Abstract
Botrytis cinerea is a primary pathogen causing stem and fruit rot during pre- and post-harvest. In the present study, the main purpose was to inquire into the antifungal activity and potential mechanisms of thymol and carvacrol against B. cinerea. During the experiment, the effects of thymol and carvacrol on physical and biochemical parameters of B. cinerea were evaluated. Results indicated that thymol and carvacrol exhibited strong antifungal activity against the targeted pathogen, with minimum inhibitory concentration and minimum fungicidal concentration of 65 mg/L and 100 mg/L for thymol, and 120 μL/L and 140 μL/L for carvacrol. Thymol and carvacrol changed obviously the morphology of B. cinerea hyphae by disrupting and distorting the mycelia through scanning electron microscopy. The membrane permeability of B. cinerea hyphae was prompted with the increment of two chemical agents’ concentration, as evidenced by extracellular conductivity increase, the release of cell constituent, and the decrease of extracellular pH. Furthermore, a marked decline in total lipid content of B. cinerea cells was induced by the two chemical agents, suggesting that the cell membrane structures were destructed. Therefore, present results indicated that thymol and carvacrol may be used as a good alternative to conventional fungicides against B. cinerea in controlling grey molds in horticultural products.
Keywords: Botrytis cinerea, Antifungal activity, Membrane permeability, Cell constituent release, Extracellular conductivity
Introduction
Botrytis cinerea seriously damaged many important economic ornamental crops, fruits and vegetables including pre- and post-harvest (Qin et al. 2010). Numerous researches have reported that numerous essential oils extracted from various plants have antifungal activity against post-harvest pathogens.
By means of adding 250 μL L−1 thyme and oregano oils to PDA medium, spore production and mycelia growth in Penicillium digitatum were repressed completely (Daferera et al. 2000). When added to the medium at 1 mL L−1 other essential oils, such as cinnamon and clove essential oils, also repressed completely P. italicum and P. digitatum (Plaza et al. 2004). Thymol (TH) and carvacrol (CAR) are two main components of thyme and oregano essential oils (EOs), and are generally recognized as healthy with no risk to the consumers by the European Commission, Food and Drug Administration (FDA, USA) (Burt et al. 2005).
The essential oil of O. compactum, which contains carvacrol (58.1%), p-cymene (11.4%) and thymol (9.0%) as major components, carvacrol and thymol were more toxic against B. cinerea (Bouchra et al. 2003). Both thymol and carvacrol had a significant antifungal activity through inhibiting the growth of postharvest pathogen such as P. digitatum, P. italicum, Fusarium spp, and Aspergillus spp, with the predominant efficacy by thymol (Pérez-Alfonso et al. 2012). Thyme oil vapor fumigation could effectively control gray mold of sweet cherries (Serrano et al. 2005) and brown rot of apricot (Liu and Chu 2002), and suppressed the mycelium growth of C. gloeosporioides originated from avocado in vivo and in vitro (Sellamuthu et al. 2013).
R-(-)-carvone was one of the very efficient volatile components from Lippia scaberrima essential oil on repressing the mycelia growth of B. cinerea isolated from table grapes (Martínez-Romero et al. 2007), Alternaria sp. isolated from mango (Regnier et al. 2008), P. digitatum isolated from oranges (Du Plooy et al. 2009), and Colletotrichum gloeosporioides isolated from avocado fruit (Combrinck et al. 2011).
Although cell wall and membrane were disrupted with morphological collapse, deformation and deterioration of the hyphae and/or conidia by thymol or carvacrol acts, the antifungal mechanism of action is not well understood (Neri et al. 2006). The anti-fungal activity of thymol and carvacrol, and their synergistic potential with antibiotics, has been depicted in many researches (Langeveld et al. 2014). However, information on the antifungal activity of thymol and carvacrol against B. cinerea is fairly limited.
The oil, carvacrol, thymol and commercial herbicide, 2,4-D, isooctyl ester showed potent hytotoxic effects against all plant species tested (Kordali et al. 2008). The use of thymol in drugs is limited by its moderate cytotoxicity that was shown both in in vitro on human cells (Suzuki et al. 1987). Moreover, carvacrol and thymol are a volatile compound which easily evaporates and/or decomposes (oxidation) during food processing owing to direct exposure to light or oxygen (Martínez-Hernández et al. 2017).
Accordingly, the purpose of the present research is to measure the antifungal efficacy of thyme and carvacrol against the mycelia growth of B. cinerea. The anti-fungal action mechanism will be evaluated by measuring (1) the membrane integrity and morphology of membrane surfaces with fluorescence microscope and scanning electron microscopy (SEM), (2) the extracellular conductivity and extracellular pH, (3) the release of 260 nm absorbing intracellular constituents, and (4) Potassium (K+) efflux and the total lipid content.
Materials and methods
Fungal species
The B. cinerea strain was generously provided by Dr. Taotao Wang from Huazhong Agricultural University, Wuhan, China. The strain was initially isolated from the decaying tomato fruits, and preserved at present in the Department of Biological and Food Engineering, Xiangtan University, Hunan. The fungus was cultured and purified at 28 ± 2 °C on PDA medium. The fungal suspensions were adjusted to approximately 5 × 105 CFU/mL with a hamacytometer, and diluted with sterile potato dextrose buffer (PDB).
Chemicals
Thymol (99%) and carvacrol (98%) were bought from TCI Company (Shanghai, China). The two chemicals were analytical reagents.
Antifungal activity of thymol and carvacrol
Effects of thymol and carvacrol on the mycelia growth of B. cinerea were tested by agar dilution method in vitro (Yahyazadeh et al. 2008). Thymol was dissolved in dimethyl sulfoxide (DMSO) solution. A certain amount of thymol and carvacrol was added to PDA mediums (0.05% Tween-80) to reach the desired concentrations of 0, 20, 40, 60, 80, and 100 mg/L, 0, 20, 40, 60, 80, 100, 120, 140 and 160 μL/L, respectively. And then, 20 mL of PDA solution was poured into sterile Petri plates (90 mm diameter). A disc of inocula (6 mm diameter) was removed from 7-day-old cultures on PDA plates with a punching bear. Soon afterwards, the disc of mycelia was placed centrally upside down on another new Petri plate. The Petri plates were cultured for 48 h at 28 ± 2 °C, and each treatment was conducted in triplicates. The percentage of inhibition of mycelial growth was calculated according to the following formula (Yahyazadeh et al. 2008).
where dc (cm) is the mean colony diameter for the control sets and dt (cm) is the mean colony diameter for the treatment sets. The minimum concentration that repressed completely fungus growth was deemed to the minimum inhibitory concentration (MIC). The minimum fungicidal concentration (MFC) was considered as the lowest concentration that guarded against pathogen growth after a following 72 h of incubation in a fresh PDA plate at 28 ± 2 °C, which indicated in excess of 99.5% killing of the initial inoculums (Talibi et al. 2012).
Scanning electron microscopy (SEM)
Fungal mycelia cultured for 6 days on PDA medium, which were handled with thymol and carvacrol at different dosages (0, MIC and MFC), were observed by utilizing SEM (Helal et al. 2007; Yahyazadeh et al. 2008). About 5 × 10 mm segments were removed from culture medium growing on PDA plates and quickly placed in vials containing glutaraldehyde (3%, v/v) in phosphate buffer (0.05 M, pH 6.8) at 4 °C. The samples were placed in the above solution for 48 h for fixation and then washed three times for 20 min each with distilled water. They were dehydrated in a graded series of ethanol (30%, 50%, 70%, and 95%, v/v) for 20 min, respectively, and treated for 45 min with absolute ethanol. Samples were then dried in liquid carbon dioxide at critical point. Fungal segments were stored in desiccators until further utilization. After drying, samples prepared were installed on standard 1/2-inch SEM stubs with double-stick adhesive tabs and coated with gold–palladium electroplating (60 s, 1.8 mA, 2.4 kV) in a Polaron SEM Coating System sputter coater. All samples were observed in JEOL JSM-6360 LV SEM (JEOL, Tokyo, Japan) operating at 2000 × levels of magnification and 25 kV. All the tests were performed in triplicate.
Release of cellular constituents
The cellular constituents in the mycelia were measured on the basis of the method of Paul et al. (2011), with minor modifications. In brief, 6-day-old B. cinerea mycelia from 100 mL PDB culture were gained through centrifugating for 20 min at 4000×g, rinsed three times, and re-suspended in 100 mL of pH 7.0 phosphate buffered saline. The above suspensions were handled with thymol and carvacrol at different concentrations (0, MIC and MFC) and incubated at 28 ± 2 °C with continuous agitation in an environmental incubator shaker (200 rpm) for 0, 30, 60 and 120 min, respectively. Subsequently, these samples (2 mL) were collected by centrifuging for 2 min at 12,000×g. In order to measure the concentration of the released components, the supernatant (1 mL) was used for determining the absorbance with the UV-2450 UV/Vis Spectrophotometer at 260 nm. Three repetitions of three independent experiments were considered.
Determination of extracellular pH and extracellular conductivity
The extracellular pH in B. cinerea cells was measured by a Delta 320 pH meter according to the instructions. The extracellular conductivity in B. cinerea cells was determined with a DDS-W conductivity meter (Shao et al. 2013). Originally, 100 μL of fungal suspensions (105 CFU/mL) was poured into 20 mL PDB medium and cultured in a moist chamber for 2 d at 28 ± 2 °C. The solutions were centrifuged for 20 min at 4000×g and the residual precipitation was collected, rinsed for 2–3 times with sterilized water, and re-suspended with 20 mL of sterilized water. After the two chemicals at MIC or MFC were added, the extracellular pH and extracellular conductivity of B. cinerea cells were measured at 0, 30, 60 and 120 min of treatment, respectively. These flasks with no the two chemicals were served as control. The results were expressed as pH in the growth media in each interval of incubation and amount of extracellular conductivity (μs/cm).
Measurement of lipid content
The total lipid content in B. cinerea cells with the two chemicals at three concentrations (0, MIC and MFC) were measured by means of the phosphor-vanillin method (Helal et al. 2007). 6-day-old mycelia were collected from 50 mL PDB medium after centrifuging for 10 min at 4000×g. And then, the samples were dried for 4 h with a vacuum freeze drier. Dry mycelia (0.1 g) were ground with liquid nitrogen and extracted with 4.0 mL of methanol-chloroform-water mixture (2:1:0.8, v/v/v) in a new test tube with violent shaking for 30 min. The solutions were centrifuged for 10 min at 4000×g. The lower phase contained lipids, which were collected. They were mixed thoroughly with 0.2 mL of saline solution and centrifuged for 10 min at 4000×g. Then, an aliquot of chloroform (0.2 mL) and lipid mixture (0.2 mL) was transferred to another new tube, and 0.5 mL H2SO4 was added, heated for 10 min in a boiling water bath. After that, 3 mL phosphovanillin was added and shaken vigorously, and then incubated at room temperature for 10 min. The absorbance at 520 nm was utilized to calculate total lipid content from the standard calibration curve with cholesterol as a standard.
Membrane fatty acid extraction and analysis
The extraction of cell membrane fatty acid was carried out owing to the method described by Folch et al. (1957) with slight modifications. The pellets (1.0 g, dry weight) were ground into homogenate by adding chloroform/methanol (1:1, v:v). The mixtures were blended by agitating for 15 min, and then rinsed with 2 mL of sterile water. The mixture was centrifuged for 15 min at 3000 rpm, after vortexing some seconds. The lipid contained in the lower chloroform phase is recovered and evaporated with a rotary vacuum evaporator. The fatty acid contents were measured by a GC Agilent 7820A equipped with a flame ionization detector and capillary column HP-5 using pure nitrogen as carrier gas at 1 mL/min in a split mode (20:1), working with the following temperature program: 150 °C for 2 min, ramp of 10 °C/min to 170 °C, and 170 °C for 0.5 min. Subsequently, increased at 5 °C/min to 200 °C and kept at 200 °C for 1 min. increased at 2 °C/min to 260 °C and kept at 260 °C for 2 min. Moreover, the molecular weights of all the fatty acid methyl esters were identified by GC–MS (QP2010 Ultra, SHIMADZU). Identification of the constituents was based on comparing their retention times with those of the authentic samples (Sigma, USA), and quantified by gas chromatography. The results were formulated as mg/g of fatty acid each, which was figured out as the peak area ratio of test samples to standard samples.
Statistical analysis
All the numerical values were described as average value ± standard deviation (SD). These experiments were conducted in three independent replicates. All data were subjected to analyses of variance (ANOVA one-way) using SPSS 15.0 software (SPSS, Chicago, IL, USA). The level of significance was performed by Duncan’s multiple range tests at p < 0.05.
Results
Antifungal assay
In our present study, all series concentration of thymol and carvacrol could effect the growth of B. cinerea. The fungicidal activity of thymol and carvacrol was investigated and the results were presented in Table 1. The effect of thymol and carvacrol on the mycelia growth of B. cinerea in vitro was determined. Thymol and carvacrol inhibited the mycelial growth of B. cinerea in a dose dependent manner. Therefore, we concluded that the MIC and MFC values of thymol were 65 mg/L and 100 mg/L, respectively. The MIC and MFC values of carvacrol were 120 μL/L and 140 μL/L, respectively.
Table 1.
Inhibitory effect of thymol and carvacrol on the mycelial growth of B. cinerea
| Thymol concentration (μL/L) | Mycelial growth (mm) | Growth inhibition (%)* | Carvacrol concentration (μL/L) | Mycelial growth (mm) | Growth inhibition (%)* |
|---|---|---|---|---|---|
| 0 | 10.2 ± 0.56b | 0 ± 2.8b | 0 | 10.2 ± 0.56b | 0 ± 2.8b |
| 10 | 10.9 ± 1.24a | − 6.9 ± 3.6**a | 20 | 11.2 ± 0.95a | − 9.8 ± 2.1**a |
| 20 | 9.5 ± 0.45c | 14.9 ± 1.4c | 40 | 9.1 ± 0.64c | 23.4 ± 1.7c |
| 30 | 8.7 ± 0.47d | 31.9 ± 2.1d | 60 | 8.6 ± 0.78d | 34.0 ± 3.2d |
| 40 | 7.4 ± 0.75e | 59.6 ± 3.2e | 80 | 8.2 ± 0.42e | 42.6 ± 2.5e |
| 50 | 6.8 ± 0.85f | 72.3 ± 2.7f | 100 | 7.6 ± 1.24f | 55.3 ± 1.6f |
| 65 | 5.5 ± 0g | 100 ± 0g | 120 | 5.5 ± 0g | 100 ± 0g |
| 100 | 5.5 ± 0g | 100 ± 0g | 140 | 5.5 ± 0g | 100 ± 0g |
a–fSignificant differences at p < 0.05 level according to Duncan’s multiple range test
*Values are average ± SD (n = 6)
**Additive concentration can promote the growth of mycelial
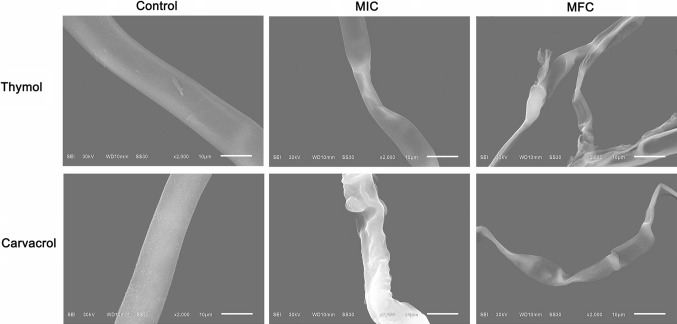
SEM
The scanning electron microscope (SEM) image (Fig. 1) clearly showed the difference between the treated and untreated B. cinerea hyphae with thymol and carvacrol. Carvacrol and thymol exhibited a prominent antifungal activity through inhibiting the mycelia growth of B. cinerea. Fungus in control groups had regular, normal, homogenous and tubular hyphae. After 2 d of treatment with thymol and carvacrol at MIC or MFC, all the fungal mycelia revealed considerable changes on hyphal morphology. The mycelia were squashed and distorted with varying degrees, which were treated with carvacrol at MIC. This phenomenon was more severely observed in these hyphae after thymol treatment. Furthermore, after treatment with thymol and carvacrol at MFC, distorted and shrunken mycelia were observed, even some of these mycelia appeared disrupted after thymol treatment. These observations were well explained that the fungicidal activity of thymol had better than that of carvacrol.
Fig. 1.
Scanning electron microphotography of B. cinerea: untreated; treated with 65 mg/L, 100 mg/L thymol, respectively; treated with 120 μL/L, 140 μL/L carvacrol, respectively
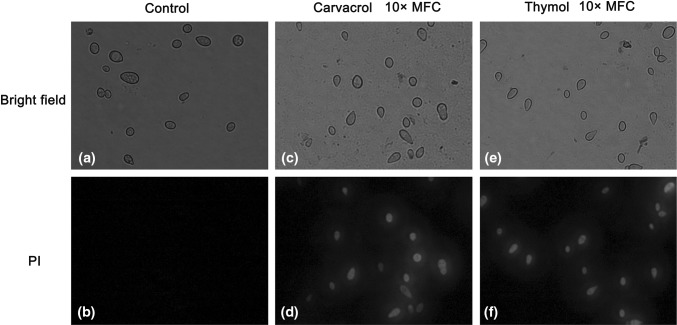
Effect of thymol and carvacrol on plasma membrane integrity of B. cinerea
Thymol and carvacrol caused the plasma membranes of B. cinerea severe damage. The membrane integrity in B. cinerea spores decreased with the extension of incubating time or amount of antifungal agents in PDB containing thymol and carvacrol. However, control preserved intact plasma membranes (Fig. 2).
Fig. 2.
Effect of 10 × MFC carvacrol and thymol on the membrane integrity of B. cinerea spores. Spore were cultured at 28 + 2 °C in PDB medium supplement with 0 (a bright field; b PI) or 10 × MFC carvacrol (c bright field; d PI) and 10 × MFC thyme (e bright field; f PI) for 2 h of incubation, spore were stained with the PI and observed with a fluorescence microscope
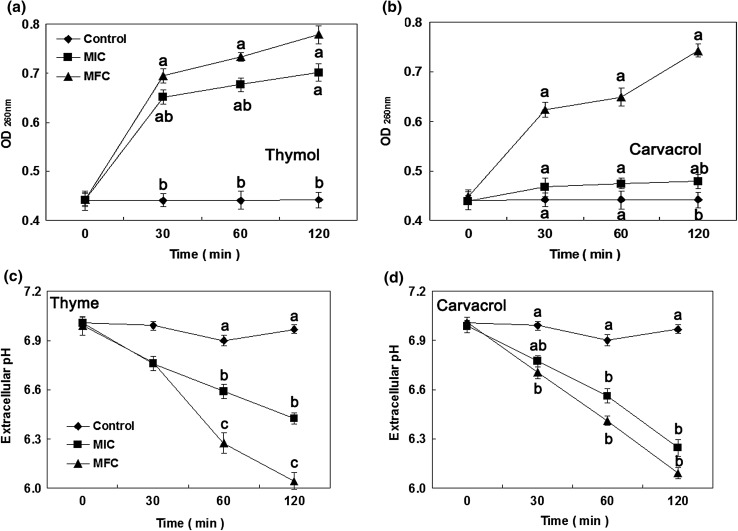
Release of cell constituents
Botrytis cinerea mycelia were treated with thymol and carvacrol for 0, 30, 60, and 120 min at various concentrations (0, MIC, and MFC), respectively. The results about the release of cell constituents were shown in Fig. 3A and B. When the fungi were treated with thymol and carvacrol at MFC, an immediately significant raise in the release of intracellular substances was found. Meanwhile, OD260nm values increased with the extension of processing time. The OD260 value in the B. cinerea suspensions exposure to thymol at MIC for 120 min reached 0.701, which was obviously higher than that in control (0.442) and carvacrol (0.479). However, during the entire treatment process, the changes in the OD260 value at MIC were not significant, compared with thymol (Fig. 3A and B). The OD260 values in B. cinerea suspensions exposure to thymol at MIC and MFC remained a sharp upward trend, whereas the OD260 values in B. cinerea suspensions exposure to carvacrol at MIC or MFC kept a relatively stable upward trend.
Fig. 3.
Effect of thymol and carvacrol on the 260 nm absorbing material release (a, b) and extracellular pH (c, d) of B. cinerea [(diamond): control; (square): MIC; (circle): MFC]. Data presented are the means of pooled data. Error bars indicate the SDs of the means (n = 3). Means with different letters in the same column were significantly different at the level of p < 0.05
Extracellular pH
The extracellular pH in B. cinerea mycelia exposed to thymol and carvacrol was showed in Fig. 3C and D. The extracellular pH sharply decreased after incubation with thymol and carvacrol at MIC or MFC. Conversely, the extracellular pH in B. cinerea suspensions in control slowly decreased during the original 60 min of exposure period, and increased slightly at 120 min of treatment. The values of extracellular pH in B. cinerea suspensions treated with thymol and carvacrol at MFC for 120 min were 6.05 and 6.09, respectively, which were remarkably below control (6.97).
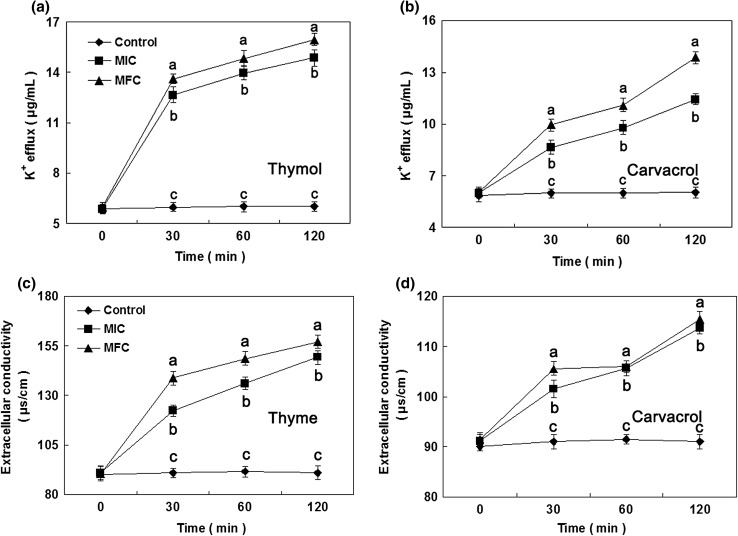
Potassium ion efflux
The leakage of potassium ions (K+) from B. cinerea mycelia incubated with thymol and carvacrol (Fig. 4A and B). The release of K+ was significantly induced by thymol at MFC during the original 30 min of exposure period. After 30 min of treatment by thymol at MFC, the K+ concentration in B. cinerea mycelia was 13.6 μg/mL. By contrast, after 30 min of treatment with carvacrol at MFC, K+ concentration in B. cinerea mycelia was 9.9 μg/mL, which was significantly less than that of thymol at MFC. After 120 min of treatment with thymol at MFC, K+ concentration presented a moderate ascending trend, and reached to 16.0 μg/mL.
Fig. 4.
Effect of thymol and carvacrol on the K+ efflux (a, b) and extracellular conductivity (c, d) of B. cinerea [(diamond): control; (square): MIC; (circle): MFC]. Data presented are the means of pooled data. Error bars indicate the SDs of the means (n = 3). Means with different letters in the same column were significantly different at the level of p < 0.05
Extracellular conductivity
The extracellular conductivity of B. cinerea mycelia treated with thymol and carvacrol at MIC or MFC for a period of 0–120 min were presented in Fig. 4C and D. After 30 min of treatment with thymol at MIC and MFC, the extracellular conductivity was 122.3 and 138.7 μs/cm, respectively, and was obviously above control (91.0 μs/cm), carvacrol at MIC (101.6 μs/cm) and MFC (105.6 μs/cm), respectively. After 120 min of treatment with thymol and carvacrol at MIC and MFC, the extracellular conductivity in B. cinerea suspensions significantly increased, and reached to 149.2 and 113.7 μs/cm for MIC, and 157.1 and 115.4 μs/cm for MFC, respectively.
Total lipid content
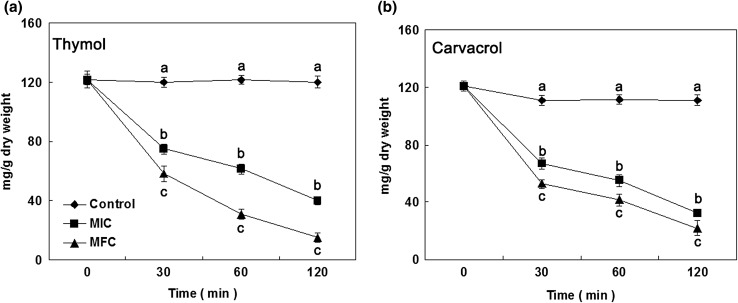
Total lipid content in B. cinerea mycelia treated with thymol and carvacrol was provided in Fig. 5. During the original 30 min of exposure, total lipid contents in B. cinerea mycelia obviously declined as the increasing concentration of thymol and carvacrol. After 30 min of exposure to thymol at MIC and MFC, the total lipid contents in B. cinerea cells were 66.8 ± 4.0 and 52.8 ± 3.2 mg/g DW, respectively, which were remarkably below control (101.0 ± 3.3 mg/g DW). Whereas the total lipid contents in B. cinerea cells treated with carvacrol at MIC and MFC for 30 min were 75.0 ± 3.6 and 58.1 ± 5.0 mg/g DW, respectively. Moreover, the total lipid contents in B. cinerea cells with thymol and carvacrol continuously decreased thereafter.
Fig. 5.
Total lipid content of B. cinerea cells in the presence of thymol (a) and carvacrol (b) [(diamond): control; (square): MIC; (circle): MFC]. Data presented are the means of pooled data. Error bars indicate the SDs of the means (n = 3). Means with different letters in the same column were significantly different at the level of p < 0.05
Fatty acid analysis
The fatty acid composition of B. cinerea mycelia treated with thymol and carvacrol at various concentrations (0, MIC and MFC) were presented in Table 2. The main compositions of fatty acids detected in control were C16:0, C17:0, C19:0, C18:2ω6, 9, C19:2ω6, 9 and C21:2ω7, 9, respectively. When the chemical stresses were applied, and especially at MFC, brought about a dramatical increase of C16:0 and C17:0. Moreover, the supplementation with thymol and carvacrol decreased some fatty acid contents, such as C19:0 and C19:2ω6, 9. As the concentration of treatment reagents increased, the fatty acids contents detected were also promoted.
Table 2.
Fatty acids composition evaluated by GC–MS in B. cinerea mycelia treated with thymol and carvacrol at different concentrations (0, MIC and MFC)
| Fatty acids | Total composition (mg/g fresh weight) in B. cinerea mycelia treated with thymol and carvacrol | ||||
|---|---|---|---|---|---|
| Control | Thymol | Carvacrol | |||
| MIC | MFC | MIC | MFC | ||
| C17:0 | 0.27 ± 0.02b | 0.23 ± 0.03b | 0.60 ± 0.04a | 0.21 ± 0.05b | 0.59 ± 0.08a |
| C19:2ω6,9 | 0.79 ± 0.03a | 0.32 ± 0.06b | 0.49 ± 0.03b | 0.31 ± 0.07b | 0.61 ± 0.02ab |
| C19:0 | 0.03 ± 0.01a | 0.04 ± 0.01a | 0.07 ± 0.02a | 0.05 ± 0.02a | 0.12 ± 0.04a |
| C16:0 | 0.10 ± 0.01a | 0.19 ± 0.04a | 0.28 ± 0.03a | – | – |
| C18:2ω6,9 | 0.31 ± 0.04 | – | – | – | – |
| C21:2ω7,9 | 0.02 ± 0.01a | 0.01 ± 0.01a | 0.04 ± 0.01a | – | – |
| C23:0 | – | – | – | 0.10 ± 0.01a | 0.14 ± 0.02a |
Values are expressed as mean ± standard deviation (n = 3). Means with different letters in the same row were significantly different at the level of p < 0.05
Discussion
In this study, thymol and carvacrol could effectively repress B. cinerea growth in vitro, and their inhibitory effect was a concentration-dependent manner. The results were accordance with previous reports described these chemicals against pathogens (Pérez-Alfonso et al. 2012; Jesus et al. 2015; Ochoa-Velasco et al. 2017; Kanchana et al. 2017). No fungal mycelium was observed with 250 μL L−1 and more of thymol or 500 μL L−1 of carvacrol on the lemon surface (Pérez-Alfonso et al. 2012). Moreover, thymol had higher antifungal effect than that of carvacrol on the basis of previous reports (Numpaque et al. 2011). This finding suggested that thymol showed the more effective anti-fungal effect, and followed by carvacrol.
The potential antimicrobial activity mechanisms of volatile compounds are because they can pierce through cell membrane, where these substances disturb intracellular metabolism (Marino et al. 2001), and modify the efflux pumps activities (Langeveld et al. 2014). Furthermore, the lipophilicity of volatile compounds increased membrane permeability and fluidity, inhibited respiration, disturbed membrane-embedded proteins, induced ions leakage and other intracellular contents, and altered transport processes of ion in fungi (Burt 2004; Fadli et al. 2012; Khan et al. 2010).
SEM examinations could reveal that rough surface and shrinkage of cells were apparent in the treated hyphae (Tao et al. 2014). SEM observations also confirmed the damage that the treated hypha demonstrated bending, entwining, shriveling, collapse and disorganization, and the breakage of the hypha was clearly visible (Jiang et al. 2015). In the present experiment, B. cinerea mycelia were treated with thymol and carvacrol, and were used for SEM observations. SEM images revealed that the two chemicals could influence the morphology of B. cinerea hyphae, and disrupt membrane integrity, as reported by damaging cell membrane of R. stolonifer (Jiang et al. 2015). Consequently, the antifungal mechanisms of thymol and carvacrol against B. cinerea were because of their actions on cellular membrane and disrupting the cytoplasm of hyphae, eventually resulting in mycelia death.
Membrane permeability parameters included potassium ions leakage, 260 nm absorbing materials loss, and change in extracellular pH. These parameters are usually adopted for indicating gross and irreversible damage happened on plasma membranes (Paul et al. 2011; Shao et al. 2013). In the present study, the release of cell constituents and extracellular conductivity were dramatically increased in a dose-dependent manner, compared with control. The intracellular proton leakages were distinctly induced by thymol and carvacrol after 30 min of treatment, as evidenced by extracellular pH decrease. The irreversible damage occurred on the cytoplasmic membranes of B. cinerea, which lead to intracellular ions leak and an imbalance in osmotic pressure between extra- and intra- cellular membrane occur. The small substances would be the leakage of intracellular contents of the cells caused by the change of cellular membrane permeability and destruction of microbiological protective barriers (Zhao et al. 2017). In addition, the antifungal effect of thymol was higher than that of carvacrol, according to previous reports (Numpaque et al. 2011; Pérez-Alfonso et al. 2012). These reports supported our current results.
Lipids on cell membrane play an important role in adjusting membrane liquidity, raising membrane stability, and decreasing water-soluble materials permeability (Helal et al. 2007). Volatile substances like terpenes reportedly could penetrate or disrupt these fungal lipid structures (Prashar et al. 2003). The total lipid content in Aspergillus flavus and Aspergillus niger cells remarkedly reduced from non-fumigated cells to fumigated cells with 1 μL/mL of Cymbopogon citratus essential oil (Helal et al. 2007). In this research, the treatment with thymol and carvacrol obviously reduced fungal total lipid content, which indicated that the two chemicals disrupted plasma membrane structure and integrity.
Conclusion
This research has revealed that thymol and carvacrol can obviously repress the mycelial growth of B. cinerea, which are promising anti-fungal compounds. The two chemicals can come into being obvious cytotoxic effect on B. cinerea cells, and the anti-fungal activity can be derived from cell membrane integrity damage and cell components leakage. These results indicated that thymol and carvacrol can be used as an alternative to traditional fungicides by fumigation to control post-harvest tomato pathogens.
Acknowledgements
This work was supported by Natural Science Foundation of Hunan Province (No. 2015JJ6108) and Ph.D. Research Fund of Xiangtan University (No. KZ08033).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bouchra C, Achouri M, Hassani LMI, Hmamouchi M. Chemical composition and antifungal activity of essential oils of seven Moroccan Labiatae against Botrytis cinerea Pers: Fr. J Ethnopharmacol. 2003;89:165–169. doi: 10.1016/S0378-8741(03)00275-7. [DOI] [PubMed] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol. 2004;94(3):223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Burt SA, Vlielander R, Haagsman HP, Veldhuizen EJ. Increase in activity of essential oil components carvacrol and thymol against Escherichia coli O157:H7 by addition of food stabilizers. J Food Protect. 2005;68(5):919–926. doi: 10.4315/0362-028X-68.5.919. [DOI] [PubMed] [Google Scholar]
- Combrinck S, Regnier T, Kamatou GPP. In vitro activity of eighteen essential oils and some major components against common postharvest fungal pathogens of fruit. Ind Crop Prod. 2011;33(2):344–349. doi: 10.1016/j.indcrop.2010.11.011. [DOI] [Google Scholar]
- Daferera DJ, Ziogas BN, Polissiou MG. GC-analysis of essential oils of some Greek aromatic plants and their fungitoxicity on P. digitatum. J Agric Food Chem. 2000;48(6):2576–2581. doi: 10.1021/jf990835x. [DOI] [PubMed] [Google Scholar]
- Du Plooy W, Regnier T, Combrinck S. Essential oil amended coatings as alternatives to synthetic fungicides in citrus postharvest management. Postharvest Biol Technol. 2009;53(3):117–122. doi: 10.1016/j.postharvbio.2009.04.005. [DOI] [Google Scholar]
- Fadli M, Saad A, Sayadi S, Chevalier J, Mezriouia NE, Pagèsc JM, Hassani L. Antibacterial activity of Thymus maroccanus and Thymus broussonetii essential oils against nosocomial infection-bacteria and their synergistic potential with antibiotics. Phytomedicine. 2012;19(5):464–471. doi: 10.1016/j.phymed.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- Helal GA, Sarhan MM, Abu SANK, Abou EEK. Effects of Cymbopogon citratus L. essential oil on the growth, morphogenesis and aflatoxin production of Aspergillus flavus ML2-strain. J Basic Microb. 2007;47(1):5–15. doi: 10.1002/jobm.200610137. [DOI] [PubMed] [Google Scholar]
- Jesus FPK, Ferreiro L, Bizzi KS, Loreto ÉS, Pilotto MB, Ludwig A, Alves SH, Zanette RA, Santurio JM. In vitro activity of carvacrol and thymol combined with antifungals or antibacterials against Pythium insidiosum. J Mycol Méd. 2015;25(2):e89–e93. doi: 10.1016/j.mycmed.2014.10.023. [DOI] [PubMed] [Google Scholar]
- Jiang XD, Feng KJ, Yang XP. In vitro antifungal activity and mechanism of action of tea polyphenols and tea saponin against Rhizopus stolonifer. J Mol Microbiol Biotechnol. 2015;25:269–276. doi: 10.1159/000430866. [DOI] [PubMed] [Google Scholar]
- Kanchana B, Noppadon K, Wannee C, Elizabeth JM, Vanee C. Antifungal effect of poly(lactic acid) films containing thymol and R-(-)-carvone against anthracnose pathogens isolated from avocado and citrus. Food Control. 2017;78:85–93. doi: 10.1016/j.foodcont.2017.02.032. [DOI] [Google Scholar]
- Khan A, Ahmad A, Akhtar F, Yousuf S, Xess I, Khan LA, Manzoor N. Ocimum sanctum essential oil and its active principles exert their antifungal activity by disrupting ergosterol biosynthesis and membrane integrity. Res Microbiol. 2010;161(10):816–823. doi: 10.1016/j.resmic.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Kordali S, Cakir A, Ozer H, Cakmakci R, Kesdek M, Mete E. Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Biores Technol. 2008;99:8788–8795. doi: 10.1016/j.biortech.2008.04.048. [DOI] [PubMed] [Google Scholar]
- Langeveld WT, Veldhuizen EJ, Burt SA. Synergy between essential oil components and antibiotics: a review. Crit Rev Microbiol. 2014;40(1):76–94. doi: 10.3109/1040841X.2013.763219. [DOI] [PubMed] [Google Scholar]
- Liu WT, Chu CL. Thymol and acetic acid vapors reduce postharvest brown rot of apricots and plums. HortScienc. 2002;37(1):151–156. doi: 10.21273/HORTSCI.37.1.151. [DOI] [Google Scholar]
- Marino M, Bersani C, Comi G. Impedance measurements to study the antimicrobial activity of essential oils from Lamiaceae and Compositae. Int J Food Microbiol. 2001;67(3):185–187. doi: 10.1016/S0168-1605(01)00447-0. [DOI] [PubMed] [Google Scholar]
- Martínez-Hernández GB, Amodio ML, Colelli G. Carvacrol-loaded chitosan nanoparticles maintain quality of fresh-cut carrots. Innov Food Sci Emerg. 2017;41:56–63. doi: 10.1016/j.ifset.2017.02.005. [DOI] [Google Scholar]
- Martínez-Romero D, Guillén F, Valverde JM, Bailén G, Zapata PJ, Serrano M, Castillo S, Valero D. Influence of carvacrol on survival of Botrytis cinerea inoculated in table grapes. Int J Food Microbiol. 2007;115(2):144–148. doi: 10.1016/j.ijfoodmicro.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Neri F, Mari M, Brigati S. Control of Penicillium expansum by plant volatile compounds. Plant Pathol. 2006;55(1):100–105. doi: 10.1111/j.1365-3059.2005.01312.x. [DOI] [Google Scholar]
- Numpaque MA, Oviedo LA, Gil JH, García CM, Durango DL. Thymol and carvacrol: biotransformation and antifungal activity against the plant pathogenic fungi Colletotricjum acutatum and Botryodiplodia theobromae. Trop Plant Pathol. 2011;36(1):3–13. doi: 10.1590/S1982-56762011000100001. [DOI] [Google Scholar]
- Ochoa-Velasco CE, Navarro-Cruz AR, Vera-López O, Palou E, Avila-Sosa R. Growth modeling to control (in vitro) Fusarium verticillioides and Rhizopus stolonifer with thymol and carvacrol. Rev Argent Microbiol. 2017;50(1):70–74. doi: 10.1016/j.ram.2016.11.010. [DOI] [PubMed] [Google Scholar]
- Paul S, Dubey RC, Maheswari DK, Kang SC. Trachyspermum ammi (L.) fruit essential oil influencing on membrane permeability and surface characteristics in inhibiting food-borne pathogens. Food Control. 2011;22(5):725–731. doi: 10.1016/j.foodcont.2010.11.003. [DOI] [Google Scholar]
- Pérez-Alfonso CO, Martínez-Romero D, Zapata PJ, Serrano M, Valero D, Castillo S. The effects of essential oils carvacrol and thymol on growth of Penicillium digitatum and P. italicum involved in lemon decay. Int J Food Microbiol. 2012;158(2):101–106. doi: 10.1016/j.ijfoodmicro.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Plaza P, Torres R, Usall J, Lamarca N, Vinas I. Evaluation of the potential of commercial post-harvest application of essential oils to control citrus decay. J Pomol Hortic Sci. 2004;79(6):935–940. [Google Scholar]
- Prashar A, Hili P, Veness RG, Evans CS. Antimicrobial action of palmarosa oil (Cymbopogon martini) on Saccharomyces cerevisiae. Phytochemistry. 2003;63(5):569–575. doi: 10.1016/S0031-9422(03)00226-7. [DOI] [PubMed] [Google Scholar]
- Qin G, Zong Y, Chen Q, Hua D, Tian S. Inhibitory effect of boron against Botrytis cinerea on table grapes and its possible mechanisms of action. Int J Food Microbiol. 2010;138(1):145–150. doi: 10.1016/j.ijfoodmicro.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Regnier T, Du Plooy W, Combrinck S, Botha B. Fungitoxicity of Lippia scaberrima essential oil and selected terpenoid components on two mango postharvest spoilage pathogens. Postharvest Biol Technol. 2008;48(2):254–258. doi: 10.1016/j.postharvbio.2007.10.011. [DOI] [Google Scholar]
- Sellamuthu PS, Sivakumar D, Soundy P, Korsten L. Essential oil vapours suppress the development of anthracnose and enhance defence related and antioxidant enzyme activities in avocado fruit. Postharvest Biol Technol. 2013;81(3):66–72. doi: 10.1016/j.postharvbio.2013.02.007. [DOI] [Google Scholar]
- Serrano M, Martínez-Romero D, Castillo S, Guillén F, Valero D. The use of antifungal compounds improves the beneficial effect of MAP in sweet cherry storage. Innov Food Sci Emerg. 2005;6(1):115–123. doi: 10.1016/j.ifset.2004.09.001. [DOI] [Google Scholar]
- Shao X, Cheng S, Wang H, Yu D, Mungai C. The possible mechanism of antifungal action of tea tree oil on Botrytis cinerea. J Appl Microbiol. 2013;114(6):1642–1649. doi: 10.1111/jam.12193. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Nakamura S, Sugiyama K, Furata H. Differences of superoxide production in blood leukocytes stimulated with thymol between human and non-human primates. Life Sci. 1987;41:1659–1664. doi: 10.1016/0024-3205(87)90735-1. [DOI] [PubMed] [Google Scholar]
- Talibi I, Askarne L, Boubaker H, Boudyach EH, Msanda F, Saadi B, Ben Aoumar AA. Antifungal activity of some Moroccan plants against Geotrichum candidum, the causal agent of postharvest citrus sour rot. Crop Prot. 2012;35(3):41–46. doi: 10.1016/j.cropro.2011.12.016. [DOI] [Google Scholar]
- Tao NG, Jia L, Zhou HE, He XL. Effect of octanal on the mycelial growth of Penicillium italicum and P. digitatum. World J Microb Biotechnol. 2014;30(4):1169–1175. doi: 10.1007/s11274-013-1539-2. [DOI] [PubMed] [Google Scholar]
- Yahyazadeh M, Omidbaigi R, Zare R, Taheri H. Effect of some essential oils on mycelial growth of Penicillium digitatum Sacc. World J Microb Biotechnol. 2008;24(8):1445–1450. doi: 10.1007/s11274-007-9636-8. [DOI] [Google Scholar]
- Zhao L, Zhang Y, Yang HS. Efficacy of low concentration neutralized electrolysed water and ultrasound combination for inactivating Escherichia coli ATCC 25922, Pichia pastoris GS115 and Aureobasidium pullulans 2012 on stainless steel coupons. Food Control. 2017;73:889–899. doi: 10.1016/j.foodcont.2016.09.041. [DOI] [Google Scholar]