Abstract
Background:
Iranian borage, Echium amoenum, is believed to improve reproduction according to folk medicine. Although E. amoenum distillate known as “Aragh Gav-zaban” is widely consumed as a safe and natural remedy, its possible effects on fertility have not yet been scientifically examined. The present study aimed to investigate the effects of borage distillate (BD) on reproductive parameters of male mice.
Methods:
In this experimental study, 30 adult male Mus musculus mice (30-35 g) were equally divided into three groups. The control group received distilled water (DW) for five weeks and the other two groups, BD1/2 and BD1/4, received borage distillate of 1/2 dilution (150±2.5 ml/kg/day) and 1/4 dilution (75±1.25 ml/kg/day), respectively, ad libitum for three weeks and DW for 2 weeks. On the day 35, mice were sacrificed, sperm analysis was performed, and sera were collected to evaluate gonadotropins, testosterone, and toxicity parameters. The left testis was excised for stereological study and the right testis was used to evaluate androgen receptor (AR) gene expression.
Results:
The administration of BD1/2 significantly increased serum FSH (P=0.004), LH (P=0.025), testosterone (P=0.014), the percentage of motile (P=0.011); slow progressive (P=0.001), coiled tail (P<0.001) sperms, and the number of Leydig cells (P=0.008) compared to the control group. Treatment with BD1/4 significantly increased sperm count (P=0.044) and motile sperms percentage (P=0.040) compared to the control group too. The administration of BD revealed no significant effects on toxicity parameters and AR gene expression.
Conclusion:
The findings of the present study showed that the consumption of borage distillate, as a safe herbal remedy, improves hormonal and sperm parameters in male mice.
Keywords: Echium , Male fertility agents , Gonadotropins , Testosterone , Semen analysis
What’s Known
Borage distillate (BD) is widely used in different areas of Iran, according to traditional Iranian medicine, for the treatment of many diseases.
Despite health beliefs in folk medicine regarding its ameliorative effect on male reproductive parameters, its potential effects and possible toxicity have not yet been investigated scientifically.
What’s New
The administration of BD in mice improved male reproductive parameters such as increasing sex hormone levels (FSH, LH, and testosterone), sperm quality, and Leydig cell number with no effect on the level of androgen receptor gene expression.
No hepatotoxicity and/or nephrotoxicity were observed for borage distillate consumption under experimental conditions.
Introduction
Human infertility, as a worldwide public health problem, has almost equally affected both male and female in 15% of the couples.1 The number of affected couples worldwide has increased from 42.0 million in 1990 to 48.5 million in 2010.2 Infertility may result in additional stress and emotional complications as well as high treatment costs.3,4 Male factors (e.g. sperm production and its overall quality) represent approximately 50% of conception issues. The reported decrease in male reproductive potential during the past 50 years may have been the result of increased psychological stress, obesity, radiations, and environmental toxins. The latter has more effects on testes than on ovaries.5-8 Moreover, reactive oxygen species (ROS) can disrupt the endocrine system and sperm function via lipid peroxidation and DNA oxidative damage.5,9 Considering the high costs of treatments and their undesirable safety and efficacy, the use of complementary and alternative medicine (CAM) has recently gained much attention.9
Herbal remedies are considered safe and natural therapeutic alternatives among the public. Medicinal plants have been utilized in the past 6,000 years.10 The World Health Organization (WHO) estimates that 80% of the world population use herbal medicine for health purposes.11 Recent studies have shown that the administration of various herbal plant extracts improve semen parameters,12 androgen status,9,13 or fertility index.14 The most common forms of medicinal plant preparations, according to traditional Iranian medicine (TIM), are extracts and distillates. Since distillates (locally named “aragh”) are easier to use, they are the most favorite drink in Iran especially in the city of Shiraz.15
Iranian borage or Echium amoenum (Gol-Gav-Zaban) belongs to genus Echium and Boraginaceae family. In a study performed in Shiraz in 2010, it was reported to be the second most used medicinal herb.10Echium amoenum has been used as anti-inflammatory, analgesic, anxiolytic, and sedative in Iranian folk medicine. It is also considered to improve fertility parameters.16 Although borage distillate (BD) has been widely used for its reproductive benefits in different areas of Iran, its possible effects on fertility have not yet been examined scientifically. Hence, the present study was designed to investigate the effects of BD on the fertility parameters in mice, including the levels of gonadotropins and testosterone, sperm analysis, stereological parameters of testis, and the level of androgen receptor gene expression. The potential hepatotoxicity and nephrotoxicity due to the distillate were also evaluated.
Materials and Methods
Preparation of Borage Distillate
The aerial parts of Iranian borage (E. amoenum) were collected from the farms around the city of Fasa (Fars province). The genus and species of the plant were authenticated and approved by an herbal specialist at the herbarium of the Biology Department of Shiraz University, Shiraz, Iran. To obtain 1 liter of BD stock, 0.2 kg of the dried plant was placed in the boiler with 1.2 liters of water and the distillate was prepared as previously described.17 Passing through the condenser duct, the outgoing steam was cooled and BD was collected and stored light-protected at 4 ºC until use.
Animals
Adult 8-week male Mus musculus mice, weighing 30-35 g, were obtained from the Animal Breeding Center, Shiraz University of Medical Science, Shiraz, Iran and kept under standard conditions (light:dark cycle 12:12 h, humidity 25-35%, and temperature 20-22 °C).18 All procedures were approved by the Institutional Animal Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran (IR.SUMS.REC).
Experimental Protocol
In this experimental study, 30 male mice were equally divided into 3 groups. The first group received distilled water (DW), ad libitum. The second and third groups were administered with one volume of BD stock diluted with one volume of water (BD1/2) and/or three volumes of water (BD1/4), ad libitum. The treatment groups were administered BD for 3 weeks and DW for 2 weeks, 5 weeks in total, which is the length of spermatogenesis in mice.3 The animals were weighed weekly and the volume of consumed distillate was also recorded to calculate the exact daily consumption of BD per kg body weight.
Sacrifice Schedule
On day 35, the mice were anesthetized with CO2, the blood samples were obtained by heart puncture and their sera were collected and kept at -80 °C for biochemical analysis. Left cauda epididymis and testes were excised for semen analysis and stereological studies, respectively. The right testes were separated and kept at -80 °C for gene expression analysis.
Evaluation of Biochemical Parameters
Hormonal Assay
Serum follicle-stimulating hormone (FSH) and luteinizing hormone (LH) assays were carried out using mouse ELISA kit (Hangzhou Eastbiopharm Co., Hangzhou, China). Testosterone assay was carried out using Biospes ELISA kit (China).
Toxicity Assay
To investigate the possible hepatotoxicity of BD, the levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured in serum using Biorexfars kit (Shiraz, Iran). Blood urea nitrogen (BUN) and creatinine were also evaluated for nephrotoxicity using Mancompany kit (Tehran, Iran) and Biorexfars kit (Shiraz, Iran), respectively.
Gene Expression Analysis
The expression level of androgen receptor (AR) gene was evaluated using quantitative RT-PCR. Total RNA was extracted using Yekta Tajhiz Azma RNA extraction mini kit (Tehran, Iran). To exclude DNA contamination, total RNA was treated with RNase-free DNase (Fermentas, Burlington, Canada) and cDNA was synthesized using reverse transcriptase (Fermentas, Burlington, Canada). RNA samples were subjected to RT-PCR using ABI 7500 real-time PCR system (USA) and SYBR Green Master Mix (Amplicon, Denmark). The CT values of AR gene were normalized to beta-actin as an internal control gene to obtain relative expression level using the ΔΔCT method. The primer pairs sequence for beta-actin and AR gene were designed using AlleleID 7.73 software and were respectively as follows:
Forward: 5’CACACCCGCCACCAGTTCG3’
Reverse: 5’ACCCATTCCCACCATCACACC3’
Forward: 5’ATGACAACAACCAACCAGATT3’
Reverse: 5’TTAGTGAAGGACCGCCAAC3’
Sperm Analysis
Immediately after sacrifice, left cauda epididymis was cut and incubated for 3 minutes in pre-warmed 37 °C normal saline. The sperm solution was placed in a hemocytometer and the heads of the sperms were counted under a light microscope. To evaluate sperm motility, 10 fields were randomly selected and sperms were categorized as different motile types or immotile.19 For the evaluation of sperm morphology, sperm smears were stained with eosin Y and sperms were classified as normal or abnormal subgroups according to the criteria of normal sperm morphology.20
Stereological Parameters
Five mice of each group were randomly chosen for stereological analysis of the testes. The left testis was excised, its primary volume was estimated by immersion in distilled water21 using Scherle’s immersion method,22 and then fixed in a buffered formaldehyde solution.
The testis volume was estimated by the isotropic Cavalieri method using orientator to obtain isotropic uniform random (IUR) sections. 23 Then, 5 and 25 μm thick sections were prepared consecutively using a microtome with a constant interval to obtain 8-12 sections per testis. Tissue sections with 25 μm thickness were stained with hematoxylin and eosin to evaluate cell number, and 5 μm thick sections were stained with azan for volume estimation.
Testis volume estimation: The Cavalieri’s volume of the testis was estimated at the final magnification of 13× using the following formula:
Where V(testis) is the exact volume of the testis, A is the area of tissue sections, and T is the distance between the sampled sections.
Tubules and interstitial tissue volume evaluation: The point counting method was applied to estimate the volume density and volume of the seminiferous tubules and interstitial tissue at the final magnification of 280×:
Vv(structure)=ΣP(structure)/ΣP(total)
Where Vv(structure) is the volume density of the structure and ΣP(structure) and ΣP(total) are the total points hitting the target structure and the testis sections, respectively.
The volume of the structure was calculated by multiplying the volume density of the structure by the testis volume using the following formula:
V(structure)=Vv(structure)×V(testis)
Testicular cell number estimation: To estimate the total number of spermatogonia A (pale-staining nucleus with a fine “dusty” distribution of heterochromatin throughout the nucleus), B (with dense clumps of heterochromatin around the periphery of the nucleus),24 Sertoli cells, spermatocytes, round and long spermatids, and Leydig cells, a Nikon E200 light microscope (Tokyo, Japan) fitted with a 60× oil objective lens was applied at the final magnification of 1,640×. An unbiased counting frame was superimposed on testis sections. The number of cells was estimated using “optical dissector” method with a microcator (MT12, Heidenhain, Traunreut, Germany) used to measure the z-axis of tissue sections. The numerical density of the cells was calculated as follows:23
Nv(cells)=[ΣQ/(ΣP×(a/f)×h)]×(t/BA)
Where Nv(cells) is the numerical density of the cells, ΣQ is the number of each cell type nuclei, a/f is the area per counting frame, ΣP is the total number of counted frames per animal; h is the height of the optical dissector measured using the microcator, t is the mean thickness of final sections, and BA is the microtome block advance.
N(cells)=NV(cells)×V(structure)
Where N(cells) is the number of the cells, V(structure) is the volume of epithelium for the cells of germinal layer, and the volume of interstitial tissue for Leydig cells.
Estimation of seminiferous tubules length: The length density of the tubules was calculated using an unbiased counting frame25 at the final magnification of 148× using the following formula:
Where ΣQ is the number of the tubule profiles counted, a/f is the area per counting frame, and ΣP is the total number of frames counted in each animal. The following formula was used to calculate the length of tubule (L) by multiplying the length density (LV) by the tubules total volume:
L=LV×V(tubule)
Statistical Analysis
Statistical analysis was performed using SPSS 16.0 and the graphs were designed by GraphPad Prism 5 (San Diego, CA, USA). For comparisons between groups, the data (shown as mean±SD) were analyzed using one-way analysis of variance (ANOVA) followed by Dunnett’s post-hoc test (n=10). For stereological parameters (n=5), comparisons between groups were performed by Kruskal-Wallis followed by Mann-Whitney U test for pairwise comparisons. P<0.05 was considered statistically significant.
Results
Daily Distillate Consumption
The mean dosage of BD stock distillate consumption was calculated by dividing the daily consumption volume of the distillate by the animal body weight. It was 150±2.5 and 75±1.25 ml/kg/day for BD1/2 and BD1/4 groups, respectively.
Biochemical Parameters of Experimental Mice
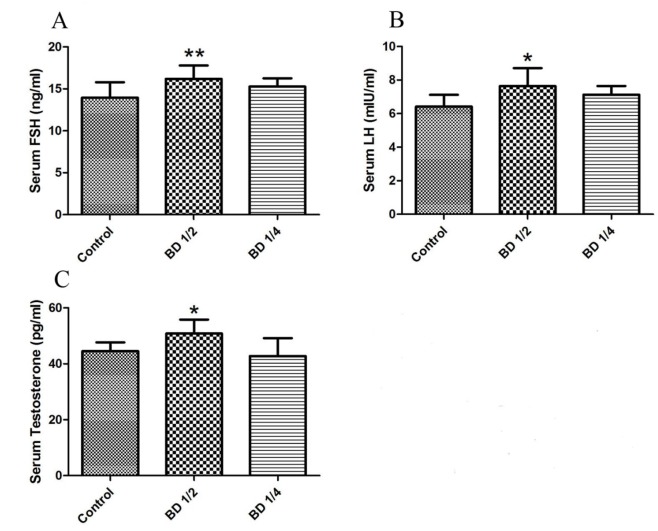
Hormone levels: The levels of serum male reproductive hormones are represented in figure 1. As shown, BD1/2 group exhibited significantly higher FSH (16.1±1.6 ng/ml), LH (7.6±1.0 mIU/ml), and testosterone (50.8±4.9 pg/ml) levels (P=0.004, P=0.025, and P=0.014, respectively) compared to the control group (FSH=13.9±1.8 ng/ml, LH=6.4±0.7 mIU/ml, testosterone=44.5±3.11 pg/ml). No significant difference was observed between hormone levels of the group receiving BD1/4 compared to the control group.
Figure1.
The level of serum male reproductive hormones in male mice (n=10) including FSH (A), LH (B), and Testosterone (C). Control group received distilled water, BD1/2 and BD1/4 groups received borage distillates of 1/2 and 1/4 dilutions, respectively. *P<0.05 and **P<0.01 vs. Control group
Toxicity: The level of ALT and AST (as the two parameters of liver function tests) and BUN and creatinine (as kidney function indicators) are shown in figure 2. As shown, administration of BD revealed no significant changes in the above-mentioned parameters in the experimental mice.
Figure2.
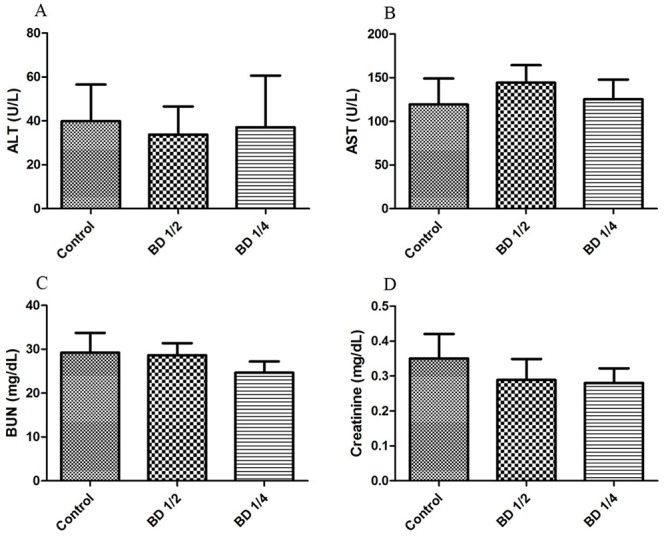
The level of serum hepatic enzymes and nephrotoxicity parameters in the male mice (n=10) including ALT (A), AST (B), BUN (C), and Creatinine (D). Control group received distilled water, BD1/2 and BD1/4 groups received borage distillates of 1/2 and 1/4 dilutions, respectively
Androgen Receptor Gene Expression
As shown in figure 3, administration of BD1/2 or BD1/4 did not significantly affect the expression of androgen receptor gene compared to the control group.
Figure3.
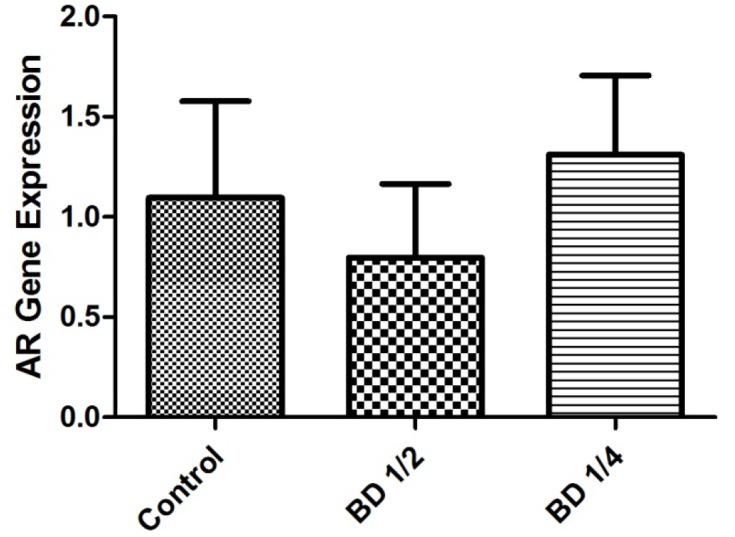
The relative expression of androgen receptor (AR) gene in the male mice (n=10). Control group received distilled water, BD1/2 and BD1/4 groups received borage distillates of 1/2 and 1/4 dilutions, respectively. Beta-actin gene expression has been used as an internal control
Semen Parameters
The values of sperm count and the percentage of normal or abnormal sperms are presented in table 1. The data indicated that the total number of sperms was significantly higher (P=0.044) in the BD1/4 group compared to the control group. The percentage of normal sperms revealed no significant difference between groups. Sperms with abnormal morphology, including big or small head, short or coiled tail, head only and tail only, revealed no significant changes except for the percentage of coiled tail sperms that was significantly higher (P<0.001) in mice receiving BD1/2 compared to the control group.
Table 1.
The values of sperm count and the percentage of normal and abnormal morphology of sperms in the experimental mice
| Groups | Sperm count (×106/ml) | Normal % | Abnormal morphology | |||||
|---|---|---|---|---|---|---|---|---|
| Big head % | Small head % | Short tail % | Coiled tail % | Head only % | Tail only % | |||
| Control | 43.6±7.7 | 95.6±2.4 | 0.2±0.1 | 0.6±0.2 | 0±0 | 0.03±0.1 | 2.2±1.4 | 1.2±0.3 |
| BD 1/2 | 48.5±18.2 | 92.7±6.8 | 0.2±0.1 | 0.4±0.3 | 0.1±0.2 | 0.6±0.4** | 1.6±1.4 | 1.6±0.8 |
| BD 1/4 | 60.2±13.6* | 95.0±2.8 | 0.5±0.6 | 0.8±0.4 | 0.2±0.4 | 0.1±0.2 | 2.3±1.4 | 0.7±0.4 |
The values (n=10) are presented as mean±SD. Control group includes mice receiving distilled water, BD1/2 group consists of mice receiving borage distillate of 1/2 dilution, and BD1/4 group are mice receiving borage distillate of 1/4 dilution.
P<0.05
P<0.01 vs. Control group
The values for sperm motility, including fast progressive, slow progressive and non-progressive sperms are shown in table 2. Results indicated a significant increase in the percentage of motile sperms in the BD1/2 (P=0.011) and BD1/4 (P=0.040) groups compared to the control group. Investigating the different aspects of sperm motility showed no significant changes between the groups except for the group receiving BD1/2, which presented a significant increase (P=0.001) in the percentage of slow progressive sperms.
Table 2.
The values of different aspects of sperm motility in the experimental mice
| Groups | Total motile % | Motile | ||
|---|---|---|---|---|
| Fast progressive % | Slow progressive % | Non-progressive % | ||
| Control | 52.4±15.2 | 45.3±15.7 | 4.3±5.9 | 2.8±1.9 |
| BD1/2 | 70.9±7.3* | 50.2±14.1 | 15.5±10.1** | 7.1±6.8 |
| BD1/4 | 69.3±9.3* | 54.1±15.0 | 9.1±5.3 | 7.0±5.6 |
The values (n=10) are presented as mean±SD. Control group includes mice receiving distilled water, BD1/2 group consists of mice receiving borage distillate of 1/2 dilution, and BD1/4 group are mice receiving borage distillate of 1/4 dilution.
P<0.05
P<0.01 vs. Control group
Stereological Parameters
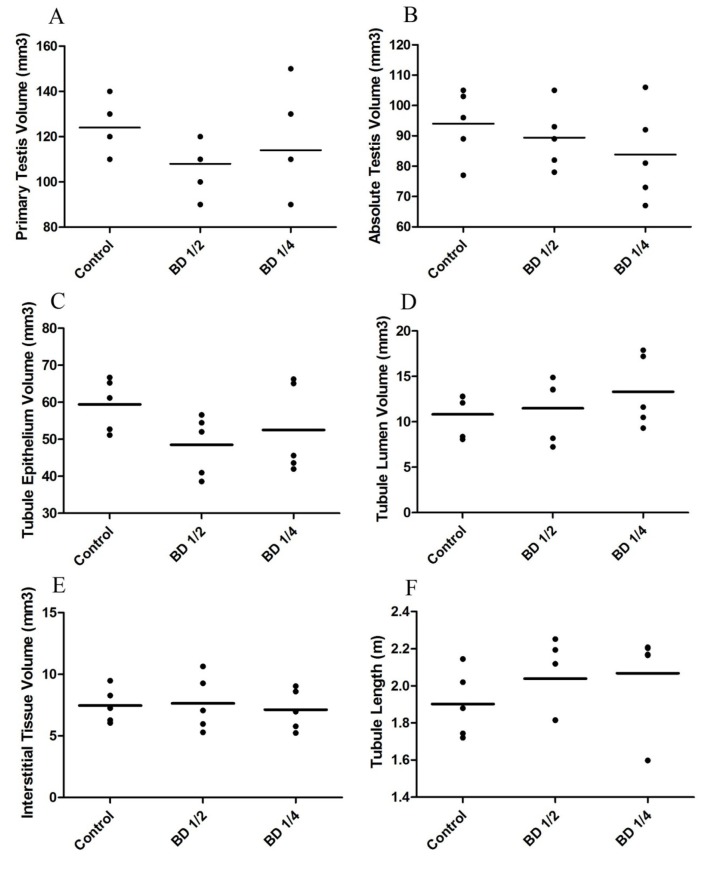
The values of primary testis volume, absolute Cavalieri testis volume, the volume of seminiferous tubule epithelium and lumen, the volume of testis interstitial tissue, and the length of seminiferous tubules are illustrated in figure 4. The results indicated that the administration of BD made no significant changes in these parameters of the study groups.
Figure4.
Stereological parameters of male mice (n=5). Control group received distilled water, BD1/2 and BD1/4 groups received borage distillates of 1/2 and 1/4 dilutions, respectively. As shown, primary testis volume (A), absolute testis volume calculated by Cavalieri’s method (B), the volume of seminiferous tubule epithelium (C), the volume of seminiferous tubule lumen (D), the volume of testis interstitial tissue (E), and the length of seminiferous tubules (F) revealed no significant changes between groups.
The numbers of different testis cell types are summarized in table 3. According to the results of this part, administration of BD1/2 resulted in a significant increase (P=0.008) in the total number of Leydig cells compared to the control group. However, no significant difference was observed in the number of spermatogonia (A and B), spermatocyte, round and long spermatids, and Sertoli cells between the experimental groups.
Table 3.
The number of different testis cell types (×106)
| Groups | Spermatogonia A | Spermatogonia B | Sertoli cell | Primary spermatocyte | Round spermatid | Long spermatid | Leydig cell |
|---|---|---|---|---|---|---|---|
| Control | 0.83±0.06 | 0.16±0.07 | 2.7±0.2 | 12.1±0.98 | 31.8±2.49 | 30.9±3.02 | 2.2±0.02 |
| BD1/2 | 0.83±0.05 | 0.13±0.05 | 2.5±0.1 | 12.0±0.80 | 31.0±2.18 | 30.6±2.66 | 2.5±0.15** |
| BD1/4 | 0.84±0.05 | 0.18±0.05 | 2.7±0.3 | 12.4±0.92 | 32.2±2.20 | 32.1±2.65 | 2.1±0.03 |
The values (n=5) are presented as mean±SD. Control group includes mice receiving distilled water, BD1/2 group consists of mice receiving borage distillate of 1/2 dilution, and BD1/4 group are mice receiving borage distillate of 1/4 dilution.
P<0.01 vs. Control group
Discussion
Despite advances in the management of subfertility, the tendency towards alternative and complementary treatments, especially herbal medicine has increased due to high costs and unwanted side effects of conventional medical treatments. Traditional Iranian medicine (TIM) has been used since the ancient times by scientists such as Ibn-Sina (Avicenna, 980-1037 CE), and Al-Razi (Rhazes, 865-925 CE).17,26 Although some herbal distillates have been traditionally utilized to improve reproductive parameters and treat sterility and subfertility, no experimental scientific study has been designed yet to evaluate their impact on reproductive parameters. To the best of our knowledge, the current study is the first experimental approach in investigating the effects of medicinal plants, especially in a distillate form, considering such various aspects and potential toxicity.
According to the results, it is revealed that the administration of E. amoenum distillate with the higher concentration (BD1/2) significantly increased the level of FSH, LH, and testosterone. The hormonal management of spermatogenesis is controlled by the hypothalamic-pituitary-gonadal axis. Secretion of gonadotropin-releasing hormone (GnRH) from hypothalamus stimulates the release of FSH and LH from pituitary gland. FSH receptor is expressed in Sertoli cells, which support germ cells during multiple stages of spermatogenesis. Stimulation of LH receptors in Leydig cells leads to the secretion of testosterone hormone.8,27 In the current study, BD1/2 increased LH and consequently testosterone hormone level, probably through affecting the hypothalamic-pituitary-gonadal axis.
Ghassemi et al. investigated the chemical composition of the volatile fraction of E. amoenum petals distillate and revealed that sesquiterpenes are the major components (49.55%) of the distillate. Other main components were n-Tricosane (33.92%), n-Pentacosane (21.09%), Palmitic acid (18.91%), Linoleic acid (3.51%), and Pulegone (2.20%).28 In addition, anti-oxidant and anti-inflammatory effects have been reported for sesquiterpenes in another study.29 Since environmental toxins accumulate in the adipose tissue all over the body, they might adversely affect reproductive hormone levels5 and the anti-oxidant effects of sesquiterpene components of BD might ameliorate these adverse effects. The main phenolic component of E. amoenum flowers extract has also been identified to be rosmarinic acid, which is frequent in Boraginaceae and Lamiaceae families and has antioxidant and hepatoprotective properties.30-32 Rosmarinic acid has shown to increase the impaired level of testosterone after exposure to electromagnetic field in rats.33,34
In the current study, consumption of BD for three weeks revealed no changes in ALT and AST level, which confirms the safety of BD for the liver. Moreover, Zamansoltani et al. indicated that administration of E. amoenum extract reduced the level of ALT, ALP, and AST after 2 weeks.32 The observed difference might be due to the different preparations of the plant. Furthermore, administration of BD made no significant difference in the creatinine and BUN level in experimental mice, which indicates no nephrotoxicity under experimental conditions.
We also observed that BD administration did not affect the level of AR gene expression. Thus, any observed changes in the fertility parameters might be due to the changes in the testosterone level and not the receptors. The present study also showed an increase in the percentage of motile and slow progressive sperms in the BD1/2 group, which might be resulted from elevated FSH level. Moreover, consumption of BD1/4 resulted in the enhanced sperm count and motility, which could be resulted from anti-oxidant components of borage distillate.
The only change in the morphology of sperms in the experimental groups was the elevated percentage of coiled tail abnormal sperms in the BD1/2 group. The methanol content of many herbal distillates, which is produced from pectin or fermentation of cellulose during the distillation of woody plants and also their accumulation in the adipose tissue around the scrotum, might be responsible for their adverse effects on spermatogenesis.5,35 Al-Alami et al. showed that rosmarinic acid increased the sperm count and motility in metronidazole-induced infertile rats as a result of its penetration into the membrane of sperms and protecting the high ratio of membrane unsaturated fatty acids from lipid peroxidation.36
In the present study, although the consumption of BD1/2 elevated the number of Leydig cells, in line with the increased testosterone level they produced, the expected volume increase in the interstitial tissue was not observed. This finding was in agreement with a previous study which demonstrated that the administration of curcumin in metronidazole-treated mice, despite the increase in the number of Leydig cells, had no significant effects on the volume of testis interstitial tissue.25 Briefly, BD in the higher concentration could significantly increase the level of serum FSH which resulted in higher sperm quality. It also elevated the level of LH and consequently increased the number of Leydig cells and testosterone hormone, probably due to its antioxidant constituents.
The present study has some limitations which should be mentioned for a better interpretation. First, as in most animal studies using a mouse model, the research presented here was limited by the insufficiency of the volume of mice serum. It limited us to evaluate the impact of BD consumption on oxidant/antioxidant status by examining the total antioxidant capacity, antioxidant enzymes, and lipid peroxidation level. Second, since the current study is the first experimental approach in investigating the effects of BD on male reproductive parameters, no all-inclusive positive control, covering all aspects of the investigation, was yet defined to include in the study. Third, the exact composition of the aerial parts of E. amoenum distillate is not defined yet. Fortunately, in a parallel study, the data revealing the constituents of the distillate are due soon. Finally, the present study aimed to investigate the ameliorative or adverse effects of BD consumption. However, due to time limitations and the fact that the effect in humans requires a longer period, the current duration was not comparable to humans.
Conclusion
The results of the present study have shown that the administration of E. amoenum distillate could improve the male reproductive hormones profile and sperm parameters. This might be due to the attenuation of oxidative stress by antioxidant components of the distillate. Based on the beneficial effects of BD and lack of toxicity, it could be suggested as a safe drug supplement to improve fertility in males. However, in the category of plant distillate consumption, the safety, standardization, dosage, efficacy, and stability of the distillates, as well as the exact mechanisms responsible for the observed effects, require further investigation. Finally, for a better understanding of BD consumption effects on the human body, it is highly recommended to perform a clinical trial on humans using larger population samples and a routine dose of BD consumption.
Acknowledgement
The present study was financially supported by a research grant (93-7389) from Shiraz University of Medical Sciences, Shiraz, Iran. This article was extracted from a part of the PhD thesis written by Shima Fakher.
Conflict of Interest:None declared.
References
- 1.Chen SR, Chen M, Deng SL, Hao XX, Wang XX, Liu YX. Sodium-hydrogen exchanger NHA1 and NHA2 control sperm motility and male fertility. Cell Death Dis. 2016;7:e2152. doi: 10.1038/cddis.2016.65. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37. doi: 10.1186/s12958-015-0032-1. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooke HJ, Saunders PT. Mouse models of male infertility. Nat Rev Genet. 2002;3:790–801. doi: 10.1038/nrg911. [DOI] [PubMed] [Google Scholar]
- 4.Sankako MK, Garcia PC, Piffer RC, Dallaqua B, Damasceno DC, Pereira OC. Possible mechanism by which zinc protects the testicular function of rats exposed to cigarette smoke. Pharmacol Rep. 2012;64:1537–46. doi: 10.1016/s1734-1140(12)70951-9. [DOI] [PubMed] [Google Scholar]
- 5.Du Plessis SS, Cabler S, McAlister DA, Sabanegh E, Agarwal A. The effect of obesity on sperm disorders and male infertility. Nat Rev Urol. 2010;7:153–61. doi: 10.1038/nrurol.2010.6. [DOI] [PubMed] [Google Scholar]
- 6.Nargund VH. Effects of psychological stress on male fertility. Nat Rev Urol. 2015;12:373–82. doi: 10.1038/nrurol.2015.112. [DOI] [PubMed] [Google Scholar]
- 7.Kumar BS, Kumar JV, Selvaraj R. Evaluation of Fertility Efficacy of Ionidium Suffruticosum Extract on Senility Induced Sterility of Male Albino Rats. Int J Curr Res Rev. 2013;5:98–103. [Google Scholar]
- 8.Ramaswamy S, Weinbauer GF. Endocrine control of spermatogenesis: Role of FSH and LH/ testosterone. Spermatogenesis. 2014;4:e996025. doi: 10.1080/21565562.2014.996025. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahangarpour A, Oroojan AA, Heidari H, Ghaedi E, Taherkhani R. Effects of Hydro-alcoholic Extract from Arctium lappa L. (Burdock) Root on Gonadotropins, Testosterone, and Sperm Count and Viability in Male Mice with Nicotinamide/ Streptozotocin-Induced Type 2 Diabetes. Malays J Med Sci. 2015;22:25–32. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 10.Beheshti-Poor N, Jamali Moghadam N, Soleimani S, Haghnegahdar A, Salehi A. Assessment of knowledge, belief and function of people about herbal medicines who referred to one of clinics dependent to medical university of Shiraz in 2010. Journal of Herbal Drugs. 2011;1:53–9. [Google Scholar]
- 11.Ligha A, Oyibo A. The effect of Indian liquorice on fertility potentials of male rats. Arch Biol Sci. 2012;64:229–37. doi: 10.2298/ABS1201229l. [DOI] [Google Scholar]
- 12.Bahmanpour S, Vojdani Z, Panjehshahin MR, Hoballah H, Kassas H. Effects of Carthamus tinctorius on Semen Quality and Gonadal Hormone Levels in Partially Sterile Male Rats. Korean J Urol. 2012;53:705–10. doi: 10.4111/kju.2012.53.10.705. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jalili C, Ahmadi S, Roshankhah S, Salahshoor M. Effect of Genistein on reproductive parameter and serum nitric oxide levels in morphine-treated mice. Int J Reprod Biomed (Yazd) 2016;14:95–102. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 14.Fallah Huseini H, Kianbakht S. Study on effects of chicory (Cichorium intybus L.), fennel (Foeniculum vulgare Mill.) and dill (Anethum graveolens L.) on fertility and neonatal gender in rats. Journal of Medicinal Plants. 2012;2:192–6. Persian. [Google Scholar]
- 15.Gohari A, Noorafshan A, Akmali M, Zamani-Garmsiri F, Seghatoleslam A. Urtica Dioica Distillate Regenerates Pancreatic Beta Cells in Streptozotocin-Induced Diabetic Rats. Iran J Med Sci. 2018;43:174–83. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 16.Asadi-Samani M, Bahmani M, Rafieian-Kopaei M. The chemical composition, botanical characteristic and biological activities of Borago officinalis: a review. Asian Pac J Trop Med. 2014;7S1:S22–8. doi: 10.1016/S1995-7645(14)60199-1. [DOI] [PubMed] [Google Scholar]
- 17.Seghatoleslam A, Mashkour N, Namavari M, Azarmehr B, Nejabat M. The potential effects of herbal distillates with hot and cold temperament on cell metabolic activity and growth: A preliminary in vitro study. J Pharm Biomed Sci. 2014;4:532–5. [Google Scholar]
- 18.Council NR. Guide for the care and use of laboratory animals. Washington: National Academies Press; 2010. [Google Scholar]
- 19.Seghatoleslam A, Ferro VA, Mansourian M, Kargar M, Hosseini MH, Manavian M, et al. Evaluation of immunocastration conjugates based on GnRH linked to carrier molecules in a male rodent model. Comparative Clinical Pathology. 2014;23:805–11. doi: 10.1007/s00580-013-1693-9. [DOI] [Google Scholar]
- 20.Otubanjo OA, Mosuro AA, Ladipo TF. An in vivo evaluation of induction of abnormal sperm morphology by ivermectin MSD (Mectizan) Pak J Biol Sci. 2007;10:90–5. doi: 10.3923/pjbs.2007.90.95. [DOI] [PubMed] [Google Scholar]
- 21.Elias H, Hyde DM. An elementary introduction to stereology (quantitative microscopy) Am J Anat. 1980;159:412–46. doi: 10.1002/aja.1001590407. [DOI] [PubMed] [Google Scholar]
- 22.Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie. 1970;26:57–60. [PubMed] [Google Scholar]
- 23.Noorafshan A, Ahmadi M, Mesbah SF, Karbalay-Doust S. Stereological study of the effects of letrozole and estradiol valerate treatment on the ovary of rats. Clin Exp Reprod Med. 2013;40:115–21. doi: 10.5653/cerm.2013.40.3.115. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Creasy DM, Chapin RE. Male reproductive system. Haschek and Rousseaux’s Handbook of Toxicologic Pathology (Third Edition) Toronto: Elsevier; 2013. pp. 2493–598. [Google Scholar]
- 25.Noorafshan A, Karbalay-Doust S, Valizadeh A, Aliabadi E. Ameliorative effects of curcumin on the structural parameters of seminiferous tubules and Leydig cells in metronidazole-treated mice: a stereological approach. Exp Toxicol Pathol. 2011;63:627–33. doi: 10.1016/j.etp.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Rashidi M, Seghatoleslam A, Namavari M, Amiri A, Fahmidehkar MA, Ramezani A, et al. Selective Cytotoxicity and apoptosis-induction of Cyrtopodion scabrum extract against digestive cancer cell lines. Int J Cancer Mana. 2017;10:e8633. doi: 10.5812/ijcm.8633. [DOI] [Google Scholar]
- 27.Kathrins M, Niederberger C. Diagnosis and treatment of infertility-related male hormonal dysfunction. Nat Rev Urol. 2016;13:309–23. doi: 10.1038/nrurol.2016.62. [DOI] [PubMed] [Google Scholar]
- 28.Ghassemi N, Sajjadi SE, Ghannadi A, Shams-Ardakani M, Mehrabani M. Volatile Constituents of Amedicinal Plant of Iran, Echium Amoenim Fisch. and CA Mey. DARU Journal of Pharmaceutical Sciences 2003;11:32–3. [Google Scholar]
- 29.Chadwick M, Trewin H, Gawthrop F, Wagstaff C. Sesquiterpenoids lactones: benefits to plants and people. Int J Mol Sci. 2013;14:12780–805. doi: 10.3390/ijms140612780. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehrabani M, Ghassemi N, Ghannadi ESA, Shams-Ardakani M. Main phenolic compound of petals of Echium amoenum Fisch. and CA Mey., a famous medicinal plant of Iran. DARU Journal of Pharmaceutical Sciences. 2005;13:65–9. [Google Scholar]
- 31.Ranjbar A, Khorami S, Safarabadi M, Shahmoradi A, Malekirad AA, Vakilian K, et al. Antioxidant Activity of Iranian Echium amoenum Fisch & C.A. Mey Flower Decoction in Humans: A cross-sectional Before/After Clinical Trial. Evid Based Complement Alternat Med. 2006;3:469–73. doi: 10.1093/ecam/nel031. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zamansoltani F, Nassiri-Asl M, Karimi R, Mamaghani-Rad P. Hepatotoxicity effects of aqueous extract of Echium amoenum in rats. Pharmacologyonline. 2008;1:432–8. [Google Scholar]
- 33.Khaki A, Imani S, Golzar FS. Effects of rosmarinic acid on male sex hormones (testosterone-FSH-LH) and testis tissue apoptosis after exposure to electromagnetic field (EMF) in rats. Afr J Pharm Pharmacol. 2012;6:248–52. [Google Scholar]
- 34.Farsi A, Khaki A, Fathiazad F, Afshari F, Hajhossini L, Kahki AA. Improvement effect of rosmarinic acid on serum testosterone level after exposing with electromagnetic fields. Int J Women’s Health Reproduction Sci Vol. 2013;1:45–50. doi: 10.15296/ijwhr.2013.08. [DOI] [Google Scholar]
- 35.Shirani K, Hassani FV, Azar-Khiavi KR, Moghaddam ZS, Karimi G. Determination of methanol in Iranian herbal distillates. J Complement Integr Med. 2016;13:123–7. doi: 10.1515/jcim-2015-0041. [DOI] [PubMed] [Google Scholar]
- 36.Al-Alami ZM, Shraideh ZA, Taha MO. Rosmarinic acid reverses the effects of metronidazole-induced infertility in male albino rats. Reprod Fertil Dev. 2017;29:1910–20. doi: 10.1071/RD16174. [DOI] [PubMed] [Google Scholar]