Abstract
Context
Foetal asphyxia, a frequent birth complication, detrimentally impacts the immature brain, resulting in neuronal damage, uncontrolled seizure activity and long-term neurological deficits. Oxytocin, a neurohormone mediating important materno-foetal interactions and parturition, has been previously suggested to modulate the immature brain’s excitability, playing a neuroprotective role. Our aim was to investigate the effects of exogenous oxytocin administration on seizure burden and acute brain injury in a perinatal model of asphyxia in rats.
Animals and methods
Asphyxia was modelled by exposing immature rats to a 90-minute episode of low oxygen (9% O2) and high CO2 (20% CO2). Control rats were kept in ambient room-air for the same time interval. In a third group of experiments, oxytocin (0.02 UI/g body weight) was nasally administered 30 minutes before the asphyxia episode. Seizure burden was assessed by the cumulative number of loss of righting reflex (LRR) over a two-hour postexposure period. Acute brain injury was assessed through hippocampal S-100 beta, a biomarker of cellular injury, 24-hours after exposure.
Results
Asphyxia increased both LRR and hippocampal S-100 beta protein compared to controls, and these effects were significantly reduced by oxytocin administration.
Conclusion
Oxytocin treatment decreased both seizure burden and hippocampal injury, supporting a potential neuroprotective role for oxytocin in perinatal asphyxia.
Keywords: perinatal asphyxia, oxytocin, hippocampus, S100B, loss of righting reflex
INTRODUCTION
The developing brain is vulnerable to the influence of different factors that may impair neurodevelopment and have long-term deleterious effects. During a pathological pregnancy or an abnormal delivery, the foetal brain may be exposed to perinatal asphyxia (PA), a severe condition with significant mortality and morbidity that has long-lasting implications for adult brain development (1). PA is characterized by impairment of exchange of the respiratory gases (oxygen and carbon dioxide) resulting in hypoxemia and hypercapnia, accompanied by metabolic acidosis (2). Acute clinical manifestations include lethargy or coma, the need for mechanical ventilation and parenteral feeding, altered tonus and seizures that are notoriously difficult to treat. Different brain regions respond in different ways to the effect of PA, with the hippocampus being one of the most vulnerable areas (3). Available medical interventions for PA are currently limited to controlled hypothermia, which aims to reduce the spread of the initial injury (4). Hence, there is a need for further research to explore novel preventive and targeted therapeutic strategies.
Oxytocin is a neurohormone, synthetized primarily by the hypothalamus, but also in other brain areas, such as the pineal gland (5), that mediates numerous physiological effects including milk ejection, parturition and maternal-foetal interactions. Recent studies have also investigated oxytocin’s direct effects on the brain during different physiological and pathological conditions (6-10). In the immature brain, oxytocin has been found to modulate brain’s excitability by promoting near-term excitatory-to-inhibitory switch in GABA action (11) and has been found to exert a prolonged neuroprotective action on hippocampal neurons subjected to an in vitro model of transient hypoxia-ischemia (12). In the adult brain, oxytocin has been shown to inhibit pentylenetetrazol (PTZ) induced seizures, further highlighting its potential as a modulator of brain’s excitability (13). To the best of our knowledge, the potential anticonvulsant and neuroprotective effects of oxytocin have not been investigated in the context of PA.
In an animal model of PA, recently developed by Helmy et al. (14) and further studied by us (15,16), exposure of postnatal day 6 (P6) rat pups to combined hypoxia and hypercapnia leads to pronounced post-asphyxic seizures, corresponding to those seen in neonates affected by birth asphyxia. In the present study we use this recently developed PA model to test the effects of oxytocin on post-asphyxic seizure burden and hippocampal injury.
MATERIALS AND METHODS
Animals and experimental conditions
Experiments were performed using Wistar rats which were kept under standard conditions, with ad libitum access to food and water and in 12 hours light-dark cycles. The experimental procedures were conducted with the consent of the local ethics committee for animal research and in accordance with the 2010/63/EU directive of the European Parliament on the protection of animals used for scientific purposes.
Postnatal day 6 (P6) pups of both genders randomly separated from 5 mothers were exposed to hypoxia (9% O2) and hypercapnia (20% CO2) for 90 minutes, to mimic the persistent impairment of gas exchange seen in PA, as previously described (14-16). Control pups were kept in room air (normoxia) for the same time interval. Immediately post-exposure, pups were returned to their mothers. Thirty minutes before PA exposure, a group of pups received oxytocin (Sigma Aldrich) nasally administered (0.02 IU/g body weight, dissolved in 2 µL sterile Ringer solution (mM: 147.1 Na+, 4 K+, 2.25 Ca2+, 155.6 Cl-, pH 7.4)), using a pipette on the rhinarium, around the nostrils, avoiding direct application into the nostrils, as described by Neumann et al. (17). All non-treated pups were subjected to nasal administration of vehicle solution (Ringer solution only), following the same conditions as oxytocin-treated pups.
Three experimental groups resulted: Control group (pups exposed to normoxia and treated with vehicle solution, n=17), PA group (pups exposed to PA and treated with vehicle solution, n=26) and PA-Oxy group (pups treated with oxytocin and exposed to PA, n=16).
Seizure burden evaluation by Loss of Righting Reflex
Seizure burden was assessed over a two hours postexposure period by the cumulative number of loss of righting reflex (LRR), as described by Helmy et al. (14). LRR, associated with tonic-clonic seizures and a highest score in a modified Racine scale, is considered a reliable tool for a reproducible quantitative analysis of the total seizure burden in various experimental paradigms (14).
Immediately after exposure, 11 pups from the PA-group, 10 from PA-Oxy group and 9 from the control group were video recorded over a time period of 2 hours with a camera placed above the pups. The video recordings were further used to assess the cumulative number of LRRs in a blind evaluation. A score of 1 was given for each LRR event, then the score was averaged per pup, and cumulative seizure burden was calculated with data points set at 1-minute intervals (14).
ELISA assessment of hippocampal injury by S100B
To measure the level of S100B, known as an early marker for brain injury, pups from PA (n=6), PA-Oxy (n=6) and control groups (n=8) were sacrificed 24 hours post-exposure and the hippocampi were dissected, isolated from the meningeal structures in ice-cold PBS (0.02 mol/L, pH=7.0-7.2), homogenized and sonicated for 10 min, then centrifuged (5000g/5 min) at 4°C. The supernatant was used for the ELISA measurements of S100B (Cusabio Biotech) and total protein content (Biosystems), as previously described (15,16). Results were expressed in pg/mg of total protein.
Statistical Analysis
The data was analyzed using GraphPad Prism 6.00 (GraphPad Software Inc.) and graphical representations were done using Microsoft Excel. The biochemical results were compared using one way measure ANOVA followed by posthoc Tuckey’s test. Data from all experiments were expressed as mean ± SEM. A two-sided p-value < 0.05 was considered statistically significant.
RESULTS
Seizure burden
The convulsive events assessed by the cumulative number of LRR per pup during the post-asphyxia 2-hour video recording was significantly increased in the PA group compared to control group (23.27 vs. 9.44 average cumulative LRRs per pup). In PA-Oxy group, oxytocin administered 30 minutes before PA significantly reduced the post-asphyxic seizure burden as shown by an average cumulative LRR per pup reduced to 14.8 (Fig. 1).
Figure 1.
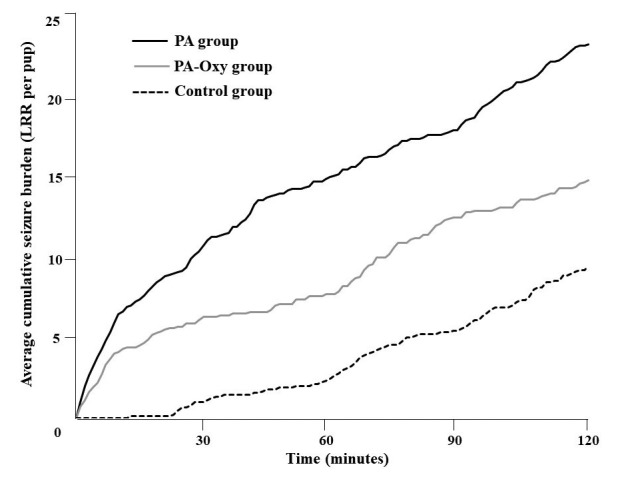
Seizure burden during 2-hours post-asphyxia. Seizure burden as reflected in the cumulative loss of righting reflex (LRR) per pup in each experimental group (PA n=11, PA-Oxy n=10, Control n=9), during a 2-hour time interval following perinatal asphyxia or normoxia.
Hippocampal injury
Exposure to PA significantly increased hippocampal level of S100B compared to control, 24 hours post-asphyxia (516 ± 83.78 vs. 185.7 ± 21.59 pg/mg of total protein, p < 0.05). In pups from PA-Oxy group, hippocampal level of S100B was significantly lower (229 ± 17.67 vs. 516 ± 83.78, pg/mg of total protein, p<0.001) as compared to PA group 24 hours after asphyxia (Fig. 2).
Figure 2.
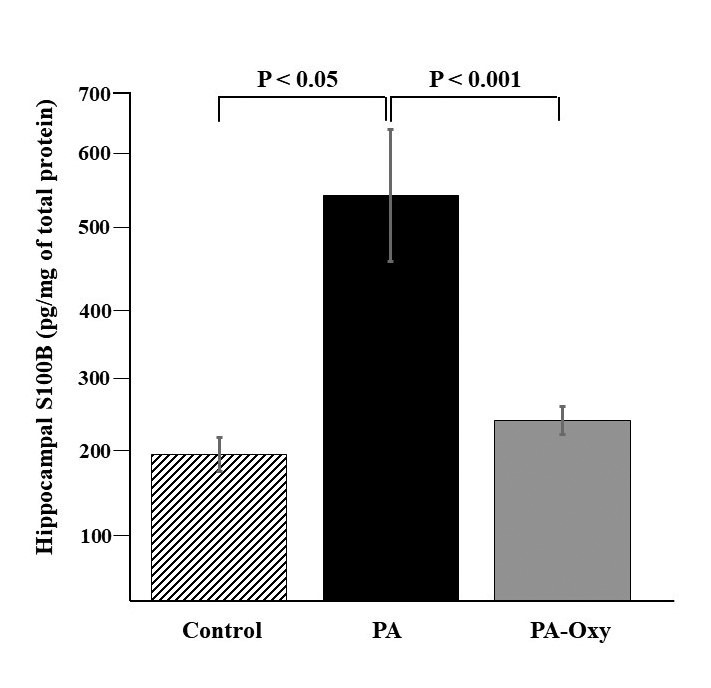
Hippocampal S-100B levels 24 hours post-asphyxia. Hippocampal levels of S-100B assessed 24 hours after a 90 minutes exposure to perinatal asphyxia (PA, n=6), oxytocin treatment and perinatal asphyxia (PA-Oxy, n=6) or normoxia (Control, n=8) in 6-day-old Wistar rat pups. Bars are mean ± SEM.
DISCUSSION
In this study we used a model of PA that induces post-asphyxic seizures to test the anticonvulsant and neuroprotective effects of oxytocin. We found that nasal administration of oxytocin 30 minutes before PA exposure is associated with a significant decrease of post-asphyxic seizures rate and hippocampal S100B level.
Oxytocin has been previously reported to reduce seizures as reflected on electroencephalographic recordings in rats exposed to PTZ (13). Also, in zebrafish, oxytocin and its non-mammalian homologue isotocin showed anticonvulsive effects leading to decrease in the number of seizures after PTZ treatment (18). Oxytocin-receptor null mice have an increased susceptibility for seizures, a characteristic that was shown to be antagonized by peripherally administered oxytocin (19).
Nasal administration of oxytocin bypasses the blood-brain barrier (20) and has been proven to be effective in delivering the neurohormone to the rat brain, with a peak level in the hippocampus at 30 to 60 minutes, changes that were followed by corresponding increases in the plasma oxytocin levels (17). The dose of oxytocin used in this study was adapted from the dosage used by Neumann et al., considering the less efficient uptake in nasal application comparing with the intranasal administration used in humans (17) Also, oxytocin was used at a dose similar to the one in the clinical settings of pregnancy, labour and delivery, considering that both the mother and the foetus are releasing oxytocin (21) and there is an oxytocin surge, especially in prolonged or difficult labour, accompanying the foetal hypoxia and foetal distress (22, 23). Oxytocin was also reported to act on the vasopressin V1a receptors (19,24) and part of its effects could be explained through the interaction with these receptors.
Perinatal asphyxia is a major cause of neonatal morbidity, including seizures. Neonatal seizures are notoriously difficult to control as many of the anti-convulsant agents used in adults are ineffective at this stage (25), and other circumstantial factors such as sex hormones and endogenous neurosteroids impact on neuronal excitability and seizure susceptibility (26). Hence, oxytocin may represent a suitable anticonvulsant alternative for the neonatal period. Moreover, intranasal administration in new-borns may be more suitable for delivering oxytocin to the brain and avoiding its potential peripheral effects.
In the present study we also quantified the hippocampal S100B to assess the level of brain injury induced by PA. S100B is an acidic calcium binding protein primarily concentrated in the glial cells of the nervous system and involved in the regulation of several intra- and extracellular processes (27). The hippocampus is one of the most vulnerable areas to hypoxia-ischemia (3). Protein S100B levels have been shown to increase in rat hippocampi after exposure to brain injury or to ischemia (28). S100B is considered a reliable marker of neuronal and glial injury after perinatal asphyxia (29) and it has been shown to remain increased at 24 - 48 hours after a traumatic event (30). Consistent with our previous studies (15,16), in the present one we found that PA is associated with hippocampal cellular injury, as shown by the significant increase in hippocampal S100B level 24 hours post-asphyxia. Administration of oxytocin prior to PA resulted in significant lower levels of hippocampal S100B compared with non-treated PA group, suggesting a potential neuroprotective effect of oxytocin. These results are in accordance with our previous study showing a protective effect of oxytocin in a model of oxygen-glucose deprivation that mimics birth ischemia in cultured immature hippocampal neurons in rats (12).
Maternal oxytocin is an essential peptide during labour and delivery and is one of the key signalling molecules between the mother and the foetus during intrauterine life. High levels of oxytocin found in maternal blood at delivery are likely to be reflected in the foetal blood and the foetal brain (30-32) and a growing body of evidence suggests that oxytocin signals the foetus to prepare for the extrauterine environment (33, 34). A potential role of oxytocin in the foetal brain is to promote a switch in the action of the neurotransmitter GABA from depolarizing to hyperpolarizing and to augment the resistance of foetal brain to the normal anoxia associated with labour and delivery (35). The exact mechanisms by which oxytocin reduced seizure burden and brain injury in our study remain to be explored and clarified by future studies. It is tempting to speculate that oxytocin administration induced a similar shift in GABA action toward more hyperpolarised levels, as previously reported during birth (35). Such a shift would increase the strength of GABAergic inhibition, which in turn could reduce seizure activity and neuronal damage. On the other hand, recent studies have reported that during the neonatal period vasopressin acts to increase interneuron activity and thus to enhance brain inhibition, irrespective of the depolarising/hyperpolarising GABA actions (36). As mentioned earlier, at high doses, oxytocin can also activate vasopressin receptors (19, 24). Hence the effects of oxytocin in our study could represent a combination of acting on oxytocin and vasopressin receptors. Regardless of the exact mechanisms, our study provides a proof of principle that oxytocin can act as a neuroprotective agent in the acute phase of asphyxia-induced brain injury. Future studies should also explore the potential of oxytocin to prevent long-term consequences of asphyxia-induced brain injury. Such studies should include behavioural and memory tests since the hippocampus is particularly susceptible during development (37).
In conclusion, the results of the present in vivo study show that oxytocin administration prior to perinatal asphyxia decreased the seizure burden and the hippocampal injury induced by perinatal asphyxia and support the potential anticonvulsant and neuroprotective effects of oxytocin in the immature brain. Further studies are necessary to clarify the exact mechanisms through which oxytocin is acting under various clinically relevant circumstances.
Conflict of interest
The authors declare that they have no competing interests.
Acknowledgements
The authors would like to thank Nikolaos Zygouropoulos for his help with some experimental procedures. This research was funded by “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania, Young Researcher Grant no. 21807/2012 and by “Maternitatea Filantropia 1883” Foundation, Bucharest, Romania.
References
- 1.Robertson CM, Finer NN. Long-term follow-up of term neonates with perinatal asphyxia. Clin Perinatol. 1993;20(2):483–500. [PubMed] [Google Scholar]
- 2.Bax MC, Flodmark O, Tydeman C. Definition and classification of cerebral palsy. Dev Med Child Neurol Suppl. 2007;109:39–41. From syndrome toward disease. [PubMed] [Google Scholar]
- 3.Barkovich AJ, Truwit CL. Brain damage from perinatal asphyxia: correlation of MR findings with gestational age. AJNR Am J Neuroradiol. 1990;11(6):1087–1096. [PMC free article] [PubMed] [Google Scholar]
- 4.Azzopardi D, Strohm B, Marlow N, Brocklehurst P, Deierl A, Eddama O, Goodwin J, Halliday HL, Juszczak E, Kapellou O, Levene M, Linsell L, Omar O, Thoresen M, Tusor N, Whitelaw A, Edwards AD, TOBY Study Group Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med. 2014;371(2):140–149. doi: 10.1056/NEJMoa1315788. [DOI] [PubMed] [Google Scholar]
- 5.Badiu C, Badiu L, Coculescu M, Vilhardt H, Møller M. Presence of oxytocinergic neuronal-like cells in the bovine pineal gland: an immunocytochemical and in situ hybridization study. J Pineal Res. 2001;31(3):273–280. doi: 10.1034/j.1600-079x.2001.310312.x. [DOI] [PubMed] [Google Scholar]
- 6.Kaneko Y, Pappas C, Tajiri N, Borlongan CV. Oxytocin modulates GABA(A)R subunits to confer neuroprotection in stroke in vitro. Sci Rep. 2016;6:35659. doi: 10.1038/srep35659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braga RI, Panaitescu A, Bădescu S, Zăgrean AM, Zăgrean L. Intranasal administration of oxytocin alters sleep architecture. Biological Rhythm Research. 2013;45(1): 69–75. [Google Scholar]
- 8.Vargas-Martínez F, Uvnäs-Moberg K, Petersson M, Olausson HA, Jiménez-Estrada I. Neuropeptides as neuroprotective agents: Oxytocin a forefront developmental player in the mammalian brain. Prog Neurobiol. 2014;123C:37–78. doi: 10.1016/j.pneurobio.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Karelina K, Stuller KA, Jarrett B, Zhang N, Wells J, Norman GJ, DeVries AC. Oxytocin mediates social neuroprotection after cerebral ischemia. Stroke. 2011;42(12):3606. doi: 10.1161/STROKEAHA.111.628008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobylinska L, Ghita MA, Caruntu C, Gabreanu G, Tataru CP, Badescu SV, Geicu O, Neagu M, Constantin C, Dobrescu I, Zagrean L. Preliminary Insights in Oxytocin Association with the Onset of Diabetic Neuropathy. Acta Endo (Buc) 2017;13(2):249–253. doi: 10.4183/aeb.2017.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tyzio R, Cossart R, Khalilov I, Minlebaev M, Hübner CA, Represa A, Ben-Ari Y, Khazipov R. Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science. 2006;314(5806):1788–1792. doi: 10.1126/science.1133212. [DOI] [PubMed] [Google Scholar]
- 12.Ceanga M, Spataru A, Zagrean AM. Oxytocin is neuroprotective against oxygen-glucose deprivation and reoxygenation in immature hippocampal cultures. Neurosci Lett. 2010;477(1):15–18. doi: 10.1016/j.neulet.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 13.Erbas O, Yılmaz M, Korkmaz HA, Bora S, Evren V, Peker G. Oxytocin inhibits pentylentetrazol-induced seizures in the rat. Peptides. 2013;40:141–144. doi: 10.1016/j.peptides.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Helmy MM, Tolner EA, Vanhatalo S, Voipio J, Kaila K. Brain alkalosis causes birth asphyxia seizures, suggesting therapeutic strategy. Ann. Neurol. 2011;69:493–500. doi: 10.1002/ana.22223. [DOI] [PubMed] [Google Scholar]
- 15.Isac S, Panaitescu AM, Spataru A, Iesanu M, Totan A, Udriste A, Cucu N, Peltecu G, Zagrean L, Zagrean AM. Trans-resveratrol enriched maternal diet protects the immature hippocampus from perinatal asphyxia in rats. Neurosci Lett. 2017;653:308–313. doi: 10.1016/j.neulet.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Isac S, Panaitescu AM, Iesanu M, Grigoras IF, Totan A, Udriste A, Cucu N, Peltecu G, Zagrean L, Zagrean AM. Maternal high-fat diet modifies the immature hippocampus vulnerability to perinatal asphyxia in rats. Neonatology. 2018 doi: 10.1159/000491383. [DOI] [PubMed] [Google Scholar]
- 17.Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38(10):1985–1993. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Braida D, Donzelli A, Martucci R, Ponzoni L, Pauletti A, Sala M. Neurohypophyseal hormones protect against pentylenetetrazole-induced seizures in zebrafish: role of oxytocin-like and V1a-like receptor. Peptides. 2012;37:327–333. doi: 10.1016/j.peptides.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, Finardi A, Donzelli A, Pattini L, Rubino T, Parolaro D, Nishimori K, Parenti M, Chini B. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry. 201;69(9):875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 20.Dhuria SV, Hanson LR, Frey WH. Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J. Pharm. Sci. 2010;99:1654–1673. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]
- 21.Kumaresan P, Han GS, Anandarangam PB, Vasicka A. Oxytocin in maternal and fetal blood. Obstet Gynecol. 1975;46(3):272–274. [PubMed] [Google Scholar]
- 22.Vasicka A, Kumaresan P, Han GS, Kumaresan M. Plasma oxytocin in initiation of labor. Am J Obstet Gynecol. 1978;130(3):263–273. [PubMed] [Google Scholar]
- 23.Ozer OF, Kacar O, Demirci O, Eren YS, Bilsel AS. Plasma Concentrations and Correlations of Natriuretic Peptides and Oxytocin During Labor and Early Postpartum Period. Acta Endo (Buc) 2017;13(1):65–71. doi: 10.4183/aeb.2017.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Summanen M, Bäck S, Voipio J, Kaila K. Surge of Peripheral Arginine Vasopressin in a Rat Model of Birth Asphyxia. Front Cell Neurosci. 2018;19(12):2. doi: 10.3389/fncel.2018.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans DJ, Levene MI, Tsakmakis M. Anticonvulsants for preventing mortality and morbidity in full term newborns with perinatal asphyxia. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD001240.pub2. (3):CD001240. [DOI] [PubMed] [Google Scholar]
- 26.Craiu D. Implications of Sex Hormones in the Treatment of Women with Epilepsy: Catamenial Epilepsy. Acta Endo (Buc) 2014;10(1):102–117. [Google Scholar]
- 27.Heizmann CW. S100B protein in clinical diagnostics: assay specificity. Clin Chem. 2004;50(1):249–251. doi: 10.1373/clinchem.2003.027367. [DOI] [PubMed] [Google Scholar]
- 28.Rothermundt M, Peters M, Prehn JH, Arolt V. S100B in brain damage and neurodegeneration. Microsc Res Tech. 2003;60(6):614–632. doi: 10.1002/jemt.10303. [DOI] [PubMed] [Google Scholar]
- 29.Gazzolo D, Li Volti G, Gavilanes AW, Scapagnini G. Biomarkers of Brain Function and Injury: Biological and Clinical Significance. Biomed Res Int. 2015;2015:389023. doi: 10.1155/2015/389023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ellis EF, Willoughby KA, Sparks SA, Chen T. S100B protein is released from rat neonatal neurons, astrocytes, and microglia by in vitro trauma and anti-S100 increases trauma-induced delayed neuronal injury and negates the protective effect of exogenous S100B on neurons. J Neurochem. 2007;101(6):1463–1470. doi: 10.1111/j.1471-4159.2007.04515.x. [DOI] [PubMed] [Google Scholar]
- 31.Chard T, Hudson CN, Edwards CR, Boyd NR. Release of oxytocin and vasopressin by the human foetus during labour. Nature. 1971;234(5328):352–354. doi: 10.1038/234352a0. [DOI] [PubMed] [Google Scholar]
- 32.Dawood MY, Khan-Dawood FS, Ayromlooi J, Tobias M. Maternal and fetal plasma oxytocin levels during pregnancy and parturition in the sheep. Am J Obstet Gynecol. 1983;147(5):584–588. doi: 10.1016/0002-9378(83)90022-4. [DOI] [PubMed] [Google Scholar]
- 33.Kenkel WM, Yee JR, Carter CS. Is oxytocin a maternal-foetal signalling molecule at birth? Implications for development. J Neuroendocrinol. 2014;26(10):739–749. doi: 10.1111/jne.12186. [DOI] [PubMed] [Google Scholar]
- 34.Chen X, Du J. Hypoxia induces oxytocin release in the rat. Neuro Endocrinol Lett. 1999;20(6): 373–378. [PubMed] [Google Scholar]
- 35.Khazipov R, Tyzio R, Ben-Ari Y. Effects of oxytocin on GABA signalling in the foetal brain during delivery. Prog Brain Res. 2008;170:243–257. doi: 10.1016/S0079-6123(08)00421-4. [DOI] [PubMed] [Google Scholar]
- 36.Spoljaric A, Seja P, Spoljaric I, Virtanen MA, Lindfors J, Uvarov P, Summanen M, Crow AK, Hsueh B, Puskarjov M, Ruusuvuori E, Voipio J, Deisseroth K, Kaila K. Vasopressin excites interneurons to suppress hippocampal network activity across a broad span of brain maturity at birth. Proc Natl Acad Sci U S A. 2017;114(50):E10819–E10828. doi: 10.1073/pnas.1717337114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voiculescu SE, Le Duc D, Roşca AE, Zeca V, Chiţimuş DM, Arsene AL, Drăgoi CM, Nicolae AC, Zăgrean L, Schöneberg T, Zagrean AM. Behavioral and molecular effects of prenatal continuous light exposure in the adult rat. Brain Res. 2016;1650:51–59. doi: 10.1016/j.brainres.2016.08.031. [DOI] [PubMed] [Google Scholar]


