Abstract
The present study aimed to differentiate Mimosa scabrella Bentham (bracatinga) honeydew honeys from blossom honeys, with and without addition of heat treatment, and bracatinga honeydew honeys adulterated with blossom honeys (5, 15 and 25% of blossom honeys), using chromatic characterization associated with chemometric analysis. Bracatinga honeydew honeys presented unusual chromatic characteristics which allowed differentiation of blossom honeys by principal components analysis. Additionally, a classification model was developed in order to establish clear rules that characterize each group of honey. The proposed model correctly classified bracatinga honeydew honey and blossom honey samples, with and without heat treatment. Only two samples adulterated with 5% blossom honey were misclassified. The chromatic analysis associated with chemometric analysis showed promising perspectives for its exploitation being able to be used for screening and selection of bracatinga honeydew honey, fresh or thermally treated as well as fraud detection.
Electronic supplementary material
The online version of this article (10.1007/s13197-019-03737-2) contains supplementary material, which is available to authorized users.
Keywords: Data-mining C&RT, Classification and regression tree, Principal component analysis, Floral honey, Botanical discrimination, CIELAB
Introduction
Honey is a sweet substance produced by bees (Apis mellifera) from the nectar of plants—blossom honey—or from secretions of living parts of plants or from excretions of plant-sucking insects—honeydew honey (European Commission 2002). Honeydew honey are usually differentiated from nectar honeys by higher values of pH, electrical conductivity, ash percentage, di-and trisaccharide contents, net absorbance, protein and lower monosaccharide content, besides the darker colour and peculiar sensory features (Escuredo et al. 2014). Because they originate from different sources, these honeys have unique characteristics being that honeydew honey generally has a strong flavour and a higher intensity of colour (Castro-Vázquez et al. 2006).
In Brazil, there is the production of a distinctive honeydew honey obtained from the mutualism between the bracatinga tree (Mimosa Scabrella Bentham) and plant-sucking insects, mainly from Tachardiella sp., but also from Stigmacoccus paranaensis Foldi (Mazuchowski et al. 2014; dos Wolff et al. 2015). These insects feed on the phloem of the bracatinga tree and release a saccharinic exudate, which is used by bees to produce bracatinga honeydew honey, highly valued mainly by the European market (Azevedo et al. 2017). This mutualism and consequently, the production of bracatinga honeydew honey, occurs only every 2 years, usually in the first semester, coinciding with even years, which corresponds to the cycle of reproduction of these insects. Still, during these months the production of this specific type of honey is favoured because this period coincides with the low availability of flowers, causing the bee to use the exudate to produce honey (Mazuchowski et al. 2014). Although its properties have not yet been well elucidated, it is recognized that Bracatinga honeydew honey possesses unique nutritional properties, such as free amino acids (Azevedo et al. 2017) and bioaccessible amounts of minerals and phenolic compounds (Seraglio et al. 2017).
The increasing demand and consumption of honeydew honeys by consumers and by the food industry due to its differentiated nutritional and possible therapeutic characteristics has raised concern about its authenticity (Castro-Vázquez et al. 2006; Bergamo et al. 2018). In order to increase the yield of the product, fraudsters can add blossom honeys with low commercial value. Therefore, several studies have been looking for the association of the physical–chemical parameters (Popek et al. 2017) as well as the identification of specific chemical markers (Castro-Vázquez et al. 2006; Simova et al. 2012) as a way to guarantee the authenticity of these honeys. Consequently, some sophisticated analytical methods and techniques have been used for honeydew honeys authentication as nuclear magnetic resonance spectroscopy (qNMR) (Simova et al. 2012), inductively coupled plasma-mass spectrometry (ICP-MS) (Madejczyk and Baralkiewicz 2008), and gas chromatography-mass spectrometry (GC–MS) (Castro-Vázquez et al. 2006), which are generally expensive, require specific structure, trained professionals and generate high amount of chemical residue. Therefore, the differentiation of honeys using a more eco-friendly and costless technology is a true challenge to be completed in the area of science, technology and food engineering.
The colour of honey is directly influenced by the botanical origin (Belay et al. 2015; Silva et al. 2016), presenting positively correlated with the antioxidant activity and mineral content of honeys (Bertoncelj et al. 2007; Kuś and van Ruth 2015). Hence, some studies have already been published exploring the differentiation of honeys using physicochemical parameters associated with the chromatic analysis (González-Miret et al. 2005; Nayik and Nanda 2016). However, the investigation of the use of only the colour parameters for the distinction between different types of honey is scarce (Tuberoso et al. 2014) mainly because the heat treatments used in the processing of honey can also affect the colour of the product (Singh and Bath 1997, 1998; Belay et al. 2015). Until the present moment, no study has reported the use of chromatic characteristics as a safe method for the classification of heat-treated honeys. Thus, it is very important that classification methods involving colour parameters should be validated with samples that have undergone heat treatment.
Among the techniques used to evaluate the honey colour, the most commonly used is based on the international CIELAB system (CIE 2004; Bertoncelj et al. 2007; Nayik and Nanda 2016). The CIELAB system evaluates the colour intensities for the lightness attribute (L*) and the coordinates a* and b*, which evaluate the intensity of the colour green to red and blue to yellow, respectively. Chroma (C*ab) represents attributes of the colour referring to the chromaticity and hue angle (h*ab) represents attributes of the colour referring to hue, both derived from the parameters a* and b* (Jha 2010; McGrath et al. 2017).
Due to the scarce studies related to the exploration of the colour parameters in the differentiation and fraud detection of honeys, principally honeydew honeys, the objective of this study was to characterize bracatinga honeydew honeys, blossom honeys, bracatinga honeydew honeys adulterated in the laboratory, with blossom honeys (5, 15 and 25%) and honeys subjected to thermal processing in relation to the attributes of colour and differentiate each group of honey using chemometric analysis.
Materials and methods
Honeydew and blossom honey samples
Honeydew honeys from bracatinga (Mimosa scabrella Bentham) and blossom honeys were randomly harvested in 2016 from different geographic locations across the state of Santa Catarina (Brazil) (Supplementary material 1). The origin botanic from the samples was obtained through declaration of the beekeepers.
Bracatinga honeydew honeys (n = 16) were obtained from local producers with vast areas of bracatinga tree cultivars and in the months with high exudate production to ensure the extreme purity of honeydew honey. Additionally, the honeycombs were collected, immediately transported (5 ± 2 °C) to the laboratory, and manually drained to avoid contamination with blossom honeys. Honeydew honeys were filtrated to removed sediments and immediately analysed for the colour parameters. Blossom honeys (n = 25) were acquired from the same and nearby localities of the collection of bracatinga honeydew honeys. The samples were sent to the laboratory and processed in the same way as honeydew honeys. In order to avoid blossom-honeydew honeys, blossom honeys were harvested in the months there was not production of exudates.
Adulterated honeydew honey samples
To make adulterated samples, one sample of pure bracatinga honeydew honey (HD-8) was mixed with three samples of blossom honey (HB-10, HB-14, HB-18) at different concentrations—95:5 (w/w), 85:15 (w/w) and 75:25 (w/w), respectively—resulting in nine adulterated samples (Supplementary material 2). Each blossom honey sample used from the mixtures was selected as a representative of different honey subgroups formed through the cluster analysis using the Ward’s method of separation, calculated using the Euclidian distance (data not shown). The same procedure was used to choose the honeydew honey sample, and the sample chosen was the one that represented an intermediate characteristic of the samples. The mixtures were analysed for the L*, a*, b*, C*ab and h*ab colour parameters.
Heat-processed honey samples
To evaluate the effects of heat on the chromatic characteristics of honey, all bracatinga honeydew honey and blossom honey samples were subjected to heating in two different processes: (a) 45 °C for 48 h, process commonly performed by beekeepers to make honey liquid and easy to handle (Escriche et al. 2009), and (b) 80 °C for 4 min, pasteurization applied mainly by the packers in order to eliminate the yeasts that cause undesired fermentation and slow the crystallization of the product (European Commission 2013; Escriche et al. 2014). The two processes were carried out in a water bath. A thermometer was used to measure the temperature of the sample. The time began to be counted from the moment the ideal temperature was reached. After cooling to room temperature (25 °C ± 2 °C), the samples were subjected to chromatic analysis.
Chromatic analysis
The honey colour was assessed by using a colorimeter (Chroma Meter CR-400, Konica Minolta, Tokyo, Japan) adjusted to operate with D65 illuminant and 2° of observation angle. The honey samples previously homogenized and without dilution were placed in polystyrene cuvettes, with an optical path of 10 mm, and the colour parameters (L*, a*, b*) were measured against a white background. The parameters Chroma (C*ab) and hue angle (h*ab) were determined as proposed by the International Commission of the Eclairage (CIE 2004; Jha 2010).
Chemometric analysis
Assays were performed in triplicate for each sample, and the data was expressed as mean ± standard deviation. The principal component analysis (PCA) was used to explain and interpret interdependence of data obtained in the chromatic analysis.
A model for the classification of the type of honey (honeydew, blossom and mixture) was developed with the use of a method of data mining through classification and regression trees (C&RT) using the parameters L*, a*, b*, C*ab and h*ab. In this model, a data set is successively split into increasingly homogeneous subsets until some specified criterion is satisfied. These splits are represented by a tree structure where each node corresponds to a split on a particular variable (Bell 1996).
The software Statistica® version 10 and Paleontological Statistics® (Past) version 2.16 were used to perform the statistical analyses.
Results and discussion
The results of the colorimetric analysis of bracatinga honeydew honeys and blossom honeys are shown in Table 1. The L*, b*, C*ab and h*ab values were generally higher in blossom honeys than in bracatinga honeydew honeys, while only parameter a* presented higher values in bracatinga honeydew honeys. These behaviours suggest a difference in colour composition of honeys, indicating that bracatinga honeydew honeys possess a specific chromatic profile possibly capable to differentiate it from blossom honeys produced in the same geographical location and at similar periods.
Table 1.
Colorimetric analysis of bracatinga honeydew honeys and blossom honeys
| Sample | L | a* | b* | C*ab | h*ab |
|---|---|---|---|---|---|
| HD-1 | 27.37 ± 0.21 | 16.82 ± 0.05 | 12.01 ± 0.22 | 20.67 ± 0.17 | 35.53 ± 0.41 |
| HD-2 | 24.48 ± 0.01 | 15.49 ± 0.16 | 11.12 ± 0.02 | 19.09 ± 0.13 | 35.77 ± 0.28 |
| HD-3 | 27.50 ± 0.47 | 17.03 ± 0.01 | 13.28 ± 0.02 | 21.60 ± 0.00 | 37.95 ± 0.05 |
| HD-4 | 26.95 ± 0.48 | 16.15 ± 0.01 | 11.93 ± 0.06 | 20.08 ± 0.03 | 36.45 ± 0.15 |
| HD-5 | 27.60 ± 0.38 | 16.56 ± 0.10 | 12.98 ± 0.36 | 21.04 ± 0.30 | 38.10 ± 0.60 |
| HD-6 | 26.28 ± 0.01 | 14.24 ± 0.03 | 12.44 ± 0.12 | 18.91 ± 0.08 | 41.13 ± 0.29 |
| HD-7 | 26.85 ± 0.00 | 11.95 ± 0.01 | 11.91 ± 0.00 | 16.87 ± 0.00 | 44.92 ± 0.01 |
| HD-8 | 28.06 ± 0.01 | 16.99 ± 0.18 | 14.79 ± 0.02 | 22.53 ± 0.14 | 41.04 ± 0.31 |
| HD-9 | 28.04 ± 0.44 | 17.28 ± 0.15 | 14.74 ± 0.02 | 22.71 ± 0.11 | 40.47 ± 0.25 |
| HD-10 | 21.01 ± 0.01 | 15.29 ± 0.04 | 12.39 ± 0.32 | 19.68 ± 0.18 | 39.00 ± 0.80 |
| HD-11 | 27.77 ± 0.01 | 16.98 ± 0.04 | 15.62 ± 0.13 | 23.07 ± 0.11 | 42.60 ± 0.22 |
| HD-12 | 22.80 ± 0.01 | 11.31 ± 0.06 | 6.60 ± 0.06 | 13.09 ± 0.07 | 30.28 ± 0.16 |
| HD-13 | 27.91 ± 0.15 | 17.56 ± 0.10 | 15.21 ± 0.25 | 23.23 ± 0.22 | 40.90 ± 0.38 |
| HD-14 | 25.98 ± 0.19 | 15.12 ± 0.11 | 9.71 ± 0.11 | 17.97 ± 0.15 | 32.69 ± 0.13 |
| HD-15 | 26.01 ± 0.18 | 13.54 ± 0.05 | 9.67 ± 0.01 | 16.64 ± 0.04 | 35.53 ± 0.11 |
| HD-16 | 27.62 ± 0.68 | 17.42 ± 0.31 | 13.64 ± 0.01 | 22.12 ± 0.24 | 38.06 ± 0.48 |
| HB-1 | 42.92 ± 0.01 | 6.71 ± 0.03 | 33.11 ± 0.98 | 33.78 ± 0.96 | 78.54 ± 0.35 |
| HB-2 | 42.61 ± 0.00 | 3.75 ± 0.07 | 30.57 ± 0.60 | 30.80 ± 0.59 | 83.00 ± 0.27 |
| HB-3 | 38.64 ± 0.65 | 8.59 ± 0.10 | 30.98 ± 1.10 | 32.14 ± 1.03 | 74.49 ± 0.69 |
| HB-4 | 43.41 ± 0.50 | 5.13 ± 0.07 | 35.21 ± 0.02 | 35.58 ± 0.02 | 81.71 ± 0.12 |
| HB-5 | 34.92 ± 0.62 | 16.24 ± 0.11 | 26.41 ± 1.04 | 31.01 ± 0.94 | 58.40 ± 0.85 |
| HB-6 | 49.88 ± 0.46 | 1.93 ± 0.06 | 30.77 ± 0.59 | 30.83 ± 0.59 | 86.40 ± 0.13 |
| HB-7 | 34.78 ± 0.21 | 12.89 ± 0.07 | 21.56 ± 0.31 | 25.12 ± 0.28 | 59.14 ± 0.33 |
| HB-8 | 44.33 ± 0.34 | 6.61 ± 0.07 | 37.43 ± 0.01 | 38.00 ± 0.01 | 79.98 ± 0.10 |
| HB-9 | 45.40 ± 0.02 | 7.02 ± 0.02 | 38.61 ± 0.20 | 39.24 ± 0.01 | 79.70 ± 0.03 |
| HB-10 | 43.00 ± 0.49 | 9.28 ± 0.08 | 37.21 ± 0.04 | 38.35 ± 0.03 | 75.99 ± 0.12 |
| HB-11 | 30.33 ± 0.71 | 15.65 ± 0.22 | 20.82 ± 0.13 | 26.05 ± 0.17 | 53.07 ± 0.43 |
| HB-12 | 33.01 ± 0.50 | 15.56 ± 0.14 | 22.79 ± 0.91 | 27.60 ± 0.83 | 55.65 ± 0.83 |
| HB-13 | 37.15 ± 0.51 | 15.14 ± 0.07 | 29.89 ± 0.98 | 30.50 ± 0.89 | 63.12 ± 0.73 |
| HB-14 | 35.24 ± 0.61 | 15.79 ± 0.11 | 26.60 ± 1.12 | 30.94 ± 1.01 | 59.28 ± 0.89 |
| HB-15 | 44.41 ± 0.30 | 6.89 ± 0.04 | 39.54 ± 0.76 | 40.14 ± 0.74 | 80.12 ± 0.24 |
| HB-16 | 40.33 ± 0.24 | 6.13 ± 0.02 | 32.79 ± 0.90 | 33.36 ± 0.89 | 79.41 ± 0.25 |
| HB-17 | 43.58 ± 0.01 | 5.47 ± 0.10 | 37.91 ± 0.01 | 38.30 ± 0.02 | 81.80 ± 0.14 |
| HB-18 | 44.16 ± 0.00 | 6.95 ± 0.05 | 33.62 ± 0.27 | 34.33 ± 0.26 | 78.32 ± 0.13 |
| HB-19 | 38.93 ± 0.38 | 13.66 ± 0.05 | 31.75 ± 0.80 | 34.56 ± 0.76 | 66.72 ± 0.46 |
| HB-20 | 39.96 ± 0.52 | 12.35 ± 0.07 | 32.72 ± 1.24 | 34.98 ± 1.18 | 69.31 ± 0.67 |
| HB-21 | 32.83 ± 0.19 | 15.27 ± 0.04 | 21.65 ± 0.18 | 26.49 ± 0.15 | 54.81 ± 0.23 |
| HB-22 | 38.82 ± 0.00 | 10.36 ± 0.06 | 31.94 ± 0.00 | 33.58 ± 0.02 | 72.04 ± 0.09 |
| HB-23 | 40.26 ± 0.00 | 11.43 ± 0.03 | 32.20 ± 0.01 | 34.17 ± 0.02 | 70.45 ± 0.04 |
| HB-24 | 35.09 ± 0.01 | 12.78 ± 0.04 | 24.42 ± 0.02 | 27.56 ± 0.03 | 62.38 ± 0.06 |
| HB-25 | 47.57 ± 0.01 | 1.07 ± 0.06 | 28.16 ± 0.01 | 28.18 ± 0.01 | 87.83 ± 0.12 |
| HDA-1 | 26.05 ± 0.01 | 15.29 ± 0.06 | 13.02 ± 0.01 | 20.08 ± 0.05 | 40.40 ± 0.10 |
| HDA-2 | 28.56 ± 0.43 | 16.72 ± 0.18 | 15.30 ± 0.01 | 22.66 ± 0.14 | 42.46 ± 0.31 |
| HDA-3 | 31.34 ± 0.20 | 17.32 ± 0.04 | 19.70 ± 0.00 | 26.23 ± 0.03 | 48.68 ± 0.08 |
| HDA-4 | 27.26 ± 0.01 | 16.71 ± 0.31 | 14.27 ± 0.01 | 21.97 ± 0.23 | 40.50 ± 0.52 |
| HDA-5 | 28.97 ± 0.01 | 16.72 ± 0.02 | 15.21 ± 0.13 | 22.60 ± 0.09 | 42.30 ± 0.24 |
| HDA-6 | 29.60 ± 0.01 | 17.21 ± 0.01 | 17.41 ± 0.08 | 24.48 ± 0.05 | 45.33 ± 0.15 |
| HDA-7 | 28.27 ± 0.79 | 16.47 ± 0.04 | 14.63 ± 0.03 | 22.03 ± 0.01 | 41.62 ± 0.13 |
| HDA-8 | 29.01 ± 0.01 | 17.11 ± 0.09 | 16.85 ± 0.02 | 24.01 ± 0.01 | 44.56 ± 0.05 |
| HDA-9 | 30.50 ± 0.01 | 18.53 ± 0.02 | 18.74 ± 0.01 | 26.35 ± 0.01 | 45.33 ± 0.04 |
HD Bracatinga honeydew honey, HB Blossom honey, HDA Bracatinga honeydew honeys adulterated with blossom honey, L* lightness, a* Intensity of green (negative values) and red colours (positive values), b* Intensity of blue (negative values) and yellow colours (positive values), C*ab Chroma, hab Hue angle
According to PCA (Supplementary material 3), the formation of two large groups is observed: one composed only by bracatinga honeydew honeys and the other composed by blossom honeys, where the principal component (PC) 1 and 2 represent 98.65% of the total variability of the data, which shows that the two honey types are well distinguished by their chromatic characteristics. The PC1 was the main factor responsible for the variance of the data (89.57%). The large dispersion of blossom honeys in the PCA is possibly related to its variable chromatic characteristics resulting from the distinct sources of nectar. Meanwhile, bracatinga honeydew honeys showed similar chromatic characteristics even though produced in distinct locations, reinforcing the idea that this characteristic is unique to this specific type of honey, enabling differentiation from other blossom honeys.
Bracatinga honeydew honeys and blossom honeys showed different chromatic characteristics, this profile was evaluated as a possible authenticity marker for bracatinga honeydew honeys. Thus, blossom honeys were added to the bracatinga honeydew honey at concentrations of 5, 10 and 15% and then chromatic characterization was performed.
The results of the colorimetric analysis of bracatinga honeydew honeys adulterated with different proportions of blossom honeys are shown in Table 1. The lightness (L*) of adulterated bracatinga honeydew honey was higher in the samples with 25% (w/w) of blossom honeys (HDA-3, HDA-6 and HAD-9 samples). This same tendency was also generally observed for the parameters a*, b*, C*ab and h*ab, where the honey samples with the highest percentage of blossom honeys presented the highest values for these parameters. This behaviour was expected once blossom honeys showed high values for almost all the chromatic parameters evaluated.
For a more robust evaluation, a classification model was constructed using the C&RT classification trees looking for the classification of honey samples in three groups: bracatinga honeydew honeys (pure and adulterated) and blossom honeys. This model has already been used successfully for the classification of blossom honeys from different botanical origins by Popek et al. (2017) using the results obtained from the physical–chemical parameters as variables. For the construction of the model, only the use of the colour parameters were necessary to ensure a good prediction model.
According to the classification model developed (Fig. 1), considering bracatinga honeydew honey, blossom honey or adulterated bracatinga honeydew honey (containing 5% or more of blossom honey), the honey sample must satisfy pre-established conditions.
Fig. 1.
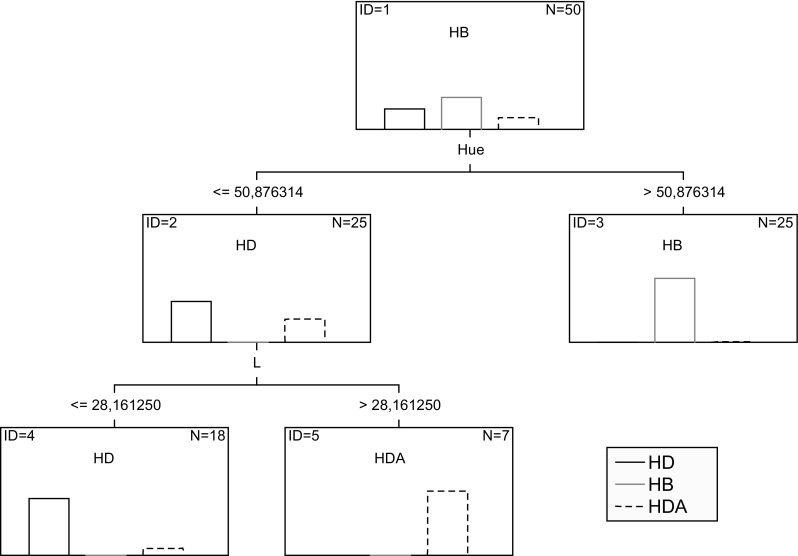
Classification model in the form of the Decision Tree using L*, a* and b* as predictor variables
A classification matrix was constructed in order to evaluate the effective representation of the structure of the data set obtained through the classification model proposed (Supplementary material 4). According to the classification matrix, it is possible to observe that the proposed classification model was able to correctly classify bracatinga honeydew honey and blossom honey samples. Only two samples (of a total of 9 samples) adulterated with 5% blossom honey were misclassified (HDA-1 and HDA-4). These results indicate that the developed model is reliable and can be successfully used to classify bracatinga honeydew honeys and blossom honeys, as well as adulterated bracatinga honeydew honeys, especially when added with contents above 15% of blossom honey.
The chromatic characterization of samples submitted to thermal processing is demonstrated in the Supplementary material 5. When analysed through the classification model developed, the heated samples were classified correctly in 100% of the cases. It is known that the HMF formation in honey is intensified by increasing the temperature and time of heating, affecting the colour of honeys (Singh and Bath 1997, 1998). The correct classification of the heated samples suggesting that the heating processes applied does not affected significatively the HMF content and, consequently, the colour of honey samples. Therefore, the classification model proposed in this work can also be used successfully for samples subjected to moderate heat.
Additionally, it is important to note that the proposed classification method can be used for authenticity and fraud verification easily and quickly through chromatic analysis. It is also worth noting that this method can be performed in any laboratory without the need for sophisticated equipment, trained technicians or use of chemical reagents and can be useful as part of the initial screening process of bracatinga honeydew honey.
Conclusion
In this study, bracatinga honeydew honeys showed peculiar chromatic characteristics that allowed the differentiation of this type of honey from blossom honeys produced in the same geographical location and harvest period as well as from bracatinga honeydew honeys containing blossom honeys. A classification model was developed, which allowed the correct classification of samples, including those that have undergone heat treatment. These results clearly demonstrate that the colour profile, associated with chemometric analysis, can be used for the authenticity and fraud verification control in honeydew honey.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. Authors also wish to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico - Brasil (CNPq), the Fundação de Amparo à Pesquisa do Estado de Santa Catarina - Brasil (FAPESC) and the participating beekeepers from the mountain plateau region of Santa Catarina state.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Azevedo MS, Seraglio SKT, Rocha G, Balderas CB, Piovezan M, Gonzaga LV, de Barcellos Falkenberg D, Fett R, de Oliveira MAL, Costa ACO. Free amino acid determination by GC-MS combined with a chemometric approach for geographical classification of bracatinga honeydew honey (<i>Mimosa scabrella Bentham) Food Control. 2017;78:383–392. doi: 10.1016/j.foodcont.2017.03.008. [DOI] [Google Scholar]
- Belay A, Solomon WKK, Bultossa G, Adgaba N, Melaku S. Botanical origin, colour, granulation, and sensory properties of the Harenna forest honey, Bale, Ethiopia. Food Chem. 2015;167:213–219. doi: 10.1016/j.foodchem.2014.06.080. [DOI] [PubMed] [Google Scholar]
- Bell JF. Application of classification trees to the habitat preference of upland birds. J Appl Stat. 1996;23(2–3):349–360. doi: 10.1080/02664769624297. [DOI] [Google Scholar]
- Bergamo G, Seraglio SKT, Gonzaga LV, Fett R, Costa ACO. Mineral profile as a potential parameter for verifying the authenticity of bracatinga honeydew honeys. LWT. 2018;97:390–395. doi: 10.1016/j.lwt.2018.07.028. [DOI] [Google Scholar]
- Bertoncelj J, Dobersek U, Jamnik M, Golob T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007;105(2):822–828. doi: 10.1016/j.foodchem.2007.01.060. [DOI] [Google Scholar]
- Castro-Vázquez L, Díaz-Maroto MC, Pérez-Coello MS. Volatile composition and contribution to the aroma of spanish honeydew honeys Identification of a new chemical marker. J Agric Food Chem. 2006;54(13):4809–4813. doi: 10.1021/jf0604384. [DOI] [PubMed] [Google Scholar]
- CIE . Colorimetry. 3. Vienna: Commission Internationale de I’Eclairage; 2004. [Google Scholar]
- dos Wolff VR, Witter S, Lisboa BB. Reporte de Stigmacoccus paranaensis Foldi (Hemiptera, Stigmacoccidae), insecto escama asociado con la producción de miel de mielato en Rio Grande do Sul, Brasil. Insecta Mundi. 2015;434:1–7. [Google Scholar]
- Escriche I, Visquert M, Juan-Borrás M, Fito P. Influence of simulated industrial thermal treatments on the volatile fractions of different varieties of honey. Food Chem. 2009;112(2):329–338. doi: 10.1016/j.foodchem.2008.05.068. [DOI] [Google Scholar]
- Escriche I, Kadar M, Juan-Borrás M, Domenech E. Suitability of antioxidant capacity, flavonoids and phenolic acids for floral authentication of honey. Impact of industrial thermal treatment. Food Chem. 2014;142:135–143. doi: 10.1016/j.foodchem.2013.07.033. [DOI] [PubMed] [Google Scholar]
- Escuredo O, Dobre I, Fernández-González M, Seijo MC. Contribution of botanical origin and sugar composition of honeys on the crystallization phenomenon. Food Chem. 2014;149:84–90. doi: 10.1016/j.foodchem.2013.10.097. [DOI] [PubMed] [Google Scholar]
- European Commission European Commission council directive 2001/110/EC of 20 December 2001 relating to honey. Off J Eur Commun. 2002;10:47. [Google Scholar]
- European Commission (2013) Final report summary—TOPHONEY (enhancing the quality attributes of processed honey and avoiding its crystallisation by the application of a non-thermal treatment process). http://cordis.europa.eu/result/rcn/149416_en.html
- González-Miret ML, Terrab A, Hernanz D, Fernández-Recamales MÁA, Heredia FJ. Multivariate correlation between color and mineral composition of honeys and by their botanical origin. J Agric Food Chem. 2005;53(7):2574–2580. doi: 10.1021/jf048207p. [DOI] [PubMed] [Google Scholar]
- Jha N. Colour measurements and modeling. In: Jha N, editor. Nondestructive evaluation of food quality. Berlin: Springer; 2010. pp. 17–40. [Google Scholar]
- Kuś PM, van Ruth S. Discrimination of Polish unifloral honeys using overall PTR-MS and HPLC fingerprints combined with chemometrics. LWT Food Sci Technol. 2015;62(1):69–75. doi: 10.1016/j.lwt.2014.12.060. [DOI] [Google Scholar]
- Madejczyk M, Baralkiewicz D. Characterization of Polish rape and honeydew honey according to their mineral contents using ICP-MS and F-AAS/AES. Anal Chim Acta. 2008;617(1–2):11–17. doi: 10.1016/j.aca.2008.01.038. [DOI] [PubMed] [Google Scholar]
- Mazuchowski JZ, Rech TD, Toresan L. Bracatinga, Mimosa scabrella Bentham: Cultivo, manejo e usos da espécie. Florianópolis: Epagri; 2014. [Google Scholar]
- McGrath JR, Beck M, Hill ME. Replicating red: analysis of ceramic slip color with CIELAB color data. J Archaeol Sci Rep. 2017;14:432–438. [Google Scholar]
- Nayik GA, Nanda V. ‘A chemometric approach to evaluate the phenolic compounds, antioxidant activity and mineral content of different unifloral honey types from Kashmir, India. LWT Food Sci Technol. 2016;74:504–513. doi: 10.1016/j.lwt.2016.08.016. [DOI] [Google Scholar]
- Popek S, Halagarda M, Kursa K. A new model to identify botanical origin of Polish honeys based on the physicochemical parameters and chemometric analysis. LWT Food Sci Technol. 2017;77:482–487. doi: 10.1016/j.lwt.2016.12.003. [DOI] [Google Scholar]
- Seraglio SKT, Valese AC, Daguer H, Bergamo G, Azevedo MS, Nehring P, Gonzaga LV, Fett R, Costa ACO. Effect of in vitro gastrointestinal digestion on the bioaccessibility of phenolic compounds, minerals, and antioxidant capacity of Mimosa scabrella Bentham honeydew honeys. Food Res Int. 2017;99:670–678. doi: 10.1016/j.foodres.2017.06.024. [DOI] [PubMed] [Google Scholar]
- Silva PM, Gauche C, Gonzaga LV, Costa ACO, Fett R. Honey: chemical composition, stability and authenticity. Food Chem. 2016;196:309–323. doi: 10.1016/j.foodchem.2015.09.051. [DOI] [PubMed] [Google Scholar]
- Simova S, Atanassov A, Shishiniova M, Bankova V. A rapid differentiation between oak honeydew honey and nectar and other honeydew honeys by NMR spectroscopy. Food Chem. 2012;134(3):1706–1710. doi: 10.1016/j.foodchem.2012.03.071. [DOI] [PubMed] [Google Scholar]
- Singh N, Bath PK. Quality evaluation of different types of Indian honey. Food Chem. 1997;58(1–2):129–133. doi: 10.1016/S0308-8146(96)00231-2. [DOI] [Google Scholar]
- Singh N, Bath PK. Relationship between heating and hydroxymethylfurfural formation in different honey types. J Food Sci Technol. 1998;35(2):154–156. [Google Scholar]
- Tuberoso CIG, Jerković I, Sarais G, Congiu F, Marijanović Z, Kuś PM. Color evaluation of seventeen European unifloral honey types by means of spectrophotometrically determined CIE L*C*abh°ab chromaticity coordinates. Food Chem. 2014;145:284–291. doi: 10.1016/j.foodchem.2013.08.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


