Abstract
Context
Pancreatic neuroendocrine tumours (PanNETs) are rare pancreatic neoplasms. PanNETs can be treated by multimodal approach including surgery, locoregional and systemic therapy.
Objective
The aim of the present study is to evaluate predictive factors of overall survival in patients with PanNETs surgically treated at a single center.
Subjects and methods
The study group consisted of 120 patients with PanNETs who had undergone surgery at the Center of Digestive Diseases and Liver Transplantation of Fundeni Clinical Institute, Bucharest, Romania. Surgical resection of the primary tumor was performed in 110 patients.
Results
Tumor size > 2 cm (p=0.048) (90% CI) lymph node involvement (p=0.048), ENET grade (p<0.001), distant metastases (p<0.001), Ki 67 index (<2%, 2-5%, 5-10%, 10-20%, >20%) (p<0.001) were identified as significant prognostic factors for OS on univariate analysis. Using multivariate Cox proportional regression model we found that distant metastases and Ki 67 index were independent risk factors for the survival outcome.
Conclusions
Surgery with curative intent should be considered in all cases if clinically appropriate and technically feasible. High grade (Ki67 index ≥10%) tumours were associated with a 2- fold increase in risk of death as compared to those with a Ki67 <10%.
Keywords: pancreatic, neuroendocrine, surgery, prognostic factors
INTRODUCTION
Pancreatic and duodenal neuroendocrine tumours (NETs) represent 18% of all gastroenteropancreatic neuroendocrine tumours (1). The reported annual incidence of the PanNETs range from 0.12 to 0.4 cases/ 100 000 in Europe and Asia (2), and account for 3 % of all pancreatic neoplasms (3, 4). However, an increased incidence of PanNETs has been described over the past years (4, 5). Although PanNETs patients have a much better prognosis than those with pancreatic ductal adenocarcinomas (PDAC), the concept that PanNETs are “more benign”, and slow-growing must be properly reconsidered in therapeutic decisions. PanNETs can be treated by multidisciplinary approach including surgery, locoregional and systemic therapy.
The outcome results seem to be influenced by the radical and aggressive surgical treatment, both in terms of primary tumours and liver metastases. Surgery is the only curative option for localized primary tumours. The management of non-functioning tumours <2 cm remains controversial (6). Unfortunately, metastatic disease is present in 64% of patients with PanNETs at the time of presentation (1). The 5-year survival for treated metastatic PanNETs is above 60% compared with 30% for untreated tumours (7, 8). Locoregional liver directed therapy (trans-arterial chemo-embolisation, transarterial embolization, radiofrequency ablation) and systemic therapies including somatostatin receptor inhibitors, chemotherapy, biological therapy (everolimus, sunitinib) is also employed in the PanNETs treatment (9).
The study reported herein evaluates predictors of overall survival in patients with PanNETs surgically treated at a single center.
MATERIALS AND METHODS
The study group consisted of 120 patients with PanNETs who had undergone surgery at the Center of Digestive Diseases and Liver Transplantation of Fundeni Clinical Institute, Bucharest, Romania, from January 2000 through July 2014. All participants provided written informed consent. The institutional review board or Ethics Committee at Fundeni Clinical Institute approved the study protocol. All patients were analyzed retrospectively regarding age, gender, functioning syndrome, tumours location (head, body, tail), T-N-M status (tumor size, lymph node status, and distance metastasis), the date and the type of surgery, resection margin (R0 or R1/R2), and surgical complications. Imaging studies included contrast enhanced tomography (CT) and magnetic resonance scan (MRI) was performed. Endoscopic ultrasonography with fine needle aspiration was performed in selected groups of patients. Ultrasonography was performed intraoperatively to exclude multiple endocrine tumours being very important for resection type selection.
Local recurrence and distant metastases were recorded. Perioperative mortality was defined as death occurring during 30 days of surgery.
Tissue samples
All samples were classified according to WHO (version 2010) (10, 11) and staged according to ENETS - TNM (European Neuroendocrine Tumor Society). Immunohistochemistry was performed on paraffin embedded specimens, interpreted by consensus of 2 gastrointestinal pathologists (H.V. and C.P). For grading, the Ki67 index was assessed using the MIB1 clone antibody (Dako, Glostrup, Denmark), as the percentage of Ki-67 positive cells among 2,000 tumor cells in areas where the highest nuclear labeling was noticed.
Statistical analysis
All statistical analyses were performed using SPSS (Statistical Packages for Social Sciences, Chicago, IL, USA) software version 16.0. OS (overall survival) was measured from time of resection until death from any cause, or last clinical contact. Survival was estimated by Kaplan-Meier method. Multivariate analyses were performed using a Cox proportional hazards model and forward stepwise procedures. The data were censored from the analysis for the surviving patients at the date of last follow-up. All differences and associations were considered statistically significant if the 2-sided p-value was below 0.05 (95% Confidence Intervals).
RESULTS
Patient clinical characteristics and pathology
A total of 120 patients with PanNETs undergoing surgical exploration at the Fundeni Clinical Institute were retrospectively reviewed. Of these, 68 were females (57%) and 52 males (43 %), with a median age at surgery of 54 years (range, 14-81 years). PanNETs tumours were located in the pancreatic head in 40 % (n=43), in pancreatic body in 34 % (n =37), in pancreatic tail in 26 % (n=28). Median tumour size was 3.5cm (range: 0.4-20 cm). Lymph nodes were found to be positive in 19 (16%) of these patients.
Synchronous distant metastases were present in 21% of patients (n=25) (ENETS stage IV) at the time of diagnostic. Enucleated PanNETs have been considered to be as N0 stage as (12) previously reported. The majority of patients, 70% of (n=59) had non-functional PanNETs tumours. A hypoglycemic syndrome was present at initial diagnosis in 35 cases.
A total of 110 patients underwent resection of the primary tumour by open or minimally invasive surgery. The first tumour robotic enucleation was done in our center in 2009. Tumour enucleation was performed in 20 % (n=22), pancreaticoduodenectomy in 35.4% (n=39), central pancreatectomy in 7.3% (n=8), distal pancreatectomy in 37.3% (n=41) patients. Portal vein resection was performed in 4 cases. Fifteen synchronous liver metastases were resected simultaneously with primary PanNETs, and en-bloc resection of adjacent organs in 5 cases. In 10 patients with synchronous liver metastases the primary tumour was not resected. Adequate samples for Ki-67 assessment were available in 102 patients. Surgical and locoregional therapy was repeated for 20 patients. Most patients received somatostatin analogues (n=35).
The clinicopathologic data of the patients included in the present study are presented in Table 1.
Table 1.
Clinicopathological characteristics of pancreatic neuroendocrine tumours
| Characteristic | Patients (N=no of patients) | |
| N | % | |
| Age, median (range) | 54 years (14 – 71) | |
| ≤ 40 years | 33 | 28% |
| 41-60 years | 57 | 48% |
| >60 years | 29 | 24% |
| Gender | ||
| Male | 52 | 43% |
| Female | 68 | 55% |
| Tumor location within pancreas | ||
| Head | 43 | 40% |
| Body | 37 | 34% |
| Tail | 28 | 26% |
| Tumor size, median (range) cm | 3.75 (0.4 – 20) | |
| ≤2 | 42 | 36% |
| >2 | 76 | 64% |
| Distant metastases (2 groups) | ||
| With metastases | 92 | 78% |
| No metastases | 25 | 22% |
| ENETS – TNM Stage | ||
| I | 29 | 25% |
| II A | 35 | 30% |
| II B | 19 | 16% |
| III A(1 patient) +III B | 11 | 9% |
| IV | 24 | 20% |
| WHO 2010 Grade | ||
| G1 | 56 | 54% |
| G2 | 31 | 30% |
| G3 | 16 | 16% |
| Node Involvement | ||
| Yes | 19 | 16% |
| No | 97 | 84% |
| Functional syndrome | ||
| Yes | 48 | 41% |
| No | 70 | 59% |
Predictors of survival
Overall survival data was available in 95% of patients (5 patients were lost on follow-up). At the time of analysis 85 patients are still alive. A total of 24 patients (21.8%) out of 110 patients with curative resection of the primary PanNETs who initially did not have distant metastases develop recurrence. The median survival was 61 months (range 1 – 243 months).
Potential factors affecting survival were evaluated using univariate analysis (long- rank test). Tumor size > 2 cm (p=0.048) (90% CI) lymph node involvement (p=0.048), ENETS grade (p<0.001), distant metastases (p<0.001), Ki 67 index (<2%, 2-5%, 5-10%, 10-20%, >20%) (p<0.001) were identified as significant prognostic factors for OS on univariate analysis.
Using multivariate Cox proportional regression model we found that distant metastases and Ki 67 index were independent risk factors for the survival outcome. The results of the multivariate analysis are shown in Table 2. High grade (Ki67 index ≥10%) tumours were associated with a 2- fold increase in risk of death than those with a Ki67 <10%.
Table 2.
Multivariate Cox regression analysis of variables associated with OS for patients with resected pancreatic neuroendocrine tumors
| Predictors | Sig. | HR | 95% CI for HR | |
| Lower | Upper | |||
| Distant metastases | .002 | 6.499 | 1.966 | 21.485 |
| Ki67 proliferative index | .046 | 3.466 | 1.020 | 11.772 |
Overall survival analysis for patients classified according to the Ki67 index (Ki67 <10% vs. Ki67 ≥10%) and WHO 2010 are shown in Figure 1A and B. Patients with Ki67 <10% in PanNET tumours had better survival than those with Ki67 index ≥10%.
Figure 1 A.
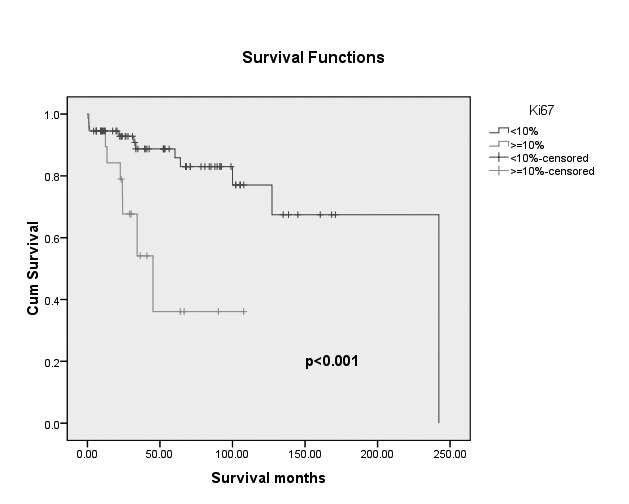
The Kaplan- Meier survival curves of patients with pancreatic neuroendocrine tumors according to Ki 67 value (p<0.001).
Figure 1 B.
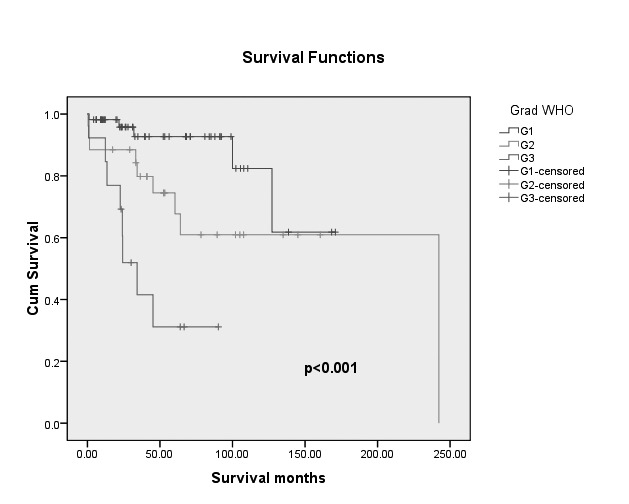
The Kaplan- Meier survival curves of patients with pancreatic neuroendocrine tumors according to WHO 2010 (p<0.001).
DISCUSSION
Previous studies reported several predictors of the PanNETs prognosis, including tumour stage (TNM: tumour size, node involvement, metastases), and tumour grade are the most important (5;11;13-15). Other studies reported as prognostic factors for patients with PanNETs: the functional status, the IHC staining (15) for CK19 (16), surgery type (17).
There is no consensus regarding the prognostic value of the lymph node involvement (18-20).
Kazanjian et al., in a study of PanNETs patients, highlighted that positive lymph nodes and the presence of liver metastases did not affect survival (21). In our study the presence of positive lymph nodes is associated with worse survival (p=0.033).
WHO classification for pancreatic endocrine tumor was associated with significant poor overall survival (22). The 2010 World Health Organization (WHO) system, classified digestive neuroendocrine tumours, including PanNETs into three main histological categories: neuroendocrine tumours grade 1, or NET G1 (Ki67 index <2%), neuroendocrine tumours grade 2 or NET G2 (Ki67 index 3-20%), neuroendocrine carcinomas or NEC of grade G3 (Ki67 index >20%) (10). The WHO 2017 classification of pancreatic neuroendocrine neoplasm included a new category PanNET G3 (Ki67 index >20%) and PanNET G1 (Ki67 index <3%), PanNET G2 (Ki67 index 3-20%) (23).
Buchler M et al. assess clinical relevance of WHO classification on 118 patients with PanNETs who underwent surgery and have shown that WHO and TNM classification can be used to predict long-term survival (24).
Rindi G. et al. suggested that the ENETS-TNM staging system is superior to the UICC/ American Joint Committee of Cancer (AJCC)/WHO 2010 TNM staging system (25). Our study confirms the findings of others (26) that ENETS III and IV had the poorest survival rate compared to patients with ENETS I and II.
Some previous studies showed that liver metastases are the only independent predictors of survival and that tumour size predicts development of liver metastases (27-30). In our study we have shown that after controlling for distant metastases, the patients with a value of Ki67 ≥10% have a 3-fold higher hazard (risk) of death than those with a value of Ki67 <10%. A Ki67 cut off of 10% is used to select patients suitable for liver transplantation according to the Milan criteria (31). There are studies suggesting a higher cut-off point for Ki67 in clinical management. A revision of the cut- off values from 2 to 5% for G1/G2 tumours and from > 20 to >15 for G3 has been recommended by some groups, as these cut off values correlated more accurately with prognosis.
Gao et at. have shown that high grade tumour (Ki67 >20%) tumours were associated with a 2-fold increase in risk of death compared with low grade (Ki67≤ 2%) tumours. Moreover, they generated a novel risk stratification system for resected PanNETs tumours to identify patients with different recurrent risk (32).
In the present study, the 5-year survival rate was 87% and 10-year survival rate is 84.7%, which is within the range of that reported by most large studies (33, 34), survival might be impacted by systemic complications of NETs (35). Watzko F et al. reported a 5-year survival rate of 90.6 and 82.4 % at 10 years. These results were obtained in 124 patients, including a high percent of insulinoma (40.9%) and a low percent of G3 grade (6.3%) (34). In our study G3 grade was assessed in 16% of patients.
In conclusion, surgery with curative intent should be considered in all cases if clinically appropriate and technically feasible. Our study identified lymph node metastases, ENET grade, Ki67, WHO 2010 grade as prognostic factors for OS in PanNETs patients.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 2.Lepage C, Bouvier AM, Phelip JM, Hatem C, Vernet C, Faivre J. Incidence and management of malignant digestive endocrine tumours in a well defined French population. Gut. 2004;53(4):549–553. doi: 10.1136/gut.2003.026401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fesinmeyer MD, Austin MA, Li CI, De Roos AJ, Bowen DJ. Differences in survival by histologic type of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1766–1773. doi: 10.1158/1055-9965.EPI-05-0120. [DOI] [PubMed] [Google Scholar]
- 4.Zhou J, Enewold L, Stojadinovic A, Clifton GT, Potter JF, Peoples GE, Zhu K. Incidence rates of exocrine and endocrine pancreatic cancers in the United States. Cancer Causes Control. 2010;21(6):853–861. doi: 10.1007/s10552-010-9512-y. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence B, Gustafsson BI, Kidd M, Pavel M, Svejda B, Modlin IM. The clinical relevance of chromogranin A as a biomarker for gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40(1):111–134. doi: 10.1016/j.ecl.2010.12.001. viii. [DOI] [PubMed] [Google Scholar]
- 6.Lee LC, Grant CS, Salomao DR, Fletcher JG, Takahashi N, Fidler JL, Levy MJ, Huebner M. Small, nonfunctioning, asymptomatic pancreatic neuroendocrine tumors (PNETs): role for nonoperative management. Surgery. 2012;152(6):965–974. doi: 10.1016/j.surg.2012.08.038. [DOI] [PubMed] [Google Scholar]
- 7.Ekeblad S, Skogseid B, Dunder K, Oberg K, Eriksson B. Prognostic factors and survival in 324 patients with pancreatic endocrine tumor treated at a single institution. Clin Cancer Res. 2008;14(23):7798–7803. doi: 10.1158/1078-0432.CCR-08-0734. [DOI] [PubMed] [Google Scholar]
- 8.Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol. 2008;19(10):1727–1733. doi: 10.1093/annonc/mdn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanno L, Mayo D, Mills S, Takhar A, Cave J, Nolan L, Stedman B, Sundram FX, Abu HM, Connor H, Pearce N, Armstrong T. Proactive multi-modality treatment of Pancreatic Neuroendocrine Tumours (PNETs): Potential survival benefits. Pancreatology. 2018;18(3):304–312. doi: 10.1016/j.pan.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Bosman FT, Carneiro RHHRH, Theise N.D. 4th edn. Lyon: IARC; 2010. WHO classification of tumors of the digestive system. [Google Scholar]
- 11.Rindi G, Kloppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, Erikssson B, Falchetti A, Falconi M, Komminoth P, Korner M, Lopes JM, McNicol AM, Nilsson O, Perren A, Scarpa A, Scoazec JY, Wiedenmann B. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449(4):395–401. doi: 10.1007/s00428-006-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsutsumi K, Ohtsuka T, Mori Y, Fujino M, Yasui T, Aishima S, Takahata S, Nakamura M, Ito T, Tanaka M. Analysis of lymph node metastasis in pancreatic neuroendocrine tumors (PNETs) based on the tumor size and hormonal production. J Gastroenterol. 2012;47(6):678–685. doi: 10.1007/s00535-012-0540-0. [DOI] [PubMed] [Google Scholar]
- 13.Ferrone CR, Tang LH, Tomlinson J, Gonen M, Hochwald SN, Brennan MF, Klimstra DS, Allen PJ. Determining prognosis in patients with pancreatic endocrine neoplasms: can the WHO classification system be simplified? J Clin Oncol. 2007;25(35):5609–5615. doi: 10.1200/JCO.2007.12.9809. [DOI] [PubMed] [Google Scholar]
- 14.Pape UF, Jann H, Muller-Nordhorn J, Bockelbrink A, Berndt U, Willich SN, Koch M, Rocken C, Rindi G, Wiedenmann B. Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer. 2008;113(2):256–265. doi: 10.1002/cncr.23549. 15; [DOI] [PubMed] [Google Scholar]
- 15.Hochwald SN, Zee S, Conlon KC, Colleoni R, Louie O, Brennan MF, Klimstra DS. Prognostic factors in pancreatic endocrine neoplasms: an analysis of 136 cases with a proposal for low-grade and intermediate-grade groups. J Clin Oncol. 2002;20(11):2633–2642. doi: 10.1200/JCO.2002.10.030. [DOI] [PubMed] [Google Scholar]
- 16.Deshpande V, Fernandez-del CC, Muzikansky A, Deshpande A, Zukerberg L, Warshaw AL, Lauwers GY. Cytokeratin 19 is a powerful predictor of survival in pancreatic endocrine tumors. Am J Surg Pathol. 2004;28(9):1145–1153. doi: 10.1097/01.pas.0000135525.11566.b4. [DOI] [PubMed] [Google Scholar]
- 17.Jarufe NP, Coldham C, Orug T, Mayer AD, Mirza DF, Buckels JA, Bramhall SR. Neuroendocrine tumours of the pancreas: predictors of survival after surgical treatment. Dig Surg. 2005;22(3):157–162. doi: 10.1159/000087148. [DOI] [PubMed] [Google Scholar]
- 18.Bilimoria KY, Talamonti MS, Tomlinson JS, Stewart AK, Winchester DP, Ko CY, Bentrem DJ. Prognostic score predicting survival after resection of pancreatic neuroendocrine tumors: analysis of 3851 patients. Ann Surg. 2008;247(3):490–500. doi: 10.1097/SLA.0b013e31815b9cae. [DOI] [PubMed] [Google Scholar]
- 19.Schurr PG, Strate T, Rese K, Kaifi JT, Reichelt U, Petri S, Kleinhans H, Yekebas EF, Izbicki JR. Aggressive surgery improves long-term survival in neuroendocrine pancreatic tumors: an institutional experience. Ann Surg. 2007;245(2):273–281. doi: 10.1097/01.sla.0000232556.24258.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bettini R, Boninsegna L, Mantovani W, Capelli P, Bassi C, Pederzoli P, le Fave GF, Panzuto F, Scarpa A, Falconi M. Prognostic factors at diagnosis and value of WHO classification in a mono-institutional series of 180 non-functioning pancreatic endocrine tumours. Ann Oncol. 2008;19(5):903–908. doi: 10.1093/annonc/mdm552. [DOI] [PubMed] [Google Scholar]
- 21.Kazanjian KK, Reber HA, Hines OJ. Resection of pancreatic neuroendocrine tumors: results of 70 cases. Arch Surg. 2006;141(8):765–769. doi: 10.1001/archsurg.141.8.765. [DOI] [PubMed] [Google Scholar]
- 22.Demir R, Pohl J, Agaimy A, Peros G, Perrakis A, Merkel S, Hohenberger W, Klein P. Necrosis and angioinvasion predict adverse outcome in pancreatic neuroendocrine tumors after curative surgical resection: results of a single-center series. World J Surg. 2011;35(12):2764–2772. doi: 10.1007/s00268-011-1262-9. [DOI] [PubMed] [Google Scholar]
- 23.Kloppel G, La Rosa S. Correction to: Ki67 labeling index: assessment and prognostic role in gastroenteropancreatic neuroendocrine neoplasms. Virchows Arch. 2018;472(3):515. doi: 10.1007/s00428-017-2283-z. [DOI] [PubMed] [Google Scholar]
- 24.Fischer L, Kleeff J, Esposito I, Hinz U, Zimmermann A, Friess H, Buchler MW. Clinical outcome and long-term survival in 118 consecutive patients with neuroendocrine tumours of the pancreas. Br J Surg. 2008;95(5):627–635. doi: 10.1002/bjs.6051. [DOI] [PubMed] [Google Scholar]
- 25.Rindi G, Falconi M, Klersy C, Albarello L, Boninsegna L, Buchler MW, Capella C, Caplin M, Couvelard A, Doglioni C, Delle Fave G, Fischer L, Fusai G, de Herder WW, Jann H, Komminoth P, de Krijger RR, La Rosa S, Luong TV, Pape U, Perren A, Ruszniewski P, Scarpa A, Schmitt A, Solcia E, Wiedenmann B. TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Natl Cancer Inst. 2012;104(10):764–777. doi: 10.1093/jnci/djs208. [DOI] [PubMed] [Google Scholar]
- 26.Roland CL, Katz MH, Gonzalez GM, Pisters PW, Vauthey JN, Wolff RA, Crane CH, Lee JE, Fleming JB. A high positive lymph node ratio is associated with distant recurrence after surgical resection of ampullary carcinoma. J Gastrointest Surg. 2012;16(11):2056–2063. doi: 10.1007/s11605-012-2015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu QD, Hill HC, Douglass HO, Jr., Driscoll D, Smith JL, Nava HR, Gibbs JF. Predictive factors associated with long-term survival in patients with neuroendocrine tumors of the pancreas. Ann Surg Oncol. 2002;9(9):855–862. doi: 10.1007/BF02557521. [DOI] [PubMed] [Google Scholar]
- 28.Dralle H, Krohn SL, Karges W, Boehm BO, Brauckhoff M, Gimm O. Surgery of resectable nonfunctioning neuroendocrine pancreatic tumors. World J Surg. 2004;28(12):1248–1260. doi: 10.1007/s00268-004-7609-8. [DOI] [PubMed] [Google Scholar]
- 29.Norton JA. Surgery for primary pancreatic neuroendocrine tumors. J Gastrointest Surg. 2006;10(3):327–331. doi: 10.1016/j.gassur.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 30.Lambrescu IM, Martin S, Cima L, Herlea V, Badiu C, Fica S. Primary hepatic neuroendocrine tumor after 4 years tumor-free follow-up. J Gastrointestin Liver Dis. 2015;24(2):241–244. doi: 10.15403/jgld.2014.1121.242.yrs. [DOI] [PubMed] [Google Scholar]
- 31.Mazzaferro V, Pulvirenti A, Coppa J. Neuroendocrine tumors metastatic to the liver: how to select patients for liver transplantation? J Hepatol. 2007;47(4):460–466. doi: 10.1016/j.jhep.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Gao H, Liu L, Wang W, Xu H, Jin K, Wu C, Qi Z, Zhang S, Liu C, Xu J, Ni Q, Yu X. Novel recurrence risk stratification of resected pancreatic neuroendocrine tumor. Cancer Lett. 2018;412:188–193. doi: 10.1016/j.canlet.2017.10.036. [DOI] [PubMed] [Google Scholar]
- 33.Kim MJ, Choi DW, Choi SH, Heo JS, Park HJ, Choi KK, Jang KT, Sung JY. Surgical strategies for non-functioning pancreatic neuroendocrine tumours. Br J Surg. 2012;99(11):1562–1568. doi: 10.1002/bjs.8892. [DOI] [PubMed] [Google Scholar]
- 34.Watzka FM, Laumen C, Fottner C, Weber MM, Schad A, Lang H, Musholt TJ. Resection strategies for neuroendocrine pancreatic neoplasms. Langenbecks Arch Surg. 2013;398(3):431–440. doi: 10.1007/s00423-012-1024-7. [DOI] [PubMed] [Google Scholar]
- 35.Zlate AC, Alexandrescu ST, Grigorie RT, Gramaticu IM, Kraft A, Dumitru R, Tomescu D, Popescu I. Role of Surgery in A Patient with Carcinoid Syndrome, Complicated by Carcinoid Heart Disease. Acta Endocrinologica-Bucharest. 2018;14(1):117–121. doi: 10.4183/aeb.2018.117. [DOI] [PMC free article] [PubMed] [Google Scholar]


