity anticoagulation
One double-blind RCT with low risk of bias presented stratified results on patients with prior venous thrombosis specifically25 (table 2). Recurrent thrombosis was more frequent in those in the higher intensity (target international normalised ratio (INR) 3–4) warfarin group than in the INR 2–3 group over a mean follow-up of 2.6 years. However, in the group of patients with a target INR 2–3, the INR was above the target range 11% of the time, within range 71% of the time and below range 19% of the time. In the high-intensity group, the corresponding figures were 17%, 40% and 43%.
Table 2.
Studies of intensity of oral anticoagulation treatment in patients with antiphospholipid syndrome and previous venous thrombosis
| Reference | Design | Comparison | Percentage with venous thrombosis history | Intervention | Control | Number of patient-years, intervention | Number of patient-years, control | Events, intervention | Events, control | Relative effect (95% CI) | Study quality |
| Recurrent thrombosis | |||||||||||
| Crowther et al 200325 | RCT | Direct | 100 | Warfarin INR 3–4 | Warfarin INR 2–3 | 113 py | 119 py | 2.6/100 py | 0.8/100 py | HR 2.9 (0.3 to 28.0) | Low risk of bias* |
| Finazzi et al 200526 | RCT | Indirect | 68 | Warfarin INR 3–4.5 | Warfarin INR 2–3 | 189 py | 181 py | 3.1/100 py | 1.6/100 py | – | High risk of bias* |
| Rosove and Brewer 199227 | Retrospective cohort | Indirect | 56 | Warfarin INR 3–4 | Warfarin INR 2–3 | 110.2 py | 40.9 py | 0 | 7/100 py | – | Intermediate quality |
| Khamashta et al 199528 | Retrospective cohort | Indirect | 54 | Warfarin ≥INR 3.0 | Warfarin INR <3.0 | 197.3 py | 141.3 py | 1.5/100 py | 23/100 py | – | High quality |
| Ames et al 200529 | Prospective cohort | Indirect | 74 | Warfarin INR 3–4 | Warfarin INR 2–3 | – | – | 10.5/100 py | 4.0/100 py | – | Intermediate quality |
| Total | Direct and indirect | RR 0.67 (0.05 to 8.20), I2=82% |
|||||||||
| Major bleeding | |||||||||||
| Crowther et al 200325 | RCT | Direct | 100 | Warfarin INR 3–4 | Warfarin INR 2–3 | 111 py | 133 py | 2.7/100 py | 3.0/100 py | HR 1.0 (0.02 to 4.8) | Low risk of bias* |
| Finazzi et al 200526 | RCT | Indirect | 68 | Warfarin INR 3–4.5 | Warfarin INR 2–3 | 189 py | 181 py | 1.0/100 py | 1.6/100 py | – | High risk of bias* |
| Khamashta et al 199528 | Retrospective cohort | Indirect | 54 | Warfarin ≥3.0 | Warfarin <3.0 | 197.3 py | 141.3 py | 1.7/100 py | 0 | – | High quality |
| Ames et al 200529 | Prospective cohort | Indirect | 74 | Warfarin INR 3–4 | Warfarin INR 2–3 | – | – | 10.5/100 py | 0.57/100 py | – | Intermediate quality |
| Direct and Indirect | RR 1.61 (0.34 to 7.56) I2=35% |
||||||||||
*Risk of bias for randomised controlled trials using the Cochrane tool.
INR, international normalised ratio; RCT, randomised controlled trial; py, person-years.
Pooled data including this RCT25 and four indirect comparison studies (one RCT, two retrospective cohorts, one prospective cohort)26–29 of variable quality did not indicate a difference in risk of recurrent thrombosis between patients on treatment with VKA with a target INR 2–3 and patients with a target INR >3 (RR 0.67 (95% CI 0.05 to 8.20), with high heterogeneity among studies, I2=82%) (table 2). The indirect studies included patients with either prior venous or arterial thromboses; the proportion with venous thromboses ranged from 54% to 74% (26–29). High-intensity INR was not significantly associated with an increased risk of major bleeding (RR 1.61; 95% CI 0.34 to 7.56); however, data on bleeding complications were not reported for patients with venous thrombosis specifically in any study.
Nine single treatment arm studies16 30–37 reported on risks of recurrent thrombosis in cohorts treated with either higher INR targets or conventional INR targets only (online supplementary table 2). Risks in these studies substantially overlapped although appeared to be somewhat lower in the higher INR target studies.
Treatment with direct oral anticoagulants (DOACs)
In a post hoc analysis of patients with APS with venous thrombosis included in three RCTs that compared dabigatran versus warfarin in patients with thrombophilia or APS, the risk of recurrent thrombosis was not significantly different between treatment groups, nor was the risk of major bleeding38 (online supplementary table 3). Risk of any bleeding was lower among patients treated with dabigatran (20%) compared with warfarin (40.3%) (HR 0.50; 95% CI 0.26 to 0.95). Another RCT compared rivaroxaban versus warfarin in 115 patients with venous thrombotic APS.39 Peak thrombin generation was lower in the rivaroxaban compared with warfarin group (56 nmol/L, 95% CI 47 to 66 vs 86 nmol/L, 72 to 102, treatment effect 0.6, 95% CI 0.5 to 0.8, p=0.0006). Although the primary endpoint was endogenous thrombin potential, no patients in either treatment group had recurrence of thrombosis or major bleeding. Minor bleeding occurred in 18% treated with rivaroxaban and 15% treated with warfarin. However, the follow-up was only 7 months, and patients with a history of arterial thrombosis were excluded from the study. A recent RCT of rivaroxaban versus warfarin that included patients with APS with arterial and/or venous thrombosis and with triple aPL positivity was terminated early due to a higher rate of thrombotic events in the rivaroxaban arm.40
Four case series reported on outcomes of patients with APS treated with DOACs but did not include a comparison group.41–44 These studies reported individual-patient data, allowing abstraction of data on patients with histories of venous thromboses, but were not restricted to patients with a first venous event only. The percentage with recurrent thrombosis ranged from 0% to 5.2% over follow-ups from 11 to 35 months. In a systematic review of case series and case reports that included 122 patients treated with DOACs, recurrent events occurred in 15.6%, and in 16.6% of those with prior venous events, while any bleeding events were reported in 4.1%.45
Duration of anticoagulation in patients with unprovoked venous thrombosis
Use of long-term anticoagulation in patients with APS was supported by two small studies (one RCT, one retrospective cohort study)46 that showed a lower risk of recurrent events among patients on long-term anticoagulation compared with patients treated with VKA for 3–6 months (RR 0.33; 95% CI 0.06 to 1.81) (online supplementary table 4). In the RCT, recurrent venous thromboembolism was reported in three patients (20%) who discontinued VKA at 6 months compared with one (5.3%) on long-term oral anticoagulation over 48 months. The risk of bias was high due to the unblinded design. The report did not comment on bleeding complications.
Three additional indirect studies,47–49 each of which reported fewer recurrences in the group that received prolonged anticoagulation, included many patients with arterial events and therefore are not directly applicable to patients with first venous thromboembolism (online supplementary table 4). Studies were not restricted to provoked or unprovoked venous thrombosis. A single treatment arm study reported outcomes of 44 patients with APS who discontinued oral anticoagulation for various reasons. Among 25 patients with solely venous events, recurrent thrombosis occurred in 6 (24%) after discontinuation of oral anticoagulation.50
Recurrent venous thrombosis despite oral anticoagulation with target INR 2–3
Treatment options for these patients include intensification of anticoagulation with VKA, switch to low molecular weight heparin (LMWH) or addition of LDA. No studies directly addressed the effectiveness and risks associated with an increase of INR target to 3–4 for patients with recurrent venous thrombosis while anticoagulated to INR 2–3. In one retrospective study, warfarin failure was the reason for switching to LMWH in 9 of 23 patients, but data on patients with venous events specifically were not reported.51 No patient had recurrent thrombosis on LMWH over a mean follow-up of 36 months, and none had major bleeding. Data from case series indicated no appreciable difference in risk of recurrences between the addition of LDA to VKA versus treatment with VKA alone.52 53 However, the timing of addition of LDA was not clearly specified and confounding by indication may have been present.
Patients with definite APS and first arterial thrombosis
Treatment with VKA vs LDA
In a prospective cohort study of patients with APS with prior ischaemic strokes, the likelihood of recurrent events over 34 months was lower among patients treated with warfarin versus those treated with LDA alone, with a wide CI (RR 0.37; 95% CI 0.05 to 2.47)54 (online supplementary table 5). INR was not reported. There was no comment regarding bleeding. Methodological quality was intermediate, with incomplete data on outcome assessment and follow-up. Another cohort study of patients with both venous (60%) and arterial (40%) events reported on recurrent thrombosis but not separately for these groups.55 Recurrences were lower among patients treated with VKA (30.6%) than those treated with LDA (57%). Data on bleeding were not reported. A single treatment arm study reported that three (37.5%) of eight patients with APS and prior ischaemic stroke who were treated with LDA had a recurrent thrombosis over a median of 8.9 years of follow-up. None had major bleeding.
The APASS study compared new cerebrovascular events over 24 months in elderly patients with recent stroke who were aPL positive on one occasion and treated with either LDA or warfarin at target INR 1.4–2.8.56 Most patients had cardiovascular risk factors (69% ever smoker; 69% hypertension; 32% diabetes). The primary outcome was death or new stroke, although deaths accounted for only a minority of events. Frequency of outcomes was similar between groups. Bleeding was not commented on. The study is not directly relevant because many patients likely did not have definite APS (single aPL measurement, low aPL titres). The relatively low target INR may have minimised differences in recurrences between treatment groups.
Treatment with VKA with INR 2–3 versus INR 3–4
Summary of an earlier SLR showed a lower risk for recurrent thrombosis in patients with high-intensity INR anticoagulation treatment versus those with a target INR of 2–3.57 Analyses of paired comparisons within studies were not reported. Recurrence risk among patients with first arterial thrombosis was not analysed specifically. One RCT with low risk of bias provided stratified data on patients with arterial thrombosis specifically (table 3). Over a mean of 2.6 years, recurrences were higher among those in the target INR 3–4 group than in the INR 2–3 group (8.6/100 py vs 2.6/100 py) but without statistical significance. Bleeding complications were only reported for the treatment groups as a whole (including patients with either venous or arterial events) and were similar. Another RCT compared patients with APS treated to a high target INR (3–4.5) versus moderate target INR,2 3 but only 32% of participants had prior arterial events. Over a median follow-up of 3.6 years, recurrent events occurred more frequently in the high INR group (3.1/100 patient-years (py) vs 1.6/100 py). Major bleeding was slightly less common in the high INR group. Results were not stratified by history of venous or arterial events, so these results are only indirectly related to the treatment of patients with arterial thrombosis. Risk of bias was rated as high (table 3).
Table 3.
Studies of intensity of oral anticoagulation treatment in patients with antiphospholipid syndrome and previous arterial thrombosis
| Reference | Design | Comparison | Percentage with arterial thrombosis history | Intervention | Control | Number of patient-years, intervention | Number of patient-years, control | Events, intervention | Events, control | Relative effect (95% CI) | Study quality |
| Recurrent thrombosis | |||||||||||
| Crowther et al 200325 | RCT | Direct | 100 | Warfarin INR 3–4 | Warfarin INR 2–3 | 35 py | 38 py | 8.6/100 py | 2.6/100 py | HR 3.1 (0.3 to 30.0) | Low risk of bias* |
| Finazzi et al 200526 | RCT | Indirect | 32 | Warfarin INR 3–4.5 | Warfarin INR 2–3 | 189 py | 181 py | 3.1/100 py | 1.6/100 py | – | High risk of bias* |
| Rosove and Brewer 199227 | Retrospective cohort | Indirect | 44 | Warfarin INR 3–4 | Warfarin INR 2–3 | 110.2 py | 40.9 py | 0 | 7/100 py | – | Intermediate quality |
| Khamashta et al 199528 | Retrospective cohort | Indirect | 46 | Warfarin ≥INR 3.0 | Warfarin INR <3.0 | 197.3 py | 141.3 py | 1.5/100 py | 23/100 py | – | High quality |
| Total | Direct and indirect | RR 0.46 (0.06 to 3.52) I2=83% |
|||||||||
| Major bleeding | |||||||||||
| Crowther et al 200325 | RCT | Direct | 100 | Warfarin INR 3–4 | Warfarin INR 2–3 | 111 py | 133 py | 2.7/100 py | 3.0/100 py | HR 1.0 (0.02 to 4.8) | Low risk of bias* |
| Finazzi et al 200526 | RCT | Indirect | 32 | Warfarin INR 3–4.5 | Warfarin INR 2–3 | 189 py | 181 py | 1.0/100 py | 1.6/100 py | – | High risk of bias* |
| Khamashta et al 199528 | Retrospective cohort | Indirect | 46 | Warfarin ≥3.0 | Warfarin <3.0 | 197.3 py | 141.3 py | 1.7/100 py | 0 | – | High quality |
| Total | Direct and indirect | RR 0.86 (0.28 to 2.67) I2=0% |
|||||||||
*Risk of bias for randomised controlled trials using the Cochrane tool.
INR, international normalised ratio; RCT, randomised controlled trial; py, patient-years.
Two additional indirect comparison studies included a mixture of patients with histories of venous and arterial events. The first study included 39 patients with venous thrombosis and 31 with arterial thrombosis. Recurrent thrombosis was not observed in the subgroup treated with warfarin INR ≥3 while the rate was 7/100 py in the subgroup of INR 2–3. Methodological quality was intermediate. In the second study of patients with APS with history of thrombosis (54% venous; 46% arterial at diagnosis), recurrent thrombosis was less frequent among patients treated with VKA at INR ≥3 than those with INR <3 (63). All bleeding complications occurred in the high-intensity group (any bleeding 7.1/100 py; major bleeding 1.7/100 py) (table 3). Data from mixed treatment and single treatment arm studies showed substantial variability in thrombosis recurrence16 33–37 58 59 (online supplementary table 6).
Treatment with DOACs
Data from a recent open-label RCT (TRAPS trial) showed that thrombosis, mainly arterial, developed in 12% of triple aPL patients with APS randomised to rivaroxaban compared with none of those randomised to warfarin, over a mean follow-up of 569 days.40 Based on these results, and the data from a previous45 and an updated systematic review60 of 447 APS cases treated with DOACs reporting that triple aPL positivity and history of arterial thrombosis was associated with higher risk of recurrent thrombosis, rivaroxaban should not be used in the treatment of patients with APS and arterial thrombosis.
Treatment in patients with recurrent arterial thrombosis despite adequate anticoagulation
No studies were identified that directly addressed the effectiveness and risks related to an increase of INR target to 3–4, or switch of VKA treatment to LMWH. Regarding the addition of LDA, a prospective cohort study with 5-year follow-up showed that among 22 patients with at least one arterial event, new thromboses occurred in 2/4 (50%) treated with VKA and one antiplatelet drug compared with 6/18 (33%) treated with VKA alone. The target INR was >3 but achieved INR was not reported, and the timing of antiplatelet treatment relative to events was not clearly specified. Confounding by indication may have been present. The role of dual antiplatelet therapy, statins, HCQ and targeted treatments including B-cell depletion therapy for the management of recurrent arterial events has been mainly examined in small case series and may reflect publication bias. Their potential effect in refractory thrombotic APS should be examined in well-designed prospective studies (Research agenda).
Obstetric APS
Treatment with LDA of pregnant women (with or without SLE) with high-risk aPL profile but no history of thrombosis or pregnancy complications
Although several studies examined the effectiveness of LDA in the treatment of pregnant women with aPL, no studies specifically examined women with high-risk aPL profiles. A double-blind RCT with high risk of bias of LDA versus placebo in six women with SLE resulted in live births in all pregnancies.61 Three studies of patients without SLE examined treatment with LDA62–64 (online supplementary table 7). One RCT included women with generally low-risk aPL profiles and 47% had a history of abortion.62 The second RCT included women with any titre of aPL and no prior pregnancy losses; 60% had low-positive anticardiolipin antibodies.63 The trial was double blind but had a high prevalence of treatment non-adherence. LDA was started between week 12 and week 32 of pregnancy, which may have influenced effectiveness. Lastly, a retrospective cohort study examined women with predominantly low-risk aPL profile (10% with lupus anticoagulant and 30%–33% with high titre aCL).64 In each study, live births occurred in more than 90% of pregnancies regardless of the use of LDA. Evidence was indirect given that none of the studies specifically enrolled women with high-risk aPL profile.
Treatment of pregnant women with a history of ‘criteria’ obstetric APS
History of three recurrent miscarriages or fetal loss
History of three recurrent spontaneous abortions < 10th week of gestation. Pooled results of one direct comparison study (RCT with high risk of bias)65 and eight supporting indirect studies32 66–72 that did not explicitly report on women with prior first trimester losses indicated a higher likelihood of live births with combination treatment with LDA and heparin compared with LDA alone (RR 1.23; 1.12 to 1.35) (table 4). Miscarriages were also less likely. There were no differences in risk of pre-term delivery, pre-eclampsia or intrauterine growth retardation (IUGR), but estimates were imprecise. Maternal thrombosis did not occur in either treatment group.
Table 4.
Studies of low-dose aspirin (LDA) with heparin compared with LDA alone in women with ≥3 recurrent spontaneous miscarriages before the 10th week of gestation
| Reference | Design | Comparison | Percentage with explicitly ≥3 early miscarriages | Intervention | Control | Number of patients, intervention | Number of patients, control | Events, intervention | Events, control | Relative effect (95% CI) | Study quality |
| Live births | |||||||||||
| Rai et al 199765 | RCT | Direct | 100 | LDA+UFH | LDA | 45 | 45 | 32 (71.1%) | 19 (42.2%) | RR 1.68 (1.14 to 2.49) | High risk of bias* |
| Lima et al 199666 | Retrospective cohort | Indirect | NR | LDA+heparin | LDA | 7 | 28 | 6 (85.7%) | 20 (71.4%) | – | Intermediate quality |
| Muñoz-Rodriguez et al 199932 | Retrospective cohort | Indirect | NR | LDA+LMWH | LDA | 12 | 18 | 10 (83%) | 14 (78%) | – | High quality |
| Franklin and Kutteh 200267 | Retrospective cohort | Indirect | 88 | LDA+LMWH | LDA | 25 | 26 | 19 (76%) | 12 (46%) | – | High quality |
| Laskin et al 200968 | RCT | Indirect | 75 | LDA+LMWH | LDA | 45 | 43 | 35 (77.8%) | 34 (79.1%) | – | High quality |
| Naru et al 201069 | Retrospective cohort | Indirect | NR | LDA+LMWH | LDA | 35 | 29 | 35 (100%) | 29 (100%) | – | High quality |
| Cohn et al 201070 | Retrospective cohort | Indirect | NR | LDA+LMWH | LDA | 62 | 98 | 49 (79%) | 61 (62%) | High quality | |
| Mohamed and Saad 201471 | Retrospective cohort | Indirect | NR | LDA+LMWH | LDA | 47 | 23 | 43 (91%) | 15 (65%) | – | Intermediate quality |
| Ruffatti et al 201472 | Retrospective cohort | Indirect | NR | LDA+LMWH | LDA | 104 | 16 | 81 (77.8%) | 11 (68.7%) | – | High quality |
| Total | Direct and indirect | 382 | 326 | 310 | 215 | RR 1.23 (1.12 to 1.35) I2=85% | |||||
| Miscarriages | |||||||||||
| Rai et al 199765 | RCT | Direct | 100 | LDA+UFH | LDA | 45 | 45 | 11 (24.4%) | 24 (53.3%) | RR 0.46 (0.26 to 0.82) | High risk of bias* |
| Cohn et al 201070 | Retrospective cohort | Indirect | NR | LDA+LMWH | LDA | 62 | 98 | 11 (16%) | 38 (37%) | – | High quality |
| Total | Direct and indirect | 107 | 143 | 22 | 62 | RR 0.46 (0.30 to 0.70) I2=0% | |||||
| Pre-term delivery | |||||||||||
| Rai et al 199765 | RCT | Direct | 100 | LDA+UFH | LDA | 45 | 45 | 8 (8.8%) | 4 (4.4%) | RR 2.00 (0.65 to 6.17) | High risk of bias* |
| Lima et al 199666 | Retrospective cohort | Indirect | NR | LDA+heparin | LDA | 7 | 28 | 2 (28.5%) | 5 (18%) | – | Intermediate quality |
| Franklin and Kutteh 200267 | Retrospective cohort | Indirect | 88 | LDA+LMWH | LDA | 25 | 26 | 2 (10.5%) | 0 | – | High quality |
| Naru et al 201069 | Retrospective cohort | Indirect | NR | LDA+UFH | LDA | 35 | 29 | 12 (34.3%) | 9 (31%) | – | High quality |
| Total | Direct and indirect | 112 | 128 | 24 | 18 | RR 1.51 (0.88 to 2.59) I2=0% | |||||
| Pre-eclampsia | |||||||||||
| Rai et al 199765 | RCT | Direct | 100 | LDA+UFH | LDA | 45 | 45 | 0 | 1 (2.2%) | RR 0.33 (0.01 to 7.97) | High risk of bias* |
| Lima et al 199666 | Retrospective cohort | Indirect | NR | LDA+heparin | LDA | 7 | 28 | 3 (43%) | 3 (11%) | – | Intermediate quality |
| Naru et al 201069 | Retrospective cohort | Indirect | NR | LDA+UFH | LDA | 35 | 29 | 10 (28.6%) | 6 (20.7%) | – | High quality |
| Mohamed and Saad 201471 | Retrospective cohort | Indirect | NR | LDA+LMWH | LDA | 47 | 23 | 3 (7%) | 9 (40%) | – | Intermediate quality |
| Total | Direct and indirect | 134 | 125 | 16 | 19 | RR 0.77 (0.45 to 1.31) I2=78% | |||||
| Intrauterine growth retardation | |||||||||||
| Rai et al 199765 | RCT | Direct | 100 | LDA+UFH | LDA | 45 | 45 | 4 (9%) | 2 (5%) | RR 2.00 (0.39 to 10.38) | High risk of bias* |
| Franklin and Kutteh 200267 | Retrospective cohort | Indirect | 88 | LMWH+LDA | LDA | 25 | 26 | 2 (10.5%) | 1 (8.3%) | – | High quality |
| Naru et al 201069 | Retrospective cohort | Indirect | NR | LDA+UFH | LDA | 35 | 29 | 8 (22.8%) | 5 (17.2%) | – | High quality |
| Mohamed and Saad 201471 | Retrospective cohort | Indirect | NR | LDA+LMWH | LDA | 47 | 23 | 6 (12%) | 8 (33%) | – | Intermediate quality |
| Total | Direct and indirect | 152 | 123 | 20 | 16 | RR 0.90 (0.51 to 1.60) I2=46% | |||||
| Maternal thrombosis | |||||||||||
| Rai et al 199765 | RCT | Direct | LDA+UFH | LDA | 45 | 45 | 0 | 0 | RR not estimable | ||
| Naru et al 201069 | Retrospective cohort | Indirect | LDA+UFH | LDA | 35 | 29 | 0 | 0 | NA | ||
| Total | Direct and indirect | 80 | 74 | 0 | 0 | RR not estimable | |||||
*Risk of bias for randomised controlled trials using the Cochrane tool.
LMWH, low molecular weight heparin; NR, not reported; RCT, randomised controlled trial; RR, risk ratio; UFH, unfractionated heparin.
Eight studies,73–80 including five RCTs, compared treatment regimens that included either LDA or heparin but also included other medications (mixed treatment studies) or tested only LDA or heparin alone (online supplementary table 8). Only one of these studies specifically examined women with a history of first trimester losses.79 In these studies, live births were generally more common among treatment arms that included heparin than treatment arms that did not include heparin. Effects on pre-term delivery or pre-eclampsia, reported in few studies, were mixed.
Twenty-two studies81–100 reported outcomes of treatment with LDA and heparin without a comparison treatment arm or compared different doses of heparin. On average, live births occurred in 82.6% of patients treated with LDA and heparin (online supplementary table 8).
A Cochrane review of the effect of LDA with or without heparin on pregnancy outcomes in women with ≥2 unexplained miscarriages with or without inherited thrombophilia was not used because data on patients with APS were not reported separately.101 A second Cochrane review was also excluded because it used data from studies of women with only one aPL measurement or only one miscarriage.102 Results of a third systemic review were also not used because the pooled analysis included both studies of women with aPL only and women with APS.103
History of fetal loss (≥ 10th week of gestation). We did not identify any studies that examined women with exclusively a history of fetal loss. Five studies (all retrospective cohort studies, four of high quality) (32, 76–79) that included a combination of types of miscarriages or fetal losses generally showed a higher likelihood of live births with combination treatment with LDA and heparin compared with treatment with LDA alone (table 5). Miscarriages were less likely among women treated with LDA and heparin. The likelihood of pre-term delivery, pre-eclampsia and IUGR did not differ between treatment groups, but estimates were again heterogeneous. The number of patients in some studies was small.
Table 5.
Studies of low-dose aspirin (LDA) with heparin compared with LDA alone in women with history of fetal loss
| Reference | Design | Comparison | Percent with explicitly fetal loss | Intervention | Control | Number of patients, intervention | Number of patients, control | Events, intervention | Events, control | Relative effect (95% CI) | Study quality |
| Live births | |||||||||||
| Muñoz-Rodriguez et al 199932 | Retrospective cohort | Indirect | NR | LDA+LMWH | LDA | 12 | 18 | 10 (83%) | 14 (78%) | – | High quality |
| Naru et al 201069 | Retrospective cohort | Indirect | NR | LDA+LMWH | LDA | 35 | 29 | 35 (100%) | 29 (100%) | – | High quality |
| Cohn et al 201070 | Retrospective cohort | Indirect | NR | LDA+LMWH | LDA | 62 | 98 | 49 (79%) | 61 (62%) | – | High quality |
| Mohamed and Saad 201471 | Retrospective cohort | Indirect | NR | LDA+LMWH | LDA | 47 | 23 | 43 (91%) | 15 (65%) | – | Intermediate quality |
| Ruffatti et al 201472 | Retrospective cohort | Indirect | NR | LDA+LMWH | LDA | 104 | 16 | 81 (77.8%) | 11 (68.7%) | – | High quality |
| Total | Indirect | 260 | 184 | 218 | 130 | RR=1.19 (1.06, 1.32) I2=88% | |||||
| Miscarriages | |||||||||||
| Cohn et al 201070 | Retrospective cohort | Indirect | NR | LDA+LMWH | LDA | 62 | 98 | 11 (16%) | 38 (37%) | – | High quality |
| Mohamed and Saad 201471 | Retrospective cohort | Indirect | NR | LDA+LMWH | LDA | 47 | 23 | 4 (9%) | 8 (35%) | – | Intermediate quality |
| Total | Indirect | 109 | 121 | 15 | 46 | RR=0.40 (0.24, 0.67) I2=0% | |||||
| Pre-term delivery | |||||||||||
| Naru et al 201069 | Retrospective cohort | Indirect | NR | LDA+LMWH | LDA | 35 | 29 | 12 (34.3%) | 9 (31%) | RR=1.10 (0.54 to 2.25) | High quality |
| Pre-eclampsia | |||||||||||
| Naru et al 201069 | Retrospective cohort | Indirect | NR | LDA+LMWH | LDA | 35 | 29 | 10 (28.6%) | 6 (20.7%) | – | High quality |
| Mohamed and Saad 201471 | Retrospective cohort | Indirect | NR | LDA+LMWH | LDA | 47 | 23 | 3 (7%) | 9 (39%) | – | High quality |
| Total | Indirect | 82 | 52 | 13 | 15 | RR=0.59 (0.32, 1.11) I2=87% | |||||
| Intrauterine growth retardation | |||||||||||
| Naru et al 201069 | Retrospective cohort | Indirect | NR | LDA+LMWH | LDA | 35 | 29 | 8 (22.8%) | 5 (17.2%) | – | High quality |
| Mohamed and Saad 201471 | Retrospective cohort | Indirect | NR | LDA+LMWH | LDA | 47 | 23 | 6 (12%) | 8 (33%) | – | Intermediate quality |
| Total | Indirect | 82 | 52 | 14 | 13 | RR=0.69 (0.36, 1.32) I2=70% | |||||
| Maternal thrombosis | |||||||||||
| Naru et al 201069 | Retrospective cohort | Indirect | NR | LDA+LMWH | LDA | 35 | 29 | 0 | 0 | RR not estimable | High quality |
LMWH, low molecular weight heparin;NR, not reported; RR, risk ratio.
Seven mixed treatment studies,73 75–80 including four RCTs, compared treatment regimens that included either LDA or heparin but also included other medications or tested only LDA or heparin alone (online supplementary table 9).
Nineteen studies22 82 86–88 90 92 94–99 104–109 reported outcomes of treatment with LDA and heparin without a comparison treatment arm or compared different doses of heparin (online supplementary table 9). Results of a systemic review were not used because it included studies of women with aPL and not women with APS.103
History of delivery <34th week of gestation due to eclampsia or severe pre-eclampsia, or recognised features of placental insufficiency
In one RCT (in which 56% of participants had preterm delivery due to eclampsia or severe pre-eclampsia)110 and one retrospective cohort study,32 the likelihood of live births did not differ between women treated with LDA and heparin and those treated with LDA alone (online supplementary table 10). Neither study reported on miscarriages. The trial reported on the likelihood of pre-term delivery, maternal thrombosis and small-for-gestational age babies, with no differences between treatment arms. The RCT was rated as having high risk of bias. Study samples were very small.
Seven studies79 82 88 92 95 98 111 reported outcomes of treatment with LDA and heparin without a comparison treatment arm, or compared heparin alone with no treatment. These studies did not only include women with prior history of severe pre-eclampsia or placental insufficiency but some proportion of patients in these studies had this history. On average, live births occurred in 76.8% of patients treated with LDA and heparin.
Treatment of women with a history of ‘non-criteria’ obstetric APS
History of two recurrent spontaneous abortions < 10th week of gestation. The comparison between treatment with LDA versus no treatment was addressed by one retrospective cohort study. Live births occurred in 89.5% of 57 women treated with LDA and 100% of 17 women not treated with LDA (RR 0.91; 95% CI 0.81 to 1.03).64 Quality was rated as high, but the sample was small. A second, single treatment arm study among women with two prior miscarriages reported live births in 83.9% of pregnancies among women treated with LDA.112
The comparison between the combination treatment with LDA and heparin versus LDA alone for this group of patients was not addressed directly by any study. One RCT found no difference in live births between these treatments (35 (77.8%) vs 34 (79.1%)) in a group of patients with two or more consecutive pregnancy losses <32 weeks’ gestation but was not limited to women with only two losses or early losses.68 The trial was rated as having high risk of bias. The sample was small. A second retrospective cohort study compared women with non-criteria obstetric APS, who were treated with LDA and heparin, with untreated women; live births occurred in 81.7% and 55% of pregnancies, respectively.80 Because these studies did not focus specifically on the population of interest, evidence for this question is indirect.
History of delivery ≥ 34 weeks due to eclampsia or severe pre-eclampsia, or placental insufficiency. One study compared 71 women with various non-criteria obstetric APS complications (two consecutive miscarriages <10 weeks, delivery ≥34 weeks, late intrauterine growth restriction, abruption placentae at term or placental haematoma), who were treated with LDA and heparin, with 20 untreated women; live births occurred in 81.7% and 55%, respectively.80 An unknown percentage of women in this study matched the population of interest.
Duration of treatment with heparin after delivery
We did not identify any studies that directly addressed the benefits and risks of continuing anticoagulation post partum. Few studies reported maternal thrombosis, and even fewer reported assessment of thrombosis specifically in the postpartum period. Twelve studies62 66 68 90 109 110 113–118 included data on both duration of heparin treatment and postpartum thrombosis, but none compared these treatment approaches (online supplementary table 11). In nine studies, treatment was continued 3 to 6 weeks post partum; thrombosis occurred in 3 of 239 patients (1.2%). In three studies,68 116 117 treatment with heparin was stopped prior to delivery. None of 104 patients in these three studies had postpartum thrombosis.
Treatment of patients with a history of recurrent pregnancy complications despite treatment with LDA and prophylactic dose heparin
Increase of heparin to therapeutic dose
No studies were identified that addressed this question.
Addition of HCQ
One study compared pregnancy losses among women with refractory obstetric APS before and after the addition of HCQ.119 Treatment also included heparin in all patients, in combination with LDA in 79%. The proportion of live births was higher in pregnancies in which HCQ was used (online supplementary table 12). Quality was rated as intermediate. The sample was very small. In a second retrospective cohort study, the addition of HCQ to LDA and heparin was associated with a lower likelihood of fetal loss and placenta-mediated complications.120 LMWH was not used by all women in either treatment group (72% and 79%, respectively).
Addition of low-dose prednisolone in the first trimester
One retrospective cohort study compared outcomes between 23 women treated with prednisolone 10 mg/day until week 14 in combination with LDA and heparin with 93 women treated with LDA and heparin.121 Live births were more common in the group treated with prednisolone (online supplementary table 12). Quality was rated as high, but the number of patients treated with prednisolone was small.
Addition of treatment with intravenous immunoglobulin (IVIG)
In three studies83 85 122 that compared pregnancy complications in patients who did or did not receive IVIG, the proportion of live births did not differ between treatment arms (RR 1.10 (95% CI 0.97 to 1.25)) (online supplementary table 12). Miscarriage frequency, pre-term delivery and IUGR also did not differ between treatment arms. The likelihood of pre-eclampsia was lower among patients who received IVIG. Quality of each study was rated as intermediate. One small RCT compared IVIG plus LDA and LMWH versus LDA and LMWH but did not study patients with recurrent pregnancy complications.105 Live births occurred in 100% of pregnancies in both treatment arms. Two other RCTs116 117 compared IVIG alone versus LDA and LMWH in women with recurrent pregnancy losses; live births were less common in the group treated with IVIG alone. Results for miscarriages were mixed.
Three other studies used various treatment regimens, including prednisolone, plasmapheresis/plasma exchange with or without IVIG.72 123 124 Two additional studies were single treatment arm studies.89 125 The proportion of live births in the IVIG treatment arms averaged 86.2% in these five studies.
Treatment of pregnant women with a history of thrombotic APS
Five studies72 86 111 115 126 provided data on outcomes of pregnancies in women with a history of thrombotic APS who were treated with LDA and heparin at therapeutic doses, but none included a comparison group (online supplementary table 13). Live births occurred in 79.1% of pregnancies on average. Only one study115 reported on maternal thrombosis (online supplementary table 13).
Discussion
This SLR provides a summary of the evidence that informed the EULAR recommendations for the management of both thrombotic and obstetric APS in adults. Data from meta-analyses and cohort studies showed that LDA is effective as primary thromboprophylaxis treatment in aPL-positive individuals. Use of VKA at INR 2–3 is protective against thrombosis recurrences in patients with venous thrombosis. In patients with prior arterial thrombosis, no difference in the risk of new thrombotic events was found between VKA treatment at INR 2–3 and INR 3–4. According to the currently available data, rivaroxaban should not be used in high-risk patients with APS such as those with triple aPL positivity. For patients with either recurrent miscarriages or fetal loss, use of combination treatment with LDA and heparin is more effective than LDA alone.
The main limitation of this SLR is that it included a few high-quality RCTs, primarily due to the rarity of the syndrome and the heterogeneity of APS subsets. Performing RCTs in obstetric APS is even more difficult because of ethical regulations of research involving pregnant women and the reluctance of patients to be randomised during pregnancy. Existing literature is limited by heterogeneous patient groups that may have different outcomes and, importantly, different responses to treatment, including, for example, patients with high-risk aPL profile compared with those with low-risk aPL profile, and patients with arterial versus venous thrombotic events. Stratifying patients by risk is crucial to avoid overtreatment and risk of bleeding in some patients, or undertreatment and risk of thrombotic recurrences in others. In addition, reporting of side effects needs improvement. Bleeding complications must be reported in all studies in order to judge the risks of treatment. In obstetric APS studies, maternal thrombosis during pregnancy or post partum is also important and not only pregnancy outcomes.127
Limitations of previous SLRs on thrombotic APS included mixed populations and the lack of separate analysis of outcomes for venous and arterial thrombosis.57 In obstetric APS, SLRs have pooled data from different patient groups such as women with aPL only and women with definite APS.99 In two Cochrane reviews, data on different obstetric APS subgroups were not reported separately, and data from studies of women with only one aPL measurement or only one miscarriage were included, respectively.101 102
One of the strengths of this SLR is that it included the most common and important questions impacting patient care. An additional strength of the SLR was the methodological rigour of the process we followed. The full-text review, the data abstraction and risk of bias assessments were independently performed by multiple reviewers (physicians in charge for literature search (LA, ML), convenor (MGGT) and methodologist (MMW)). In addition, a detailed presentation of the results from both direct and indirect comparison studies in the tables can help the readers of the SLR (clinicians and researchers) to better interpret the separately published EULAR recommendations for the management of APS in adults.
Acknowledgments
The panel is grateful for the administrative and logistical support from EULAR Executive secretariat and especially from Patrizia Jud, Εxecutive Αssistant.
Footnotes
Contributors: The full-text review, data abstraction and risk-of-bias assessments were independently performed by the literature reviewers (LA, ML), the convenor (MGGT) and the methodologist (MMW). MMW prepared the evidence report including summary of finding tables. MMW, MGGT and AT contributed to the interpretation of the data. MGGT drafted the manuscript and all authors reviewed and approved the final version.
Funding: This project was funded by the European League Against Rheumatism. MMW was funded by the Intramural Research Program, National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: The datasets generated for this study are available on request to the corresponding author.
References
- 1.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306. 10.1111/j.1538-7836.2006.01753.x [DOI] [PubMed] [Google Scholar]
- 2.Tektonidou MG, Andreoli L, Limper M, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W–94. 10.7326/0003-4819-151-4-200908180-00136 [DOI] [PubMed] [Google Scholar]
- 4.Sackett DL, Richardson WS, Rosenberg W, et al. Evidence based medicine—how to practice and teach EBM. London: Churchill Livingstone, 1997. [Google Scholar]
- 5.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm
- 7.Arnaud L, Mathian A, Ruffatti A, et al. Efficacy of aspirin for the primary prevention of thrombosis in patients with antiphospholipid antibodies: an international and collaborative meta-analysis. Autoimmun Rev 2014;13:281–91. 10.1016/j.autrev.2013.10.014 [DOI] [PubMed] [Google Scholar]
- 8.Arnaud L, Mathian A, Devilliers H, et al. Patient-level analysis of five international cohorts further confirms the efficacy of aspirin for the primary prevention of thrombosis in patients with antiphospholipid antibodies. Autoimmun Rev 2015;14:192–200. 10.1016/j.autrev.2014.10.019 [DOI] [PubMed] [Google Scholar]
- 9.Mustonen P, Lehtonen KV, Javela K, et al. Persistent antiphospholipid antibody (aPL) in asymptomatic carriers as a risk factor for future thrombotic events: a nationwide prospective study. Lupus 2014;23:1468–76. 10.1177/0961203314545410 [DOI] [PubMed] [Google Scholar]
- 10.Forastiero R, Martinuzzo M, Pombo G, et al. A prospective study of antibodies to beta2-glycoprotein I and prothrombin, and risk of thrombosis. J Thromb Haemost 2005;3:1231–8. 10.1111/j.1538-7836.2005.01295.x [DOI] [PubMed] [Google Scholar]
- 11.Erkan D, Harrison MJ, Levy R, et al. Aspirin for primary thrombosis prevention in the antiphospholipid syndrome: a randomized, double-blind, placebo-controlled trial in asymptomatic antiphospholipid antibody–positive individuals. Arthritis Rheum 2007;56:2382–91. 10.1002/art.22663 [DOI] [PubMed] [Google Scholar]
- 12.Hereng T, Lambert M, Hachulla E, et al. Influence of aspirin on the clinical outcomes of 103 anti-phospholipid antibodies-positive patients. Lupus 2008;17:11–15. 10.1177/0961203307084724 [DOI] [PubMed] [Google Scholar]
- 13.Tektonidou MG, Laskari K, Panagiotakos DB, et al. Risk factors for thrombosis and primary thrombosis prevention in patients with systemic lupus erythematosus with or without antiphospholipid antibodies. Arthritis Rheum 2009;61:29–36. 10.1002/art.24232 [DOI] [PubMed] [Google Scholar]
- 14.Ruffatti A, Del Ross T, Ciprian M, et al. Risk factors for a first thrombotic event in antiphospholipid antibody carriers: a prospective multicentre follow-up study. Ann Rheum Dis 2011;70:1083–6. 10.1136/ard.2010.142042 [DOI] [PubMed] [Google Scholar]
- 15.Pengo V, Ruffatti A, Legnani C, et al. Incidence of a first thromboembolic event in asymptomatic carriers of high-risk antiphospholipid antibody profile: a multicenter prospective study. Blood 2011;118:4714–8. 10.1182/blood-2011-03-340232 [DOI] [PubMed] [Google Scholar]
- 16.Tarr T, Lakos G, Bhattoa HP, et al. Analysis of risk factors for the development of thrombotic complications in antiphospholipid antibody positive lupus patients. Lupus 2007;16:39–45. 10.1177/0961203306074767 [DOI] [PubMed] [Google Scholar]
- 17.Fasano S, Pierro L, Pantano I, et al. Longterm hydroxychloroquine therapy and low-dose aspirin may have an additive effectiveness in the primary prevention of cardiovascular events in patients with systemic lupus erythematosus. J Rheumatol 2017;44:1032–8. 10.3899/jrheum.161351 [DOI] [PubMed] [Google Scholar]
- 18.Erkan D, Merrill JT, Yazici Y, et al. High thrombosis rate after fetal loss in antiphospholipid syndrome: effective prophylaxis with aspirin. Arthritis Rheum 2001;44:1466–7. [DOI] [PubMed] [Google Scholar]
- 19.Cervera R, Khamashta MA, Shoenfeld Y, et al. Morbidity and mortality in the antiphospholipid syndrome during a 5-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis 2009;68:1428–32. 10.1136/ard.2008.093179 [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Zamora MA, Peralta S, Creus M, et al. Risk of thromboembolic events after recurrent spontaneous abortion in antiphospholipid syndrome: a case–control study. Ann Rheum Dis 2012;71:61–6. 10.1136/ard.2011.153817 [DOI] [PubMed] [Google Scholar]
- 21.Ruffatti A, Del Ross T, Ciprian M, et al. Risk factors for a first thrombotic event in antiphospholipid antibody carriers. A multicentre, retrospective follow-up study. Ann Rheum Dis 2009;68:397–9. 10.1136/ard.2008.096669 [DOI] [PubMed] [Google Scholar]
- 22.Lefèvre G, Lambert M, Bacri J-L, et al. Thrombotic events during long-term follow-up of obstetric antiphospholipid syndrome patients. Lupus 2011;20:861–5. 10.1177/0961203310397080 [DOI] [PubMed] [Google Scholar]
- 23.Cuadrado MJ, Bertolaccini ML, Seed PT, et al. Low-dose aspirin vs low-dose aspirin plus low-intensity warfarin in thromboprophylaxis: a prospective, multicentre, randomized, open, controlled trial in patients positive for antiphospholipid antibodies (ALIWAPAS). Rheumatology 2014;53:275–84. 10.1093/rheumatology/ket313 [DOI] [PubMed] [Google Scholar]
- 24.Gris J-C, Bouvier S, Molinari N, et al. Comparative incidence of a first thrombotic event in purely obstetric antiphospholipid syndrome with pregnancy loss: the NOH-APS observational study. Blood 2012;119:2624–32. 10.1182/blood-2011-09-381913 [DOI] [PubMed] [Google Scholar]
- 25.Crowther MA, Ginsberg JS, Julian J, et al. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N Engl J Med 2003;349:1133–8. 10.1056/NEJMoa035241 [DOI] [PubMed] [Google Scholar]
- 26.Finazzi G, Marchioli R, Brancaccio V, et al. A randomized clinical trial of high-intensity warfarin vs. conventional antithrombotic therapy for the prevention of recurrent thrombosis in patients with the antiphospholipid syndrome (WAPS). J Thromb Haemost 2005;3:848–53. 10.1111/j.1538-7836.2005.01340.x [DOI] [PubMed] [Google Scholar]
- 27.Rosove MH, Brewer PM. Antiphospholipid thrombosis: clinical course after the first thrombotic event in 70 patients. Ann Intern Med 1992;117:303–8. 10.7326/0003-4819-117-4-303 [DOI] [PubMed] [Google Scholar]
- 28.Khamashta MA, Cuadrado MJ, Mujic F, et al. The management of thrombosis in the antiphospholipid-antibody syndrome. N Engl J Med 1995;332:993–7. 10.1056/NEJM199504133321504 [DOI] [PubMed] [Google Scholar]
- 29.Ames PRJ, Ciampa A, Margaglione M, et al. Bleeding and re-thrombosis in primary antiphospholipid syndrome on oral anticoagulation: an 8-year longitudinal comparison with mitral valve replacement and inherited thrombophilia. Thromb Haemost 2005;93:694–9. 10.1160/TH04-11-0723 [DOI] [PubMed] [Google Scholar]
- 30.Derksen RH, de Groot PG, Kater L, et al. Patients with antiphospholipid antibodies and venous thrombosis should receive long term anticoagulant treatment. Ann Rheum Dis 1993;52:689–92. 10.1136/ard.52.9.689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulman S, Svenungsson E, Granqvist S. Anticardiolipin antibodies predict early recurrence of thromboembolism and death among patients with venous thromboembolism following anticoagulant therapy. Duration of Anticoagulation Study Group. Am J Med 1998;104:332–8. 10.1016/S0002-9343(98)00060-6 [DOI] [PubMed] [Google Scholar]
- 32.Muñoz-Rodriguez FJ, Font J, Cervera R, et al. Clinical study and follow-up of 100 patients with the antiphospholipid syndrome. Semin Arthritis Rheum 1999;29:182–90. 10.1016/S0049-0172(99)80029-8 [DOI] [PubMed] [Google Scholar]
- 33.Krnic-Barrie S, O'Connor CR, Looney SW, et al. A retrospective review of 61 patients with antiphospholipid syndrome. Analysis of factors influencing recurrent thrombosis. Arch Intern Med 1997;157:2101–8. [PubMed] [Google Scholar]
- 34.Wittkowsky AK, Downing J, Blackburn J, et al. Warfarin-related outcomes in patients with antiphospholipid antibody syndrome managed in an anticoagulation clinic. Thromb Haemost 2006;96:137–41. [PubMed] [Google Scholar]
- 35.Girón-González JA, García del Río E, Rodríguez C, et al. Antiphospholipid syndrome and asymptomatic carriers of antiphospholipid antibody: prospective analysis of 404 individuals. J Rheumatol 2004;31:1560–7. [PubMed] [Google Scholar]
- 36.Petrović R, Petrović M, Novicić-Sasić D, et al. Anticardiolipin antibodies and clinical spectrum of antiphospholipid syndrome in patients with systemic lupus erythematosus. Vojnosanit Pregl 1998;55(2 Suppl):23–8. [PubMed] [Google Scholar]
- 37.Vlachoyiannopoulos PG, Tsiakou E, Chalevelakis G, et al. Antiphospholipid syndrome: clinical and therapeutic aspects. Lupus 1994;3:91–6. 10.1177/096120339400300206 [DOI] [PubMed] [Google Scholar]
- 38.Goldhaber SZ, Eriksson H, Kakkar A, et al. Efficacy of dabigatran versus warfarin in patients with acute venous thromboembolism in the presence of thrombophilia: findings from RE-COVER®, RE-COVER™ II, and RE-MEDY™. Vasc Med 2016;21:506–14. 10.1177/1358863X16668588 [DOI] [PubMed] [Google Scholar]
- 39.Cohen H, Hunt BJ, Efthymiou M, et al. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol 2016;3:e426–36. 10.1016/S2352-3026(16)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pengo V, Denas G, Zoppellaro G, et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood 2018;132:1365–71. 10.1182/blood-2018-04-848333 [DOI] [PubMed] [Google Scholar]
- 41.Noel N, Dutasta F, Costedoat-Chalumeau N, et al. Safety and efficacy of oral direct inhibitors of thrombin and factor Xa in antiphospholipid syndrome. Autoimmun Rev 2015;14:680–5. 10.1016/j.autrev.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 42.Haładyj E, Olesińska M. Rivaroxaban – a safe therapeutic option in patients with antiphospholipid syndrome? our experience in 23 cases. Rheumatology 2016;3:146–9. 10.5114/reum.2016.61217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malec K, Góralczyk T, Undas A. The use of direct oral anticoagulants in 56 patients with antiphospholipid syndrome. Thromb Res 2017;152:93–7. 10.1016/j.thromres.2016.12.009 [DOI] [PubMed] [Google Scholar]
- 44.Resseguier AS, Pereira B, Rieu V, et al. Direct oral anticoagulants: an alternative treatment for thrombotic antiphospholipid syndrome? Lupus 2017;26:1297–303. 10.1177/0961203317701841 [DOI] [PubMed] [Google Scholar]
- 45.Dufrost V, Risse J, Zuily S, et al. Direct oral anticoagulants use in antiphospholipid syndrome: are these drugs an effective and safe alternative to warfarin? A systematic review of the literature. Curr Rheumatol Rep 2016;18 10.1007/s11926-016-0623-7 [DOI] [PubMed] [Google Scholar]
- 46.Ginsberg JS, Wells PS, Brill-Edwards P, et al. Antiphospholipid antibodies and venous thromboembolism. Blood 1995;86:3685–91. [PubMed] [Google Scholar]
- 47.Pengo V, Ruffatti A, Legnani C, et al. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J Thromb Haemost 2010;8:237–42. 10.1111/j.1538-7836.2009.03674.x [DOI] [PubMed] [Google Scholar]
- 48.Hernández-Molina G, Espericueta-Arriola G, Cabral AR. The role of lupus anticoagulant and triple marker positivity as risk factors for rethrombosis in patients with primary antiphospholipid syndrome. Clin Exp Rheumatol 2013;31:382–8. [PubMed] [Google Scholar]
- 49.Taraborelli M, Reggia R, Dall’Ara F, et al. Long-term outcome of patients with primary antiphospholipid syndrome: a retrospective multicenter study. J Rheumatol 2017;44:1165–72. 10.3899/jrheum.161364 [DOI] [PubMed] [Google Scholar]
- 50.Comarmond C, Jego P, Veyssier-Belot C, et al. Cessation of oral anticoagulants in antiphospholipid syndrome. Lupus 2017;26:1291–6. 10.1177/0961203317699285 [DOI] [PubMed] [Google Scholar]
- 51.Vargas-Hitos JA, Ateka-Barrutia O, Sangle S, et al. Efficacy and safety of long-term low molecular weight heparin in patients with antiphospholipid syndrome. Ann Rheum Dis 2011;70:1652–4. 10.1136/ard.2011.150268 [DOI] [PubMed] [Google Scholar]
- 52.Asherson RA, Baguley E, Pal C, et al. Antiphospholipid syndrome: five year follow up. Ann Rheum Dis 1991;50:805–10. 10.1136/ard.50.11.805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turiel M, Sarzi-Puttini P, Peretti R, et al. Thrombotic risk factors in primary antiphospholipid syndrome: a 5-year prospective study. Stroke 2005;36:1490–4. 10.1161/01.STR.0000170645.40562.09 [DOI] [PubMed] [Google Scholar]
- 54.Verro P, Levine SR, Tietjen GE. Cerebrovascular ischemic events with high positive anticardiolipin antibodies. Stroke 1998;29:2245–53. 10.1161/01.STR.29.11.2245 [DOI] [PubMed] [Google Scholar]
- 55.Wang C-R, Liu M-F. Rituximab usage in systemic lupus erythematosus-associated antiphospholipid syndrome: a single-center experience. Semin Arthritis Rheum 2016;46:102–8. 10.1016/j.semarthrit.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 56.Levine SR, Brey RL, Tilley BC, et al. Antiphospholipid antibodies and subsequent thrombo-occlusive events in patients with ischemic stroke. JAMA 2004;291:576–84. 10.1001/jama.291.5.576 [DOI] [PubMed] [Google Scholar]
- 57.Ruiz-Irastorza G, Hunt BJ, Khamashta MA. A systematic review of secondary thromboprophylaxis in patients with antiphospholipid antibodies. Arthritis Rheum 2007;57:1487–95. 10.1002/art.23109 [DOI] [PubMed] [Google Scholar]
- 58.Rivier G, Herranz MT, Khamashta MA, et al. Thrombosis and antiphospholipid syndrome: a preliminary assessment of three antithrombotic treatments. Lupus 1994;3:85–90. 10.1177/096120339400300205 [DOI] [PubMed] [Google Scholar]
- 59.Ruiz-Irastorza G, Khamashta MA, Hunt BJ, et al. Bleeding and recurrent thrombosis in definite antiphospholipid syndrome: analysis of a series of 66 patients treated with oral anticoagulation to a target international normalized ratio of 3.5. Arch Intern Med 2002;162:1164–9. [DOI] [PubMed] [Google Scholar]
- 60.Dufrost V, Risse J, Reshetnyak T, et al. Increased risk of thrombosis in antiphospholipid syndrome patients treated with direct oral anticoagulants. Results from an international patient-level data meta-analysis. Autoimmun Rev 2018;17:1011–21. 10.1016/j.autrev.2018.04.009 [DOI] [PubMed] [Google Scholar]
- 61.Kaaja R, Julkunen H, Viinikka L, et al. Production of prostacyclin and thromboxane in lupus pregnancies: effect of small dose of aspirin. Obstet Gynecol 1993;81:229–31. 10.1016/0020-7292(93)90340-3 [DOI] [PubMed] [Google Scholar]
- 62.Cowchock S, Reece EA. Do low-risk pregnant women with antiphospholipid antibodies need to be treated? Organizing group of the antiphospholipid antibody treatment trial. Am J Obstet Gynecol 1997;176:1099–100. [DOI] [PubMed] [Google Scholar]
- 63.Kahwa EK, Sargeant LA, McCaw-Binns A, et al. Anticardiolipin antibodies in Jamaican primiparae. J Obstet Gynaecol 2006;26:122–6. 10.1080/01443610500443352 [DOI] [PubMed] [Google Scholar]
- 64.Del Ross T, Ruffatti A, Visentin MS, et al. Treatment of 139 pregnancies in antiphospholipid-positive women not fulfilling criteria for antiphospholipid syndrome: a retrospective study. J Rheumatol 2013;40:425–9. 10.3899/jrheum.120576 [DOI] [PubMed] [Google Scholar]
- 65.Rai R, Cohen H, Dave M, et al. Randomised controlled trial of aspirin and aspirin plus heparin in pregnant women with recurrent miscarriage associated with phospholipid antibodies (or antiphospholipid antibodies). BMJ 1997;314:253–7. 10.1136/bmj.314.7076.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lima F, Khamashta MA, Buchanan NM, et al. A study of sixty pregnancies in patients with the antiphospholipid syndrome. Clin Exp Rheumatol 1996;14:131–6. [PubMed] [Google Scholar]
- 67.Franklin RD, Kutteh WH. Antiphospholipid antibodies (APA) and recurrent pregnancy loss: treating a unique APA positive population. Hum Reprod 2002;17:2981–5. 10.1093/humrep/17.11.2981 [DOI] [PubMed] [Google Scholar]
- 68.Laskin CA, Spitzer KA, Clark CA, et al. Low molecular weight heparin and aspirin for recurrent pregnancy loss: results from the randomized, controlled HepASA trial. J Rheumatol 2009;36:279–87. 10.3899/jrheum.080763 [DOI] [PubMed] [Google Scholar]
- 69.Naru T, Khan RS, Ali R. Pregnancy outcome in women with antiphospholipid syndrome on low-dose aspirin and heparin: a retrospective study. East Mediterr Health J 2010;16:308–12. 10.26719/2010.16.3.308 [DOI] [PubMed] [Google Scholar]
- 70.Cohn DM, Goddijn M, Middeldorp S, et al. Recurrent miscarriage and antiphospholipid antibodies: prognosis of subsequent pregnancy. J Thromb Haemost 2010;8:2208–13. 10.1111/j.1538-7836.2010.04015.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mohamed KAA, Saad AS. Enoxaparin and aspirin therapy for recurrent pregnancy loss due to anti-phospholipid syndrome (APS). Middle East Fertil Soc J 2014;19:176–82. 10.1016/j.mefs.2013.12.004 [DOI] [Google Scholar]
- 72.Ruffatti A, Salvan E, Del Ross T, et al. Treatment strategies and pregnancy outcomes in antiphospholipid syndrome patients with thrombosis and triple antiphospholipid positivity. A European multicentre retrospective study. Thromb Haemost 2014;112:727–35. 10.1160/TH14-03-0191 [DOI] [PubMed] [Google Scholar]
- 73.Cowchock FS, Reece EA, Balaban D, et al. Repeated fetal losses associated with antiphospholipid antibodies: a collaborative randomized trial comparing prednisone with low-dose heparin treatment. Am J Obstet Gynecol 1992;166:1318–23. 10.1016/0002-9378(92)91596-3 [DOI] [PubMed] [Google Scholar]
- 74.Pattison NS, Chamley LW, Birdsall M, et al. Does aspirin have a role in improving pregnancy outcome for women with the antiphospholipid syndrome? A randomized controlled trial. Am J Obstet Gynecol 2000;183:1008–12. 10.1067/mob.2000.106754 [DOI] [PubMed] [Google Scholar]
- 75.Stephenson MD, Ballem PJ, Tsang P, et al. Treatment of antiphospholipid antibody syndrome (APS) in pregnancy: a randomized pilot trial comparing low molecular weight heparin to unfractionated heparin. J Obstet Gynaecol Can 2004;26:729–34. 10.1016/S1701-2163(16)30644-2 [DOI] [PubMed] [Google Scholar]
- 76.Ghosh K, Shetty S, Vora S, et al. Successful pregnancy outcome in women with bad obstetric history and recurrent fetal loss due to thrombophilia: effect of unfractionated heparin and low-molecular weight heparin. Clin Appl Thromb Hemost 2008;14:174–9. 10.1177/1076029607306400 [DOI] [PubMed] [Google Scholar]
- 77.Dadhwal V, Sharma AK, Deka D, et al. The obstetric outcome following treatment in a cohort of patients with antiphospholipid antibody syndrome in a tertiary care center. J Postgrad Med 2011;57:16–19. 10.4103/0022-3859.74285 [DOI] [PubMed] [Google Scholar]
- 78.Alalaf S. Bemiparin versus low dose aspirin for management of recurrent early pregnancy losses due to antiphospholipid antibody syndrome. Arch Gynecol Obstet 2012;285:641–7. 10.1007/s00404-011-2055-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rodger MA, Hague WM, Kingdom J, et al. Antepartum dalteparin versus no antepartum dalteparin for the prevention of pregnancy complications in pregnant women with thrombophilia (TIPPS): a multinational open-label randomised trial. The Lancet 2014;384:1673–83. 10.1016/S0140-6736(14)60793-5 [DOI] [PubMed] [Google Scholar]
- 80.Alijotas-Reig J, Esteve-Valverde E, Ferrer-Oliveras R, et al. Comparative study between obstetric antiphospholipid syndrome and obstetric morbidity related with antiphospholipid antibodies. Medicina Clínica 2018;151:215–22. 10.1016/j.medcli.2017.11.017 [DOI] [PubMed] [Google Scholar]
- 81.Backos M, Rai R, Baxter N, et al. Pregnancy complications in women with recurrent miscarriage associated with antiphospholipid antibodies treated with low dose aspirin and heparin. BJOG 1999;106:102–7. 10.1111/j.1471-0528.1999.tb08208.x [DOI] [PubMed] [Google Scholar]
- 82.Pauzner R, Dulitzki M, Langevitz P, et al. Low molecular weight heparin and warfarin in the treatment of patients with antiphospholipid syndrome during pregnancy. Thromb Haemost 2001;86:1379–84. [PubMed] [Google Scholar]
- 83.Diejomaoh MF, Al-Azemi MM, Bandar A, et al. A favorable outcome of pregnancies in women with primary and secondary recurrent pregnancy loss associated with antiphospholipid syndrome. Arch Gynecol Obstet 2002;266:61–6. 10.1007/s004040100179 [DOI] [PubMed] [Google Scholar]
- 84.Noble LS, Kutteh WH, Lashey N, et al. Antiphospholipid antibodies associated with recurrent pregnancy loss: prospective, multicenter, controlled pilot study comparing treatment with low-molecular-weight heparin versus unfractionated heparin. Fertil Steril 2005;83:684–90. 10.1016/j.fertnstert.2004.11.002 [DOI] [PubMed] [Google Scholar]
- 85.Jeremic K, Pervulov M, Gojnic M, et al. Comparison of two therapeutic protocols in patients with antiphospholipid antibodies and recurrent miscarriages. Vojnosanit Pregl 2005;62:435–9. 10.2298/VSP0506435J [DOI] [PubMed] [Google Scholar]
- 86.Stone S, Hunt BJ, Khamashta MA, et al. Primary antiphospholipid syndrome in pregnancy: an analysis of outcome in a cohort of 33 women treated with a rigorous protocol. J Thromb Haemost 2005;3:243–5. 10.1111/j.1538-7836.2005.01185.x [DOI] [PubMed] [Google Scholar]
- 87.A/Magid YM, Elussein EA, Omer MM, et al. Heparin and aspirin in pregnant Sudanese women with recurrent miscarriage associated with antiphospholipid antibodies. Afr J Reprod Health 2007;11:95–8. [PubMed] [Google Scholar]
- 88.Glasnović M, Bosnjak I, Vcev A, et al. Antibody profile of pregnant women with antiphospholipid syndrome and pregnancy outcome after treatment with low dose aspirin and low-weight-molecular heparin. Coll Antropol 2007;31:173–7. [PubMed] [Google Scholar]
- 89.Heilmann L, von Tempelhoff GF, Kuse S. The influence of antiphospholipid antibodies on the pregnancy outcome of patients with recurrent spontaneous abortion. Clin Appl Thromb Hemost 2001;7:281–5. 10.1177/107602960100700405 [DOI] [PubMed] [Google Scholar]
- 90.Serrano F, Nogueira I, Borges A, et al. Primary antiphospholipid syndrome: pregnancy outcome in a Portuguese population. Acta Reumatol Port 2009;34:492–7. [PubMed] [Google Scholar]
- 91.Mo D, Saravelos S, Metwally M, et al. Treatment of recurrent miscarriage and antiphospholipid syndrome with low-dose enoxaparin and aspirin. Reprod Biomed Online 2009;19:216–20. 10.1016/S1472-6483(10)60075-2 [DOI] [PubMed] [Google Scholar]
- 92.Fawad S. Role of anti-thrombotic therapy for recurrent pregnancy loss due to anti-phospholipid syndrome. J Ayub Med Coll Abbottabad 2010;22:197–200. [PubMed] [Google Scholar]
- 93.Fouda UM, Sayed AM, Ramadan DI, et al. Efficacy and safety of two doses of low molecular weight heparin (enoxaparin) in pregnant women with a history of recurrent abortion secondary to antiphospholipid syndrome. J Obstet Gynaecol 2010;30:842–6. 10.3109/01443615.2010.518651 [DOI] [PubMed] [Google Scholar]
- 94.Simchen MJ, Dulitzki M, Rofe G, et al. High positive antibody titers and adverse pregnancy outcome in women with antiphospholipid syndrome. Acta Obstet Gynecol Scand 2011;90:1428–33. 10.1111/j.1600-0412.2011.01236.x [DOI] [PubMed] [Google Scholar]
- 95.De Carolis S, Botta A, Santucci S, et al. Complementemia and obstetric outcome in pregnancy with antiphospholipid syndrome. Lupus 2012;21:776–8. 10.1177/0961203312444172 [DOI] [PubMed] [Google Scholar]
- 96.Mekinian A, Loire-Berson P, Nicaise-Roland P, et al. Outcomes and treatment of obstetrical antiphospholipid syndrome in women with low antiphospholipid antibody levels. J Reprod Immunol 2012;94:222–6. 10.1016/j.jri.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 97.Bouvier S, Cochery-Nouvellon E, Lavigne-Lissalde G, et al. Comparative incidence of pregnancy outcomes in treated obstetric antiphospholipid syndrome: the NOH-APS observational study. Blood 2014;123:404–13. 10.1182/blood-2013-08-522623 [DOI] [PubMed] [Google Scholar]
- 98.Cetkovic A, Kastratovic B, Novakovic I. Prospective study of perinatal outcome in pregnancies with primary antiphospholipid syndrome. Vojnosanitetski pregled 2014;71:742–5. 10.2298/VSP1408742C [DOI] [PubMed] [Google Scholar]
- 99.Rezk M, Dawood R, Badr H. Maternal and fetal outcome in women with antiphospholipid syndrome: a three-year observational study. J Matern Fetal Neonatal Med 2016;29:4015–9. 10.3109/14767058.2016.1152254 [DOI] [PubMed] [Google Scholar]
- 100.Latino JO, Udry S, Aranda FM, et al. Pregnancy failure in patients with obstetric antiphospholipid syndrome with conventional treatment: the influence of a triple positive antibody profile. Lupus 2017;26:983–8. 10.1177/0961203317692432 [DOI] [PubMed] [Google Scholar]
- 101.de Jong PG, Kaandorp S, Di Nisio M, et al. Aspirin and/or heparin for women with unexplained recurrent miscarriage with or without inherited thrombophilia. Cochrane Database Syst Rev 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Empson MB, Lassere M, Craig JC, et al. Prevention of recurrent miscarriage for women with antiphospholipid antibody or lupus anticoagulant. Cochrane Database Syst Rev 2005;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.de Jesus GR, Agmon-Levin N, Andrade CA, et al. 14th International Congress on antiphospholipid antibodies Task Force report on obstetric antiphospholipid syndrome. Autoimmunity Reviews 2014;13:795–813. 10.1016/j.autrev.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 104.Font J, Loapez-Soto A, Cervera R, et al. The ‘primary’ antiphospholipid syndrome: antiphospholipid antibody pattern and clinical features of a series of 23 patients. Autoimmunity 1991;9:69–75. 10.3109/08916939108997126 [DOI] [PubMed] [Google Scholar]
- 105.Branch DW, Peaceman AM, Druzin M, et al. A multicenter, placebo-controlled pilot study of intravenous immune globulin treatment of antiphospholipid syndrome during pregnancy. the pregnancy loss Study Group. Am J Obstet Gynecol 2000;182:122–7. 10.1016/S0002-9378(00)70500-X [DOI] [PubMed] [Google Scholar]
- 106.Venkat-Raman N, Backos M, Teoh TG, et al. Uterine artery Doppler in predicting pregnancy outcome in women with antiphospholipid syndrome. Obstet Gynecol 2001;98:235–42. [DOI] [PubMed] [Google Scholar]
- 107.Bats A-S, Lejeune V, Cynober E, et al. Antiphospholipid syndrome and second- or third-trimester fetal death: follow-up in the next pregnancy. EJOG 2004;114:125–9. 10.1016/j.ejogrb.2003.09.040 [DOI] [PubMed] [Google Scholar]
- 108.Calderon ACS, Berezowski AT, Marcolin AC, et al. Ultrasonography in pregnant women with antiphospholipid syndrome using salicylic acid and heparin. Arch Gynecol Obstet 2011;284:79–84. 10.1007/s00404-010-1612-0 [DOI] [PubMed] [Google Scholar]
- 109.Ruffatti A, Gervasi MT, Favaro M, et al. Adjusted prophylactic doses of nadroparin plus low dose aspirin therapy in obstetric antiphospholipid syndrome. A prospective cohort management study. Clin Exp Rheumatol 2011;29:551–4. [PubMed] [Google Scholar]
- 110.van Hoorn ME, Hague WM, van Pampus MG, et al. Low-molecular-weight heparin and aspirin in the prevention of recurrent early-onset pre-eclampsia in women with antiphospholipid antibodies: the FRUIT-RCT. EJOG 2016;197:168–73. 10.1016/j.ejogrb.2015.12.011 [DOI] [PubMed] [Google Scholar]
- 111.Saccone G, Berghella V, Maruotti GM, et al. Antiphospholipid antibody profile based obstetric outcomes of primary antiphospholipid syndrome: the PREGNANTS study. Am J Obstet Gynecol 2017;216:525.e1–525.e12. 10.1016/j.ajog.2017.01.026 [DOI] [PubMed] [Google Scholar]
- 112.Sugiura-Ogasawara M, Ozaki Y, Nakanishi T, et al. Occasional antiphospholipid antibody positive patients with recurrent pregnancy loss also merit aspirin therapy: a retrospective cohort–control study. Am J Obstet Gynecol 2008;59:235–41. [DOI] [PubMed] [Google Scholar]
- 113.Kutteh WH, Ermel LD. A clinical trial for the treatment of antiphospholipid antibody-associated recurrent pregnancy loss with lower dose heparin and aspirin. Am J Reprod Immunol 1996;35:402–7. 10.1111/j.1600-0897.1996.tb00501.x [DOI] [PubMed] [Google Scholar]
- 114.Granger KA, Farquharson RG. Review: Obstetric outcome in antiphospholipid syndrome. Lupus 1997;6:509–13. 10.1177/096120339700600606 [DOI] [PubMed] [Google Scholar]
- 115.Fischer-Betz R, Specker C, Brinks R, et al. Pregnancy outcome in patients with antiphospholipid syndrome after cerebral ischaemic events: an observational study. Lupus 2012;21:1183–9. 10.1177/0961203312451335 [DOI] [PubMed] [Google Scholar]
- 116.Triolo G, Ferrante A, Ciccia F, et al. Randomized study of subcutaneous low molecular weight heparin plus aspirin versus intravenous immunoglobulin in the treatment of recurrent fetal loss associated with antiphospholipid antibodies. Arthritis & Rheumatism 2003;48:728–31. 10.1002/art.10957 [DOI] [PubMed] [Google Scholar]
- 117.Dendrinos S, Sakkas E, Makrakis E. Low-molecular-weight heparin versus intravenous immunoglobulin for recurrent abortion associated with antiphospholipid antibody syndrome. Int J Gynaecol Obstet 2009;104:223–5. 10.1016/j.ijgo.2008.11.010 [DOI] [PubMed] [Google Scholar]
- 118.Branch DW, Silver RM, Blackwell JL, et al. Outcome of treated pregnancies in women with antiphospholipid syndrome: an update of the Utah experience. Obstet Gynecol 1992;80:614–20. [PubMed] [Google Scholar]
- 119.Mekinian A, Lazzaroni MG, Kuzenko A, et al. The efficacy of hydroxychloroquine for obstetrical outcome in anti-phospholipid syndrome: data from a European multicenter retrospective study. Autoimmun Rev 2015;14:498–502. 10.1016/j.autrev.2015.01.012 [DOI] [PubMed] [Google Scholar]
- 120.Sciascia S, Hunt BJ, Talavera-Garcia E, et al. The impact of hydroxychloroquine treatment on pregnancy outcome in women with antiphospholipid antibodies. Am J Obstet Gynecol 2016;214:273.e1–273.e8. 10.1016/j.ajog.2015.09.078 [DOI] [PubMed] [Google Scholar]
- 121.Bramham K, Thomas M, Nelson-Piercy C, et al. First-trimester low-dose prednisolone in refractory antiphospholipid antibody-related pregnancy loss. Blood 2011;117:6948–51. 10.1182/blood-2011-02-339234 [DOI] [PubMed] [Google Scholar]
- 122.Heilmann L, Schorch M, Hahn T, et al. Pregnancy outcome in women with antiphospholipid antibodies: report on a retrospective study. Semin Thromb Hemost 2008;34:794–802. 10.1055/s-0029-1145261 [DOI] [PubMed] [Google Scholar]
- 123.Vaquero E, Valensise H, MENGHINI S, et al. Pregnancy outcome in recurrent spontaneous abortion associated with antiphospholipid antibodies: a comparative study of intravenous immunoglobulin versus prednisone plus low-dose aspirin. Am J Reprod Immunol 2001;45:174–9. 10.1111/j.8755-8920.2001.450309.x [DOI] [PubMed] [Google Scholar]
- 124.Ruffatti A, Favaro M, Brucato A, et al. Apheresis in high risk antiphospholipid syndrome pregnancy and autoimmune congenital heart block. Transfus Apher Sci 2015;53:269–78. 10.1016/j.transci.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 125.Marzusch K, Dietl J, Klein R, et al. Recurrent first trimester spontaneous abortion associated with antiphospholipid antibodies: a pilot study of treatment with intravenous immunoglobulin. Acta Obstet Gynecol Scand 1996;75:922–6. 10.3109/00016349609055029 [DOI] [PubMed] [Google Scholar]
- 126.Bramham K, Hunt BJ, Germain S, et al. Pregnancy outcome in different clinical phenotypes of antiphospholipid syndrome. Lupus 2010;19:58–64. 10.1177/0961203309347794 [DOI] [PubMed] [Google Scholar]
- 127.Andreoli L, Bertsias GK, Agmon-Levin N, et al. EULAR recommendations for women's health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann Rheum Dis 2017;76:476–85. 10.1136/annrheumdis-2016-209770 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2019-000924supp002.docx (36.1KB, docx)
rmdopen-2019-000924supp001.docx (89.2KB, docx)