Abstract
Background:
Research on a putative link between apolipoprotein-ε4 allele (APOE-ε4) and schizophrenia has been inconclusive. However, prior studies have not investigated the association between APOE-ε4 and symptom trajectories, nor has the existing literature taken into account the potentially moderating effect of age in genetic association studies.
Methods:
The association between APOE-ε4 and four symptom dimensions was investigated in a longitudinal study of 116 individuals with schizophrenia initially assessed during their first admission for psychosis and evaluated five times over the following 20 years. A meta-analysis identified 29 case-control studies of APOE-ε4 allele frequency in schizophrenia, which were analyzed using random-effects meta-regression to test the potentially moderating effect of age.
Results:
Longitudinal models identified a specific association between APOE-ε4 and symptom trajectories, showing that APOE-ε4 portends worsening severity of hallucinations and delusions in late adulthood among people with schizophrenia, at a rate of a 0.46 standard deviation increase per decade. Meta-analysis showed a significant effect of age: the association between APOE-ε4 and schizophrenia was not detectable in younger people but became pronounced with age, such that APOE-ε4 increased the odds of diagnosis by 10% per decade.
Conclusions:
Taken together, the meta-analysis and longitudinal analysis implicate APOE-ε4 as an age-related risk factor for worsening hallucinations and delusions, and suggest APOE-ε4 may play an age-mediated pathophysiological role in schizophrenia. The presence of an APOE-ε4 allele may also identify a subgroup of patients who require intensive monitoring and additional targeted interventions, especially in mid-to late-life.
Keywords: apolipoprotein e, schizophrenia, psychosis, hallucinations, delusions
1. Introduction
A large body of research has focused on the predictors of illness chronicity in schizophrenia (Ram et al., 1992). Most studies have found that early age of onset, male sex, poor premorbid adjustment, duration of untreated psychosis, negative symptoms, and persistence of symptoms following first episode all forecast poor prognosis (Austin et al., 2013; Haro et al., 2011; Harrison et al., 2001). However, apart from neurodevelopmental processes that are presumably reflected in age of onset, these predictors provide limited insight into mechanisms of illness, as they are difficult to distinguish from the core deficits of the diagnosis itself. Consequently, research is increasingly focused on identifying biomarkers of disease risk and progression. Identification of such objective markers would provide clinicians with a tool to predict prognosis and plan treatment accordingly.
Two years after the initial publication showing that APOE-ε4 is a critical risk factor for Alzheimer’s disease (Strittmatter et al., 1993), Harrington et al. (1995) presented evidence that it was also associated with schizophrenia (OR=2.7; 95% CI=[1.8–4.0], p<0.0001). A subsequent meta-analysis of 17 case-control studies published between 1995 and 2004 supported the increased prevalence of schizophrenia in ε4 carriers but observed significant heterogeneity among studies, and concluded that the effect may not be robust (Xu et al., 2006). The most recent meta-analysis of 28 case-control studies conducted between 1995 and 2011 detected no association (González-Castro et al., 2015).
Conclusion of no relationship between APOE-ε4 and schizophrenia is premature for two reasons. First, APOE-ε4’s effects are known to be moderated by age, as demonstrated by its association with Alzheimer’s disease. Given the variation in the ages of the samples reported in case-control studies of schizophrenia, it is important to test whether age moderates the association between APOE-ε4 and diagnosis before dismissing it.
Second, it is possible that APOE-ε4 affects the manifestation or progression of specific symptoms of schizophrenia. Schizophrenia is a heterogeneous disorder, with core symptoms including hallucinations and delusions, disorganization, and negative symptoms of avolition and inexpressivity (American Psychiatric Association, 2013; Blanchard and Cohen, 2006; Kring et al., 2013; Strauss et al., 2013, 2012). The presence and severity of these symptoms vary between individuals, and within individuals over time. A handful of cross-sectional studies have examined whether APOE-ε4 is associated with specific symptoms or diagnostic subtypes. Two groups reported associations between APOE-ε4 and negative symptoms (Martorell et al., 2001; Pickar et al., 1997). Another group identified a link with positive symptoms (Al-Asmary et al., 2015). More commonly, findings linking APOE-ε4 and diagnostic subtypes have been null (Akanji et al., 2009; Arnold et al., 1997; Durany et al., 2000; Jönsson et al., 1996; Kecmanović et al., 2010), although this literature is likely limited by the poor reliability of diagnostic subtypes (Tandon et al., 2013). However, no prior study has examined whether APOE-ε4 is associated with symptom trajectories as participants age.
The present study investigated the role of age in the relationship of APOE-ε4 with schizophrenia by first examining its associations with trajectories of symptoms (hallucinations/delusions, disorganization, avolition, and inexpressivity) during the 20 years following first hospitalization. We hypothesize that, given the link between APOE-ε4 and progressive dementias, the effect of APOE-ε4 will emerge with increasing age. In addition, we extended prior meta-analyses by conducting a meta-regression of 29 case-control studies to test whether age explains the heterogeneity of effects among previous studies of APOE-ε4 and schizophrenia.
2. Materials & Methods
2.1. Longitudinal Study
2.1.1. Design & Sample.
Data were drawn from the Suffolk County Mental Health Project, a longitudinal first-admission study of psychosis. Participants were recruited from the 12 inpatient facilities of Suffolk County, New York. The Stony Brook University Committee on Research Involving Human Subjects and the review boards of participating hospitals approved the protocol annually. To be eligible for study inclusion, participants with psychosis had to reside in Suffolk County, be between ages 15–60, speak English, and have IQ > 70. Additionally, participants had to have their first admission for psychosis within the past 6 months, with no apparent medical etiology to explain their psychotic symptoms, and have the capacity to provide informed consent.
A total of 628 participants met inclusion criteria. Follow-up interviews were conducted at 6 months, 24 months, 48 months, 10 years, and 20 years after baseline. Of the 536 who survived at the 20 year follow-up, 373 were interviewed, including 135 with a 20 year consensus diagnosis of DSM-IV schizophrenia (diagnostic procedure described in Bromet et al., 2016). Among them, 116 provided DNA samples as part of the Genomics Psychiatry Cohort collaborative (Pato et al., 2013). Demographic variables did not differ between participants for whom DNA was available and those for whom it was not, and clozapine prescription rates did not differ. However, the groups differed in the early stages of the study in terms of avolition (baseline and 48 month Cohen’s d = 0.22 and −0.28, respectively, both p < 0.05) and prescription of antipsychotic medication generally (24 and 48 month Cohen’s d = 0.20 and 0.18, respectively, both p < 0.05). Sample sizes at each time point were as follows: 6 month (N = 116); 24 month (N = 115); 48 month (N = 116); 10 year (N = 111); 20 year (N = 111).
In addition to the longitudinal clinical cohort, at the 20 year follow-up, a comparison group of 261 (206 provided DNA) never psychotic adults was recruited using random digit dialing within zip codes where participants from the original cohort resided (Velthorst et al., 2016). Rate of participation in the comparison group was 67%. The comparison group was frequency-matched to the longitudinal cohort on sex and age.
2.1.2. Measures.
Symptoms were rated using the Scale for the Assessment of Positive Symptoms (SAPS Andreasen, 1984) and the Scale for the Assessment of Negative Symptoms (SANS Andreasen, 1989). The ratings were made by master’s level interviewers after an extensive semi-structured diagnostic interview with the participant, interviews with significant others, and medical record reviews. SAPS and SANS ratings were scored into four factor-analytically derived subscales: reality distortion (hallucinations and delusions; alpha = 0.85), disorganization (alpha = 0.77) from the SAPS, and avolition/apathy (alpha = 0.87) and inexpressivity (alpha = 0.90) from the SANS (Kotov et al., 2016).
Following venipuncture, DNA was extracted from peripheral lymphocytes and sent to the Rutgers University Cell and DNA Repository, where it was genotyped using the Illumina PsychArray-8 platform containing 571,054 markers. Standard quality control procedures were performed to exclude SNPs with minor allele frequency (MAF) <1%, genotyping failure >5%, Hardy-Weinberg equilibrium p<10−6, mismatch between recorded and genotyped sex, as well as related individuals (>.20, in which case the relative with the lower call rate was dropped).
Mean call rate was 99.8%. SNP imputation was conducted with IMPUTE2 (Howie et al. 2009) against the full 1000 Genomes phase 3 reference panel (1000 Genomes Project Consortium, 2012). The imputed SNPs underwent another round of quality control and SNPs with missing data >5% and imputation information score <0.8 were excluded, yielding 6.87M high quality biallelic SNPs. All genomic data analysis was performed using the SVS software, version 8.7.0 (Golden Helix, Inc., Bozeman, MT, USA). APOE-ε4 status was defined in the standard manner using genotypes at two functional polymorphisms, rs429358 and rs7412, where the presence of the C at both positions indicates an ε4 carrier, and the presence of two T’s at the same positions indicates an ε2 allele. There were too few individuals who were homozygous for the APOE-ε4 allele to model trajectory of this group separately (N = 5), so the sample was divided based on the presence of one or two ε4 alleles (N = 31) or no ε4 allele (N = 85). Similarly, there were no ε2 homozygotes, so the sample was divided into carriers (N=32) and non-carriers (N=84). To control for population stratification due to ancestry, all analyses were covaried on the first ten principal components of genetic covariance (Price et al., 2006).
2.1.3. Statistical Analysis.
To estimate the effect of APOE genotype over time, we used multi-level spline regression models in which ratings on the four domains of the SAPS and SANS were regressed on participant age, prescription of antipsychotic medication (yes/no), APOE genotype (ε4 or ε2 carrier/noncarrier), and the interaction of APOE genotype and age. These models estimate each person’s intercept and slope, thus modeling individual trajectories. They also allowed for non-linear trajectories by estimating a point in time when the average trajectory changes. The placement of the change point was estimated by alternatively placing the change point at each 5-year interval from ages 25 and 55 (this range was chosen to ensure at least 10% of the sample was on either side of the change point). The fit of competing change points was compared via the Bayesian Information Criterion (Schwarz, 1978). Full information maximum likelihood estimation was used, which uses all information, including partial cases, to arrive at unbiased parameter estimates.
Since the baseline assessment was during hospitalization, baseline symptoms were qualitative different from the subsequent time-points. Therefore, baseline scores were analyzed separately, and trajectory analyses examined course from month 6 to year 20.
2.2. Meta-Analysis
2.2.1. Data.
We searched MEDLINE (PubMed) and PsycInfo (EBSCO) up to May 2018 for articles with title, abstract or subject terms for “APOE,” “apoprotein e,” or “apolipoprotein e” as well as “schizophrenia,” “schizophrenic,” “psychosis,” “psychotic,” “hallucination,” “hallucinations,” “delusion” or “delusions.” We also identified studies from two previous meta-analyses of APOE genotype and schizophrenia (González-Castro et al., 2015; Xu et al., 2006). In addition, to identify studies completed since the publication of the last meta-analysis, we searched among papers citing González-Castro and colleagues (2015) for relevant studies. After removal of duplicates, 293 titles and abstracts were screened by 2 reviewers. Full text was reviewed if title and abstract were unclear and discrepancies in rating were resolved by consensus, yielding 25 studies (one of these studies, Liu 2003, comprised three cohorts of different ages, which were treated as independent samples for the purposes of the meta-analysis). Studies were included if they used a case-control design, reported allele frequencies for APOE-ε4 in subjects with schizophrenia, and reported the mean age of the schizophrenia group. We limited the analysis to studies of adults, thus excluding one study in which the mean age of cases was <18 (Fernandez et al., 1999).
We also included data from the Suffolk County Mental Health Project cohort and controls, described above, as well as unpublished data from H. Haroutunian (personal correspondence; clinical data from a subset of H. Haroutunian’s sample was reported in Harvey et al., 2000, 1997). Table 1 presents the details of the samples included in the meta-regression, which amounted to N=4,297 cases and N=6,252 controls (total N=10,529). This represents a 24% increase in cases and 33% increase in the number of controls compared to the previous meta-analysis (González-Castro et al., 2015).
Table 1.
Studies Included in Meta-Analysis
| Author | Year | Race | Diagnosis | Sample | Cases | Controls | Case Age | % Male | Cases-ε4 | Controls-ε4 | OR | 95%LL | 95%UL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vila-Rodríguez et al. | 2017 | Caucasian | DSM-IV | Out. | 86 | 39 | 20.41 | 68.6 | 15 | 8 | 2.00 | 0.74 | 5.43 |
| Liu 1967–1998 sample | 2003 | Asian | DSM-IV | Inp. & Out. | 102 | 112 | 20.4 | Unk. | 12 | 8 | 1.73 | 0.68 | 4.43 |
| Moberg et al. | 2006 | Caucasian | DSM-IV | Out. | 28 | 26 | 29 | 50.0 | 17 | 5 | 3.40 | 1.16 | 9.97 |
| Lee et al. | 2001 | Asian | DSM-IV | Inp. | 60 | 60 | 32 | 65.0 | 4 | 10 | 0.38 | 0.12 | 1.24 |
| Zhu et al. | 1996 | Caucasian | DSM-III-R | Inp. | 98 | 98 | 32.9 | 62.2 | 18 | 16 | 1.15 | 0.55 | 2.42 |
| Saiz et al. | 2002 | Caucasian | DSM-IV | Out. | 106 | 250 | 35.1 | 56.6 | 19 | 31 | 1.49 | 0.82 | 2.70 |
| Igata-Yi et al. | 1997 | Asian | DSM-IV | Inp. & Out. | 54 | 33 | 36 | 27.8 | 8 | 10 | 0.45 | 0.17 | 1.20 |
| Akanji et al. | 2009 | Arab | DSM-IV | Out. | 207 | 165 | 36 | Unk. | 42 | 29 | 1.17 | 0.71 | 1.93 |
| Kecmanovic et al. | 2010 | Caucasian | DSM-IV | Out. | 76 | 82 | 37.2 | 43.4 | 20 | 15 | 1.51 | 0.74 | 3.06 |
| Thibaut et al. | 1999 | Caucasian | DSM-III-R | Out. | 106 | 98 | 38 | 72.6 | 28 | 33 | 0.49 | 0.28 | 0.85 |
| Hammer | 2014 | Caucasian | DSM-IV | Inp. & Out. | 875 | 1269 | 38.66 | 72.8 | 223 | 308 | 1.07 | 0.87 | 1.30 |
| Al-Asmary et al. | 2015 | Arab | DSM-IV | Out. | 180 | 200 | 39 | 28.9 | 27 | 17 | 1.83 | 0.98 | 3.41 |
| Kimura et al. | 2000 | Asian | DSM-IV | Inp. | 314 | 188 | 40 | 51.0 | 65 | 41 | 0.90 | 0.60 | 1.36 |
| Joober et al. | 1996 | Caucasian | Unspecified | Unk. | 51 | 35 | 41 | Unk. | 8 | 7 | 1.01 | 0.33 | 3.05 |
| Thibaut et al. | 1998 | Caucasian | DSM-III-R | Out. | 134 | 103 | 42 | 67.2 | 39 | 36 | 0.81 | 0.50 | 1.34 |
| Town et al. | 1997 | Caucasian | DSM-III-R | Unk. | 63 | 301 | 42 | Unk. | 18 | 82 | 1.06 | 0.61 | 1.83 |
| Arnold et al. | 1997 | Caucasian | DSM-III-R | Out. & P-M | 93 | 106 | 42 | 53.8 | 29 | 25 | 1.24 | 0.69 | 2.20 |
| Dean et al. | 2003 | Caucasian | DSM-IV | P-M | 23 | 47 | 42 | 65.2 | 7 | 14 | 1.03 | 0.38 | 2.75 |
| Vila-Rodríguez et al. | 2011 | Caucasian | DSM-IV | P-M | 35 | 35 | 42.6 | 74.3 | 9 | 9 | 1.09 | 0.40 | 2.93 |
| Kampman et al. | 2004 | Caucasian | DSM-IV | Inp. | 94 | 98 | 45.9 | 48.9 | 43 | 35 | 1.36 | 0.83 | 2.25 |
| Jonsson et al. | 1996 | Caucasian | DSM-III-R | Unk. | 87 | 57 | 46 | 64.4 | 32 | 22 | 0.94 | 0.52 | 1.72 |
| Kimura et al. | 1997 | Asian | DSM-III-R | Inp. | 122 | 126 | 47 | 49.2 | 21 | 26 | 0.82 | 0.45 | 1.50 |
| Suffolk County | 2013 | White | DSM-IV | Out. | 116 | 204 | 48.35 | 62.9 | 51 | 46 | 0.99 | 0.64 | 1.53 |
| Liu 1948–67 sample | 2003 | Asian | DSM-IV | Inp. & Out. | 301 | 147 | 55.3 | Unk. | 37 | 13 | 1.44 | 0.74 | 2.81 |
| Martorell et al. | 2001 | Caucasian | ICD-9 | Inp. | 365 | 548 | 56 | 60.8 | 68 | 108 | 1.02 | 0.74 | 1.40 |
| Durany et al. | 2000 | Caucasian | DSM-IV | Unk. | 114 | 94 | 56 | 43.0 | 24 | 16 | 1.20 | 0.59 | 2.43 |
| Harrington et al. | 1995 | Caucasian | Unspecified | P-M | 42 | 131 | 65 | 42.9 | 22 | 37 | 2.16 | 1.19 | 3.92 |
| Liu 1920–47 sample | 2003 | Asian | DSM-IV | Inp. & Out. | 176 | 1269 | 69 | Unk. | 24 | 74 | 2.55 | 1.56 | 4.16 |
| Haroutonian | Unp. | Caucasian | DSM-III-R | P-M | 189 | 331 | 81.08 | 52.9 | 56 | 64 | 1.76 | 1.16 | 2.66 |
Note. Studies included in meta-analysis, sorted by mean age of cases.
Defined by majority of sample.
Unp. = previously unpublished data; Unk. = unknown; Inp. = inpatient; Out. = outpatient; P-M = post-mortem.
2.2.2. Statistical Analysis.
Random-effects meta-regression was used to determine the extent to which the association between APOE-ε4 and schizophrenia (compared to healthy controls) was moderated by the age of cases. Meta-regression seeks to model reasons for heterogeneity among observed effect sizes based on between-study covariates (Harbord and Higgins, 2008). Random-effects allow for differences among effects due random between-study differences (Cornell et al., 2014). Together, a random-effects meta-regression can identify whether effect-size heterogeneity is due to systematic or random differences between studies. We followed the Hartung-Knapp-Sidik-Jonkman (HKSJ) method for meta-analysis because it consistently outperforms other estimators in small-n studies (IntHout et al., 2014). Supporting the use of random-effects meta-regression, between-study heterogeneity (τ2) was larger than zero (Q=42.52, p=0.029).
3. Results
3.1. APOE Genotype and Symptom Trajectories
Table 3 reports the associations between APOE-ε4 and symptom domains at baseline, as well as symptom trajectories from 6 months to 20 years. APOE-ε4 was not associated with any of the symptom scores at baseline, controlling for age, prescription of an antipsychotic,1 and the first ten principal components of genetic covariance. Consistent with this, the trajectory analyses found that the APOE genotype was not associated with mean symptom levels (intercepts). Furthermore, APOE-ε4 was not associated with the course (slopes) of inexpressivity, avolition/apathy, or disorganization.
Table 3.
The Association between APOE- ε4 and Symptoms in Schizophrenia
| β | p-value | |
|---|---|---|
| Baseline Associations | ||
| Reality Distortion (Hallucinations/Delusions) | −0.08 | 0.73 |
| Disorganization | 0.01 | 0.95 |
| Avolition/Apathy | 0.06 | 0.78 |
| Inexpressivity | 0.07 | 0.74 |
| Longitudinal Associations | ||
| Reality Distortion (Hallucinations/Delusions) | ||
| APOE-ε4 | 0.22 | 0.12 |
| APOE-ε4 x Slope (prior to age 40) | −0.10 | 0.46 |
| APOE-ε4 x Slope (post age 40) | 0.52 | < 0.0001 |
| Disorganization | ||
| APOE-ε4 | 0.15 | 0.43 |
| APOE-ε4 x Slope (prior to age 40) | −0.04 | 0.81 |
| APOE-ε4 x Slope (post age 40) | 0.11 | 0.34 |
| Avolition/Apathy | ||
| APOE-ε4 | 0.02 | 0.76 |
| APOE-ε4 x Slope (prior to age 50) | −0.24 | 0.10 |
| APOE-ε4 x Slope (post age 50) | 0.07 | 0.09 |
| Inexpressivity | ||
| APOE-ε4 | −0.06 | 0.92 |
| APOE-ε4 x Slope (prior to age 25) | −0.24 | 0.08 |
| APOE-ε4 x Slope (post age 25) | −0.20 | 0.49 |
Note. Analyses controlled for use of antipsychotics (time-varying covariate) and the first 10 principal components of genetic covariance. Baseline analyses were also controlled for age.
However, APOE-ε4 was significantly associated with an increasing severity of hallucinations and delusions (e.g., reality distortion) with age. After age 40, those with an APOE-ε4 allele experienced an escalation of psychotic symptoms (β = 0.52, p < 0.0001). Figure 1 depicts the mean trajectory of changes in severity of hallucinations and delusions with age, moderated by APOE-ε4. Age 40 is the inflection point at which the severity of hallucinations and delusions increases for those with one or more alleles, escalating at a rate of a 0.46 standard deviations per decade.
Fig. 1.
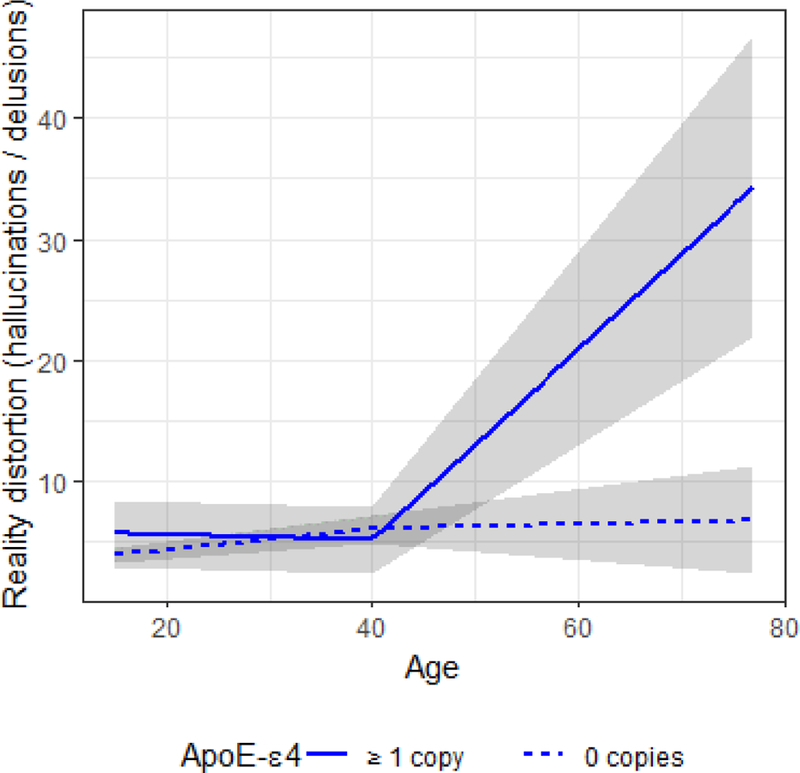
Note. Reality distortion (hallucinations and delusions) as a function of age in APOE-ε4 carriers (solid line) and non-carriers (dotted line). The surrounding gray area represents the standard error of the effect.
The APOE-ε2 allele was investigated as a post-hoc follow-up analysis. The APOE-ε2 allele was not a predictor of any domain of schizophrenia symptoms, either as a risk factor for mean symptom severity, or symptom course.
3.2. Meta-Analysis of Case-Control Studies
Figure 2 depicts the meta-regression of effect sizes, weighted by variance of the observed effects, on the mean age of cases (see Table 1 for details of included studies). Regressing observed effect sizes on age revealed a significant effect of case age on the magnitude of the association between APOE-ε4 and schizophrenia (OR=1.01 [1.00–1.02]; p<0.05). Specifically, the effect of APOE-ε4 was not significant for participants younger than 45, but for those over 45, APOE-ε4 was associated with schizophrenia, such that carriers were 10% more likely to have the diagnosis with every passing decade.2 Neither race nor sample type (inpatient, outpatient, or post-mortem) moderated the effect. Gender balance of cases did moderate the effect, such that studies with a larger proportion of male cases observed smaller effect sizes (OR=0.99 [0.98–0.99]; p<0.05), but the addition of this covariate did not diminish the effect of age or change its significance (adjusted OR=1.01 [1.00–1.02]; p<0.05).
Fig. 2.
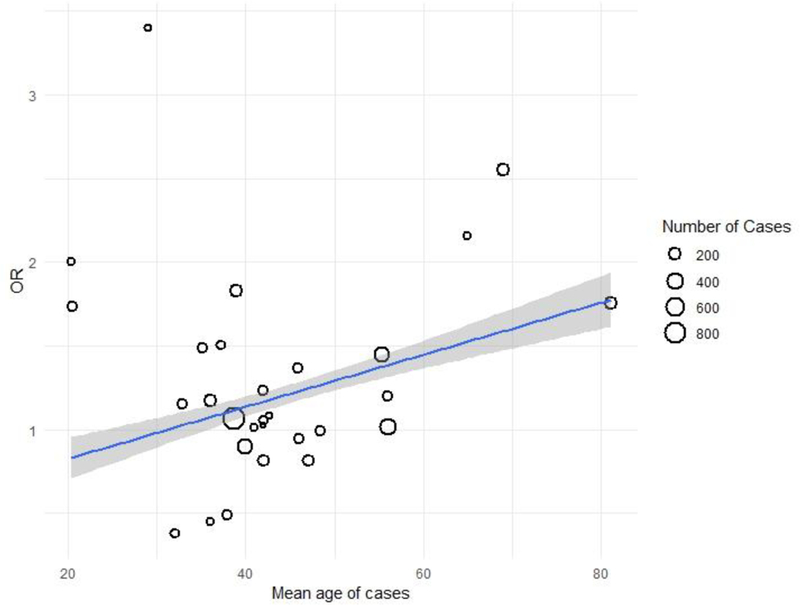
Note. Association between APOE-ε4 and schizophrenia diagnosis as a function of mean age of cases. The blue line represents the meta-regression of the effect sizes of individual studies on age. The surrounding gray area represents the standard error of the effect.
4. Discussion
This study sought to determine whether APOE-ε4, a genetic variant long known to influence risk of a number of chronic diseases including cardiovascular disease and dementia (Eichner et al., 2002; Poirier et al., 1993), shapes the course of psychosis. First, we examined contributions of this polymorphism to the course of schizophrenia tracked over 20 years, and found that participants with APOE-ε4 experienced a progressive increase in reality distortion (hallucinations and delusions) after age 40, while those without the allele had no increase in this symptom domain. Of note, the effect was specific to hallucinations and delusions, with an effect size twice as large as any other that was detected. Second, we performed a meta-analysis that revealed a significant association between APOE-ε4 and schizophrenia in participants after midlife, such that carriers’ odds of diagnosis increased 10% per decade. An effect of APOE-ε4 in schizophrenia was not previously detected because other meta-analyses did not consider age. Taken together, these results argue for an important role of this polymorphism in older people with schizophrenia.
4.1. Implications
These results suggest that APOE genotype may be useful in predicting the course of illness among individuals with schizophrenia. Hallucinations and delusions are critical symptoms for this population. For example, delusions predict medication non-adherence (Verdoux et al., 2000), which in turn increases the likelihood of another psychotic episode (Coldham et al., 2002). Those with the APOE-ε4 allele may be at increased risk for entering this feedforward loop, even if psychotic symptoms have been controlled in the past. Genetic screening may provide patients, families, and clinicians with a better sense of prognosis in schizophrenia, and help to identify patients who would benefit the most from support in maintaining medication adherence as they age.
This study was concerned only with individuals with schizophrenia. Whether APOE-ε4 is a risk factor for a trajectory of worsening psychotic symptoms in other psychotic disorders or patients with dementia remains unknown (Shah et al., 2017). Genetic liabilities for Alzheimer’s disease with psychosis do not appear to increase liability for psychosis outside of dementia (Bacanu et al., 2005; Hollingworth et al., 2012), and many have suggested that psychosis in dementia is largely distinct from other psychotic disorders (Jeste and Finkel, 2000). These two forms of psychosis may be mediated by different biological pathways (DeMichele-Sweet and Sweet, 2010; DeMichele-Sweet et al., 2011; Sweet et al., 2003). Alternatively, the effect of APOE-ε4 may be mediated through its association with reduced hippocampal volume (Khan et al., 2017; Li et al., 2016; Plassman et al., 1997), also observed in schizophrenia (Heckers Stephan, 2001; Lieberman et al., 2018), through the same pathways that lead to cognitive impairment in both diseases (Martins et al., 2005; Rapp et al., 2010), or through interaction with pathways that are up- and downregulated over the course of illness in schizophrenia (Narayan et al., 2008; Tang et al., 2011). Regardless, just as APOE-ε4’s effect on psychotic symptoms has gone undetected because it is dependent on age, it may be that neurodegenerative processes shared by schizophrenia and Alzheimer’s disease have gone undetected due to the shortened life expectancy of people with schizophrenia (Saha et al., 2007). Resolution of this conundrum will require a longitudinal study using of early imaging and behavioral markers of Alzheimer’s disease in people with schizophrenia and age-matched controls.
Of note, these results cannot conclusively determine whether APOE-ε4 is enriched in late-onset schizophrenia, as suggested by meta-regression findings, or if it instead predicts an exacerbation of hallucinations and delusions among those who have schizophrenia with typical age of onset, as suggested by longitudinal analyses. The effect of APOE-ε4 was specific to hallucinations and delusions, which are insufficient for a diagnosis of schizophrenia, and meta-regression found no association between APOE-ε4 and age of onset. Therefore, it seems likely that the association between APOE-ε4 and psychotic symptoms manifests as differences in case-control status due to attrition. Specifically, a worsening course of hallucinations and delusions among individuals with schizophrenia who are APOE-ε4 carriers, such as we observed, could increase involvement with psychiatric services relative to other cases, increasing enrollment of carriers into studies. This mechanism, however, remains to be tested.
4.2. Strengths and Limitations
To our knowledge, this is the first study of APOE-ε4 as a predictor of longitudinal changes in schizophrenia symptom severity. It is especially novel in that participants were followed for two decades after first hospitalization. While this study can clarify the role of genetic liabilities in individuals with psychosis as they age, it is not without important limitations. One limitation is the substantial attrition from the genetic assays. The patterns of missing data suggest that in the early phases of the study, participants for whom genetic information was available were more symptomatic than those for whom it was not. However, the effect of ApoE- ε4 was only observed after age 40, at which point there were no differences between these two groups. Sample size was modest, but the repeated assessment of symptoms over time increased the precision of the estimates beyond what would be obtained in a cross-sectional design, partially mitigating this limitation. Another point of concern may be that only the APOE gene was tested. The reason these analyses were constrained to APOE is that it was identified a priori as a gene of interest in this sample (see MH094398). Furthermore, only one symptom domain out of four was associated with APOE-ε4 genotype. However, the association between APOE-ε4 and Alzheimer’s disease (Poirier et al., 1993) and the high incidence of hallucinations and delusions in Alzheimer’s disease (20.1% over one year (Paulsen et al., 2000) make the link between APOE-ε4 and psychosis plausible. After correction for multiple comparisons (corrected α = 0.003), this sample of 116 had 84% power to detect an effect of the observed magnitude.
A limitation of meta-regression is that between-study covariates, in this case age, are a blunt approximation of the true effect of interest, which is the moderating effect of age on the observed association between APOE-ε4 and schizophrenia. However, these preliminary findings suggest the importance of considering age when designing future case-control genetic association studies, particularly for those genes whose products are known to exert their effect within a specific developmental window. A second limitation of the meta-regression is the dependence on convenience samples, which were not always demographically matched to cases. Lastly, in both the longitudinal analyses and meta-analysis, there was insufficient information to distinguish APOE-ε4 homozygotes and heterozygotes. Although this is common practice in even the largest studies of APOE-ε4 (Hammer et al., 2014; Khan et al., 2017), future studies should examine whether APOE-ε4 has differential effects for these two groups.
4.3. Conclusions
In sum, APOE-ε4 carriers show a pronounced increase in the severity of delusions and hallucinations later in life, while those without the allele do not. This escalation in reality distortion attributable to APOE-ε4 may explain discrepancies in the case-control literature. Prior studies and meta-analyses of APOE-ε4 and schizophrenia did not account for age, and therefore missed a genetic association that emerges in late adulthood. By understanding the function of APOE-ε4, future research may discover modulatory pathways for psychosis. APOE-ε4 might function as a marker to identify people with schizophrenia who need increased support and intervention in late adulthood.
Table 2.
Descriptive Characteristics of the Twenty Year Follow-Up of the Suffolk County Mental Health Project
| N (%) | |
|---|---|
| Gender | |
| Male | 73(62.9) |
| Female | 43(37.1) |
| Race/Ethnicity | |
| Caucasian | 85(79.4) |
| African-American | 14(13.1) |
| Other | 8(7.5) |
| APOE-ε4 alleles | |
| 0 | 85(73.3) |
| 1 | 26(22.4) |
| 2 | 5(4.3) |
| Prescribed antipsychotics | |
| Baseline | 100(86) |
| 6 months | 99(85) |
| 24 months | 97(84) |
| 48 months | 88(76) |
| 10 years | 100(90) |
| 20 years | 90(81) |
| Age at baseline | |
| 10–20 | 21(18.1) |
| 20–30 | 52(44.8) |
| 30–40 | 34(29.3) |
| 40–50 | 9(7.8) |
| Age at 20-year follow up | |
| 30–40 | 26(22.4) |
| 40–50 | 48(41.4) |
| 50–60 | 36(31.0) |
| 60–70 | 6(5.2) |
Note. Total N=116.
5.4. Acknowledgements
The authors gratefully acknowledge the support of the participants and mental health community of Suffolk County for contributing their time and energy to this project. They are also indebted to the study coordinators for their dedicated efforts, the interviewers for their careful assessments, and the psychiatrists who derived the consensus diagnoses.
5.1 Funding Body Agreements and Policies
This work was supported by the National Institutes of Health (MH44801 to EB, MH094398 to RK, MH085548 to Carlos Pato, subcontract to EB, and MH085542 to Carlos Pato, subcontract to EB, and MH117646 to TL); and by the Brain and Behavior Research Foundation (NARSAD Young Investigator Grant to RK).
Footnotes
Author Disclosures
Conflicts of Interest
The authors (KJ, SC, KL, LF, TL, AM, DC, GP, EB, & RK) have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Removing antipsychotic prescriptions from the regression did not change the observed pattern of effects.
In the subset of studies for which age of onset was reported (12 papers), age of onset did not predict the relationship between APOE genotype and diagnosis (OR=1.01 [0.97–1.05]; p=0.65).
6. References
- 1000 Genomes Project Consortium, 2012. An integrated map of genetic variation from 1,092 human genomes. Nature 491 (7422) 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akanji AO, Ohaeri JU, Al-Shammri SN, Fatania HR, 2009. Apolipoprotein E polymorphism and clinical disease phenotypes in Arab patients with schizophrenia. Neuropsychobiology 60 (2) 67–72. [DOI] [PubMed] [Google Scholar]
- Al-Asmary SM, Kadasah S, Arfin M, Tariq M, Al-Asmari A, 2015. Apolipoprotein E polymorphism is associated with susceptibility to schizophrenia among Saudis. Arch. Med. Sci. AMS 11 (4) 869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders, 5th edition. ed. Washington, DC. [Google Scholar]
- Andreasen NC, 1989. Scale for the Assessment of Negative Symptoms (SANS). Br. J. Psychiatry [PubMed]
- Andreasen NC, 1984. Scale for the assessment of positive symptoms (SAPS) University of Iowa Iowa City. [Google Scholar]
- Arnold SE, Joo E, Martinoli M-G, Roy N, Trojanowski JQ, Gur RE, Cannon T, Price RA, 1997. Apolipoprotein E genotype in schizophrenia: frequency, age of onset, and neuropathologic features. Neuroreport 8 (6) 1523–1526. [DOI] [PubMed] [Google Scholar]
- Austin SF, Mors O, Secher RG, Hjorthøj CR, Albert N, Bertelsen M, Jensen H, Jeppesen P, Petersen L, Randers L, 2013. Predictors of recovery in first episode psychosis: the OPUS cohort at 10 year follow-up. Schizophr. Res 150 (1) 163–168. [DOI] [PubMed] [Google Scholar]
- Bacanu S-A, Devlin B, Chowdari KV, DeKosky ST, Nimgaonkar VL, Sweet RA, 2005. Heritability of psychosis in Alzheimer disease. Am. J. Geriatr. Psychiatry 13 (7) 624–627. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Cohen AS, 2006. The structure of negative symptoms within schizophrenia: Implications for assessment. Schizophr. Bull 32 (2) 238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromet EJ, Kotov R, Fochtmann LJ, Carlson GA, Tanenberg-Karant M, Ruggero C, Chang S, 2011. Diagnostic shifts during the decade following first admission for psychosis. Am. J. Psychiatry 168 (11) 1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coldham EL, Addington J, Addington D, 2002. Medication adherence of individuals with a first episode of psychosis. Acta Psychiatr. Scand 106 (4) 286–290. [DOI] [PubMed] [Google Scholar]
- Cornell JE, Mulrow CD, Localio R, Stack CB, Meibohm AR, Guallar E, Goodman SN, 2014. Random-effects meta-analysis of inconsistent effects: a time for change. Ann. Intern. Med 160 (4) 267–270. [DOI] [PubMed] [Google Scholar]
- DeMichele-Sweet MA, Sweet RA, 2010. Genetics of Psychosis in Alzheimer Disease: A Review. J. Alzheimers Dis. JAD 19 (3) 761–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMichele-Sweet MAA, Lopez OL, Sweet RA, 2011. Psychosis in Alzheimer’s disease in the National Alzheimer’s Disease Coordinating Center Uniform Data Set: Clinical correlates and association with Apolipoprotein E. Int. J. Alzheimers Dis 2011. [DOI] [PMC free article] [PubMed]
- Durany N, Riederer P, Cruz-Sánchez FF, 2000. Apolipoprotein E genotype in Spanish schizophrenic patients. Psychiatr. Genet 10 (2) 73–77. [DOI] [PubMed] [Google Scholar]
- Eichner JE, Dunn ST, Perveen G, Thompson DM, Stewart KE, Stroehla BC, 2002. Apolipoprotein E polymorphism and cardiovascular disease: a HuGE review. Am. J. Epidemiol 155 (6) 487–495. [DOI] [PubMed] [Google Scholar]
- Fernandez T, Yan WL, Hamburger S, Rapoport JL, Saunders AM, Schapiro M, Ginns EI, Sidransky E, 1999. Apolipoprotein E alleles in childhood-onset schizophrenia. Am. J. Med. Genet 88 (2) 211–213. [PubMed] [Google Scholar]
- González-Castro TB, Tovilla-Zárate CA, Hernández-Díaz Y, Fresán A, Juárez-Rojop IE, Ble-Castillo JL, López-Narváez L, Genis A, Hernández-Alvarado MM, 2015. No association between ApoE and schizophrenia: Evidence of systematic review and updated meta-analysis. Schizophr. Res 169 (1) 355–368. [DOI] [PubMed] [Google Scholar]
- Hammer C, Zerche M, Schneider A, Begemann M, Nave K-A, Ehrenreich H, 2014. Apolipoprotein E4 carrier status plus circulating anti-NMDAR1 autoantibodies: association with schizoaffective disorder. Mol. Psychiatry 19 (10) 1054–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbord RM, Higgins J, 2008. Meta-regression in Stata. Meta 8 (4) 493–519. [Google Scholar]
- Haro JM, Novick D, Bertsch J, Karagianis J, Dossenbach M, Jones PB, 2011. Cross-national clinical and functional remission rates: Worldwide Schizophrenia Outpatient Health Outcomes (W-SOHO) study. Br. J. Psychiatry 199 (3) 194–201. [DOI] [PubMed] [Google Scholar]
- Harrison G, Hopper K, Craig T, Laska E, Siegel C, Wanderling J, Dube K, Ganev K, Giel R, Der Heiden W, 2001. Recovery from psychotic illness: a 15-and 25-year international follow-up study. Br. J. Psychiatry 178 (6) 506–517. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Jacobsen H, Mancini D, Parrella M, White L, Haroutunian V, Davis KL, 2000. Clinical, cognitive and functional characteristics of long-stay patients with schizophrenia: a comparison of VA and state hospital patients. Schizophr. Res 43 (1) 3–9. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Powchik P, Parrella M, White L, Davidson M, 1997. Symptom severity and cognitive impairment in chronically hospitalised geriatric patients with affective disorders. Br. J. Psychiatry 170 (4) 369–374. [DOI] [PubMed] [Google Scholar]
- Heckers Stephan, 2001. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus 11 (5) 520–528. [DOI] [PubMed] [Google Scholar]
- Hollingworth P, Sweet R, Sims R, Harold D, Russo G, Abraham R, Stretton A, Jones N, Gerrish A, Chapman J, Ivanov D, Moskvina V, Lovestone S, Priotsi P, Lupton M, Brayne C, Gill M, Lawlor B, Lynch A, Craig D, McGuinness B, Johnston J, Holmes C, Livingston G, Bass NJ, Gurling H, McQuillin A, Holmans P, Jones L, Devlin B, Klei L, Barmada MM, Demirci FY, DeKosky ST, Lopez OL, Passmore P, Owen MJ, O’Donovan MC, Mayeux R, Kamboh MI, Williams J, 2012. Genome-wide association study of Alzheimer’s disease with psychotic symptoms. Mol. Psychiatry 17 (12) 1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IntHout J, Ioannidis JP, Borm GF, 2014. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med. Res. Methodol 14 (1) 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste DV, Finkel SI, 2000. Psychosis of Alzheimer’s Disease and Related Dementias: Diagnostic Criteria for a Distinct Syndrome. Am. J. Geriatr. Psychiatry 8 (1) 29–34. [DOI] [PubMed] [Google Scholar]
- Jönsson E, Sedvall G, Lannfelt L, Engvall B, 1996. Lack of association between schizophrenia and the apolipoprotein E ε4 allele. Eur. Arch. Psychiatry Clin. Neurosci 246 (4) 182–184. [DOI] [PubMed] [Google Scholar]
- Kecmanović M, Dobričić V, Dimitrijević R, Keckarević D, Savić-Pavićević D, Keckarević-Marković M, Ivkovic M, Romac S, 2010. Schizophrenia and apolipoprotein E gene polymorphism in Serbian population. Int. J. Neurosci 120 (7) 502–506. [DOI] [PubMed] [Google Scholar]
- Khan W, Giampietro V, Banaschewski T, Barker GJ, Bokde AL, Büchel C, Conrod P, Flor H, Frouin V, Garavan H, 2017. A multi-cohort study of ApoE ɛ4 and Amyloid-β effects on the hippocampus in Alzheimer’s disease. J. Alzheimers Dis 56 (3) 1159–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Foti D, Li K, Bromet EJ, Hajcak G, Ruggero CJ, 2016. Validating dimensions of psychosis symptomatology: Neural correlates and 20-year outcomes. J. Abnorm. Psychol 125 (8) 1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP, 2013. The clinical assessment interview for negative symptoms (CAINS): final development and validation. Am. J. Psychiatry 170 (2) 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Shi J, Gutman BA, Baxter LC, Thompson PM, Caselli RJ, Wang Y, Initiative ADN, 2016. Influence of APOE genotype on hippocampal atrophy over time-An N= 1925 surface-based ADNI study. PloS One 11 (4) e0152901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman J, Girgis R, Brucato G, Moore H, Provenzano F, Kegeles L, Javitt D, Kantrowitz J, Wall M, Corcoran C, 2018. Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention. Mol. Psychiatry [DOI] [PMC free article] [PubMed]
- Martins CAR, Oulhaj A, de Jager CA, Williams JH, 2005. APOE alleles predict the rate of cognitive decline in Alzheimer disease. Neurology 65 (12) 1888–1893. [DOI] [PubMed] [Google Scholar]
- Martorell L, Virgos C, Valero J, Coll G, Figuera L, Joven J, Pocoví M, Labad A, Vilella E, 2001. Schizophrenic women with the APOE [straight epsilon]4 allele have a worse prognosis than those without it. Mol. Psychiatry N. Y 6 (3) 307–10. [DOI] [PubMed] [Google Scholar]
- Narayan S, Tang B, Head SR, Gilmartin TJ, Sutcliffe JG, Dean B, Thomas EA, 2008. Molecular profiles of schizophrenia in the CNS at different stages of illness. Brain Res 1239 235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pato MT, Sobell JL, Medeiros H, Abbott C, Sklar BM, Buckley PF, Bromet EJ, Escamilla MA, Fanous AH, Lehrer DS, 2013. The genomic psychiatry cohort: partners in discovery. Am. J. Med. Genet. B Neuropsychiatr. Genet 162 (4) 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen JS, Salmon DP, Thal LJ, Romero R, Weisstein–Jenkins C, Galasko D, Hofstetter CR, Thomas R, Grant I, Jeste DV, 2000. Incidence of and risk factors for hallucinations and delusions in patients with probable AD. Neurology 54 (10) 1965–1971. [DOI] [PubMed] [Google Scholar]
- Pickar D, Malhotra AK, Rooney W, Breier A, Goldman D, 1997. Apolipoprotein E ε4 and clinical phenotype in schizophrenia. The Lancet 350 (9082) 930–931. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Welsh-Bohmer K, Bigler E, Johnson S, Anderson C, Helms M, Saunders A, Breitner J, 1997. Apolipoprotein E ϵ4 allele and hippocampal volume in twins with normal cognition. Neurology 48 (4) 985–988. [DOI] [PubMed] [Google Scholar]
- Poirier J, Bertrand P, Kogan S, Gauthier S, Davignon J, Bouthillier D, 1993. Apolipoprotein E polymorphism and Alzheimer’s disease. The Lancet 342 (8873) 697–699. [DOI] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D, 2006. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet 38 (8) 904. [DOI] [PubMed] [Google Scholar]
- Ram R, Bromet EJ, Eaton WW, Pato C, Schwartz JE, 1992. The natural course of schizophrenia: a review of first-admission studies. Schizophr. Bull 18 (2) 185–207. [DOI] [PubMed] [Google Scholar]
- Rapp MA, Schnaider-Beeri M, Purohit DP, Reichenberg A, McGurk SR, Haroutunian V, Harvey PD, 2010. Cortical neuritic plaques and hippocampal neurofibrillary tangles are related to dementia severity in elderly schizophrenia patients. Schizophr. Res 116 (1) 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Chant D, McGrath J, 2007. A systematic review of mortality in schizophrenia: Is the differential mortality gap worsening over time? Arch. Gen. Psychiatry 64 (10) 1123–1131. [DOI] [PubMed] [Google Scholar]
- Schwarz G, 1978. Estimating the dimension of a model. Ann. Stat 6 (2) 461–464. [Google Scholar]
- Shah C, DeMichele-Sweet MAA, Sweet RA, 2017. Genetics of psychosis of Alzheimer disease. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet 174 (1) 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Hong LE, Gold JM, Buchanan RW, McMahon RP, Keller WR, Fischer BA, Catalano LT, Culbreth AJ, Carpenter WT, 2012. Factor structure of the brief negative symptom scale. Schizophr. Res 142 (1) 96–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Horan WP, Kirkpatrick B, Fischer BA, Keller WR, Miski P, Buchanan RW, Green MF, Carpenter WT, 2013. Deconstructing negative symptoms of schizophrenia: avolition–apathy and diminished expression clusters predict clinical presentation and functional outcome. J. Psychiatr. Res 47 (6) 783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD, 1993. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci 90 (5) 1977–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet RA, Nimgaonkar VL, Devlin B, Jeste DV, 2003. Psychotic symptoms in Alzheimer disease: evidence for a distinct phenotype. Mol. Psychiatry 8 (4) 383–392. [DOI] [PubMed] [Google Scholar]
- Tandon R, Gaebel W, Barch DM, Bustillo J, Gur RE, Heckers S, Malaspina D, Owen MJ, Schultz S, Tsuang M, 2013. Definition and description of schizophrenia in the DSM-5. Schizophr. Res 150 (1) 3–10. [DOI] [PubMed] [Google Scholar]
- Tang B, Dean B, Thomas E, 2011. Disease-and age-related changes in histone acetylation at gene promoters in psychiatric disorders. Transl. Psychiatry 1 (12) e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velthorst E, Fett A-KJ, Reichenberg A, Perlman G, van Os J, Bromet EJ, Kotov R, 2016. The 20-year longitudinal trajectories of social functioning in individuals with psychotic disorders. Am. J. Psychiatry 174 (11) 1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoux H, Lengronne J, Liraud F, Gonzales B, Assens F, Abalan F, Os J van, 2000. Medication adherence in psychosis: predictors and impact on outcome.A 2-year follow-up of first-admitted subjects. Acta Psychiatr. Scand 102 (3) 203–210. [DOI] [PubMed] [Google Scholar]
- Xu M-Q, St Clair D, He L, 2006. Meta-analysis of association between ApoE ε4 allele and schizophrenia. Schizophr. Res 84 (2) 228–235. [DOI] [PubMed] [Google Scholar]


