Abstract
Background
Single‐dose, postoperative intravesical chemotherapy reduces the risk of bladder cancer recurrence after transurethral resection of bladder tumours. However, there is limited evidence whether single‐dose intravesical chemotherapy is similarly effective at preventing bladder cancer recurrence after nephroureterectomy.
Objectives
To assess the effects of single‐dose intravesical chemotherapy instillation after nephroureterectomy for upper tract urothelial carcinoma.
Search methods
We performed a comprehensive literature search using multiple databases (MEDLINE, Cochrane Library, Embase, Scopus, Web of Science, and LILACS), trials registries, other sources of grey literature, and conference proceedings published up to April 15 2019, with no restrictions on language or status of publication.
Selection criteria
We included randomised controlled trials in which participants either received or did not receive single‐dose intravesical chemotherapy instillation after nephroureterectomy.
Data collection and analysis
Two review authors screened and independently assessed studies and extracted data from included studies. We performed statistical analyses using a random‐effects model. We rated the certainty of evidence according to the GRADE approach.
Main results
The search identified two studies (a multicenter study from Japan and the United Kingdom) with 361 participants.
Primary outcomes
Our results indicate that single‐dose intravesical chemotherapy instillation may reduce the risk of bladder cancer recurrence over time compared to no instillation (hazard ratio [HR]: 0.51, 95% confidence interval [CI]: 0.32 to 0.82, low‐certainty evidence). After 12 months follow‐up, this would result in 127 fewer bladder cancer recurrences (95% CI: 182 to 44 fewer bladder cancer recurrences) per 1000 participants. We downgraded the certainty of evidence by two levels due to study limitations and imprecision.
We found no trials that reported on the outcomes of time to death from upper tract urothelial carcinoma. The effect of single‐dose intravesical chemotherapy instillation on serious adverse events is uncertain (risk ratio [RR]: not estimable, 95% CI: not estimable, there were no events, very low‐certainty evidence). We downgraded the certainty of evidence by one level due to study limitations and by two levels due to imprecision.
Secondary outcomes
We found no trials that reported on the outcomes of time to death from any cause and participants’ disease‐specific quality of life. The effect of single‐dose intravesical chemotherapy instillation on minor adverse events is uncertain (risk ratio [RR]: not estimable, 95% CI: not estimable, there were no events, very low‐certainty evidence). We downgraded the certainty of evidence by one level due to study limitations and by two levels due to imprecision.
Authors' conclusions
For patients who have undergone nephroureterectomy for upper tract urothelial carcinoma, single‐dose intravesical chemotherapy instillation may reduce bladder cancer recurrence after nephroureterectomy. However, we are uncertain as to the risk of serious (and minor) adverse events. We found no evidence for the outcome of time to death from upper tract urothelial carcinoma. We were unable to conduct any of the preplanned subgroup analyses, particularly those based on operative approach, pathologic stage, and method of bladder cuff excision.
Plain language summary
Should we administer single‐dose chemotherapy to the bladder after removing the kidney and ureter for the treatment of renal pelvis and ureter cancer?
Review question
In people with cancer of the inner lining of their kidney and ureter (the tube that transports urine from the kidney to the bladder) who are having surgery to remove the kidney and ureter, what are the effects of a one‐time dose of chemotherapy into their bladder after surgery.
Background
In people with cancer of the inner lining of the bladder, one‐time chemotherapy put into the bladder (after the tumour has been removed) is helpful in making the cancer less likely to come back. We don't know whether the same is true for people in whom the same type of cancer is found in the inner lining of the kidney and ureter. Even if it does, it may also make these people have serious unwanted effects. We performed this study to summarise the best available evidence on the effects of one‐time dose of chemotherapy in these people after removal of the kidney and ureter for cancer.
Study characteristics
We found two randomised controlled studies (RCTs), with a total of 361 participants that compared a single‐dose chemotherapy placed in the bladder to no chemotherapy in people having their kidney and ureter removed for cancer of the inner lining of the kidney or ureter, or both. These findings are based on a literature search up to April 15, 2019.
Key results
We found that a one‐time dose of chemotherapy put into the bladder after surgery may reduce the risk of this type of tumor coming back in the bladder over time compared to no chemotherapy. We found no evidence whether this affects the time to death from this type of cancer. Serious unwanted effects appear to be rare and not increased with chemotherapy, but we are uncertain of this finding.
Certainty of the evidence
Our confidence in the evidence for the effect on the risk of recurrence within the bladder is low. This means that the true effect may be very different from what this review shows. The certainty of evidence for the effects of one‐time chemotherapy put into the bladder on serious unwanted effects was very low. This means that we are very uncertain about this result.
Summary of findings
Summary of findings for the main comparison. Single‐dose intravesical chemotherapy instillation versus placebo or observation after nephroureterectomy for upper tract urothelial carcinoma.
| Single‐dose intravesical chemotherapy instillation versus placebo or observation after nephroureterectomy for upper tract urothelial carcinoma | |||||
| participants: people who received nephroureterectomy due to UTUC Setting: a multicenter study from Japan and the United Kingdom (likely inpatients) Intervention: single‐dose intravesical chemotherapy instillation (pirarubicin and mitomycin) Comparison: no instillation (observation) | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with no instillation (observation) | Risk difference with single‐dose intravesical chemotherapy instillation | ||||
|
Time to bladder cancer recurrence (absolute effect size estimates based on recurrence rate at 12 months) Follow‐up: median 12 to 24.9 months |
311 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | HR 0.51 (0.32 to 0.82) | Study population | |
| 283 per 1,000 3 | 127 fewer per 1,000 (182 fewer to 44 fewer) | ||||
| High | |||||
| 500 per 1,000 4 | 202 fewer per 1,000 (301 fewer to 66 fewer) | ||||
| Low | |||||
| 150 per 1,000 4 | 70 fewer per 1,000 (99 fewer to 25 fewer) | ||||
|
Time to death from UTUC5 Follow‐up: median 12 to 24.9 months |
Not reported | ‐ | ‐ | Study population | |
| ‐ | ‐ | ||||
| Serious adverse events Follow‐up: median 12 to 24.9 months | 311 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 6 | No events | Study population | |
| 0 per 1,000 | 0 fewer per 1,000 (0 fewer to 0 fewer) | ||||
|
Time to death from any cause5 Follow‐up: median 12 to 24.9 months |
Not reported | ‐ | ‐ | Study population | |
| ‐ | ‐ | ||||
| Minor adverse events Follow‐up: median 12 to 24.9 months | 72 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 6 | No events | Study population | |
| 0 per 1,000 | 0 fewer per 1,000 (0 fewer to 0 fewer) | ||||
|
Disease‐specific quality of life5 Follow‐up: median 12 to 24.9 months |
Not reported | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; HR: Hazard ratio; RR: Risk ratio; RCT: Randomised controlled trial; UTUC: Upper tract urothelial carcinoma | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded by one level for study limitations: unclear or high risk of bias in one or more domains
2 Downgraded by one level for imprecision: confidence interval crossed assumed threshold of clinical importance
3 Baseline risk for bladder cancer recurrence in the no instillation (observation) group was assumed to be 28.3% (moderate risk) at 12 months based on pooled estimates from the two included studies
4 Baseline risk for bladder cancer recurrence in the no instillation (observation) group was assumed to be 15% (low risk) and 50% (high risk) at 12 months as reported by Azémar 2011 and Xylinas 2013, respectively (both observational studies)
5 Time to death from upper tract urothelial carcinoma; time to death from any cause; disease‐specific quality of life: no available data
6 Downgraded by two level for imprecision: no events
Background
Description of the condition
Upper tract urothelial carcinoma (UTUC) is an urothelial malignancy involving the renal pelvis or ureter. It is a relatively rare disease and accounts for approximately 5% to 10% of all urothelial carcinomas (Siegel 2014). Ureteral tumours are even less common and have an incidence of approximately one‐quarter that of renal pelvic tumours (Hall 1998). UTUC is mostly found in people from the age of 50 to 80 years, and its incidence is twice as high in men than in women (Siegel 2014). UTUC and bladder carcinomas have common pathogenic mechanisms and show analogous tumour characteristics with similar prognostic risk factors (Novara 2007; Sylvester 2006). Hence, much of the clinical decision‐making for UTUC is extrapolated from the larger evidence base on bladder cancer (Green 2013; Kim 2015). The cause of UTUC is still not known, but many environmental factors, such as smoking cigarettes, medication (e.g. Chinese herbs and aristolochic acid), chronic infection, exposure to carcinogenic chemicals, and occupational carcinogenesis, have been linked to the development of UTUC (Colin 2009).
Given that symptoms of both localised diseases (hematuria, dysuria) and advanced diseases (weight loss, fatigue, anaemia, bone pain) are similar to those of bladder cancer, their diagnostic approaches also overlap. The recommended evaluation of UTUC includes computed tomography urography (CTU) or magnetic resonance urography (usually only performed when CTU is contraindicated), cystoscopy, and urine cytology (NCCN Guideline 2018; Rouprêt 2018). Other commonly used local imaging modalities include retrograde ureteropyelography or ureteroscopy. Chest radiography and CT chest and bone scans are often also part of the diagnostic pathway for the staging of the disease to rule out metastatic spread (NCCN Guideline 2018). The stages of UTUC are classified as follows: Stage 0a: TaN0M0, Stage 0is: TisN0M0, Stage I: T1N0M0, Stage II: T2N0M0, Stage III: T3N0M0 and Stage IV: T4NXM0, or Any T N1M0, Any T N2M0, Any T, and Any N M1 (Amin 2017).
Currently, the gold standard of treatment for UTUC is radical nephroureterectomy (RNU) with bladder cuff excision. This procedure is associated with a risk of chronic kidney disease due to the loss of a kidney. This procedure is generally performed in cases of high‐risk UTUC with a normal contralateral kidney (NCCN Guideline 2018; Rouprêt 2018). Kidney‐sparing surgeries, such as ureteroscopic and percutaneous tumour removal (e.g. endoscopic tumour ablation or resection) and segmental ureterectomy, are alternatives to RNU when patients have impaired renal function, low‐risk disease, or prohibitive surgical risks (Bagrodia 2013; Oya 2015).
Following RNU, there is a risk of intravesical recurrence (IVR; recurrence in the bladder), which is estimated to occur in 22% to 47% of patients within an approximate two‐year postoperative time period (Cho 2014; Lee 2017; Xylinas 2014). Several studies have elucidated the identification of postoperative prognostic factors in order to be able to propose adjuvant intravesical chemotherapy and risk‐based surveillance to patients at high risk of disease recurrence (Azémar 2011; Lee 2017; Mbeutcha 2017). The proposed prognostic factors of IVR are aggressiveness of the tumour (i.e. advanced stage), tumour size, a tumour located in the ureter, laparoscopic surgical approaches, and positive surgical margins (Azémar 2011; Lee 2017; Mbeutcha 2017). Given that these factors are derived from retrospective studies, the true value of these factors remains unclear but provides the rationale of adjuvant intravesical chemotherapy (Mbeutcha 2017).
In this Cochrane Review, we define IVR as bladder cancer recurrence after RNU for UTUC. IVR is managed in the same way as recurrent bladder tumours in other settings, namely by transurethral resection, intravesical instillation immunotherapy or chemotherapy, and close surveillance using cystoscopy and urinary cytology (Griffiths 2013). This results in substantial economic costs due to the continued surveillance, diagnosis, and treatment of bladder cancer recurrences (Svatek 2014). This societal cost is compounded by the decrease in productivity of participants and their time lost from work (Svatek 2014).
Description of the intervention
Following the transurethral resection of bladder tumour (TURBT), the single‐dose intravesical instillation of chemotherapy has been shown to decrease the risk of recurrence if a noninvasive disease is suspected (Abern 2013; Perlis 2013; Sylvester 2004). Mitomycin C (MMC) is the most commonly used intravesical chemotherapeutic agent in this setting, but epirubicin and pirarubicin (THP) have also been shown to be beneficial in reducing the risk of recurrence (NCCN Guideline 2018; Sylvester 2016). The rationale and explanation for its efficacy is thought to be based on its antitumour effect, as it can destroy tumour cells floating in the irrigation fluid and urine after TURBT, and its ablative effect on residual tumour cells at sites of resection and on small overlooked tumours (Sylvester 2016). These, or similar agents, may be used in a similar context to prevent IVR after RNU.
For intravesical chemotherapy instillation, a two‐way catheter is inserted into the bladder in a sterile manner inside the operative field at the beginning of the surgical procedure. After surgery, when the bladder has been completely drained, chemotherapeutic agents (e.g. 40 mg of MMC in 40 mL of sterile water or 30 mg of THP in 30 mL of sterile water) are passed into the bladder through the catheter and the catheter is then clamped. After a certain period of time (typically 30 to 60 minutes with or without position changes), the catheter is unclamped, and the chemotherapeutic agents are allowed to drain passively. The catheter bag is then discarded as cytotoxic waste. The bladder is occasionally irrigated with saline at the physician's discretion. Post‐RNU intravesical instillation is usually recommended within 24 to 72 hours post‐operation (NCCN Guideline 2018; Rouprêt 2018), but can also be performed later (up to a week after RNU).
Adverse event of the intervention
Adverse events can be categorised as local or systemic. The incidence of local adverse events related to single‐dose intravesical installations is approximately 10%, according to perioperative trials conducted after the TURBT, with the most common adverse events being increased urinary frequency, urinary urgency, dysuria, hematuria, and bladder or pelvic pain and prostatitis. These adverse events are usually self‐limiting (Sylvester 2004; Williams 2010). The most severe adverse events related to this setting are extravasation from the bladder with local toxicity in the pelvis, peritoneum, or both. Even though the bladder wall is usually closed post‐RNU, this site is potentially more vulnerable to extravasation after intravesical chemotherapy administration. Other systemic and serious local adverse events, such as perivesical fat necrosis, bladder ulceration, perirectal fat necrosis with abscesses, and myelosuppression, are relatively rare (Griffin 2013; Lu 2017).
How the intervention might work
There are two main theories that may explain the occurrence of IVR after RNU. The first theory suggests that preoperative carcinogen exposure in the entire urothelium accounts for independent tumour development following RNU. Alternatively, the intraluminal seeding and implantation theory proposes that the bladder is continuously exposed to cancer cells dropping from the upper urinary tract before and during RNU, which may be responsible for IVR (Habuchi 1993; Jones 2005). The mechanism of action for intravesical chemotherapy involves the delivery of high concentrations of anticancer drugs to the bladder, potentially destroying circulating tumour cells within the urine that remain after RNU and preventing the seeding of cancer cells shed from UTUC. Moreover, intravesical chemotherapy may suppress the implantation of cancer cells, thereby reducing the likelihood of IVR following RNU.
Why it is important to do this review
There is limited evidence regarding the effectiveness of single‐dose intravesical chemotherapy in preventing IVR after RNU. Although several systematic reviews and meta‐analyses have been conducted on this topic (Fang 2013; Wu 2015; Yuan 2015), there is still considerable uncertainty in this area. In addition, none of the aforementioned published reviews have been conducted using rigorous methodologies, nor have they used the GRADE methodology to rate the certainty of evidence. This systematic review evaluates the best available evidence on the effectiveness of single‐dose intravesical chemotherapy in preventing IVR after nephroureterectomy that exists to date and includes an independent assessment of the risks of bias and the rating of the certainty of evidence using the GRADE methodology. A survey of urologic oncologists regarding the use of intravesical chemotherapy after nephroureterectomy reported that almost half of the included urologists (44%) did not use intravesical chemotherapy due to the lack of supporting data (Lu 2017). We expect this review to be helpful for clinicians', guideline developers’ and policy‐makers' decisions seeking to establish the current role for single‐dose intravesical chemotherapy after RNU.
Objectives
To assess the effects of single‐dose intravesical chemotherapy instillation after nephroureterectomy for upper tract urothelial carcinoma.
Methods
Criteria for considering studies for this review
Types of studies
This review is based on a previously published protocol (Hwang 2018). For details on the differences between that protocol and this review, please refer to the 'Differences between protocol and review' section. We included randomised controlled trials (RCTs), as they offer the most reliable results. We excluded quasi‐randomised and non‐randomised studies, cohort studies, case series, cross‐over trials, and cluster‐randomised trials. We did not exclude studies on the basis of publication status or language.
Types of participants
We included studies that used participants with localised or locally‐advanced UTUC (Stage 0a, Stage 0is, and Stages I ‐ IV) (Amin 2017), as determined via cross‐sectional imaging, biopsy, or both. We excluded trials that had participants with known metastatic diseases. We also excluded trials that had participants who underwent kidney‐sparing surgery, segmental ureterectomy, or endoscopic tumour removal (e.g. ureteroscopic tumour removal or ablation), and those that had participants with a history of bladder tumours or intravesical chemotherapy. We included studies using diverse methods of bladder cuff management due to the lack of a gold standard. We included studies irrespective of intravesical chemotherapy instillation timing, the duration of how long the chemotherapeutic agent remains in the bladder, and the change of the participants' position after instillation.
Types of interventions
We planned to investigate the following experimental and comparator intervention comparisons.
Experimental interventions
Single dose of any intravesical chemotherapeutic agent instillation (e.g. mitomycin, epirubicin, pirarubicin, gemcitabine, etc.) after RNU (Rouprêt 2018)
Comparator interventions
Observation
Placebo
Concomitant interventions had to be the same in the experimental and comparator groups to ensure fair comparisons.
Types of outcome measures
We did not exclude trials if they met inclusion criteria but did not report one or several of our primary or secondary outcomes.
Primary outcomes
Time to bladder cancer recurrence (time‐to‐event outcome)
Time to death from UTUC (time‐to‐event outcome)
Serious adverse events (dichotomous outcome)
Secondary outcomes
Time to death from any cause (time‐to‐event outcome)
Minor adverse events (dichotomous outcome)
Disease‐specific quality of life (continuous outcome)
We used a previously‐reported minimum clinically important difference (MCID) to assess participants’ disease‐specific quality of life (e.g. European Organisation for Research and Treatment of Cacer core quality of life questionnaire version 3.0 (EORTC QLQ‐C30 v. 3.0) > 10 points) in order to rate the certainty of evidence for imprecision, and these results can be found in our 'Table 1' (Cocks 2008; Johnston 2013). We could not find any published information about a clinically important difference for time‐to‐event outcomes (i.e. time to bladder cancer recurrence, time to death from UTUC and from any cause) and dichotomous outcomes (i.e. adverse events). We, therefore, used a relative risk reduction (RRR), risk ratio (RR), or hazard ratio (HR) of at least 25%, based on the guidance in Guyatt 2011a.
Method and timing of outcome measurement
-
Time to bladder cancer recurrence: as measured from the time of randomisation to the time of the first confirmed bladder cancer recurrence
Definition of recurrence: judged based on the cystoscopic visual appearance of the tumour or histopathologic proof of recurrence
Time to death from UTUC: as measured from the time of randomisation to the time of death due to UTUC
Time to death from any cause: as measured from the time of randomisation to the time of death due to any cause
Adverse events: determined by the Common Terminology Criteria for Adverse Events (CTCAE). We classified grade 3 or higher complications as serious (e.g. bladder perforation and the need for invasive intervention, gross hematuria, and the need for hospitalisation), and grade 1 and 2 complications as minor (e.g. dysuria, hematuria, and the need for bladder irrigation). If the authors did not use the CTCAE, we graded the adverse events as described in their respective studies.
We considered adverse events that appeared within six months after randomisation. If we were unable to retrieve the necessary information to assess time‐to‐event outcomes, we tried to assess the number of events per the total number of included participants in each relevant study for dichotomised outcomes at 12 months, 24 months, 36 months, and 60 months for bladder cancer recurrence, death from UTUC, and death from any cause.
Search methods for identification of studies
We performed a comprehensive literature search with no restrictions on language or the status of publication. We planned to rerun searches within three months prior to the anticipated publication of the review.
Electronic searches
We searched the following sources for relevant literature that was published since the inception of each database (Appendix 1). The date of the last search for all databases was April 15, 2019.
MEDLINE via Ovid (from 1946);
Cochrane Library (Issue 4, April 2019);
Embase (Elsevier, 1947 ‐ present);
Scopus (1966 ‐ present);
Web of Science (1900 ‐ present);
LILACS (Latin American and the Caribbean Health Sciences Literature; www.bireme.br/; 1982 ‐ present).
We also searched the following.
ClinicalTrials.gov (www.clinicaltrials.gov/);
World Health Organization (WHO) International Clinical Trials Registry Platform search portal (apps.who.int/trialsearch/);
'Grey literature' repository from the current Grey Literature Report (www.greylit.org/).
If we detected additional relevant key words during our literature search, we modified our electronic search strategies to incorporate these terms and documented the changes.
Searching other resources
We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of included trials, reviews, and meta‐analyses. We also contacted the authors of included trials to identify any further studies that we may have missed. We searched the abstract proceedings of any relevant meetings conducted during the last three years (2016 to 2018) by the American Urological Association, European Association of Urology, and American Society of Clinical Oncology to search for unpublished studies.
Data collection and analysis
In this review, we followed the methodological recommendations given by Cochrane (Higgins 2017a).
Selection of studies
We used EndNote reference management software to identify and remove potential duplicate records. Two review authors (ECH and JHJ) independently assessed abstracts and titles to determine which studies should be assessed further using Covidence software. Two review authors (ECH and JHJ) investigated all potentially relevant records, such as full texts, mapped records to studies, and classified them as included studies, excluded studies, studies awaiting classification, or ongoing studies in accordance with the criteria provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017a). We resolved any discrepancies through consensus or by recourse to a third review author (PD). If a resolution was not possible, we designated the study as 'awaiting classification'. We documented the reasons for the exclusion of studies in the 'Characteristics of excluded studies' table. We presented an adapted PRISMA flow diagram showing the process of study selection (Liberati 2009).
Data extraction and management
Two review authors (ECH and NS) independently extracted relevant data using a data extraction form. We based this form on the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017a) and pilot tested it before using it for our analysis. We resolved any potential disagreements by consensus or through discussion with a third review author (PD). In addition, when necessary, we contacted the original study authors. We collected and used the most detailed numerical data that might facilitate the similar analyses of included studies. When studies reported the median and range rather than the mean and standard deviation for continuous outcomes, we used the method provided by Hozo 2005. We detailed all characteristics of the included studies in the ‘Characteristics of included studies’ table.
Record citation (e.g. authors’ names and article title).
Details of methods: study design and date when the study was conducted.
Details of participants: setting; country; number of included participants; age; sex; inclusion and exclusion criteria; participants’ risk factors for bladder cancer recurrence, death from UTUC, and death from any cause; and previous or concomitant bladder tumours, including information on tumour stage (T category), tumour grade, tumour location (ureteral), the presence of concurrent carcinoma in situ, tumour multifocality, and the use of the bladder cuff excision method (Mbeutcha 2017).
Details of interventions: the number of participants randomly assigned to each intervention group and drug use, including dosage and dilution details, and the time point of instillation.
Details of outcomes: outcomes included in this review that were assessed in each study, including how each was measured and the times at which they were measured.
Study funding sources.
Declarations of interest among the primary study authors.
Dealing with duplicate and companion publications: In the event of duplicate publications, companion documents or multiple reports for a primary study, we maximised the yield of information by mapping all publications to unique studies and collating all available data. We used the most complete data set aggregated across all known publications. In case of doubt, we gave priority to the publication reporting the longest follow‐up associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
Two review authors (ECH and NS) independently assessed the risks of bias for each included study. We resolved disagreements by consensus, or by consulting with a third review author (PD). We used the Cochrane 'Risk of bias' assessment tool for the following domains (Higgins 2017b).
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other potential sources of bias (e.g. baseline imbalance).
We judged 'Risk of bias' domains as ’low risk,’ ’high risk’ or ’unclear risk’. We presented the results of this assessment graphically. For selection bias (random sequence generation and allocation concealment) and reporting bias (selective reporting), we evaluated the risks of bias at a trial level.
For performance bias (blinding of participants and personnel), we defined all outcomes as similarly susceptible to performance bias and assessed them in one group.
For detection bias (blinding of outcome assessments), we grouped outcomes as susceptible to detection bias (subjective) or not susceptible to detection bias (objective) outcomes. We defined the following outcome measures as subjective:
time to bladder cancer recurrence
time to death from UTUC
serious and minor adverse events
disease‐specific quality of life.
We defined the following outcome as objective:
time to death from any cause.
We assessed attrition bias (incomplete outcome data) by outcome. We summarised the risk of attrition bias across domains for each outcome in each included study, as well as across the studies and domains for each outcome, in accordance with the approach for the summary assessment of the risk of bias presented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017b).
Measures of treatment effect
When at least two included trials were available for a given outcome, we expressed dichotomous data as RRs with a 95% confidence interval (CI). For continuous outcomes measured on the same scale, we estimated the intervention effect using the mean difference (MD) with a 95% CI. For continuous outcomes measuring the same underlying concept (e.g. disease‐specific quality of life), but using different measurement scales, we planned to calculate the standardised mean difference (SMD). We expressed time‐to‐event data as HRs with 95% CIs or used an indirect estimation method if HRs were not given (Parmar 1998; Tierney 2007).
Unit of analysis issues
The units of analysis were each individual participant. If we had identified trials with more than two intervention groups for inclusion in this review, we would have handled these in accordance with guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
We planned to obtain missing data from the original authors of each included study, if feasible, and planned to perform intention‐to‐treat analyses if data were available. Otherwise, we performed available‐case analyses. We investigated attrition rates (e.g. dropouts, losses to follow‐up, and withdrawals) and critically appraised issues of missing data. We did not plan to impute missing data.
Assessment of heterogeneity
We identified heterogeneity (inconsistency) through the visual inspection of forest plots to assess the amount of overlap between 95% CIs and used the I2 statistic, which quantifies inconsistency across studies, to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003); we interpreted I2 as follows (Deeks 2017).
0% to 40%: may not be important.
30% to 60%: may indicate moderate heterogeneity.
50% to 90%: may indicate substantial heterogeneity.
75% to 100%: may indicate considerable heterogeneity.
When we identified heterogeneity, we attempted to determine the possible reasons for it by examining individual study and subgroup characteristics.
Assessment of reporting biases
We tried to obtain study protocols to assess selective outcome reporting. As we included only two studies for comparison in our review, we could not use funnel plots to assess any small study effects. Please refer to Differences between protocol and review.
Data synthesis
We performed data synthesis using Review Manager 5 (RevMan) software, provided by Cochrane (Review Manager 2014) in accordance with the guidelines contained in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017a). In the meta‐analyses, we used a random‐effects model. For dichotomous outcomes, we used the Mantel‐Haenszel method. For continuous outcomes, we used the inverse variance method. For time‐to‐event outcomes, we used the generic inverse variance method.
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to introduce clinical heterogeneity and planned to carry out subgroup analyses to investigate interactions.
Operative approach (open RNU versus laparoscopic RNU).
Pathologic stage (localised (Tis, Ta, T1, T2) versus locally advanced (T3, T4)).
Bladder cuff excision method (endoscopic excision versus extravesical or intravesical excision).
These subgroup analyses were based on the following observations:
High IVR rates are possibly associated with laparoscopic RNU, advanced tumour stage, and endoscopic bladder cuff excision (Xylinas 2014).
However, we could not perform any subgroup analyses due to the lack of relevant data.
Sensitivity analysis
We planned to perform sensitivity analyses in order to explore the influence of the following factors on effect size, if applicable:
Restricting the analysis by taking the risk of bias into account and excluding studies classified as having a high risk or unclear risk of bias.
However, we could not perform any sensitivity analyses due to the lack of relevant data.
'Summary of findings' table
Main outcomes for 'Summary of findings' table
We present a 'Table 1' that reports on the following outcome measures listed according to priority. One review author (PD) determined outcome measure priority using content expertise:
Time to bladder cancer recurrence
Time to death from UTUC
Serious adverse events
Time to death from any cause
Minor adverse events
Disease‐specific quality of life
We present the findings and the certainty of the available evidence according to the GRADE methodology (Schünemann 2017).
We assessed the overall certainty of evidence for each outcome according to the GRADE approach, which takes into account five criteria related, not only to internal validity (risk of bias, inconsistency, imprecision, and publication bias), but also to external validity (directness of results) (Guyatt 2008). Two review authors (ECH, JHJ) independently rated the certainty of evidence for each outcome as 'high', 'moderate', 'low', or 'very low'. We resolved discrepancies by consensus, or, if needed, by the arbitration of a third review author (PD). We present a summary of the evidence for the main outcomes in the 'Table 1', which we generated using the Gradepro GDT (gradepro.org/); This table provides key information about the best estimate of the magnitude of an effect in relative terms and presents absolute differences for each relevant comparison of alternative management strategies; numbers of participants and studies addressing each important outcome; and the rating of our overall confidence in the effect estimates for each outcome (Guyatt 2011b; Schünemann 2017).
Results
Description of studies
Results of the search
Our literature search yielded 121 references, to which we added an additional 25 studies that we identified by searching trial registries (Figure 1). After the exclusion of duplicates, we screened 97 references at the title and abstract stage. Of these 97 references, 12 references that were mapped to 10 unique studies entered the full‐text screening stage. We ultimately included two studies in the quantitative analyses. The reasons for exclusion at the full‐text screening stage are summarised in the PRISMA flow diagram (Figure 1), with further details provided in the ‘Characteristics of excluded studies’ table.
1.
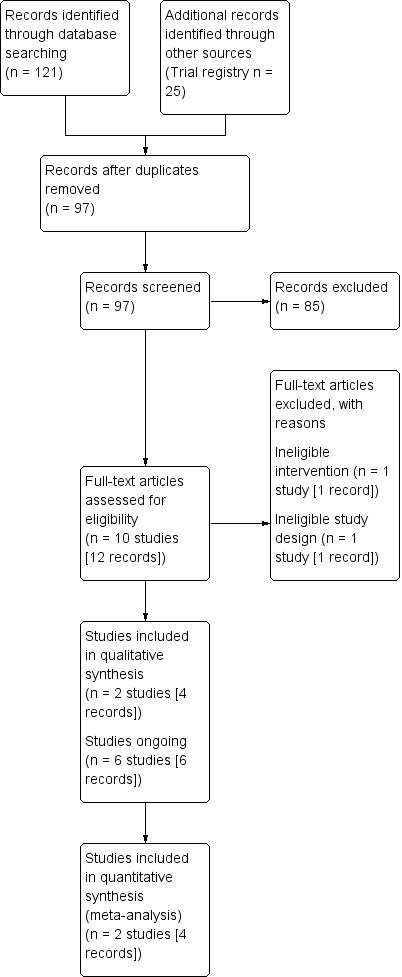
Study flow diagram.
Included studies
The details of the included studies are presented in the 'Characteristics of included studies' table; Table 2; Table 3.
1. Baseline characteristics of included study.
| Study name | Trial period (year to year) | Setting/Country | Description of participants | Intervention(s) and comparator(s) | Duration of follow‐up | Age | Gender |
| Ito 2013 | 2005 to 2008 | Multicentre/Japan | Participants with UTUC who underwent nephroureterectomy | Intervention: pirarubicin 30 mg in 30 mL of normal saline | 24.9 months (range: 2.6 to 39.3 months) | < 69 years; n = 18 (50%) ≥ 69 years; n = 18 (50%) |
Male: n = 22 (61.1%) Female: n = 14 (38.9%) |
| Comparator: no instillation | 13.7 months (range: 2.8 to 34.1 months) | < 69 years; n = 19 (52.8%), ≥ 69 years; n = 17 (47.2%) |
Male: n = 21 (58.3%) Female: n = 15 (41.7%) |
||||
| O'Brien 2011 | 2000 to 2006 | Multicentre/United Kingdom | Participants with UTUC who underwent nephroureterectomy | Intervention: mitomycin 40 mg in 40 mL of normal saline | 12 months | median 70 years (range: 44 to 87) | not reported |
| Comparator: no instillation | median 71 years (range: 36 to 90) | not reported |
UTUC: upper tract urothelial carcinoma
2. Participants in included study.
| Study name | Intervention(s) and comparator(s) | Screened/eligible (N) | Randomised (N) | Analysed (N): efficacya | Analysed (N): safetyb | Finishing trial (N (%)) |
| Ito 2013 | Intervention: pirarubicin 30mg in 30 mL of normal saline | N/A/77 | 39 | 36 | 36 | 32 |
| Comparator: no instillation | 38 | 36 | 36 | 31 | ||
| Total | 77 | 72 | 72 | 63 | ||
| O'Brien 2011 | Intervention:mitomycin 40 mg in 40 mL of normal saline | N/A/284 | 144 | 120 | 120 | 92 |
| Comparator: no instillation | 140 | 119 | 119 | 95 | ||
| Total | 284 | 239 | 239 | 187 | ||
| Grand total | 361 | 311 | 311 | 250 | ||
N/A: not available
a: The number of participants analysed for bladder cancer recurrence
b: The number of participants with adverse events
Source of data
All included trials were identified through the literature search (Ito 2013; O'Brien 2011).
Study design and settings
All included studies were parallel group RCTs (Ito 2013; O'Brien 2011). Both trials were open‐label, multicentred, and likely conducted in an inpatient setting. The included studies were performed in Japan (Ito 2013) and the UK (O'Brien 2011). Accrual periods ranged from 2000 to 2008.
Participants
This review included a total of 361 randomised participants with UTUC, of which 250 completed the trials. The median follow‐up period and age of participants ranged from 12 to 24.9 months (Ito 2013; O'Brien 2011) and 36 to 90 years old (O'Brien 2011), respectively. Participants were required to have an adequate functional status, as defined by the Eastern Cooperative Oncology Group as a score of less or equal to 2, and a life expectancy of more than one year. Prior or existing bladder cancer was not allowed in the included studies.
Interventions, comparators, and comparisons
The included trials administered single‐dose intravesical chemotherapy. However, these trials used different drugs and doses and were administered at different time periods. The Ito 2013 study used 30 mg of THP with 30 mL of normal saline, which was administered within 48 hours after RNU, while the O'Brien 2011 study used 40 mg of MMC with 40 mL of normal saline, which was administered at various time periods after RNU due to their concerns over the extravasation of chemotherapy.
The comparator in the included trials was no chemotherapy instillation (observation).
Outcomes
The predefined primary outcomes of time to bladder cancer recurrence and serious adverse events were identified in both included studies, while minor adverse events were only available in one of the included trials (Ito 2013). However, we were unable to evaluate time to death from UTUC, time to death from any cause, and disease‐specific quality of life because these outcomes were not investigated in the included studies.
Funding sources and conflicts of interest
All included studies reported receiving funding from multiple sources, including hospitals, pharmaceutical companies, and their respective governments (Ito 2013; O'Brien 2011). Conflicts of interests were reported as 'none' in the included studies.
Excluded studies
We excluded two studies on the basis that one had an ineligible intervention (multiple instillation (28 times) of chemotherapy) (Sakamoto 2001) and the other was a published trial protocol (not full text) with an ineligible study design (Van Doeveren 2018). The details of these excluded studies are presented in the ‘Characteristics of excluded studies’ table.
Studies awaiting classification and ongoing trials
There were no studies awaiting classification. We found six ongoing studies which did not provide usable outcome data at the time this review was written (JPRN‐UMIN000009682; Miyamoto 2018; NCT02547350; NCT02923557; NCT03062059; NCT03209206) (see ‘Characteristics of ongoing studies’ table).
Risk of bias in included studies
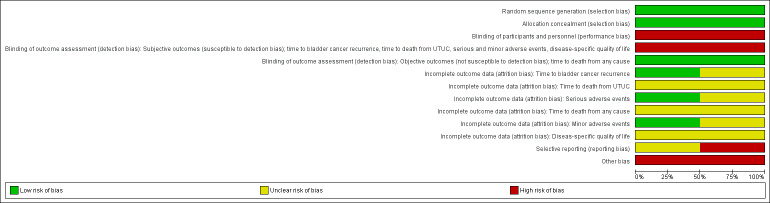
The detailed results of the 'risk of bias' assessment are provided in Figure 2 and Figure 3, and the judgements regarding the individual domains are provided in the ‘Characteristics of included studies’ table.
2.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Both included studies reported sufficient detail to provide the assurance of an adequate method of sequence generation and were rated as having a low risk of bias.
Allocation concealment
We rated all trials as having a low risk of bias as group allocation was performed centrally in both studies.
Blinding
Blinding of participants and personnel
We rated the included trials as having a high risk of performance bias because the participants and personnel were not blinded in either study.
Blinding of outcome assessments
We distinguished between the outcomes in which the blinding of outcome assessors appeared relevant (subjective outcomes; susceptible to detection bias) versus those in which it was not (objective outcomes; not susceptible to detection bias).
The subjective outcomes were time to bladder cancer recurrence, time to death from UTUC, serious and minor adverse events, and disease‐specific quality of life. We rated the included studies as having a high risk of detection bias because unblinded assessors were responsible for these outcomes. The objective outcome, time to death from any cause, was rated as having a low risk of detection bias in the included trials because blinding was unlikely to influence this outcome in either of the studies.
Incomplete outcome data
Time to bladder cancer recurrence and serious adverse events
We rated the Ito 2013 study as having a low risk of attrition bias regarding these outcomes, while the O'Brien 2011 study was rated as having an unclear risk of attrition bias due to a moderate number of participants lost to follow‐up.
Time to death from UTUC, time to death from any cause, and disease‐specific quality of life
We did not rate these domains because these outcomes were not investigated in the trials. We report the risk of bias as unclear in the tables and figures only because this is the default value.
Minor adverse events
We rated one study as having a low risk of attrition bias (Ito 2013) regarding this outcome. We did not rate the other study as it did not investigate this outcome (O'Brien 2011). Therefore, the risk of bias is reported as unclear in the table and figures.
Selective reporting
We rated one study as having an unclear risk of reporting bias (Ito 2013) and one as having a high risk of reporting bias (O'Brien 2011), since several outcomes were not predefined in the protocol or were not analysed as intended.
Other potential sources of bias
We rated the included trials as having a high risk of other potential biases due to imbalances of baseline characteristics, mainly with regard to the proportion of participants with carcinoma in situ, as well as different tumor grades and stages.
Effects of interventions
See: Table 1
Please refer to Analysis 1.1 to Analysis 1.3 and Table 1 for the main comparison.
1.1. Analysis.
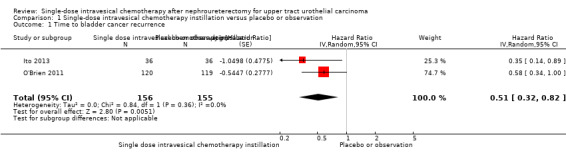
Comparison 1 Single‐dose intravesical chemotherapy instillation versus placebo or observation, Outcome 1 Time to bladder cancer recurrence.
1.3. Analysis.
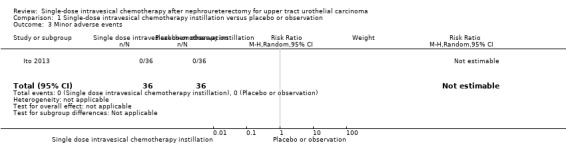
Comparison 1 Single‐dose intravesical chemotherapy instillation versus placebo or observation, Outcome 3 Minor adverse events.
Single‐dose intravesical chemotherapy instillation versus placebo or observation
Primary outcomes
Time to bladder cancer recurrence
Single‐dose intravesical chemotherapy instillation may reduce the risk of bladder cancer recurrence over time compared to no instillation (HR: 0.51, 95% CI: 0.32 to 0.82, two studies, 311 participants, Analysis 1.1, low‐certainty evidence). Based on the control event risk taken from the trials included in this analysis and 12 months follow‐up, this corresponds to 127 fewer bladder cancer recurrences (95% CI: 182 fewer to 44 fewer) per 1000 participants for those that undergo single‐dose intravesical chemotherapy. We rated the certainty of evidence as low due to study limitations and imprecision.
Based on low‐risk and high‐risk control groups as drawn from separate observational studies (Azémar 2011; Xylinas 2013) also at 12 months follow‐up, single‐dose intravesical chemotherapy may result in 70 fewer bladder cancer recurrences (95% CI: 99 fewer to 25 fewer) per 1000 participants or 202 fewer bladder cancer recurrences (95% CI: 301 fewer to 66 fewer) per 1000 participants, respectively.
Time to death from UTUC
We found no studies that reported this outcome.
Serious adverse events
We are uncertain whether single‐dose intravesical chemotherapy instillation has little to no effect on serious adverse events compared to no instillation as there were no serious adverse events in either group (RR: not estimable, 95% CI: not estimable, two studies, 311 participants, Analysis 1.2, very low‐certainty evidence). We downgraded the certainty of evidence by one level due to study limitations and by two levels for imprecision.
1.2. Analysis.
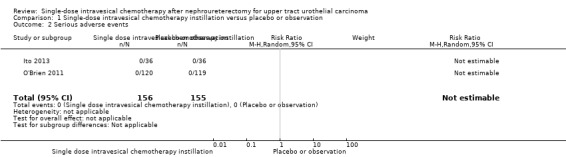
Comparison 1 Single‐dose intravesical chemotherapy instillation versus placebo or observation, Outcome 2 Serious adverse events.
Secondary outcomes
Time to death from any cause
We found no studies that reported this outcome.
Minor adverse events
We are uncertain whether single‐dose intravesical chemotherapy instillation has little to no effect on minor adverse events compared to no instillation as there were no minor adverse events in either group (RR: not estimable, 95% CI: not estimable, one study, 72 participants, Analysis 1.3, very low‐certainty evidence). We downgraded the certainty of evidence by one level due to study limitations and by two levels for imprecision.
Disease‐specific quality of life
We found no studies that reported this outcome.
Subgroup analysis
We were unable to perform any of the predefined subgroup analyses based on operative approach, pathologic stage and method of bladder cuff excision.
Sensitivity analysis
We rated both of the included studies as having a high or unclear risk of bias overall and were therefore unable to perform a meaningful sensitivity analysis.
Discussion
Summary of main results
We included two RCTs with 361 participants. The findings of this systematic review indicate that single‐dose intravesical chemotherapy instillation may increase the time to bladder cancer recurrence compared to no chemotherapy installation. We found no evidence on the risk of death from UTUC. We are uncertain whether single‐dose intravesical chemotherapy instillation has little or no effect on serious (and minor) adverse events.
We also found no evidence about the effect of single‐dose intravesical chemotherapy instillation on the time to death from any cause and disease‐specific quality of life.
Overall completeness and applicability of evidence
The following issues deserve consideration:
Findings of this review were based on only two, relatively small studies, which limits the generalisability of its findings.
Information on time‐to recurrence was limited to 12 months; therefore, is of very short‐term nature.
We stipulated that factors such as surgical approach, pathological stage, and technique of managing the bladder cuff could be important effect modifiers but were unable to conduct any relevant subgroup analyses.
The included studies used two different chemotherapeutic agents and instillation time periods (please refer to the ‘Characteristics of included studies’ table). However, this review is unable to address whether one drug is more effective than another and what the optimal timing of instillation should be.
Findings of this systematic review were limited to evidence from randomised controlled trials that yielded low quality at best. The consideration of non‐randomised controlled trials may have provided some evidence for additional outcomes such as adverse events (Schünemann 2013). Also, while we believe this to be unlikely, it is possible that they could have provided higher quality for time‐to‐recurrence.
Quality of the evidence
We rated the certainty of evidence as low to very low. The reasons for downgrading the certainty of evidence were as follows:
Study limitations: Neither of the studies blinded participants or personnel, which raises concerns about performance bias. For the subjective outcomes, there is also a similar concern over detection bias. In conjunction with incomplete outcome data and the concerns over other sources of bias (i.e. baseline imbalances in each group), this prompted us to downgrade the certainty of evidence.
Imprecision: The finding of wide confidence intervals that crossed the thresholds of clinical relevance, rare events, or both led to the downgrading of the certainty of evidence.
Selective reporting bias: We rated one study as unclear, the other as high risk of bias due to discordances between planned and actual outcome reporting and/or analyses.
Potential biases in the review process
Despite our comprehensive literature searching strategy without any publication status or language restrictions, there is a possibility that we may have missed studies that were published in a language other than English, published in non‐indexed journals, or not published at all.
The number of studies included in this review was insufficient to generate funnel plots. Therefore, we may have underestimated the risk of publication bias.
We contacted study authors on several occasions and they provided feedback to some of our queries, but only one (O'Brien 2011) provided the additional data we requested, which may also be a potential source of bias.
Agreements and disagreements with other studies or reviews
We identified existing systematic reviews on this topic (Deng 2014; Fang 2013; Wu 2015; Yuan 2015). Similar to our results, all of these reviews reported that intravesical chemotherapy reduces bladder cancer recurrence and causes little to no minor adverse events, even though they pooled single and multiple chemotherapy instillations and different study designs.
However, only our review applied the necessary methodological rigor. Unlike this review, previous systematic reviews did not publish protocols nor did they rate the certainty of evidence (Deng 2014, Fang 2013, Wu 2015, Yuan 2015). We believe that three studies did not apply the 'risk of bias' tool (Fang 2013; Wu 2015; Yuan 2015) appropriately and that two studies have unit of analysis errors (Deng 2014, Fang 2013). In addition, none of the existing systematic reviews included a certainty of evidence rating. We therefore believe that our systematic review provides the most reliable summary of evidence on this topic to date, thereby fulfilling an important role in guiding evidence‐based decision‐making.
Authors' conclusions
Implications for practice.
Single‐dose intravesical chemotherapy instillation after nephroureterectomy for UTUC may reduce the risk of recurrence over time. However, we are very uncertain as to the risk of serious (and minor) adverse events. This major uncertainty surrounding this outcome that is critical to the trade‐off of desirable and undesirable effects of this treatment approach relates to the small number of included studies, their small sample size, and the possibility of selective reporting bias for harm outcomes. We also found no RCT evidence for other patient‐important outcomes such as disease‐specific survival, overall survival and quality of life.
Implications for research.
Our knowledge on this topic can be improved by focusing on the following issues:
The body of evidence in this review comes from relatively small studies of limited methodological quality. More rigorous, adequately powered trials are necessary.
It is important that future trials assess the head‐to‐head comparisons of chemotherapeutic drugs, as well as the evidence for optimal chemotherapy instillation time periods. Moreover, recent evidence suggests that gemcitabine is more effective than mitomycin in preventing bladder cancer recurrence in non‐muscle invasive bladder cancer (Addeo 2010). Future research should ascertain the efficacy and safety of gemcitabine instillation for preventing bladder cancer recurrence after nephroureterectomy for UTUC.
Future studies with longer‐term data (beyond 12 months) should also provide data on disease‐specific survival, overall survival, and quality of life.
There is a need for both randomised trials as well as prospective observational studies that assess the true burden of this intervention in terms of side effects and quality of life impact.
Notes
Parts of the Methods section of this protocol are based on a standard template developed by the Cochrane Metabolic and Endocrine Disorders Group, which has been modified and adapted for use by Cochrane Urology.
Acknowledgements
We acknowledge the support received from the author of one included study, Tim O'Brien, who provided additional, unpublished data.
We are very grateful to Alon Weizer and George Thalmann for having served as peer reviewers.
We thank Cochrane Urology, our contact editor Andrew Shepherd, and Managing Editor Robert Lane for the support we received.
Appendices
Appendix 1. Search strategy
| MEDLINE | |
| 1 | exp NEPHRECTOMY/ |
| 2 | Kidney Neoplasms/su [Surgery] |
| 3 | Ureteral Neoplasms/su [Surgery] |
| 4 | (Nephrectom$ or Nephroureterectom$ or Nephro‐ureterectom$ or Ureteronephrectom$).tw. |
| 5 | 1 or 2 or 3 or 4 |
| 6 | exp DOXORUBICIN/ |
| 7 | (23214‐92‐8 or 25316‐40‐9).rn,tw. |
| 8 | (Doxorubicin$ or Caelyx or Doxil or Myocet or Adriblastin$ or Adriablastin$ or Doxolem or Adrimedac or Farmiblastina or Ribodoxo or DOXO‐cell or Onkodox).nm,tw. |
| 9 | exp EPIRUBICIN/ |
| 10 | (56390‐09‐1 or 56420‐45‐2).rn,tw. |
| 11 | (Epirubicin$ or Farmorubicin$ or Pharmorubicin$ or IMI28 or Ellence or Epidoxorubicin or Epi‐Doxorubicin or Epiadriamycin or Epi‐Adriamycin or EPI‐cell or EPIcell or Epilem).nm,tw. |
| 12 | exp MITOMYCIN/ |
| 13 | (1404‐00‐8 or 50‐07‐7 or 74349‐48‐7).rn,tw. |
| 14 | (Mitomycin$ or Mitomicin$ or Mitocin$ or Ametycin$ or Mutamycin$).nm,tw. |
| 15 | exp THIOTEPA/ |
| 16 | 52‐24‐4.rn,tw. |
| 17 | (Thiotepa or Thio‐tepa or Tespa$ or Thiophosphamide or Girostan).nm,tw. |
| 18 | (Gemcitabin$ or Gemcetabin$ or Gemcatabin$ or Gemzar$).nm,tw. |
| 19 | 103882‐84‐4.rn,tw. |
| 20 | 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 |
| 21 | exp Administration, Intravesical/ |
| 22 | ((Bladder or Intravesical) adj2 (Administration$ or Injection$ or Instillation$)).tw. |
| 23 | exp Urinary Bladder Neoplasms/su [Surgery] |
| 24 | exp Urinary Bladder Neoplasms/pc [Prevention & Control] |
| 25 | 21 or 22 or 23 or 24 |
| 26 | 20 and 25 |
| 27 | 5 and 26 |
| 28 | randomized controlled trial.pt. |
| 29 | controlled clinical trial.pt. |
| 30 | randomized.ab. |
| 31 | placebo.ab. |
| 32 | drug therapy.fs. |
| 33 | randomly.ab. |
| 34 | trial.ab. |
| 35 | groups.ab. |
| 36 | 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 |
| 37 | exp animals/ not humans.sh. |
| 38 | 36 not 37 |
| 39 | 27 and 38 |
| Cochrane Library | |
| 1 | MeSH descriptor: [Nephrectomy] explode all trees |
| 2 | MeSH descriptor: [Kidney Neoplasms] explode all trees and with qualifier(s): [surgery ‐ SU] |
| 3 | MeSH descriptor: [Ureteral Neoplasms] explode all trees and with qualifier(s): [surgery ‐ SU] |
| 4 | (Nephrectom* OR Nephroureterectom* OR Nephro‐ureterectom* OR Ureteronephrectom*):ti,ab,kw |
| 5 | #1 OR #2 OR #3 OR #4 |
| 6 | MeSH descriptor: [Doxorubicin] explode all trees |
| 7 | ('23214 92 8' OR '25316 40 9'):ti,ab,kw |
| 8 | (Doxorubicin* OR Caelyx OR Doxil OR Myocet OR Adriblastin* OR Adriablastin* OR Doxolem OR Adrimedac OR Farmiblastina OR Ribodoxo OR DOXO‐cell OR Onkodox):ti,ab,kw |
| 9 | MeSH descriptor: [Epirubicin] explode all trees |
| 10 | ('56390 09 1' OR '56420 45 2'):ti,ab,kw |
| 11 | (Epirubicin* OR Farmorubicin* OR Pharmorubicin* OR IMI28 OR Ellence OR Epidoxorubicin OR Epi‐Doxorubicin OR Epiadriamycin OR Epi‐Adriamycin OR EPI‐cell OR EPIcell OR Epilem):ti,ab,kw |
| 12 | MeSH descriptor: [Mitomycins] in all MeSH products |
| 13 | ('1404 00 8' OR '50 07 7' OR '74349 48 7'):ti,ab,kw |
| 14 | (Mitomycin* OR Mitomicin* OR Mitocin* OR Ametycin* OR Mutamycin*):ti,ab,kw |
| 15 | MeSH descriptor: [Thiotepa] explode all trees |
| 16 | ('52 24 4'):ti,ab,kw |
| 17 | (Thiotepa OR Thio‐tepa OR Tespa* OR Thiophosphamide OR Girostan):ti,ab,kw |
| 18 | (Gemcitabin* OR Gemcetabin* OR Gemcatabin* OR Gemzar*):ti,ab,kw |
| 19 | ('103882 84 4'):ti,ab,kw |
| 20 | #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 |
| 21 | MeSH descriptor: [Administration, Intravesical] explode all trees |
| 22 | ((Bladder OR Intravesical) near/2 (Administration* OR Injection* OR Instillation*)):ti,ab,kw |
| 23 | MeSH descriptor: [Urinary Bladder Neoplasms] explode all trees and with qualifier(s): [prevention & control ‐ PC, surgery ‐ SU] |
| 24 | #21 OR #22 OR #23 |
| 25 | #5 AND #20 AND #24 |
| Embase | |
| 1 | 'nephrectomy'/exp |
| 2 | 'kidney tumor'/exp/dm_su |
| 3 | 'ureter tumor'/exp/dm_su |
| 4 | (Nephrectom* OR Nephroureterectom* OR Nephro‐ureterectom* OR Ureteronephrectom*):ab,ti |
| 5 | #1 OR #2 OR #3 OR #4 |
| 6 | doxorubicin'/exp |
| 7 | ('23214 92 8' OR '25316 40 9'):rn |
| 8 | (Doxorubicin* OR Caelyx OR Doxil OR Myocet OR Adriblastin* OR Adriablastin* OR Doxolem OR Adrimedac OR Farmiblastina OR Ribodoxo OR DOXO‐cell OR Onkodox):ab,ti |
| 9 | 'epirubicin'/exp |
| 10 | ('56390 09 1' OR '56420 45 2'):rn |
| 11 | (Epirubicin* OR Farmorubicin* OR Pharmorubicin* OR IMI28 OR Ellence OR Epidoxorubicin OR Epi‐Doxorubicin OR Epiadriamycin OR Epi‐Adriamycin OR EPI‐cell OR EPIcell OR Epilem):ab,ti,tn |
| 12 | 'mitomycin'/exp |
| 13 | ('1404 00 8' OR '50 07 7' OR '74349 48 7'):rn |
| 14 | (Mitomycin* OR Mitomicin* OR Mitocin* OR Ametycin* OR Mutamycin*):ab,ti,tn |
| 15 | thiotepa'/exp |
| 16 | 52 24 4':rn |
| 17 | (Thiotepa OR Thio‐tepa OR Tespa* OR Thiophosphamide OR Girostan):ab,ti,tn |
| 18 | gemcitabine'/exp |
| 19 | (Gemcitabin* OR Gemcetabin* OR Gemcatabin* OR Gemzar*):ab,ti,tn |
| 20 | '103882 84 4':rn |
| 21 | #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 |
| 22 | intravesical drug administration'/exp |
| 23 | ((Bladder OR Intravesical) NEAR/2 (Administration* OR Injection* OR Instillation*)):ab,ti |
| 24 | bladder tumor'/exp/dm_pc,dm_su |
| 25 | #22 OR #23 OR #24 |
| 26 | #5 AND #21 AND #25 |
| 27 | ''crossover procedure':de OR 'double‐blind procedure':de OR 'randomized controlled trial':de OR 'single‐blind procedure':de OR random*:de,ab,ti OR factorial*:de,ab,ti OR crossover*:de,ab,ti OR ((cross NEXT/1 over*):de,ab,ti) OR placebo*:de,ab,ti OR ((doubl* NEAR/1 blind*):de,ab,ti) OR ((singl* NEAR/1 blind*):de,ab,ti) OR assign*:de,ab,ti OR allocat*:de,ab,ti OR volunteer*:de,ab,ti |
| 28 | ('animals'/exp) NOT ('humans'/exp and 'animals'/exp) |
| 29 | #27 NOT #28 |
| 30 | #26 AND #29 |
| Scopus | |
| 1 | TITLE‐ABS‐KEY ( ( nephrectom* OR nephroureterectom* OR nephro‐ureterectom* OR ureteronephrectom* ) ) |
| 2 | TITLE‐ABS‐KEY ( ( doxorubicin* OR caelyx OR doxil OR myocet OR adriblastin* OR adriablastin* OR doxolem OR adrimedac OR farmiblastina OR ribodoxo OR doxo‐cell OR onkodox OR epirubicin* OR farmorubicin* OR pharmorubicin* OR imi28 OR ellence OR epidoxorubicin OR epi‐doxorubicin OR epiadriamycin OR epi‐adriamycin OR epi‐cell OR epicell OR epilem OR mitomycin* OR mitomicin* OR mitocin* OR ametycin* OR mutamycin* OR thiotepa OR thio‐tepa OR tespa* OR thiophosphamide OR girostan OR gemcitabin* OR gemcetabin* OR gemcatabin* OR gemzar* ) ) |
| 3 | CASREGNUMBER ( "23214 92 8" OR "25316 40 9" OR "56390 09 1" OR "56420 45 2" OR "1404 00 8" OR "50 07 7" OR "74349 48 7" OR "52 24 4" OR "103882 84 4" ) |
| 4 | #2 OR #3 |
| 5 | TITLE‐ABS‐KEY ( ( ( bladder OR intravesical ) W/2 ( administration* OR injection* OR instillation* ) ) ) |
| 6 | #1 AND #2 AND #3 |
| 7 | ( INDEXTERMS ( "clinical trials" OR "clinical trials as a topic" OR "randomized controlled trial" OR "Randomized Controlled Trials as Topic" OR "controlled clinical trial" OR "Controlled Clinical Trials" OR "random allocation" OR "Double‐Blind Method" OR "Single‐Blind Method" OR "Cross‐Over Studies" OR "Placebos" OR "multicenter study" OR "double blind procedure" OR "single blind procedure" OR "crossover procedure" OR "clinical trial" OR "controlled study" OR "randomisation" OR "placebo" ) ) OR ( TITLE‐ABS‐KEY ( ( "clinical trials" OR "clinical trials as a topic" OR "randomized controlled trial" OR "Randomized Controlled Trials as Topic" OR "controlled clinical trial" OR "Controlled Clinical Trials as Topic" OR "random allocation" OR "randomly allocated" OR "allocated randomly" OR "Double‐Blind Method" OR "Single‐Blind Method" OR "Cross‐Over Studies" OR "Placebos" OR "cross‐over trial" OR "single blind" OR "double blind" OR "factorial design" OR "factorial trial" ) ) ) OR ( TITLE‐ABS ( clinical trial* OR trial* OR rct* OR random* OR blind* ) ) |
| 8 | #4 AND #5 |
| Web of Science | |
| 1 | TS= ((Nephrectom* OR Nephroureterectom* OR Nephro‐ureterectom* OR Ureteronephrectom*)) |
| 2 | TS= ((Doxorubicin* OR Caelyx OR Doxil OR Myocet OR Adriblastin* OR Adriablastin* OR Doxolem OR Adrimedac OR Farmiblastina OR Ribodoxo OR DOXO‐cell OR Onkodox OR Epirubicin* OR Farmorubicin* OR Pharmorubicin* OR IMI28 OR Ellence OR Epidoxorubicin OR Epi‐Doxorubicin OR Epiadriamycin OR Epi‐Adriamycin OR EPI‐cell OR EPIcell OR Epilem OR Mitomycin* OR Mitomicin* OR Mitocin* OR Ametycin* OR Mutamycin* OR Thiotepa OR Thio‐tepa OR Tespa* OR Thiophosphamide OR Girostan OR Gemcitabin* OR Gemcetabin* OR Gemcatabin* OR Gemzar* OR "23214 92 8" OR "25316 40 9" OR "56390 09 1" OR "56420 45 2" OR "1404 00 8" OR "50 07 7" OR "74349 48 7" OR "52 24 4" OR "103882 84 4")) |
| 3 | TS= (((Bladder OR Intravesical) NEAR/2 (Administration* OR Injection* OR Instillation*))) |
| 4 | #1 AND #2 AND #3 |
| 5 | TS= clinical trial* OR TS=research design OR TS=comparative stud* OR TS=evaluation stud* OR TS=controlled trial* OR TS=follow‐up stud* OR TS=prospective stud* OR TS=random* OR TS=placebo* OR TS=(single blind*) OR TS=(double blind*) |
| 6 | #3 AND #4 |
| LILACS | |
| 1 | (mh:("Nephrectomy" OR "Kidney Neoplasms/SU" OR "Ureteral Neoplasms/SU")) OR (tw:(Nephrectom* or Nephroureterectom* or Nephro‐ureterectom* or Ureteronephrectom*)) |
| 2 | (mh:("Doxorubicin" OR "Epirubicin" OR "Mitomycin" OR "Thiotepa")) OR (tw:(Doxorubicin* OR Caelyx OR Doxil OR Myocet OR Adriblastin* OR Adriablastin* OR Doxolem OR Adrimedac OR Farmiblastina OR Ribodoxo OR DOXO‐cell OR Onkodox OR Epirubicin* OR Farmorubicin* OR Pharmorubicin* OR IMI28 OR Ellence OR Epidoxorubicin OR Epi‐Doxorubicin OR Epiadriamycin OR Epi‐Adriamycin OR EPI‐cell OR EPIcell OR Epilem OR Mitomycin* OR Mitomicin* OR Mitocin* OR Ametycin* OR Mutamycin* OR Thiotepa OR Thio‐tepa OR Tespa* OR Thiophosphamide OR Girostan OR Gemcitabin* OR Gemcetabin* OR Gemcatabin* OR Gemzar* OR 23214‐92‐8 OR 25316‐40‐9 OR 56390‐09‐1 OR 56420‐45‐2 OR 1404‐00‐8 OR 50‐07‐7 OR 74349‐48‐7 OR 52‐24‐4 OR 103882‐84‐4)) |
| 3 | (mh:("Administration, Intravesical")) OR (mh:("Urinary Bladder Neoplasms/SU")) OR (mh:("Urinary Bladder Neoplasms/PC")) OR (tw:("Bladder Drug Administration" OR "Bladder Instillation" OR "Intravesical Administration" OR "Intravesical Drug Administration" OR "Intravesical Injection" OR "Intravesical Instillation")) |
| 4 | ((PT:"randomized controlled trial" OR PT:"controlled clinical trial" OR PT:"multicenter study" OR MH:"randomized controlled trials as topic" OR MH:"controlled clinical trials as topic" OR MH:"multicenter study as topic" OR MH:"random allocation" OR MH:"double‐blind method" OR MH:"single‐blind method") OR ((ensaio$ OR ensayo$ OR trial$) AND (azar OR acaso OR placebo OR control$ OR aleat$ OR random$ OR enmascarado$ OR simpleciego OR ((simple$ OR single OR duplo$ OR doble$ OR double$) AND (cego OR ciego OR blind OR mask))) AND clinic$)) AND NOT (MH:animals OR MH:rabbits OR MH:rats OR MH:primates OR MH:dogs OR MH:cats OR MH:swine OR PT:"in vitro") |
| 5 | 1 AND 2 AND 3 AND 4 |
| ClinicalTrials.gov | |
| 1 | (Nephrectomy OR Nephroureterectomy OR Nephro‐ureterectomy OR Ureteronephrectomy) |
| 2 | (Bladder OR Intravesical) |
| 3 | 1 AND 2 |
| World Health Organization (WHO) International Clinical Trials Registry Platform search portal | |
| 1 | In the title = (Nephrectom* OR Nephroureterectom* OR Nephro‐ureterectom* OR Ureteronephrectom*) AND In the intervention= (Bladder OR Intravesical) |
| Grey Literature (Open Grey) | |
| 1 | (Nephrectom* OR Nephroureterectom* OR Nephro‐ureterectom* OR Ureteronephrectom*) AND (Bladder OR Intravesical) |
Appendix 2. Survey of trial investigators providing information on included trials
| Study | Date trial author contacted (first) | Date trial author provided data (latest) | Data trial author provided (short summary) |
| O'Brien 2011 | 3 Nov 2018 | 5 Nov 2018 | Random sequence generation method and baseline characteristics |
Data and analyses
Comparison 1. Single‐dose intravesical chemotherapy instillation versus placebo or observation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to bladder cancer recurrence | 2 | 311 | Hazard Ratio (Random, 95% CI) | 0.51 [0.32, 0.82] |
| 2 Serious adverse events | 2 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Minor adverse events | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ito 2013.
| Methods |
Study design: Prospective randomised phase II study Statistical design: N/A Setting/Country: Multicentre/Japan Dates when study was conducted: December 2005 to November 2008 |
|
| Participants |
Ethnicity: likely Japanese Inclusion criteria
Exclusion criteria
Total number of participants randomly assigned:
Group A (THP instillation)
Group B (No instillation)
|
|
| Interventions |
Group A: Single‐dose THP 30 mg in 30 mL of normal saline was delivered into the bladder through a catheter within 48 hours after nephroureterectomy and was retained for 30 minutes. Group B: No instillation Follow‐up: median 24.9 months (range: 2.6 to 39.3 months) in group A; median 13.7 months (range: 2.8 to 34.1 months) in group B |
|
| Outcomes |
Primary outcome
Safety outcome
Subgroup: none |
|
| Funding Sources | Supported in part by a Grant‐In‐Aid for Scientific Research from the Ministry of Education, Science and Culture (Grant No. 21390437) of the Japanese government (Y.A.) | |
| Declarations of interest | None | |
| Notes |
Protocol: UMIN Clinical Trials Registry: Trial number UMIN000004039 Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Randomly assigned using a minimization method" Comment: This method of random sequence generation was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Enrolled patients were stratified at University Hospital Medical Information Network Clinical Trials Registry according to institution, sex, location of urothelial tumour, and operative method and then randomly assigned". Comment: Central registration. This method may ensure allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Quote from publication: Open‐label trial (reported in the study protocol) Comment: Participants and personnel were not blinded; therefore risk of performance bias was considered to be high. |
| Blinding of outcome assessment (detection bias) Subjective outcomes (susceptible to detection bias); time to bladder cancer recurrence, time to death from UTUC, serious and minor adverse events, disease‐specific quality of life | High risk |
Quote from publication: Open‐label trial (reported in the study protocol) Comment: Outcome assessor was not blinded; therefore, risk of detection bias was considered to be high. |
| Blinding of outcome assessment (detection bias) Objective outcomes (not susceptible to detection bias); time to death from any cause | Low risk | Comment: Objective outcomes were not likely affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Time to bladder cancer recurrence | Low risk | Comment: 3/39 (7.6%) in intervention arm and 2/38 (5.3%) in control arm were excluded from the analysis. Owing to the small number of participants lost to follow‐up, risk of attrition bias was considered to be low. |
| Incomplete outcome data (attrition bias) Time to death from UTUC | Unclear risk | Comment: This study did not address this outcome. |
| Incomplete outcome data (attrition bias) Serious adverse events | Low risk | Comment: 3/39 (7.6%) in intervention arm and 2/38 (5.3%) in control arm were excluded from the analysis. Owing to the small number of participants lost to follow‐up, risk of attrition bias was considered to be low. |
| Incomplete outcome data (attrition bias) Time to death from any cause | Unclear risk | Comment: This study did not address this outcome. |
| Incomplete outcome data (attrition bias) Minor adverse events | Low risk | Comment: 3/39 (7.6%) in intervention arm and 2/38 (5.3%) in control arm were excluded from the analysis. Owing to the small number of participants lost to follow‐up, risk of attrition bias was considered to be low. |
| Incomplete outcome data (attrition bias) Diseas‐specific quality of life | Unclear risk | Comment: This study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Protocol was provided (https://upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000004865; UMIN Clinical Trials Registry: Trial number UMIN000004039) but toxicity outcomes were not predefined. |
| Other bias | High risk | Comment: Difference in median follow‐up between the groups (24.9 vs 13.7 months) and there was baseline imbalance in carcinoma in situ and tumour grade. |
O'Brien 2011.
| Methods |
Study design: Prospective randomised nonblinded study Statistical design: N/A Setting/Country: Multicentre/United Kingdom Dates when study was conducted: July 2000 to December 2006 |
|
| Participants |
Ethnicity: likely English Inclusion criteria
Exclusion criteria
Total number of participants randomly assigned:
Group A (MMC instillation)
Group B (No instillation)
|
|
| Interventions |
Group A: Single‐dose MMC 40 mg in 40 mL of normal saline was delivered into the bladder prior to removal of the urethral catheter and was retained for 1 hour (the timing of the administration of the intravesical chemotherapy was chosen to minimise the risk of extravasation). Group B: No instillation Follow‐up: 12 months |
|
| Outcomes |
Primary outcome
Secondary outcome
Safety outcome
Subgroup
|
|
| Funding Sources | The trial was funded through Guys and St Thomas’ Hospitals Urology Research Fund. Kyowa Hakko gave two unrestricted donations totaling £7000 to offset some administrative expenses. No payments were made to recruiting centres. None of the team at Guys Hospital had financial links with Kyowa. | |
| Declarations of interest | None | |
| Notes |
Protocol: ISRCTN36343644 Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Randomisation stated and trial author provided random sequence generation method "used ‘Tombola’ blinded selection of treatment from within the block"; therefore selection bias was considered to have low risk of bias. |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Randomisation was performed at Guys Hospital following the nephroureterectomy and was by means of sealed envelopes in blocks of 20". Comment: Since this study was multicentre and allocation was performed by central allocation (randomisation was performed at Guys Hospital with sealed envelopes), we assumed that this method may ensure allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Quote from publication: "nonblinded trial" Comment: Participants and personnel were not blinded; therefore risk of performance bias was considered to be high. |
| Blinding of outcome assessment (detection bias) Subjective outcomes (susceptible to detection bias); time to bladder cancer recurrence, time to death from UTUC, serious and minor adverse events, disease‐specific quality of life | High risk |
Quote from publication: "nonblinded trial" Comment: Participants and personnel were not blinded; therefore risk of performance bias was considered to be high. |
| Blinding of outcome assessment (detection bias) Objective outcomes (not susceptible to detection bias); time to death from any cause | Low risk | Comment: Objective outcomes were not likely to be affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Time to bladder cancer recurrence | Unclear risk | Comment: 24/144 (16.6%) in intervention arm and 21/140(15%) in control arm were excluded from the analysis; owing to the moderate number of participants lost to follow‐up (> 10%), risk of attrition bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) Time to death from UTUC | Unclear risk | Comment: This study did not address this outcome. |
| Incomplete outcome data (attrition bias) Serious adverse events | Unclear risk | Comment: 24/144 (16.6%) in intervention arm and 21/140(15%) in control arm were excluded from the analysis; owing to the moderate number of participants lost to follow‐up (> 10%), risk of attrition bias was considered to be unclear. |
| Incomplete outcome data (attrition bias) Time to death from any cause | Unclear risk | Comment: This study did not address this outcome. |
| Incomplete outcome data (attrition bias) Minor adverse events | Unclear risk | Comment: This study did not address this outcome. |
| Incomplete outcome data (attrition bias) Diseas‐specific quality of life | Unclear risk | Comment: This study did not address this outcome. |
| Selective reporting (reporting bias) | High risk | Comment: Protocol was provided (ISRCTN36343644) but secondary outcomes were not reported and subgroup analyses were not predefined. |
| Other bias | High risk | Comment: There was baseline imbalance in Ta disease and high grade tumour. |
ALT: alanine aminotransferase AST: aspartate aminotransferase ECOG PS: Eastern Cooperative Oncology Group performance status F: female Hb: haemoglobin M: male M1: metastasis MMC: mitomycin n: number of participants N1: lymph node involvement N/A: not available N/R: not reported PLT: platelet THP: pirarubicin UTUC: upper tract urothelial carcinoma WBC: white blood cell
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Sakamoto 2001 | Ineligible intervention; multiple instillation (28 times) of chemotherapy |
| Van Doeveren 2018 | Published study protocol, not full‐text, and ineligible study design |
Characteristics of ongoing studies [ordered by study ID]
JPRN‐UMIN000009682.
| Trial name or title | Randomised controlled trial of a single postoperative intravesical instillation of THP for prevention of intravesical recurrence after nephroureterectomy |
| Methods | Open‐label randomised parallel group trial |
| Participants |
Estimated enrollment: 90 participants Inclusion Criteria
Exclusion Criteria
|
| Interventions |
Group A
Group B
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | August 22, 2012; Expected date of completion: June 2020 |
| Contact information | shingoy@hyo‐med.ac.jp; tosuzuki@hyo‐med.ac.jp |
| Notes | Funding source: Self funding |
Miyamoto 2018.
| Trial name or title | A Phase III trial of a single early intravesical instillation of pirarubicin to prevent bladder cancer recurrence after radical nephroureterectomy for upper tract urothelial carcinoma (JCOG1403, UTUC THP Phase III) |
| Methods | A multi‐institutional open‐label randomised phase III study |
| Participants |
Estimated enrollment: 310 participants Inclusion Criteria First registration (before undergoing radical nephrectomy)
Second registration (after undergoing radical nephrectomy)
Exclusion Criteria
|
| Interventions |
Group A
Group B:
|
| Outcomes |
Primary outcomes
Secondary outcomes
Follow‐up: at least 3 years after the patient recruitment is completed |
| Starting date | October 03, 2016; Expected date of completion: October 2025 |
| Contact information | itoaki@uro.med.tohoku.ac.jp |
| Notes | Funding source: National Cancer Center Research and Development Fund (26‐A‐4) |
NCT02547350.
| Trial name or title | Prophylactic intravesical chemotherapy to prevent bladder tumors after nephroureterectomy for primary upper urinary tract urothelial carcinomas |
| Methods | Open‐label randomised parallel group phase II study |
| Participants |
Estimated enrollment: 200 participants Inclusion Criteria
Exclusion Criteria
|
| Interventions |
Group A
Group B
Group C
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | September 2015; Expected date of completion: December 2020 |
| Contact information | Xuesong Li, Professor, Peking University First Hospital |
| Notes | Funding source: Not available |
NCT02923557.
| Trial name or title | Prophylactic intravesical chemotherapy to prevent bladder cancer recurrence after nephroureterectomy for primary upper tract urothelial carcinoma patients |
| Methods | Single‐blinded randomised parallel group phase II study |
| Participants |
Estimated enrollment: 200 participants Inclusion Criteria
Exclusion Criteria
|
| Interventions |
Group A
Group B
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | November 2016; Expected date of completion: November 2021 |
| Contact information | Xuesong Li, Associated Professor, Peking University First Hospital |
| Notes | Funding source: Not available |
NCT03062059.
| Trial name or title | The effectiveness and safety of intravesical gemcitabine instillation during operation to prevent intravesical recurrence after radical nephroureterectomy in upper urinary tract urothelial carcinoma: prospective, phase II Study |
| Methods | A multicentre open‐label randomised parallel group phase II study |
| Participants |
Estimated enrollment: 134 participants Inclusion Criteria
Exclusion Criteria
|
| Interventions |
Group A
Group B
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | March 1, 2018; Expected date of completion: December 31, 2022 |
| Contact information | seohk@ncc.re.kr; 12754@ncc.re.kr |
| Notes | Funding source: Chong Kun Dang Pharmaceutical Corp |
NCT03209206.
| Trial name or title | The effectiveness and safety of intravesical docetaxel instillation after operation to prevent intravesical recurrence after radical nephroureterectomy or distal ureterectomy in upper urinary tract urothelial carcinoma: a prospective study |
| Methods | Open‐label randomised parallel group phase II study |
| Participants |
Estimated enrollment: 84 participants Inclusion Criteria
Exclusion Criteria
|
| Interventions |
Group A
Group B
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | June 28, 2017; Expected date of completion: April 1, 2022 |
| Contact information | randyku@hanmail.net |
| Notes | Funding source: Seoul National University Hospital |
ANC: absolute neutrophil count ALP: alkaline phosphatase ALT: alanine aminotransferase AST: aspartate aminotransferase CT: computed tomography ECOG PS: Eastern Cooperative Oncology Group performance status Hb: haemoglobin PLT: platelet THP: pirarubicin UTUC: upper tract urothelial carcinoma WBC: white blood cell
Differences between protocol and review
This review is based on a previously published protocol (Hwang 2018). There were no relevant departures from this protocol.
Contributions of authors
Eu Chang Hwang (ECH): study selection, extracting data, assessing risk of bias, performing data analysis, interpretation of data, and drafting the review.
Niranjan Sathianathen (NS): extracting data, assessing risk of bias, performing data analysis, and providing clinical advice and critical content.
Jae Hung Jung (JHJ): study selection and interpretation of data.
Myung Ha Kim (MHK): creating search strategies and searching for trials.
Philipp Dahm (PD): providing clinical and methodological guidance for this review.
Michael C Risk (MR): conception and study design, providing clinical and methodological advice on the review, and final approval.
Sources of support
Internal sources
-
Chonnam National University Hwasun Hospital, Korea, South.
Salary support for Eu Chang Hwang
-
Yonsei University Wonju College of Medicine, Korea, South.
Salary support for Jae Hung Jung
-
Yonsei University Wonju College of Medicine, Korea, South.
Salary support for Myung Ha Kim
-
Minneapolis Veterans Administration Medical Center, USA.
Salary support for Philipp Dahm
-
Minneapolis Veterans Administration Medical Center, USA.
Salary support for Michael C Risk
Department of Urology, Chonnam National University Medical School, Korea, South.
Department of Urology, Yonsei University Wonju College of Medicine, Korea, South.
Department of Urology, University of Minnesota, USA.
External sources
No sources of support supplied
Declarations of interest
ECH: none known.
NS: none known.
JHJ: none known.
MHK: none known.
PD: serves as Co‐ordinating Editor of Cochrane Urology. However, he was not involved in the editorial processing or decision‐making for this review. Other editors of Cochrane Urology managed the editorial process, including final sign‐off for this review through the Cancer Network.
MR: none known.
New
References
References to studies included in this review
Ito 2013 {published data only}
- Ito A, Satoh M, Shintaku I, Ishidoya S, Arai Y. Prospective randomized phase II trial of single early intravesical instillation of (2'r)‐4'‐o‐Tetrahydropyranyl‐doxorubicin (THP) for prevention of bladder recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma (UUT‐UC). Journal of Urology 2011;185(4):e270‐1. [DOI: 10.1016/j.juro.2011.02.1599] [DOI] [Google Scholar]
- Ito A, Shintaku I, Satoh M, Ioritani N, Aizawa M, Tochigi T, et al. Prospective randomized phase II trial of a single early intravesical instillation of pirarubicin (THP) in the prevention of bladder recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma: the THP monotherapy study group trial. Journal of Clinical Oncology 2013;31(11):1422‐7. [PUBMED: 23460707] [DOI] [PubMed] [Google Scholar]
- Ito A, Shintaku I, Satoh M, Ioritani N, Tochigi T, Numata I, et al. Intravesical seeding of upper urinary tract urothelial carcinoma cells during nephroureterectomy: an exploratory analysis from the THPMG trial. Japanese Journal of Clinical Oncology 2013;43(11):1139‐44. [PUBMED: 24006504] [DOI] [PubMed] [Google Scholar]
O'Brien 2011 {published data only}
- O'Brien T, Ray E, Singh R, Coker B, Beard R, British Association of Urological Surgeons Section of Oncology. Prevention of bladder tumours after nephroureterectomy for primary upper urinary tract urothelial carcinoma: a prospective, multicentre, randomised clinical trial of a single postoperative intravesical dose of mitomycin C (the ODMIT‐C Trial). European Urology 2011;60(4):703‐10. [PUBMED: 21684068] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Sakamoto 2001 {published data only}
- Sakamoto N, Naito S, Kumazawa J, Ariyoshi A, Osada Y, Omoto T, et al. Prophylactic intravesical instillation of mitomycin C and cytosine arabinoside for prevention of recurrent bladder tumors following surgery for upper urinary tract tumors: a prospective randomized study. International Journal of Urology 2001;8(5):212‐6. [PUBMED: 11328420] [DOI] [PubMed] [Google Scholar]
Van Doeveren 2018 {published data only}
- Doeveren T, Leeuwen PJ, Aben KKH, Aa M, Barendrecht M, Boevé ER, et al. Reduce bladder cancer recurrence in patients treated for upper urinary tract urothelial carcinoma: the REBACARE‐trial. Contemporary Clinical Trials Communications 2018;9:121‐9. [PUBMED: 29696234] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
JPRN‐UMIN000009682 {unpublished data only}
- JPRN‐UMIN000009682. Randomized control trial of a single postoperative intravesical instillation of THP for prevention of intravesical recurrence after nephroureterectomy. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000009682 (first received 4 January 2013). [JPRN‐UMIN000009682]
Miyamoto 2018 {published and unpublished data}
- Miyamoto K, Ito A, Wakabayashi M, Eba J, Arai Y, Nishiyama H, et al. A phase III trial of a single early intravesical instillation of pirarubicin to prevent bladder recurrence after radical nephroureterectomy for upper tract urothelial carcinoma (JCOG1403, UTUC THP Phase III). Japanese Journal of Clinical Oncology 2018;48(1):94‐7. [DOI: 10.1093/jjco/hyx158; PUBMED: 29136187] [DOI] [PubMed] [Google Scholar]
NCT02547350 {unpublished data only}
- NCT02547350. Prophylactic intravesical chemotherapy to prevent bladder tumors after nephroureterectomy for primary upper urinary tract urothelial carcinomas. clinicaltrials.gov/ct2/show/record/NCT02547350 (first received 11 September 2015). [NCT02547350]
NCT02923557 {unpublished data only}
- NCT02923557. Prophylactic intravesical chemotherapy to prevent bladder recurrence after nephroureterectomy for primary upper tract urothelial carcinoma patients. clinicaltrials.gov/ct2/show/record/NCT02923557 (first received 4 October 2016). [NCT02923557]
NCT03062059 {unpublished data only}
- NCT03062059. The effectiveness and safety of intravesical gemcitabine instillation to prevent intravesical recurrence [The effectiveness and safety of intravesical gemcitabine instillation during operation to prevent intravesical recurrence after radical nephroureterectomy in upper urinary tract urothelial carcinoma: prospective, phase II study]. clinicaltrials.gov/ct2/show/NCT03062059 (first received 23 February 2017). [NCT03062059]
NCT03209206 {unpublished data only}
- NCT03209206. The effectiveness and safety of intravesical docetaxel instillation for prevent bladder recurrence [The effectiveness and safety of intravesical docetaxel instillation after operation to prevent intravesical recurrence after radical nephroureterectomy or distal ureterectomy in upper urinary tract urothelial carcinoma: a prospective study]. clinicaltrials.gov/ct2/show/NCT03209206 (first received 6 July 2017). [NCT03209206]
Additional references
Abern 2013
- Abern MR, Owusu RA, Anderson MR, Rampersaud EN, Inman BA. Perioperative intravesical chemotherapy in non‐muscle‐invasive bladder cancer: a systematic review and meta‐analysis. Journal of the National Comprehensive Cancer Network 2013;11(4):477‐84. [PUBMED: 23584348] [DOI] [PubMed] [Google Scholar]
Addeo 2010
- Addeo R, Caraglia M, Bellini S, Abbruzzese A, Vincenzi B, Montella L, et al. Randomized phase III trial on gemcitabine versus mytomicin in recurrent superficial bladder cancer: evaluation of efficacy and tolerance. Journal of Clinical Oncology 2010;28(4):543‐8. [PUBMED: 19841330] [DOI] [PubMed] [Google Scholar]
Amin 2017
- Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer Staging Manual. 8th Edition. New York: Springer, 2017. [Google Scholar]
Azémar 2011
- Azémar MD, Comperat E, Richard F, Cussenot O, Rouprêt M. Bladder recurrence after surgery for upper urinary tract urothelial cell carcinoma: frequency, risk factors, and surveillance. Urologic Oncology 2011;29(2):130‐6. [PUBMED: 19762256] [DOI] [PubMed] [Google Scholar]
Bagrodia 2013
- Bagrodia A, Kuehhas FE, Gayed BA, Wood CG, Raman JD, Kapur P, et al. Comparative analysis of oncologic outcomes of partial ureterectomy vs radical nephroureterectomy in upper tract urothelial carcinoma. Urology 2013;81(5):972‐7. [PUBMED: 23523292] [DOI] [PubMed] [Google Scholar]
Cho 2014
- Cho YH, Seo YH, Chung SJ, Hwang I, Yu HS, Kim SO, et al. Predictors of intravesical recurrence after radical nephroureterectomy for upper urinary tract urothelial carcinoma: an inflammation‐based prognostic score. Korean Journal of Urology 2014;55(7):453‐9. [PUBMED: 25045443] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cocks 2008
- Cocks K, King MT, Velikova G, Fayers PM, Brown JM. Quality, interpretation and presentation of European Organisation for Research and Treatment of Cancer quality of life questionnaire core 30 data in randomised controlled trials. European Journal of Cancer 2008;44(13):1793‐8. [PUBMED: 18599286] [DOI] [PubMed] [Google Scholar]
Colin 2009
- Colin P, Koenig P, Ouzzane A, Berthon N, Villers A, Biserte J, et al. Environmental factors involved in carcinogenesis of urothelial cell carcinomas of the upper urinary tract. BJU International 2009;104(10):1436‐40. [PUBMED: 19689473] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation. Covidence. Version accessed 26 May 2018. Melbourne, Australia: Veritas Health Innovation, 2017.
CTCAE
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed 05 June 2018).
Deeks 2017
- Deeks JJ, Higgins JPT, Altman DG (editors), Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Deng 2014
- Deng X, Yang X, Cheng Y, Liu X, Wu B, Wang Z, et al. Prognostic value and efficacy valuation of postoperative intravesical instillation in primary urothelial carcinomas of upper urinary tract. International Journal of Clinical and Experimental Medicine 2014;7(12):4734‐46. [PUBMED: 25663970] [PMC free article] [PubMed] [Google Scholar]
EndNote [Computer program]
- Clarivate Analytics. EndNote. Version 7.5. Clarivate Analytics, 2016.
Fang 2013
- Fang D, Li XS, Xiong GY, Yao L, He ZS, Zhou LQ. Prophylactic intravesical chemotherapy to prevent bladder tumors after nephroureterectomy for primary upper urinary tract urothelial carcinomas: a systematic review and meta‐analysis. Urologia Internationalis 2013;91(3):291‐6. [PUBMED: 23948770] [DOI] [PubMed] [Google Scholar]
Green 2013
- Green DA, Rink M, Xylinas E, Matin SF, Stenzl A, Roupret M, et al. Urothelial carcinoma of the bladder and the upper tract: disparate twins. Journal of Urology 2013;189(4):1214‐21. [PUBMED: 23023150] [DOI] [PubMed] [Google Scholar]
Griffin 2013
- Griffin JG, Holzbeierlein J. Side effects of perioperative intravesical treatment and treatment strategies for these side effects. Urologic Clinics of North America 2013;40(2):197‐210. [PUBMED: 23540778] [DOI] [PubMed] [Google Scholar]
Griffiths 2013
- Griffiths TR. Current perspectives in bladder cancer management. International Journal of Clinical Practice 2013;67(5):435‐48. [PUBMED: 23137019] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Habuchi 1993
- Habuchi T, Takahashi R, Yamada H, Kakehi Y, Sugiyama T, Yoshida O. Metachronous multifocal development of urothelial cancers by intraluminal seeding. Lancet 1993;342(8879):1087‐8. [PUBMED: 8105314] [DOI] [PubMed] [Google Scholar]
Hall 1998
- Hall MC, Womack S, Sagalowsky AI, Carmody T, Erickstad MD, Roehrborn CG. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30‐year experience in 252 patients. Urology 1998;52(4):594‐601. [PUBMED: 9763077] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Deeks JJ, Altman DG, editor(s), Cochrane Statistical Methods Group. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017a
- Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2017b
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [PUBMED: 15840177] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnston 2013
- Johnston BC, Patrick DL, Busse JW, Schunemann HJ, Agarwal A, Guyatt GH. Patient‐reported outcomes in meta‐analyses ‐ Part 1: assessing risk of bias and combining outcomes. Health and Quality of Life Outcomes 2013;11:109. [PUBMED: 23815754] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2005
- Jones TD, Wang M, Eble JN, MacLennan GT, Lopez‐Beltran A, Zhang S, et al. Molecular evidence supporting field effect in urothelial carcinogenesis. Clinical Cancer Research 2005;11(18):6512‐9. [PUBMED: 16166427] [DOI] [PubMed] [Google Scholar]
Kim 2015
- Kim M, Jeong CW, Kwak C, Kim HH, Ku JH. Are urothelial carcinomas of the upper urinary tract a distinct entity from urothelial carcinomas of the urinary bladder? Behavior of urothelial carcinoma after radical surgery with respect to anatomical location: a case control study. BMC Cancer 2015;15:149. [PUBMED: 25886012] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2017
- Lee CH, Ku JY, Jeong CW, Ku JH, Kwak C, Kim HH, et al. Predictors for intravesical recurrence following radical nephroureterectomy for upper tract urothelial carcinoma: a national multicenter analysis. Clinical Genitourinary Cancer 2017;15(6):e1055‐61. [PUBMED: 28802888] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 2009;6(7):e1000100. [PUBMED: 19621070] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2017
- Lu DD, Boorjian SA, Raman JD. Intravesical chemotherapy use after radical nephroureterectomy: a national survey of urologic oncologists. Urologic Oncology 2017;35(3):113.e1‐7. [PUBMED: 27884539] [DOI] [PubMed] [Google Scholar]
Mbeutcha 2017
- Mbeutcha A, Roupret M, Kamat AM, Karakiewicz PI, Lawrentschuk N, Novara G, et al. Prognostic factors and predictive tools for upper tract urothelial carcinoma: a systematic review. World Journal of Urology 2017;35(3):337‐53. [PUBMED: 27101100] [DOI] [PubMed] [Google Scholar]
NCCN Guideline 2018
- Bladder Cancer, NCCN Clinical Practice Guidelines in Oncology. https://www.nccn.org/professionals/physician_gls/default.aspx#site (accessed 05 June, 2018), issue 3.
Novara 2007
- Novara G, Marco V, Gottardo F, Dalpiaz O, Bouygues V, Galfano A, et al. Independent predictors of cancer‐specific survival in transitional cell carcinoma of the upper urinary tract: multi‐institutional dataset from 3 European centers. Cancer 2007;110(8):1715‐22. [PUBMED: 17724728] [DOI] [PubMed] [Google Scholar]
Oya 2015
- Oya M, Kikuchi E, Japanese Urological Association. Evidenced‐based clinical practice guideline for upper tract urothelial carcinoma (summary). International Journal of Uology 2015;22(1):3‐13. [PUBMED: 25243652] [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [PUBMED: 9921604] [DOI] [PubMed] [Google Scholar]
Perlis 2013
- Perlis N, Zlotta AR, Beyene J, Finelli A, Fleshner NE, Kulkarni GS. Immediate post‐transurethral resection of bladder tumor intravesical chemotherapy prevents non‐muscle‐invasive bladder cancer recurrences: an updated meta‐analysis on 2548 patients and quality‐of‐evidence review. European Urology 2013;64(3):421‐30. [PUBMED: 23830475] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rouprêt 2018
- Rouprêt M, Babjuk M, Comperat E, Zigeuner R, Sylvester RJ, Burger M, et al. European Association of Urology Guidelines on upper urinary tract urothelial carcinoma: 2017 update. European Urology 2018;73(1):111‐22. [PUBMED: 28867446] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann HJ, Tugwell P, Reeves BC, Akl EA, Santesso N, Spencer FA, et al. Non‐randomized studies as a source of complementary, sequential or replacement evidence for randomized controlled trials in systematic reviews on the effects of interventions. Research Synthesis Methods 2013;4(1):49‐62. [PUBMED: 26053539] [DOI] [PubMed] [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Akl E, Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Siegel 2014
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA 2014;64(1):9‐29. [PUBMED: 24399786] [DOI] [PubMed] [Google Scholar]
Svatek 2014
- Svatek RS, Hollenbeck BK, Holmang S, Lee R, Kim SP, Stenzl A, et al. The economics of bladder cancer: costs and considerations of caring for this disease. European Urology 2014;66(2):253‐62. [PUBMED: 24472711] [DOI] [PubMed] [Google Scholar]
Sylvester 2004
- Sylvester RJ, Oosterlinck W, Meijden AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta‐analysis of published results of randomized clinical trials. Journal of Urology 2004;171(6 Pt 1):2186‐90. [PUBMED: 15126782] [DOI] [PubMed] [Google Scholar]
Sylvester 2006
- Sylvester RJ, Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. European Urology 2006;49(3):466‐77. [PUBMED: 16442208] [DOI] [PubMed] [Google Scholar]
Sylvester 2016
- Sylvester RJ, Oosterlinck W, Holmang S, Sydes MR, Birtle A, Gudjonsson S, et al. Systematic review and individual patient data meta‐analysis of randomized trials comparing a single immediate instillation of chemotherapy after transurethral resection with transurethral resection alone in patients with stage pTa‐pT1 urothelial carcinoma of the bladder: which patients benefit from the instillation?. European Urology 2016;69(2):231‐44. [PUBMED: 26091833] [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [PUBMED: 17555582] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2010
- Williams SK, Hoenig DM, Ghavamian R, Soloway M. Intravesical therapy for bladder cancer. Expert Opinion on Pharmacotherapy 2010;11(6):947‐58. [PUBMED: 20205607] [DOI] [PubMed] [Google Scholar]
Wu 2015
- Wu P, Zhu G, Wei D, Liu S, Walsh K, Li D, et al. Prophylactic intravesical chemotherapy decreases bladder tumor recurrence after nephroureterectomy for primary upper tract urothelial carcinoma: a systematic review and meta‐analysis. Journal of Balkan Union of Oncology 2015;20(5):1229‐38. [PUBMED: 26537069] [PubMed] [Google Scholar]
Xylinas 2013
- Xylinas E, Shariat SF. Words of wisdom: re: Prospective randomized phase II trial of a single early intravesical instillation of pirarubicin (THP) in the prevention of bladder recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma: the THP Monotherapy Study Group trial. European Urology 2013;64(4):683‐4. [PUBMED: 23998501] [DOI] [PubMed] [Google Scholar]
Xylinas 2014
- Xylinas E, Kluth L, Passoni N, Trinh QD, Rieken M, Lee RK, et al. Prediction of intravesical recurrence after radical nephroureterectomy: development of a clinical decision‐making tool. European Urology 2014;65(3):650‐8. [PUBMED: 24070577] [DOI] [PubMed] [Google Scholar]
Yuan 2015
- Yuan H, Mao X, Bai Y, Li H, Liu L, Pu C, et al. The effect of intravesical chemotherapy in the prevention of intravesical recurrence after nephroureterectomy for upper tract urothelial carcinoma: a meta‐analysis. Journal of Chemotherapy 2015;27(4):195‐200. [PUBMED: 25968487] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hwang 2018
- Hwang EC, Sathianathen NJ, Jung JH, Kim MH, Dahm P, Risk MC. Single‐dose intravesical chemotherapy after nephroureterectomy for upper tract urothelial carcinoma. Cochrane Database of Systematic Reviews 2018, Issue 10. [DOI: 10.1002/14651858.CD013160] [DOI] [PMC free article] [PubMed] [Google Scholar]