Abstract
Introduction
The polyglandular autoimmune syndrome (PAS) type III is a rare condition defined as the coexistence of autoimmune thyroid disorder with other endocrine autoimmune diseases, including type 1 diabetes, without adrenal dysfunction. PAS may associate with other non-endocrine autoimmune diseases, overlapping with the multiple autoimmune syndromes (MAS). We present a case of PAS III/ MAS type 3, including autoimmune thyroiditis, autoimmune diabetes, vitiligo, lupus erythematosus, associated with adult-onset atopic dermatitis, a combination not reported previously.
Case report
A 40 years old woman, registered as nurse working in dialysis unit, previously diagnosed with vitiligo, euthyroid autoimmune thyroiditis and disseminated granuloma annulare, with personal and familial history of atopic disorders, presented in our clinic for disseminated eczematous and lichenoid cutaneous rashes. She was tested positive for antinuclear, anti-double stranded DNA and anti-histone antibodies, with inflammatory syndrome and marginal lymphopenia and she was diagnosed with systemic lupus erythematosus (SLE). Subsequently, moderate hyperglycemia, positive anti-glutamic acid decarboxylase antibodies and low C-peptide level prompted the diagnosis of autoimmune diabetes. Recurrent flexural eczematous rashes, with negative epicutaneous tests but positive specific IgE tests for common allergens fulfilled the clinical criteria for the diagnosis of atopic dermatitis. The clinical, immunological and glycemic status were controlled with low doses of oral prednisone (<0.5 mg/kg), methotrexate (10mg/week), antimalarials, metformin, emollients and photoprotection. After changing her workplace, the immunosuppressive treatment could be discontinued, and the patient maintained normal immunological and biochemical profile at 6 months follow-up.
This case brings a unique perspective on the evolution, associations spectrum and the management challenges of endocrine polyautoimmunity associated with atopic diathesis.
Keywords: polyglandular autoimmune syndrome, multiple autoimmune syndrome, latent autoimmune diabetes of the adult, atopic dermatitis
INTRODUCTION
The polyglandular autoimmune syndromes (PAS) represent a heterogeneous group of rare conditions, defined by the coexistence of at least two endocrine autoimmune disorders. Its taxonomy and classification has evolved in time (1, 2), and currently several types are described. PAS type I has an autosomal recessive inheritance and is defined by the coexistence of oral candidiasis, hypoparathyroidism, and Addison’s disease in association with mutations in the AIRE gene (2). PAS type II, also known as Schmidt’s syndrome, after the author of the first case report in 1926, is currently defined as a combination of Addison’s disease with autoimmune thyroiditis or/and type 1 diabetes (T1D)(1, 2) and has a complex polygenic foundation. PAS type III is defined as the coexistence of autoimmune thyroid disorder (AITD) with other endocrine autoimmune condition, including T1D, but without adrenal dysfunction (2). It may be associated with other non-endocrine autoimmune conditions. More recently, PAS type IV has been described as the association of two or more other organ specific autoimmune disorders (2, 3). However, PAS II-IV are regarded by some authors as different manifestations of the same polyautoimmunity syndrome (2, 3). PAS may occur in association with other non-endocrine autoimmune diseases, so there is a significant overlap with the described multiple autoimmune syndromes (MAS), defined as the coexistence of 3 or more well-defined autoimmune diseases (4). MAS were described in 1988 by Humbert and Dupond (4) and their classification, based on clustering of particular diseases has been subsequently revised (3). The original classification distinguished: the type 1 of MAS, including myasthenia gravis, thymoma, dermatopolymyositis, and autoimmune myocarditis; the type 2 of MAS, including Sjögren’s syndrome (SS), rheumatoid arthritis, primary biliary cirrhosis, systemic sclerosis, and autoimmune thyroid disease (AITD); MAS-3 group consisting of AITD, myasthenia gravis and/or thymoma, SS, pernicious anemia, idiopathic thrombocytopenic purpura, Addison’s disease, type 1 diabetes (T1D), vitiligo, autoimmune hemolytic anemia, and systemic lupus erythematosus (SLE)(4). PAS types II-IV and MAS are currently regarded as different forms of polyautoimmunity.
Atopic dermatitis (AD) is a polymorphous chronic inflammatory disorder of the skin, conditioned by the interplay of multiple genetic and environmental factors (5, 6), and whose central mechanisms consist in the genetic-conditioned alteration of the skin barrier and the Th-2-mediated immune dysfunction. The immune abnormalities in the atopic diathesis and its extreme form the atopic dermatitis are continuously unravelling and provide the substrate for the association of AD with multiple atopic and non-atopic disorders, including autoimmune diseases (5, 7-9). While associations of AD with thyroid disease (5, 7), or with vitiligo (5, 8) have been long documented, the coexistence of MAS/or PAS with AD appears to be less common. Moreover, AD seems to occur in divergent pathways with some particular autoimmune endocrine disorders like type 1 diabetes(10).
We present an unusual case of PAS III/MAS-3 including autoimmune thyroiditis, adult onset type 1 diabetes, vitiligo, lupus erythematosus, who associated adult onset atopic dermatitis.
CASE REPORT
A 40-year-old woman, normoponderal, registered nurse working in a dialysis unit, with previous diagnoses of vitiligo, disseminated granuloma annulare, and euthyroid autoimmune thyroiditis presented in our clinic in October 2013 for a recurrent, pruritic, polymorphous skin eruption, with eczematous and lichenoid aspects, involving thorax, arms and legs (Fig. 1). The diagnosis of autoimmune thyroiditis had been set in 2010, based on elevated titers of anti-thyroglobulin antibodies (ATG) (110.6 U/mL, N<13.6 U/mL), with heterogeneous echostructure and increased vascularisation revealed on thyroid ultrasound, but with normal levels of thyroid stimulating hormone (TSH), thyroid hormones (T3, T4, free T3,T4) and anti-thyroid-peroxidase antibodies (ATPO). She reported no family history of autoimmune or endocrine disease, but personal and family history of atopic manifestations (urticaria, asthma, rhinitis). The physiological history was normal, with 2 normal births and no pregnancy or menstrual-cycle-related disorders. No inducing agent could be established for the skin changes. Standard epicutaneous test was negative. The patient denied administration of any chronic medication. The only recent change in her environment was the start of work in the dialysis unit, 6 months previously. Laboratory work-up on presentation revealed elevated titers of antinuclear (ANA 1/320) and anti-double stranded DNA antibodies (aDNAds) (425.5 U/mL), elevated erythrocyte sedimentation rate (50 mm/h) and reactive C protein serum levels (6.72 mg/L), slight lymphopenia (lymphocytes 1.26*1000/µL), and slightly elevated ATG titers (34 U/mL, <10 U/mL normal range) with normal TSH, T3,T4, free T4. Skin biopsies on the thorax and legs revealed non-specific features of spongiotic dermatitis and drug-induced eruption respectively, without clear-cut lupus erythematosus-related changes. In the absence of other clinical or laboratory abnormalities, the diagnosis of systemic lupus erythematosus met only 3 of the 4 necessary diagnostic criteria. The skin changes were interpreted as systemic contact dermatitis, without the possibility to identify a drug-related trigger. Emollients, topical steroids and usual allergens avoidance led to partial improvement of skin status.
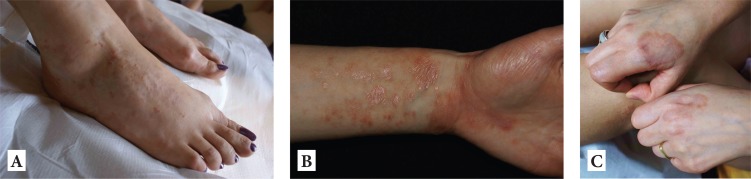
Figure 1.
Skin changes upon first presentation. A, B: polymorphous lichenoid and eczematous changes on extremities. C. granuloma annulare.
Two months later, an altered fasting serum glucose level was followed by a positive glucose tolerance test, so the patient was initially started on low carbohydrates diet. Persisting altered glycemia (135 mg/dL) and glycated hemoglobin (7.8%) values after further two months prompted screening for T1D and elevated titers for anti-glutamic acid decarboxylase antibodies (25 IE/mL) with low C-peptide level (0.5 ng/mL) were found. Thus the diagnosis of latent autoimmune diabetes of the adult (LADA) was set in the diabetes specialized clinic, and the patient was started on metformin 1000 mg/day. Basic endocrine screening work-up (prolactin, cortisol, progesterone, estradiol, FSH, LH, TSH, T3, T4, free T4) revealed normal values and in the absence of other biochemical or clinical abnormalities, the endocrinology specialist did not recommend further dynamic tests of the adrenal axis at that point. In parallel, the immunological reevaluation revealed persistent high anti-DNAds titers (333 U/mL), present serum cryoglobulins and reduced C3 complement fraction, along with persistent eczematous and lichenoid skin changes. Under multidisciplinary care of rheumatology, dermatology and metabolic diseases the patient was started on oral prednisone (30 mg/day), hydroxychloroquine 400 mg/day, and metformin 1000 mg/day. Under this regimen the cutaneous status improved significantly, and the glycemic values were controlled. Over the following 12 months, the patients’ conditions were controlled with this regimen, with decreasing doses of prednisone. Glycemic values were normal, aDNAds titer decreased slowly to 225 U/mL, the other immunological and hematological parameters normalized. The only clinical complaint remained the cutaneous eczematous rashes, recurring at any attempt to lower prednisone doses below 10 mg/day.
In April 2015 at a new attempt to taper off prednisone, the patient developed a new pruritic eczematous rash on neck, eyelids and face, upper limbs, especially in folds (Fig. 2). Patch tests were negative and no specific contact trigger could be identified. Total IgE levels were normal but specific IgE tests were positive for food (blue-cheese, eggs and fish), and drug antigens (cephalosporins, trimethoprim and pyrazolam). These changes together with the clinical and evolutive aspect of the skin rash in the absence of identifiable contact allergen, allowed the clinical diagnosis of atopic dermatitis, according the diagnosis criteria of Hanifin and Rajka (5). Concomitantly anti-histone, anti-nucleosome, anti-Sm antibodies tested positive. In this context methotrexate 10 mg/week was added to Prednisone 30 mg/day, combined with local treatment with topical calcineurin inhibitors, emollients, photoprotection and allergen avoidance. Metformin and hydroxychloroquine doses were maintained. The response was favorable, so that over two months the prednisone was completely tapered off, and the methotrexate dose was slowly reduced. At 6 months follow-up, in 11/2015, the patient was normoglycemic, HbA1c 6%, had normal TSH, haematological and biochemical profile, and decreased aDNAds titer (165 U/mL), persistent positive anti-histone antibodies, with minimal pruritic skin lesions in cubital folds, under methotrexate 2.5 mg/week (Fig. 3).
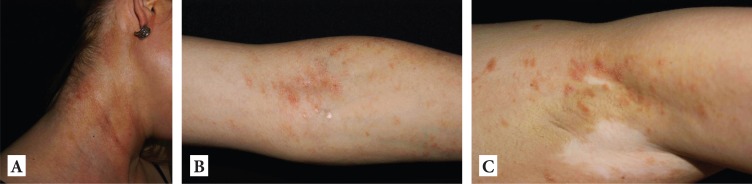
Figure 2.
Persistent eczematous lesions supporting the diagnosis of atopic dermatitis laterocervical (A), cubital folds (B) and axillar folds superposed on vitiligo patches (C).
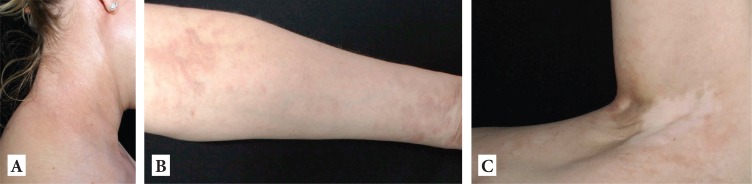
Figure 3.
A-C: Significantly improved skin status, under treatment with methotrexate 2.5mg/week and antimalarials.
In January 2016 the patient changed her work place to an office-based work and shortly afterwards the methotrexate was discontinued, upon decreasing of aDNAds below normal threshold. In July 2016 at the last follow-up in or clinic, the patient was on systemic treatment with metformin and hydroxychloroquine 200 mg/day, her immunological, biochemical and endocrine work-up was normal, she presented residual skin hyperpigmentation, xerosis cutis and unchanged vitiligo patches. She remains in regular monitoring in multidisciplinary care.
DISCUSSION
We present a patient with an unusual combination of autoimmune, endocrine and non-endocrine diseases including autoimmune thyroid disease, autoimmune diabetes, systemic lupus erythematosus, vitiligo, who associated atopic diathesis and atopic dermatitis. This complex case can be classified both as PAS type III or IV, and as MAS type 3 and is highly illustrative for the concept of polyautoimmunity, which sets in relation diverse autoimmune conditions and diverse disease phenotypes with a common genotypic background (3, 11, 12). The coexistence of 4 autoimmune disorders has been documented in less than 25% of MAS patients(3), with SLE, AITD and vitiligo occurring the most frequently in different combinations (3, 12-15). However, the coexistence of the particular four autoimmune diseases of our patient is rare.
Moreover, to our knowledge, this is the first report in the literature of the association between MAS/PAS and atopic dermatitis. In exchange, the association between the atopic diathesis or atopic dermatitis with autoimmune thyroid (5, 7) disease has been well documented, as well as the relationship between atopy and vitiligo (5, 8). The coexistence of SLE and atopic dermatitis was rarely reported until recently, when epidemiological studies found a 2 times higher probability of patients with SLE to develop AD (9), as well as a higher risk of AD patients to develop SLE (OR: 2.05, 95% CI: 1.53-2.76), especially in women (16). Th2-mediated immunity may be the common pathogenic ground for this risk, but new insights in the auto-antigenic role of proteins modified in AD like filaggrin may further expand this view.
The relationship of AD and T1D is controversial. Atopic dermatitis was shown recently to be associated with a lower risk of T1D than the non-atopic patients (RR: 0.72; 95% CI, 0.53-0.998)(17), and the age- and sex-adjusted risk of atopic dermatitis was decreased in subjects with type 1 diabetes compared with nondiabetic subjects, (odds ratio (OR)= 0.23 (0.07-0.71), P = 0.011) in a twin population (10). These findings support the Th1 vs. Th2 cell dichotomy for type 1 diabetes and atopic dermatitis, and the divergence of the genetic factors for these disorders (6). However, in our patient adult onset of atopic dermatitis overlapped with the onset of diabetes with the typical features of latent autoimmune diabetes of the adult (LADA)(18). The late onset of AD is rare, less than 2% of AD patients are reported to present onset beyond the age of 20 (5, 19, 20). In contrast, LADA is considered the most prevalent form of autoimmune diabetes (18).
An important particularity of the case consists in its evolution, as it combined the histopathologic features of eczema and drug-induced dermatitis, the positive anti-histone antibodies titer suggestive for drug-induced SLE, the chronological overlapping of the activity peak of skin manifestations and of a multitude of autoimmunity serological abnormalities with the period the patient worked in the dialysis unit, with significant improvement under mild immunosuppressant treatment and quasi-normalisation after workplace change, sustained even after discontinuation of treatment. These elements together make the triggering role of an environmental factor probable. The patient was not on any chronic medication and denied contact with toxic substances, so the pathogenic role of an element encountered at her workplace may be considered, although not possible to prove, as no definitive histopathological or biochemical markers exist and the diagnosis of drug-induced changes has to rely on clinical and anamnestic clues.
It is also noteworthy that the persistent polymorphous skin lesions were the reason for the patient’s presentation and the extensive laboratory work-up, which prompted the early diagnosis of SLE and further of T1D. Moreover, the skin changes were the only clinical manifestation along the disease’s course, in contrast with the diverse serological abnormalities. Since the patient had no clinical and biochemical abnormalities suggestive for adrenal dysfunction, her condition could be classified as PAS III-IV. Still, she needs careful monitoring of the adrenal axis function in the future. The atypical aspect and course of the cutaneous lesions emphasize the impact of the complex background of atopy and autoimmunity on the skin reaction patterns..
In conclusion, our case illustrates the overwhelming heterogeneity of manifestations of the polyautoimmunity phenotype, the diagnostic and therapeutic challenges in a patient who joins a complex polyglandular autoimmune background with an atopic diathesis, and the complexity of the interplay between predisposing genotype and environment factors in this category of patients.
The physicians’ knowledge of the clinical associations, shared risks and pathogenic mechanisms of PAS and MAS are essential for the quality medical care of these patients who need: close monitoring for the early diagnosis of further associated autoimmune disease or adrenal dysfunction; the monitoring and counseling for their families, as polyautoimmunity has strong familiar aggregation; and particular attention to possible environmental and iatrogenic triggers or aggravating factors, to which these patients are more susceptible.
Conflict of interest
The authors declare that they have no conflict of interest concerning this article.
References
- 1.Carpenter CC, Solomon N, Silverberg SG, Bledsoe T, Northcutt RC, Klinenberg JR, Bennett IL, Harvey AM., Jr Schmidt’s Syndrome (Thyroid and Adrenal Insufficiency) A Review of the Literature and a Report of Fifteen New Cases Including Ten Instances of Coexistent Diabetes Mellitus. Medicine (Baltimore) 1964;43:153–180. [PubMed] [Google Scholar]
- 2.Eisenbarth GS, Gottlieb PA. Autoimmune polyendocrine syndromes. N Engl J Med. 2004;350(20):2068–2079. doi: 10.1056/NEJMra030158. [DOI] [PubMed] [Google Scholar]
- 3.Anaya JM, Castiblanco J, Rojas-Villarraga A, Pineda-Tamayo R, Levy RA, Gómez-Puerta J, Dias C, Mantilla RD, Gallo JE, Cervera R, Shoenfeld Y, Arcos-Burgos M. The multiple autoimmune syndromes. A clue for the autoimmune tautology. Clin Rev Allergy Immunol. 2012;43(3):256–264. doi: 10.1007/s12016-012-8317-z. [DOI] [PubMed] [Google Scholar]
- 4.Humbert P, Dupond JL. [Multiple autoimmune syndromes] Ann Med Interne (Paris) 1988;139(3):159–168. [PubMed] [Google Scholar]
- 5.Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387(10023):1109–1122. doi: 10.1016/S0140-6736(15)00149-X. [DOI] [PubMed] [Google Scholar]
- 6.Brown SJ. Molecular mechanisms in atopic eczema: insight gained from genetic studies. J Pathol. 2016 doi: 10.1002/path.4810. [DOI] [PubMed] [Google Scholar]
- 7.Pedullà M, Miraglia Del Giudice M, Fierro V, Arrigo T, Gitto E, Salpietro A, Lionetti E, Salpietro V, Leonardi S, Santaniello F, Perrone L. Atopy as a risk factor for thyroid autoimmunity in children. J Biol Regul Homeost Agents. 2012;26(1 Suppl):S9–14. [PubMed] [Google Scholar]
- 8.Ezzedine K, Diallo A, Léauté-Labrèze C, Seneschal J, Boniface K, Cario-André M, Prey S, Ballanger F, Boralevi F, Jouary T, Mossalayi D, Taieb A. Pre- vs. post-pubertal onset of vitiligo: multivariate analysis indicates atopic diathesis association in prepubertal onset vitiligo. Br J Dermatol. 2012;167(3):490–495. doi: 10.1111/j.1365-2133.2012.11002.x. [DOI] [PubMed] [Google Scholar]
- 9.Wu LC, Hwang CY, Chung PI, Hua TC, Chen YD, Chu SY, Lee DD, Chang YT, Wang WJ, Liu HN, Chen CC. Autoimmune disease comorbidities in patients with atopic dermatitis: a nationwide case-control study in Taiwan. Pediatr Allergy Immunol. 2014;25(6):586–592. doi: 10.1111/pai.12274. [DOI] [PubMed] [Google Scholar]
- 10.Thomsen SF, Duffy DL, Kyvik KO, Skytthe A, Backer V. Relationship between type 1 diabetes and atopic diseases in a twin population. Allergy. 2011;66(5):645–647. doi: 10.1111/j.1398-9995.2010.02517.x. [DOI] [PubMed] [Google Scholar]
- 11.Tomer Y, Dolan LM, Kahaly G, Divers J, D’Agostino RB, Jr, Imperatore G, Dabelea D, Marcovina S, Black MH, Pihoker C, Hasham A, Hammerstad SS, Greenberg DA, Lotay V, Zhang W, Monti MC, Matheis N, SEARCH for Diabetes in Youth Study Genome wide identification of new genes and pathways in patients with both autoimmune thyroiditis and type 1 diabetes. J Autoimmun. 2015;60:32–39. doi: 10.1016/j.jaut.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castiblanco J, Sarmiento-Monroy JC, Mantilla RD, Rojas-Villarraga A, Anaya JM. Familial Aggregation and Segregation Analysis in Families Presenting Autoimmunity, Polyautoimmunity, and Multiple Autoimmune Syndrome. J Immunol Res. 2015;2015:572353. doi: 10.1155/2015/572353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bumbacea R, Popa L, Orzan O, Voiculescu V, Giurcaneanu C. Clinical and therapeutical implications of the association between chronic urticaria and autoimmune thyroiditis. Acta Endocrinol Buch. 2014;10(4):595–604. [Google Scholar]
- 14.Antonelli A, Ferrari SM, Corrado A, Di Domenicantonio A, Fallahi P. Autoimmune thyroid disorders. Autoimmun Rev. 2015;14(2):174–180. doi: 10.1016/j.autrev.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Gill L, Zarbo A, Isedeh P, Jacobsen G, Lim HW, Hamzavi I. Comorbid autoimmune diseases in patients with vitiligo: A cross-sectional study. J Am Acad Dermatol. 2016;74(2):295–302. doi: 10.1016/j.jaad.2015.08.063. [DOI] [PubMed] [Google Scholar]
- 16.Hsiao YP, Tsai JD, Muo CH, Tsai CH, Sung FC, Liao YT, Chang YJ, Yang JH. Atopic diseases and systemic lupus erythematosus: an epidemiological study of the risks and correlations. Int J Environ Res Public Health. 2014;11(8):8112–8122. doi: 10.3390/ijerph110808112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitt J, Schwarz K, Baurecht H, Hotze M, Fölster-Holst R, Rodríguez E, Lee YA, Franke A, Degenhardt F, Lieb W, Gieger C, Kabesch M, Nöthen MM, Irvine AD, McLean WH, Deckert S, Stephan V, Schwarz P, Aringer M, Novak N, Weidinger S. Atopic dermatitis is associated with an increased risk for rheumatoid arthritis and inflammatory bowel disease, and a decreased risk for type 1 diabetes. J Allergy Clin Immunol. 2016;137(1):130–136. doi: 10.1016/j.jaci.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 18.Laugesen E, Ostergaard JA, Leslie RD. Latent autoimmune diabetes of the adult: current knowledge and uncertainty. Diabet Med. 2015;32(7):843–852. doi: 10.1111/dme.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lancrajan C, Bumbacea R, Giurcaneanu C. Erythrodermic atopic dermatitis with late onset--case presentation. J Med Life. 2010;3(1):80–83. [PMC free article] [PubMed] [Google Scholar]
- 20.Zeppa L, Bellini V, Lisi P. Atopic dermatitis in adults. Dermatitis. 2011;22(1):40–46. [PubMed] [Google Scholar]