Abstract
Hawthorn fruits are rich in nutrients and antioxidant compounds. Dehydration is the major processing and preservation method for hawthorn fruits. The rates of moisture loss; polyphenol, flavonoid and triterpenoid acid contents; and antioxidant activities and their relationships were investigated in hawthorn slices that were dried by four dehydration techniques (microwave drying, solar drying, hot air drying and freeze drying) under different operation conditions. The results showed that both the drying method and the processing parameter affected the antioxidants. Microwave drying and hot air drying at high temperatures (≥ 80 °C) resulted in the degradation of the polyphenols, flavonoids and triterpenoid acids in the hawthorn slices. These antioxidant compounds were best preserved by freeze drying and hot air drying at 60 °C. Epicatechin and chlorogenic acid were the major phenolic compounds identified in this research, and these compounds were significantly affected during processing. The antioxidant activities of the hawthorn fruits were significantly related to the total polyphenol, flavonoid and triterpenoid acid contents. Hot air drying at proper temperatures could be suitable for hawthorn slice dehydration processing that conserves the antioxidant properties of the fruit.
Keywords: Hawthorn slices, Drying methods, Antioxidant compounds, Antioxidant activities
Introduction
Hawthorn (Crataegus pinnatifida Bge.), belonging to the Rosaceae family, is an important horticultural product in fresh and processed forms with excellent flavour, attractive colour and high nutrient contents. Recently, hawthorn has attracted increasing amounts of attention in the fields of nutraceuticals and medicine due to the high nutrient contents and health benefits of its leaves, flowers, pollen and berries (Coklar et al. 2018). As a commonly used medicinal plant, hawthorn is believed to reduce the risk of atherosclerosis due to its hypolipidaemic and vascular protective effects (Coklar et al. 2018; Kwok et al. 2013). These positive effects are mainly attributed to the high antioxidant activities of hawthorns. Phenolics, flavonoids and triterpenoid acids are important groups of natural antioxidant compounds present in hawthorns (Li et al. 2013).
Dehydration is a preferred processing method for hawthorn fruits, and dried hawthorn slices have been traditionally used for medicinal purposes to improve digestion and increase appetite (Kwok et al. 2013). The main aim of dehydration processing is to reduce moisture, minimize microbial growth and deterioration, and obtain a good quality dried product. The quality of the dried product is strongly dependent on the drying methods and the processing conditions (Pereira et al. 2007). Solar drying is the most frequently used method for hawthorn fruits, but this method takes a long time and is dependent on the environment, resulting in variable quality. In addition to conventional solar drying, hawthorn fruits can be processed at temperatures above 180 °C by stir-frying or roasting as described in Chinese Pharmacopoeia (2010 edn) to improve the functional characteristics of the fruit. Several research studies have revealed the effects of stir-frying or roasting on the nutritional and sensory qualities of hawthorn fruits (Zhang et al. 2017; Zhong 2012).
Fruit dehydration processing always affects the physicochemical and nutritional properties of the fruits. This method has significant effects on the bioactive compounds and antioxidant activities (Wojdyło et al. 2009) of fruits. As mentioned above, the functional and therapeutic properties of hawthorn fruits are related to antioxidant compounds, which are not stable and will degrade when exposed to oxygen, light and heat during long drying hours. The degradation of these compounds should be considered when optimizing the processing parameters. Several research studies have investigated the effects of different drying methods (hot air drying, microwave drying, freeze drying, and vacuum drying) on the drying kinetics, colour, polyphenols and antioxidant activities of hawthorn fruits (Aral and Bese 2016; Saadatian et al. 2016; Coklar et al. 2018). However, minimal information is available regarding the effects of different drying methods with different experimental parameters on the antioxidants of hawthorn slices. In addition, the effects of different dehydration methods on the total flavonoids and triterpenoid acids of hawthorn fruits and their relationships with antioxidant activities have not yet been reported. A convenient and energy-saving dehydration method for hawthorn slices has not been clearly suggested. The objective of this study was to evaluate the drying time and the quality of the preservation of hawthorn fruit slices by freeze drying, solar drying, microwave drying with different powers, and hot air drying with different temperatures. Understanding the modifications of the bioactive components and antioxidant activities in hawthorn fruits dehydrated by different processes could be helpful for determining the proper methods to dehydrate hawthorn slices.
Materials and methods
Hawthorn materials
Hawthorn (Crataegus pinnatifida Bge.) cultivar “Dajinxing”, a variety typically cultivated to produce table hawthorns, was used for the samples in this study. Hawthorn fruits at the same maturity stage were picked by hand in the orchard of Dengfeng Sanzhahong Wine Co. Ltd. (Dengfeng, Henan) in September and were immediately transported to the laboratory. Each hawthorn fruit was crosscut into 3 slices with a thickness of approximately 5–7 mm per slice, and the cores were removed.
Chemicals
HPLC grade methanol and phosphoric acid from Merck (Darmstadt, Germany) were used. Ellagic acid, chlorogenic acid, epicatechin, rutin, protocatechuic acid, Trolox, DPPH (1,1-diphenyl-2-picrylhydrazyl), and Folin–Ciocalteu reagent were purchased from Sigma Chemical Co. (St. Louis, MO, USA). The other chemical reagents used were of analytical grade.
Drying process
The hawthorn slices were dehydrated by four drying techniques, and the experimental conditions were as follows. (1) Hot air drying (HAD): the experiments were conducted in a dryer oven (GS101-2EB, Chongqing SD Experiment Instrument Co., Ltd) with hot air (constant air flow rate of 1.2 m/s) at 60 °C, 80 °C, 100 °C and 120 °C for 12.0, 8.0, 6.5 and 2.5 h, respectively. (2) Microwave drying (MD): the hawthorn slices were dried in a microwave oven (WD 800G, Guangdong Galanz Enterprises Co., Ltd, China) with a maximum output of 800 W at 2450 MHz. The output power was set to 400 W, 640 W, 800 W for 19, 11 and 10 min, respectively, with a 5 s pause every 1 min. (3) Solar natural drying (SND): the slices were placed outdoors in sunshine and natural wind at temperatures ranging between 13 and 26 °C during September 29th to October 1st, 2016 in Zhengzhou. (4) Freeze drying (FD): the hawthorn slices were initially frozen at − 80 °C for 8 h. Then, the frozen samples were immediately transferred into a freeze-dryer (Alpha 1-2 LD plus, CHRIST, Germany) under a vacuum of 0.10 mbar with an ice condenser at − 42 °C. The freeze drying time was approximately 12 h to obtain the desired moisture. The drying continued until the samples reached a moisture content of < 12% as described in the Chinese Pharmacopoeia (2010 edn). Each treatment was conducted in triplicate, and each replicate contained 500 randomly selected hawthorn slices. All the experiments were performed in the dark except for the solar drying.
Moisture content
The moisture contents of the samples were calculated by the oven method at 70 °C under pressure ≤ 100 mm Hg until the weight was constant according to AOAC Official Method 934.06. During the drying process, approximately 20 slices of each replicate were removed at regular time intervals and then dried to a constant weight in the oven. In this study, the average initial moisture content of the fresh hawthorn slices was 69.58 ± 2.36 g/100 g fresh weight (FW).
Total phenolic, flavonoid and triterpenoid acid contents
Preparation of hawthorn extracts
The bioactive extracts of 30 hawthorn slices of fresh samples (RF) and dried samples were prepared using ultrasonication as described by Coklar et al. (2018) with slight modifications. Briefly, 2.0 g of ground hawthorn samples were submerged in 10 mL of aqueous methanol (methanol: water = 8:2 v/v) for 10 min at 40 °C in an ultrasonic bath. The mixture was centrifuged at 8000×g for 10 min, and the supernatants were recovered. This extraction was repeated twice, and the supernatants were pooled and evaporated at 40 °C to dry. The dry matter was dissolved in pure methanol to a final volume of 10 mL and stored at − 80 °C until analysis. All the steps were performed with protection from light.
Total phenolic content (TPC)
TPC was measured using the Folin–Ciocalteu method (Singleton and Rossi 1965). First, 0.5 mL of diluted methanol extract was mixed with 2.5 mL of Folin–Ciocalteu solvent (prediluted 50-fold with distilled water). The mixture was incubated at 50 °C for 5 min. Then, the test tube was cooled to room temperature, and 2.0 mL of sodium carbonate (75 g/L) was added. The absorbance was measured at 760 nm with a spectrophotometer (Specord 50, Analytic Jena, Germany) after standing for 30 min. Gallic acid was used as a standard (ranging from 0 to 100 μg/mL), and the result was expressed as g of gallic acid equivalent per 100 g of dry weight hawthorn (g GAE/100 g DW). The analytical data were given on a dry weight basis to compare hawthorn fruits with different moisture contents.
Total flavonoid content (TFC)
TFC in the hawthorn fruits were analysed with the colourimetric method described by Jia et al. (1999). Briefly, 1.0 mL of diluted hawthorn extract sample was reacted with 0.3 mL of 50 g/L sodium nitrite for 6 min. Then, 0.3 mL of 100 g/L aluminium nitrate was added and maintained for 6 min. Finally, 2.4 mL of 40 g/L sodium hydroxide was added. The absorbance was measured at 510 nm after a reaction time of 15 min. Rutin was used as a standard, and the TFC was reported as g rutin equivalent per 100 g of dry weight hawthorn (g RE/100 g DW).
Total triterpenoid acid content (TAC)
TAC was determined using the method of Fan and He (2006) with certain modifications. A sample of 1.0 mL of methanol extract was placed in a boiling water bath to evaporate the solvent. Then, 0.4 mL of vanillin/glacial acetic acid (5 g/L) and 1.6 mL of perchloric acid were added. The mixture was incubated at 70 °C for 15 min. After cooling in ice-water, 8.0 mL of ethyl acetate was added. The absorbance values were measured at 560 nm with a spectrophotometer. Oleanolic acid was used as a standard. The results were expressed as g oleanolic acid equivalent per 100 g dry weight hawthorn (g OAE/100 g DW).
HPLC analysis of phenolic compounds
To determine the phenolic compound contents of the hawthorn extracts, HPLC was performed according to the method reported by Liu et al. (2013). A HPLC system (Waters CO., USA) was equipped with a 2515 solvent delivery pump, a 2707 auto-sample injector, a thermostated column compartment, a 2998 diode-array detector and a C18 column (150 mm*4.6 mm, 5.0 μm, Waters Symmetry). The mobile phase consisted of methanol (A) and deionized water with phosphoric acid adjusted to pH 2.6 (B). The programme began with a linear gradient elution as follows: 0 min, 15% A, 85% B; 15 min, 15% A, 85% B; 25 min, 25% A, 75% B; 65 min, 60% A, 40% B; 67 min, 100% A; 73 min, 100% A; and 75 min, 15% A, 85% B. The flow rate was kept at 0.6 mL/min. The column was operated at a constant 30 °C, and the injection volume was 10 μL. Before the HPLC analysis, the hawthorn methanol extracts were filtered through a 0.45 μm membrane filter. The photodiode array spectra were measured over the wavelength range of 210–400 nm with a resolution of 1.2 nm. The phenols were monitored at 280 nm and 320 nm. The identification of the phenolic compounds in the hawthorn fruit was achieved by comparing the retention times and the UV-absorption spectra with those of phenolic standards. The quantification of the phenolic compounds (gallic acid, protocatechuic acid, catechinic acid, chlorogenic acid, caffeic acid, epicatechin, ferulic acid, rutin, ellagic acid, phlorizin, cinnamic acid, and quercetin) was carried out by an external standard method using calibration curves, ranging from 5 to 200 mg/L. The results were expressed as mg/100 g DW.
Antioxidant activity of hawthorn extracts
1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity
The scavenging activity of DPPH free radicals was assessed in the hawthorn extracts according to reported methods (Brand-Williams et al. 1995). Diluted extract (0.1 mL) was added to 2.4 mL of DPPH solution (0.1 mmol/L). After allowing the mixture to stand in the dark for 30 min, the absorbance was measured at 517 nm. The standard calibration curve was plotted using 0.01–1.20 mmol/L Trolox (R2 = 0.9994), and the results were expressed as mmol Trolox equivalent of dry weight hawthorn (mmol TE/100 g DW) in terms of Trolox equivalent antioxidant capacity (TEAC).
Ferric reducing antioxidant power (FRAP)
FRAP assay of the hawthorn extracts was based on the methods of Benzie and Strain (1996). Briefly, FRAP working solution comprising 10 mmol/L 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ) dissolved in 40 mmol/L HCl, 20 mmol/L FeCl3·6H2O and 300 mmol/L acetate buffer (pH 3.6) at a ratio of 1:1:25. The sample extract (0.1 mL) was mixed with 2.9 mL of FRAP reagent. Then, the mixture was incubated at 37 °C for 5 min, and the absorbance was measured at 593 nm. The standard calibration curve was plotted using 0.005–0.4 mmol/L Trolox (R2 = 0.9998). The results were expressed as mmol Trolox equivalent of dry weight hawthorn (mmol TE/100 g DW) for TEAC.
Statistical analysis
All the experiments were conducted three independent times, and the data were expressed as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was carried out by Duncan’s multiple range test to determine the significant differences (P < 0.05) by SAS 9.2. Pearson’s correlation coefficients were calculated to estimate the correlations between the total polyphenols, flavonoids, triterpenoid acids, phenolic compounds and antioxidant activities by SPSS 13.0 (P < 0.05).
Results and discussion
Moisture content
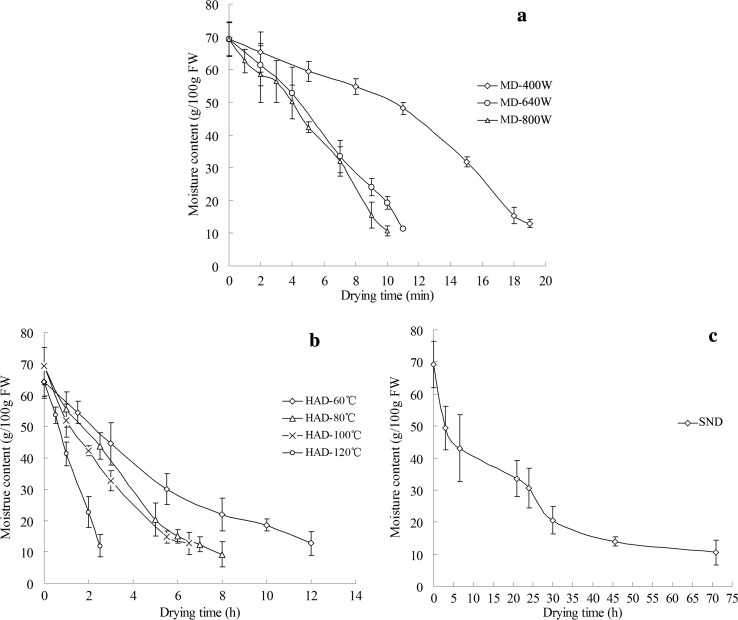
The modifications of the moisture contents of the hawthorn fruit slices during dehydration by hot air drying (HAD), microwave drying (MW) and solar natural drying (SND) are presented in Fig. 1. In this study, we used fixed parameters for the freeze drying, and the moisture changes were not investigated. The time costs for reducing the moisture contents of the hawthorn slices to lower than 12% in ascending order were microwave drying, hot air drying, freeze drying, and solar natural drying. The hawthorn slices that were microwave dried at 800 W yielded the shortest time (10 min) to a final moisture content of 11.93% (Fig. 1a, MW-800 W), while the time was prolonged to approximately three days for solar natural drying (Fig. 1c). Convective hot air drying (Fig. 1b) took 2.5–12 h. Microwaves can directly penetrate the samples and heat the entire sample in a fast and uniform manner, leading to rapid evaporation of water and creating an outward flux of rapidly escaping vapor (Hu et al. 2006). For the conventional solar drying and hot air drying methods, the heat was transferred from the surface to the interior of the materials and required longer time. The outer layer of the product dried first and formed a poor heat conductor that prevented the dehydration process.
Fig. 1.
Changes of the moisture contents during dehydration of hawthorn slices. a Microwave drying, b hot air drying, c solar natural drying. HAD hot air drying, MD microwave drying, SND solar natural drying. Data are the mean of triplicates; vertical bars indicate standard error
As shown in Fig. 1a, microwave drying treatment with high power resulted in faster moisture loss and shorter dehydration time. The drying time was shortened from 19 min to 10 min as the microwave power increased from 400 W (MW-400 W) to 800 W (MW-800 W). Similar results were obtained in several research studies on other fruit dehydration processes (Pereira et al. 2007; Wojdyło et al. 2009; Chaikham et al. 2013). The internal heat quickly generated by a higher microwave energy resulted in a fast increase in the material temperature and a large vapor difference between the centre and surface of the product, leading to faster moisture removal (Pereira et al. 2007). Though microwave drying at high power conditions took less time to decrease the moisture, this method was not satisfactory for hawthorn slice dehydration processing. In the present work, when the moisture content was approximately 35%, the outer pericarp of the hawthorn slices showed brown speckling after microwave drying treatment at 800 W. The same phenomenon was observed in banana slices dried by microwave when the moisture content reached 0.67 kg moisture/kg dry matter (Pereira et al. 2007).
The drying time decreased as the hot air temperature increased, as shown in Fig. 1b (r = − 0.986, P < 0.05). The drying process required approximately 12 h at 60 °C compared with 2.5 h at 120 °C. Temperatures ranging from 50 to 90 °C have been commonly reported for convective air drying of other berries (Alfaro et al. 2014; López et al. 2010). However, in Chinese traditional medicine preparations, hawthorn fruits have been processed at higher temperatures (even those higher than 200 °C). The fried hawthorn fruits were considered to be good for the stomach, reducing the raw acidity and helping assimilation. Thus, the effects of hot air drying at 100 and 120 °C on bioactive compounds were investigated in this study. The hawthorn slices were found to be scorched when dried at 120 °C. The fast hot air transport made the sample outer layer immediately shrink and harden, which prevented the evaporation of water from the sealed inner space. A high temperature with a long drying time damaged appearance of the sample.
The solar drying (Fig. 1c) took very long time in the present research, though this method saves energy and is environmentally friendly. This method takes several days to dry foods outdoors and would be unsafe. The cool night air could condense and add moisture to the food and slow the drying process.
TPC, TFC and TAC
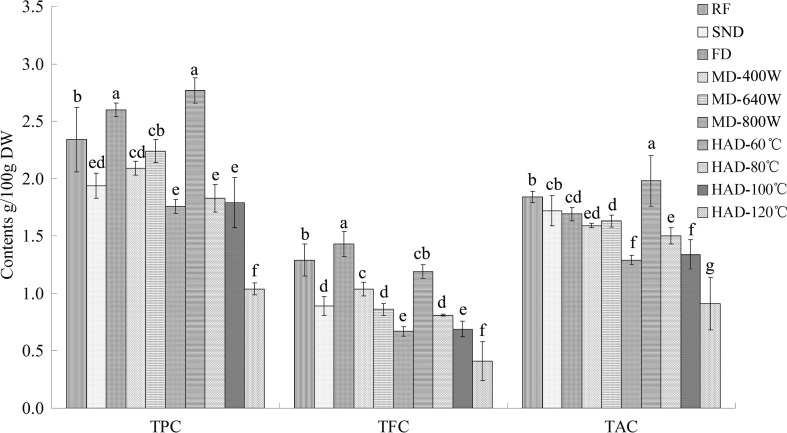
Polyphenols are a major group of phytochemicals ubiquitously distributed in all higher plants that are subclassified into two main groups: the phenolics and the flavonoids (Singh et al. 2016). The results of the total phenolic contents, flavonoid contents and triterpenoid acid contents of the fresh and dried hawthorn slices are summarized in Fig. 2. The drying process significantly affected the bioactive compounds of the hawthorn slices. According to the results, different drying techniques affected different bioactive compounds, which is similar to results reported for other fruits and medicinal plants (Hsu et al. 2003; Komes et al. 2011). The hawthorn slices dried by hot air at 60 °C possessed the highest contents of polyphenols (2.77 g GAE/100 g DW) and triterpenoid acids (1.98 g OE/100 g DW). The highest content of total flavonoids (1.43 g/100 g DW) was found in the freeze-dried hawthorn slices.
Fig. 2.
TPC, TFC, TAC of fresh and dried hawthorn fruit slices. TPC total phenol contents (g GAE/100 g DW), TFC total flavonoid contents (g RE/100 g DW), TAC total triterpenoid acid contents (g OAE/100 g DW), RF raw fresh fruit, HAD hot air drying, FD freeze drying, MD microwave drying, SND solar natural drying. Data are the mean of triplicates; vertical bars indicate standard error. Different letters within each bar indicate a significant difference (P < 0.05)
As shown in Fig. 2, the freeze-dried hawthorn slices had higher contents of polyphenols and flavonoids than the fresh hawthorn fruits. Several research studies found that freeze-dried samples contained significantly higher polyphenol contents than fresh, hot air-dried (65 °C and 80 °C) and microwave-dried samples (Alfaro et al. 2014; Komes et al. 2011). The increase in the antioxidant compounds in freeze-dried hawthorns could be ascribed to the modification and chemical degradation of the cell wall structure associated with freezing and subsequent sublimation. The disruption of the cell walls was concomitant with the breakdown of insoluble bound phenolics, increasing the extractability of these compounds (Alfaro et al. 2014). Meanwhile, the freezing and vacuum conditions prevented the oxidation and thermal degradation of the phenolics during the dehydration of the hawthorn slices. However, the use of freeze-drying technology is still limited, as the equipment is expensive, and the drying time is usually dozens of hours.
The TPC and TAC of the hawthorn slices dried by hot air at 60 °C were significantly (P < 0.05) higher than those of the raw hawthorn fruit (Fig. 2). Chang et al. (2006) found that the total phenolic content in hot air-dried tomatoes was significantly higher than that in fresh samples. The phenolic compounds are located in the vacuoles and are separated from the oxidative enzymes in fresh fruit. Dehydration processing destroys the structure and results in the release of more phenolic compounds, which facilitates the extraction of polyphenols (Toor and Savage 2006). Dehydration processing by hot air also induces the expression of the polyphenol pathway gene, resulting in a significant increase in total polyphenols during grape dehydration (Sanctis et al. 2012). Another possibility is that enzymes protective against oxidation are simulated by moderate heat treatments (Vicente et al. 2006).
On the other hand, the TPC, TFC and TAC decreased significantly as the hot air-drying temperature increased from 60 °C to 120 °C, as shown in Fig. 2 (r = − 0.953 to − 0.984, P < 0.05). The oxidative and thermal degradation of phenolic compounds accelerates with increasing heat intensity (Wojdyło et al. 2009). The remaining TPC of the hawthorn slices dried at 80, 100 and 120 °C decreased to 1.83, 1.79 and 1.04 g GAE/100 g DW, respectively. Hot air was reported to induce a decrease of total polyphenols in strawberry dehydration processing (Wojdyło et al. 2009). The decrease of total polyphenols at higher temperature may be due to the thermal degradation of these heat sensitive compounds and the generation of smaller molecules, which affects the aromatic ring activity and the measurement of polyphenols by the Folin–Ciocalteu method (Alfaro et al. 2014).
Poor preservation of TPC, TFC and TAC was found in the microwave-dried hawthorn slices (Fig. 2) compared with those of the fresh, freeze-dried and 60 °C hot air-dried samples. These results differed from the research of Wojdyło et al. (2009), who reported that vacuum-microwave drying could inactivate the oxidative and hydrolytic enzymes and protect the phenols from further decomposition. This difference might be due to oxidation protection in vacuum conditions. In addition, the TPC, TFC and TAC of the hawthorns dried by microwave at higher power (800 W) were 1.76 g GAE/100 g DW, 0.67 g GE/100 g DW and 1.29 g OAE/100 g DW, respectively, which were significantly lower (P < 0.05) than those of microwave drying at 400 W (2.09 g GAE/100 g DW, 1.04 g GE/100 g DW, and 1.59 g OAE/100 g DW, respectively). These results indicate that certain phenolic compounds may degrade under high-power microwave treatment. During the dehydration of longan, the total phenolic contents were found to decrease with increasing microwave power (Chaikham et al. 2013). Combined with the phenomenon of brown speckling under these conditions, the quantitative results indicated that drying at higher microwave power (800 W) had certain negative effects on the hawthorn slices.
TFC losses (7.75–68.22%) were higher than the TPC losses (4.27–55.55%) in samples after dehydration by solar drying, microwave drying and hot air drying (except at 60 °C) than in raw fresh samples, indicating that flavonoids were more sensitive to the dehydration conditions than other phenolic components. The loss of flavonoid compounds might be due to the harsh drying conditions, in particular, the temperature and duration used (Baslar et al. 2014). The freeze dried samples possessed significantly higher (P < 0.05) TFC compared with the fresh samples and samples dried by other technologies. Certain biotic and abiotic stress factors such as low temperature, wounding and pathogen attacks may trigger the synthesis of phenylpropanoids such as flavonoids, isoflavonoids and phenolic acids (Dixon and Paiva 1995). The hawthorn slices, in response to the stress of moisture loss, may form flavonoids at cold temperature. In addition, more flavonoids were preserved by hot air drying at lower temperature (60 °C) and at lower microwave power (400 W) than by the other conditions in this study.
Triterpenic compounds are a group of nonphenolic active components in hawthorn fruits that are reported to possess pharmacological properties (Cui et al. 2006). However, there is relatively little information and research about the modifications of these compounds during fruit processing compared with the abundant studies on polyphenols and flavonoids. As shown in Fig. 2, the highest contents of triterpenoid acids were observed in hot air drying at 60 °C (1.98 g OAE/100 g DW), and the lowest were observed in the 120 °C hot air-dried hawthorns (0.91 g OAE/100 g DW). The TAC decreased with increasing temperature (r = − 0.984, P < 0.05). These results were consistent with those of TPC and TFC. The microwave drying treatments could not properly preserve the triterpenic acids, as the values ranged from 1.63 to 1.29 g OAE/100 g DW, which was significantly lower (P < 0.05) than that of the raw fresh hawthorn slices.
Phenolic compound profile
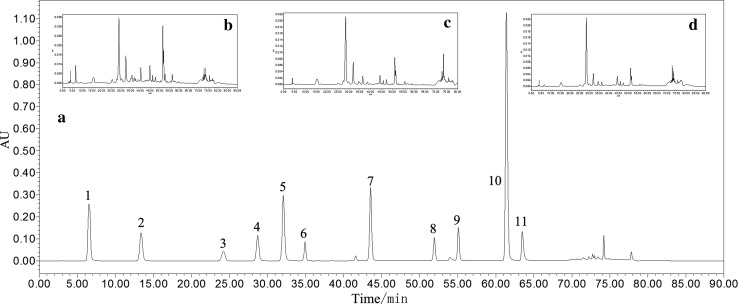
Figure 3 shows the HPLC chromatograms of the phenolic standards (Fig. 3a), 80% methanol extracts of freeze-dried hawthorn slices (Fig. 3b), samples hot air-dried at 60 °C (Fig. 3c) and samples microwave-dried at 800 W (Fig. 3d) at a wavelength of 280 nm. A total of 5 peaks were identified and quantified in this research, including chlorogenic acid, epicatechin, rutin, ellagic acid and protocatechuic acid, by comparing with the retention time and UV spectrogram of the standards by a DAD detector. Further studies (HPLC–MS and other analysis techniques) are needed to identify other phenolic components.
Fig. 3.
HPLC profiles of phenolic compounds of standards and hawthorn slices detected at 280 nm. a HPLC chromatogram of phenolic standards with peaks noted as 1: gallic acid; 2: protocatechuic acid; 3: catechinic acid; 4: chlorogenic acid; 5: caffeic acid; 6: epicatechin; 7: ferulic acid; 8: rutin; 9: ellagic acid; 10: phlorizin; 11: cinnamic acid; 12: quercetin. b, c and d, HPLC chromatograms of phenolic acids in hawthorn slices dehydrated by freeze drying, hot air drying at 60 °C and microwave drying at 800 W, respectively
The quantitative results listed in Table 1 show that epicatechin was the most abundant phenolic compound identified, which agreed with the results of Li et al. (2013), followed by chlorogenic acid and rutin. Compared with the fresh samples (547.06 mg/100 g DW), the epicatechin contents in all the hawthorns dried by hot air treatments significantly decreased (P < 0.05), indicating that this component is sensitive to thermal treatment. On the other hand, the ellagic acid and rutin contents of the hot air-dried hawthorns were higher than those of the fresh fruit. The degradation of certain phenolic compounds during dehydration processing might contribute to the formation of ellagic acid and rutin.
Table 1.
Contents of phenolic compounds (mg/100 g DW) of fresh and dried hawthorn slices
| Treatment conditions | Chlorogenic acid | Epicatechin | Rutin | Ellagic acid | Protocatechuic acid |
|---|---|---|---|---|---|
| RF | 354.99 ± 41.42b | 547.06 ± 10.05a | 106.10 ± 6.57e | 12.02 ± 1.62d | 42.34 ± 6.78cb |
| SND | 348.28 ± 30.66b | 416.64 ± 15.96c | 107.60 ± 6.77e | 22.01 ± 1.62bc | 54.42 ± 3.67a |
| FD | 482.14 ± 6.30a | 517.00 ± 18.09b | 143.18 ± 7.04cb | 21.41 ± 1.01bc | 32.13 ± 1.95d |
| MW-400 W | 368.76 ± 42.35b | 564.83 ± 14.29a | 141.13 ± 16.50cb | 9.31 ± 0.88de | 38.82 ± 4.03c |
| MW-640 W | 295.29 ± 10.58c | 444.61 ± 22.28c | 150.86 ± 1.78b | 19.54 ± 1.96c | 59.23 ± 3.84a |
| MW-800 W | 284.77 ± 6.31c | 365.90 ± 20.36d | 123.59 ± 4.19d | 7.13 ± 1.34e | 57.14 ± 1.71a |
| HAD-60 °C | 368.64 ± 7.64b | 417.79 ± 15.49c | 187.14 ± 10.15a | 39.85 ± 3.32a | 45.68 ± 2.82b |
| HAD-80 °C | 346.53 ± 21.86b | 376.98 ± 7.66d | 124.17 ± 9.96d | 23.33 ± 3.01b | 53.25 ± 1.06a |
| HAD-100 °C | 253.44 ± 41.60c | 276.19 ± 18.51f | 132.40 ± 5.40 cd | 20.15 ± 1.49cb | 39.85 ± 3.44cb |
| HAD-120 °C | 296.66 ± 9.41c | 320.20 ± 15.93e | 142.69 ± 4.05cb | 22.14 ± 1.54cb | 37.66 ± 2.03dc |
RF raw fresh fruit, HAD hot air drying, FD freeze drying, MW microwave drying, SND solar natural drying
Reported values were expressed as mean ± SD (n = 3). Different letters within each bar mean a statistical difference (P < 0.05)
The highest contents of chlorogenic acid (482.14 mg/100 g DW) were found in the freeze-dried hawthorns. These results were similar to those reported by Coklar et al. (2018) in freeze-dried hawthorn fruits. Hung and Duy (2012) reported that the amounts of certain free phenols in freeze-dried vegetables were significantly higher than those in hot air-dried samples. Methanol extraction was not able to extract the bound phenolic esters linked to fibre, which represented a large fraction of the antioxidants (Fares et al. 2010). However, after the drying process, the cell wall microstructure was destroyed, and the liberation of bound phenolic compounds might be accelerated due to the breakdown of the cellular constituents (Wojdyło et al. 2009). Moreover, depolymerization could promote the conversion of proanthocyanidins into elementary units in fruit (Wojdyło et al. 2009). The chlorogenic acids were intermediary catabolites of anthocyanin degradation from temperature and enzymatic activity (Piga et al. 2003).
However, epicatechin and chlorogenic acid, which represented the major phenolic acids in the hawthorns, showed similar decreasing tendencies (r = − 0.942 to − 1.000, P < 0.05) with increasing temperature from 60 to 100 °C for hot air drying and with increasing power for microwave drying. These results indicate that high temperature and high microwave power treatments may lead to degradation of chlorogenic acid and epicatechin. Similar results were reported in oven dried apples (Joshi et al. 2011) and microwave dried sour cherries (Wojdyło et al. 2014). The chlorogenic acid content in the hawthorn slices dried by hot air at 100 °C was only 68.75% of that dried at 60 °C. For microwave drying, the chlorogenic acid content decreased 22.78% when the microwave power increased from 400 to 800 W. The epicatechin content of the hawthorn slices dried at 100 °C was 33.89% lower than those dried at 60 °C and decreased by 35.22% when the microwave power increased from 400 to 800 W. The losses of phenolic compounds could be affected by the temperature and time used in the drying techniques (Mohd-Zainol et al. 2009).
Antioxidant properties
DPPH radical scavenging activity
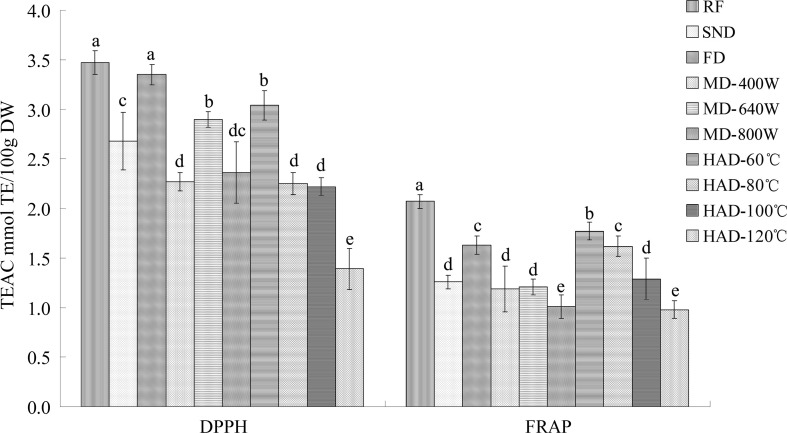
As shown in Fig. 4, the DPPH scavenging capacity of the fresh hawthorn slices was 3.48 mmol TE/100 g DW, which was significantly higher (P < 0.05) than that of the dried hawthorn slices except for the freeze-dried samples. The results were similar to those in freeze-dried strawberries (Wojdyło et al. 2009). The vacuum conditions may allow water to be evaporated at low temperature and prevent oxidation of the antioxidant components.
Fig. 4.
TEAC of fresh and dried hawthorn fruit slices measured by DPPH and FRAP assays. TEAC trolox equivalent antioxidant capacity, DPPH 1,1-diphenyl-2-picrylhydrazyl, FRAP ferric reducing antioxidant power, RF raw fresh fruit, HAD hot air drying, FD freeze drying, MD microwave drying, SND solar natural drying. Data are the mean of triplicates; vertical bars indicate standard error. Different letters within each bar indicate a significant difference (P < 0.05)
The DPPH scavenging activity was significantly correlated with the total polyphenol, flavonoid, and triterpenoid acid contents (P < 0.01) and the epicatechin contents, as shown in the correlation analysis results (Table 2). Meanwhile, the lowest TEAC by DPPH assay was found in the samples hot air-dried at 120 °C (accounting for 40.03% of the original fresh materials), which also possessed the lowest epicatechin content. As shown in Table 2, the correlation between the epicatechin content and the DPPH scavenging activity was significant (P < 0.05). The TEAC of the hawthorn slices dried by hot air at 80 °C and 100 °C showed no significant differences (P > 0.05), but both were lower than that of the hawthorns dried at 60 °C (P < 0.05). The results were consistent with the changes in the total polyphenol (Fig. 2), rutin and ellagic acid (Table 2) contents. The TEAC of the microwave dried hawthorn slices measured by DPPH assay ranged from 2.27 to 2.36 mmol TE/100 g DW. There were no significant differences (P > 0.05) between the 400 W and 800 W microwave dried samples, which were both lower than 640 W. The results were in agreement with that of the ellagic acid content (Table 1). The DPPH scavenging activity of the hawthorns was affected by these components as reported by other research studies (Li et al. 2013; Coklar et al. 2018).
Table 2.
Pearson’s correlation analysis of antioxidant compounds and activities for fresh and dried hawthorn slices
| TPC | TFC | TAC | CA | EP | RT | EA | PA | DPPH | FRAP | |
|---|---|---|---|---|---|---|---|---|---|---|
| TPC | 1 | |||||||||
| TFC | 0.908** | 1 | ||||||||
| TAC | 0.926** | 0.859** | 1 | |||||||
| CA | 0.622 | 0.819* | 0.545 | 1 | ||||||
| EP | 0.622 | 0.796* | 0.632* | 0.695* | 1 | |||||
| RT | 0.373 | 0.134 | 0.167 | 0.142 | − 0.048 | 1 | ||||
| EA | 0.315 | 0.143 | 0.316 | 0.191 | − 0.273 | 0.659* | 1 | |||
| PA | − 0.057 | − 0.311 | 0.093 | − 0.454 | − 0.218 | − 0.174 | − 0.061 | 1 | ||
| DPPH | 0.897** | 0.895** | 0.875** | 0.576 | 0.642* | 0.019 | 0.109 | 0.011 | 1 | |
| FRAP | 0.685* | 0.774** | 0.739* | 0.520 | 0.463 | 0.007 | 0.319 | 0.221 | 0.746* | 1 |
TPC total phenol contents, TFC total flavoniod contents, TAC total triterpenoid acid contents, CA chlorogenic acid, EP epicatechin, RT rutin, EA ellagic acid, PA protocatechuic acid, DPPH 1,1-diphenyl-2-picrylhydrazyl, FRAP ferric reducing antioxidant power
**Correlation is significant at the 0.01 level; *correlation is significant at the 0.05 level
Ferric reducing antioxidant power
The antioxidant activities of the dehydrated and fresh hawthorn fruits measured by FRAP assay were different from those of the DPPH assay (Fig. 4). The results of the correlations between the FRAP and the antioxidants (Table 2) showed that the FRAP was significantly correlated with the total polyphenols, flavonoids and triterpenoid acids (P < 0.05) but had no significant relationship with the individual phenolic compounds (P > 0.05). In addition, all the dried hawthorn slices showed significantly lower FRAP values than the fresh materials (2.07 mmol TE/100 g DW). No significant difference was found between the samples microwave dried at 400 W and 640 W (P > 0.05), which both were higher than that dried at 800 W (P < 0.05). There were differences between the FRAP of the hawthorns dried by hot air at 80 °C and 100 °C (P < 0.05). As shown in Fig. 4, the antioxidant activities of different treatments determined by DPPH method were all higher than those measured by FRAP method, indicating that the antioxidant activity depend not only on the chemical structure of antioxidants but also strongly on the model system employed. The varying responses of different antioxidant activity assays for fruits and vegetables had also been observed by Singh et al. (2016).
The hawthorns dried by hot air at 60 °C possessed higher TEAC by FRAP assay (1.77 mmol TE/100 g DW) than the other drying methods. These results might be due to the significantly higher total polyphenol, triterpenoid acid, rutin and ellagic acid contents in the hawthorns dried at 60 °C, as shown in Fig. 2 and Table 1. In addition, stable and mild temperatures may result in the formation of certain new compounds such as Maillard reaction products with antioxidant power by a chain breaking-type mechanism (Manzocco et al. 2011). Similar results have been observed during the dehydration of strawberries dried at 70 °C (Wojdyło et al. 2009) and prunes dried at 85–90 °C (Piga et al. 2003). Proper temperature in the hot air-drying process deactivates the oxidative and hydrolytic enzymes and prevents antioxidant actions (Wojdyło et al. 2009).
However, the ferric reducing antioxidant power and DPPH radical scavenging ability of the hot air-dried hawthorn slices were all lower than those of the fresh materials. The increase in antioxidant activities resulting from the Maillard reaction could not compensate for the destruction of certain phenolics and vitamins at high temperature (Wojdyło et al. 2009; Piga et al. 2003). As Saadatian et al. (2016) found, the vitamin C content in dehydrated hawthorn fruits decreased. As shown in Table 1, the major phenolic compound of hawthorn fruits, epicatechin, was significantly higher in the fresh flesh than in the hot air-dried samples (P < 0.05). Considering the decrease of individual polyphenolic compounds in hot air-drying processes, the reduction in antioxidant activities could be due to the changes in the phenolic compounds (Coklar et al. 2018). As reported by Toor and Savage (2006), the antioxidant activities of semidry tomatoes decreased by 28–38% compared with those of fresh tomatoes due to the destruction of ascorbic acid and phenolic compounds.
The correlations of the antioxidant activities with the identified phenolic acids were not significant, as shown in Table 2 (P > 0.05), except for that between epicatechin and DPPH scavenging ability (P < 0.05). However, significant correlations could be observed among TPC, TFC, DPPH and FRAP antioxidant activities (Table 2). These results suggested that the antioxidant abilities in the hawthorn fruits could be due to the combined reactions of the total phenolics but not certain individual phenolic components. Previous researches also revealed the positive relations amongst TPC, TFC and antioxidant activities in fruits and vegetables (Singh et al. 2016). But the connections between the antioxidants and antioxidant capacities were still uncertain based only on the quantitative results (López et al. 2010). The antioxidant abilities could be influenced by not only the level of antioxidants but also a synergy with the other constituents (Capecka et al. 2005). In further research, other phenolics, vitamins and triterpene components should be identified and quantified to determine their correlations with the antioxidant activities of hawthorn fruits.
Conclusion
The bioactive components and antioxidant activities of hawthorn slices dehydrated by different drying techniques were evaluated. Microwave drying drastically shortened the dehydration time to 10 min, though the product quality was poor. The bioactive components of phenolics and flavonoids were effectively preserved by the freeze-drying technology. The hawthorn slices dehydrated by hot air drying at 60 °C possessed higher contents of polyphenols (2.77 GAE g/100 g DW), triterpenoid acids (1.98 OAE g/100 g DW) and antioxidant activities (3.04 mmol TE/100 g DW for DPPH assay and 1.77 mmol TE/100 g DW for FRAP assay) than those of other drying methods, making hot air drying at this temperature a potentially desirable method for hawthorn slice dehydration. Chlorogenic acid and epicatechin were the major phenolic components detected in the hawthorn fruit slices. More studies are needed to identify other bioactive compositions and their contributions to the antioxidant activities.
Acknowledgements
This work was supported by the Agricultural Science and Technology Innovation Program (ASTIP) of Chinese Academy of Agricultural Sciences(CAAS-ASTIP-ZFRI) and Science and Technique Program of Henan Province (122300410125).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alfaro S, Mutis A, Quiroz A, Seguel I, Scheuermann E. Effects of drying techniques on murtilla fruit polyphenols and antioxidant activity. J Food Res. 2014;3(5):73–82. [Google Scholar]
- Aral S, Beşe AV. Convective drying of hawthorn fruit (Crataegus spp.). Effect of experimental parameters on drying kinetics, color, shrinkage, and rehydration capacity. Food Chem. 2016;210:577–584. doi: 10.1016/j.foodchem.2016.04.128. [DOI] [PubMed] [Google Scholar]
- Baslar M, Karasu S, Kilicli M, Us AA, Sagdic O. Degradation kinetics of bioactive compounds and antioxidant activity of pomegranate arils during the drying process. Int J Food Eng. 2014;10(4):839–848. [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–78. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28(1):25–30. [Google Scholar]
- Capecka E, Mareczek A, Leja M. Antioxidant activity of fresh and dry herbs of some Lamiaceae species. Food Chem. 2005;93(2):223–226. [Google Scholar]
- Chaikham P, Kreungngern D, Apichartsrangkoon A. Combined microwave and hot air convective dehydration on physical and biochemical qualities of dried longan flesh. Int Food Res J. 2013;20(5):2145–2151. [Google Scholar]
- Chang CH, Lin HY, Chang CY, Liu YC. Comparisons on the antioxidant properties of fresh, freeze-dried and hot-air-dried tomatoes. J Food Eng. 2006;77(3):478–485. [Google Scholar]
- Coklar H, Akbulut M, Kilinc S, Yildirim A, Alhassan I. Effect of freeze, oven and microwave pretreated oven drying on color, browning index, phenolic compounds and antioxidant activity of hawthorn (Crataegus orientalis) fruit. Not Bot Horti Agrobot. 2018;46(2):449–456. [Google Scholar]
- Cui T, Li JZ, Kayahara H, Ma L, Wu LX, Nakamura K. Quantification of the polyphenols and triterpene acids in Chinese hawthorn fruit by high-performance liquid chromatography. J Agric Food Chem. 2006;54:4574–4581. doi: 10.1021/jf060310m. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Paiva NL. Stress-induced phenylpropanoid metabolism. Plant Cell. 1995;7(7):1085–1097. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan JP, He CH. Simultaneous quantification of three major bioactive triterpene acids in the leaves of Diospyros kaki by high-performance liquid chromatography method. J Pharm Biomed Anal. 2006;41:950–956. doi: 10.1016/j.jpba.2006.01.044. [DOI] [PubMed] [Google Scholar]
- Fares C, Platani C, Baiano A, Menga V. Effect of processing and cooking on phenolic acid profile and antioxidant capacity of drum wheat pasta enriched with debranning fractions of wheat. Food Chem. 2010;119(3):1023–1029. [Google Scholar]
- Hsu CL, Chen WL, Weng YM, Tseng CY. Chemical composition, physical properties, and antioxidant activities of yam flours as affected by different drying methods. Food Chem. 2003;83:85–92. [Google Scholar]
- Hu Q, Zhang M, Mujumdar AS, Xiao G, Sun J. Drying of edamames by hot air and vacuum microwave combination. J Food Eng. 2006;77(4):977–982. [Google Scholar]
- Hung PV, Duy TL. Effects of drying methods on bioactive compounds of vegetables and correlation between bioactive compounds and their antioxidants. Int Food Res. 2012;J19(1):327–332. [Google Scholar]
- Jia Z, Tang M, Wu J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. [Google Scholar]
- Joshi A, Rupasinghe H, Khanizadeh S. Impact of drying processes on bioactive phenolics, vitamin C and antioxidant capacity of red-fleshed apple slices. J Food Process Preserv. 2011;35(4):453–457. [Google Scholar]
- Komes D, Belščak-Cvitanović A, Horžić D, Marković K, Konačević GK (2011) Characterisation of pigments and antioxidant properties of three medicinal plants dried under different drying conditions. In: Proceedings of the 11th international congress on engineering and food. Atena, Grčka, vol 05, pp 22–26
- Kwok CY, Li C, Cheng HL, Ng YF, Chan TY, Kwan YW, Leung GPH, Lee SMY, Mok DKW, Yu PHF, Chan SW. Cholesterol lowering and vascular protective effects of ethanolic extract of dried fruit of Crataegus pinnatifida, hawthorn (Shan Zha), in diet-induced hypercholesterolaemic rat model. J Funct Foods. 2013;5(3):1326–1335. [Google Scholar]
- Li T, Zhu J, Guo L, Shi X, Liu Y, Yang X. Different effects of polyphenols-enriched extracts from hawthorn fruit peels and fleshes on cell cycle and apoptosis in human MCF-7 breast carcinoma cells. Food Chem. 2013;141(2):1008–1018. doi: 10.1016/j.foodchem.2013.04.050. [DOI] [PubMed] [Google Scholar]
- Liu H, Jiao ZG, Liu JC, Zhang CL, Zheng XW, Lai SJ, Chen FS, Yang HS. Optimization of supercritical fluid extraction of phenolics from date seeds and characterization of its antioxidant activity. Food Anal Method. 2013;6(3):781–788. [Google Scholar]
- López J, Uribe E, Vega-Gálvez A, Miranda M, Vergara J, González E, Di Scala K. Effect of air temperature on drying kinetics, vitamin c, antioxidant activity, total phenolic content, non-enzymatic browning and firmness of blueberries variety O’Neil. Food Bioprocess Tech. 2010;3(5):772–777. [Google Scholar]
- Manzocco L, Calligaris S, Mastrocola D, Nicoli MC, Lerici CR. Review of non-enzymatic browning and antioxidant capacity in processed foods. Trends Food Sci Tech. 2011;11(9–10):340–346. [Google Scholar]
- Mohd-Zainol M, Abdul-Hamid A, Abu-Bakar F, Pak-Dek S. Effect of different drying methods on the degradation of selected flavonoids in Centella asiatica. Int Food Res J. 2009;16:531–537. [Google Scholar]
- Pereira NR, Marsaioli A, Ahrné LM. Effect of microwave power, air velocity and temperature on the final drying osmotically dehydrated bananas. J Food Eng. 2007;81(1):79–87. [Google Scholar]
- Piga A, Del Caro A, Corda G. From plums to prunes: influence of drying parameters on polyphenols and antioxidant activity. J Agric Food Chem. 2003;51(12):3675–3681. doi: 10.1021/jf021207+. [DOI] [PubMed] [Google Scholar]
- Saadatian M, Najda A, Jasour MS. Drying process affects bioactive compounds in hawthorn species. Acta Sci Pol Hortorum Cultus. 2016;15(4):3–16. [Google Scholar]
- Sanctis FD, Silvestrini MG, Luneia R, Botondi R, Bellincontro A, Mencarelli F. Postharvest dehydration of wine white grapes to increase genistein, daidzein and the main carotenoids. Food Chem. 2012;135(3):1619–1625. doi: 10.1016/j.foodchem.2012.05.092. [DOI] [PubMed] [Google Scholar]
- Singh JP, Kaur A, Shevkani K, Singh N. Composition, bioactive compounds and antioxidant activity of common Indian fruits and vegetables. J Food Sci Technol. 2016;53(11):4056–4066. doi: 10.1007/s13197-016-2412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton V, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-photungstic acid reagents. Am J Enol Vitic. 1965;16(3):144–158. [Google Scholar]
- Toor RK, Savage GP. Effect of semi-drying on the antioxidant components of tomatoes. Food Chem. 2006;94(1):90–97. [Google Scholar]
- Vicente AR, Martínez GA, Chaves AR, Civello PM. Effect of heat treatment on strawberry fruit damage and oxidative metabolism during storage. Postharvest Biol Technol. 2006;40(2):116–120. [Google Scholar]
- Wojdyło A, Figiel A, Oszmiański J. Effect of drying methods with the application of vacuum microwaves on the bioactive compounds, color and antioxidant activity of strawberry fruits. J Agric Food Chem. 2009;57(4):1337–1343. doi: 10.1021/jf802507j. [DOI] [PubMed] [Google Scholar]
- Wojdyło A, Figiel A, Lech K, Nowicka P, Oszmiański J. Effect of convective and vacuum-microwave drying on the bioactive compounds, color, and antioxidant capacity of sour cherries. Food Bioprocess Technol. 2014;7(3):829–841. [Google Scholar]
- Zhang HK, Guo CD, Gao GB, Huang YY, Gao YJ, Hao TF. Processing technology study of Crataegi Fructus charcoal based on pilot-scale level and multi-component quantification by HPLC. Chin J Mod Appl Pharm. 2017;34(10):1413–1417. [Google Scholar]
- Zhong MF. Impact analysis of the processing against organic acid content in hawthorn. Clin J Chin Med. 2012;4(14):27–28. [Google Scholar]