Abstract
Nowadays, the interest in manufacturing non-alcoholic or low alcoholic content beverages from alcoholic beverages is a current challenge for food technologists; this is due to the fact that huge consumption of alcoholic beverages may produce health problems in the costumers. In principle, the post-fermentation ethanol removal from alcoholic beverages is carried out by means of evaporation or distillation. Such current dealcoholization methodologies are efficiently removing the ethanol, however, some organoleptic compounds can also be lost during the process. This makes the dealcoholization process highly sensitive in order to preserve the quality properties of the beverages. Thereby, membrane-based technologies, which use perm-selective barriers for the separation, have been highly promoted for such purpose. Pervaporation (PV) technology is indeed one of these technologies aimed for ethanol removal. Herein, the goal of this review is to provide a compelling overview of the most relevant findings for the production of non-alcoholic beverages (such as beer and wine) by means of PV. Particular attention is paid to experimental results which provide compelling feedback about the accurate ethanol removal and minimal changes on physicochemical properties of the beverages. Moreover, some theoretical basis of such technology, as well as key criteria for a more efficient dealcoholization, are also given.
Keywords: Non-alcoholic beverages, Dealcoholization, Membranes, Pervaporation, Ethanol removal
Introduction
As it is well-known, the alcoholic beverages consumption has extremely increased according to the World Health Organization (WHO) (WHO 2011). Indeed, the current global consumption of alcoholic beverages is around 54.2 billion liters per year (Statista 2018) that includes mainly beer and wine products, this consumption is expected to increase in coming years (Solov 2018). Typically, several types of drinks can be found in the category of alcoholic beverages, such as beer, spirits, wines (e.g. fortified wines, rice wine or other fermented beverages based on sorghum, millet, maize) and some other traditional high-alcohol content beverages (Cleophas 1999); among all these products, the highest consumption regards to the beer and wine. However, in the last decades, the market for non-alcoholic beverages, especially for beer and wine, has increased its demand based on the regulations to prevent health issues. It is well documented that high and excessive consumption of alcoholic beverages may result in different types of diseases, such as pancreas cancer, pancreatitis, hepatitis, fatty degradation of liver, cirrhosis, peptic ulcers, allergenic induction, increase of uric acid in plasma, promoting obesity and some other derivative harmful effects; especially, pancreatitis and cirrhosis in their acute form are frequently caused by high huge consumption of alcoholic beverages (Costanzo et al. 2010; Partanen et al. 1997; Sohrabvandi et al. 2012). In fact, these diseases are directly attributed to the alcohol content of such beverages. On the other hand, such beverages can also provide some positive benefits into human health related to their nutritional benefits, including hypolipidemic effect, anti-mutagenic and anti-carcinogenic effects, reduction of cardiovascular disease (cardioprotective effect), immune system stimulation, anti-osteoporosis effect, and reducing risk of dementia (Sohrabvandi et al. 2012).
The production of non-alcoholic beverages can be carried out by altering the fermentation process (Purwasasmita et al. 2015), or by using special and immobilized yeasts (Strejc et al. 2013); unfortunately, to break the fermentation process restricts the production of some desirable compounds. In fact, at the brewery industry, the fermentation step is fundamental for the flavor formation and quality control of beers (Olaniran et al. 2017). Thereby, this practice is not so recommendable alternative for the production of non-alcoholic beverages, At this point, it is needed to carry out the ethanol removal after the complete beverage fermentation step; this may imply a dealcoholization through a downstream process.
The dealcoholization of beverages is a promising alternative for the production of non-alcoholic drinks which can preserve most of the organoleptic and nutritional values of the original beverages. Basically, the dealcoholization process mainly involves physical separation processes based on different driving force, such as (1) temperature (e.g. evaporation and distillation) (Andrés-Iglesias et al. 2016; Belisario-Sánchez et al. 2012), (2) pressure in which different membrane-based processes can be found (e.g. pervaporation (PV), nanofiltration, reverse osmosis), (3) pressure and temperature simultaneously (e.g. PV, membrane distillation), and (4) concentration (e.g. dialysis, membrane contactors, osmotic distillation, and diafiltration). All these membrane processes have been widely sought by food research community according to their advantages over conventional dealcoholization procedures (Lipnizki 2014; Mangindaan et al. 2018). Importantly, membrane-based processes are becoming emerging alternatives for the treatment and processing of food systems (Cassano et al. 2018; Castro-Muñoz et al. 2017, 2018a; Castro-Muñoz and Fíla 2018; Galanakis 2013; Galanakis et al. 2015), as well as the recovery of high-added value compounds from natural food products (Castro-Muñoz et al. 2016, 2018b; Galanakis 2013, 2015; Galanakis et al. 2015). In particular, PV is a potential candidate for efficient and selective ethanol removal from alcoholic beverages. Thereby, the aim of this review paper is to provide a perspective about the literature findings for the dealcoholization of alcoholic beverages (e.g. beer and wines) by means of PV, aiming the production of non-alcoholic beverages.
Particular attention is paid to experimental results which provide compelling ethanol removal and its effect on the physicochemical properties of the beverages. Moreover, some theoretical basis of such technology, as well as key criteria for a more efficient and selective dealcoholization, are also given.
Ethanol removal using pervaporation: theoretical background and key features
Pervaporation (PV) is considered as a suitable and effective membrane-based technology to perform the separation of similar boiling points components contained in an ‘‘azeotropic mixture’’, where phase change from liquid to vapor takes place. Today, PV is considered an alternative to traditional processes, such as simple distillation, vacuum distillation, fractional distillation and steam distillation (Afonso et al. 2015). Table 1 displays the most relevant advantages and disadvantages of PV technology, highlighting that its low energy consumption and non-use of additional solvents encourage its use (Figoli et al. 2015).
Table 1.
Advantages and disadvantages of PV technology.
| Advantages | Disadvantages |
|---|---|
| Less energy consumption compared to traditional distillation | High investment costs (e.g. membranes, devices, installations) |
| Minimal possibility of product contamination | Operating temperature range 50–100 °C |
| High separation efficiency for purification | Components with high boiling points make pervaporation more difficult |
| Non-use of additional solvents/phases | Removes the minor component |
| Competitive for removal of volatile organic compounds with carbon adsorption | Needs pure phases (i.e. solvents) to avoid membrane fouling/pollution |
| Ability to be coupled with other processes (e.g. distillation) | Low permeation rates at low temperatures |
| Multi-component mixtures even with just small differences in boiling points can be effectively dehydrated | Scarce membrane market |
| PV systems are easy to operate |
In principle, a binary or a multi-component liquid mixture is separated by partial vaporization using a dense non-porous membrane. The liquid feed mixture is in direct contact with the “selective” side of the membrane, while the permeate (collected at the other side of the membrane) is in a vapor phase, enriched by the species with a higher affinity with the membrane (hydrophilic or hydrophobic type). In theory, the transport of the permeating species takes place due to the driving force applied, which could be (1) vacuum or (2) sweeping gas (like nitrogen) and (3) temperature, and then condensed and recovered. Indeed, PV is combining two different separation processes, such as “permeation” and “evaporation” (Crespo and Brazinha 2015). The transport mechanism through dense polymeric membranes is described by the so-called solution-diffusion model (Crespo and Brazinha 2015; Wijmans and Baker 1995), which comprises three main steps: (1) adsorption of the target component from the mixture to the membrane “selective” layer on the basis of its chemical affinity, (2) diffusion of the target component through the membrane as a result of the concentration gradient, (3) desorption of the component at the permeate side of the membrane (Wee et al. 2008). The mass transport is governed by the chemical potential (μi) gradient, the physical properties of the permeating component (1) and its concentration in feed and permeate side.
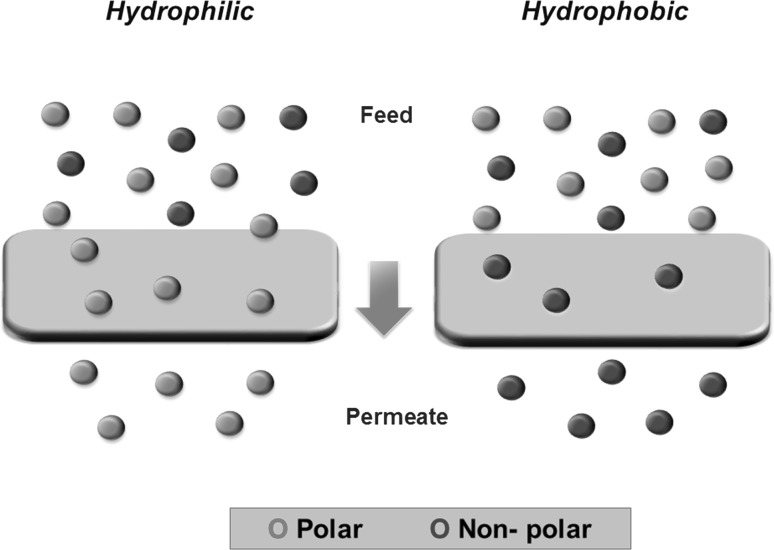
To date, PV has been efficient in separating different types of water-organic, organic–organic and organic–water mixtures (Castro-Muñoz et al. 2018b, c). Importantly, the membrane material plays an important role in the separation by PV, as Fig. 1 depicts.
Fig. 1.
General drawing of hydrophilic and hydrophobic membranes for PV applications
The alcohol removal crucially comprises the use of non-porous hydrophobic membranes, which are able to remove organics (non-polar or less polar compounds), such as alcohols (e.g. ethanol, propanol, isobutanol, and isoamyl alcohol), aldehydes (e.g. acetaldehyde) and esters (e.g. ethyl acetate and isoamyl acetate). Indeed, PV finds its main application in food and cosmetic industries for the extraction of aroma compounds (Lipnizki et al. 2002; Raisi et al. 2009; Saffarionpour and Ottens 2018). When dealing with the dealcoholization of beverages, this implies an organic–water separation, it means, removing the ethanol from a complex aqueous solution. Actually, PV uses highly selective membranes removing the minor component in the mixture only, i.e. the ethanol is commonly the minor compound in alcoholic beverages.
To date, several hydrophobic nature polymers for the preparation of membranes have been proposed for the ethanol removal in aqueous solution models (Castro-Muñoz et al. 2018c). Table 2 displays some of these hydrophobic membranes and their performance.
Table 2.
Hydrophobic membranes tested for the ethanol recovery by PV.
Adapted from Castro-Muñoz et al. (2018c)
| Feed concentration | Polymeric membrane | Manufacture | Operating conditions | Total flux values (kg/m2/h) | Selectivity (separation factor) | References |
|---|---|---|---|---|---|---|
| 5 wt% ethanol | PEBA | Homemade |
40 °C Vacuum pressure 1.5 mbar. |
0.70 | 3.0 | Gu et al. (2009) |
| 10 wt% ethanol | PDMS | Homemade |
40 °C Vacuum pressure 13 mbar. |
0.50 | 5.0 | Huang et al. (2009) |
| 10 wt% ethanol | PTMSP | Homemade |
50 °C Vacuum pressure 0.04 mbar. |
3.5 | 12 | Claes et al. (2010) |
| 10 wt% ethanol | Pervatech PDMS | Pervatech BV (The Netherlands) |
50 °C Vacuum pressure 0.04 mbar. |
3.3 | 6 | |
| 10 wt% ethanol | PERVAP 4060 | Sulzer ChemTech (Switzerland) |
50 °C Vacuum pressure 0.04 mbar. |
1.9 | 7 | |
| 5 wt% ethanol | Pebax | Homemade |
25 °C Vacuum pressure 0 bar. |
0.080 | 2.5 | Le et al. (2011) |
|
1 wt% butanol 0.5 wt% acetone 0.15 wt% ethanol |
PDMS | Homemade |
50 °C Vacuum pressure 2.8 mbar. |
0.275 | 10 | Zhou et al. (2011) |
| 5 wt% ethanol | Cross-linked PDMS | Homemade | 40 °C | 0.080 | 8.5 | Zhan et al. (2012) |
| 5 wt% ethanol | PDMS | Homemade |
60° Vacuum pressure 0 bar |
0.060 | 15 | Sun et al. (2013) |
| 4 wt% ethanol | PDMS | Homemade |
25 °C Vacuum pressure 20 mbar |
0.100 | 8 | Yadav et al. (2013) |
| 5 wt% ethanol | PDMS | Homemade |
50 °C Vacuum pressure 330 mbar |
0.800 | 5 | Li et al. (2014) |
| 5 wt% ethanol | PDMS | Homemade |
50 °C Vacuum pressure 2 mbar |
0.090 | 5 | Liu et al. (2015) |
| 5 wt% ethanol | PDMS | Homemade |
70 °C Vacuum pressure 10.1 mbar |
1.5 | 7.5 | Zhang et al. (2015) |
| 5 wt% ethanol | Butyl acrylate-styrene copolymer | Homemade |
30 °C Vacuum pressure 1.3 mbar |
0.300 | 16 | Samanta and Ray (2015) |
| 10 wt% ethanol | PBZ | Homemade | 70 °C Vacuum pressure 13 mbar | 0.035 | 10,000 | Chuntanalerg et al. (2016) |
| 6 wt% ethanol | PDMS | Homemade |
40 °C Vacuum pressure < 1 mbar |
0.900 | 8.7 | Naik et al. (2016a, b) |
| 6% wt% ethanol | PDMS | Homemade |
40 °C Vacuum pressure < 1 mbar |
0.35 | 8 | Naik et al. (2016a, b) |
| 3 wt% ethanol | PDMS | Homemade |
30 °C Vacuum pressure 8–10 mbar |
0.90 | 2.0 | Mohammadi et al. (2005) |
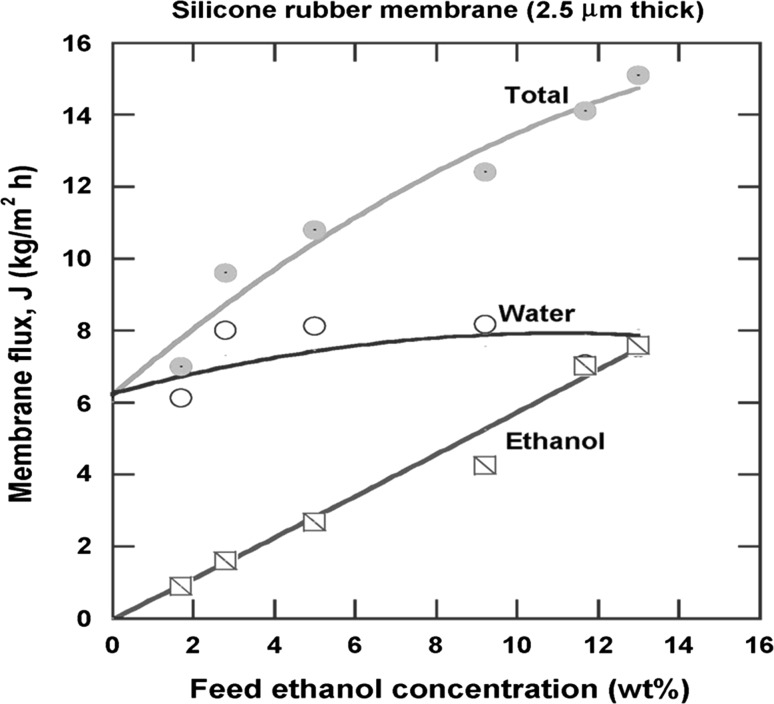
As can be seen, polydimethylsiloxane (PDMS) based membranes have been the most sought for such separation. In fact, hydrophobic membranes are also well recognized as organophilic, for their ability to remove organics. Generally, the membranes cannot display high productivities in terms of permeate flux; however, they were highly selective displaying selectivity values between 2–15 towards ethanol, whereas the total fluxes were considerably low (0.060–3.3 kg/m2/h). At this point, polyether block amide (PEBA) and poly [1-(trimethylsilyl)-1-propyne] (PTMSP) polymers provide higher total fluxes. On the other hand, it is likely that membranes based on polybenzoxazines (PBZ) and butyl acrylate-styrene copolymer tend to provide better selective properties, having selectivities of about 10,000 and 16, respectively. Of course, as in all membrane-based technologies, several factors are playing an important role on the separation performance of membranes, such as operating conditions (e.g. temperature, vacuum pressure, feed flow rate, feed concentration) and membrane properties (e.g. nature, thickness) (Baker et al. 2010). For instance, the permeation flux of ethanol tends to increase usually with the increase of feed concentration, similar behavior has been seen for the selectivity as well (Gu et al. 2009). This is because the flux of organic solvents (e.g. ethanol, butanol, acetone) is a result of increasing of the activity and partial pressure, hence enhancing the driving force for permeation, and thus allows higher fluxes (Zhou et al. 2011). Figure 2 shows clearly the effect of feed ethanol concentration on the performance of a hydrophobic membrane during the removal of ethanol.
Fig. 2.
Effect of feed ethanol concentration on the pervaporation performance of a hydrophobic membrane.
Taken from Baker et al. (2010)
On the other hand, the increase in temperature also enhances the permeation rates of polymeric membranes; however, the selectivity usually decreases. Temperature indeed causes a polymer chains motion in membranes; this promotes the permeation of other molecules across the membranes, and thus reducing the selective membrane properties (Baker et al. 2010).
Non-alcoholic beverage production through PV
Nutritional value of alcoholic beverages
This review addresses the most consumed beverages worldwide, such as beer and wine that have been dealcoholized by PV. In principle, the dealcoholization process seems to be an easy task; however, the organic compounds (related to nutrients) contained in such products make difficult the ethanol removal. For instance, the beer contains several nutritional compounds, including proteins, phenolics (e.g. antioxidants), certain minerals, anthocyanins, dietary fibers, some prebiotic compounds and B vitamins (Sohrabvandi et al. 2010a, b, 2012). Table 3 shows the most common nutrients that can be found in beer.
Table 3.
Nutrients contained in a normal beer.
Adapted from Sohrabvandi et al. (2012)
| Vitamins | Minerals |
|---|---|
| Biotin (2–7 µg/L) | Calcium (25–120 mg/L) |
| Vitamin B12, B6 (5–10 mg/L) | Phosphorus (11 mg/L) |
| Vitamin A, C, D, E, K (< 0.06 µg/L) | Magnesium (50–90 mg/L) |
| Niacin (16 mg/L) | Potassium (200–450 mg/L) |
| Folate (20 µg/L) | Sodium (20–350 mg/L) |
| Thiamine (0.2–7 mg/L) | Iron (1–5 mg/L) |
| Riboflavin (1–61 mg/L) | Zinc (0.07–12 mg/L) |
| Selenium (0.7–13 µg/L) |
Moreover, a beer contains multiple desirable organoleptic compounds (e.g. alcohols, esters, carbonyl compounds, vicinal ketones) (Olaniran et al. 2017); finally, beer contains more protein and B vitamins than wine (Sohrabvandi et al. 2012); however, the wine, as the main winemaking product, contains much more bioactive compounds (e.g. polyphenols, anthocyanins) than beer, e.g. red wines contain about 1720 mg polyphenols L−1 (Lugasi and Hóvári 2003), which definitely provide biological benefits to the costumers, this is because such compounds are well-recognized for their associated antioxidant capacity (Cartron et al. 2003; Paixão et al. 2007). Certainly, minimal content of phenolic compounds have been quantified in beers compared to wine (Bartolomé et al. 2000); however, more than 35 phenolic compounds (e.g. tyrosol, ferulic acid, HMF, trytophol) can be found in the beer (about 80–90% from malt and 10–20% from hops) (Sohrabvandi et al. 2010a, b); whereas a huge range of phenolic compounds are contained in wines, such as catechin, epicatechin, p-coumaric acid, caffeic acid, gallic acid, fertaric acid, to mention just a few (Paixão et al. 2007). Table 4 displays the main phenolic compounds that have been quantified in the wine:
Table 4.
Phenolic compounds contained in red wine.
Adapted from Cartron et al. (2003)
| Phenolic compound | Concentration (mg/L) |
|---|---|
| Catechin | 89 |
| Epicatechin | 94 |
| Dimer B1 | 69 |
| Dimer B2 | 54 |
| Dimer B3 | 79 |
| Dimer B4 | 96 |
| Trimer C1 | 41 |
| Trimer C2 | 74 |
| Gallic acid | 43 |
| Protocatechuic acid | 12 |
| Caftaric acid | 55 |
| Gentisic acid | 54 |
| Caffeic acid | 8 |
The variety of phenolic compounds indeed depends on the kind of wine (e.g. red, white, sparkling, sherry, port, fruit, and brandy). Particularly, red wine is a complex mixture of flavonoids (e.g. anthocyanins and flavan-3-ols) and nonflavonoids (e.g. resveratrol, cinnamates, and gallic acid), while flavan-3-ols are the most abundant together with polymeric procyanidins composing up to 50% of the total phenolic constituents (Guilford and Pezzuto 2011). It has been reported that phenolic compounds provided by beer and wine are able to be absorbed and extensively metabolized by the human body (Nardini et al. 2006, 2009). Thanks to this, beneficial effects have been attributed to the consumption of beer and wine, such as anti-mutagenic and anti-carcinogenic effects, cardioprotective effect, immunomodulation, and anti-osteoporosis effect (Sohrabvandi et al. 2012). Finally, the balance between alcohol and phenolic compounds in wine and beer may be critical in determining their antioxidant potential due to the fact that the alcohol displays pro-oxidant effects (van Golde et al. 1999). Clearly, the ethanol removal from such beverages needs a highly selective technique, like pervaporation, to separate the alcohol without altering strongly the nutritional properties. The next section provides the state of the art of the literature findings in the field.
Non-alcoholic beverages production through pervaporation
Through traditional techniques, the dealcoholization pretends the ethanol removal from alcoholic beverages (Lipnizki 2014), without altering the sensory and nutritional properties of the original products. However, unfortunately, some of the valuable compounds can be considerably lost. For instance, Table 5 shows a comparison of the polyphenols profile and associated substances in alcohol-free and standard beers.
Table 5.
Comparison of the polyphenols content and its derivatives in alcohol-free and standard commercial beers.
Adapted from Bartolomé et al. (2000)
| Compound | Alcohol-free beers | Standard beers |
|---|---|---|
| HMF | 1.65 | 2.57 |
| p-hydroxybenzoic acid | 0.073 | 0.092 |
| Tyrosol | 2.78 | 11.83 |
| Catechin | 0.641 | 0.463 |
| 2,3-Dihydroxy-1-guaiacylpropan-1-one | 0.025 | 0.034 |
| Vanillic acid | 0.347 | 0.477 |
| Caffeic acid | 0.045 | 0.074 |
| Vanillin | 0.048 | 0.028 |
| p-Coumaric acid | 0.576 | 0.773 |
| Ferulic acid | 0.718 | 1.305 |
| Sinapic acid | 0.073 | 0.090 |
| Tryptophol | 0.242 | 0.368 |
Importantly, such non-alcoholic beers are nowadays produced by means of different approaches in order to suppress the alcohol production in the beer (Lehnert et al. 2009; Schmidtke et al. 2012; Sohrabvandi et al. 2010a, b; Strejc et al. 2013), such as:
the use of special strains of fermenting yeasts,
reducing fermentable fractions to non-fermentable fractions,
reducing glucose content in the wort,
heating of fermenting wort,
high-temperature mashing,
pressurization during fermentation,
cold contact procedure, and
periodic aeration of fermenting wort.
While the vacuum evaporation and vacuum distillation are used as post-fermentation technologies for removing out the ethanol (Andrés-Iglesias et al. 2015; Blanco et al. 2016; Lipnizki 2014). All these techniques and procedures are definitely producing changes in nutritional value as well as the sensorial characteristics of the beverages (Brányik et al. 2012). This is, therefore, the main reason that makes to the industry of looking for highly selective techniques to carry out the dealcoholization. According to Sohrabvandi et al. (2010a, b), several techniques are currently being proposed for the dealcoholization of beverages (e.g., beers and wines), such as vacuum distillation, water vapor-/gas-stripping under vacuum, adsorptive alcohol removal, dialysis, reverse osmosis, and osmotic distillation; while some of membranes processes, like dialysis, and reverse osmosis, are already industrial methods for beverage dealcoholization (especially beer).
In the case of pervaporation (PV) has not been widely studied for such purpose, but it has been proved to be a potential candidate for the partial dealcoholization of beverages (Blanco et al. 2016). For instance, Catarino et al. (2009) developed a process to extract aromas from an original beer through a polyoctylmethylsiloxane membrane. Several compounds were identified in beer, such as alcohols (e.g. ethanol, propanol, isobutanol, and isoamyl alcohol), esters (e.g. ethyl acetate and isoamyl acetate) and aldehydes (e.g. acetaldehyde). Similarly, Olmo et al. (2014) also demonstrated that PV meets the requirements for the recovery of aroma compounds from beer, e.g. isobutyl alcohol increased up to16% in the in alcohol-free beer, while ethyl acetate in low-alcohol beer increased up to 35.72%. In this sense, the extraction of beer aromas could be useful to adjust the aroma profile of the dealcoholized beers, which can, unfortunately, be lost during the processing.
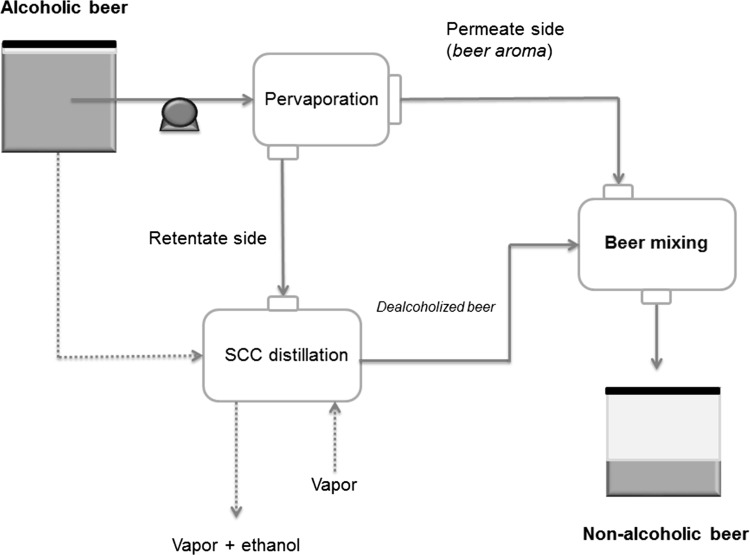
Regarding the beverage dealcoholization by PV, Catarino and Mendes (2011b) investigated the production of low-alcohol content beer by means of an industrial plant. Basically, the plant involved a hybrid process, which included the integration of PV and distillation units, as Fig. 3 shows.
Fig. 3.
Integrated dealcoholization process proposed for the non-alcoholic beer.
Adapted from Catarino and Mendes (2011b)
The aroma compounds (e.g. amyl alcohol, ethyl acetate, isoamyl acetate, and acetaldehyde) were recovered using a PV polyoctylmethylsiloxane membrane (organophilic) (Catarino and Mendes 2011b). Such aroma compounds were then incorporated into the dealcoholized beer (produced by conventional distillation). In such a way, this industrial methodology allowed to obtain a non-alcoholic beer containing less than 0.5 vol.% ethanol, and also a good flavor profile. It is important to mention that PV technology does not require in principle temperature to carried out the separation; nonetheless, the use of temperature may help to improve the permeate fluxes with a possible effect on components selectivity. At this point, the PV process at room temperature could help to avoid any compound degradation, as well as contribute to obtain higher selective performance.
As introduced previously, the wine has been also dealcoholized by membrane-based technologies, to produce non-alcoholic wine. Since wine contains more nutritional compounds than beer, the post-fermentation removal of ethanol has to be carefully performed, minimizing any possible degradation of bioactive compounds (e.g. polyphenols, antioxidants, etc.) (Gómez-Plaza et al. 1999); actually, wine also contains higher ethanol content compared to the beer. To date, different types of wines have been dealcoholized by means of PV; for instance, Tan et al. (2003) used a PV-PDMS membrane for wine dealcoholization. The dealcoholization process was conducted at 40 °C. The process was able to produce wine with 3–7% of ethanol while the average permeate flux was 1.5 kg/m2/h. This flux is indeed in agreement with reported values for ethanol removal using PDMS membranes in diluted ethanol solutions (see Table 2).
Takács et al. (2007) reported the dealcoholization of semi-sweet Tokaji Harslevelu type wines (alcohol content 13.11% ABV) using pervaporation PERVAP membranes. Such commercial membranes are well recognized by its organophilic nature. The authors reported that temperature influences in PV performance in terms of flux and separation ability, e.g. higher permeate fluxes were commonly obtained at higher temperatures; whereas the ethanol selectivity of the membranes tended to decrease. No details about the physico-chemical properties of the dealcoholized wines were provided. On the other hand, Catarino and Mendes (2011a) used another PV organophilic (polyoctylmethylsiloxane supported in polyetherimide) membrane to recover the aroma compounds from wine. Afterward, the aroma compounds were added back to the dealcoholized wines, which was partially dealcoholized by means of nanofiltration (NF). Herein, the authors tested four different NF membranes (same molecular weight cut-off of 200 Da), which were manufactured by different membrane materials. In general, the integrated membrane processes were able to provide a high-quality and low alcohol content wine (ca. 7–8 vol.%) from a standard alcoholic wine (ca. 12 vol.% of ethanol).
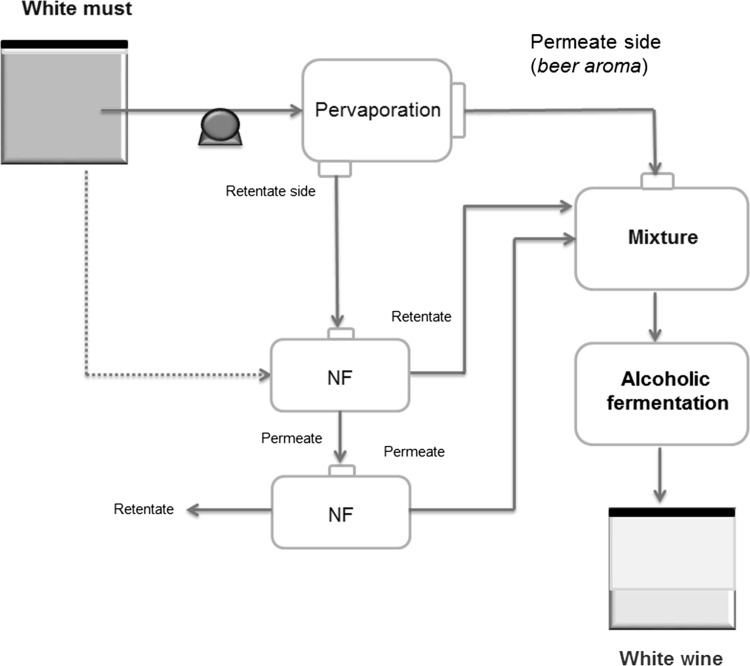
In a different approach but still using integrated membrane-based systems, Salgado et al. (2017) proposed PV and NF steps for the preparation of flavored white wines (see Fig. 4), which contained low alcohol contents.
Fig. 4.
Integrated dealcoholization process proposed for the production of low alcoholic wines.
Adapted from Salgado et al. (2017)
The production process basically involved two NF processes for the sugar reduction of must, while PV was used for the aroma recovery. Such a procedure allowed to obtain a wine containing a final alcoholic degree between 10.2–10.5 vol.%. The conventional production commonly produces white wine containing ca. 12 vol.% ethanol concentration, it means, a small reduction in the alcohol content was observed; but definitely, a concentration of the aroma compounds has been noticed (Salgado et al. 2017). Particularly, this commercial organophilic (PERVATECH PV-SR1 spiral wound) membrane displayed low total flux (0.073 kg/h/m2); but it was able to recover some specific compounds, such as isoamyl alcohol, hexanal, 2-phenylethanol and benzaldehyde. This membrane was indeed highly selective to the alcohols compared to the aldehydes.
Economic framework in beverages dealcoholization by means of PV technology
To provide an economic assessment about the involvement of PV itself in dealcoholization process is a difficult task due to the fact that most of the ongoing research has been done at lab scale. Moreover, the use of PV technology at large scale is usually combined with the distillation process. In this regard, Hoof et al. (2004) provided a theoretical economic evaluation of a hybrid distillation/pervaporation unit for used for specific separation (isopropanol-water mixtures), which resulted in the following costs:
Operational costs mainly related to energy: ~ 17 €/ton product
Investment costs: ~ 40 €/ton product
Maintenance, including membrane replacement: ~ 13 €/ton product
Furthermore, they stated that the costs, for draining solvents that form an azeotrope, using such a hybrid distillation-pervaporation process, can be around half of those associated with conventional distillation. In addition, considering the limited implementation, no investment costs can be mentioned for a full-scale installation. However, there is an increasing importance of ethanol removal from alcoholic beverages. Moreover, the high worldwide consumption of these beverages may minimize the final total investment cost.
On the other hand, PV process can also be useful for the recovery of aromas, in which beer and wine have been also considered as a source. For instance, the worldwide market for flavors and nutraceutical ingredients has been estimated about € 13 billion in 2006, while more recently the US market has been projected around € 5.5 billion in 2014 (food 36%, cosmetics and toiletries 27%, beverages 15%), which is expected to rise 3% per year (Brazinha and Crespo 2014; Castro-Muñoz et al. 2018a). In this regard, the food processing companies could extend the use of PV technology for other purposes contributing to decrease the final investment cost.
Future directions and challenges in beverages dealcoholization by PV
To date, it is likely that beer and wine are the main alcoholic beverages that have been subjected to dealcoholization. This is probably due to the fact that they are the most consumed products worldwide. Definitely, their expected consumption and high nutritional content may play a crucial role in the implementation of pervaporation in dealcoholization processes (Mangindaan et al. 2018). Moreover, the ability of PV in the recovery of aroma compounds could be an alternative to develop more efficient processes. Indeed, if the loss of aroma and some other organoleptic compounds are continued being an issue in beverage dealcoholization, the use of PV can be strongly needed for meeting the quality parameters of the non-alcoholic beverages.
Actually, it is challenging that most of the organic compounds are ethanol soluble meaning that there is no process which can guarantee the complete ethanol removal without altering the composition of the other organics (Müller et al. 2017). At this point, it is needed to start the use of other highly ethanol selective membrane materials, such as PBZ and butyl acrylate-styrene copolymer. In this way, low temperatures may provide higher selectivity as well as minimal degradation for highly sensitive compounds.
When dealing with the integration of PV into other technologies, such technology has proven to be able to be coupled with the conventional distillation, but also to other membrane-based technologies proposed for alcohol removal, such as osmotic distillation, nanofiltration, reverse osmosis and membrane contactors (Liguori et al. 2016, 2018; Mangindaan et al. 2018). Interestingly, the coupling of such processes could produce high-quality and low-alcohol content beverages (Blanco et al. 2016; Brányik et al. 2012). Finally, it is clear that further research is required in order to adopt pervaporation in dealcoholization of beverages.
Concluding remarks
This review has shown the role of the pervaporation technology during the dealcoholization of beverages. Apparently, dealcoholization procedure seems to be a simple process; however, the alcoholic beverages (e.g. beer, wine), as multicomponent colloidal solutions, make complicated the removal of alcohol. Also, the high affinity of the organics in ethanol produces strong changes on the organoleptic features of the final products. In this way, pervaporation could be used not only in dealcoholization, but also in the field of aroma recovery. Actually, it is quite possible that PV is going to be implemented in any dealcoholization process based on its ability to recover aromas, and thus meet the quality parameters of the non-alcoholic beverages.
Moreover, if the removal of ethanol is attempted to be highly efficient, the use of hydrophobic membranes is crucially needed in PV technology, these membranes can partially remove the alcohol from the drinks, but they can also perform the separation of the ethanol from other polar compounds. Finally, the food technicians should take into account other highly selective membrane materials toward ethanol, e.g. the ones provided in Table 1. Moreover, it is recommendable to operate the PV at the low temperatures which generally provides higher selectivity.
Acknowledgements
R. Castro-Muñoz acknowledges the European Commission—Education, Audiovisual and Culture Executive Agency (EACEA) for his PhD scholarship under the program: Erasmus Mundus Doctorate in Membrane Engineering—EUDIME (FPA No 2011-0014, Edition V, http:/eudime.unical.it).
Compliance with ethical standards
Conflict of interest
The author declares no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Afonso C, Crespo J, Anastas P (2015) Green separation processes: fundamentals and applications, 1st edn. Wiley, Weinheim, pp 1–383
- Andrés-Iglesias C, García-Serna J, Montero O, Blanco CA. Simulation and flavor compound analysis of dealcoholized beer via one-step vacuum distillation. Food Res Int. 2015;76:751–760. doi: 10.1016/j.foodres.2015.07.017. [DOI] [PubMed] [Google Scholar]
- Andrés-Iglesias C, Blanco CA, García-Serna J, Pando V, Montero O. Volatile compound profiling in commercial lager regular beers and derived alcohol-free beers after dealcoholization by vacuum distillation. Food Anal Methods. 2016;9(11):3230–3241. [Google Scholar]
- Baker RW, Wijmans JG, Huang Y. Permeability, permeance and selectivity: a preferred way of reporting pervaporation performance data. J Membr Sci. 2010;348(1–2):346–352. [Google Scholar]
- Bartolomé B, Peña-Neira A, Gómez-Cordovés C. Phenolics and related substances in alcohol-free beers. Eur Food Res Technol. 2000;210(6):419–423. [Google Scholar]
- Belisario-Sánchez YY, Taboada-Rodríguez A, Marín-Iniesta F, Iguaz-Gainza A, López-Gómez A. Aroma recovery in wine dealcoholization by SCC distillation. Food Bioprocess Technol. 2012;5(6):2529–2539. [Google Scholar]
- Blanco CA, Andrés-Iglesias C, Montero O. Low-alcohol beers: flavor compounds, defects, and improvement strategies. Crit Rev Food Sci Nutr. 2016;56(8):1379–1388. doi: 10.1080/10408398.2012.733979. [DOI] [PubMed] [Google Scholar]
- Brányik T, Silva DP, Baszczyňski M, Lehnert R, Almeida E. A review of methods of low alcohol and alcohol-free beer production. J Food Eng. 2012;108(4):493–506. [Google Scholar]
- Brazinha C, Crespo JG. Valorization of food processing streams for obtaining extracts enriched in biologically active compounds. In: Cassano A, Drioli E, editors. Integrated membrane operations in the food production. 1. Berlin: Degruyter; 2014. pp. 295–310. [Google Scholar]
- Cartron E, Fouret G, Carbonneau MA, Lauret C, Michel F, Monnier L, et al. Red-wine beneficial long-term effect on lipids but not on antioxidant characteristics in plasma in a study comparing three types of wine—description of two O-methylated derivatives of gallic acid in humans. Free Radic Res. 2003;37(9):1021–1035. doi: 10.1080/10715760310001598097. [DOI] [PubMed] [Google Scholar]
- Cassano A, Conidi C, Ruby-Figueroa R, Castro-Muñoz R. Nanofiltration and tight ultrafiltration membranes for the recovery of polyphenols from agro-food by-products. Int J Mol Sci. 2018;19(2):351. doi: 10.3390/ijms19020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Muñoz R, Fíla V. Membrane-based technologies as an emerging tool for separating high-added-value compounds from natural products. Trends Food Sci Technol. 2018;82:8–20. [Google Scholar]
- Castro-Muñoz R, Yáñez-Fernández J, Fíla V. Phenolic compounds recovered from agro-food by-products using membrane technologies: an overview. Food Chem. 2016;213:753–762. doi: 10.1016/j.foodchem.2016.07.030. [DOI] [PubMed] [Google Scholar]
- Castro-Muñoz R, Fíla V, Barragán-Huerta BE, Yáñez-Fernández J, Piña-Rosas JA, Arboleda-Mejía J. Processing of Xoconostle fruit (Opuntia joconostle) juice for improving its commercialization using membrane filtration. J Food Process Preserv. 2017 [Google Scholar]
- Castro-Muñoz R, Conidi C, Cassano A. Membrane-based technologies for meeting the recovery of biologically active compounds from foods and their by-products. Crit Rev Food Sci Nutr. 2018 doi: 10.1080/10408398.2018.1478796. [DOI] [PubMed] [Google Scholar]
- Castro-Muñoz R, De La Iglesia Ó, Fila V, Téllez C, Coronas J. Pervaporation-assisted esterification reactions by means of mixed matrix membranes. Ind Eng Chem Res. 2018;57:15998–16011. [Google Scholar]
- Castro-Muñoz R, Galiano F, Fíla V, Drioli E, Figoli A. Mixed matrix membranes (MMMs) for ethanol purification through pervaporation: current state of the art. Rev Chem Eng. 2018 [Google Scholar]
- Catarino M, Mendes A. Dealcoholizing wine by membrane separation processes. Innov Food Sci Emerg Technol. 2011;12(3):330–337. [Google Scholar]
- Catarino M, Mendes A. Non-alcoholic beer—a new industrial process. Sep Purif Technol. 2011;79(3):342–351. [Google Scholar]
- Catarino M, Ferreira A, Mendes A. Study and optimization of aroma recovery from beer by pervaporation. J Membr Sci. 2009;341(1–2):51–59. [Google Scholar]
- Chuntanalerg P, Kulprathipanja S, Chaisuwan T, Aungkavattana P, Hemra K, Wongkasemjit S. Performance polybenzoxazine membrane and mixed matrix membrane for ethanol purification via pervaporation applications. J Chem Technol Biotechnol. 2016;91(4):1173–1182. [Google Scholar]
- Claes S, Vandezande P, Mullens S, Leysen R, De Sitter K, Andersson A, et al. High flux composite PTMSP-silica nanohybrid membranes for the pervaporation of ethanol/water mixtures. J Membr Sci. 2010;351(1–2):160–167. [Google Scholar]
- Cleophas TJ. Wine, beer and spirits and the risk of myocardial infarction: a systematic review. Biomed Pharmacother. 1999;53(9):417–423. doi: 10.1016/S0753-3322(99)80121-8. [DOI] [PubMed] [Google Scholar]
- Costanzo S, Di Castelnuovo A, Donati MB, Iacoviello L, de Gaetano G. Alcohol consumption and mortality in patients with cardiovascular disease. A meta-analysis. J Am Coll Cardiol. 2010;55(13):1339–1347. doi: 10.1016/j.jacc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Crespo J, Brazinha C. Fundamentals of pervaporation. In: Basile A, Figoli A, Khayet M, editors. Pervaporation, vapour permeation and membrane distillation: principles and applications. Cambridge: Elsevier; 2015. pp. 1–17. [Google Scholar]
- Figoli A, Santoro S, Galiano F, Basile A. Pervaporation membranes: preparation, characterization, and application. In: Basile A, Figoli A, Khayet M, editors. Pervaporation, vapour permeation and membrane distillation. 1. Cambridge: Elsevier; 2015. pp. 281–304. [Google Scholar]
- Galanakis CM. Emerging technologies for the production of nutraceuticals from agricultural by-products: a viewpoint of opportunities and challenges. Food Bioprod Process. 2013;91(4):575–579. [Google Scholar]
- Galanakis CM. Separation of functional macromolecules and micromolecules: from ultrafiltration to the border of nanofiltration. Trends Food Sci Technol. 2015;42(1):44–63. [Google Scholar]
- Galanakis CM, Kotanidis A, Dianellou M, Gekas V. Phenolic content and antioxidant capacity of Cypriot wines. Czech J Food Sci. 2015;33(2):126–136. [Google Scholar]
- Gómez-Plaza E, López-Nicolás JM, López-Roca JM, Martínez-Cutillas A. Dealcoholization of wine. Behaviour of the aroma components during the process. LWT Food Sci Technol. 1999;32(6):384–386. [Google Scholar]
- Gu J, Shi X, Bai Y, Zhang H, Zhang L, Huang H. Silicalite-filled polyether-block-amides membranes for recovering ethanol from aqueous solution by pervaporation. Chem Eng Technol. 2009;32(1):155–160. [Google Scholar]
- Guilford JM, Pezzuto JM. Wine and health: a review. Am J Enol Viticult. 2011;62(4):471–486. [Google Scholar]
- Hoof V, Van Abeele L, Buekenhoudt A, Dotremont C, Leysen R. Economic comparison between azeotropic distillation and different hybrid systems combining distillation with pervaporation for the dehydration of isopropanol. Sep Purif Technol. 2004;37:33–49. [Google Scholar]
- Huang Y, Zhang P, Fu J, Zhou Y, Huang X, Tang X. Pervaporation of ethanol aqueous solution by polydimethylsiloxane/polyphosphazene nanotube nanocomposite membranes. J Membr Sci. 2009;339(1–2):85–92. [Google Scholar]
- Le NL, Wang Y, Chung TS. Pebax/POSS mixed matrix membranes for ethanol recovery from aqueous solutions via pervaporation. J Membr Sci. 2011;379(1–2):174–183. [Google Scholar]
- Lehnert R, Novák P, Macieira F, Kuřec M, Teixeira JA, Brányik T. Optimisation of lab-scale continuous alcohol-free beer production. Czech J Food Sci. 2009;27(4):267–275. [Google Scholar]
- Li Y, Wee LH, Martens J, Vankelecom IFJ. ZIF-71 as a potential filler to prepare pervaporation membranes for bio-alcohol recovery. J Mater Chem A. 2014;2:10034–10040. [Google Scholar]
- Liguori L, De Francesco G, Russo P, Perretti G, Albanese D, Di Matteo M. Quality attributes of low-alcohol top-fermented beers produced by membrane contactor. Food Bioprocess Technol. 2016;9(1):191–200. [Google Scholar]
- Liguori L, Russo P, Albanese D, Di Matteo M. Production of low-alcohol beverages: current status and perspectives, food processing for increased quality and consumption. Amsterdam: Elsevier Inc.; 2018. [Google Scholar]
- Lipnizki F. Beer dealcoholization. In: Drioli E, Giorno L, editors. Encyclopedia of membranes. 1. Berlin: Springer; 2014. pp. 1–2. [Google Scholar]
- Lipnizki F, Olsson J, Trägårdh G. Scale-up of pervaporation for the recovery of natural aroma compounds in the food industry part 2: optimisation and integration. J Food Eng. 2002;54(3):197–205. [Google Scholar]
- Liu X, Hu D, Li M, Zhang J, Zhu Z, Zeng G, et al. Preparation and characterization of silicalite-1/PDMS surface sieving pervaporation membrane for separation of ethanol/water mixture. J Appl Polym Sci. 2015;132(34):1–11. [Google Scholar]
- Lugasi A, Hóvári J. Antioxidant properties of commercial alcoholic and nonalcoholic beverages. Nahrung/Food. 2003;47(2):79–86. doi: 10.1002/food.200390031. [DOI] [PubMed] [Google Scholar]
- Mangindaan D, Khoiruddin K, Wenten IG. Beverage dealcoholization processes: past, present, and future. Trends Food Sci Technol. 2018;71:36–45. [Google Scholar]
- Mohammadi T, Aroujalian A, Bakhshi A. Pervaporation of dilute alcoholic mixtures using PDMS membrane. Chem Eng Sci. 2005;60(7):1875–1880. [Google Scholar]
- Müller M, Bellut K, Tippmann J, Becker T. Physical methods for dealcoholization of beverage matrices and their impact on quality attributes. ChemBioEng Rev. 2017;5:310–326. [Google Scholar]
- Naik PV, Verlooy PLH, Smet S, Martens JM, Vankelecom IFJ. PDMS mixed matrix membranes filled with novel PSS-2 nanoparticles for ethanol/water separation by pervaporation. RSC Adv. 2016;6:78648–78651. [Google Scholar]
- Naik PV, Kerkhofs S, Martens JA, Vankelecom IFJ. PDMS mixed matrix membranes containing hollow silicalite sphere for ethanol/water separation by pervaporation. J Membr Sci. 2016;502:48–56. [Google Scholar]
- Nardini M, Natella F, Scaccini C, Ghiselli A. Phenolic acids from beer are absorbed and extensively metabolized in humans. J Nutr Biochem. 2006;17(1):14–22. doi: 10.1016/j.jnutbio.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Nardini M, Forte M, Vrhovsek U, Mattivi F, Viola R, Scaccini C. White wine phenolics are absorbed and extensively metabolized in humans. J Agric Food Chem. 2009;57(7):2711–2718. doi: 10.1021/jf8034463. [DOI] [PubMed] [Google Scholar]
- Olaniran AO, Hiralal L, Mokoena MP, Pillay B. Flavour-active volatile compounds in beer: production, regulation and control. J Inst Brew. 2017;123(1):13–23. [Google Scholar]
- Olmo Á, Blanco CA, Palacio L, Prádanos P, Hernández A. Pervaporation methodology for improving alcohol-free beer quality through aroma recovery. J Food Eng. 2014;133:1–8. [Google Scholar]
- Paixão N, Perestrelo R, Marques JC, Câmara JS. Relationship between antioxidant capacity and total phenolic content of red, rosé and white wines. Food Chem. 2007;105(1):204–214. [Google Scholar]
- Partanen TJ, Vainio HU, Ojajärvi IA, Kauppinen TP. Pancreas cancer, tobacco smoking and consumption of alcoholic beverages: a case-control study. Cancer Lett. 1997;116(1):27–32. doi: 10.1016/s0304-3835(97)04744-7. [DOI] [PubMed] [Google Scholar]
- Purwasasmita M, Kurnia D, Mandias FC, Wenten K. Beer dealcoholization using non-porous membrane distillation. Food Bioprod Process. 2015;94:180–186. [Google Scholar]
- Raisi A, Aroujalian A, Kaghazchi T. A predictive mass transfer model for aroma compounds recovery by pervaporation. J Food Eng. 2009;95(2):305–312. [Google Scholar]
- Saffarionpour S, Ottens M. Recent advances in techniques for flavor recovery in liquid food processing. Food Eng Rev. 2018;10(2):81–94. [Google Scholar]
- Salgado CM, Fernández-Fernández E, Palacio L, Carmona FJ, Hernández A, Prádanos P. Application of pervaporation and nanofiltration membrane processes for the elaboration of full flavored low alcohol white wines. Food Bioprod Process. 2017;101:11–21. [Google Scholar]
- Samanta HS, Ray SK. Separation of ethanol from water by pervaporation using mixed matrix copolymer membranes. Sep Purif Technol. 2015;146:176–186. [Google Scholar]
- Schmidtke LM, Blackman JW, Agboola SO. Production technologies for reduced alcoholic wines. J Food Sci. 2012;77(1):25–41. doi: 10.1111/j.1750-3841.2011.02448.x. [DOI] [PubMed] [Google Scholar]
- Sohrabvandi S, Mousavi SM, Razavi SH, Mortazavian AM, Rezaei K. Alcohol-free beer: methods of production, sensorial defects, and healthful effects. Food Rev Int. 2010;26(4):335–352. [Google Scholar]
- Sohrabvandi S, Mousavi S, Razavi S, Mortazavian A, Issn S, Amir M. Bacteria in beer viability of probiotic bacteria in low alcohol-and non-alcoholic beer during refrigerated storage. Philipp Agric Sci. 2010;93(1):104–108. [Google Scholar]
- Sohrabvandi S, Mortazavian AM, Rezaei K. Health-related aspects of beer: a review. Int J Food Prop. 2012;15(2):350–373. [Google Scholar]
- Solov AM. Global production and consumption of alcoholic beverages. Stud Russ Econ Dev. 2018;29(1):102–108. [Google Scholar]
- Statista (2018) Alcoholic beverages consumption. Retrieved September 15, 2018, from https://www.statista.com/
- Strejc J, Siristova L, Karabin M, Almeida JB, Branyik T. Production of alcohol-free beer with elevated amounts of flavouring compounds using lager yeast mutants. J Inst Brew. 2013;119(3):149–155. [Google Scholar]
- Sun D, Li BB, Xu ZL. Pervaporation of ethanol/water mixture by organophilic nano-silica filled PDMS composite membranes. Desalination. 2013;322:159–166. [Google Scholar]
- Takács L, Vatai G, Korány K. Production of alcohol free wine by pervaporation. J Food Eng. 2007;78(1):118–125. [Google Scholar]
- Tan SJ, Xiao ZY, Li L, Wu FW, Xu ZH, Zhang ZB. Experimental research on dealcoholization of wine by pervaporation. Jingxi Huagong/Fine Chem. 2003;20(2):69–79. [Google Scholar]
- van Golde P, Sloots L, Vermeulen W, Wielders J, Hart H, Bonno B, van de Wiel A. The role of alcohol in the anti low density lipoprotein oxidation activity of red wine. Atherosclerosis. 1999;147:365–370. doi: 10.1016/s0021-9150(99)00206-3. [DOI] [PubMed] [Google Scholar]
- Wee SL, Tye CT, Bhatia S. Membrane separation process-pervaporation through zeolite membrane. Sep Purif Technol. 2008;63(3):500–516. [Google Scholar]
- WHO (2011) Global status report on alcohol and health. Geneva, Switzerland
- Wijmans JG, Baker RW. The solution–diffusion model: a review. J Membr Sci. 1995;107(1–2):1–21. [Google Scholar]
- Yadav A, Lind ML, Ma X, Lin YS. Nanocomposite silicalite-1/polydimethylsiloxane membranes for pervaporation of ethanol from dilute aqueous solutions. Ind Eng Chem Res. 2013;52:5207–5212. [Google Scholar]
- Zhan X, Lu J, Tan T, Li J. Mixed matrix membranes with HF acid etched ZSM-5 for ethanol/water separation: preparation and pervaporation performance. Appl Surf Sci. 2012;259:547–556. [Google Scholar]
- Zhang G, Li J, Wang N, Fan H, Zhang R, Zhang G, Ji S. Enhanced flux of polydimethylsiloxane membrane for ethanol permselective pervaporation via incorporation of MIL-53 particles. J Membr Sci. 2015;492:322–330. [Google Scholar]
- Zhou H, Su Y, Chen X, Wan Y. Separation of acetone, butanol and ethanol (ABE) from dilute aqueous solutions by silicalite-1/PDMS hybrid pervaporation membranes. Sep Purif Technol. 2011;79(3):375–384. [Google Scholar]