Abstract
In this work, the nano composites of carrageenan/AgNPs/Laponite were prepared and coated on the oxygen plasma surface modified polypropylene film to enhance the barrier and adhesion properties. The mechanical, barrier, adhesion and antimicrobial properties were also studied to use for food packaging applications. The polypropylene film was surface modified with oxygen plasma treatment for 60 s. The AgNPs are prepared by green synthesis method from the Digitalis purpurea plant. Then the carrageenan based nanocomposites were coated by roller coating method with the thickness of 24 μm. By using scanning electron microscopy, the morphology of the coating was investigated. The Laponite and AgNPs dispersion was analyzed by X-ray diffraction analysis. The tensile and adhesion strength of the coated film was increased and the OTR and WVTR were decreased after the incorporation of Laponite and AgNPs. It exhibited the strong antimicrobial activity against the E. coli and S. aureus.
Electronic supplementary material
The online version of this article (10.1007/s13197-019-03735-4) contains supplementary material, which is available to authorized users.
Keywords: Carrageenan, Laponite, AgNPs, Plasma treatment, Adhesion
Introduction
In the food packaging sector, one of the most challenging research subjects is to develop the packaging materials with enhanced property to secure the safety and extend the shelf life of food (Duncan 2011). The bionanocomposite is one of the promising technologies for food packaging due to their functionality, uniqueness and excellent properties (Reddy et al. 2013; Armentano et al. 2013). Bionanocomposite is a hybrid class material consists of biopolymer matrix and nanoscale reinforcement material, in which one or more dimensions are in the order of less than 100 nm (Ojijo and Ray 2013). When the nanosize particles were dispersed in the biopolymer matrix, it markedly improved the mechanical, barrier and other properties with very low filler loading when compared with the pure polymer (Rhim and Wang 2014). Several types of nanofillers have been used to prepare the bionanocomposite by mixing with various types of biopolymers such as polysaccharides, proteins (Zolfi et al. 2015). In addition, several natural or synthetic antimicrobial agents have been used to develop the packaging materials with antimicrobial properties (Jafarzadeh et al. 2017). The use of more than one antimicrobial agent may give the combined or synergistic effect of their antimicrobial activity. As compared to organic antimicrobial materials, inorganic materials are greater in thermal stability, chemical durability and selectivity (Vishnuvarthanan and Rajeswari 2017). Antimicrobial nanocomposite systems are very effective because of the high surface to volume ratio and improve the surface reactivity of nano antimicrobial agents which are very useful to prevent the microbial contamination for the shelf life extension and food safety (Arfat et al. 2015). Among the inorganic materials, Silver Nanoparticles (AgNPs) have attracted more attention in food packaging applications because of the unique physical and chemical properties, high thermal stability, especially the good antimicrobial activity as well as the low toxicity (Bora and Mishra 2016).
The AgNPs are prepared by various complex synthesis methods or by reduction procedures (Ebrahimiasl and Rajabpour 2015). The reduction procedure consists of photochemical and sonochemical reduction, laser ablation, UV irradiation, reduction by biological systems and plant extracts. In this, the plant extract method is very environmental friendly, efficient and economical (Vishnuvarthanan and Rajeswari 2017). If the AgNPs are used alone, it has a tendency to agglomerate which causes deterioration of chemical activities and reduce the antimicrobial properties. To solve this problem, the nanocomposites are prepared with Ag/clay, in which the clay supported the AgNPs within the intermellar spaces or on its external surfaces (Paula et al. 2015; Lamareerat et al. 2018). Laponite is synthetic clay which leads to novel properties. It has not been investigated with carrageenan based nanocomposites. Laponite is a synthetic hydrous magnesium lithium silicate which consists of two tetrahedral silica sheets. It has a great capacity for exfoliation and the diameter and thickness of individual platelets are 30 nm and 1 nm (Gaharwar et al. 2011). Several polysaccharides have been used as biopolymer matrices and also as carriers of nanoparticles. As one of such polysaccharides, carrageenan has been used for food packaging applications since it forms strong gels, water holding capacity, good barrier properties (Rhim and Wang 2013). It also has been used as a stabilizing agent for the nanoparticles such as clay minerals, metallic oxides and nanometals (Kanmani and Rhim 2014; Llorens et al. 2012). Compared to other materials, having a good barrier is not only enough for food packaging applications (Nagarajan et al. 2015). Many strategies were developed to utilize the carrageenan sustainability and improve the properties. These include the blending with synthetic or biopolymer and coating on polyolefin surfaces. However, the blending strategy is limited by the incompatibility with the polymer or thermal stability during the extrusion process. For that, surface modification and coating are more desirable in terms of stability and adhesiveness on the plastic film (Vishnuvarthanan and Rajeswari 2015). In the environmental aspect, biopolymer coatings may be helpful, since the identification and layer separation for the synthetic film recycling are difficult and the multilayer structures for various applications add up to a amount of waste.
In this study, the Laponite and silver nanoparticles loaded carrageenan coatings on oxygen plasma surface modified PP films were developed and investigated the effect of nanocomposite coatings on barrier, adhesion, mechanical and antimicrobial properties.
Materials and methods
Materials
The PP film used in this study was purchased from Jayanthi Plastics, Erode, India. It has the thickness of 0.048 ± .003 mm and density of 0.92 g/cm3. The κ-carrageenan (300,000 g/mol) of food grade was purchased from Sigma Aldrich, India. The Laponite was obtained from Rock wood additives, England and its cation exchange capacity (CEC) is 60 meq/100 g. The glycerol was obtained from S.K. Chemicals, Ltd. The microorganisms of gram-negative E. coli (MTCC 1652) and gram-positive S. aureus (MTCC 3160) were procured from Microbial Type Culture Collection and Gene Bank (MTCC), India.
Oxygen plasma treatment and green synthesis of silver nanoparticle (AgNPs)
The detailed procedure and the results of the oxygen plasma treatment of PP films were discussed and the procedure was followed (Vishnuvarthanan and Rajeswari 2015). The AgNPs were green synthesized by Digitalis purpurea plant and the characterization and properties were investigated (Vishnuvarthanan and Rajeswari 2017).
Preparation of carrageenan/AgNPs/Laponite nanocomposites
The carrageenan nanocomposites were prepared using the Laponite with silver nanoparticles (AgNPs) by green synthesis. The method of preparation is as follows: The carrageenan and Laponite used in this study was pre-dried in hot air oven for about 6 h. 3 grams of carrageenan powder was dissolved in 100 mL of double distilled water along with 0.5 mL of glycerol as plasticizer. It should be mixed thoroughly for 30 min at 60 °C by magnetic stirrer in a hot plate. Then, the Laponite with different wt% of 1, 3, 5 and 7 was dispersed each separately in 100 mL of deionized water. The solutions were stirred by magnetic stirrer for 12 h and then they were homogenized for 15 min at 5000 rpm. The prepared Laponite solutions were mixed with the prepared solutions of carrageenan and stirred for 15 min at 60 °C. Afterwards, the solution of 20 µg of AgNPs was added dropwise to the carrageenan/Laponite solutions. Finally, all the prepared solutions were thoroughly mixed and stirred for 30 min at 60 °C. Then, the solutions were sonicated for 45 min to avoid agglomeration. The prepared carrageenan nanocomposites with different wt% of 0, 1, 3, 5 and 7 using Laponite nanoclay was designated as CL0, CL1, CL3, CL5 and CL7 respectively.
Characterization of carrageenan nanocomposites coated PP
The FTIR analyses were carried out using 66 V/S (Bruker) spectrometer. The spectra were investigated in the wavenumber range of 4000–400 cm−1 at a resolution of 4 cm-1 with 16 scans. Wide angle X-ray diffraction were obtained using Bruker AXS D8 advance X-ray diffractometer utilizing nickel filter. By using the LEO 1430VP SEM instrument, Scanning electron microscopy (SEM) analysis was performed. The Tensile strength of the nanocomposite coated PP films were investigated by Universal Testing Machine (UTM, H10KS, Tinius Olsen, UK) at 23 °C by the standard of ASTM D638. The standard T—peel test method of ASTM D 1876-93 was followed and the scotch tape was used to find the adhesion strength of the samples. The Oxygen Transmission Rate (OTR) of the nanocomposite coated PP films were calculated by OXTRAN Oxygen Permeability Tester (noselab ATS) at 23 °C under the condition of 0% RH at 1 atm by the standard of ASTM D3985. The measurements were taken at three times at different places of the film and the average value was calculated. The Watervapour Transmission Rate (WVTR) of nanocomposite coated PP films were calculated by nissy, according to the standard of ASTM F 1249-90. Under the condition of 100% RH the tests were carried out at 35 °C at ambient conditions and it was repeated for three times and the average mean values were calculated. The antimicrobial activity of nanocomposite coated PP films was tested against gram-negative E. coli and gram-positive S. aureus bacteria by using the agar disk diffusion method. After the incubation period, the zone of inhibition (in mm) was observed and tabulated.
Results and discussion
FTIR spectra
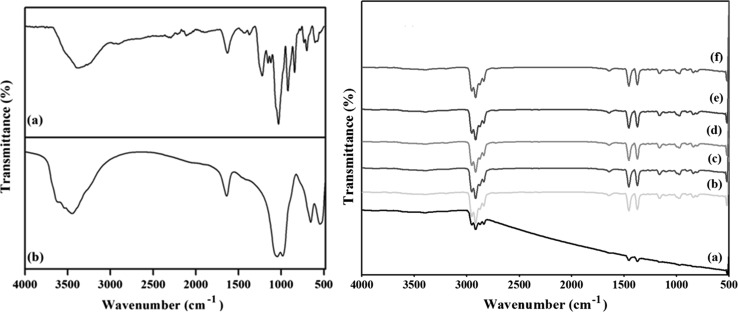
The functional nature of Carrageenan, Laponite and carrageenan nanocomposite coated PP films were identified using FTIR analysis and it is shown in the Fig. 1. The peaks at 2938 cm−1 and 2871 cm−1 indicates the presence of CH2 and CH3 stretching vibration of the plasma treated PP. In the carrageenan, the O–H stretching exhibits the characteristic peak in the range of 3200–3500 cm−1 and the peak at 2955 cm−1 and 2904 cm−1 corresponds to the C-H stretching vibration of the alkane group. The sulfate ester group of the carrageenan corresponds to the peak at 1185 cm−1. The characteristic peaks were observed at 970 cm−1 and 845 cm−1 indicates the presence of 3, 6-anhydro-d-galactose and galactose-4-sulfate (Hezaveh and Muhamad 2013). The peak at 1645 cm−1 shows the presence of amide I in the carrageenan based nanocomposite coated films.
Fig. 1.
FTIR spectra of a Carrageenan, b Laponite, c Carrageenan nanocomposite coated films. (a) CL0 (b) CL1 (c) CL3 (d) CL5 (e) CL7 (f) CL-AgNPs
The FTIR spectrum of the Laponite exhibits a peak around 3700–3400 cm−1 and this is due to the bending and stretching vibrations of sorbed water and surface hydroxyl groups. The peak at 1010 cm−1 and 545 cm−1 indicates the presence of Si–O bond and Si–O bending vibration of Laponite. At 770 cm−1, a peak was observed and indicates the presence of Mg-O bonding and the peak 1639 cm−1 indicates the Si–O stretching vibrations. A peak at 3670 cm−1 was observed due to the presence of hydroxyl groups in Laponite. The Si–O peak at 1010 cm−1 was observed in the carrageenan/Laponite nanocomposite coated films and indicates the dispersion of Laponite in the carrageenan and also the exfoliation of carrageenan between the interlayer spacing of Laponite. It was also observed that, there was no new peak formation by the incorporation of AgNPs in the carrageenan/Laponite nanocomposite coating which indicates there was no chemical bond formation.
XRD analysis
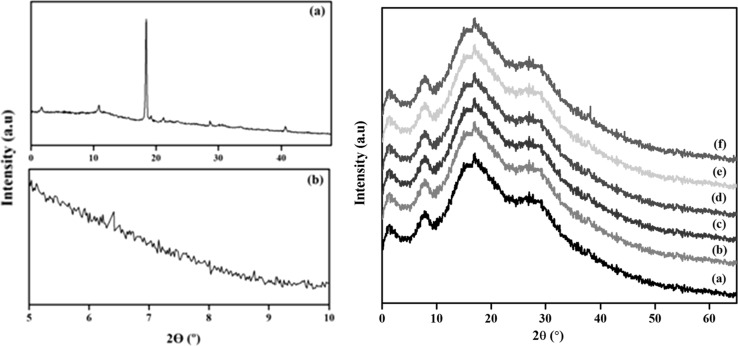
The clay dispersion within the carrageenan matrix has been characterized by XRD. It allows the interpretation of the nanocomposite structure depends on the intensity, shape and also used to measure the interlayer spacing of layered silicate layers. Based on the disappearance and the peak shifts to the lower angles represents the exfoliated and intercalated structure. The XRD analysis of the carrageenan and carrageenan based nanocomposite coated PP are shown in the Fig. 2.
Fig. 2.
XRD of a Carrageenan, b Laponite, c XRD of Carrageenan nanocomposite coated films. (a) CL1 (b) CL3 (c) CL5 (d) CL7 (e) CL-AgNPs
In the carrageenan, the peaks were observed commonly at 2θ around 1.68°, 10.87°, 18.45°, 21.04°, 28.62° and 40.77°. The peak at 6.4° in Laponite corresponds to the basal spacing of 13.810 Å. Furthermore, peaks were also observed at 19.7° (4.506 Å) 20.2° (4.395 Å), 27.6° (3.231 Å) and 33.8° (2.651 Å). The XRD analysis of the nanocomposite coating of carrageenan for the Laponite from 1 to 7 wt% indicates the absence of peak at 6.4° and it indicates the Laponite is highly exfoliated in the carrageenan matrix. It also indicates the polymer chains can enter the silicate layers resulting in a higher degree of disorder and exfoliation. The aggregate layered structure was induced at 10% of Laponite. The higher clay content not only led to the lesser silicate layer separation or lower d-spacing but also causes the aggregation rather than random distribution. The incorporation of AgNPs into the carrageenan/Laponite nano composite coating shows similar profiles and the peaks are observed at 38.2°, 44.4° and 64.4° respectively. These peaks were corresponds to the (111), (200), (222) crystallographic planes of silver. As a consequence, the composite materials have both intercalated and stacked clay layers (Kasirga et al. 2012). In general, at lower wt% of clay content the exfoliation system is more possible and for the higher wt% of clay content the intercalation and the aggregation are more frequently observed.
SEM analysis
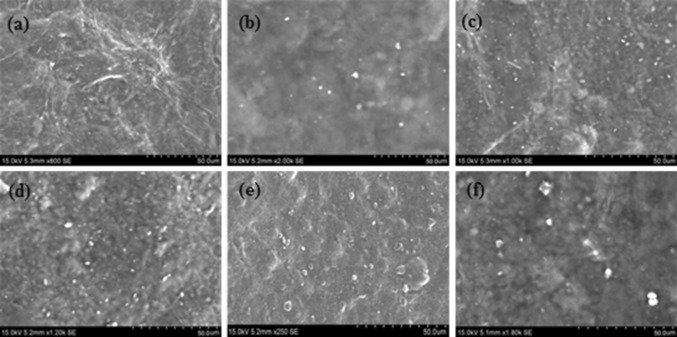
The surface morphology of the uncoated oxygen plasma modified PP and the carrageenan based nanocomposite coated PP was analyzed by SEM. Figure 3 shows the SEM images of the carrageenan nanocomposites coated PP film.
Fig. 3.
SEM images of Carrageenan nanocomposite coated films. a CL0, b CL1, c CL3, d CL5, e CL7, f CL-AgNPs
The PP surface was well covered by the carrageenan nanocomposite coating. The carrageenan nanocomposite coating showed a smooth appearance and exhibited a good adherence on the oxygen plasma surface modified PP. The results of the SEM images showed a difference in Laponite between the neat carrageenan and the clay with the AgNPs based carrageenan and there was a uniform dispersion of nanoclay and AgNPs in the carrageenan matrix. Higher the degree of dispersion was obtained up to 7 wt% of Laponite in the carrageenan matrix and shows the most of the clay particles were exfoliated. By adding more amounts of Laponite in the carrageenan matrix, there was a significant change in the morphological structure.
Barrier properties
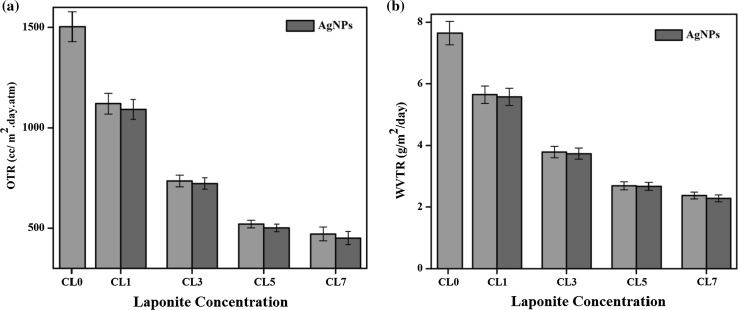
To maintain the quality of the packaged food, the oxygen and watervapour barrier property of the coated film is very important in deciding the suitability for various applications (Vishnuvarthanan and Rajeswari 2015). The oxygen transmission rate and watervapour transmission rate of the uncoated oxygen plasma treated PP film and the carrageenan nanocomposite coated films are shown in the Fig. 4.
Fig. 4.
a OTR of Carrageenan nanocomposite coated films. b WVTR of Carrageenan nanocomposite coated films
The oxygen transmission rate of the uncoated oxygen plasma treated film was 1853.32 cc. (m2 day atm)−1. After the carrageenan coating, the OTR was significantly decreased to 1499.81 cc. (m2 day atm)−1 By the addition of 1 wt% of Laponite with carrageenan, the OTR was decreased to 1120.35 cc. (m2 day atm)−1. After the incorporation of AgNPs with 1 wt% of Laponite, the OTR was decreased to 1091.42 cc. (m2 day atm)−1. The OTR was still decreased to 735.35 cc. (m2 day atm)−1 for 3wt % of Laponite and it was further decreased to 722.42 cc. (m2 da atm)−1 after the incorporation of AgNPs. The WVTR of the uncoated PP film was 9.62 g/m2/day and after the coating of carrageenan it was decreased to 7.65 g/m2/day. By the addition of 1 wt% of Laponite in carrageenan, the value of WVTR was 5.65 g/m2/day. After the incorporation of AgNPs, the values of WVTR were further decreased to 5.58 g/m2/day. The decrease of WVTR was due to the effect of nanoclays and nanosilver. In the case of 3 wt% of Laponite, the WVTR values were 3.44 g/m2/day. After the incorporation of AgNPs, it shows the higher values of 3.37 g/m2/day. The efficient reduction of watervapour values were obtained for the carrageenan with 7 wt% of Laponite and the value was 2.02 g/m2/day.
In the previous literature, it has been reported that the incorporation of nanoclay or metal nanoparticles in the polymeric matrices affects the gas and watervapour permeability (Orsuwan et al. 2017). These materials have the ability to act as a barrier for oxygen and watervapour to control the transport of both to extend the shelf life of the food. The OTR and WVTR were continuously decreased after the addition of nanoclay and the AgNPs with carrageenan. Here, the materials were acted as a discontinuous barrier for the diffusion of watervapour in the film matrix and thus the path length of the diffusion got decreased. There was a large reduction in OTR and WVTR after the addition of Laponite in Carrageenan and this may be due to the existence of more tortuous path in the diffusing molecules and that can be go around the impenetrable platelets. The formation of tortuous path in the carrageenan was by the presence of Laponite which decreases the permeation of oxygen and watervapour. After the incorporation of AgNPs, it was noticed that further decrease of OTR and WVTR values as compared with Laponite incorporated carrageenan. It was because of the shape and structure of the nanofillers, i.e., layered plate-like structure clay minerals and the spherical shape of the AgNPs. The dispersion of clay mineral in the polymer matrix with the AgNPs may also develop further efficient tortuous path for oxygen transmission. The aspect ratio of the layered silicates is very high as compared to the other fillers used for the preparation of nanocomposites (Martins et al. 2013). The effective aspect ratio of the layered silicate is reduced because of agglomeration and decrease in length of the diffusion path.
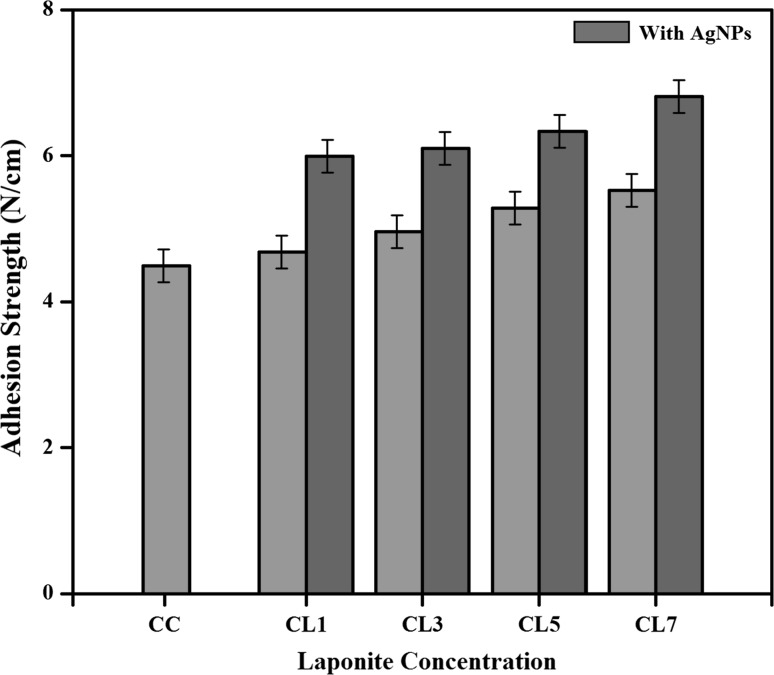
Adhesion strength
The peel strength is an important property to characterize the adhesion behavior of any material. The T-peel strength of the uncoated and the carrageenan nanocomposites coated PP were determined and the results are shown in Fig. 5. The peel strength of the uncoated oxygen plasma surface modified PP is 3.48 N cm−1. After the carrageenan coating it increased to 4.49 N cm−1. Subsequently, the value increased to 4.68 N/cm after the incorporation of 1 wt% of Laponite. After the incorporation of AgNPs with Laponite, the value of the peel strength was 5.99 N/cm.
Fig. 5.
Adhesion strength of Carrageenan nanocomposite coated films
This is because of the surface roughness and increase in surface energy due to oxygen plasma treatment. The surface roughness is one of the main keys for adhesion strength. The oxygen plasma treatment made the PP surface polar in nature and thus created the adhesion between the PP surface and the coated carrageenan nanocomposites due to the van der waals interaction. It was noticed that, the peel strength was not changed significantly with increase in clay concentration in carrageenan. Moreover, the addition of AgNPs showed no significant effect on peel strength. Furthermore, the interlocking of the polymer matrix, nanoclay and AgNPs into the cavities, pores and asperities of the solid surface also be the main factor in determining the adhesive strength. The mechanical interlocking can be attributed by the increase in interfacial area due to surface roughness caused by the plasma treatment and it created the pitted structure on the polymer surface.
Antimicrobial properties
The antimicrobial activity of carrageenan based nanocomposites coated on the oxygen plasma surface modified PP was investigated against the both gram-positive and gram-negative organisms using the agar well diffusion method. The micro organisms used were E. coli and S. aureus and the antimicrobial activities were determined by the diameter of the inhibition zone. The neat carrageenan coated oxygen plasma surface modified PP film didn’t show any inhibitory activity against the both microorganisms. As expected after the addition of Laponite also, no zone of inhibition was observed. The clear zone of inhibition was occurred after the mixing of AgNPs with the carrageenan nanocomposites. It gave more pronounced activity. For the laponite, the antimicrobial activity is attributed to their structure and the antimicrobial action mode as well as the difference in the cell wall structure of indicator microorganisms (Abreu et al. 2015) The gram-positive bacteria’s are composed of three dimensional thick peptidoglycan layers consists of linear polysaccharide chains cross linked with short peptides to form a complex wall structure which might obstruct the clays and Silver nanoparticles to penetrate (Vishnuvarthanan and Rajeswari 2018). The thin peptidoglycan layer is coated on the outer membrane which are negatively charged might facilitate the clays and AgNPs to break through more easily into the Gram-negative bacteria (Morones et al. 2005). Many mechanistic actions for the antimicrobial activity of silver nanoparticles have been reported, though the exact mechanism has not been established yet (Alboofetileh et al. 2016). The interaction of silver nanoparticles disrupts the cell membrane preventing the replication of DNA and leads to the cell death (Singh et al. 2016). The cell wall and surface protein disruption is caused by the binding of the negatively charged bacterial cell membrane with the positively charged AgNPs which leads to the cell death. The enzyme inactivation is caused by the AgNPs penetration into the bacteria and produces H2O2 and leads to the cell death. Though the carrageenan nanocomposite coated PP films, composed of Laponite have not exhibited the antimicrobial property. The use of AgNPs has greatly improved the antimicrobial property in the carrageenan coating against the both Gram-positive and Gram-negative organisms. However the addition of Laponite has been significantly improved the barrier properties. These combinations are expected to have a high potential for the food packaging applications.
Conclusion
The carrageenan nanocomposites were prepared and coated on the oxygen plasma surface modified PP. The successful coating of carrageenan/AgNPs/Laponite on PP was characterized by various methods. The obtained results showed better barrier, adhesion and mechanical properties. The carrageenan nanocomposites with AgNPs showed the excellent antimicrobial activity against the both Gram-negative E. coli and Gram-positive S. aureus. The results showed a good prospective to obtain the improved packaging materials for food packaging applications.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abreu AS, Oliveira M, De Sa A, Rodrigues RM, Cerqueira MA, Vicente AA, Machado AV. Antimicrobial nanostructured starch based films for packaging. Carbohydr Polym. 2015;129:127–134. doi: 10.1016/j.carbpol.2015.04.021. [DOI] [PubMed] [Google Scholar]
- Alboofetileh M, Rezaei M, Hosseini H, Abdollahi M. Efficacy of activated alginate-based nanocomposite films to control Listeria monocytogenes and spoilage flora in rainbow trout slice. J Food Sci Technol. 2016;53(1):521–530. doi: 10.1007/s13197-015-2015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfat YA, Benjakul S, Vongkamjan K, Sumpavapol P, Yarnpakdee S. Shelf-life extension of refrigerated sea bass slices wrapped with fish protein isolate/fish skin gelatin-ZnO nanocomposite film incorporated with basil leaf essential oil. J Food Sci Technol. 2015;52(10):6182–6193. doi: 10.1007/s13197-014-1706-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentano I, Bitinis N, Fortunati E, Mattioli S, Rescignano N, Verdejo R, Lopez-Manchado MA, Kenny JM. Multifunctional nanostructured PLA materials for packaging and tissue engineering. Prog Polym Sci. 2013;38:1720–1747. doi: 10.1016/j.progpolymsci.2013.05.010. [DOI] [Google Scholar]
- Bora A, Mishra P. Characterization of casein and casein-silver conjugated nanoparticle containing multifunctional (pectin–sodium alginate/casein) bilayer film. J Food Sci Technol. 2016;53(10):3704–3714. doi: 10.1007/s13197-016-2343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan TV. Applications of nanotechnology in food packaging and food safety: barrier materials, antimicrobials and sensors. J Colloid Interface Sci. 2011;363:1–24. doi: 10.1016/j.jcis.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimiasl S, Rajabpour A. Synthesis and characterization of novel bactericidal Cu/HPMC BNCs using chemical reduction method for food packaging. J Food Sci Technol. 2015;52(9):5982–5988. doi: 10.1007/s13197-014-1615-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaharwar AK, Rivera CP, Wu CJ, Schmidt G. Transparent, elastomeric and tough hydrogels from poly(ethylene glycol) and silicate nanoparticles. Acta Biomater. 2011;7:4139–4148. doi: 10.1016/j.actbio.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Hezaveh H, Muhamad II. Modification and swelling kinetic study of kappa-carrageenan-based hydrogel for controlled release study. J Taiwan Inst Chem Eng. 2013;44:182–191. doi: 10.1016/j.jtice.2012.10.011. [DOI] [Google Scholar]
- Jafarzadeh S, Alias AK, Ariffin F, Mahmud S, Najafi A, Ahmad M. Fabrication and characterization of novel semolina-based antimicrobial films derived from the combination of ZnO nanorods and nanokaolin. J Food Sci Technol. 2017;54(1):105–113. doi: 10.1007/s13197-016-2441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmani P, Rhim JW. Physical, mechanical and antimicrobial properties of gelatin based active nanocomposite films containing AgNPs and nanoclay. Food Hydrocolloids. 2014;35:644–652. doi: 10.1016/j.foodhyd.2013.08.011. [DOI] [Google Scholar]
- Kasirga Y, Oral A, Caner C. Preparation and characterization of chitosan/montmorillonite/K10 nanocomposites films for food packaging applications. Polym Compos. 2012;33:1874–1882. doi: 10.1002/pc.22310. [DOI] [Google Scholar]
- Lamareerat B, Singh M, Sadiq MB, Anal AK. Reinforced cassava starch based edible film incorporated with essential oil and sodium bentonite nanoclay as food packaging material. J Food Sci Technol. 2018;55(5):1953–1959. doi: 10.1007/s13197-018-3100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens A, Lloret E, Picouet PA, Trbojevich R, Fernandez A. Metallic-based micro and nanocomposites in food contact materials and active food packaging. Trends Food Sci Technol. 2012;24:19–29. doi: 10.1016/j.tifs.2011.10.001. [DOI] [Google Scholar]
- Martins JT, Bourbon AI, Pinheiro AC, Souza BW, Cerqueira MA, Vicente AA. Biocomposite films based on κ-carrageenan/locust bean gum blends and clays: physical and antimicrobial properties. Food Bioprocess Technol. 2013;6:2081–2092. doi: 10.1007/s11947-012-0851-4. [DOI] [Google Scholar]
- Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, Yacaman MJ. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- Nagarajan M, Benjakul S, Prodpran T, Songtipya P. Properties and characteristics of nanocomposite films from tilapia skin gelatin incorporated with ethanolic extract from coconut husk. J Food Sci Technol. 2015;52(12):7669–76682. doi: 10.1007/s13197-015-1905-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojijo V, Ray SS. Processing strategies in bionanocomposites. Prog Polym Sci. 2013;38:1543–1589. doi: 10.1016/j.progpolymsci.2013.05.011. [DOI] [Google Scholar]
- Orsuwan A, Shankar S, Wang LF, Sothornvit R, Rhim JW. One-step preparation of banana powder/silver nanoparticles composite films. J Food Sci Technol. 2017;54(2):497–506. doi: 10.1007/s13197-017-2491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paula GA, Benevides NM, Cunha AP, de Oliveira AV, Pinto AM, Morais JPS, Azeredo HM. Development and characterization of edible films from mixtures of κ-carrageenan, ι-carrageenan, and alginate. Food Hydrocolloids. 2015;47:140–145. doi: 10.1016/j.foodhyd.2015.01.004. [DOI] [Google Scholar]
- Reddy MM, Vivekanandhan S, Misra M, Bhatia SK, Mohanty AK. Biobased plastics and bionanocomposites: current status and future opportunities. Prog Polym Sci. 2013;38:1653–1689. doi: 10.1016/j.progpolymsci.2013.05.006. [DOI] [Google Scholar]
- Rhim JW, Wang LF. Mechanical and water barrier properties of agar/κ-carrageenan/konjac glucomannan ternary blend biohydrogel films. Carbohydr Polym. 2013;96:71–81. doi: 10.1016/j.carbpol.2013.03.083. [DOI] [PubMed] [Google Scholar]
- Rhim JW, Wang LF. Preparation and characterization of carrageenan-based nanocomposite films reinforced with clay mineral and silver nanoparticles. Appl Clay Sci. 2014;97:174–181. doi: 10.1016/j.clay.2014.05.025. [DOI] [Google Scholar]
- Singh PK, Jairath G, Ahlawat SS. Nanotechnology: a future tool to improve quality and safety in meat industry. J Food Sci Technol. 2016;53(4):1739–1749. doi: 10.1007/s13197-015-2090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishnuvarthanan M, Rajeswari N. Effect of mechanical, barrier and adhesion properties on oxygen plasma surface modified PP. Innov Food Sci Emerg Technol. 2015;30:119–126. doi: 10.1016/j.ifset.2015.05.007. [DOI] [Google Scholar]
- Vishnuvarthanan M, Rajeswari N. Plant mediated greener approach for synthesis of silver nanoparticles from Digitalis purpurea plant and its antibacterial activity. Int J Nanopart. 2017;9:166–179. doi: 10.1504/IJNP.2017.089196. [DOI] [Google Scholar]
- Vishnuvarthanan M, Rajeswari N. Food packaging: pectin–laponite–Ag nanoparticle bionanocomposite coated on polypropylene shows low O2 transmission, low Ag migration and high antimicrobial activity. Environ Chem Lett. 2018 [Google Scholar]
- Zolfi M, Khodaiyan F, Mousavi M, Hashemi M. Characterization of the new biodegradable WPI/clay nanocomposite films based on kefiran exopolysaccharide. J Food Sci Technol. 2015;52(6):3485–3493. doi: 10.1007/s13197-014-1407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.