Abstract
Vascular endothelial growth factor (VEGF) stimulates endothelial cells to promote developmental and pathological angiogenesis. It also targets tumor cells, especially cancer stem cells (CSCs), to affect tumor initiation, progression and recurrence directly. Recent work has uncovered the importance of YAP/TAZ, transcriptional co-activators, in mediating VEGF signaling. One theme that has emerged from this work is that VEGF signaling impacts Rho family GTPase activity and cytoskeletal dynamics, which contribute to YAP/TAZ activation, and that YAP/TAZ-mediated transcriptional changes sustain Rho family GTPase activity and cytoskeletal dynamics to impact vascular growth and remodeling in endothelial cells and the acquisition of stem-like traits in tumor cells. These findings are significant because of their pathophysiological importance and their connection to other receptor-mediated pathways. This review discusses these findings and highlights areas for future study.
Gloss
Vascular endothelial growth factor (VEGF) is essential for angiogenic processes including the vascularization of organs during development and nascent tumors, which fuels their growth and dissemination. These processes are mediated by VEGF receptors expressed on endothelial cells. Some tumor cells also express VEGF receptors and VEGF signaling in these cells is associated with aggressive behavior, including the acquisition of stem-like traits. Understanding how VEGF impacts endothelial and tumor cells is a problem of paramount importance given the potential benefit of inhibiting this signaling as a therapeutic strategy. In this review with one figure and 60 references, Elaimy and Mercurio describe recent work revealing the importance of YAP and TAZ, transcriptional effectors in the Hippo pathway, in mediating VEGF signaling. The confluence of VEGF and YAP/TAZ signaling results in transcriptional and cytoskeletal alterations that promote angiogenesis and the acquisition of stem-like traits in tumor cells.
Introduction
Vascular endothelial growth factor (VEGF) was identified and isolated as an endothelial cell-specific mitogen that has the capacity to induce developmental and pathological angiogenesis (1, 2). More recent work has revealed the ability of VEGF to target tumor cells, especially cells with stem-like traits, referred to as cancer stem cells (CSCs) and, consequently, contribute to tumor initiation, progression and recurrence directly (3). These vital functions of VEGF are mediated by specific receptors expressed on endothelial and tumor cells including receptor tyrosine kinases (VEGFR1 and VEGFR2) and the neuropilins (NRPs), which function as VEGF co-receptors (4–8). The mechanisms by which these VEGF receptors execute these diverse functions are of paramount importance because of their potential as therapeutic targets. Not surprisingly, VEGF-mediated signaling events have been studied intensely but much remains to be learned. In particular, a better understanding of how VEGF signaling impacts the cell biology that underlies vascular growth and remodeling (angiogenesis) and self-renewal (CSCs) is needed. In this direction, recent work has uncovered a convergence of VEGF and Hippo signaling that has the potential to provide considerably new insight into angiogenesis and CSC function.
The Hippo pathway is critical for development because it restricts proliferation and controls organ size (9). Inhibition of this pathway results in the activation of YAP and TAZ, transcriptional co-activators that have profound effects on cell behavior. Active YAP and TAZ reside in the nucleus where they associate with the TEA domain (TEAD) family of transcription factors and regulate the expression of numerous target collectively known as a YAP/TAZ “signature”. Several cues, such as high cell density and polarity, can activate the core Hippo tumor suppressor pathway and thereby inhibit YAP/TAZ. This pathway consists of a kinase cascade mediated by the MST 1/2 kinases, which phosphorylate and activate the LATS 1/2 kinases (10). Activated LATS 1/2 directly phosphorylates YAP/TAZ at several conserved residues, with serine 127 of YAP and serine 89 of TAZ being the major and most heavily studied LATS 1/2 phosphorylation sites. YAP/TAZ phosphorylation creates 14–3-3 binding sites, which results in their cytoplasmic retention, separation from the TEAD family of transcription factors and functional inactivation (10, 11). In addition to core MST/LATS kinase Hippo signaling, YAP/TAZ activity can be controlled by several other molecular factors termed the “extended” Hippo pathway. Although the core Hippo pathway and its extended components are well-characterized (12), a rigorous understanding of signaling at the cell surface that leads to inactivation of the Hippo pathway and contributes to enhanced YAP/TAZ activity is still emerging. What has been shown recently in several studies is that VEGF receptor signaling can promote YAP/TAZ activation in endothelial and tumor cells. A common theme that has emerged from these studies is that VEGF signaling impacts Rho family GTPase activity and cytoskeletal dynamics, which contribute to YAP/TAZ activation, and that YAP/TAZ-mediated transcriptional changes sustain GTPase activity and cytoskeletal dynamics to affect vascular growth and remodeling in endothelial cells and the acquisition of stem-like traits in tumor cells (Figure 1). These findings are significant because of their pathophysiological importance and their connection to other receptor-mediated pathways. This review discusses these findings and highlights areas for future study.
Figure 1.
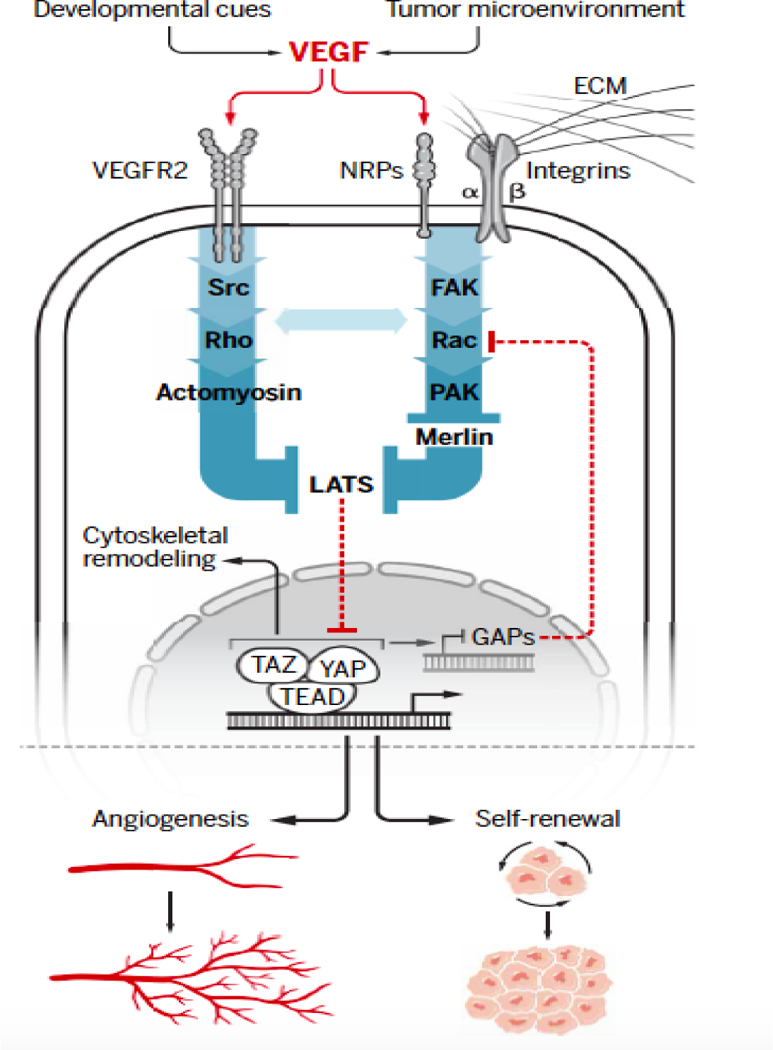
Schematic depicting the confluence of VEGF and YAP/TAZ signaling that has been described in endothelial and tumor cells. The central theme that has emerged is that VEGF signaling mediated by VEGFR2 and NRPs increases the activity of Rho family GTPases resulting in LATS inhibition and YAP/TAZ activation. Integrins have a key role in this signaling by associating with NRPs and engaging the extracellular matrix (ECM). VEGF signaling can involve Src-mediated Rho activation and the consequent activation of YAP/TAZ by cytoskeletal (CSK) dynamics. VEGF signaling can also inhibit LATS by a Rac1-dependent mechanism that involves PAK inhibition of Merlin. An important issue is whether these two mechanisms function in concert to promote YAP/TAZ activation in response to VEGF signaling. YAP/TAZ-mediated transcription can alter the expression of genes involved in CSK remodeling and Rho and Rac1 activation, establishing a positive feedback loop.
YAP/TAZ as Effectors of VEGF Signaling in Developmental Angiogenesis
The vascularization of tissues during development is a precisely orchestrated angiogenic process mediated primarily by VEGF that involves profound changes in endothelial cells including proliferation, sprouting, directed migration and re-organization of cell-cell junctions (13). These diverse functions of endothelial cells are mediated, in part, by transcriptional and cytoskeletal alterations, but the mechanisms responsible for these alterations are still being elucidated. For this reason, recent reports describing VEGF-mediated YAP/TAZ activation in promoting developmental angiogenesis are a significant advance. One study observed VEGF-mediated YAP/TAZ activation in a variety of endothelial cells in vitro (14). They also performed endothelial-cell specific deletions of YAP/TAZ in mice and demonstrated their importance in embryonic and postnatal vascular development. YAP/TAZ deletion resulted in an impaired vascular response to VEGF indicating that YAP/TAZ are important regulators of angiogenesis downstream of VEGF. The mechanism proposed by which VEGF activates YAP/TAZ involves VEGFR2 activation of Src family kinases and subsequent cytoskeletal rearrangements mediated by Src activation of Rho family GTPases. Inhibiting VEGF-VEGFR2-Src-Rho GTPase signaling promoted LATS-mediated YAP/TAZ phosphorylation. This finding is consistent with reports that cytoskeletal by Rho GTPases mediated by upstream G-protein coupled receptor signaling (GPCR) activates YAP/TAZ (15–17), which can be due to inhibition of LATS kinases (16, 17). A positive feedback loop enabled by YAP/TAZ-dependent transcriptional changes was also described that sustains cytoskeletal alterations and Rho GTPase activity. More specifically, chromatin immunoprecipitation sequencing (ChIP-Seq) analysis revealed that the VEGF-VEGFR2-Src-Rho GTPase signaling axis promotes YAP/TAZ dependent expression of several cytoskeletal remodeling genes, including myosin 1C (14). This finding is significant because myosin 1C has been implicated in VEGFR2 trafficking from the Golgi apparatus to the plasma membrane (18). Consequently, enhanced YAP/TAZ activity was shown to be critical in retaining VEGFR2 on the cell surface and promoting a feedforward loop sustaining VEGF-VEGFR2-Src-Rho family GTPase activation of YAP/TAZ that contributes to developmental angiogenesis (14).
Another interesting study concluding that VEGF activates YAP/TAZ in endothelial cells focused more on the cell biology of how YAP/TAZ influence angiogenesis (19). Analysis of mice with endothelial-specific deletion of YAP/TAZ revealed defects in sprouting at the vascular front where tip endothelial cells are exposed to VEGF, which was associated with defective filopodia and lumen formation. A reduction and disarrangement of endothelial cell junctions was also observed in the YAP/TAZ knock-out mice. This phenotype was attributed to the ability of YAP/TAZ to modulate the actomyosin cytoskeleton in response to VEGF by sustaining activation of a Rho family GTPase, CDC42. Although the mechanism by which YAP/TAZ contribute to CDC42 activation was not addressed, it was presumed to involve transcriptionally competent YAP/TAZ in the nucleus. This point is mentioned because another study, which did not consider VEGF signaling, concluded that cytoplasmic YAP regulates CDC42 in the migration of endothelial tip cells (20). This observation is somewhat surprising but a prior study not involving VEGF signaling found that cytoplasmic YAP influences cytokinesis through its regulation of Rho GTPases and Rho GTPase regulatory proteins (21). It will be important to assess whether VEGF signaling affects cytoplasmic YAP and to understand its role in angiogenesis. A broader issue is the role of nuclear vs. cytoplasmic YAP in the regulation of Rho GTPases and their regulation by both transcriptional and non-transcriptional mechanisms. Also, it is likely that YAP and TAZ differ in this regard. Although both YAP and TAZ are prone to degradation in the cytoplasm, cytoplasmic TAZ may not have a non-transcriptional function because it has a significantly shorter half-life than YAP and is highly unstable in the cytoplasm (9, 22, 23).
Other recent studies have used different approaches to arrive at the conclusion that YAP/TAZ are key effectors of VEGF-mediated angiogenesis. The use of a novel bioluminescence-based biosensor that monitors LATS kinase activity in combination with a library of small molecule kinase inhibitors revealed that VEGFR signaling inhibits LATS activity and, consequently, promotes YAP/TAZ activation in endothelial cells (24). The mechanism identified involves VEGFR-mediated PI3K/MAPK activation that suppresses activation of MST1/2, which impedes their ability to activate the LATS kinases. Based on this information, this study also demonstrated that YAP/TAZ are critical for VEGF-induced angiogenesis in vivo. They also found that YAP/TAZ control the expression of angiopoietin-2 and CYR61, proteins that had been previously implicated in angiogenesis (25). Another study observed that VEGF stimulation of endothelial cells reduced YAP phosphorylation and increased its nuclear localization, hallmarks of YAP activation (26). This result prompted the generation of transgenic mice (Tie2Cre-YAPTg) in which YAP was overexpressed in endothelial cells resulting in increased retinal angiogenesis. Interestingly, YAP was shown to partner with STAT3 and promote nuclear STAT3 localization, and evidence was provided that the YAP/STAT3 complex enhances angiogenic functions in vitro. One mechanism proposed is that YAP increases the STAT3-mediated transcription of angiopoietin-2, which is related to the conclusion of the previous study (14).
The studies described demonstrating that VEGF signaling activates YAP/TAZ in endothelial cells have proposed different signaling mechanisms (Src-Rho family GTPase, PI3K/MAPK and STAT3). Future work should focus on whether these pathways can be integrated into a unified mechanism for VEGF-VEGFR-mediated YAP/TAZ activation in endothelial cells. The possibility that a central tenet of this mechanism involves VEGF-mediated activation of Rho family GTPases and the consequent impact on cytoskeletal dynamics and mechanical tension seems likely, especially given the existing literature on YAP/TAZ activation by mechanical forces (27). Nonetheless, it is premature to exclude other modes of YAP/TAZ activation. It is also important to note that other studies had implicated Hippo signaling in angiogenesis (28), but they did not assess the potential role of VEGF signaling in regulating this pathway. This issue is timely because a recent study implicated BMP signaling and discounted the involvement of VEGF in YAP/TAZ regulation of sprouting angiogenesis in the mouse retina (29). Clearly, more work is needed to define the contribution of VEGF signaling to YAP/TAZ activation in developmental angiogenesis and to assess its relationship to other signaling pathways. The possibility that YAP/TAZ can be activated in endothelial cells by blood flow independently of VEGF and Hippo signaling also needs to be considered (30).
Role of VEGF-mediated YAP/TAZ Activation in Cancer Cells
The seminal finding that VEGF-mediated angiogenesis is a hallmark of cancer sparked intense interest in the identification of signaling pathways in tumor-associated endothelial cells that are driven by VEGF and how they can be exploited for therapeutic purposes (31). The importance of VEGF in cancer has been amplified by the realization that VEGF signaling is critical for the function of some cancer cells, especially CSCs. CSCs are defined as a subpopulation of cells that exhibit properties of stem cells including self-renewal and function in tumor initiation, differentiation into heterogeneous cancer cell lineages, and therapy resistance (32). Autocrine VEGF signaling has emerged as an essential pathway for sustaining self-renewal and other CSC functions in several types of cancer (3). Interestingly, many of these studies, but not all, have highlighted a key role for the NRPs (NRP1 and NRP2) in mediating this signaling and discounted the contribution of VEGFRs (33–36). This latter observation has significant implications for therapy because the most common anti-VEGF drug (bevacizumab) blocks the binding of VEGF to receptor tyrosine kinases but not to NRPs (37). This observation may explain, in part, the dismal efficacy of bevacizumab for many cancers (38, 39), and it highlights the potential benefit of targeting the NRPs directly as an approach to inhibiting VEGF signaling in CSCs. For this reason, understanding how VEGF-NRP signaling impacts CSCs is a timely and significant problem, which sets the stage for the recent work implicating YAP/TAZ in this mechanism.
There is now compelling evidence that YAP/TAZ are essential for the function of CSCs, as well as other aspects of aggressive tumor behavior (40). This finding raises the important issue of how YAP/TAZ activity is regulated to impact CSCs and the behavior of aggressive cancers. Presumably, this regulation occurs by factors and conditions in the tumor microenvironment but not much is known about the nature of these signals. There is evidence, for example, that the matrix protein laminin 511 is a component of a CSC niche and that the engagement of this laminin with a splice variant of the α6β1 integrin (α6Bβ1) sustains TAZ activity in breast CSCs (41).
The recent report that autocrine VEGF signaling mediated by NRP2 promotes the self-renewal of breast CSCs by sustaining TAZ activation is significant because it links a key component of the tumor microenvironment (VEGF) with TAZ activation as a mechanism that underlies CSC function (42). This study highlighted a critical role of Rac1 in TAZ activation by VEGF-NRP2 signaling, which is consistent with the key role for Rho family GTPases in promoting YAP/TAZ activation observed in endothelial cells (19, 26). One mechanism proposed by which Rac1 contributes to TAZ activation involves p21-activated kinase (PAK), a Rac-activated kinase, that phosphorylates Merlin, the protein encoded by the Neurofibromatosis Type 2 gene, on Ser518 (43–45). This phosphorylation is known to inhibit LATS phosphorylation (12, 46–48) and, consequently, facilitate YAP/TAZ activation (42, 49). An essential component of this mechanism is the repression of β2-Chimaerin, a Rac GAP, by TAZ and the consequent activation of Rac1 resulting in a positive feedback loop driven by VEGF-NRP2 signaling that sustains TAZ activation. This study also found that a TAZ/TEAD complex binds to the promoter region of the β2-Chimaerin gene and contributes to its repression. Another study in gastric cancer, which did not involve VEGF signaling, found that the expression of ARHGAP29, a Rho GAP, is controlled by YAP, which modulates Rho signaling to promote a metastatic phenotype (50). These observations reveal the ability of YAP/TAZ-dependent transcription to impact CSCs and promote metastasis by regulating the expression of Rho family GAPs.
Interestingly, the available data, albeit limited, indicate that VEGF-mediated YAP/TAZ activation in CSCs can be mediated by NRPs and not involve VEGFRs (42, 51). This conclusion is substantiated by a study on prostate cancer cells, which found that VEGF-NRP-mediated Rac1 activation is VEGFR-independent (52). As alluded to above, however, there is evidence that VEGF signaling in CSCs can be mediated by VEGFRs but these studies did not involve an assessment of YAP/TAZ activation (53–55). Given that NRPs function as co-receptors and lack intrinsic signaling properties (56–58), the issue of how NRPs activate YAP/TAZ arises. One mechanism involves the ability of NRP2 to function as a co-receptor for the α6β1 integrin. This integrin associates with NRP2 in breast cancer cells and this interaction facilitates the signaling potential of this integrin, including its ability to activate FAK (34, 59), which is consistent with the report that VEGF-NRP2 activates Rac1 in a FAK-dependent manner (42). This finding is consistent with the observation that NRP2 and the α6β1 integrin are markers of breast CSCs (34, 60). A more recent study confirmed the importance of NRPs in regulating α6 integrin signaling in epidermal CSCs and the lack of VEGFR involvement (51). This study also found that VEGF-NRP signaling activates YAP by FAK/Src inhibition of LATS phosphorylation. In contrast to the previous report on the α6β1 integrin, however, this study argued that the α6β4 integrin interacts with NRP1 on the surface of CSCs and that this interaction promotes YAP activation. This discrepancy may reflect differences in α6 integrin expression between breast and epidermal CSCs. Nonetheless, these studies demonstrate that NRPs can promote YAP/TAZ activation without VEGFR involvement by co-opting integrin signaling. More work is needed, however, to exclude the involvement of VEGFRs in VEGF-mediated YAP/TAZ activation definitively, especially in light of their key role in YAP/TAZ activation in endothelial cells and other reports on cancer cells.
Concluding Comments
The role of YAP/TAZ activation in executing the functional consequences of VEGF signaling in endothelial and CSCs that has become apparent is providing new insight into the mechanisms that underlie angiogenesis and the acquisition of stem-like traits. A central theme that has emerged from these studies is the critical role of the Rho family of small GTPases in mediating the signaling events initiated by VEGF to activate YAP/TAZ. These GTPases contribute to YAP/TAZ activation indirectly by altering cytoskeletal dynamics as well as directly by inhibiting LATS phosphorylation. The transcriptional alterations that result from YAP/TAZ activation can initiate a positive feedback loop that sustains Rho GTPase activation. Although aspects of this signaling network had been established previously, the novelty of the recent studies highlighted in this review is the ability of VEGF and VEGF receptors to orchestrate this network. This mode of YAP/TAZ regulation is significant because VEGF signaling itself is tightly regulated during development and aberrantly activated by the tumor microenvironment, which provides a pathophysiological context for YAP/TAZ activation. Looking forward, a better understanding of how the different types of VEGF receptors (receptor tyrosine kinases and NRPs) contribute to YAP/TAZ activation and their interaction with other surface receptors is needed. These studies should consider the role of mechanical forces imposed by the extracellular matrix and tissue microenvironment in VEGF receptor-mediated YAP/TAZ activation (Figure 1). Other areas ripe for investigation that are depicted in Figure 1 include delineating the specific contributions of different Rho GTPase family members to YAP/TAZ activation in the context of VEGF signaling. Also, much more needs to be learned about how YAP and TAZ affect gene expression to execute VEGF-mediated functions in endothelial and tumor cells and how YAP and TAZ may differ in this regard. The possibility that cytoplasmic YAP is regulated by VEGF signaling and contributes to angiogenesis and CSC function should also be considered (Figure 1). The impact of this work is likely to be substantial given the intense interest in targeting VEGF signaling as a therapeutic approach for inhibiting angiogenesis and CSC function.
Acknowledgments
Funding
This work was supported by NIH Grants CA168464 and CA203439 (A.M.M). Ameer L. Elaimy was supported by a Ruth L. Kirschstein National Research Service Award from the NCI (F30 CA206271).
Footnotes
Competing Interests
The authors of this manuscript do not have any competing interests.
References
- 1.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N, Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246, 1306–1309 (1989). [DOI] [PubMed] [Google Scholar]
- 2.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF, Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219, 983–985 (1983). [DOI] [PubMed] [Google Scholar]
- 3.Goel HL, Mercurio AM, VEGF targets the tumour cell. Nat Rev Cancer 13, 871–882 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goel HL, Mercurio AM, Enhancing integrin function by VEGF/neuropilin signaling: implications for tumor biology. Cell Adh Migr 6, 554–560 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neufeld G, Kessler O, Herzog Y, The interaction of Neuropilin-1 and Neuropilin-2 with tyrosine-kinase receptors for VEGF. Adv Exp Med Biol 515, 81–90 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M, Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 92, 735–745 (1998). [DOI] [PubMed] [Google Scholar]
- 7.Sulpice E, Plouet J, Berge M, Allanic D, Tobelem G, Merkulova-Rainon T, Neuropilin-1 and neuropilin-2 act as coreceptors, potentiating proangiogenic activity. Blood 111, 2036–2045 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Koch S, van Meeteren LA, Morin E, Testini C, Westrom S, Bjorkelund H, Le Jan S, Adler J, Berger P, Claesson-Welsh L, NRP1 presented in trans to the endothelium arrests VEGFR2 endocytosis, preventing angiogenic signaling and tumor initiation. Dev Cell 28, 633–646 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Piccolo S, Dupont S, Cordenonsi M, The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev 94, 1287–1312 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Meng Z, Moroishi T, Guan KL, Mechanisms of Hippo pathway regulation. Genes Dev 30, 1–17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanai F, Marignani PA, Sarbassova D, Yagi R, Hall RA, Donowitz M, Hisaminato A, Fujiwara T, Ito Y, Cantley LC, Yaffe MB, TAZ: a novel transcriptional co-activator regulated by interactions with 14–3-3 and PDZ domain proteins. EMBO J 19, 6778–6791 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plouffe SW, Meng Z, Lin KC, Lin B, Hong AW, Chun JV, Guan KL, Characterization of Hippo Pathway Components by Gene Inactivation. Mol Cell 64, 993–1008 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haigh JJ, Role of VEGF in organogenesis. Organogenesis 4, 247–256 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Freire Valls A., Schermann G, Shen Y, Moya IM, Castro L, Urban S, Solecki GM, Winkler F, Riedemann L, Jain RK, Mazzone M, Schmidt T, Fischer T, Halder G, Ruiz de Almodovar C, YAP/TAZ Orchestrate VEGF Signaling during Developmental Angiogenesis. Dev Cell 42, 462–478 e467 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Feng X, Degese MS, Iglesias-Bartolome R, Vaque JP, Molinolo AA, Rodrigues M, Zaidi MR, Ksander BR, Merlino G, Sodhi A, Chen Q, Gutkind JS, Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell 25, 831–845 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW, Meng Z, Lin KC, Yu FX, Alexander CM, Wang CY, Guan KL, Alternative Wnt Signaling Activates YAP/TAZ. Cell 162, 780–794 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, Fu XD, Mills GB, Guan KL, Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 150, 780–791 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiwari A, Jung JJ, Inamdar SM, Nihalani D, Choudhury A, The myosin motor Myo1c is required for VEGFR2 delivery to the cell surface and for angiogenic signaling. Am J Physiol Heart Circ Physiol 304, H687–696 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim J, Kim YH, Kim J, Park DY, Bae H, Lee DH, Kim KH, Hong SP, Jang SP, Kubota Y, Kwon YG, Lim DS, Koh GY, YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. J Clin Invest 127, 3441–3461 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakabe M, Fan J, Odaka Y, Liu N, Hassan A, Duan X, Stump P, Byerly L, Donaldson M, Hao J, Fruttiger M, Lu QR, Zheng Y, Lang RA, Xin M, YAP/TAZ-CDC42 signaling regulates vascular tip cell migration. Proc Natl Acad Sci U S A 114, 10918–10923 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bui DA, Lee W, White AE, Harper JW, Schackmann RC, Overholtzer M, Selfors LM, Brugge JS, Cytokinesis involves a nontranscriptional function of the Hippo pathway effector YAP. Sci Signal 9, ra23 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu CY, Zha ZY, Zhou X, Zhang H, Huang W, Zhao D, Li T, Chan SW, Lim CJ, Hong W, Zhao S, Xiong Y, Lei QY, Guan KL, The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase. J Biol Chem 285, 37159–37169 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang W, Lv X, Liu C, Zha Z, Zhang H, Jiang Y, Xiong Y, Lei QY, Guan KL, The N-terminal phosphodegron targets TAZ/WWTR1 protein for SCFbeta-TrCP-dependent degradation in response to phosphatidylinositol 3-kinase inhibition. J Biol Chem 287, 26245–26253 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azad T, Janse van Rensburg HJ, Lightbody ED, Neveu B, Champagne A, Ghaffari A, Kay VR, Hao Y, Shen H, Yeung B, Croy BA, Guan KL, Pouliot F, Zhang J, Nicol CJB, Yang X, A LATS biosensor screen identifies VEGFR as a regulator of the Hippo pathway in angiogenesis. Nat Commun 9, 1061 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi HJ, Zhang H, Park H, Choi KS, Lee HW, Agrawal V, Kim YM, Kwon YG, Yes-associated protein regulates endothelial cell contact-mediated expression of angiopoietin-2. Nat Commun 6, 6943 (2015). [DOI] [PubMed] [Google Scholar]
- 26.He J, Bao Q, Zhang Y, Liu M, Lv H, Liu Y, Yao L, Li B, Zhang C, He S, Zhai G, Zhu Y, Liu X, Zhang K, Wang XJ, Zou MH, Zhu Y, Ai D, Yes-Associated Protein Promotes Angiogenesis via Signal Transducer and Activator of Transcription 3 in Endothelial Cells. Circ Res 122, 591–605 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Totaro A, Panciera T, Piccolo S, YAP/TAZ upstream signals and downstream responses. Nat Cell Biol 20, 888–899 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park JA, Kwon YG, Hippo-YAP/TAZ signaling in angiogenesis. BMB Rep 51, 157–162 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neto F, Klaus-Bergmann A, Ong YT, Alt S, Vion AC, Szymborska A, Carvalho JR, Hollfinger I, Bartels-Klein E, Franco CA, Potente M, Gerhardt H, YAP and TAZ regulate adherens junction dynamics and endothelial cell distribution during vascular development. Elife 7, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakajima H, Yamamoto K, Agarwala S, Terai K, Fukui H, Fukuhara S, Ando K, Miyazaki T, Yokota Y, Schmelzer E, Belting HG, Affolter M, Lecaudey V, Mochizuki N, Flow-Dependent Endothelial YAP Regulation Contributes to Vessel Maintenance. Dev Cell 40, 523–536 e526 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Ferrara N, VEGF as a therapeutic target in cancer. Oncology 69 Suppl 3, 11–16 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Batlle E, Clevers H, Cancer stem cells revisited. Nat Med 23, 1124–1134 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Beck B, Driessens G, Goossens S, Youssef KK, Kuchnio A, Caauwe A, Sotiropoulou PA, Loges S, Lapouge G, Candi A, Mascre G, Drogat B, Dekoninck S, Haigh JJ, Carmeliet P, Blanpain C, A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature 478, 399–403 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Goel HL, Pursell B, Chang C, Shaw LM, Mao J, Simin K, Kumar P, Vander Kooi CW, Shultz LD, Greiner DL, Norum JH, Toftgard R, Kuperwasser C, Mercurio AM, GLI1 regulates a novel neuropilin-2/alpha6beta1 integrin based autocrine pathway that contributes to breast cancer initiation. EMBO Mol Med 5, 488–508 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegle JM, Basin A, Sastre-Perona A, Yonekubo Y, Brown J, Sennett R, Rendl M, Tsirigos A, Carucci JA, Schober M, SOX2 is a cancer-specific regulator of tumour initiating potential in cutaneous squamous cell carcinoma. Nat Commun 5, 4511 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snuderl M, Batista A, Kirkpatrick ND, Ruiz de Almodovar C, Riedemann L, Walsh EC, Anolik R, Huang Y, Martin JD, Kamoun W, Knevels E, Schmidt T, Farrar CT, Vakoc BJ, Mohan N, Chung E, Roberge S, Peterson T, Bais C, Zhelyazkova BH, Yip S, Hasselblatt M, Rossig C, Niemeyer E, Ferrara N, Klagsbrun M, Duda DG, Fukumura D, Xu L, Carmeliet P, Jain RK, Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell 152, 1065–1076 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geretti E, van Meeteren LA, Shimizu A, Dudley AC, Claesson-Welsh L, Klagsbrun M, A mutated soluble neuropilin-2 B domain antagonizes vascular endothelial growth factor bioactivity and inhibits tumor progression. Mol Cancer Res 8, 1063–1073 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergers G, Hanahan D, Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 8, 592–603 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lambrechts D, Lenz HJ, de Haas S, Carmeliet P, Scherer SJ, Markers of response for the antiangiogenic agent bevacizumab. J Clin Oncol 31, 1219–1230 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Zanconato F, Cordenonsi M, Piccolo S, YAP/TAZ at the Roots of Cancer. Cancer Cell 29, 783–803 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang C, Goel HL, Gao H, Pursell B, Shultz LD, Greiner DL, Ingerpuu S, Patarroyo M, Cao S, Lim E, Mao J, McKee KK, Yurchenco PD, Mercurio AM, A laminin 511 matrix is regulated by TAZ and functions as the ligand for the alpha6Bbeta1 integrin to sustain breast cancer stem cells. Genes Dev 29, 1–6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elaimy AL, Guru S, Chang C, Ou J, Amante JJ, Zhu LJ, Goel HL, Mercurio AM, VEGF-neuropilin-2 signaling promotes stem-like traits in breast cancer cells by TAZ-mediated repression of the Rac GAP beta2-chimaerin. Sci Signal 11, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kissil JL, Johnson KC, Eckman MS, Jacks T, Merlin phosphorylation by p21-activated kinase 2 and effects of phosphorylation on merlin localization. J Biol Chem 277, 10394–10399 (2002). [DOI] [PubMed] [Google Scholar]
- 44.Shaw RJ, Paez JG, Curto M, Yaktine A, Pruitt WM, Saotome I, O’Bryan JP, Gupta V, Ratner N, Der CJ, Jacks T, McClatchey AI, The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev Cell 1, 63–72 (2001). [DOI] [PubMed] [Google Scholar]
- 45.Xiao GH, Beeser A, Chernoff J, Testa JR, p21-activated kinase links Rac/Cdc42 signaling to merlin. J Biol Chem 277, 883–886 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Li W, Cooper J, Zhou L, Yang C, Erdjument-Bromage H, Zagzag D, Snuderl M, Ladanyi M, Hanemann CO, Zhou P, Karajannis MA, Giancotti FG, Merlin/NF2 loss-driven tumorigenesis linked to CRL4(DCAF1)-mediated inhibition of the hippo pathway kinases Lats1 and 2 in the nucleus. Cancer Cell 26, 48–60 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li W, You L, Cooper J, Schiavon G, Pepe-Caprio A, Zhou L, Ishii R, Giovannini M, Hanemann CO, Long SB, Erdjument-Bromage H, Zhou P, Tempst P, Giancotti FG, Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell 140, 477–490 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yin F, Yu J, Zheng Y, Chen Q, Zhang N, Pan D, Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell 154, 1342–1355 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sabra H, Brunner M, Mandati V, Wehrle-Haller B, Lallemand D, Ribba AS, Chevalier G, Guardiola P, Block MR, Bouvard D, beta1 integrin-dependent Rac/group I PAK signaling mediates YAP activation of Yes-associated protein 1 (YAP1) via NF2/merlin. J Biol Chem 292, 19179–19197 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qiao Y, Chen J, Lim YB, Finch-Edmondson ML, Seshachalam VP, Qin L, Jiang T, Low BC, Singh H, Lim CT, Sudol M, YAP Regulates Actin Dynamics through ARHGAP29 and Promotes Metastasis. Cell Rep 19, 1495–1502 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Grun D, Adhikary G, Eckert RL, NRP-1 interacts with GIPC1 and alpha6/beta4-integrins to increase YAP1/Np63alpha-dependent epidermal cancer stem cell survival. Oncogene, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goel HL, Pursell B, Shultz LD, Greiner DL, Brekken RA, Vander Kooi CW, Mercurio AM, P-Rex1 Promotes Resistance to VEGF/VEGFR-Targeted Therapy in Prostate Cancer. Cell Rep 14, 2193–2208 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu K, Hao M, Ouyang Y, Zheng J, Chen D, CD133(+) cancer stem cells promoted by VEGF accelerate the recurrence of hepatocellular carcinoma. Sci Rep 7, 41499 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamerlik P, Lathia JD, Rasmussen R, Wu Q, Bartkova J, Lee M, Moudry P, Bartek J Jr., Fischer W, Lukas J, Rich JN, Bartek J, Autocrine VEGF-VEGFR2-Neuropilin-1 signaling promotes glioma stem-like cell viability and tumor growth. J Exp Med 209, 507–520 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao D, Pan C, Sun J, Gilbert C, Drews-Elger K, Azzam DJ, Picon-Ruiz M, Kim M, Ullmer W, El-Ashry D, Creighton CJ, Slingerland JM, VEGF drives cancer-initiating stem cells through VEGFR-2/Stat3 signaling to upregulate Myc and Sox2. Oncogene 34, 3107–3119 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Parker MW, Guo HF, Li X, Linkugel AD, Vander Kooi CW, Function of members of the neuropilin family as essential pleiotropic cell surface receptors. Biochemistry 51, 9437–9446 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geretti E, Shimizu A, Klagsbrun M, Neuropilin structure governs VEGF and semaphorin binding and regulates angiogenesis. Angiogenesis 11, 31–39 (2008). [DOI] [PubMed] [Google Scholar]
- 58.Parker MW, Xu P, Li X, Vander Kooi CW, Structural basis for selective vascular endothelial growth factor-A (VEGF-A) binding to neuropilin-1. J Biol Chem 287, 11082–11089 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goel HL, Pursell B, Standley C, Fogarty K, Mercurio AM, Neuropilin-2 regulates alpha6beta1 integrin in the formation of focal adhesions and signaling. J Cell Sci 125, 497–506 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goel HL, Gritsko T, Pursell B, Chang C, Shultz LD, Greiner DL, Norum JH, Toftgard R, Shaw LM, Mercurio AM, Regulated splicing of the alpha6 integrin cytoplasmic domain determines the fate of breast cancer stem cells. Cell Rep 7, 747–761 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]


