Abstract
In the present study, various phytoconstituents of methanolic extract of Foeniculum vulgare were identified using gas-chromatography mass spectrometry (GC–MS) method. GC–MS method was also applied for the analysis of biomarker fenchone in extract and eight different commercial formulations. The mass of prepared extract and formulations A–D and H (commercial herbal mixtures and commercial extract) used for the analysis of fenchone was 10 g. However, the mass of formulations E–G (soft gelatin capsules) was 100 mg. Fifty seven different phytoconstituents were identified in the methanolic extract of F. vulgare using GC–MS technique. The main compounds identified were trans-anethole (31.49%), 2-pentanone (25.01%), fenchone (11.68%) and benzaldehyde-4-methoxy (8.01%). Several other compounds were also identified in higher amounts and some compounds were identified in trace amounts. Many compounds have been reported for the first time in the methanolic extract of F. vulgare. The amount of fenchone was found to be maximum in plant extract (9.789 mg/g) in comparison with other commercial formulations by the proposed GC–MS technique. In three different commercial formulations (F, G and H), the amount of fenchone was obtained as more than 1.0 mg/g. However, in five different commercial formulations (A, B, C, D and E), the amount of fenchone was recorded as less than 0.1 mg/g. This method could be utilized for the analysis of fenchone contents in the commercial formulations containing fenchone as an active ingredient. The results obtained in this work could be useful in standardization of commercial formulations containing fenchone.
Electronic supplementary material
The online version of this article (10.1007/s13197-019-03695-9) contains supplementary material, which is available to authorized users.
Keywords: Fenchone, Foeniculum vulgare, Gas-chromatography mass-spectrometer, Phytoconstituents, Standardization
Introduction
Foeniculum vulgare Mill. [chemical structure: supplementary Fig. 1 (Figure S1)] is a biennial or perennial medicinal crop/herb which belongs to the family Apiaceae (Bahmani et al. 2015). Essential oils are the main constituents of F. vulgare seed which are being used as flavoring agents in various food, cosmetic and pharmaceutical products (Piccaglia and Marotti 2001; Shahat et al. 2011; Bahmani et al. 2015). Various therapeutic activities such as hepatoprotective (Ozbek et al. 2003), antispasmodic (Reynolds 1982), diuretic (Shahat et al. 2011), anti-inflammatory, analgesic, antioxidant (Choi and Hwang 2004; Badgujar et al. 2014; Kontogiorgis et al. 2016; Majdoub et al. 2017), antibacterial (Elagayyar et al. 2001; Diao et al. 2014; Shahat et al. 2011), antimicrobial (Roby et al. 2013), antifungal (Singh et al. 2006), anti-diabetic (Saleem et al. 2017), anti-neurological (Cioanca et al. 2016) and anticancer activity (Anand et al. 2008) have been reported in essential oils of F. vulgare.
Several studies have been carried out to determine the different phytoconstituents of F. vulgare in literature (Diaz-Maroto et al. 2006; Ozcan et al. 2006; Mojab et al. 2007; Cosge et al. 2009; Renjie et al. 2010; Anubhuti et al. 2011; Shahat et al. 2011; Hammouda et al. 2013; Rodriguez-Solana et al. 2014; Acimovic et al. 2015; Bahmani et al. 2015; Shojaiefar et al. 2015; Upadhyay 2015; Abdel Karm et al. 2017; Shahmokhtar and Armand 2017; Ahmad et al. 2018). The main components which have been reported in literature are phenylpropanoid derivatives and monoterpenoids in essential oils of F. vulgare (Diaz-Maroto et al. 2006; Ozcan et al. 2006; Renjie et al. 2010; Shahat et al. 2011). In literature, various compounds such as trans-anithole, fenchone, α-pinene, β-pinene and camphene have been reported as the biomarkers of the essential oils of F. vulgare (Renjie et al. 2010; Shahat et al. 2011; Bahmani et al. 2015; Upadhyay 2015; Abdel Karm et al. 2017; Ahmad et al. 2018).
Gas-chromatography mass-spectrometry (GC–MS) technique has been reported as one of the most important analytical techniques for the identification of phytoconstituents of plant materials (Renjie et al. 2010; Shahat et al. 2011; Acimovic et al. 2015; Bahmani et al. 2015; Upadhyay 2015; Ahmad et al. 2018). It offers several advantages over other analytical techniques for profiling of chemical compositions of plant materials (Renjie et al. 2010; Shahat et al. 2011; Bahmani et al. 2015). Fenchone (Figure S2) is one of the biomarkers of essential oils of F. vulgare which is present in various commercially available formulations. Chemical composition of essential oils of F. vulgare using GC–MS technique had been reported extensively in literature (Cosge et al. 2009; Renjie et al. 2010; Anubhuti et al. 2011; Shahat et al. 2011; Hammouda et al. 2013; Acimovic et al. 2015; Bahmani et al. 2015; Upadhyay 2015; Abdel Karm et al. 2017; Shahmokhtar and Armand 2017). Chiral GC method has been reported for the analysis of fenchone in its essential oils (Ravid et al. 1992). Nevertheless, the analysis of its biomarker fenchone in various commercial formulations has not been reported in literature. Therefore, in this work, different phytoconstituents of methanolic extract of F. vulgare were determined using GC–MS technique. The proposed GC–MS technique was applied further for the analysis of fenchone contents in extract and eight different commercial formulations.
Materials and methods
Materials
Standard fenchone, ethanol, ethyl acetate (EA) and hexane were obtained from “Sigma-Aldrich (St. Louis, MO, USA)”. Herbal mixtures and various commercial formulations were purchased from local market in Riyadh, Saudi Arabia and Alexandria, Egypt. HNO3, perchloric acid and hydrogen peroxide were obtained from “E-Merck (Hamburg, Germany)”. Water was collected from “Milli-Q Water Purification Unit” in the Laboratory. All the solvents were of chromatography grade and other chemicals used were of analytical reagent (AR) grade.
Plant material
The seeds of F. vulgare Mill. were purchased from the local market of “Al-Kharj, Saudi Arabia”. The seeds were identified by comparison with voucher specimen at the “Medicinal, Aromatic and Poisonous Plants Research Center (MAPPRC), College of Pharmacy, King Saud University, Riyadh, Saudi Arabia”.
Preparation of standard solutions and calibration
Accurately weighed 10 mg of standard fenchone (purity 99%) was dissolved in methanol in a 10 ml volumetric flask. About 1.0 ml of accurately measured standard was transferred to another 10 ml volumetric flask and completed the volume with methanol in order to obtain standard concentration of 100 μg/ml. Serial dilutions were made from standard solution in order to obtain the concentrations of fenchone in the range of 1–100 μg/ml. The GC–MS response of each concentration was recorded and calibration curve was plotted between the concentration of fenchone and GC–MS response. The experiments were performed in triplicates.
Sample preparation for the analysis of fenchone in the methanolic extract of fennel seeds and herbal mixtures
For seed extract, 10 g of fennel seeds were extracted to exhaustion with methanol at room temperature. After filtration, the combined methanol extract was evaporated under reduced pressure using rotary vacuum evaporator. The resulted extracts were separately transferred to 50 ml volumetric flasks and completed the volume to 50 ml.
For marketed herbal mixtures (A–D), 10 g of each mixture were separately extracted to exhaustion with methanol at room temperature. After filtration, the combined methanol extracts were evaporated under reduced pressure using rotary vacuum evaporator. The resulted extracts were separately transferred to 10 ml volumetric flasks and completed the volume to 10 ml. All the sample matrices were investigated in triplicates.
Sample preparation for the analysis of fenchone in soft gelatin capsules
HPLC syringe was used to obtain the oily contents of the capsules. From each formulation (E–G), 100 mg samples were taken and dissolved in 10 ml of methanol in volumetric flasks. The volume was completed to 10 ml. These experiments were performed in triplicates.
Sample preparation for the analysis of fenchone in baby instant drink
From the instant baby drink granules (H), 10 g of samples were taken and dissolved in 50 ml of methanol, filtered and completed the volume to 50 ml in volumetric flask. Experiments were performed in triplicates.
GC–MS analysis of methanolic extract of F. vulgare
Around 2 μl of sample extract was injected into the system with the split mode (split ratio 1:20). To perform the analysis, “Perkin Elmer GC–MS coupled with Clarus 600 T mass Spectrometer (USA)” was used. The system was composed of an auto-sampler unit, auto-injector unit and a gas chromatograph Clarus 600 coupled with a single quadrupole mass spectrometer. For the analysis of the samples, “TurboMass Solution Software Version 5.4” was used in GC–MS analysis. The samples were separated on Elite 5 MS (30 m × 0.25 mm i.d., 0.25 μm film thickness) capillary CG column (Perkin Elmer, USA). Analyses were performed using helium as a carrier gas at a constant pressure mode (65.2 kPa). The separation was carried out in a gradient temperature program. The oven temperature was maintained at 40 °C for 2 min, ramped to 100 °C at 5 °C/min for 2 min and again increased at a rate of 5 °C/min to 300 °C, ramped with the grade 5 °C/min and held for 5 min. The total run time was 61 min. The injector, ion source and interface temperature were set at 280 °C, 240 °C and 220 °C, respectively. The electron energy was set at 70 eV. The unknown components were identified using “National Institute of Standard and Technology (NIST, 2005) Library” and “WILEY, 2006, Library”.
GC–MS analysis of standard fenchone
Around 2 μl of standard sample of fenchone was injected into the system with the split mode (split ratio 1:20). To perform the analysis, “Perkin Elmer GC–MS coupled with Clarus 600 T mass spectrometer (USA)” was used. The samples were separated on Elite 5 MS (30 m × 0.25 mm i.d., 0.25 μm film thickness) capillary CG column (Perkin Elmer, USA). Analyses were performed with the helium as a carrier gas at a constant pressure mode (65.2 kPa). The separation was carried out in a gradient temperature program. The oven temperature was maintained at 50 °C for 1 min, ramped to 200 °C at 5 °C/min for 1 min and again increased at a rate of 5 °C/min to 300 °C, ramped with the grade 5 °C/min and held for 2 min. The total run time was 14 min. The injector, ion source and interface temperature were set at 280 °C, 240 °C and 220 °C, respectively. The ionization voltage was 70 eV. Using same methodology, GC–MS response of different concentrations of fenchone was recorded and calibration curve was constructed between the concentration and GC–MS response. The concentration of fenchone in fennel extract and various commercial formulations was determined from the calibration curve plotted between concentration and GC–MS response. The experiments were performed in triplicates.
Results and discussion
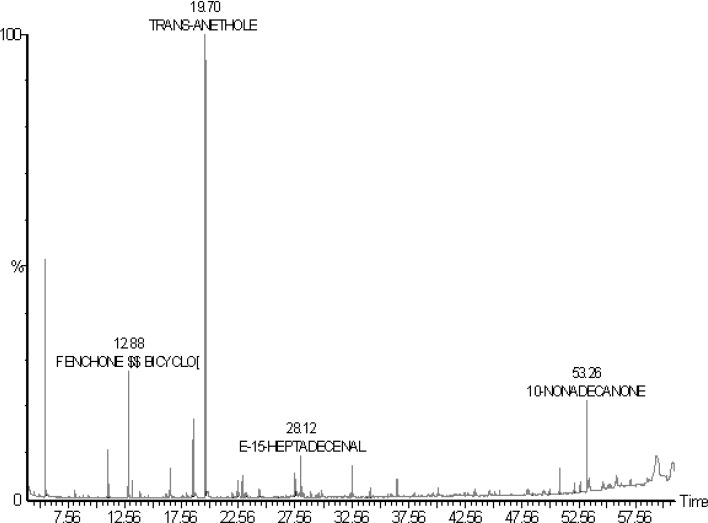
The medicinal properties of F. vulgare have been utilized in our country (Saudi Arabia). Various phytoconstituents have been reported in F. vulgare which are responsible for different therapeutic activities of fennel fruits (Mojab et al. 2007; Renjie et al. 2010; Shahat et al. 2011; Bahmani et al. 2015). In this work, phytoconstituents of methanolic extract of F. vulgare were identified by GC–MS method. The proposed GC–MS method was also applied for the determination of fenchone contents in plant extract and various commercial formulations. The results of GC–MS profiling of methanolic extract of F. vulgare are listed in Table 1. Various identified compounds are presented in (%) along with their retention times (Table 1). By GC–MS analysis of unknown mixtures, various compounds are identified using NIST and WILEY library. These libraries provide information about various compounds based on their respective retention times and GC–MS response. Based on GC–MS response of various compounds, the amount of compounds can be calculated in % as reported in literature (Mojab et al. 2007; Cosge et al. 2009; Renjie et al. 2010; Acimovic et al. 2015). The calculation of these compounds in masses is not possible by NIST and WILEY libraries. Therefore, the amount of identified compounds is presented in (%) along with their retention times in Table 1. The representative GC–MS chromatogram of methanolic extract of F. vulgare is shown in Fig. 1. Results showed significant amount of the important phytoconstituents identified in the methanolic extract of F. vulgare. Fifty seven different phytoconstituents were identified in methanolic extract of F. vulgare (Table 1). The most abundant includes trans-anethole (31.49%), 2-pentanone (25.01%), fenchone (11.68%) and benzaldehyde-4-methoxy (8.01%). Some other phytoconstituents such as 10-nonadecanone (2.97%), DL-limonene (1.76%), benzene-1-methoxy-4-(2-propene) (1.52%), hexatriacontane (1.44%), 1-heptadecanol (1.12%) and E-15-heptadecenal (1.04%) were also present in good amounts. Some phytoconstituents such as tetradecane, 8-heptadecene, 4-hexen-2-one, 3-hydroxytetrahydropyran, delta-3-carene, acetic acid, sabinene, 1,3,8-p-menthatriene, trans-p-mentha-2,8-dienol, 1,1′-bicyclohexyl, 1-undecanol, fenchyl acetate, cyclohexane, 4-pentyloxy-2,3-dicyanophenyl, 4-germacrane-1,7-dimethyl, cis-2,3-epoxy-2,3,4,5,6-pentam, 1-tetradecene, tetradecane, hydroxymethylcyclododecane, 1-methoxy-3-(ethenylcarbonyl)B, 10-oxoundecyl acetate, pentadecane, diphenyl methanone, menthol-1′-(butyn-3-one-1-yl), cyclohexane eicosyl, 8-pentadecanone, 1-undecene-9-methyl, dotriacontane, 1,2-benzenedicarboxylic acid, 9-octadecenoic acid, 3-Eicosene-(e)-(CAS), hexadecanoic acid, octadecanal, 3-eicosene, di(2-ethylhexyl)adipate, decanal, 14-heptadecenal and 1-octadecanol were present in moderate to low amounts. On the other hands, the phytoconstituents such as camphor, 1-methyl-2-methylene-4-isopropyl, l-histidine, 1-hexyl-1-nitrocyclohexane, p-methoxybenzamide, 11-tricosene, 1-tetradecanol, 1,19-eicosadiene and eicosyl acetate were identified in trace amounts (less than 0.1%). The variety of compounds was identified in the methanolic extracts of F. vulgare which could be responsible for different therapeutic activities of fennel fruit. Hence, methanolic of F. vulgare could be used for the treatment of various diseases.
Table 1.
Components identified in methanolic extract of F. vulgare seeds by GC-MS technique
| Component name | RT (Min) | Area | Area (%) |
|---|---|---|---|
| 4-Hexen-2-one | 4.54 | 111042 | 0.20 |
| 3-Hydroxytetrahydropyran | 5.02 | 300106 | 0.55 |
| 2-Pentanone | 5.56 | 13563417 | 25.01 |
| Delta-3-carene | 8.16 | 228785 | 0.42 |
| Acetic acid | 8.90 | 55015 | 0.10 |
| Sabinene | 9.34 | 91076 | 0.17 |
| 1,3,8-P-menthatriene | 10.94 | 62420 | 0.12 |
| DL-Limonene | 11.08 | 954384 | 1.76 |
| Fenchone | 12.88 | 6333210 | 11.68 |
| Tetradecane | 13.26 | 490277 | 0.90 |
| Trans-p-mentha-2,8-dienol | 13.90 | 95814 | 0.18 |
| Camphor | 14.64 | 49474 | Trace |
| 1,1′-Bicyclohexyl | 16.16 | 73771 | 0.14 |
| 1-Undecanol | 16.32 | 80699 | 0.15 |
| Benzene-1-methoxy-4-(2-propene) | 16.54 | 825098 | 1.52 |
| 1-Methyl-2-methylene-4-isopropyl | 17.58 | 30132 | Trace |
| Fenchyl acetate | 17.74 | 57226 | 0.11 |
| Cyclohexane | 18.06 | 167539 | 0.31 |
| l-Histidine | 18.20 | 38121 | Trace |
| Benzaldehyde-4-methoxy | 18.58 | 4344178 | 8.01 |
| Trans-anethole | 19.70 | 17075762 | 31.49 |
| 1-Hexyl-1-nitrocyclohexane | 21.04 | 22706 | Trace |
| 4-Pentyloxy-2,3-dicyanophenyl | 21.58 | 56719 | 0.10 |
| 4-Germacrane-1,7-dimethyl | 22.08 | 151976 | 0.28 |
| Cis-2,3-epoxy-2,3,4,5,6-pentam | 22.52 | 379565 | 0.70 |
| 1-Tetradecene | 22.92 | 347801 | 0.64 |
| Tetradecane | 23.16 | 111364 | 0.21 |
| Hydroxymethylcyclododecane | 23.28 | 72483 | 0.13 |
| 1-Methoxy-3-(ethenylcarbonyl)B | 24.44 | 387111 | 0.71 |
| P-methoxybenzamide | 24.76 | 48233 | Trace |
| 11-Tricosene | 26.38 | 49001 | Trace |
| 10-Oxoundecyl acetate | 26.78 | 54200 | 0.10 |
| 1-Tetradecanol | 27.16 | 37634 | Trace |
| 1-Heptadecanol | 27.56 | 607788 | 1.12 |
| E-15-heptadecenal | 28.12 | 562459 | 1.04 |
| Pentadecane | 28.30 | 99652 | 0.18 |
| Diphenyl methanone | 28.94 | 359540 | 0.66 |
| Menthol-1′-(butyn-3-one-1-yl) | 29.46 | 75867 | 0.14 |
| Cyclohexane eicosyl | 29.62 | 105029 | 0.19 |
| 8-Pentadecanone | 29.92 | 174330 | 0.32 |
| 1-Undecene-9-methyl | 32.44 | 105299 | 0.19 |
| 8-Heptadecene | 32.58 | 491381 | 0.91 |
| Dotriacontane | 32.74 | 63920 | 0.12 |
| 1,19-Eicosadiene | 33.94 | 23330 | Trace |
| 1,2-Benzenedicarboxylic acid | 35.76 | 268694 | 0.50 |
| 9-Octadecenoic acid | 36.00 | 76572 | 0.14 |
| 3-Eicosene-(e)-(CAS) | 36.56 | 283988 | 0.52 |
| Hexadecanoic acid | 37.10 | 60932 | 0.11 |
| Octadecanal | 38.06 | 53211 | 0.10 |
| 3-Eicosene | 40.20 | 230501 | 0.43 |
| Di(2-ethylhexyl)adipate | 43.38 | 167431 | 0.31 |
| Eicosyl acetate | 46.66 | 51181 | Trace |
| Decanal | 48.00 | 89560 | 0.17 |
| 14-Heptadecenal | 50.02 | 139144 | 0.26 |
| Hexatriacontane | 50.58 | 780581 | 1.44 |
| 1-Octadecanol | 52.70 | 219018 | 0.40 |
| 10-Nonadecanone | 53.26 | 1608688 | 2.97 |
The compound present in < 0.1% amount (trace)
Fig. 1.
Gas chromatogram of methanolic extract of F. vulgare seeds
GC–MS method was applied as standard method for the identification of extract. The contents of extract may vary from region to region depending upon seasonal and environmental conditions. The objective of this work was to identify the contents of fennel extract from the sample available in Saudi Arabia. The novelty of this method includes the application of proposed method in determination of standard fenchone in various commercial formulations. Many of the phytoconstituents identified in this work have been reported previously in literature (Diaz-Maroto et al. 2006; Ozcan et al. 2006; Mojab et al. 2007; Cosge et al. 2009; Renjie et al. 2010; Shahat et al. 2011). However, the amount of each compound could be different with those reported in literature. The variation in amounts of each compound depends upon various factors such as temperature, humidity, climate conditions and collection time for plant (Shahat et al. 2011). Several phytoconstituents such as trans-anethole, fenchone, sabinine, camphor, fenchyl acetate, bicyclohexyl and tetradecane etc. have been reported very well in literature (Ozcan et al. 2006; Renjie et al. 2010; Shahat et al. 2011; Acimovic et al. 2015; Bahmani et al. 2015). However, many phytoconstituents such as 4-hexen-2-one, 3-hydroxytetrahydropyran, 2-pentanone, 1,3,8-p-menthatriene, tetradecane, 1,1′-bicyclohexyl, benzene-1-methoxy-4-(2-propene), 1-methyl-2-methylene-4-isopropyl, l-histidine, 1-hexyl-1-nitrocyclohexane, 4-pentyloxy-2,3-dicyanophenyl, 4-germacrane-1,7-dimethyl, cis-2,3-epoxy-2,3,4,5,6-pentam, 1-tetradecene, tetradecane, hydroxymethylcyclododecane, 1-methoxy-3-(ethenylcarbonyl)B, p-methoxybenzamide, 11-tricosene, 10-oxoundecyl acetate, 1-tetradecanol, 1-heptadecanol, E-15-heptadecenal, pentadecane, diphenyl methanone, 8-pentadecanone, 1-undecene-9-methyl, 8-heptadecene, dotriacontane, 1,2-benzenedicarboxylic acid, 3-eicosene-(e)-(CAS), octadecanal and 10-nonadecanone have been reported for the first time in this work. Different species/varieties of extract were not investigated in this work. The most abundant phytoconstituents such as trans-anethole, 2-pentanone, fenchone and benzaldehyde-4-methoxy recorded in this study have great therapeutic applications (Anwar et al. 2009; Shahat et al. 2011; Mazaheri et al. 2013; Badgujar et al. 2014; Walker and Mills 2014). Trans-anethole has been investigated as anti-oxidant, antimicrobial, estrogenic and anti-inflammatory agent (Shahat et al. 2011; Mazaheri et al. 2013; Badgujar et al. 2014). 2-Pentanone, also known as methyl propyl ketone is used as a metabolic product of Penicillium mold growth (Walker and Mills 2014). Fenchone is found in almost all species of Foeniculum and reported as anti-oxidant, antimicrobial and anti-inflammatory agent (Anwar et al. 2009; Shahat et al. 2011; Mazaheri et al. 2013). Benzaldehyde-4-methoxy, also known as p-anisaldehyde has been investigated as anti-oxidant agent (Anwar et al. 2009).
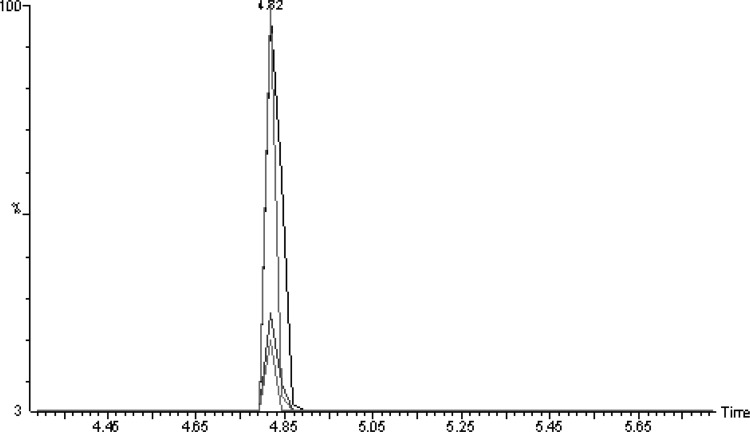
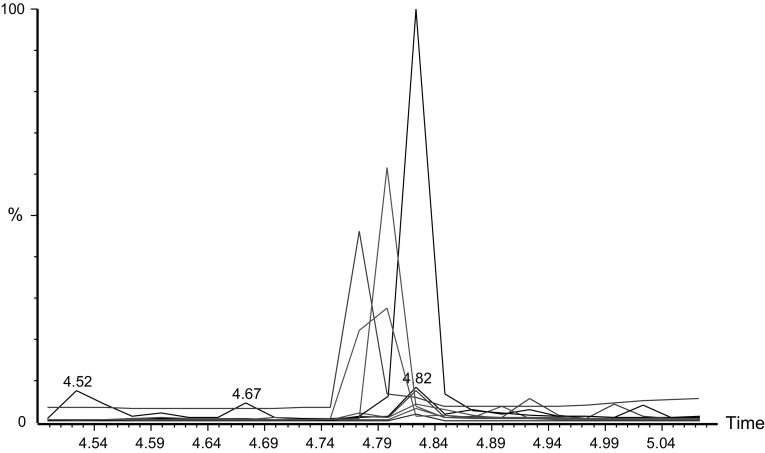
The proposed GC–MS method was also applied for the analysis of fenchone in methanolic extract of F. vulgare and various commercial formulations. For this application, the calibration curve of standard fenchone was plotted between the concentration of standard fenchone and GC–MS response. The calibration curve of fenchone was found to be linear in the range of 1-100 µg/g with correlation coefficient of 0.9955. The regression equation for calibration curve was obtained as y = 4842.8x + 26887; in which x is the concentration of fenchone and y is the GC–MS response for fenchone. The overlaid GC–MS chromatograms of different concentrations of fenchone are shown in Fig. 2 which suggested uniformity in GC–MS response of fenchone at each concentration evaluated. From calibration curve of standard fenchone plotted between GC–MS response and concentration, the amount of fenchone (mg/g) in extract and various commercial formulations was determined. The mass of extract and formulations (A-D and H) used for the analysis of fenchone was 10 g. However, the mass of formulation E–G (soft gelatin capsules) was 100 mg. Soft gelatin capsules are not available in larger doses in the market. Therefore, the mass of formulations E–G was 100 mg for soft gelatin capsules. The results of fenchone contents in extract and various commercial formulations are presented in Table 2. The overlaid GC–MS chromatograms of various commercial formulations are presented in Fig. 3. The maximum amount of fenchone was recorded in fennel extract (9.789 mg/g) in comparison with various commercial formulations investigated. Among various commercial formulations investigated, the maximum amount of fenchone was obtained in formulation F (1.164 mg/g). The amount of fenchone in commercial formulations F, G and H were obtained in similar magnitude and were not statistically different (P > 0.05). However, the amount of fenchone in commercial formulations A, B, C, D was recorded as negligible (less than 0.1 mg/g). Overall, the proposed GC–MS method was found to be suitable for the analysis of fenchone contents in extract and various commercial formulations.
Fig. 2.
Overlaid GC peaks of standard fenchone at different concentrations
Table 2.
Analysis of fenchone contents in fennel extract and different commercial formulation by proposed GC–MS technique
| Formulation code | Composition claimed | Fenchone found (mg/g) ± SD |
|---|---|---|
| Fennel extract* | Chamomile, fennel, anise, caraway | 9.789 ± 0.250 |
| A* | Chamomile, fennel, anise, caraway | 0.006 ± 0.001 |
| B* | Tilia, guava, verbascum, marjouram, peppermint, fennel, licorice | 0.004 ± 0.000 |
| C* | Thyme, fennel, salvia, anise, licorice, guava, melissa | 0.002 ± 0.000 |
| D* | – | 0.004 ± 0.000 |
| E# | α-Pinene, β-pinene, camphene, borneol, anethol, fenchone, cineol | 0.002 ± 0.000 |
| F# | α-Pinene, β-pinene, camphene, borneol, anethol, fenchone, cineol | 1.164 ± 0.004 |
| G# | Peppermint oil, fennel oil, ginger oil, caraway oil, chamomile oil | 1.149 ± 0.003 |
| H* | Fennel extract | 1.103 ± 0.002 |
*The mass of extract and formulations A–D and H used for the analysis of fenchone content was 10 g
#The mass of formulations E–G (soft gelatin capsules) used for the analysis of fenchone content was 100 mg
Fig. 3.
Overlaid GC peaks of fenchone extract and various commercial formulations
Based on GC–MS profile of various phytoconstituents identified in the methanolic extract of F. vulgare, it can be concluded that this plant could be explored in the treatment of different diseases. Moreover, the proposed GC–MS method could be applied for the analysis of fenchone contents in the commercial formulations containing fenchone as an active ingredient.
Conclusion
In the present work, various phytoconstituents in the methanolic extract of F. vulgare were identified using GC–MS method. The proposed GC–MS technique was applied for the analysis of biomarker fenchone in extract and eight different commercial formulations. The main compounds identified in the methanolic extract of F. vulgare were trans-anethole, 2-pentanone, fenchone and benzaldehyde-4-methoxy. Several compounds were detected in higher amounts and some in trace amounts. Many compounds were identified for the first time in F. vulgare by GC–MS technique. The proposed method was applied in the analysis of fenchone contents in extract and various commercial formulations. The maximum amount of fenchone was obtained in extract in comparison with commercial formulations. Many phytoconstituents of methnolic extract of F. vulgare have been reported for the first time in this work. The results obtained in this work could be useful in standardization of commercial formulations containing fennel extract or fenchone.
Electronic supplementary material
Below is the link to the electronic supplementary material.
This article contains supplementary information which can be found online. Figure S1 shows F. vulgare Mill. in its natural habitat conditions. Figure S2 presents chemical structure of fenchone (DOCX 107 kb)
Acknowledgement
This project was financially supported by King Saud University, Vice Deanship of Research Chairs, Kayyali Chair for Pharmaceutical industry through the Grant Number FN-2018.
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest associated with this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdel Karm M, Ayda A, Khalid MS. GC-MS analysis and antimicrobial activity of Saudi Foeniculum vulgare Mill. (Apiaceae) fixed oil. Int J Adv Res. 2017;5:1523–1528. doi: 10.21474/IJAR01/4866. [DOI] [Google Scholar]
- Acimovic M, Tesevic V, Todosijevic M, Djisalov J, Oljaca S. Compositional characteristics of the essential oil of Pimpinella anisum and Foeniculum vulgare grown in Serbia. Bot Serb. 2015;39:9–14. [Google Scholar]
- Ahmad BS, Talou T, Saad Z, Hijazi A, Cerny M, Kanaan H, Chokr A, Merah O. Fennel oil and by-products seed characterization and their potential applications. Ind Crops Prod. 2018;111:92–98. doi: 10.1016/j.indcrop.2017.10.008. [DOI] [Google Scholar]
- Anand P, Kunnumakara A, Sundaram C, Harikumar K, Tharakan S, Lai O, Sung B, Aggarwal B. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25:2097–2116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anubhuti P, Rahul S, Kant KC. Standardization of fennel (Foeniculum vulgare), its oleoresin and marketed ayurvedic dosage forms. Int J Pharm Sci Drug Res. 2011;3:265–269. [Google Scholar]
- Anwar F, Ali M, Hussain AI, Shahid M. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare Mill.) seeds from Pakistan. Flavour Fragr J. 2009;24:170–176. doi: 10.1002/ffj.1929. [DOI] [Google Scholar]
- Badgujar SM, Patel VV, Bandivdekar AH. Foeniculum vulgare mill: a review of its botany, phytochemistry, pharmacology, contemporary application, and toxicity. Biomed Res Int. 2014;2014:E8426674. doi: 10.1155/2014/842674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahmani K, Darbandi AI, Ramshini HA, Moradi N, Akbari A. Agro-morphological and phytochemical diversity of various Iranian fennel landraces. Ind Crops Prod. 2015;77:282–294. doi: 10.1016/j.indcrop.2015.08.059. [DOI] [Google Scholar]
- Choi E, Hwang J. Antiinflammatory, analgesic and antioxidant activities of the fruit of Foeniculum vulgare. Fitoter. 2004;75:557–565. doi: 10.1016/j.fitote.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Cioanca O, Hancianu M, Mircea C, Trifan A, Hritcu L. Essential oils from Apiaceae as valuable resources in neurological disorders: Foeniculum vulgare aetheroleum. Ind Crops Prod. 2016;88:51–57. doi: 10.1016/j.indcrop.2016.02.064. [DOI] [Google Scholar]
- Cosge B, Ipek A, Gurbuz B. Gas chromatography/mass spectrometry analysis of essential oil from different vegetative organs and fruits of Foeniculum vulgare Mill. var. vulgare growing in Turky. Asian J Chem. 2009;21:4081–4087. [Google Scholar]
- Diao WR, Hu QP, Zhang H, Xu JG. Chemical composition: antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.) Food Control. 2014;35:109–116. doi: 10.1016/j.foodcont.2013.06.056. [DOI] [Google Scholar]
- Diaz-Maroto MS, Perez-Coello S, Esteban J, Sanz J. Comparison of the volatile composition of wild fennel samples (Foeniculum vulgare Mill.) from central Spain. J Agric Food Chem. 2006;54:6814–6818. doi: 10.1021/jf0609532. [DOI] [PubMed] [Google Scholar]
- Elagayyar M, Draughon FA, Golden DA. Antimicrobial activity of essential oil from plants against selected pathogenic and saprophytic microorganisms. J Food Prot. 2001;64:1019–1024. doi: 10.4315/0362-028X-64.7.1019. [DOI] [PubMed] [Google Scholar]
- Hammouda FM, Saleh MA, Abdel-Azim NS, Shams KA, Ismail SI, Shahat AA, Saleh IA. Evaluation of the essential oil of Foeniculum vulgare Mill (fennel) fruits extracted by three different extraction methods by GC/MS. Afr J Trad Compl Alt Med. 2013;11:277–279. doi: 10.4314/ajtcam.v11i2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontogiorgis C, Deligiannidou GE, Hadjipavlou-Litina D, Lazari D, Papadopoulos A. Antioxidant protection: the contribution of proper preparation of fennel (Foeniculum vulgare Mill.) beverage. Ind Crops Prod. 2016;79:57–62. doi: 10.1016/j.indcrop.2015.10.020. [DOI] [Google Scholar]
- Majdoub N, El-Guendouz S, Rezgui M, Carlier J, Costa C, Kaab LBB, Miguel MG. Growth, photosynthetic pigments, phenolic contants and biological activities of Foeniculum vulgare Mill., Anethum greolens L. and Pimpinella anisum L. (Apiaceae) in response to zinc. Ind Crops Prod. 2017;109:627–636. doi: 10.1016/j.indcrop.2017.09.012. [DOI] [Google Scholar]
- Mazaheri S, Nematbakhsh M, Bahadorani M, Pezeshki Z, Talebi A, Ghannadi AR, Ashrafi F. Effects of fennel essential oils on cisplatin-induced nephrotoxicity overiectomized rats. Toxicol Int. 2013;20:138–145. doi: 10.4103/0971-6580.117256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojab F, Javidnia K, Nickavar B, Yazdani D. GC-MS analysis of the essential oils of roots and leaves of Foeniculum vulgare Mill. J Essent Oil Bearing Plants. 2007;19:36–40. doi: 10.1080/0972060X.2007.10643516. [DOI] [Google Scholar]
- Ozbek H, Ugras S, Dulger H, Bayram I, Tuncer I, Ozturk G. Hepatoprotective effect of Foeniculum vulgare essential oil. Fitoter. 2003;74:317–319. doi: 10.1016/S0367-326X(03)00028-5. [DOI] [PubMed] [Google Scholar]
- Ozcan MM, Chalchat JC, Arslan D, Ate A, Unver A. Comparative essential oil composition and antifungal effect of bitter fennel (Foeniculum vulgare ssp. piperitum) fruit oils obtained during different vegetation. J Med Food. 2006;9:552–561. doi: 10.1089/jmf.2006.9.552. [DOI] [PubMed] [Google Scholar]
- Piccaglia R, Marotti M. Characterization of some Italian types of wild fennel (Foeniculum vulgare Mill) J Agric Food Chem. 2001;49:239–244. doi: 10.1021/jf000636+. [DOI] [PubMed] [Google Scholar]
- Ravid U, Putievsky E, Katzir I, Ikan R. Chiral gc analysis of enantiomerically pure fenchone in essential oils. Flavor Fragr J. 1992;7:169–172. doi: 10.1002/ffj.2730070314. [DOI] [Google Scholar]
- Renjie L, Zhenhong L, Shidi S. GC-MS analysis of fennel essential oil and its effect on microbiology growth in rats’ intestine. Afr J Microbiol Res. 2010;4:1319–1323. [Google Scholar]
- Reynolds JEF. Essential oils and aromatic carminatives, martindale-the extra. Pharmacopeia. 1982;670:676. [Google Scholar]
- Roby MHH, Sarhan MA, Selim KAH, Khalel KA. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare L.) and chamomile (Matricaria chamomilla L.) Ind Crops Prod. 2013;44:437–445. doi: 10.1016/j.indcrop.2012.10.012. [DOI] [Google Scholar]
- Rodriguez-Solana R, Salgado JM, Dominguez JM, Cortes-Dieguez S. Characterization of fennel extracts and quantification of estragole: optimization and comparison of accelerated solvent extraction and Soxhlet techniques. Ind Crops Prod. 2014;52:528–536. doi: 10.1016/j.indcrop.2013.11.028. [DOI] [Google Scholar]
- Saleem F, Sarkar D, Ankolekar C, Shetty K. Phenolic bioactives and associated antioxidant and anti-hyperglycemic functions of select species of Apiaceae family targeting for type 2 diabetes relevant nutraceuticals. Ind Crops Prod. 2017;107:518–525. doi: 10.1016/j.indcrop.2017.06.023. [DOI] [Google Scholar]
- Shahat AA, Ibrahim AY, Hendawy SF, Omer EA, Hammouda FM, Abdel-Rahman FH, Saleh MA. Chemical composition, antimicrobial and antioxidant activities of essential oils from organically cultivated fennel cultivars. Molecules. 2011;16:1366–1377. doi: 10.3390/molecules16021366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahmokhtar MK, Armand S. Phytochemical and biological studies of fennel (Foeniculum vulgare Mill.) from the South Western region of Iran (Yasouj) Nat Prod Chem Res. 2017;5:1–4. doi: 10.4172/2329-6836.1000267. [DOI] [Google Scholar]
- Shojaiefar S, Mirlohi A, Sabzalian MR, Yaghini M. Seed yield and essential oil content of fennel influenced by genetic variation and genotype x year interaction. Ind Crops Prod. 2015;71:97–105. doi: 10.1016/j.indcrop.2015.03.055. [DOI] [Google Scholar]
- Singh G, Maurya S, Lampasona MP, Catalan C. Chemical constituents: antifungal and antioxidative potential of Foeniculum vulgare volatile oil and its acetone extract. Food Control. 2006;17:745–752. doi: 10.1016/j.foodcont.2005.03.010. [DOI] [Google Scholar]
- Upadhyay RK. GC-MS analysis and in vitro antimicrobial susceptibility of Foeniculum vulgare seed essential oil. Am J Plant Sci. 2015;6:1058–1068. doi: 10.4236/ajps.2015.67110. [DOI] [Google Scholar]
- Walker V, Mills GA. 2-Pentanone production from hexanoic acid by Penicillium roqueforti from blue cheese: is this the pathway used in humans? Sci World J. 2014;2014:E215783. doi: 10.1155/2014/215783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article contains supplementary information which can be found online. Figure S1 shows F. vulgare Mill. in its natural habitat conditions. Figure S2 presents chemical structure of fenchone (DOCX 107 kb)