Abstract
The quince fruit can be an alternative for producing healthy tea because it is a rich source of bioactive compounds. This study was conducted to evaluate the effects of heating (for 20, 40 and 60 min) on the bioactive compounds of quince and quality attributes its tisanes. To this aim, phenolic content of the treated sample (for 40 min according to sensory analysis) was evaluated by HPLC and antioxidant activity was measured using DPPH method. The analysis of sensory evaluation data demonstrated that the heating process significantly improved the color and taste of the quince tisanes. Thermal treatment (180 °C) for 40 min had maximum acceptability. Although, results confirmed negative effects of the heating process on phenolic content, it showed an increase in antioxidant activity. The HPLC data displayed significant reduction in total amount of five identified phenolic compounds from 460.51 to 25.49 mg/25 g extract by heat treatment. In contrast the roasted sample exhibited lower IC50 (663.88 µg/ml) than the unprocessed sample (1098.66 µg/ml). Considering the elevated antioxidant activity achieved through the heating process, the quince tisane can be considered as a healthy beverage.
Keywords: Quince tea, Phenolic compounds, HPLC, Sensory evaluation, Antioxidant activity
Introduction
Tea is ranked as the world’s most popular beverage after water. Though it is true that tea consumption is a healthy habit, other points should also be considered including the formation of anti-nutritive compounds such as oxalate (Brzezicha-Cirocka et al. 2016), and raising the potential hazard of liver damage (Mazzanti et al. 2015). In addition, excess intake of some trace elements in green tea like copper (Cu) as well as aluminum (Al) cause serious health concerns for human. High levels of aluminum have been linked with diseases like Alzheimer. Alternatively, while the results highlighted the antioxidant activity and functional properties of fruits and vegetables (Singh et al. 2016a), their infusions have a growing popularity as delivering pleasant sensory characteristics. Also, it has been indicated that the absorption rate of active ingredients of the fruits increases through consumption their tisane (Sohrabvandi et al. 2013). The beneficial effects specified, have encouraged the researchers to develop these products. Quince fruit can be a suitable candidate for the production of a palatable healthy tea.
Quince fruit (Cydonia oblonga Miller, Rosaceae family) is cultivated extensively in the South-West Asia, Armenia, Turkey, Georgia, and Iran. Quince fruit possesses potent bioactive constituents with health-promoting effects, such as phenolics and flavonoids. Besides, it is also a source of alkaloids, organic acids, sugars, crude fiber, amino acids and inorganic elements. Previous studies have suggested that it might improve heart and brain health (del Castillo et al. 2002; Szychowski et al. 2014). However, It’s not appropriate for fresh consumption, mainly due to its hardness, fibrous consistency, sourness, bitterness, and astringent taste. On the other hand, it is of great interest to be consumed when cooked or processed (Hečimović et al. 2011; Silva et al. 2004a, b). Therefore, quince tea may be a pleasant beverage since it has a desirable aroma and is a low-cost beverage which suits the nutritional needs of the public (Gheisari and Abhari 2014; Silva et al. 2004a, b). Previous researches have indicated that quince’s phenolic compounds can noticeably decrease the risk of diseases like certain cancers, cardiovascular diseases, hemorrhoids, bronchial asthma plus inflammatory disorders, as well as osteoporosis in women owing to their high ability to prevent oxidative stress (Chipurura et al. 2010; Gheisari and Abhari 2014).
While it is known that thermal processes manipulate the physicochemical and biological characteristics of phytochemicals (Nicoli et al. 1999), so far, the impacts of roasting on the quality of quince fruit have not been reported. Hence, the phenolic contents in Iranian quince fruit together with the impact of heat processing on the quality quince tea were investigated. Initially, the sensory analysis was carried out to determine the best time for quince roasting. Subsequently, the best treatment together with the unroasted sample (control) was examined for their phenolic compounds and antioxidant activity.
Materials and methods
Chemicals and reagents
Reagents and chemicals consumed in the current research were purchased from Sigma-Aldrich and Merck.
Samples preparation
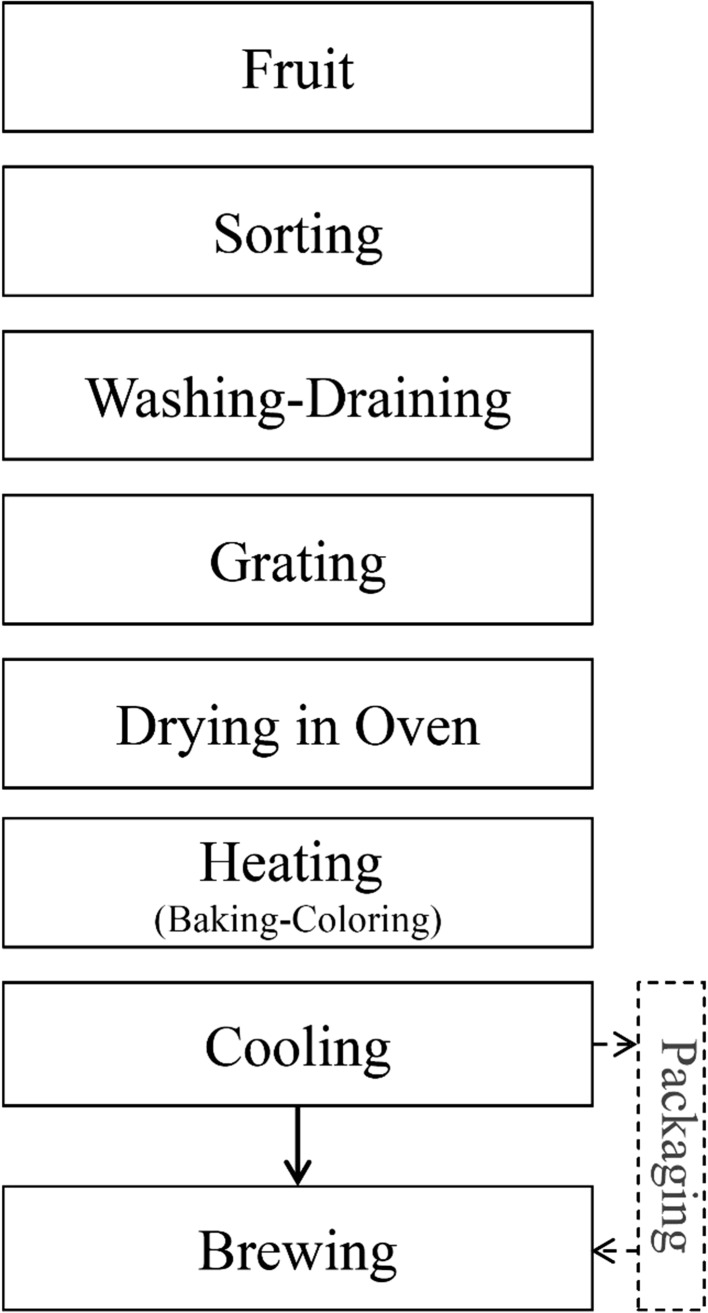
Iranian quince fruit was purchased from Yazd city. After discarding defective ones, each fruit was completely rinsed with cold water and drained; then quinces were grated and dried under the ambient temperature for 48 h (Fig. 1). The quince pieces were homogenized and subdivided randomly into four groups. To obtain different degrees of roasting, three various times of heating (20, 40 and 60 min) were considered for three separate batches by spreading on a clean tray on a stove (180 °C). After roasting, the samples were left at 20 °C until subsequent assays. An unroasted sample (the fourth group) was considered as control. Furthermore, sensory analysis was done on 4 specimens and chemical analysis was carried out on 2 samples (The best sample in regard to the sensory analysis and unroasted sample).
Fig. 1.
The process diagram for developing tisane from quine fruit
Extraction method
Briefly, 10 g of each dry sample was mixed with 100 ml of ethanol (70%)/water mixture (80:20). The resulting mixture was stirred at the ambient temperature (25 °C) for 6 h and filtered with No. 1 Whatman (Buckinghamshire, UK) under vacuum; afterwards, the solvents were separated by a rotary evaporator (R-200, Buchi, Switzerland) under reduced pressure, at low temperature (40 °C), with a rotating speed of 180 rpm. The sample extract was concentrated using a freeze drier (FDB-5503, Operon, Korea) and then the lyophilized sample extract was placed at − 18 °C for the next analysis.
Characterization of phenolic profile
The phenolics of quince extracts were separated and specified by HPLC equipped with Empower Software (Waters Corp., Milford, USA), 2800 PDA UV detector (Schambeck SFD GmbH, Bad Honnef, Germany) and a Model 8300 HPLC pump system from Knauer Gradient (Knauer, Bad Homburg, Germany) (Karar et al. 2014). The HPLC separation of individual phenolic acids was performed on a Waters C18 spherisorb (5 µm ODS2; 250 × 4.6 mm i.d.) at ambient temperature. Prior to HPLC injection, the extract of each sample was centrifuged (10,000 rpm) for 2 min at 4 °C. Then, clarification of the collected supernatants was done using a Sep-Pak C18 cartridge (Millipore, Milford, USA). A Millipore Swinnex type filter (pore size = 0.45 μm) was used to remove the particles. Subsequently, 20 μL of clarified sample was injected into the system with three replicates. The separations were done using gradient method with the following solvent system: CH3OH: 0.05% trifluoroacetic acid (A) and H2O: 0.05% trifluoroacetic acid (B). Gradient profile was as follow: 80–70% B from 0 to 10 min, 70–40% B from 10 to 40 min, 40–20% B from 40 to 45 min, 20–0% B from 45 to 50 min, 0% B from 50 to 55 min, 0–80% B from 55 to 60 min. The elution of compounds was performed at a solvent flow rate of 0.5 ml min−1, and the UV–Vis spectra was recorded in the 200–400 nm range of λ. Phenolic acids were identified and estimated by the retention times and spectral data from peaks relative to external standards. Finally, total phenolic content was calculated as the sum of individual identified phenolic acid.
Total antioxidant capacity
Antiradical activity of the freeze-dried extracts based on inhibition of DPPH radical was determined using spectrophotometer (T80+, PG Instruments Limited, UK). Briefly, 30 mg of each freeze-dried sample was mixed into 6 ml of ethanol. For each quince extract, a dilution series was prepared by ethanol, then 1 ml of DPPH ethanolic solution (0.2 mM) was added to 2 ml of each diluted sample. All the mixtures absorbance was recorded against ethanol blank by UV–visible spectrophotometer at 517 nm after at least 60 min of reaction. The antioxidant ability was specified as an inhibition percentage of the DPPH radical, defined as:
where Aextract—the absorbance of the solution containing quince extract, Acontrol—the absorbance of the solution without quince extract.
Sensory evaluation
A party consisting of 10 trained panelists, aged 26–50 years, took part in the sensory evaluation of the samples. Quality indicators of the quince tea, such as color, aroma and taste and overall acceptability, were evaluated after preparation by panelists. Sensory evaluation was performed with the addition of ≅ 2 g of each sample to 200 ml of boiling water in a teapot and applying different brewing time (5, 10 and 15 min). The panel members received encoded samples of quince tea with three-digit random numbers individually. They then performed sensory analysis and judged the key attributes of each samples using the rating scale with five-point (dislike to like). According to these attributes, the overall quality of each sample was also scored using the similar five-point scale.
Statistical analysis
All tests were done in triplicate and results were reported as mean ± SD. SPSS 19.0 software (SPSS Inc., Chicago, USA) was used to analyze the experimental data using one-way ANOVA. LSD test was applied to compare means at p ≤ 0.05.
Results and discussion
Phenolic compounds analysis
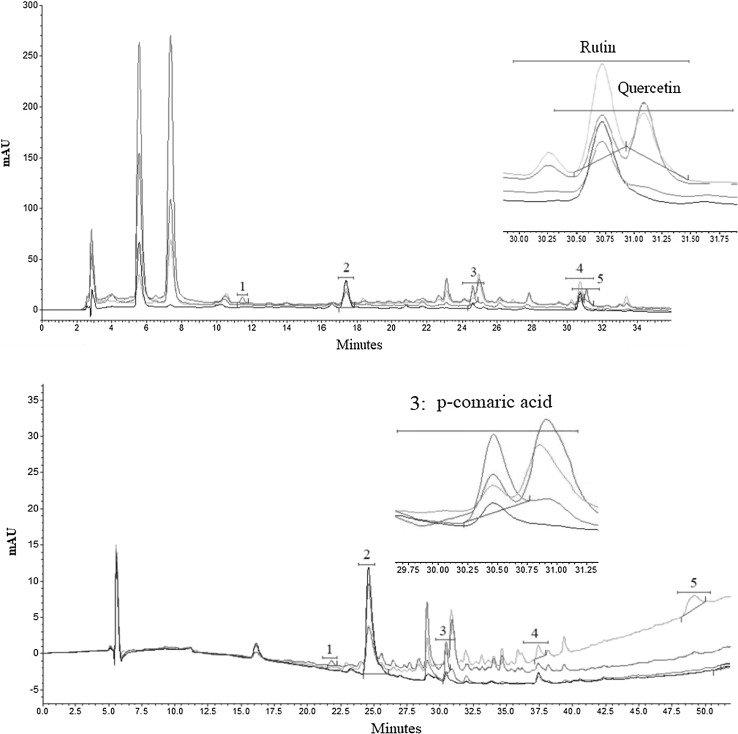
So far, many studies have been conducted on phenolic compounds due to their interesting role as antimicrobials and antioxidants (Singh et al. 2016b), and key contributors to organoleptic properties (taste and color) (Karar et al. 2014; Szychowski et al. 2014). HPLC methodology was used to specify these compounds in quince sample and to characterize their thermal durability. Five main peaks were found in the HPLC chromatograms (Fig. 2).
Fig. 2.
Representative chromatograms of the detected phenolic compounds in quince pulp extract a roasted sample; b unroasted sample. 1: Catechin, 2: chlorogenic acid, 3: p-coumaric acid, 4: rutin, 5: quercetin
Chlorogenic acid (5-O-caffeoylquinic acid) was the primary phenolic compound in both the samples, followed by quercetin, catechin, rutin and p-coumaric acid (Table 1).
Table 1.
Phenolic compounds of quince samples expressed in mg/25 g extract
| Phenolic compounds | Roasted sample | Unroasted sample |
|---|---|---|
| Catechin | 4.17 ± 0.07 | 23.08 ± 0.03 |
| Chlorogenic acid | 12.37 ± 0.00 | 346.26 ± 0.01 |
| p-Coumaric acid | 1.75 ± 0.02 | 11.54 ± 0.01 |
| Rutin | 2.3 ± 0.05 | 23.08 ± 0.02 |
| Quercetin | 4.9 ± 0.00 | 56.55 ± 0.01 |
| Total | 25.49 ± 0.07 | 460.51 ± 0.08 |
The phenolic profile obtained in this study was consistent with the previous studies (Fattouch et al. 2007; Karar et al. 2014; Silva et al. 2004a, b, 2005). As reported earlier the presence of significant amounts of chlorogenic acid and quercetin was distinguished feature in the phenolic profile of quince fruit that were low in apple and pear puree (Sut et al. 2018; Schieber et al. 2001). Although, different levels of these phenolic compounds have been reported in fruits and vegetables, but each herb has a unique combination and amount of them. To this extent, total phenolic compounds of following fruit pulps can be mentioned; pomegranate (1639.7 mg GAE/100 g), Mango (440.6 mg GAE/100 g), Banana (362.4 mg GAE/100 g), and Sapodilla (413.9 mg GAE/100 g) (Singh et al. 2016a).
The sum of all identified phenolic compounds in the unroasted sample (460.51 ± 0.08 mg/100 g, respectively) was more than those produced in other geographical regions (Andrade et al. 1998; Legua et al. 2013; Silva et al. 2005; Szychowski et al. 2014). However, the total phenolic content observed in this study was less than that found in some varieties. The specified results concurred with earlier results that the concentration of phenolic compounds depends on variety (Wojdyło et al. 2013).
Moreover, the content found in the unroasted sample was greater than reported for apple and pear puree (301.41 and 483.36 mg/100 g, respectively), which clearly appeared in its slight astringent taste. It has been confirmed that the application of products containing quince polyphenolics, can be helpful for health promotion (Andrade et al. 1998; Wojdyło et al. 2013).
According to the results of HPLC, total phenolic content was significantly higher in the unroasted sample (control) compared to the roasted sample extract. The roasting process had an impactable adverse effect on the phenolic content in quince sample (by 94.46%, respectively). This effect can be attributed to reactions, such as the degradation, oxidation or polymerization of phenolic compounds and the generation of complexes with proteins and carbohydrates during roasting (Hečimović et al. 2011; Pelvan et al. 2012; Wang et al. 2000). As reported by previous researchers, dehydration and the onset of chemical reactions caused by application of heat treatment to plant foods can modify the lignocellulosic and protein structure, which may raise the presence of phytochemicals in the matrix (Xu and Chang 2008). Phenolic compounds, especially chlorogenic acid, are considered as a favorite substrate of the catecholase activity of polyphenol oxidase. Thus, oxidation process may affect the concentration of these compounds. Moreover, oxidation products can co-oxidize other compounds and generate colored products. These observations also appeared in flavor of tea prepared with the roasted sample. The roasted quince tea had a less taste of astringency than the tea prepared with the control sample that was consistent with previous findings (Wang et al. 2000). However, in relation to thermal processing effects, previous researchers reported an increase in concentration of phenolics of roasted bitter melon fruits by 5.4% (Choi et al. 2012). This increase was attributed to the separation of phenolic compounds from cell matrix. This suggests that the type of plant food and thermal treatment affect the concentration of phenolics (Chipurura et al. 2010; Choi et al. 2012).
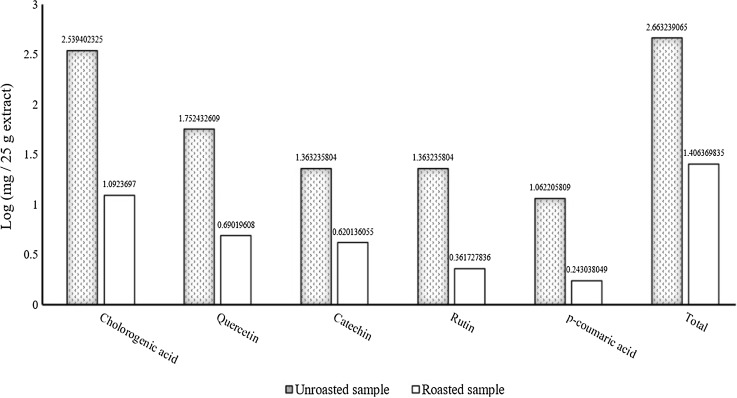
The obtained data demonstrated that roasting process decreased all individual (five) phenolic contents (Fig. 3). The chlorogenic acid, an esterification product of caffeic acid and quinic acid, has well-known functional properties such as anti-carcinogenic, anti-mutagenic, antispasmodic, antibacterial and glucose-6-phosphatase regulator. It also has cholesterol-reducing characteristics, which reduces the risks of cardiovascular diseases (Cho et al. 2010; Farah et al. 2005; Karar et al. 2014).
Fig. 3.
Content of phenolic compounds (log mg/25 g) in unroasted and roasted quince pulp
The remarkable reduction of chlorogenic acid content reveals high susceptibility of the esterified bond to heat which corroborates the findings of previous study (Schieber et al. 2001). Similar investigations with other foods demonstrated that the high temperature of the roasting process causes chemical reactions, such as isomerization and decomposition reactions (hydrolysis of the carbon–carbon bonds of chlorogenic acid), as well as disparting lactone ring from quinic acid because of dehydration (Farah et al. 2005). Hydroxycinnamic acid derivatives (p-coumaric acids) have an aromatic ring with a three-carbon chain attached to this ring. The glycosylated form of these compounds can be found in plants (Cho et al. 2010). The reduction of p-coumaric acid content after roasting can be attributed to the sensitivity of glycosidic bonds to thermal decomposition and their release from attached forms (Lemos et al. 2012; Pelvan et al. 2012). Previous studies showed that flavonols, such as quercetin and rutin are thermolabile. Their findings are in line with the current research (Wojdyło et al. 2013). Similarly, a significant alteration was observed in catechin concentration which can be linked with its highly hydroxylated structure. This structure is sensitive to redox reactions, resulting in losses during heat treatment.
Antioxidant activity
The antioxidant activity of samples was specified by DPPH radical test. This method is usually used to assess the antioxidant activity of herbal extracts. The statistical difference (p < 0.05) was specified among the mean values of percentage inhibition for the ethanolic dilutions of extracts prepared from roasted and unprocessed samples.
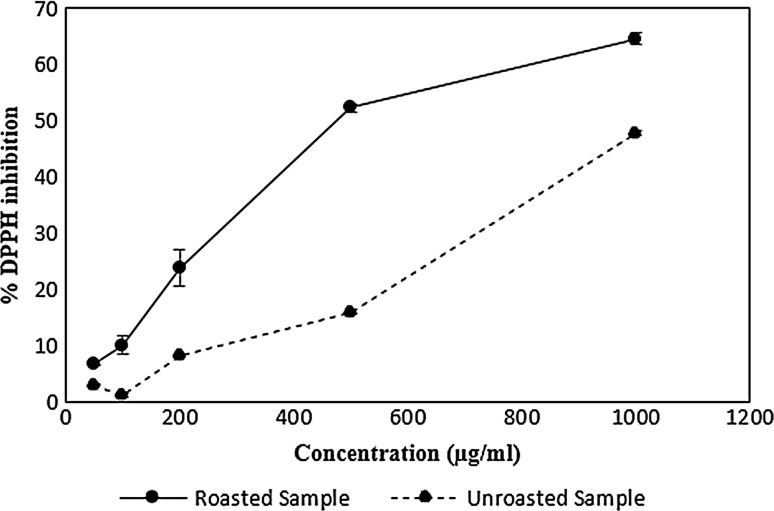
The IC50 value for the unprocessed sample extract (fruit pieces with skin) was 1098.66 µg/ml, respectively which was more than the value reported for quince pulp by previous researchers, (1700 µg/ml, respectively) (Silva et al. 2004a, b). This difference in IC50 is related to their different phenolic contents. Percentage inhibition of ethanolic extracts was dose-dependent (Fig. 4). The previous researchers indicated the positive correlation between antioxidant activity and total phenolic content in quince fruit (Lemos et al. 2012). They reported quince pulp antioxidant activity depends mainly on the value of chlorogenic acid derivatives (Gheisari and Abhari 2014; Legua et al. 2013; Silva et al. 2004a, b).
Fig. 4.
DPPH radical scavenging activities of quince samples
As compared to the unprocessed sample, the roast processing caused a significant increase in antioxidant activity of quince sample. The roasted sample exhibited lower IC50 (663.88 µg/ml) than the unprocessed sample. In the DPPH method, the IC50 value is inversely related to the antioxidant activity (Gheisari and Abhari 2014). This increase in activity was not consistent with phenolics content. Indeed, although phenolic compounds considerably decreased as a result of roasting, the total antioxidant properties of the sample were stable or even elevated. This finding was consistent with previous investigations (del Castillo et al. 2002; Satoh et al. 2005). The resulting data could be clarified by the generation of stable intermediate products and/or non-phenolic compounds deriving from pyrolysis, Maillard reaction and/or carbohydrate caramelization, when thermal process is applied (del Castillo et al. 2002; Kim et al. 2011; Silva et al. 2004a, b; Turkmen et al. 2005). Breaking chemical bonds of higher molecular weight polyphenols into soluble low molecular weight polyphenols (for instance, breaking of 3-p-coumaroylquinic acid to quinic acid), and interconversion of the phenolic compounds (like the interconversion between 5-O-caffeoylquinic acid and 4-O-caffeoylquinic acid) could be the cause of this result (Baroni et al., 2018). It also suggested that products of the Millard reaction and phenolic compounds in the intermediate oxidation situation have robust antioxidant properties (Nicoli et al. 1999).
However previous researchers reported significant losses of antiradical activity after roasting of baru nuts with peels which were attributed to the pronounced decline in the content of some phenolic compounds (Lemos et al. 2012). According to previous researches, it is suggested that antioxidant activity behaviors, similar to phenolic compounds, depend on media and heat treatment (Lemos et al. 2012; Choi et al. 2012; Kim et al. 2011; Chipurura et al. 2010).
Sensory analysis
The quality of herbal infusion was determined by the color and, in particular, by the flavor which comprises aroma and taste. Based on the consumer’s opinion, a moderate aroma and taste of astringency, bitterness, and a clear, red or brown color are presumed for a suitable tea product that has been known to be very pleasant. The results pointed out that the overall acceptability and other attributes of quince tea were significantly affected by roasting (Table 2) whereas brewing time was ineffective.
Table 2.
Effect of the roasting and brewing time on the sensory characteristics of quince tea
| Roasting time | Brewing time | Appearance | Aroma | Taste | Overall acceptability |
|---|---|---|---|---|---|
| 0 | 5 | 2 ± 0.48ef | 2 ± 0.50d | 1.88 ± 0.40cd | 1.66 ± 0.35f |
| 10 | 2.05 ± 0.48ef | 2.55 ± 0.50cd | 1.77 ± 0.40d | 1.88 ± 0.35ef | |
| 15 | 1.55 ± 0.48f | 2.33 ± 0.50cd | 1.77 ± 0.40d | 1.88 ± 0.35ef | |
| 20 | 5 | 2.66 ± 0.48de | 3 ± 0.50bc | 2.11 ± 0.40cd | 2.22 ± 0.35def |
| 10 | 2.88 ± 0.48cde | 3.11 ± 0.50bc | 3 ± 0.40cd | 2.44 ± 0.35de | |
| 15 | 3.11 ± 0.48cd | 3.55 ± 0.50ab | 2.66 ± 0.40bc | 2.88 ± 0.35cd | |
| 40 | 5 | 4.38 ± 0.48ab | 4.11 ± 0.50a | 3.55 ± 0.40a | 3.88 ± 0.35ab |
| 10 | 4.5 ± 0.48ab | 4.27 ± 0.50a | 4.05 ± 0.40a | 4.16 ± 0.35a | |
| 15 | 3.77 ± 0.48bc | 3.61 ± 0.50ab | 3.38 ± 0.40ab | 3.55 ± 0.35abc | |
| 60 | 5 | 4.55 ± 0.48ab | 3.88 ± 0.50ab | 3.33 ± 0.40ab | 3.94 ± 0.35ab |
| 10 | 4.77 ± 0.48a | 4.22 ± 0.50a | 3.5 ± 0.40a | 3.83 ± 0.35ab | |
| 15 | 4.16 ± 0.48ab | 3.55 ± 0.50ab | 3.27 ± 0.40ab | 3.38 ± 0.35bc |
Different letters in the same column indicate significant differences between mean values (p < 0.05)
Among the qualitative characteristics, color is the first and most determining parameters determining tea infusion acceptance. The color score was more and more improved with increasing the roasting time and was the highest at roasting time of 60 min. Quince samples progressively turned dark with the enhancement in the roasting and brewing time (from light to dark brown). The perceived color change can be attributed to pyrolysis and browning products which were formed during roasting.
Based on the evaluation results of the taste and aroma, with increasing roasting time, the samples were more palatable. Therefore, the flavor in quince tea can be developed by the roasting process. The flavor is the result of the participation of volatile and nonvolatile chemical components. These compounds are either metabolites accumulated during maturation or products of different reactions during processing. Similar to the color, products of Maillard reactions, formed during roasting process, have a significant impact on the aroma of quince tea (Maietta et al. 2018; Sohrabvandi et al. 2013).
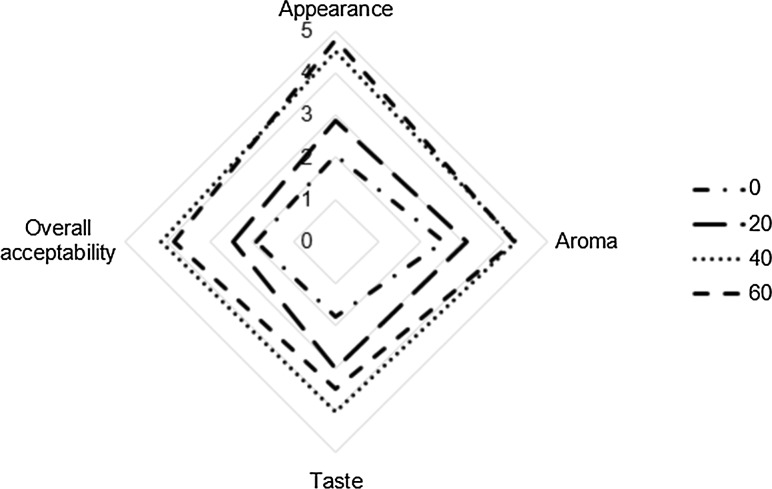
Finally, based on the overall acceptance, the tea prepared using the sample roasted for 40 min and brewing time of 10 min had the highest scores. Yet, no statistical difference between time roasting of 40 and 60 min was found. The panelists determined the lowest scores for the control sample (the tea prepared using the unroasted sample) in all features. The mean scores of evaluation of the quince tea samples for brewing time of 10 min on a scale from 1 (poor) to 5 (excellent) are pictured graphically in Fig. 5.
Fig. 5.
Radar plot for sensory attributes of tea infusions prepared using the roasted and unroasted quince for brewing time of 10 m s
According to the assessment results, the duration of 40 min is recommended as the appropriate time to roast the quince samples. Developing the brown color, agreeable aroma and taste, as well as the overall acceptance of quince tea can be attributed to various physicochemical changes (like water loss, flavor development, color change, and so on). Based on findings from previous studies, these changes may be caused by the transfer of heat and mass during the roasting process.
Conclusion
Being high in phenolic compounds and robust in antioxidant activity, C. oblonga employed to produce an introducible healthy beverage. Roasting process could affect the nutritional and sensory properties of products. As roasting time increased, despite reduction of the phenolic compounds, a significant increase appeared in antioxidant activity, and sensorial properties of the tisane, including color and taste. Accordingly, the roasted sample had lower phenolics content and higher total antiradical activity than the unroasted type. Besides, desirably the color intensity and other attributes in quince fruit tea improved with the increasing roasting time. Considering the elevated antioxidant activity achieved through the heating process, quince fruit can be considered as a healthy option for the production of a functional beverage.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Andrade P, Carvalho A, Seabra R, Ferreira M. A previous study of phenolic profiles of quince, pear, and apple purees by HPLC diode array detection for the evaluation of quince puree genuineness. J Agric Food Chem. 1998;46:968–972. doi: 10.1021/jf970571j. [DOI] [Google Scholar]
- Baroni MV, Gastaminza J, Podio NS, Lingua MS, Wunderlin DA, Rovasio JL, Dotti R, Rosso JC, Ghione S, Ribotta PD. Changes in the antioxidant properties of quince fruit (Cydonia oblonga Miller) during jam production at industrial scale. J Food Qual. 2018;1:1–19. doi: 10.1155/2018/1460758. [DOI] [Google Scholar]
- Brzezicha-Cirocka J, Grembecka M, Szefer P. Oxalate, magnesium and calcium content in selected kinds of tea: impact on human health. Eur Food Res Technol. 2016;242:383–389. doi: 10.1007/s00217-015-2548-1. [DOI] [Google Scholar]
- Chipurura B, Muchuweti M, Manditseraa F. Effects of thermal treatment on the phenolic content and antioxidant activity of some vegetables. Asian J Clin Nutr. 2010;2:93–100. doi: 10.3923/ajcn.2010.93.100. [DOI] [Google Scholar]
- Cho AS, Jeon SM, Kim MJ, Yeo J, Seo KI, Choi MS, Lee MK. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem Toxicol. 2010;48:937–943. doi: 10.1016/j.fct.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Choi JS, Kim HY, Seo WT, Lee JH, Cho KM. Roasting enhances antioxidant effect of bitter melon (Momordica charantia L.) increasing in flavan-3-ol and phenolic acid contents. Food Sci Biotechnol. 2012;21:19–26. doi: 10.1007/s10068-012-0003-7. [DOI] [Google Scholar]
- del Castillo MD, Ames JM, Gordon MH. Effect of roasting on the antioxidant activity of coffee brews. J Agric Food Chem. 2002;50:3698–3703. doi: 10.1021/jf011702q. [DOI] [PubMed] [Google Scholar]
- Farah A, de Paulis T, Trugo LC, Martin PR. Effect of roasting on the formation of chlorogenic acid lactones in coffee. J Agric Food Chem. 2005;53:1505–1513. doi: 10.1021/jf048701t. [DOI] [PubMed] [Google Scholar]
- Fattouch S, Caboni P, Coroneo V, Tuberoso CI, Angioni A, Dessi S, Marzouki N, Cabras P. Antimicrobial activity of Tunisian quince (Cydonia oblonga Miller) pulp and peel polyphenolic extracts. J Agric Food Chem. 2007;55:963–969. doi: 10.1021/jf062614e. [DOI] [PubMed] [Google Scholar]
- Gheisari HR, Abhari KH. Drying method effects on the antioxidant activity of quince (Cydonia oblonga Miller) tea. Acta Sci Pol Technol Aliment. 2014;13:129–134. doi: 10.17306/J.AFS.2014.2.2. [DOI] [PubMed] [Google Scholar]
- Hečimović I, Belščak-Cvitanović A, Horžić D, Komes D. Comparative study of polyphenols and caffeine in different coffee varieties affected by the degree of roasting. Food Chem. 2011;129:991–1000. doi: 10.1016/j.foodchem.2011.05.059. [DOI] [PubMed] [Google Scholar]
- Karar MGE, Pletzer D, Jaiswal R, Weingart H, Kuhnert N. Identification, characterization, isolation and activity against Escherichia coli of quince (Cydonia oblonga) fruit polyphenols. Food Res Int. 2014;65:121–129. doi: 10.1016/j.foodres.2013.10.040. [DOI] [Google Scholar]
- Kim HG, Kim GW, Oh H, Yoo SY, Kim YO, Oh MS. Influence of roasting on the antioxidant activity of small black soybean (Glycine max L. Merrill) LWT Food Sci Technol. 2011;44:992–998. doi: 10.1016/j.lwt.2010.12.011. [DOI] [Google Scholar]
- Legua P, Serrano M, Melgarejo P, Valero D, Martínez JJ, Martínez R, Hernández F. Quality parameters, biocompounds and antioxidant activity in fruits of nine quince (Cydonia oblonga Miller) accessions. Sci Hortic. 2013;154:61–65. doi: 10.1016/j.scienta.2013.02.017. [DOI] [Google Scholar]
- Lemos MRB, de Almeida Siqueira EM, Arruda SF, Zambiazi RC. The effect of roasting on the phenolic compounds and antioxidant potential of baru nuts [Dipteryx alata Vog.] Food Res Int. 2012;48:592–597. doi: 10.1016/j.foodres.2012.05.027. [DOI] [Google Scholar]
- Maietta M, Colombo R, Corana F, Papetti A. Cretan tea (Origanum dictamnus L.) as a functional beverage: an investigation on antiglycative and carbonyl trapping activities. Food Funct J. 2018;9:1545–1556. doi: 10.1039/C7FO01930K. [DOI] [PubMed] [Google Scholar]
- Mazzanti G, Di Sotto A, Vitalone A. Hepatotoxicity of green tea: an update. Arch Toxicol. 2015;89:1175–1191. doi: 10.1007/s00204-015-1521-x. [DOI] [PubMed] [Google Scholar]
- Nicoli M, Anese M, Parpinel M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci Technol. 1999;10:94–100. doi: 10.1016/S0924-2244(99)00023-0. [DOI] [Google Scholar]
- Pelvan E, Alasalvar C, Uzman Sh. Effects of roasting on the antioxidant status and phenolic profiles of commercial Turkish hazelnut varieties (Corylus avellana L.) J Agric Food Chem. 2012;60:1218–1223. doi: 10.1021/jf204893x. [DOI] [PubMed] [Google Scholar]
- Satoh E, Tohyama N, Nishimura M. Comparison of the antioxidant activity of roasted tea with green, oolong, and black teas. Int J Food Sci Nutr. 2005;56:551–559. doi: 10.1080/09637480500398835. [DOI] [PubMed] [Google Scholar]
- Schieber A, Keller P, Carle R. Determination of phenolic acids and flavonoids of apple and pear by high-performance liquid chromatography. J Chromatogr A. 2001;910:265–273. doi: 10.1016/S0021-9673(00)01217-6. [DOI] [PubMed] [Google Scholar]
- Silva BM, Andrade PB, Gonçalves AC, Seabra RM, Oliveira MB, Ferreira MA. Influence of jam processing upon the contents of phenolics, organic acids and free amino acids in quince fruit (Cydonia oblonga Miller) Eur Food Res Technol. 2004;218:385–389. doi: 10.1007/s00217-003-0845-6. [DOI] [Google Scholar]
- Silva BM, Andrade PB, Valentão P, Ferreres F, Seabra RM, Ferreira MA. Quince (Cydonia oblonga Miller) fruit (pulp, peel, and seed) and jam: antioxidant activity. J Agric Food Chem. 2004;52:4705–4712. doi: 10.1021/jf040057v. [DOI] [PubMed] [Google Scholar]
- Silva BM, Andrade PB, Martins RC, Valentão P, Ferreres F, Seabra RM, Ferreira MA. Quince (Cydonia oblonga Miller) fruit characterization using principal component analysis. J Agric Food Chem. 2005;53:111–122. doi: 10.1021/jf040321k. [DOI] [PubMed] [Google Scholar]
- Singh JP, Kaur A, Shevkani K, Singh N. Composition, bioactive compounds and antioxidant activity of common Indian fruits and vegetables. J Food Sci Technol. 2016;53(11):4056–4066. doi: 10.1007/s13197-016-2412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh JP, Kaur A, Singh N, Nim L, Shevkani K, Kaur H, Arora DS. In vitro antioxidant and antimicrobial properties of jambolan (Syzygium cumini) fruit polyphenols. LWT Food Sci Technol. 2016;65:1025–1030. doi: 10.1016/j.lwt.2015.09.038. [DOI] [Google Scholar]
- Sohrabvandi S, Oroognia P, Mortazavian AM, Khoshfarjham M, Ahmadi N. Determination of some nutritional value and organoleptic properties in fruity teas. J Paramed Sci. 2013;4:78–84. [Google Scholar]
- Sut S, Dall’Acqua S, Poloniato G, Maggi F, Malagoli M. Preliminary evaluation of quince (Cydonia oblonga Mill.) fruit as extraction source of antioxidant phytoconstituents for nutraceutical and functional food applications. J Sci Food Agric. 2018;99(3):1046–1054. doi: 10.1002/jsfa.9271. [DOI] [PubMed] [Google Scholar]
- Szychowski PJ, Munera-Picazo S, Szumny A, Carbonell-Barrachina ÁA, Hernández F. Quality parameters, bio-compounds, antioxidant activity and sensory attributes of Spanish quinces (Cydonia oblonga Miller) Sci Hortic. 2014;165:163–170. doi: 10.1016/j.scienta.2013.11.028. [DOI] [Google Scholar]
- Turkmen N, Sari F, Velioglu YS. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem. 2005;93:713–718. doi: 10.1016/j.foodchem.2004.12.038. [DOI] [Google Scholar]
- Wang LF, Kim DM, Lee CY. Effects of heat processing and storage on flavanols and sensory qualities of green tea beverage. J Agric Food Chem. 2000;48:4227–4232. doi: 10.1021/jf0003597. [DOI] [PubMed] [Google Scholar]
- Wojdyło A, Oszmiański J, Bielicki P. Polyphenolic composition, antioxidant activity, and polyphenol oxidase (PPO) activity of quince (Cydonia oblonga Miller) varieties. J Agric Food Chem. 2013;61:2762–2772. doi: 10.1021/jf304969b. [DOI] [PubMed] [Google Scholar]
- Xu B, Chang SK. Total phenolics, phenolic acids, isoflavones, and anthocyanins and antioxidant properties of yellow and black soybeans as affected by thermal processing. J Agric Food Chem. 2008;56:7165–7175. doi: 10.1021/jf8012234. [DOI] [PubMed] [Google Scholar]