Summary
The acquisition of the oral microbiome is a complex process. We examined how the timing of microbial exposure alters bacterial colonization of the tooth surface. Germ-free mice were conventionalized by exposure to specific pathogen-free (SPF) mice to acquire a commensal microbiome over three distinct 4-week periods, 0-4 weeks of age (Conv0-4w), 4-8 weeks (Conv4-8w), or 8-12 weeks (Conv8-12w). Bacterial DNA was extracted from the tooth surface and analyzed by 16S rDNA sequencing. Total bacteria and inflammatory cytokine expression in gingiva were determined by quantitative real-time polymerase chain reaction. After co-housing with SPF mice, Conv0-4w and Conv4-8w mice had low bacterial diversity, whereas Conv8-12w mice had high bacterial diversity that was similar to that of SPF donor mice, as determined by both operational taxonomic units and the Shannon Index. Cluster analysis with unweighted Unifrac distance also supported these trends. This was surprising as the amount of maturation time, 4 weeks, was equal in all conventionalized mice and tooth eruption was largely completed by 4 weeks. This suggests that host factors that occur after tooth eruption have a significant effect on the microbial tooth colonization.
Keywords: 16S rDNA, age, gnotobiotic mice, inflammatory response, oral microbiota, sequencing
1 ∣. INTRODUCTION
Approximately 10% of cells in the human body are from the host, whereas the majority originate from the microbiota.1 As the initial point of the digestive tract, the oral cavity houses over 700 bacterial species.2-4 The oral microbiome not only contributes to oral homeostasis and pathology,5 but may also contribute as a risk factor for other diseases including cancer,6 diabetes mellitus,7 cardiovascular diseases,8 and preterm birth/low birthweight.9,10
The acquisition of oral bacteria has a strong influence on the host. Gnotobiotic (axenic and defined flora) mice have proven to be an extremely useful model system for understanding the interactions between the host and the microbiome.11 For example, they have been used to study the dynamics of IgA immune responses and the role of commensal bacteria in modulating alveolar bone levels.12,13
With the increased use of gnotobiotic mice, a large number of studies indicate that exposure to microbes at critical periods will influence immune system development, the maintenance of immune health, and the development of immune-mediated diseases.14-16 Marra et al. demonstrated that microbial exposure in early postnatal life is important for the proper development of immune-regulatory mechanisms, and so prevents inappropriate T-cell responses and inflammatory diseases later in life.17
The first encounter of a newborn with microbial colonizers occurs at birth and in the subsequent hours after delivery. Only a subset of microbes is capable of colonizing the early infant oral microbiome. The early colonizers are thought to establish an environment suitable for later colonizers. During the first year of life, the infant oral cavity progresses from a sterile environment to one that has considerable complexity.18-20 Our understanding of how the timing of microbial exposure alters bacterial colonization of the oral cavity and the host response to this bacterial community is still incomplete. For this purpose, we colonized germ-free (GF) mice at different time-points with microbes from specific pathogen-free (SPF) mice to investigate the acquisition of a commensal microbiota in the oral cavity. We expected bacterial diversity to be dependent on tooth eruption and to differ substantially between the period when tooth eruption occurs and after tooth eruption was largely completed. However, this was not the case, as bacterial diversity during and immediately after tooth eruption was similar. However, after tooth eruption there were significant differences in bacterial transfer from SPF mice to GF mice of age 4-8 weeks compared with mice that were 8-12 weeks old. Also surprising was that the amount of bacteria transferred in very young mice was greater than the transfer to older mice, despite the fact that in very young mice teeth were erupting. This greater bacterial colonization coincided with higher cytokine levels in the adjacent gingiva of very young mice.
2 ∣. METHODS
2.1 ∣. Animals and sample collection
Germ-free BALB/c mice were reared in flexible film isolators under germ-free conditions in the Medical University of South Carolina Gnotobiotic Animal Core and fed sterilized Tekl and diet 8656 (Harlan Laboratories Inc., Indianapolis, IN). At age 0 weeks (neonates), 4 weeks, or 8 weeks GF mice were co-housed with 9- to 13-week-old SPF female mice for 4 weeks. Hence, GF mice were conventionalized by co-housing with SPF mice to acquire a commensal microbiome at 0-4 weeks of age (Conv0-4w), at 4-8 weeks of age (Conv4-8w), or at 8-12 weeks (Conv8-12w) and compared with SPF mice. In addition, GF mice were inoculated with fecal contents from donor SPF mice once close to the start of co-housing. Approximately 0.1 g feces was homogenized in 1 mL of PBS and 200 μL was inoculated into the oral cavity and 800 μL placed at sites that are frequently contacted during self-grooming such as the fore paws and posterior of the ears. Dirty bedding was also carried over to the clean cage during the weekly cage change. For the Conv0-4w group, the GF pregnant dams were removed from the gnotobiotic isolators and co-housed with SPF females 4-5 days before the actual delivery date. Therefore, GF neonates were delivered into a non-GF environment and were exposed at day 0 to the SPF donor. In addition, a fetal inoculum was placed once on the nursing dams mammary tissue and onto the neonate’s skin once, 1-2 weeks after delivery. Mice were fed the same diet regardless of group. Each group consisted of five to ten mice and that were divided into four groups: Conv0-4w (male pre-pubescent recipients), Conv4-8w (female post-pubescent recipients), Conv8-12w (female post-pubescent recipients), and SPF (female donors).
Mice were euthanized by carbon dioxide followed by cervical dislocation after 4 weeks of co-housing. Maxilla and mandible were dissected free under sterile conditions and specimens were stored in RNAlater. The Institutional Animal Care and Use Committees of the University of Pennsylvania and Medical University of South Carolina approved all procedures.
To obtain tooth-associated bacteria, incisor and molar crowns were cut along the edge of the alveolar bone crest using a sterile blade under a stereoscopic microscope after the removal of free gingiva, and bacteria were separated from the tooth surface in cell lysis buffer with a DNeasy kit (Qiagen, Valencia, CA) with bead beating (Polysciences, Philadelphia, PA). After a 60-second vortex, DNA present in the buffer was isolated using a DNeasy kit and quantified with a spectrophotometer (Tecan, Männedorf, Switzerland).
2.2 ∣. 16S rDNA analysis
Amplification of the V4 region of 16S ribosomal DNA (16S rDNA) (IDT, Coralville, IA) was performed in 50-μL reactions and then purified with both Agencourt XP DNA purification beads (Beckman Coulter, Beverly, MA) and QiaAmp DNA Mini Spin columns (Qiagen). The sequencing was performed using two-pair end MiSeq, and data were analyzed using QIIME software.21 The trimmed reads were clustered into operational taxonomic units (OTUs) at 97% identity level over an alignment and assigned to the respective genus-level taxa. α Rarefaction was performed using Observed Species and Shannon index. β Diversity was estimated by computing unweighted UniFrac distances between samples using QIIME.22 The relative abundance of each taxon was then computed for each mock community sample at each taxonomic level.
2.3 ∣. Quantitative real-time polymerase chain reaction
The total number of bacteria on the tooth surface was determined by quantitative real-time polymerase chain reaction (PCR) of the 16S rRNA gene (total bacteria) in the ABI 7500 Fast system (Applied Biosystems, Foster City, CA).21 The primer set used to enumerate total bacterial load was as follows23:16S rRNA (Universal; total bacterial load) 5′-TCCTACGGGAGGCAGCAGT-3′, 5′-GGACTACCAGGGTATCTAATCCTGTT-3′.
2.4 ∣. RNA extraction and quantitative real-time polymerase chain reaction
Gingiva surrounding the molar teeth were separated and total RNA was extracted from gingival tissue using an RNeasy kit (Qiagen) followed by reverse transcription. Total RNA (1 μg) was reverse transcribed using High Capacity RNA-to-cDNA kit (Applied Biosystems). The target genes interleukin-1β (IL-1β) and IL- 12β Taqman primers and probes were designed using the Universal Probe Library Assay Design Center (Roche, Basel, Switzerland). The gingiva collected from the 4-week-old GF mice (GF4w), as the control group, and the Conv0-4w mice were gender matched. Results were normalized with respect to the value obtained for the housekeeping gene RPL32, a ribosomal protein. Each experiment was performed two to four times with similar results.
2.5 ∣. Statistical analysis
Statistical analyses were performed using GraphPad Prism (Version 5.0) software (GraphPad, San Diego, CA). All data were expressed as mean±SE. One-way analysis of variance was used to compare the difference of observed OTUs, Shannon index, and inflammatory cytokines expression. P<.05 was considered statistically significant.
3 ∣. RESULTS
3.1 ∣. Recipient mouse age influences oral microbial diversity during conventionalization of GF mice
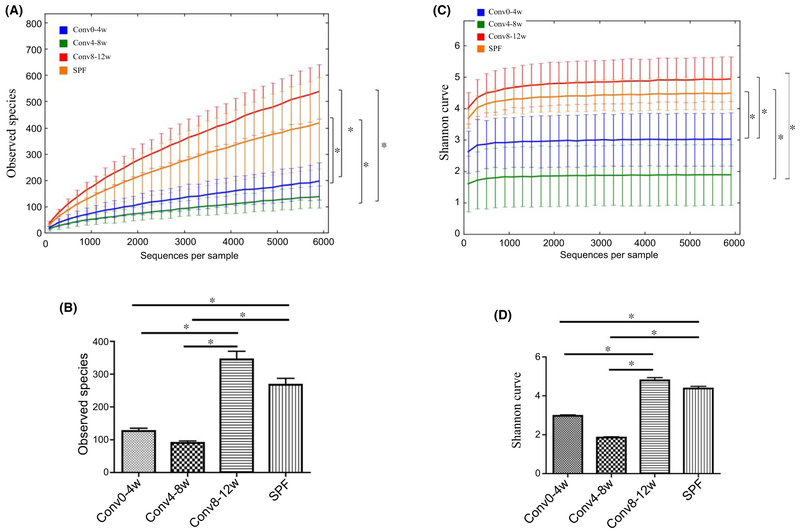
α-Diversity statistics estimated by the number of OTUs and Shannon Index, and β-diversity statistics estimated by unweighted UniFrac distance are shown in Figure 1 and Figure 2, respectively. After co-housing with SPF mice for 4 weeks, the number of OTUs in the Conv0-4w and Conv4-8w recipient bacterial communities was significantly lower compared with the SPF donor group (P<.05). However, there was no significant difference in the number of OTUs between the Conv8-12w recipient and SPF donor bacterial communities (P>.05) (Figure 1A). Similar results were obtained when the data were analyzed by Shannon Index (Figure 1C, D). The Shannon diversity index for the SPF donor bacterial communities was significantly higher compared with the Conv0-4w and Conv4-8w bacterial communities (P<.05) but similar to the Conv8-12w bacterial communities (P>.05) (Figure 1C, D).
FIGURE 1.
Age influences the acquisition of the oral microbiota as measured by alpha-diversity. A & B. Alpha diversity was assessed by the number of OTUs. C & D. Alpha diversity was assessed by the Shannon Index. (*P<.05) between the indicated groups
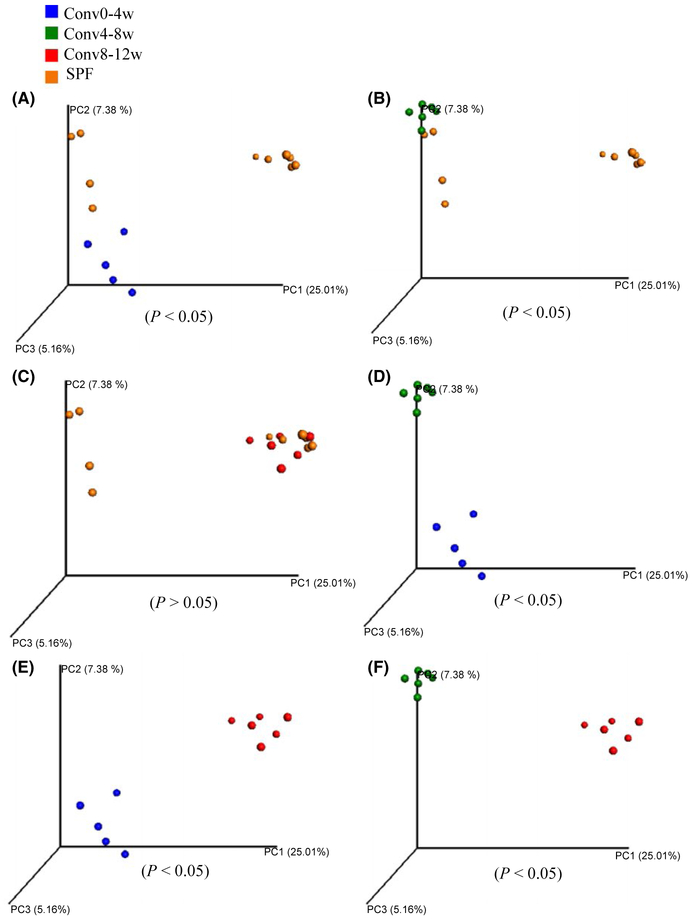
FIGURE 2.
Recipient mouse age influences oral microbial beta-diversity during conventionalization of germ-free mice. Results of Principal Component Analysis with unweighted UniFrac distance. (A) Comparison between Conv0-4w mice and specific pathogen-free (SPF) mice (P<.05). (B) Comparison between Conv4-8w mice and SPF mice (P<.05), (C) Comparison between Conv8-12w mice and SPF mice (P>.05). (D) Comparison between Conv0-4w mice and Conv4-8w mice (P<.05). (E) Comparison between Conv0-4w mice and Conv8-12w mice (P<.05). (F) Comparison between Conv4-8w mice and Conv8-12w mice (P<.05)
Unlike α-diversity estimates, β-diversity is a measure of the degree of similarity (e.g. phylogenetic relatedness) between pairs of communities. In this study, unweighted Unifrac distance was performed to compare the degree of phylogenetic overlap in the overall microbial community in different groups. Principal coordinate analysis plots depicted the distances among communities based on qualitative community metrics. Results showed that co-housing resulted in separate clustering of bacteria communities from Conv0-4w mice and SPF donor mice (Figure 2A) (P<.05). Similarly, bacterial communities within the oral cavity of Conv4-8w mice also did not cluster with SPF donor bacterial communities (Figure 2B) (P<.05). However, significant clustering of bacteria communities was observed between Conv8-12w and SPF donor samples (P>.05) (Figure 2C). Principal coordinate analysis of the recipient oral samples (Conv0-4w, Conv4-8w and Conv8-12w) clustered separately from each of the other recipient groups (Figure 2D-F) (P<.05).
3.2 ∣. Recipient mouse age influences oral microbial composition during conventionalization of GF mice
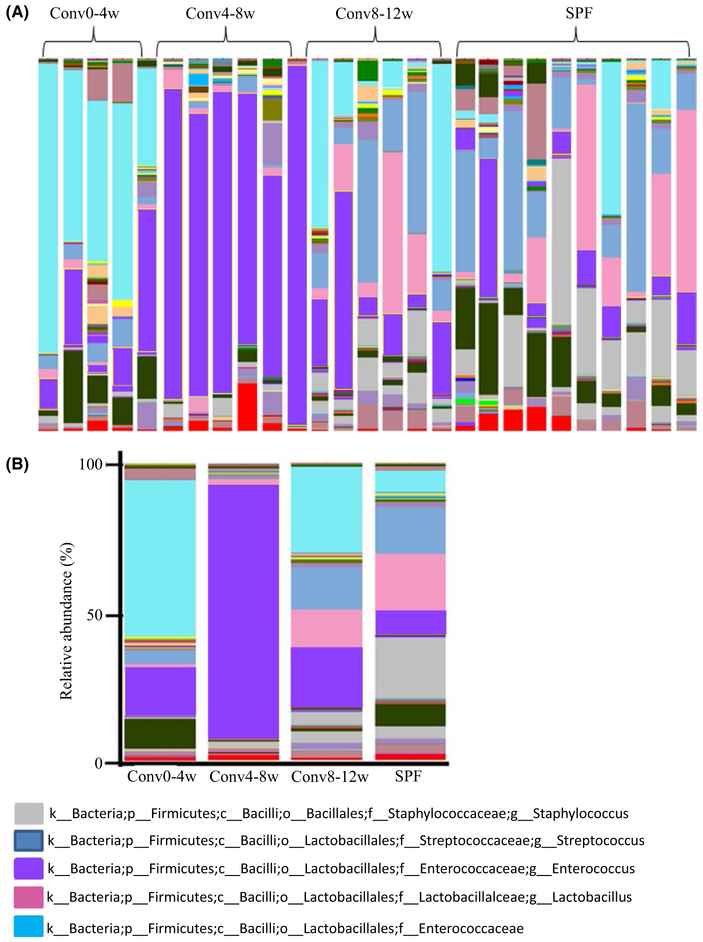
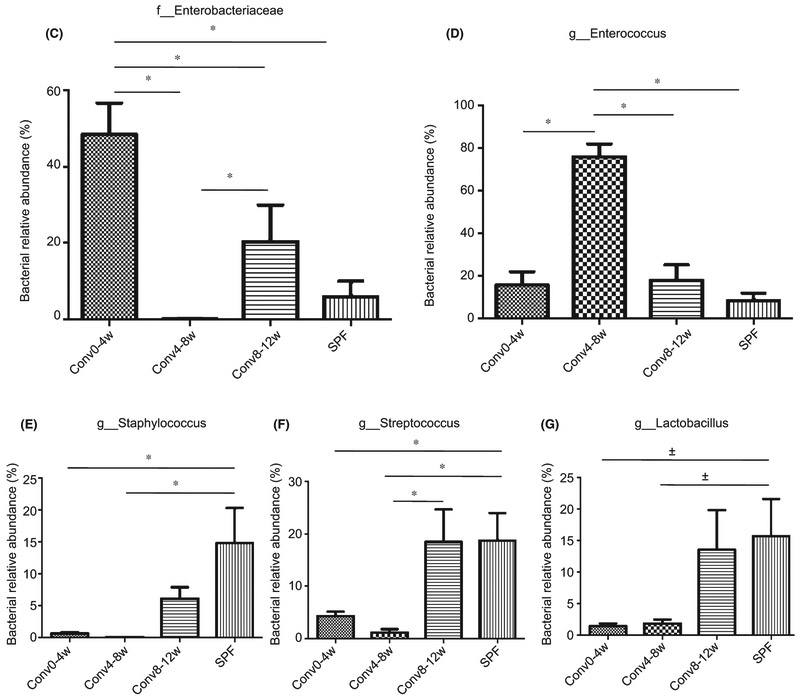
To further analyze the microbial community associated with the tooth surface, the relative abundance of microbial taxonomic groups at the family or genus levels was examined (Figure 3A). For the top five abundant OTUs (Staphylococcus, Streptococcus, Lactobacillus, Enterococcus and Enterobacteriaceae) a distinct pattern was observed (Figure 3B-G). Following co-housing with SPF mice, Conv0-4w mice acquired a higher proportion of Enterobacteriaceae at an abundance of 48.9%, which was approximately eight-fold higher than the levels found in the SPF donor group (P<.05) (Figure 3C). In the Conv4-8w group, the predominant bacterial genus was Enterococcus with an average abundance of 76%, which was nine-fold higher than the levels found in the SPF donor group (P<.05) (Figure 3D). Staphylococcus and Streptococcus did not transfer efficiently from the SPF donor mice to the Conv0-4w or Conv4-8w recipient mice (P<.05) (Figure 3E, F). In contrast, the constituent ratios and relative abundance of the top five bacteria at the family and/or genus-level in Conv8-12w mice was similar to the SPF donor mice (P>.05) (Figure 3B). The relative abundance of Lactobacillus in Conv8-12w mice and SPF donor mice was higher compared with Conv0-4w mice and Conv4-8w mice but the difference did not reach significance (P=0.06) (Figure 3G).
FIGURE 3.
Recipient mouse age influences oral microbial composition during conventionalization of germ-free mice. (A) Area charts show the relative abundance of the predominant bacteria in each group. (B) The relative abundance ratio of the top five bacteria observed in different groups. (C-G) Relative abundance of Enterobacteriaceae, Enterococcus, Staphylococcus, Streptococcus and Lactobacillus, respectively. *Significant difference (P<.05) between the indicated groups, ± indicates the difference between the indicated groups is P=0.06.
3.3 ∣. Reconventionalization of neonatal GF mice induces elevated inflammatory cytokine expression
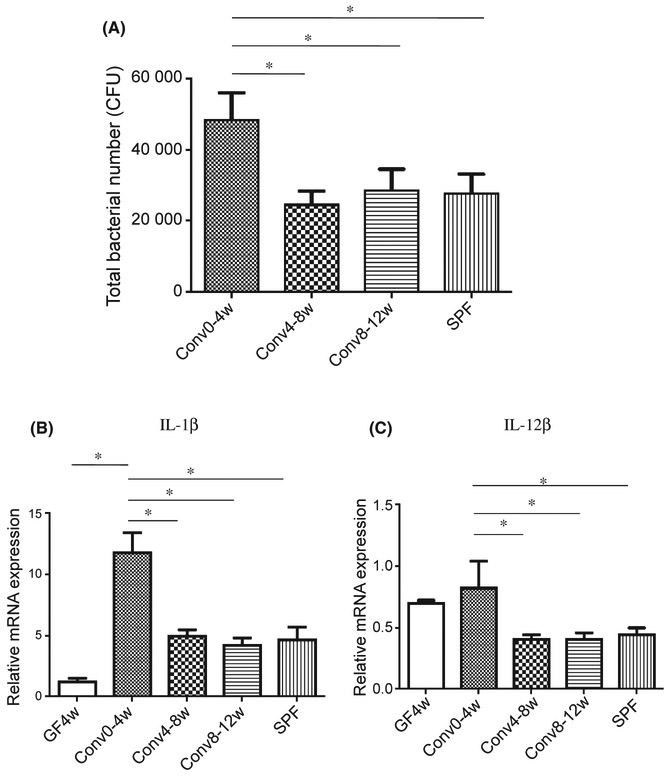
Co-housing of SPF mice with GF mice resulted in differences in total bacterial levels on the tooth surface that varied depending on the age of the GF mouse. The total bacterial load in the Conv0-4w group was two-fold higher when compared with other recipient and donor groups (P<.05), whereas the tooth surface bacterial loads in Conv4-8w, Conv8-12w and SPF mice were similar (P>.05) (Figure 4A).
FIGURE 4.
Conventionalization of neonatal germ-free mice induces elevated inflammatory cytokine expression. (A) The number of total oral bacteria on the tooth surface was determined using quantitative real-time polymerase chain reaction of the 16S rRNA gene. (B) Interleukin-1β (IL-1β) expression in the gingival tissue around molar teeth determined by quantitative real-time PCR. (C) IL-12β expression in the gingival tissue around molar teeth determined by quantitative real-time PCR. *Significant difference (P<.05) between the indicated groups
To investigate potential mechanisms for the higher bacterial burden in the Conv0-4w group the level of inflammation was examined. Quantitative real-time PCR analysis of RNA from gingival tissue demonstrated that IL-1β and IL-12β were two- to three-fold higher in Conv0-4w mice compared with the other three groups (P<.05). There was no significant difference among Conv4-8w, Conv8-12w and SPF mice (P>.05). The GF control group expressed lower levels of IL-1β and IL-12β compared with ConvD0-4w mice (Figure 4B, C). The results suggest a relationship between higher bacterial levels in Conv0-4w mice and higher inflammatory cytokine levels in this group.
4 ∣. DISCUSSION
The top five abundant OTUs in the oral cavity of the mice in our study were Staphylococcus, Streptococcus, Lactobacillus, Enterococcus and Enterobacteriaceae. The result was consistent with previous reports that the composition consists mainly of Streptococcus, Staphylococcus, Lactobacillus, Halomonas and Enterococcus.24,25 Enterobacteriaceae were found as a greater proportion in Conv0-4w mice than the other groups, whereas Enterococcus bacteria were found in greater proportions in Conv4-8w mice compared with others. The constituent ratios of the five bacteria in Conv8-12w mice were similar to those in the SPF donor mice. Rodrigue et al. found that oral cavities of the murine SPF mice were colonized by Staphylococci followed by Lactobacillus immediately after birth.26 Although these bacteria were early colonizers found in relatively high abundance, they were not dominant at the earliest time-points in our study. This may reflect different environments, difference sources for the SPF mice and the fact that our recipient mice were germ-free rather than SPF. Rodrigue et al. proposed that regardless of the first colonizing strains the bacterial population evolves towards the proportions observed in adult mice due to homeostatic forces, similar to our results.26
We found that there were substantial differences in diversity but the periods when diversity changed were somewhat surprising. Tooth eruption occurs during weeks 0-4 and eruption of the first two molars is largely completed by week 4, so we expected there to be a substantial difference in bacterial diversity between 0-4 weeks and 4-8 weeks. However, no significant difference was noted in the number of bacterial taxa identified between 0-4 and 4-8 weeks. Instead, the largest difference in diversity occurred between weeks 4-8 and weeks 8-12. These differences and similarities were shown by both α diversity and β diversity. The Conv8-12w bacterial and SPF bacterial communities were approximately two-fold more diverse than the Conv0-4w and Conv4-8w recipient bacterial communities, as determined by OTUs and Shannon diversity index. Cluster analysis with unweighted Unifrac distance also supported these trends, showing a significant difference between bacteria from Conv0-4w and SPF mice and between Conv4-8w and SPF mice but no difference between Conv8-12w and SPF mice. The results are not simply due to biofilm maturation since the maturation period, 4 weeks, was the same. This suggests that factors that occur after tooth eruption have a significant effect on the microbial tooth colonization. We were not able to isolate the aspects of the host that are responsible for these differences but it is possible that they include factors related to immune system maturity. Our experiments were generally carried out with female donors and recipients except for the pre-pubescent Conv0-4w group, which consisted of males. Interestingly, Yurkovetskiy et al.27 showed that the number of microbial species in gut samples was not significantly different between male and female 4-week-old recipients, whereas post-pubescent mice harbored gender-biased microbial composition.27 Hence, it is possible that gender-based differences need to be taken into account when interpreting the results of experiments with the oral microbiome, particularly in post-pubescent groups when hormonal changes associated with gender may affect microbial composition.
Pro-inflammatory cytokines IL-1β and IL-12β are important mediators in the initiation and enhancement of gingival inflammation.28-30 We found a positive correlation between bacterial burden and the inflammatory cytokine expression, which is consistent with other studies. Abusleme et al. demonstrated that increased inflammation in periodontal tissue may be due to an overall greater bacterial challenge, and the relationship between these two factors is reciprocal. Higher microbial load may represent a greater challenge to the host, which responds with increased inflammation, which in turn results in increased biomass through greater supply of host-derived nutrients.31 Other studies have demonstrated that the inflammatory environment of periodontal tissue is favorable for bacterial growth since degraded host proteins and hemin within the gingival inflammatory exudate are a rich source of nutrients. This, moreover, can alter the composition of microbiota, favoring the growth of inflammation-related bacteria. These changes result in even higher inflammation and tissue resorption, leading to increased niche space for the bacteria..12,32 Additionally, it has been reported that the relative salivary volume of 12- to 20-week-old mice is lower than those of 6-week-old C57BL/6 mice.33 Saliva is widely recognized as a primary source of carbon and nitrogen for growth of the oral bacterial community and contains anti-bacterial factors that modulate bacterial growth.34,35 It is possible that differences in saliva affect the higher bacterial burden in Conv0-4w mice compared with the other groups. It is worth noting that the high level of cytokine expression in the youngest group of GF mice is unlikely due to tooth eruption alone as the matched control group had low levels of IL-1β and IL-12β.
The Conv0-4w mice had a microbial composition that was also different when compared with the other groups. Enterobacteriaceae was the most abundant taxa in Conv0-4w mice and may be associated with a higher degree of inflammation. This interpretation agrees with Lupp et al.,36 who found that host-mediated inflammation disrupts the murine intestinal microbiota and supports the growth Enterobacteriaceae. In humans, Enterobacteriaceae are increased in individuals with poor oral hygiene and chronic gingivitis.37-39
Enterococcus was the predominant bacterium found in Conv4-8w mice. The explanation for the striking difference in Enterococcus between Conv0-4w and Conv4-8w mice is not entirely clear but could be related to tooth eruption or to weaning, both of which are largely completed by day 28.40,41 Other studies have demonstrated that some species of Enterococcus (Enterococcus faecalis and Enterococcus coli) appear in the oral cavity due to the appearance of the teeth.26 Abundant health-related oral taxa in mature mice are Streptococcus and Lactobacillus,24-26 which we found were relatively abundant in Conv8-12w and SPF mice. The shift in microbial composition reflects a more diverse bacterial community representative of a healthy ecosystem.42,43
In conclusion, we found that the ecological succession of the bacterial communities and the inflammatory response to the acquired microbes depended on the timing of microbial exposure. Surprisingly, bacterial diversity was not strictly related to tooth eruption, indicating that other factors related to the host such as immune maturity may play an important role. Furthermore, there may be a reciprocal relationship between increased bacterial load and greater gingival inflammation in the transfer of bacteria to the Conv0-4w group.
ACKNOWLEDGEMENTS
The studies were funded by grants from the NIDCR R01DE021921 and R01DE017732. We would like to thank Zena Khorfan for assistance in bacterial analysis. There are no financial conflicts of interest to report in this manuscript.
Funding information
National institute of Dental and Craniofacial Research, Grant/Award Number: R01DE017732 and R01DE021921
REFERENCES
- 1.Shoemark DK, Alen SJ. The microbiome and disease: Reviewing the links between the oralmicrobiome, aging, and Alzheimer’s disease. J Alzheimers Dis. 2015;43:725–738. [DOI] [PubMed] [Google Scholar]
- 2.Brown J, Wang H, Suttles J, Graves DT, Martin M. mTORC2 negatively regulates the toll-likereceptor 4-mediated inflammatory response via FoxO1. J Biol Chem. 2011;286:44295–44305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dewhirst FE, Chen T, Izard J, et al. The human oral microbiome. J Bacteriol. 2010;192:5002–5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colombo AP, Boches SK, Cotton SL, et al. Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J Periodontol. 2009;80:1421–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schindler D, Gutierrez M, Beineke A, et al. Dendritic cells are central coordinators of the host immune response to Staphylococcus aureus bloodstream infection. Am J Pathol. 2012;181:1327–1337. [DOI] [PubMed] [Google Scholar]
- 6.Kang KSLN, Shin MS, Kim SD, et al. An altered relationship of influenza vaccine-specific IgG responses with T cell immunity occurs with aging in humans. Clin Immunol. 2013;147:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Queiroz-Junior CM, Maldeira M, Coelho FM, et al. Experimental arthritis triggers periodontal disease in mice: Involvement of TNF-α and the oral microbiota. J Immunol. 2011;187:3821–3830. [DOI] [PubMed] [Google Scholar]
- 8.Xiao W, Dong G, Pacios S, et al. FOXO1 deletion reduces dendritic cell function and enhances susceptibility to periodontitis. Am J Pathol. 2014;185:1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callaway DA, Jiang JX. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J Bone Miner Metab. 2015;33:359–370. [DOI] [PubMed] [Google Scholar]
- 10.Singh T, Newman A. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koller MM, Purushotham KR, Maeda N, Scarpace PJ, Humphreys-Beher MG. Desipramine induced changes in salivary proteins, cultivable oral microbiota and gingival health in aging female NIA Fischer 344 rats. Life Sci. 2000;68:445–455. [DOI] [PubMed] [Google Scholar]
- 12.Hajishengallis G, Liang S, Payne MA, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irie K, Novince CM, Darveau RP. Impact of the oral commensal flora on alveolar bone homeostasis. J Dent Res. 2014;93:801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reading NC, Kasper DL. The starting lineup: Key microbial players in intestinal immunity and homeostasis. Front Microbiol. 2011;2:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan JL, Shi HN, Walker WA. The role of microbes in developmental immunologic programming. Pediatr Res. 2011;69:465–472. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto M, Yamaguchi R, Munakata K, et al. A microarray analysis of gnotobiotic mice indicating that microbial exposure during the neonatal period plays an essential role in immune system development. BMC Genom. 2012;13:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marra F, Lynd L, Coombes M, et al. Does antibiotic exposure during infancy lead to development of asthma?: A systematic review and metaanalysis. Chest. 2006;129:610–618. [DOI] [PubMed] [Google Scholar]
- 18.Sampaio-Maia B, Monteiro-Silva F. Acquisition and maturation of oral microbiome through childhood: An update. Dent Res J (Isfahan). 2014;11:291–301. [PMC free article] [PubMed] [Google Scholar]
- 19.Zaura E, Nicu EA, Krom BP, Keijser BJ. Acquiring and maintaining a normal oral microbiome: Current perspective. Front Cell Infect Microbiol. 2014;4:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sulyanto R. The natural history of oral bacteria acquisition in the developing infant. Bol Med Hosp Infant Mex. 1960;17:139–156.13817636 [Google Scholar]
- 21.Wu Y, Dong G, Xiao W, et al. Effect of aging on periodontal inflammation, microbial colonization, and disease susceptibility. J Dent Res. 2016;95:460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chehoud C, Rafail S, Tyldsley AS, Seykora JT, Lambris JD, Grice EA. Complement modulates the cutaneous microbiome and inflammatory milieu. Proc Natl Acad Sci USA. 2013;110:15061–15066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McIntosh ML, Hajishengallis G. Inhibition of Porphyromonas gingivalis-induced periodontal bone loss by CXCR4 antagonist treatment. Mol Oral Microbiol. 2012;27:449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chun J, Kim KY, Lee JH, Choi Y. The analysis of oral microbial communities of wild-type and toll-like receptor 2-deficient mice using a 454 GS FLX Titanium pyrosequencer. BMC Microbiol. 2010;10:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trudel L, St-Amand L, Bareil M, Cardinal P, Lavoie MC. Bacteriology of the oral cavity of BALB/c mice. Can J Microbiol. 1986;32:673–678. [DOI] [PubMed] [Google Scholar]
- 26.Rodrgue L, Barras MJ, Marcotte H, Lavoie MC. Bacterial colonization of the oral cavity of the BALB/c mouse. Microb Ecol. 1993;26: 267–275. [DOI] [PubMed] [Google Scholar]
- 27.Yurkovetskiy L, Burrows M, Khan AA, et al. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39:400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Velusamy SK, Ganeshnarayan K, Markowitz K, et al. Lactoferrin knockout mice demonstrates greater susceptibility to Aggregatibacter actinomycetemcomitans-induced periodontal disease. J Periodontol. 2013;84:1690–1701. [DOI] [PubMed] [Google Scholar]
- 29.Delima A, Spyros K, Amar S, Graves DT. Inflammation and tissue loss caused by periodontal pathogens is reduced by IL-1 antagonists. J Infect Dis. 2002;186:511–516. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Zhang Q. Periodontitis aggravated pancreatic β-cell dysfunction in diabetic mice through interleukin-12 regulation on Klotho. J Diabetes Investig. 2016;7:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abusleme L, Dupuy AK, Dutzan N, et al. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013;7:1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hajishengallis G. The inflammophilic character of the periodontitis-associated microbiota. Mol Oral Microbiol. 2014;29:248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato M, Kuroda S, Mansjur KQ, et al. Low-intensity pulsed ultrasound rescues insufficient salivary secretion in autoimmune sialadenitis. Arthritis Res Ther. 2015;17:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Y, Yang J, Zhang L, Zhou X, Cisar JO, Palmer RJ Jr. Differential utilization of basic proline-rich glycoproteins during growth of oral bacteria in saliva. Appl Environ Microbiol. 2016;82:5249–5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiang TK, Ensom MH. A qualitative review on the pharmacokinetics of antibiotics in saliva: Implications on clinical pharmacokinetic monitoring in humans. Clin Pharmacokinet. 2016;55:313–358. [DOI] [PubMed] [Google Scholar]
- 36.Lupp C, Robertson ML, Wickham ME, et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:119–129. [DOI] [PubMed] [Google Scholar]
- 37.Conti S, dos Santo SS, Koga-Ito CY, Jorge AO. Enterobacteriaceae and Pseudomonadaceae on the dorsum of the human tongue. J Appl Oral Sci. 2009;17:375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldberg S, Cardash H, Browning H, Sahly H, Rosenberg M. Isolation of Enterobacteriaceae from the mouth and potential association with malodor. J Dent Res. 1997;76:1770–1775. [DOI] [PubMed] [Google Scholar]
- 39.Gamboa F, García DA, Acosta A, et al. Presence and antimicrobial profile of gram-negative facultative anaerobe rods in patients with chronic periodontitis and gingivitis. Acta Odontol Latinoam. 2013;26:24–30. [PubMed] [Google Scholar]
- 40.Lungová V, Radlanski RJ, Tucker AS, Renz H, Míšek I, Matalovtá E. Tooth-bone morphogenesis during postnatal stages of mouse first molar development. J Anat. 2011;218:699–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li LL, Liu PH, Xie XH, et al. Loss of epithelial FAM20A in mice causes amelogenesis imperfecta, tooth eruption delay gingival overgrowth. Int J Oral Sci. 2016;8:98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griffen AL, Beall C, Campbell JH, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pryosequencin. ISME J. 2012;66:1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colombo AP, Bennet S, Cotton SL, et al. Impact of periodontal therapy on the subgingival microbiota of severe periodontitis: Comparison between good responders and individuals with refractory periodontitis using the human oral microbe identification microarray. J Periodontol. 2012;83:1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]