Abstract
The effect of harvest periods on total phenol, antioxidant activity, individual phenolic compounds of fruit and leaves of Tavşan Yüreği, Memecik, Edremit, Ayvalık and Gemlik olive varieties grown in Turkey were investigated. The highest total phenol (317.70 mg/100 g and 2657.81 mg/100 g) were observed in Tavşan Yüreği olive fruit and Ayvalık leaves harvested in December, respectively. The highest antioxidant activities (83.84%) were determined in Edremit fruit harvested in August and 83.33% in either Edremit olive leaves harvested in November and Tavşan Yüreği leaves harvested in December. The olive fruit contained gallic acid ranging from 7.18 mg/100 g (August) to 35.85 mg/100 g (December) in case of Ayvalık and 2.09 mg/100 g (November) to 21.62 mg/100 g (December) in Edremit. Gemlik olives showed higher gallic acid contents compared to the other varieties, however it depended significantly on harvest time in all cases. 3,4-Dihydroxybenzoic acid contents ranged from 33.11 mg/100 g (October) to 25.17 mg/100 g (September) in Memecik olives; 12.17 mg/100 g (August) to 33.11 mg/100 g (December) in case of Tavşan Yüreği olives depending on harvest time. The 3,4-dihydroxybenzoic acid contents of Memecik leaves ranged between 122.25 mg/100 g (September) to 196.58 mg/100 g (August) and that of Tavşan Yüreği leaves changed between 99.38 mg/100 g (November) and 179.90 mg/100 g (August). The leaves of these two varieties contained significantly (p < 0.01) higher 3,4-dihydroxybenzoic acid contents than other varieties. The highest gallic acid (144.83 mg/100 g) was detected in Memecik leaves (September) whereas lowest were found in Gemlik leaves collected in October.
Keywords: Olive varieties, Fruit and leave, Harvest time, Total phenol, Antioxidant activity, Phenolic component, HPLC
Introduction
Olive (Oleaeuropaea L.) tree is one of the major crop used for edible oil and olive fruit production in Spain, Italy and Greece which are also principal olive oil producers around the globe (Salido et al. 2015; Ilarioni and Proietti 2014). Olive products such as virgin olive oil, table olives and by-products have raised particular attention in recent years (Sousa et al. 2014). Several metabolic processes influence the profile and amounts of olive bioactive compounds including phenols, tocopherols, chlorophylls and carotenoids, fatty acids and sterols during ripening (Matos et al. 2007). The effects of maturation process on composition and contents of phenolic compounds in olive fruits have been studied in several olive varieties and countries (Rotondi et al. 2004). Oleuropein is the main phenolic compound in green olive fruits and is responsible for their characteristic bitterness (Andrews et al. 2003). The fruit and oil of olive contain simple phenolic components such as vanillic, gallic, coumaric, and cafeic acids, tyrosol, hydroxytyrosol as well as more complex components such as secoiridoids (Tanilgan et al. 2007; Lee et al. 2009; Kaeidi et al. 2011; Özcan and Matthäus 2017). The herbal teas produced from olive tree leaf has also attracted scientific attention as they are being used traditionally. The health benefits of olive leaves and their use in cure of many diseases have been debated (Lee-Huang et al. 2003; El and Karakaya 2009; Ghanema and Sadek 2012). Micronutrients, including minerals, and phenolic compounds and their derivatives are known to play important role for the biological effects of olive leaf. Phenolic compounds have complex structure and form an important group of phytochemicals (Silva et al. 2006). Olive leaves could be used not only in medicines, cosmetics, and pharmaceuticals, but they can also be used to improve the shelf life of foods and to develop functional foods (Ranalli et al. 2003). Also, phenolic extracts of olive leaves have been used commercially in dietetic products and/or food integrators (Briante et al. 2002a, b). Olive leaves mainly contain five different phenolic groups which are oleuropeosit, flavonoids, flavonols, flavan-3-ols and phenolic acids. The most two important phenolic compounds present in olive are oleuroepin and hydtoxytyrasol (3p, 4p-dihydroxyphenilatonole) (Benavente-Garcia et al. 2000; Petridis et al. 2012). Beside these phenolic compounds, olive contains cafeic acid, p-coumaric acid, vanillic acid, vaniline, luteolin, diosmetin, rutin, luteolin 7-glucoside, apigenin 7-glucoside, and diosmetin 7-glucoside (Lee et al. 2009). The presence of antioxidants in olives and their antioxidant activities have been previously reported (Siger et al. 2008; Özcan and Matthäus 2017). Edremit, Gemlik, Ayvalık, Memecik, and Tavşan Yüreği olive varieties are the most common olive varieties grown commercially in Turkey. The current study is designed to evaluate effects of variety and harvest time on the total phenol, antioxidant activity and individual phenolic compounds in olive fruits and leaves from commonly available Ayvalık, Gemlik, Edremit, Memecik and Tavşan Yüreği varieties in Turkey which were obtained from various locations of Mediterranean, Aegean and Marmara Regions.
Material and methods
Material
Fruit and leaves from five different olive (Olea europaea L.) varieties (Edremit, Gemlik, Ayvalık, Memecik, and Tavşan Yüreği) cultivated in Marmara, Aegean, and Mediterranean Regions in Turkey, were obtained. The olive fruits differ with respect to size, shape and flesh contents whereas olive leaves generally different in length and shape. Memecik and Tavşan Yüreği fruit from Mediterranean Region is bigger and fleshy and leaves are long, thin, and thornlike. Ayvalık and Edremit olives belonging to Aegean Region are smaller and fleshless compared to the other cultivars and their leaves are short and large. Gemlik fruit from Marmara is bigger and fleshy and leaves are short and large. Leaves and fruit from these olives were harvested during five different harvest time periods [1st period (August 1–August 10), 2nd period (September 1–September 10), 3rd period (October 1–October 10) 4th period (November 1–November 10), 5th period (December 1–December 10)].
Method
Fruit samples were stored at − 10 °C and crushed in garlic press before analysis. Olive leaf were dried in atmosphere and ground using grinding machine. Olive fruits and leaves obtained after grinding process were weighed and prepared for extraction process.
Preparation of extract from olive and olive leaf
In order to obtain phenolic extracts of olive fruit, extraction was carried out as explained by Arslan (2010) after some modification. A sample of 1.5 g from the liquid part of crushed olive fruit were added to 20 ml of methanol and mixed using vortex mixture for 1 min followed by sonication in ultrasonic bath for 10 min. The samples were then centrifuged at 6000 rpm for 10 min. Supernatant was mixed with 20 ml of hexane in a separatory funnel and lower phase was taken and passed through PTFE 0.45 µm filter (AIM Syringe Filter PTFE). The clear solution obtained was transferred to tubes and made ready for analysis.
A modified method of Talhaoui et al. (2014) was used for preparing leaf phenolic extract. A sample of 0.5 g ground olive leaves was mixed with 10 ml of methanol/water (80/20) using vortex for 1 min and sonicated in ultrasonic bath for 10 min followed by 10 min centrifugation. The extract was then transferred to flasks and the same process was repeated thrice by adding methanol/water mix (80/20). The extract was finally separated from solvent using rotary evaporator and transferred to 25 ml flask and volume was made with methanol before analysis.
Total phenolic contents
The total phenolic contents were determined as gallic acid equivalent using Yoo et al. (2004) method which involves Folin–Ciocalteu reagent. The analysis was carried out with two parallel experiments. Diluted Folin–Ciocalteu (1:10) and 7.5% sodium carbonate (Na2CO3) solutions were prepared. The extract sample (0.5 ml) was added to 2.5 ml Folin–Ciocalteu’s reagent (10%; v/v) and the mixture was vortexed for 15 s and allowed to stand for 5 min. Afterwards 2 ml of 7.5% sodium carbonate (Na2CO3) solution was added along with mixing. The mixture was stored in dark for 2 h followed by addition of 5 ml of purified measurement of absorbance at 725 nm using a spectrophotometer. The total phenolic content was calculated using following equation:
Antioxidant activity
The antioxidant activities of olive fruit and leaf samples were measured using scavenging of stable DPPH (1,1-diphenyl-2-picrylhydrazyl) radical method of Lee et al. (1998). The analysis was carried out in three parallel experiments. The aqueous extract (100 µl) was added to 900 µl buffer solution and 2 ml of DPPH solution. The mixture was left to stand for 30 min in the dark and the absorbance was measured at 517 nm using a spectrophotometer. The antioxidant activities of samples were calculated using the following equation where the absorbance values for control sample were 0.549 and 0.602.
Determination of individual phenolic compounds using HPLC
Determination of phenolic compounds of the olive and leave extracts were measured using HPLC according to the method of Kara et al. (2014). The analysis was carried out by performing three parallel experiments. The extract was filtered through a 0.45 μm disposable membrane syringe filter and 200 μl of the extract was placed in 1.5 ml vial. HPLC analyses of phenolic compounds were performed using a Shimadzu-HPLC equipped with a PDA detector and an Inertsil ODS-3 (5 µm; 4.6 × 250 mm) column. The mobile phase was a mixture of 0.05% acetic acid in water (A) and acetonitrile (B). The flow rate of the mobile phase was 1 ml/min at 30 °C and the injection volume was 20 µl. The peaks were recorded at 280 nm using a PDA detector. The total running time per sample was 45 min. The gradient programme was as follows.
The quantification of phenolic compounds was carried out using the same HPLC–PDA method applied fort he analysis with the respective standard. The linearity of standard curve was expressed in terms of the determination coefficient plots of the integrated peaks area versus concentration of the same standard. All experiments were performed three times and presented as mean ± SD.
Statistical analysis
Microsoft Office Excel 2007 and MINITAB 16 Statistics Package for Windows were used for data analysis. The significance of the differences among means was evaluated using ANOVA test. Tukey test was applied for the groups turning out significant, and p < 0.01 was accepted statistically significant. All data obtained were expressed as mean ± standard deviation (Püskülcü and İkiz 1989).
Results and discussion
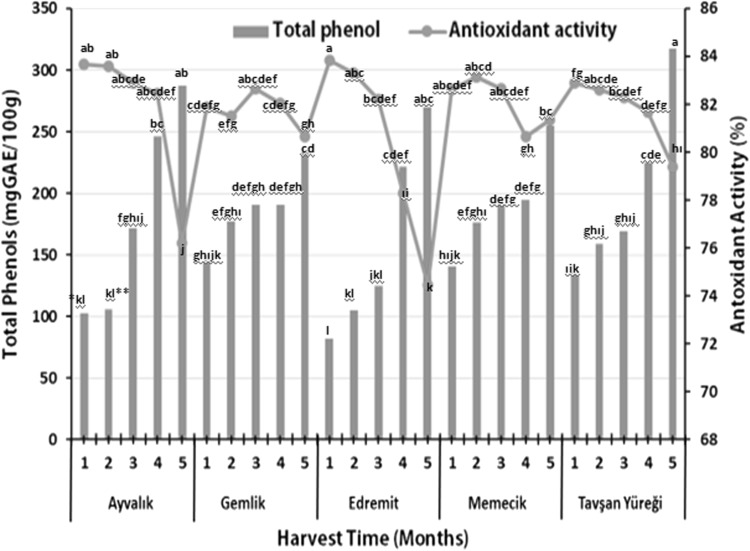
The total phenolic contents and antioxidant activities of five olive fruit cultivars harvested at different times are given in Fig. 1. The total phenolic contents of olive fruits ranged between 102.30 mg GAE/100 g (August) and 287.29 mg GAE/100 g (December) for Ayvalık fruit; 145.21 mg GAE/100 g (August) and 233.13 mg GAE/100 g (December) in case of Gemlik olives; 82.29 mg GAE/100 g (August) to 270.0 mg GAE/100 g (December) for Edremit fruit; 140.62 mg GAE/100 g (August) and 254.58 mg GAE/100 g (December) in case of Memecik and 133.96 mg GAE/100 g (August) and 225.0 mg GAE/100 g (December) in case of fruit from Tavşan Yüreği. It can be observed from data in Fig. 1 that the total phenolic contents of olive fruits increased depending on harvest time and maturity. Generally, total phenolic contents of Gemlik and Memecik fruits were found partly high compared to other three olive varieties (Ayvalık, Edremit and Tavşan Yüreği). The highest total phenolic content (317.70 mg GAE/100 g) was detected in Tavşan Yüreği fruit harvested in December (p < 0.01). There were statistically significant differences among total phenol contents of olive varieties depending on harvest times (p < 0.01). But, statistically differences among total phenol contents of Edremit, Memecik and Tavşan Yüreği olive varieties during 1, 2 and 3 harvest times were not observed.
Fig. 1.
Total phenol contents and antioxidant activity of olive fruits (n:3)
The antioxidant activities of fruits ranged between 88.885% (August) and 76.190% (December) for Ayvalık, 82.670% (October) and 80.640% (December) for Gemli, 83.841% (August) and 73.473% (December) for Edremit, 83.138% (September) and 80.640% (November) for Memecik and 82.904% (August) and 79.391% (December) for Tavşan Yüreği fruits depending on maturation and harvest times. In relation to harvest times, the highest antioxidant activity values were determined in Edremit (83.84%), Ayvalık (93.68%) and Tavşan Yüreği (82.90%) in August. In case of Memecik fruit, highest (83.13%) activity was detected in September harvest while Gemlik fruit showed maximum activity (82.67%) in October harvest (p < 0.01). Sevim and Tuncay (2012) reported that total phenolic contents of olive fruits depended on harvest time and climatic conditions. Singh et al. (2016) reported that while total phenolic contents (TPC) and antioxidant activity (ABTS and DPPH) values of different fruits change between 354.9 and 1639.7 mg GAE/100 g, 2.6–5.5 and 3.0–6.3 mM TE/g, respectively, total phenolic contents (TPC) and antioxidant activity (ABTS and DPPH) values of different vegetables ranged from 179.3 to 1028.6 mg GAE/100 g, 2.1–4.7 and 2.0–5.0 mM TE/g, respectively. Cerretani et al. (2006) observed that total phenolic contents of olive fruits could change with cultivars and type, climatic and environmental conditions and agricultural practices and technologies. It was observed in the current study that there was a significant increase in the total phenol contents in different varieties while harvest time/maturation period of olive fruits was increased from August to December. Tavşan Yüreği variety contained higher amount of total phenol compared to other cultivars. Depending on harvest time, the highest antioxidant activity value was 83.84% in Edremit olive fruits harvested in August. The results for total phenolic contents show conformance to the reports of Sevim and Tuncay (2012). Antioxidant activities of olive varieties decreased from August to December (except Gemlik cv.). But some differences in antioxidant values of olive fruits were observed depending on harvest time. These differences are thought to be related to maturity of the olive fruits during the harvest time, maturity and the orientation (the position and direction of the olive fruit according to daylight).
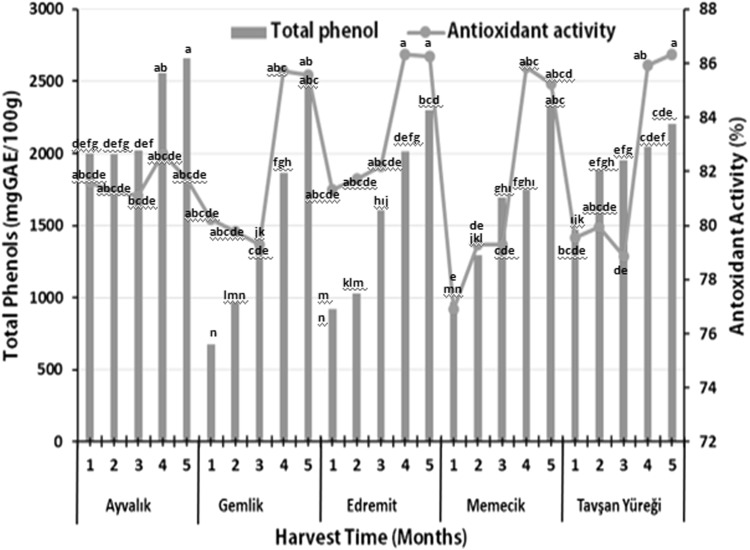
The total phenolic contents and antioxidant activities of the leaves of five olive varieties collected at the different harvest periods are presented in Fig. 2. The total phenol content of Ayvalık, Gemlik, Edremit, Memecik and Tavşan Yüreği olive leaves changed between 1995.31 mg GAE/100 g (September) and 2657.81 mg GAE/100 g (December), 965.62 mg GAE/100 g (August) and 3534.37 mg GAE/100 g (December), 917.19 mg GAE/100 g (August) and 2299.99 mg GAE/100 g (December), 965.62 mg GAE/100 g (August) and 2346.87 mg GAE/100 g (December), and 1471.87 mg GAE/100 g (September) and 2203.13 mg GAE/100 g (December), respectively. The highest total phenol contents were determined in the Ayvalık leaves harvested in December (p < 0.01). But, statistically differences among antioxidant activity values of Ayvalık, Gemlik and Tavşan Yüreği olive varieties during 1, 2, 3 and 4 harvest times were not observed. The antioxidant activity values ranged between 81.11% (October) and 82.67% (November) Ayvalık, 79.313% (October) and 85.174% (November) in Gemlik, 81.343% (August) and 86.339% (November) in Edremit, 76.893% (August) and 85.87% (November) in Memecik and 78.845% (October) and 86.339% (December) in Tavşan Yüreği leaves. It was observed that the highest antioxidant activities of 86.339% (Edremit) and 86.261% (Tavşan Yüreği) were observed in leaves harvested during November and December, respectively. Sevim and Tuncay (2012) compared the total phenolic contents of the leaves of Ayvalık and Memecik olive cultivars at different harvest times, and the total phenol contents of Memecik leaves were found higher compared to Ayvalık cultivar. In previous study, Keçeli and Büyükarslan (2008) determined antioxidant effect of Halhalı and Gemlik olive leaves. The antioxidant activity of Halhalı cv leaves concurrently decreased with maturity, it did not vary much more in Gemlik leave with maturation (Keçeli and Büyükarslan 2008). Al-Rimawi et al. (2014) determined antioxidant activities of olive leaves collected in Palestine at different harvest times. They identified that olive leaves harvested in June had higher antioxidant activity compared to those collected in January. Furthermore, olive leaves collected from different regions of Palestine also showed differences in antioxidant activity (Al-Rimawi et al. 2014). In other study, Keçeli and Harp (2014) reported that the leaves of Domat and Adana Topağı olives cultivars had higher antioxidant activity compared to those from Yerli and Gemlik cultivars. It was observed that total phenol contents of different olive varieties were not significantly affected by harvest time and the leaves of Ayvalık cultivar had higher total phenolic contents compared to the other varieties. The result about total phenol in leaves studied in the present study show similarity with reports of Sevim and Tuncay (2012). In addition, depending on harvest times, an increase in antioxidant activities of leaves of Edremit, Memecik and Tavşan Yüreği varieties was observed from August to December. The results of antioxidant activity showed differed from those of Al-Rimawi et al. (2014) who observed that olive leaves collected in winter months had more antioxidant activity compared to those of summer months. These differences can be probably due to climatic conditions as stated in the study of Sevim and Tuncay (2012). Among different olive leaves, Edremit variety had higher antioxidant activity compared to the other cultivars (p < 0.01).
Fig. 2.
Total phenol contents and antioxidant activity of olive leaves (n:3)
The chromatographic data about the contents of different individual phenolic compounds in five different olive fruits are shown in Table 1. The results showed that the gallic acid contents varied between 7.18 mg/100 g (August) and 35.85 mg/100 g (December) for Ayvalık; 2.09 mg/100 g (November) and 21.62 mg/100 g (December) Edremit; 26.61 mg/100 g gallic acid (September) and 5.52 mg/100 g gallic acid (October) for Gemlik; 18.23 mg/100 g (August) and 8.74 mg/100 g (September) for Memecik and 10.49 mg/100 g (November) and 17.44 mg/100 g (December) for Tavşan Yüreği olive fruits depending on harvest times. The fruit from Gemlik olive variety had higher amount of gallic acid compared to the others depending on harvet times (p < 0.01). The 3,4-dihydroxybenzoic acid contents of olive fruits varied between 5.42 mg/100 g (September) and 29.74 mg/100 g (December) in Ayvalık; 1.58 mg/100 g (October) and 24.98 mg/100 g (December) in Edremit; 15.86 mg/100 g (October) and 32.09 mg/100 g (December) in Gemlik; 33.11 mg/100 g (October) and 25.17 mg/100 g (September) Memecik and 12.17 mg/100 g (August) and 33.11 mg/100 g (December) in Tavşan Yüreği varieties depending on harvest times. The Gemlik olive variety had higher amount of 3,4-dihydroxybenzoic acid compared to the other olive varieties (p < 0.01). The catechin contents of olive fruits ranged between 8.46 mg/100 g (October) and 52.45 mg/100 g (December) Ayvalık; 3.08 mg/100 g (October) and 46.42 mg/100 g (December) in Edremit; 12.80 mg/100 g (October) and 42.16 mg/100 g (September) in Gemlik; 25.41 mg/100 g (December) and 41.61 mg/100 g (September) in Memecik and 10.67 mg/100 g (September) and 30.52 mg/100 g (November) in Tavşan Yüreği varieties depending on harvet times. Memecik olive fruits had higher amount of (+)-catechin compared to the other olive fruits. The highest 1,2-dihydroxybenzene (75.47 mg/100 g) was identified in Edremit variety harvested in December (p < 0.01). The 1,2-dihydroxybenzene contents of olive fruits changed between 6.21 mg/100 g (October) and 51.93 mg/100 g (December) in case of Ayvalık, 11.97 mg/100 g (October) and 49.74 mg/100 g (August); 14.53 mg/100 g (November) and 44.52 mg/100 g (September) in Memecik and 1.87 mg/100 g (September) and 68.00 mg/100 g (December) in case of Tavşan Yüreği varieties depending on harvest times. In case of syringic acid, the highest contents (20.47 mg/100 g) was observed in Edremit fruit harvested in December. The maximum p-coumaric acid (1.3 mg/100 g) was deteced in Memecik fruit harvested in October. The trans-ferulic acid contents of olive fruits varied between 0.26 mg/100 g (November) and 6.15 mg/100 g (December) in Ayvalık; 0.13 mg/100 g (October) and 10.09 mg/100 g (December) in Edremit; 0.16 mg/100 g (October) and 6.82 mg/100 g (December) in Gemlik; 2.13 mg/100 g (December) and 9.03 mg/100 g (September) in Memecik and 0.45 mg/100 g (October) and 13.18 mg/100 g (December) in case of Tavşan Yüreği varieties depending on harvest times (p < 0.01). It was observed that Tavşan Yüreği olive cultivar had higher t-ferulic acid compared to the other varieties (p < 0.01). The oleuropein contents of olive fruits showed a change between 0.06 mg/100 g (August) and 1.60 mg/100 g (December) in Ayvalık; 0.11 mg/100 g (November) and 1.45 mg/100 g (December) in Edremit; 0.17 mg/100 g (September) and 2.15 mg/100 g (November) in Gemlik; 1.20 mg/100 g (December) and 3.98 mg/100 g (October) in Memecik and 0.30 mg/100 g (August) and 3.68 mg/100 g (December) in Tavşan Yüreği vartieis depending on harvest times. The Memecik variety among all olive varieties had higher oleuropein compared to the other varieties (p < 0.01). Similarly, the quercetin contents in olive fruits change between 0.40 mg/100 g (August) and 8.78 mg/100 g (September) in Ayvalık; 0.78 mg/100 g (September and October) and 6.49 mg/100 g (December) in Gemlik and 3.09 mg/100 g (August) and 11.74 mg/100 g (November) in Tavşan Yüreği. The fruits from different olive varieties contained 0.64 mg/100 g (Ayvalık) to 18.11 mg/100 g (Tavşan Yüreği) of kaempferol and 1.02 mg/100 g (Ayvalık) to 9.48 mg/100 g (Tavşan Yüreği) of isorhamnetin. Generally, isorhamnetin contents of Tavşan Yüreği olive fruits were found higher compared to other varieties (p < 0.01). The Tavşan Yüreği olive fruit harvested in December had highest 3,4-dihydroxybenzoic acid value (p < 0.01). The highest 1,2-dihydroxybenzene and syringic acid contents were observed in Edremit fruit collected during December (p < 0.01). The highest p-coumaric acid, oleuropein and (+)-catechin contents were observed in Memecik fruit harvested in October (p < 0.01). The higher contents of trans-ferulic acid and apigenin-7-gycoside were detected in Tavşan Yüreği fruit harvested in December while compared to other olive fruits (p < 0.01). Generally, t-ferulic acid, apigenin-7-glycoside, quercetin, t-cinnamic acid, naringenin, kaempherol and isorhamnetin contents of Tavşan Yüreği olive fruits were found partly higher compared to other olive varieties. The Tavşan Yüreği fruit generally showed the highest total phenol in December and the antioxidant activity value of Edremit olive fruit harvested in August were found higher compared to the other varieties (p < 0.01). But, statistically differences among phenolic compounds of olive varieties during 1, 2 and 3 harvest times were not observed. In addition, statistically differences among syringic and caffeic acid contents of all olive varieties during harvest times were not observed. Sousa et al. (2014) analyzed the phenolic compounds of olive fruits harvested in September, October and November and they reported 439–672 mg/kg of hydroxytyrosol, 22–788 mg/kg of chlorogenic acid, 126–32,938 mg/kg of oleuropein, 9–250 mg/kg of rutin, 88–131 mg/kg of apigenin-7-d-glucoside and 48–53 mg/kg of luteolin. The observed that harvest time changed the phenolic compounds contents and rutin increased with delaying the harvest time. As generally understood that olive fruits are main food from olive tree and used for human consumption, olive leaves are also a rich source of the same types of valuable phenolic compounds. Among phenolic compounds oleuropein and hydroxytyrosol are the most abundant phenolic compounds found in olive leaves (El and Karakaya 2009). Phenolic compounds identified in olive meals include secoiridoids, iridoids, oleuropein, tyrosol, gallic, protocatechuic, vanillic, caffeic, syringic, p-coumaric, ferulic and cinnamic acids (Servili et al. 1999; Soler-Rivas et al. 2000; Dağdelen et al. 2013). Romani et al. (1999) established differences in the phenolic compounds of Frantoio, Rossellino, Ciliegino, Cuoricino and Grossolano in Toskana region. In previous study, phenolic components of Erkence, Memecik, Domat, Nizip-Yağlık, Gemlik and Ayvalık olive fruits showed differences depending on different harvest periods (2005–2006) (Ocakoglu et al. 2009). Current study resembled partially with other reports however various differences are also observed which may be attributed to variations in location, olive fruit cultivars, cultural activities, climatic conditions, plant diseases and collection time.
Table 1.
The phenolic components of olive fruits (mg/100 g)
| Olive variety | Harvest Months | Gallic acid | 3,4-Dihydroxybenzoic acid (+) | (+)-Catechin | 1,2-Dihydroxybenzene | Syringic acid | Caffeic acid | Routine trihydrate | p-coumaric acid |
|---|---|---|---|---|---|---|---|---|---|
| Ayvalık | 1 | 7.18 ± 0.01*cd | 9.76 ± 0.00defg | 10.68 ± 0.00ef | 8.93 ± 0.01fgh | 2.67 ± 0.01b | 0.91 ± 0.00cd | 0.14 ± 0.00c | 0.04 ± 0.00e |
| 2 | 11.14 ± 0.00bcd** | 5.42 ± 0.00efg | 23.88 ± 0.00abcdef | 19.72 ± 0.00defgh | 2.30 ± 0.00b | 2.10 ± 0.00bcd | 3.24 ± 0.00c | 0.24 ± 0.00bcde | |
| 3 | 14.54 ± 4.44bcd | 11.97 ± 3.32bcdefg | 8.46 ± 0.85ef | 6.21 ± 4.79gh | 5.92 ± 3.99b | 6.12 ± 5.77abcd | 3.50 ± 1.61c | 0.44 ± 0.03bcde | |
| 4 | 8.50 ± 5.77cd | 6.73 ± 0.92defg | 21.70 ± 1.78bcdef | 16.39 ± 0.33efgh | 4.61 ± 1.99b | 1.91 ± 1.77bcd | 0.81 ± 0.90c | 0.06 ± 0.00de | |
| 5 | 35.85 ± 0.35a | 29.74 ± 3.31ab | 52.45 ± 7.42a | 51.93 ± 22.73abc | 8.91 ± 0.49b | 3.79 ± 2.59abcd | 6.15 ± 2.08abc | 0.76 ± 0.32abcde | |
| Edremit | 1 | 11.70 ± 5.74bcd | 11.79 ± 0.47bcdefg | 15.44 ± 0.25cdef | 9.35 ± 0.41fgh | 2.93 ± 0.64b | 1.41 ± 0.74cd | 2.57 ± 1.35c | 0.44 ± 0.04bcde |
| 2 | 11.07 ± 1.26bcd | 14.77 ± 3.07abcdefg | 27.28 ± 0.62abcdef | 24.46 ± 1.10cdefgh | 7.80 ± 1.87b | 2.39 ± 0.01abcd | 4.07 ± 0.44bc | 0.56 ± 0.01abcde | |
| 3 | 10.96 ± 1.64bcd | 1.58 ± 0.42g | 3.08 ± 0.23f | 4.70 ± 0.28gh | 0.47 ± 0.07b | 0.43 ± 0.30d | 0.14 ± 0.00c | 0.07 ± 0.01cde | |
| 4 | 2.09 ± 1.88d | 4.09 ± 2.67fg | 5.05 ± 0.35f | 5.86 ± 1.67gh | 1.78 ± 1.36b | 0.57 ± 0.16d | 1.85 ± 1.43c | 0.17 ± 0.00bcde | |
| 5 | 21.62 ± 4.41abc | 24.98 ± 1.16abcd | 46.42 ± 3.65ab | 75.47 ± 14.95a | 20.74 ± 1.92a | 5.98 ± 1.30abcd | 12.61 ± 1.74ab | 0.63 ± 0.11abcde | |
| Gemlik | 1 | 15.24 ± 0.45bcd | 27.12 ± 3.34abc | 29.15 ± 6.04abcdef | 49.74 ± 7.01abcd | 10.34 ± 0.71b | 14.03 ± 0.18a | 7.21 ± 0.04abc | 0.77 ± 0.21abcde |
| 2 | 26.61 ± 3.92ab | 23.60 ± 0.17abcde | 42.16 ± 10.30abc | 35.66 ± 6.45cdefg | 9.26 ± 1.84b | 8.33 ± 5.02abcd | 7.38 ± 4.07abc | 0.85 ± 0.18abcd | |
| 3 | 5.52 ± 7.02cd | 15.86 ± 19.71abcdefg | 12.80 ± 15.96def | 11.97 ± 11.87fgh | 1.42 ± 1.53b | 0.92 ± 0.52cd | 0.17 ± 0.06c | 0.06 ± 0.07de | |
| 4 | 20.31 ± 8.78abc | 22.80 ± 1.73abcdef | 34.37 ± 17.35abcde | 33.91 ± 4.85cdefg | 9.85 ± 0.95b | 5.88 ± 1.24abcd | 6.54 ± 1.61abc | 0.32 ± 0.39bcde | |
| 5 | 21.35 ± 4.20abc | 32.09 ± 4.82a | 36.94 ± 7.35abcde | 13.02 ± 11.60efgh | 10.25 ± 0.35b | 9.97 ± 6.83abcd | 12.98 ± 4.61a | 0.52 ± 0.04abcde | |
| Memecik | 1 | 18.23 ± 0.10bcd | 21.33 ± 2.36abcdef | 30.23 ± 1.57abcdef | 16.11 ± 4.29efgh | 7.36 ± 0.59b | 13.46 ± 0.40ab | 2.84 ± 0.31c | 0.89 ± 0.08ab |
| 2 | 8.74 ± 4.86cd | 25.17 ± 0.64abc | 41.61 ± 3.75abcd | 44.52 ± 1.05abcde | 5.74 ± 5.18b | 4.92 ± 1.78abcd | 7.19 ± 0.27abc | 0.90 ± 0.17ab | |
| 3 | 11.21 ± 0.66bcd | 14.74 ± 0.00abcdefg | 31.36 ± 8.03abcdef | 25.84 ± 0.79cdefgh | 8.99 ± 0.89b | 5.93 ± 1.46abcd | 1.31 ± 0.42c | 1.30 ± 0.56a | |
| 4 | 13.78 ± 1.02bcd | 21.31 ± 6.26abcdef | 41.11 ± 11.61abcd | 14.53 ± 3.95efgh | 6.12 ± 3.25b | 12.67 ± 1.17abc | 8.26 ± 3.37abc | 0.78 ± 0.34abcde | |
| 5 | 19.86 ± 2.74abc | 16.25 ± 1.63abcdefg | 25.41 ± 2.42abcdef | 22.09 ± 6.21cdefgh | 6.40 ± 2.12b | 2.85 ± 0.18abcd | 3.77 ± 1.09bc | 0.48 ± 0.10bcde | |
| Tavşan Yüreği | 1 | 11.54 ± 1.81bcd | 12.17 ± 4.31bcdefg | 21.62 ± 4.30bcdef | 39.83 ± 5.05bcdef | 5.67 ± 4.40b | 3.02 ± 2.26abcd | 0.14 ± 0.08c | 0.07 ± 0.01de |
| 2 | 13.20 ± 0.54bcd | 24.10 ± 2.18abcde | 10.67 ± 8.36ef | 1.87 ± 1.27h | 6.02 ± 4.58b | 4.25 ± 5.25abcd | 3.75 ± 4.26bc | 0.65 ± 0.20abcde | |
| 3 | 11.47 ± 0.33bcd | 24.99 ± 0.10abcd | 22.39 ± 4.40bcdef | 10.43 ± 12.41fgh | 4.71 ± 0.07b | 2.58 ± 2.12abcd | 0.93 ± 0.05c | 0.87 ± 0.17abc | |
| 4 | 10.49 ± 1.09bcd | 23.58 ± 3.42abcde | 30.52 ± 3.76abcdef | 9.36 ± 8.40fgh | 8.09 ± 0.27b | 12.13 ± 5.81abcd | 12.60 ± 5.54ab | 0.55 ± 0.10abcde | |
| 5 | 17.44 ± 9.25bcd | 33.11 ± 0.81a | 28.97 ± 10.62abcdef | 68.00 ± 6.11ab | 8.61 ± 6.24b | 3.18 ± 3.19abcd | 2.85 ± 1.12c | 0.79 ± 0.23abcde |
| Olive | Months | t-ferulic acid | Apigenin 7 glycoside | Oleuropein | Quercetin | t-cinnamic acid | Naringenin | Kaempherol | Isorhamnetin |
|---|---|---|---|---|---|---|---|---|---|
| Ayvalık | 1 | 0.42 ± 0.00*bc | 0.38 ± 0.00e | 0.06 ± 0.00d | 0.40 ± 0.00a | 0.04 ± 0.00c | 0.42 ± 0.00e | 0.64 ± 0.00f | 1.02 ± 0.00h |
| 2 | 4.04 ± 0.00abc** | 4.04 ± 0.00abcde | 0.55 ± 0.00d | 8.78 ± 0.00a | 0.11 ± 0.00bc | 1.06 ± 0.00de | 1.23 ± 0.00def | 2.04 ± 0.00defgh | |
| 3 | 0.84 ± 0.66bc | 0.60 ± 0.10de | 0.48 ± 0.39d | 1.35 ± 0.47a | 0.26 ± 0.08bc | 0.39 ± 0.03e | 0.92 ± 0.10f | 1.07 ± 0.04h | |
| 4 | 0.26 ± 0.14bc | 0.53 ± 0.41e | 0.36 ± 0.31d | 0.97 ± 0.04a | 0.05 ± 0.03c | 0.58 ± 0.03e | 1.04 ± 0.19f | 1.11 ± 0.16gh | |
| 5 | 9.43 ± 7.23abc | 5.32 ± 1.41abcde | 1.60 ± 0.3abcd | 6.06 ± 2.49a | 0.63 ± 0.13bc | 1.41 ± 0.13de | 4.56 ± 0,45bcdef | 5.80 ± 0.08abcdef | |
| Edremit | 1 | 0.69 ± 0.29bc | 0.48 ± 0.00e | 0.14 ± 0.06d | 1.98 ± 0.28a | 0.28 ± 0.08bc | 0.76 ± 0.01e | 1.85 ± 0.52def | 2.81 ± 0.61cdefgh |
| 2 | 5.35 ± 0.16abc | 3.60 ± 0.62bcde | 0.89 ± 0.01cd | 3.47 ± 1.29a | 0.10 ± 0.08bc | 1.46 ± 0.39de | 1.03 ± 0.53f | 1.17 ± 0.38gh | |
| 3 | 0.13 ± 0.09c | 0.32 ± 0.04e | 0.14 ± 0.06d | 2.50 ± 0.71a | 0.10 ± 0.01bc | 0.48 ± 0.39e | 1.77 ± 0.25def | 1.10 ± 0.05h | |
| 4 | 0.77 ± 0.00bc | 0.92 ± 0.12cde | 0.11 ± 0.03d | 0.57 ± 0.40a | 0.07 ± 0.02bc | 0.30 ± 0.21e | 1.19 ± 0.05ef | 1.11 ± 0.41gh | |
| 5 | 10.09 ± 1.54ab | 7.73 ± 0.27ab | 1.45 ± 0.43bcd | 1.64 ± 0.00a | 0.71 ± 0.06bc | 3.71 ± 0.58abcde | 4.18 ± 0.45cdef | 6.34 ± 0.06abcd | |
| Gemlik | 1 | 5.03 ± 0.33abc | 3.05 ± 1.03bcde | 1.00 ± 0.17cd | 6.49 ± 0.07a | 0.48 ± 0.10bc | 3.19 ± 0.16bcde | 3.13 ± 1.09cdef | 8.37 ± 0.83ab |
| 2 | 0.46 ± 0.40bc | 0.40 ± 0.36e | 0.17 ± 0.03d | 0.78 ± 0.41a | 0.05 ± 0.01bc | 0.31 ± 0.13e | 1.31 ± 0.44def | 1.35 ± 0.01abc | |
| 3 | 0.16 ± 0.03c | 0.40 ± 0.36e | 0.17 ± 0.03d | 0.78 ± 0.41a | 0.05 ± 0.01bc | 0.31 ± 0.13e | 1.31 ± 0.44def | 1.35 ± 0.01fgh | |
| 4 | 6.82 ± 2.26abc | 7.37 ± 2.81ab | 1.66 ± 0.74abcd | 3.92 ± 0.14a | 0.90 ± 0.07bc | 0.99 ± 0.61e | 3.85 ± 0.86cdef | 5.32 ± 0.96abcdefgh | |
| 5 | 6.12 ± 0.14abc | 5.88 ± 0.03abcd | 2.15 ± 0.22abcd | 6.49 ± 0.52a | 0.84 ± 0.02bc | 3.02 ± 1.33bcde | 6.85 ± 0.33bc | 4.63 ± 1.03bcdefgh | |
| Memecik | 1 | 2.97 ± 0.47bc | 1.08 ± 0.69cde | 3.15 ± 0.35abc | 1.84 ± 0.05a | 0.60 ± 0.01bc | 4.82 ± 1.84abcd | 8.70 ± 1.70b | 6.12 ± 0.93abcde |
| 2 | 9.03 ± 1.60abc | 8.27 ± 0.92ab | 1.53 ± 0.05bcd | 4.22 ± 0.08a | 0.73 ± 0.00bc | 2.70 ± 0.74cde | 4.69 ± 0.83bcdef | 4.39 ± 0.01bcdefgh | |
| 3 | 5.63 ± 4.77abc | 3.98 ± 2.88bcde | 3.98 ± 0.00a | 3.73 ± 1.91a | 0.60 ± 0.04bc | 1.69 ± 1.09de | 3.87 ± 0.24cdef | 5.27 ± 0.18abcdefgh | |
| 4 | 7.39 ± 4.43abc | 7.78 ± 0.79ab | 2.23 ± 0.04abcd | 3.36 ± 1.41a | 0.94 ± 0.67a | 0.99 ± 1.34e | 2.67 ± 0.69def | 2.66 ± 1.16cdefgh | |
| 5 | 2.13 ± 0.36bc | 4.44 ± 1.84abcde | 1.20 ± 0.19cd | 3.50 ± 0.62a | 0.32 ± 0.08b | 0.73 ± 0.11e | 0.97 ± 0.54f | 1.57 ± 0.18efgh | |
| Tavşan Yüreği | 1 | 0.45 ± 0.50bc | 0.51 ± 0.11e | 0.30 ± 0.24d | 3.09 ± 0.86a | 0.52 ± 0.56bc | 3.98 ± 0.34abcde | 4.13 ± 0.64cdef | 2.33 ± 0.18defgh |
| 2 | 7.91 ± 0.49abc | 8.24 ± 1.84ab | 1.63 ± 0.35abcd | 3.25 ± 0.72a | 4.44 ± 1.95bc | 7.14 ± 0.76a | 18.11 ± 3.78a | 9.48 ± 1.84a | |
| 3 | 4.39 ± 2.50abc | 5.46 ± 1,98sbcde | 3.68 ± 2.18ab | 5.94 ± 0.06a | 1.84 ± 0.11 | 5.60 ± 0.48abc | 4.66 ± 0.25 | 8.90 ± 1.07ab | |
| 4 | 8.52 ± 4.55abc | 6.17 ± 2,93abc | 1.82 ± 0.71abcd | 11.74 ± 12.64a | 1.02 ± 0.06 | 6.81 ± 0.04ab | 5.36 ± 0.03 | 5.81 ± 3.78abcdef | |
| 5 | 13.18 ± 3.54a | 9.30 ± 0,82a | 3.82 ± 1.39ab | 6.33 ± 4.48a | 0.94 ± 0.40 | 6.44 ± 3.28abc | 5.19 ± 1.74 | 5.68 ± 1.81abcdefg |
*The data show mean (n:3) ± standard deviation
**The different letters in the same column show statistically significant differences according to the Tukey test (p < 0.01)
The chromatographic results for phenolic compounds of leaves from five olive cultivars collected at the different periods (from August to December) are shown in Table 2. The 3,4-dihydroxybenzoic acid contents ranged between 122.25 mg/100 g (September) and 196.58 mg/100 g (August) for Memecik and that of Tavşan Yüreği leaves were from 99.38 mg/100 g (November) to 179.90 mg/100 g (August) depending on harvest times. The 3,4-dihydroxybenzoic acid contents of other olive cultivars were found partly lower compared to Memecik and Tavşan Yüreği leaves (p < 0.01). The highest and lowest gallic acids (144.83 and 9.10 mg/100 g) were determined in Memecik and Gemlik olive leaves collected in September and October, respectively. The catechin contents were found between 16.33 mg/100 g (December) and 219.60 mg/100 g (September) in Ayvalık; 16.71 mg/100 g (August) and 248.18 mg/100 g (November) in Edremit; 24.90 mg/100 g (October) and 231.23 mg/100 g (November) in Gemlik; 158.18 mg/100 g (November) and 329.03 mg/100 g (September) in Memecik and 102.08 mg/100 g (November) and 201.20 mg/100 g (August) in Tavşan Yüreği leaves. The leaves of Memecik variety had higher (+)-catechin compared to the other olive leaves. The 1,2-dihydroxybenzene contents varied between 12.39 mg/100 g (December) and 264.90 mg/100 g (August) in Ayvalık; 6.18 mg/100 g (August) and 228.38 mg/100 g (November) in Edremit; 6.17 mg/100 g (October) and 263.18 mg/100 g 1,2-dihydroxybenzene (November) in Gemlik; 32.93 mg/100 g (November) and 266.70 mg/100 g (September) in Memecik and 97.13 mg/100 g (September) and 338.78 mg/100 g (December) in Tavşan Yüreği leaves. The Tavşan Yüreği leaves had higher 1,2-dihydroxybenzene compared to the other olive leaves (p < 0.01). The highest syringic and caffeic acids (116.33 and 85.05 mg/100 g) were determined in Ayvalık and Memecik olive leaves collected in November and October, respectively. The caffeic acid contents changed between 1.41 mg/100 g (December) and 74.78 mg/100 g (August) in Ayvalık; 2.07 mg/100 g (September) and 42.38 mg/100 g (November) in Edremit; 10.00 mg/100 g (October) and 24.15 mg/100 g caffeic acid (September) in Gemlik and 6.21 mg/100 g (October) and 66.90 mg/100 g (December) in Tavşan Yüreği leaves. Generally, Memecik leaves had higher caffeic acid compared to the other varieties. The highest t-ferulic acid contents of olive leaves was found in Tavşan Yüreği leaves collected in September (79.05 mg/100 g). The oleuropein contents changed between 0.82 mg/100 g (September) and 43.58 mg/100 g (November) in Ayvalık; 1.83 mg/100 g (August) and 69.60 mg/100 g (December) in Edremit; 4.93 mg/100 g (December) and 51.90 mg/100 g (August) in Gemlik; 6.91 mg/100 g (December) and 12.69 mg/100 g (September) (p < 0.01) in Memecik and 4.24 mg/100 (September) and 18.68 mg/100 g (December) in Tavşan Yüreği leaves. The Edremit leaves showed higher oleuropein values compared to the other cultivars depending on harvest times. The highest and lowest quercetin contents of olive leave samples were determined in Tavşan Yüreği (273.6 mg/100 g) and Ayvalık (8.22 mg/100 g) collected in August and September, respectively. The naringenin contents ranged between 3.09 mg/100 g (December) and 44.40 mg/100 g (August) in Ayvalık and 2.97 mg/100 g (November) and 277.50 mg/100 g (October) in Tavşan Yüreği leaves. The highest kaempherol and isorhamnetin were determined in Tavşan Yüreği olive leaves (886.58 and 241.65 mg/100 g) collected in October (p < 0.01). Depending on the harvest time, the highest total phenols were detected in Ayvalık leaves harvested in December (p < 0.01). In addition, Edremit and Tavşan Yüreği leaves from November and December harvest, respectively showed highest antioxidant activity. Among cultivars, the highest total phenol was observed in the leaves of Memecik cultivar (p < 0.01). The highest 3,4-dihydroxybenzoic acid was detected in Gemlik leaves collected in August (p < 0.01). The highest 1,2-dihydroxybenzene was found in the leaves of Tavşan Yüreği collected in December. Statistically differences among phenolic compounds of leaves of olive varieties during 1, 2 and 3 harvest times were not observed. But, some varieties showed statistically differences among phenolic compounds of leave extracts during 4 harvest. But, some results were found similar statistically. Statistically these similarities were found more in 3,4-dihydroxybenzoic acid, (+)-catechin, 1,2-dihydroxybenzene and isorhamnetin compounds. In previous study, Sevim and Tuncay (2012) reported that Ayvalık and Memecik olive leaves and fruits contained 279.39 and 198.87 mg GAE/100 g total phenol and 349.72 and 253.40 mg GAE/100 g total phenol, respectively. However, they did not observe any significant differences between the leaves of Ayvalık and Memecik olive cultivars in terms of total phenolic contents depending on harvest times. In other study, olive leave and olive fruit extracts contained 0.54–0.076 mg/g hydroxytyrosol, 0.058–0.032 mg/g vanillic acid, 217–0.081 mg/g caffeic acid, 0.57–0.072 mg/g vanillin, 0.83–0.003 mg/g verbascoside, 0.13–0.002 mg/g rutin, 6.53–0.042 mg/g oleuropein (Xie et al. 2015). Brahmi et al. (2013) studied phenolic compounds in olive leaves collected from Maladia Region (Center of Tunisia) and observed that harvest times affected phenolic compounds of olive leaves. They observed that the phenolic acid contents of olive leaves obtained from different olive cultivars during January were found higher compared to other harvest periods (Brahmi et al. 2013). Al-Rimawi et al. (2014) determined total flavonoid contents of olive leaves harvested during different time from variable geographic regions in Palestine and found that leaves harvested in June had higher total flavonoid contents than those harvested during winter. Hence it can be observed that the bioactive contents in olive fruit and leaves are affected significantly by the type of cultivar, geographical area and harvest time.
Table 2.
The phenolic components of olive leaves (mg/100 g)
| Olive variety | Months | Gallic acid | 3,4-Dihydroxybenzoic acid (+) | (+)-Catechin | 1,2-Dihydroxybenzene | Syrinic acid | Caffeic acid | Routine trihydrate | p-coumaric acid |
|---|---|---|---|---|---|---|---|---|---|
| Ayvalık | 1 | 90.00 ± 6.15*ab | 170.70 ± 23.33ab | 80.18 ± 83.47cd | 264.90 ± 98.43ab | 64.20 ± 15.70abcd | 74.78 ± 56.53a | 3.54 ± 2.65b | 8.00 ± 4.21b |
| 2 | 82.28 ± 4.35ab** | 56.93 ± 29.59bcde | 219.60 ± 33.09abcd | 132.53 ± 33.41bcd | 40.20 ± 13.58bcd | 18.68 ± 1.38a | 9.59 ± 5.26b | 6.10 ± 7.04b | |
| 3 | 95.25 ± 5.30ab | 128.55 ± 13.36abcde | 216.60 ± 2.97abcd | 179.10 ± 112.43abcd | 26.63 ± 0.53bcd | 33.60 ± 16.12a | 3.44 ± 0.26b | 49.05 ± 35.21a | |
| 4 | 96.08 ± 6.26ab | 90.75 ± 35.64abcde | 153.75 ± 13.36abcd | 124.13 ± 19.20bcd | 116.33 ± 41.90a | 27.75 ± 12.73a | 31.88 ± 18.99ab | 9.97 ± 5.16b | |
| 5 | 89.33 ± 49.53ab | 20.10 ± 15.48de | 16.33 ± 3.00d | 12.39 ± 3.45d | 1.73 ± 1.46d | 1.41 ± 0.46a | 5.17 ± 4.86b | 2.96 ± 0.88b | |
| Edremit | 1 | 80.25 ± 69.58ab | 163.20 ± 40.31ab | 16.71 ± 10.31d | 6.18 ± 6.92d | 5.19 ± 1.27cd | 2.92 ± 2.79a | 2.07 ± 0.79b | 0.78 ± 0.69b |
| 2 | 72.45 ± 25.24ab | 11.48 ± 6.03e | 19.95 ± 12.31d | 14.71 ± 11.01d | 4.81 ± 0.29cd | 2.07 ± 0.66a | 0.54 ± 0.04b | 0.44 ± 0.13b | |
| 3 | 120.98 ± 0.95a | 116.18 ± 5.41abcde | 52.95 ± 27.79cd | 40.73 ± 24.71cd | 54.60 ± 28.64abcd | 4.01 ± 1.66a | 1.62 ± 0.04b | 0.56 ± 0.45b | |
| 4 | 92.03 ± 1899ab | 97.28 ± 72.44abcde | 248.18 ± 111.26abc | 228.38 ± 62.05abc | 45.30 ± 19.09bcd | 38.10 ± 7.42a | 36.68 ± 14.53ab | 3.27 ± 2.00b | |
| 5 | 116.03 ± 26.62ab | 139.65 ± 3.82abcde | 235.58 ± 14.32abc | 178.95 ± 76.79abcd | 33.68 ± 19.62bcd | 42.38 ± 7.11a | 21.45 ± 25.88ab | 8.97 ± 5.91b | |
| Gemlik | 1 | 115.43 ± 12.83ab | 207.38 ± 102.78a | 315.75 ± 63.64a | 244.88 ± 88.57ab | 18.76 ± 9.95bcd | 12.00 ± 0.23a | 36.08 ± 11.56ab | 3.56 ± 3.79b |
| 2 | 85.58 ± 8.59ab | 90.90 ± 18.24abcde | 52.20 ± 48.79cd | 178.65 ± 7.21abcd | 34.80 ± 0.85bcd | 24.15 ± 7.85a | 5.76 ± 0.18b | 0.81 ± 0.01b | |
| 3 | 9.10 ± 1.49b | 27.38 ± 9.86cde | 24.90 ± 4.03d | 6.17 ± 2.33d | 10.08 ± 0.90cd | 10.00 ± 1.80a | 0.77 ± 0.61b | 0.34 ± 0.11b | |
| 4 | 82.20 ± 13.58ab | 152.70 ± 21.00abcd | 231.23 ± 97.05abc | 263.18 ± 80.93ab | 32.70 ± 7.64bcd | 37.20 ± 0.00a | 1.50 ± 1.45b | 0.43 ± 0.30b | |
| 5 | 81.98 ± 5.20ab | 181.05 ± 14.21ab | 164.18 ± 2.44abcd | 191.78 ± 35.53abcd | 35.03 ± 13.89bcd | 19.06 ± 7.83a | 5.12 ± 1.03b | 5.86 ± 0.56b | |
| Memecik | 1 | 84.98 ± 12.62ab | 196.58 ± 6.89a | 318.38 ± 69.90a | 26.43 ± 4.14ab | 18.90 ± 16.12bcd | 7.19 ± 0.07a | 9.29 ± 4.73b | 14.22 ± 0.16b |
| 2 | 144.83 ± 78.59a | 122.25 ± 31.18abcde | 329.03 ± 4.77a | 266.70 ± 15.70ab | 59.25 ± 7.64abcd | 72.15 ± 47.94a | 29.33 ± 13.47ab | 4.52 ± 1.00b | |
| 3 | 93.90 ± 4.03ab | 156.90 ± 43.49abc | 166.95 ± 5.73abcd | 189.60 ± 4.03abcd | 71.55 ± 8.06abc | 85.05 ± 25.67a | 18.46 ± 16.32ab | 7.41 ± 1.45b | |
| 4 | 125.48 ± 27.47a | 181.35 ± 12.52ab | 158.18 ± 19.41abcd | 155.93 ± 22.38abcd | 85.35 ± 18.03ab | 42.23 ± 2.65a | 63.83 ± 44.87a | 5.74 ± 0.23b | |
| 5 | 110.48 ± 23.44ab | 140.48 ± 6.05abcde | 180.00 ± 33.73abcd | 32.93 ± 5.20d | 52.80 ± 30.97abcd | 57.30 ± 59.40a | 18.98 ± 3.92ab | 3.33 ± 2.42b | |
| Tavşan Yüreği | 1 | 98.93 ± 2.65ab | 179.70 ± 17.39ab | 301.20 ± 105.01ab | 334.73 ± 2.23a | 70.43 ± 5.41abc | 66.90 ± 52.40a | 46.95 ± 0.42ab | 8.13 ± 8.78b |
| 2 | 88.05 ± 8.06ab | 151.20 ± 35.64abcd | 165.08 ± 79.87abcd | 97.13 ± 25.14bcd | 68.55 ± 2.55abcd | 23.18 ± 6.68a | 24.68 ± 11.56ab | 6.45 ± 3.45b | |
| 3 | 141.45 ± 3.18a | 169.88 ± 4.99ab | 104.70 ± 39.67bcd | 108.60 ± 8.49bcd | 56.93 ± 27.05abcd | 6.21 ± 1.53a | 15.45 ± 12.09ab | 5.17 ± 0.67b | |
| 4 | 68.03 ± 3.71ab | 99.38 ± 27.68abcde | 102.08 ± 11.14bcd | 137.63 ± 33.20bcd | 50.55 ± 12.09abcd | 18.30 ± 1.48a | 29.10 ± 4.88ab | 3.61 ± 0.79b | |
| 5 | 109.20 ± 15.27ab | 162.75 ± 13.15ab | 297.98 ± 2.86ab | 338.78 ± 27.68a | 32.40 ± 10.60bcd | 36.15 ± 7.64a | 36.83 ± 3.92ab | 10.53 ± 0.37b |
| Olive | Months | t-ferulic acid | Apigenin 7 glycoside | Oleuropein | Quercetin | t-cinnamic acid | Naringenin | Kaempherol | Isorhamnetin |
|---|---|---|---|---|---|---|---|---|---|
| Ayvalık | 1 | 73.65 ± 13.15*a | 10.83 ± 10.14c | 18.00 ± 0.00bcd | 142.88 ± 16.44bcd | 40.95 ± 0.21cde | 44.40 ± 0.85def | 48.08 ± 5.41cd | 43.80 ± 8.70c |
| 2 | 2.68 ± 1.31c** | 1.55 ± 0.19c | 0.82 ± 0.00d | 28.62 ± 28.68de | 2.07 ± 0.54f | 7.34 ± 2.24f | 16.58 ± 0.95d | 23.70 ± 4.67c | |
| 3 | 3.86 ± 3.77c | 6.75 ± 6.30c | 13.33 ± 3.00cd | 22.81 ± 21.63de | 31.95 ± 23.55cdef | 22.28 ± 3.50ef | 127.05 ± 134.28cd | 45.15 ± 0.85c | |
| 4 | 52.13 ± 21.96abc | 37.65 ± 0.85bc | 43.58 ± 34.05abc | 241.80 ± 44.55ab | 3.12 ± 2.25f | 29.40 ± 15.27ef | 43.58 ± 15.17cd | 49.43 ± 3.29c | |
| 5 | 2.76 ± 1.58c | 9.35 ± 6.18c | 3.91 ± 1.02d | 30.38 ± 9.23de | 0.73 ± 0.15f | 3.09 ± 1.30f | 4.95 ± 2.04d | 26.40 ± 9.76c | |
| Edremit | 1 | 1.57 ± 0.48c | 1.27 ± 0.88c | 1.83 ± 0.65d | 46.51 ± 58.75cde | 3.73 ± 0.85f | 41.40 ± 36.91def | 70.58 ± 67.35cd | 75.90 ± 77.22bc |
| 2 | 4.19 ± 4.21c | 6.89 ± 3.11c | 3.33 ± 3.13d | 8.22 ± 7.92e | 8.98 ± 9.37ef | 47.40 ± 9.12def | 30.08 ± 15.80cd | 32.55 ± 2.33c | |
| 3 | 6.66 ± 4.23bc | 7.26 ± 6.05c | 2.21 ± 1.11d | 27.15 ± 5.94de | 66.90 ± 12.73bc | 117.75 ± 2.12c | 434.33 ± 49.32b | 77.70 ± 5.52bc | |
| 4 | 9.00 ± 4.46bc | 80.55 ± 21.00ab | 3.53 ± 0.07d | 32.10 ± 10.61de | 0.58 ± 0.35f | 5.70 ± 0.77f | 3.91 ± 2.50d | 9.05 ± 2.26c | |
| 5 | 45.83 ± 25.77abc | 6.48 ± 0.51c | 69.60 ± 5.73a | 45.83 ± 7.32cde | 0.49 ± 0.26f | 2.87 ± 1.37f | 4.12 ± 0.09d | 6.63 ± 0.23c | |
| Gemlik | 1 | 40.50 ± 31.82abc | 15.08 ± 9.01bc | 51.90 ± 15.06ab | 124.43 ± 15.59bcde | 7.59 ± 0.42ef | 65.40 ± 25.03cdef | 79.43 ± 0.11cd | 104.78 ± 16.23bc |
| 2 | 2.31 ± 0.15c | 1.97 ± 0.69c | 4.76 ± 3.16d | 12.87 ± 1.48e | 2.25 ± 0.99f | 18.60 ± 2.97ef | 22.80 ± 2.33d | 31.88 ± 7.32c | |
| 3 | 3.19 ± 1.53c | 2.27 ± 1.06c | 5.65 ± 0.71d | 26.39 ± 17.19de | 6.51 ± 0.64ef | 4.59 ± 1.41f | 19.73 ± 3.08d | 25.05 ± 1.91c | |
| 4 | 71.63 ± 38.50a | 7.06 ± 2.73c | 6.23 ± 7.30d | 2.29 ± 2.09e | 14.01 ± 3.31ef | 16.39 ± 8.86ef | 28.50 ± 16.55cd | 8.21 ± 3.11c | |
| 5 | 4.86 ± 5.09c | 8.63 ± 10.71c | 4.93 ± 5.41d | 40.73 ± 21.96cde | 9.53 ± 13.25ef | 19.43 ± 5.20ef | 7.70 ± 5.77d | 5.85 ± 4.59c | |
| Memecik | 1 | 77.10 ± 30.76a | 61.13 ± 9.02bc | 8.01 ± 4.31cd | 28.58 ± 18.35de | 51.90 ± 1.27cd | 405.15 ± 3.18a | 119.93 ± 1.17cd | 89.33 ± 9.44bc |
| 2 | 62.85 ± 2.33abc | 146.03 ± 66.29a | 12.69 ± 5.17cd | 25.73 ± 0.95de | 8.29 ± 0.18ef | 11.02 ± 3.55ef | 30.30 ± 8.49cd | 37.80 ± 25.24c | |
| 3 | 9.07 ± 6.93bc | 45.45 ± 1.06bc | 31.95 ± 15.70bcd | 101.85 ± 98.00abc | 24.38 ± 11.77def | 101.10 ± 54.73cd | 139.73 ± 56.75cd | 150.45 ± 78.06ab | |
| 4 | 62.85 ± 5.73abc | 51.30 ± 14.21bc | 20.24 ± 10.19bcd | 162.23 ± 55.47abc | 5.83 ± 1.25ef | 24.15 ± 9.12ef | 37.43 ± 1.59cd | 28.50 ± 1.91c | |
| 5 | 3.66 ± 2.18c | 5.68 ± 3.42c | 6.91 ± 4.22d | 59.48 ± 24.08cde | 1.87 ± 1.29f | 6.33 ± 1.00f | 29.03 ± 13.05cd | 5.89 ± 3.16c | |
| Tavşan Yüreği | 1 | 6.51 ± 8.33bc | 42.75 ± 3.82bc | 6.40 ± 3.21d | 273.60 ± 19.52a | 24.90 ± 1.48def | 77.70 ± 11.46cde | 59.55 ± 17.18cd | 55.88 ± 3.71bc |
| 2 | 79.05 ± 20.58a | 47.40 ± 5.73bc | 4.24 ± 4.15d | 131.63 ± 26.83bcde | 109.20 ± 12.52a | 110.18 ± 29.17cd | 227.78 ± 52.50c | 84.23 ± 22.59bc | |
| 3 | 19.15 ± 6.86abc | 57.83 ± 34.05bc | 11.17 ± 3.27cd | 97.88 ± 43.17cde | 94.88 ± 23.44ab | 277.50 ± 28.00b | 886.58 ± 164.51a | 241.65 ± 53.88a | |
| 4 | 75.98 ± 17.71a | 8.91 ± 8.08c | 15.75 ± 1.06bcd | 22.51 ± 14.84de | 1.01 ± 0.17f | 2.97 ± 1.92f | 10.51 ± 0.19d | 5.21 ± 0.94c | |
| 5 | 68.63 ± 3.92ab | 58.95 ± 17.61bc | 18.68 ± 0.11bcd | 78.98 ± 10.92cde | 3.89 ± 0.58f | 26.70 ± 2.12ef | 53.25 ± 7.85cd | 33.00 ± 0.00c |
*The data show mean (n:3) ± standard deviation
**The different letters in the same column show statistically significant differences according to the Tukey test (p < 0.01)
Conclusion
The time of harvest and type of olive plant significantly affect total phenol, antioxidant activity and phenolic compounds of fruit and leaves. The highest gallic acid and (+)-catechin were detected in Ayvalık olive fruit harvested in December. In case of leaves from different varieties, the total phenol, antioxidant activity and phenolic compounds were also affected by olive varieties and harvet times significantly. Depending on the harvest time, the highest p-coumaric acid and ferulic acid contents were identified in Ayvalık and Tavşan Yüreği olive leaves collected in October and September, respectively. Hence, the total phenol, antioxidant activity and phenolic compounds of olive fruit and leaves from different varieties harvested at different times showed significant differences. Generally, phenolic compounds and antioxidant activity values of olive fruits were found low at the beginning of harvest. In general, phenolic compounds were partly higher in leaves collected in August. The study shows relationship of important bioactive compounds of olive fruits and leaves with different stages of maturity and cultivars.
Acknowledgements
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding the Research Group No. (RG-1435-049).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al-Rimawi F, Odeh I, Bisher A, Abbadi J, Qabbajeh M. Effect of geographical region and harvesting date on antioxidant activity, phenolic and flavonoid content of olive leaves. J Food Nutr Res. 2014;2(12):925–930. doi: 10.12691/jfnr-2-12-11. [DOI] [Google Scholar]
- Andrews P, Busch JLHC, Joode T, Groenewegen A, Alexandre H. Sensory properties of virgin olive oil polyphenols: identification of deacetoxy-ligstroside aglycon as a key contributor to pungency. J Agric Food Chem. 2003;51:1415–1420. doi: 10.1021/jf026042j. [DOI] [PubMed] [Google Scholar]
- Arslan D (2010) Güney Anadolu’da Yetisen Bazı Yaglık Zeytin Çesitlerinin ve Yaglarının Fiziksel ve Biyokimyasal Özellikleri Üzerine Lokasyon ve Hasat Zamanının Etkisi. Doktora Tezi. Selçuk Üniversitesi, Fen Bilimleri Enstitüsü, Gıda Mühendisligi Anabilim Dalı, p 249, Konya
- Benavente-Garcıa O, Castillo J, Lorente J, Ortuno A, Del Rio J. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000;68(4):457–462. doi: 10.1016/S0308-8146(99)00221-6. [DOI] [Google Scholar]
- Brahmi F, Mechri B, Dhibi M, Hammami M. Variations in phenolic compounds and antiradical scavenging activity of Olea europaea leaves and fruits extracts collected in two different seasons. Ind Crops Prod. 2013;49:256–264. doi: 10.1016/j.indcrop.2013.04.042. [DOI] [Google Scholar]
- Briante R, La Cara F, Febbraio F, Patumi M, Nucci R. Bioactive derivatives from oleuropein by a biotransformation on Olea europaea leaf extracts. J Biotechnol. 2002;93:109–119. doi: 10.1016/S0168-1656(01)00387-X. [DOI] [PubMed] [Google Scholar]
- Briante R, Patumi M, Terenziani S, Bismuto E, Febbraio F, Nucci R. Olea europaea L. leaf extract and derivatives: antioxidant properties. J Agric Food Chem. 2002;50:4934–4940. doi: 10.1021/jf025540p. [DOI] [PubMed] [Google Scholar]
- Cerretani L, Bendini A, Del Caro A, Piga A, Vacca V, Caboni MF, Toschi TG. Preliminary characterization of virgin olive oils obtained from different cultivars in Sardinia. Eur Food Res Technol. 2006;222(3–4):354–361. doi: 10.1007/s00217-005-0088-9. [DOI] [Google Scholar]
- Dağdelen A, Tumen G, Özcan MM, Dundar E. Phenolics profiles of olive fruits (Olea europaea L.) and oils from Ayvalik, Domat and Gemlik varieties at different ripening stages. Food Chem. 2013;136:41–45. doi: 10.1016/j.foodchem.2012.07.046. [DOI] [PubMed] [Google Scholar]
- El SN, Karakaya S. Olive tree (Olea europaea) leaves: potential beneficial effects on human health. Nutr Rev. 2009;67:632–638. doi: 10.1111/j.1753-4887.2009.00248.x. [DOI] [PubMed] [Google Scholar]
- Ghanema IIA, Sadek KM. Olive leaves extract restored the antioxidant perturbations in red blood cells hemolysate in streptozotocin induced diabetic rats. Int J Animal Vet Sci. 2012;6(4):124–130. [Google Scholar]
- Ilarioni L, Proietti P. Olive tree cultivars. In: Peri C, editor. the extra-virgin olive oil handbook. Oxford: Wiley; 2014. pp. 59–68. [Google Scholar]
- Kaeidi A, Esmaeili-Mahani S, Sheibani V, Abbasnejad M, Rasoulian B, Hajializadeh Z, Afrazi S. Olive (Olea europaea L.) leaf extract attenuates early diabetic neuropathic pain through prevention of high glucose-induced apoptosis: in vitro and in vivo studies. J Ethnopharm. 2011;136(1):188–196. doi: 10.1016/j.jep.2011.04.038. [DOI] [PubMed] [Google Scholar]
- Kara M, Sahin H, Turumtay H, Dinç S, Gümüşçü A. The phenolic composition and antioxidant activity of tea with different parts of Sideritis condensate at different steeping conditions. J Food Nutr Res. 2014;2(5):258–262. doi: 10.12691/jfnr-2-5-8. [DOI] [Google Scholar]
- Keçeli T, Büyükaslan Y (2008) Hatay’da Yetiştirilen Bazı Zeytinlerin Antioksidan Etkilerinin Belirlenmesi. Türkiye 10. Gıda Kongresi 21–23 May 2008 Erzurum, Turkey
- Keçeli T, Harp F. The effect of olive leaves and their harvest time on radical scavenging activity and oxidative stability of refined olive oil. Qual Assur Saf Crops Foods. 2014;6(2):141–149. doi: 10.3920/QAS2013.0305. [DOI] [Google Scholar]
- Lee SK, Mbwambo ZH, Chung HS, Luyengi L, Games EJC, Mehta RG. Evaluation of the antioxidant potential of natural products. Comb Chem High Through Screen. 1998;1:35–46. [PubMed] [Google Scholar]
- Lee OH, Lee BY, Lee J, Lee HB, Son J-Y, Park CS, Shetty K, Kim YC. Assessment of phenolics-enriched extract and fractions of olive leaves and their antioxidant activities. Biores Technol. 2009;100(23):6107–6113. doi: 10.1016/j.biortech.2009.06.059. [DOI] [PubMed] [Google Scholar]
- Lee-Huang S, Zhang L, Huang PL, Chang Y-T, Huang PL. Anti-HIV activity of olive leaf extract (OLE) and modulation of host cell gene expression by HIV-1 infection and OLE treatment. Biochem Biophys Res Commun. 2003;307(4):1029–1037. doi: 10.1016/S0006-291X(03)01292-0. [DOI] [PubMed] [Google Scholar]
- Matos LC, Cunha SC, Amaral JS, Pereira JA, Andrade PB, Seabra RM, Oliveira MB. Chemometric characterization of three varietal olive oils (Cvs. Cobrançosa, Madural and Verdeal Transmontana) extracted from olives with different maturation indices. Food Chem. 2007;102:406–414. doi: 10.1016/j.foodchem.2005.12.031. [DOI] [Google Scholar]
- Ocakoglu D, Tokatli F, Ozen B, Korel F. Distribution of simple phenols, phenolic acids and flavonoids in Turkish monovarietal extra virgin olive oils for two harvest years. Food Chem. 2009;113(2):401–410. doi: 10.1016/j.foodchem.2008.07.057. [DOI] [Google Scholar]
- Özcan MM, Matthäus B. A review: benefit and bioactive properties of olive (Olea europaea L.) leaves. Eur Food Res Technol. 2017;243(1):89–99. doi: 10.1007/s00217-016-2726-9. [DOI] [Google Scholar]
- Petridis A, Therios I, Samouris G, Tananaki C. Salinity-induced changes in phenolic compounds in leaves and roots of four olive cultivars (Olea europaea L.) and their relationship to antioxidant activity. Environ Exp Bot. 2012;79:37–43. doi: 10.1016/j.envexpbot.2012.01.007. [DOI] [Google Scholar]
- Püskülcü H, İkiz F. Introduction to statistic. Bornova: Bilgehan Press; 1989. p. 333. [Google Scholar]
- Ranalli A, Gomes T, Delcuratolo D, Contento S, Lucera L. Improving virgin olive oil quality by means of innovative extracting biotechnologies. J Agric Food Chem. 2003;51:2597–2602. doi: 10.1021/jf026176x. [DOI] [PubMed] [Google Scholar]
- Romani A, Mulinacci N, Pinelli P, Vincieri FF, Cimato A. Polyphenolic content in five tuscany cultivars of Olea europaea L. J Agric Food Chem. 1999;47(3):964–967. doi: 10.1021/jf980264t. [DOI] [PubMed] [Google Scholar]
- Rotondi A, Bendini A, Cerretani L, Mari M, Lercker G, Toschi GT. Effect of olive ripening degree on the oxidative stability and organoleptic properties of cv. Nostrana di Brisighella extra virgin olive oil. J Agric Food Chem. 2004;52:3649–3654. doi: 10.1021/jf049845a. [DOI] [PubMed] [Google Scholar]
- Salido S, Perez-Bonilla M, Adams RP, Altarejos J. Phenolic components and antioxidant activity of wood extracts from 10 main Spanish olive cultivars. J Agric Food Chem. 2015;63:6493–6500. doi: 10.1021/acs.jafc.5b02979. [DOI] [PubMed] [Google Scholar]
- Servili M, Baldioli M, Selvaggini R, Miniati E, Macchioni A, Montedoro G. High-performance liquid chromatography evaluation of phenols in olive fruit, virgin olive oil, vegetation waters, and pomace and 1D- and 2D-nuclear magnetic resonance characterization. J Am Oil Chem Soc. 1999;76:873–882. doi: 10.1007/s11746-999-0079-2. [DOI] [Google Scholar]
- Sevim D, Tuncay Ö. Ayvalik ve Memecik zeytin Çeşitlerinin yapraği ve meyvelerinin toplam fenolik madde miktarı ve antioksidan Aktiviteleri. Gıda Derg. 2012;37(4):219–226. [Google Scholar]
- Siger A, Nogala-Kalucka M, Lampart-Szczapa E. The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J Food Lipids. 2008;15:137–149. doi: 10.1111/j.1745-4522.2007.00107.x. [DOI] [Google Scholar]
- Silva S, Gomes L, Leitao F, Coelho A, Boas LV. Phenolic compounds and antioxidant activity of Olea europaea L. fruits and leaves. Food Sci Technol Int. 2006;12(5):385–395. doi: 10.1177/1082013206070166. [DOI] [Google Scholar]
- Singh JP, Kaur A, Shevkani K, Singh N. Composition, bioactive compounds and antioxidant activity of common Indian fruits and vegetables. J Food Sci Technol. 2016;53(11):4056–4066. doi: 10.1007/s13197-016-2412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler-Rivas C, Espin JC, Wichers HJ. Oleuropein and related compounds. J Sci Food Agric. 2000;80:1013–1023. doi: 10.1002/(SICI)1097-0010(20000515)80:7<1013::AID-JSFA571>3.0.CO;2-C. [DOI] [Google Scholar]
- Sousa A, Malheiro R, Casal S, Bento A. Antioxidant activity and phenolic composition of Cv. Cobrançosa olives affected through the maturation process. J Funct Foods. 2014;11:20–29. doi: 10.1016/j.jff.2014.08.024. [DOI] [Google Scholar]
- Talhaoui N, Gomez-Caravana AM, Leon L, De la Rosa R, Segura-Carretero A, Fernandez-Gutierrez A. Determination of phenolic compounds of ‘Sikitita’ olive leaves by HPLC-DAD-TOF-Ms. Comparison with its parents ‘Arbequina’ and ‘Picual’ olive leaves. LWT-Food Sci Technol. 2014;58:28–34. doi: 10.1016/j.lwt.2014.03.014. [DOI] [Google Scholar]
- Tanilgan K, Özcan MM, Ünver A. Physical and chemical characteristics of five Turkish olive (Olea europea L.) varieties and their oils. Grasas Aceites. 2007;58(2):142–147. [Google Scholar]
- Xie P-J, Huang L-X, Zhang C-H, Zhang YL. Phenolic compositions, and antioxidant performance of olive leaf and fruit (Olea europaea L.) extracts and their structure-activity relationships. J Funct Foods. 2015;16:460–471. doi: 10.1016/j.jff.2015.05.005. [DOI] [Google Scholar]
- Yoo KM, Lee KW, Park JB, Lee HJ, Hwang IK. Variation in major antioxidants and total antioxidant activity of Yuzu (Citrus junos SiebexTanaka) during maturation and between cultivars. J Agric Food Chem. 2004;52:5907–5913. doi: 10.1021/jf0498158. [DOI] [PubMed] [Google Scholar]