Abstract
The TTC12-ANKK1-DRD2 gene-cluster has been implicated in adult smoking. Here, we investigated the contribution of individual genes in the TTC12-ANKK1-DRD2 cluster in smoking and their association with smoking-associated reward processing in adolescence. A meta-analysis of TTC12-ANKK1-DRD2 variants and self-reported smoking behaviours was performed in four European adolescent cohorts (N = 14,084). The minor G-allele of rs2236709, mapping TTC12, was associated with self-reported smoking (p = 5.0 × 10−4) and higher plasma cotinine levels (p = 7.0 × 10−5). This risk allele was linked to an increased ventral-striatal blood-oxygen level-dependent (BOLD) response during reward anticipation (n = 1,263) and with higher DRD2 gene expression in the striatum (p = 0.013), but not with TTC12 or ANKK gene expression. These data suggest a role for the TTC12-ANKK1-DRD2 gene-cluster in adolescent smoking behaviours, provide evidence for the involvement of DRD2 in the early stages of addiction and support the notion that genetically-driven inter-individual differences in dopaminergic transmission mediate reward sensitivity and risk to smoking.
Keywords: fMRI, Genetics, IMAGEN-ALSPAC-NFBC, Meta-analysis, Risk taking, Smoking
Introduction
Smoking is one of the leading causes of premature death (World Health Organization, 2015). The majority of adult smokers (80–90%) initiate smoking during adolescence (Wittchen et al., 2008) and genetic factors were found to explain 44% of the individual differences (Vink et al., 2005). Adolescence is thus associated with an increased risk of developing long-lasting dependencies to nicotine and other substances (Van De Ven et al., 2010), rendering this developmental period critical for the investigation of genetic risk factors and the resulting neurobehavioral mechanisms implicated in tobacco smoking. However, our knowledge on how genes influence brain mechanisms in smoking is limited, and existing findings are often based on sample sizes not large enough to yield conclusive results (Munafo and Flint, 2011). Moreover, the investigation of smoking in adolescents is often hampered by reporting biases resulting from self-report measures (Kandel et al., 2006), thus requiring complementation by biologically-verified markers of nicotine use such as cotinine (Keskitalo et al., 2009).
Smoking in adolescence, in particular smoking initiation, is influenced by behaviours such as risk taking and impulsiveness, which reflect dissociation between the development of the subcortical reward system and a comparatively delayed maturation of cortical inhibitory functions characteristic for this developmental period (Lydon et al., 2014). In adult smokers this maturation is complete, together with the direct consequence of chronic tobacco exposure, resulting in a neurobehavioral context distinct from that in adolescents. This suggests that genetic factors and the biological processes mediating smoking behaviour may be different in adolescents and adults. For example in adults, it has previously shown that a gene-cluster containing the dopamine receptor 2 (DRD2), the ankyrin repeat and kinase domain containing 1 (ANKK1) and the tetratricopeptide repeat domain 12 (TTC12) genes, is associated with tobacco smoking (Ducci et al., 2011, Gelernter et al., 2006). Similar results were found in adolescents with associations stronger in adolescence than in mid-adulthood (Ducci et al., 2011). While this observation supports the notion of shifting biological processes underlying tobacco smoking across the life span, the mechanisms by which variations in this gene-cluster exert their biological effect on smoking behaviour and smoking initiation in adolescents have not yet been elucidated.
TTC12, ANKK1 and DRD2 are located in close proximity on chromosome 11 in a region of high linkage disequilibrium (LD). In addition to nicotine, the gene-cluster has been implicated in alcohol and opiate addiction (Nelson et al., 2013, Xu et al., 2004, Yang et al., 2007), suggesting that its influence on smoking may be exerted through a mechanism which is not substance-specific. TTC12 encodes for the tetratricopeptide repeat domain 12 protein, which is implicated in dopaminergic transmission and neurodevelopment (Castelo-Branco and Arenas, 2006). ANKK1 is hypothesized to encode a signalling protein which mediates the expression of DRD2 (Huang et al., 2009). DRD2 in turn has a central role in regulating the dopamine reward system that mediates the reinforcing effects of all known addictive substances including nicotine mainly through striatal dopaminergic transmission (Sweitzer et al., 2012). While other dopamine-related genes have been associated to smoking behaviours (Herman et al., 2014), we were interested in further investigating the role of this genetic region in early smoking initiation (Mayhew et al., 2000) and in characterising the molecular and neurological mechanisms underlying this association.
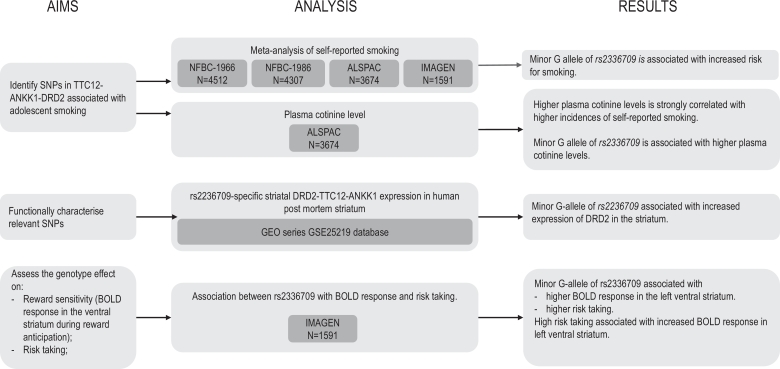
Specifically, we aimed (i) to identify single nucleotide polymorphisms (SNPs) in the TTC12-ANKK1-DRD2 gene-cluster, associated with self-reported cigarette smoking in adolescents through meta-analysis of smoking data from 14,084 youths and blood cotinine levels, an objective measure of nicotine exposure, in a subset of 2,540 youths, (ii) to functionally characterize relevant SNPs by measuring allele-specific gene expression in human post-mortem striatal brain tissue and (iii) to assess the genotype effect on reward sensitivity by analysing its relation with the blood-oxygen level-dependent (BOLD) response in the ventral striatum during reward anticipation, risk taking and tobacco smoking in a subset of adolescents from the IMAGEN sample (Fig. 1).
Fig. 1.
Study aims, statistical analysis strategy, and results.
Experimental procedures
Participants
We included 4512 adolescents from the Northern Finland Birth Cohort NFBC1966 (Sabatti et al., 2009), 4307 from NFBC1986 (Vaarasmaki et al., 2009), 3674 from the Avon Longitudinal Study of Parents and Children (ALSPAC) (Golding, 1990), and 1591 (of which 1263 had data on functional Magnetic Resonance Imaging (fMRI) data and 1085 on risk taking) from IMAGEN (Schumann et al., 2010). Demographic characteristics and phenotypic distribution of the samples are reported in Table 1.
Table 1.
Demographic characteristics and phenotypic distribution of the study samples.
| NFBC1966 | NFBC1986 | ALSPAC | IMAGEN |
|||
|---|---|---|---|---|---|---|
| Whole sample | fMRI sample | Risk-taking sample | ||||
| Total (N) | 4512 | 4307 | 3674 | 1591 | 1263 | 1085 |
| Male (in %) | 47.3 | 47.6 | 51 | 49.6 | 48.9 | 48.9 |
| Age in years | 14 | 16 | 15 | 14 | 14 | 14 |
| Country | Finland | Finland | U.K | Germany, U.K., France, Ireland | Germany, U.K., France, Ireland | Germany, U.K., France, Ireland |
| Year of assessment | 1980 | 2002 | 2006 | 2008 | 2008 | 2008 |
| Never-Tried | ||||||
| n | 1436 | 1539 | 1935 | 1139 | 914 | 771 |
| % | 31.83 | 35.73 | 52.67 | 71.59 | 72.40 | 71.10 |
| Ever-Tried | ||||||
| n | 3076 | 2768 | 1739 | 452 | 349 | 314 |
| % | 68.17 | 64.26 | 47.33 | 28.41 | 27.60 | 28.90 |
| Smokers | ||||||
| n | 757 | 982 | 891 | 189 | 146 | 133 |
| % | 16.78 | 22.8 | 24.25 | 11.88 | 11.40 | 12.30 |
| Weekly-smokers | ||||||
| n | 309 | 844 | 371 | 100 | 71 | 65 |
| % | 6.85 | 19.60 | 10.10 | 6.29 | 5.60 | 6.00 |
Notes: The total sample size N was decomposed into participants, who never tried smoking (Never-Tried) as contrasted to those who have tried smoking at least once (Ever-Tried); this comparison is mutually exclusive. Out of those Ever-Tried participants, smokers and weekly smokers were taken; percentages refer to the total sample size (N).
Smoking-related phenotypes
Lifetime smoking, measuring the number and occasions of cigarette smoking adapted for adolescents who have lesser degree of exposure to smoke than adults, was self-reported in each cohort (see Supplementary-Material for details in each cohort; Ducci et al., 2011), and recoded into four categories: Never-Tried (never smoked), Ever-Tried (smoked at least once), Smokers (smoked more than once in the last 30 days), as previously used (Ducci et al., 2011) and Weekly-Smokers (smoked at least once a week in the last month). We used Never-Tried as our reference group to contrast with Ever-Tried, and subsequently validated our results by further contrasting with adolescents who smoke occasionally (Smokers) and regularly (Weekly-Smokers).
Genetic data
We chose 33 SNPs based on data availability in the cohort that was genotyped first, i.e. NFBC1966. Not all of these 33 SNPs were available in each sample. (SNPs and LD structures for each sample can be found in Supplementary-Fig. 1, MAF of the separated cohorts can be found in Supplementary Table 1).
Cotinine level
Plasma cotinine and genotypic data were available in n = 2540 ALSPAC participants (see Supplementary-Material).
Brain genotype and gene expression
We extracted gene expression levels of TTC12, ANKK1 and DRD2 in the striatum from the GEO series GSE25219 database (Kang et al., 2011). Probe clusters assessing expression across the entire transcript of TTC12, ANKK1 and DRD2 were extracted (Transcript Cluster IDs 3349453, 3349535 and 3391653, respectively). We independently replicated the association between DRD2 expression levels (Transcript exon probe ID 3391671) and rs2236709 genotypes in 93 post-mortem cortical brain tissue samples of European ethnicity, SNPExpress (Heinzen et al., 2008).
Functional MRI
Brain activation during reward anticipation was measured using a modified monetary incentive delay (MID) fMRI task (Knutson et al., 2000) in a sample of 1263 individuals from the IMAGEN sample. Gender, recruitment site and handedness were included as covariates. Based on previous findings (Yacubian et al., 2006), we focused on the ventral striatum (bilateral) as regions of interest (ROIs) (family-wise-error (FWE)-corrected, p < 0.05) as 9-mm spheres from the contrast ‘anticipation of large reward > anticipation of no reward’ (± 15, 9, −9 in Montreal Neurological Institute (MNI) space) (see Supplementary-Materials for more details).
Risk taking
We used the Cambridge Gambling Task (CGT) from the Cambridge Cognition Neuropsychological Test Automated Battery (CANTAB; Cambridge Cognition http://www.cambridgecognition.com/).
Statistical analyses
Genetic-association analyses and meta-analysis
Genetic data analysis used Plink v1.07 (http://pngu.mgh.harvard.edu/∼purcell/plink/) if not otherwise indicated. Single marker association analyses were conducted using logistic or linear regression (for binary or quantitative traits, respectively) (see also Supplementary-Materials). Each genotype was coded as the number of copies of the minor allele in all cohorts. Analyses controlled for gender, (all samples) age and site (IMAGEN).
Meta-analysis was performed using the meta-analysis procedure from Plink. Significant results after Bonferroni-correction were investigated further (see also Table 2).
Table 2.
Association between SNPs spanning the TTC12-ANKK1-DRD2 gene-cluster and smoking for Ever-Tried vs Never-Tried.
| BP | Gene | SNP | Location | MAF | Ref allele/ |
N |
P |
OR |
Q | I2 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Other allele | Samples | Individuals | (FEM) | (REM) | (FEM) | (REM) | |||||||
| 112685412 | TTC12 | rs4517559 | Flanking 5′UTR | 0.3736 | C/T | 4 | 13553 | 0.002 | 1.08 | 0.27 | 23.84 | ||
| 112691986 | TTC12 | rs2236709 | Intron | 0.2588 | G/A | 4 | 14081 | 0.0005* | 1.1 | 0.66 | 0 | ||
| 112693407 | TTC12 | rs7927508 | Intron | 0.3667 | G/A | 3 | 9762 | 0.009 | 1.08 | 0.15 | 46.93 | ||
| 112694133 | TTC12 | rs2156486 | Intron | 0.1814 | G/T | 4 | 13555 | 0.008 | 0.92 | 0.83 | 0 | ||
| 112699378 | TTC12 | rs723077 | Coding, Met73Leu | 0.4894 | C/A | 3 | 9771 | 0.06 | 0.94 | 0.63 | 0 | ||
| 112704356 | TTC12 | rs10502172 | Intron | 0.4596 | T/C | 4 | 13675 | 0.004 | 0.93 | 0.16 | 41.71 | ||
| 112705919 | TTC12 | rs2303380 | Intron splice site | 0.3819 | G/A | 3 | 9771 | 0.49 | 1.02 | 0.18 | 41.76 | ||
| 112716539 | TTC12 | rs2288159 | Intron | 0.1561 | T/G | 3 | 9768 | 0.13 | 1.06 | 0.63 | 0 | ||
| 112721133 | TTC12 | rs4987094 | Intron | 0.1004 | A/G | 3 | 9777 | 0.12 | 1.07 | 0.67 | 0 | ||
| 112735810 | TTC12 | rs2276070 | Intron | 0.1541 | T/C | 3 | 9774 | 0.13 | 1.06 | 0.65 | 0 | ||
| 112739889 | TTC12 | rs719802 | Intron | 0.3953 | T/C | 3 | 9772 | 0.36 | 1.03 | 0.33 | 8.76 | ||
| 112739985 | TTC12 | rs719804 | Intron | 0.249 | G/A | 3 | 9759 | 0.06 | 1.07 | 0.76 | 0 | ||
| 112749387 | TTC12 | rs2282511 | Flanking 3′UTR | 0.3455 | A/C | 3 | 11764 | 0.11 | 1.08 | 0.09 | 57.82 | ||
| 112754346 | TTC12 | rs754672 | Flanking 3′UTR | 0.4894 | T/C | 4 | 13552 | 0.002 | 0.92 | 0.48 | 0 | ||
| 112761718 | ANKK1 | rs877138 | Flanking 5′UTR | 0.3431 | G/A | 3 | 9777 | 0.25 | 1.04 | 0.37 | 0 | ||
| 112768580 | ANKK1 | rs4590907 | Intron | 0.1436 | G/T | 4 | 13577 | 0.23 | 1.04 | 0.76 | 0 | ||
| 112772031 | ANKK1 | rs7118900 | Coding, Ala239Thr | 0.1896 | A/G | 3 | 9767 | 0.24 | 0.95 | 0.88 | 0 | ||
| 112775225 | ANKK1 | rs4938016 | Coding, Gly442Arg | 0.4452 | G/C | 3 | 11966 | 0.002 | 1.09 | 0.5 | 0 | ||
| 112775370 | ANKK1 | rs2734849 | Coding, His490Arg | 0.4886 | G/A | 4 | 13580 | 0.01 | 0.94 | 0.3 | 18.9 | ||
| 112776038 | ANKK1 | rs1800497 | Coding, Glu713Lys, TaqIA | 0.2026 | A/G | 4 | 13549 | 0.47 | 0.98 | 0.71 | 0 | ||
| 112788669 | DRD2 | rs6277 | Coding, Pro219Pro | 0.12201 | A/G | 3 | 11981 | 0.004 | 0.92 | 0.57 | 0 | ||
| 112801119 | DRD2 | rs1076563 | Intron | 0.4127 | C/A | 3 | 9773 | 0.07 | 0.95 | 0.78 | 0 | ||
| 112803549 | DRD2 | rs2471857 | Intron | 0.1577 | T/C | 3 | 9772 | 0.38 | 0.96 | 0.7 | 0 | ||
| 112815891 | DRD2 | rs7125415 | Intron | 0.09914 | T/C | 3 | 9775 | 0.09 | 1.08 | 0.43 | 0 | ||
| 112818599 | DRD2 | rs4648318 | Intron | 0.2538 | C/T | 3 | 9768 | 0.02 | 1.08 | 0.53 | 0 | ||
| 112824662 | DRD2 | rs4274224 | Intron | 0.4823 | G/A | 3 | 9771 | 0.48 | 1.02 | 0.42 | 0 | ||
| 112829684 | DRD2 | rs4581480 | Intron | 0.09919 | C/T | 3 | 9768 | 0.42 | 1.04 | 0.28 | 20.71 | ||
| 112834984 | DRD2 | rs7131056 | Intron | 0.4205 | C/A | 3 | 9762 | 0.14 | 1.05 | 0.37 | 0 | ||
| 112846601 | DRD2 | rs4938019 | Intron | 0.1447 | C/T | 4 | 13588 | 0.29 | 1.04 | 0.41 | 0 | ||
| 112852165 | DRD2 | rs12364283 | Flanking 5′UTR | 0.07563 | G/A | 3 | 9765 | 0.94 | 0.1 | 0.98 | 0 | ||
| 112857971 | DRD2 | rs10891556 | Intergenic | 0.1758 | T/G | 3 | 9772 | 0.51 | 1.03 | 0.35 | 5.8 | ||
| 112860946 | DRD2 | rs6589377 | Intergenic | 0.3776 | G/A | 4 | 13581 | 0.66 | 1.01 | 0.34 | 10.46 | ||
| 112863421 | DRD2 | rs4482060 | Intergenic | 0.30032 | T/A | 3 | 11955 | 0.39 | 1.03 | 0.48 | 0 | ||
Notes: Association between SNPs spanning the TTC12-ANKK1-DRD2 gene-cluster and self-reported smoking behaviour. Ever-Tried (N = 8722) are compared to Never-Tried (N = 6049) (binary logistic regression). Results from NFBC1966, ALSPAC, NFBC1986 and IMAGEN have been combined using meta-analysis. FEM = Fixed effect model, REM = Random effect model, Q = Cochrane's Q statistic, I2 = heterogeneity index (0–100), N = number of study samples. Significant p values are in bold, of these those that remain significant after Bonferroni correction for multiple testing are indicated by an asterisk. Thick lines indicate boundary between Linkage Disequilibrium blocks.
Association of genotype and BOLD responses in the ventral striatum, brain activation and risk taking in the IMAGEN cohort
We carried forward the results of the confirmatory analysis to investigate associations with brain activation and behavioural phenotypes underlying reward processing. ANOVAs comparing extracted mean ventral striatum BOLD responses across genotypes for those SNPs showing associations to self-reported smoking and cotinine were carried out in SPSS version 20.0 (IBM Corp., Armonk, NY). We explored associations of the smoking risk variants within TTC12-ANKK1-DRD2 gene-cluster with risk taking. We expected to find positive associations between genetic risk for smoking and risk taking and tested for this association one-tailed.
Results
A summary of the main findings is illustrated in Fig. 1.
Meta-analysis exploring the association between TTC12-ANKKI-DRD2 and smoking
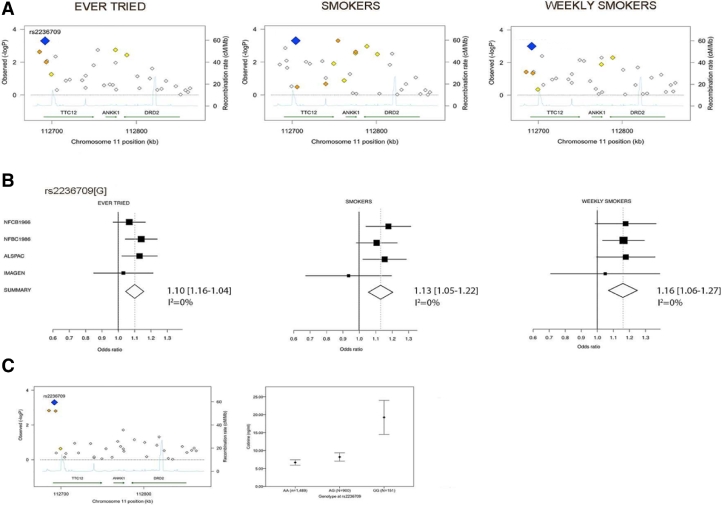
Comparing adolescents who Never-Tried smoking with those who Ever-Tried across four cohorts, we identified a significant association with rs2236709 (Risk allele G; Odds ratio (OR) = 1.10, 95% confidence interval (CI): 1.04–1.16, p = 5.0 × 10−4) (Fig. 2 and Table 2). The minor G-allele of rs2236709 was linked to increased risk for smoking amongst subgroups of regular smokers (Smokers vs. Never-Tried: OR = 1.13, 95% CI: 1.05–1.22, p = 0.001; Weekly-Smokers vs. Never-Tried: OR = 1.16, 95% CI: 1.06–1.27, p = 0.001) (Fig. 2B; Supplementary-Material Tables 2 and 3).
Fig. 2.
Association between 33 single nucleotide polymorphisms (SNPs) covering the chromosome 11q23 TTC12-ANKK1-DRD2 gene-cluster and self-reported smoking behavior in adolescence. Results of the meta-analyses across the NFBC1966, NFBC1986, ALSPAC and IMAGEN cohorts are reported. p-values were meta-p-values computed under fixed effect models. Given the multiple testing correction for all SNPs a significance threshold of 0.0015 was used. The blue diamond indicates the most significantly associated SNP. This was rs2236709 with p = 5.0 × 10−4 for Ever-Tried vs. Never-Tried, rs2236709 with p = 0.001 for Smokers vs. Never-Tried and rs2236709 with p = 0.001 for Weekly Smokers vs. Never-Tried. For other SNPs, diamonds are colored in a white-to-red scale corresponding to R2 values from 0 to 1 with the most significant SNPs. The SNP position refers to National Center for Biotechnology Information build 35. Estimated recombination rates are from HapMap and gene annotations are from UCSC genome browser with build. (B) Forest plots for rs2236709 for the comparisons of the reference group (i.e. Never-Tried) with Ever-Tried, Smokers and Weekly-Smokers. Box areas are proportional to the weight of the individual study. The overall summary odds ratios (OR) computed with a fixed effect model are represented by a diamond whose width indicate the 95% confidence interval (CI). Results of the analyses were as follows: Ever-Tried vs. Never-Tried OR = 1.10, 95%CI 1.04–1.16, p = 0.5.0 × 10−4; Smokers vs. Never-Tried OR = 1.13, 95% CI 1.05–1.22, p = 0.001 and Weekly-Smokers vs. Never-Tried OR = 1.16, 95% CI 1.06–1.27, p = 0.001 on the left, middle and right forest plots, respectively. (C, left plot) Association between 33 SNPs and cotinine level in the ALSPAC cohort. Blue diamond indicates the most significantly associated SNP (rs2236709), as determined by linear regression analyses. Other diamonds are color-coded in a white-to-orange scale corresponding to increasing R2 values of the respective SNP with rs2236709. The SNP position refers to National Center for Biotechnology Information build 35. Estimated recombination rates are from HapMap and gene annotations are from UCSC genome browser with build 35 coordinates. (C, right plot) Mean cotinine level (SE) according to rs2236709 genotypes (linear regression analysis: β = 0.11, p = 0.5.0 × 10−4). MeanAA = 6.35, SE = 0.72, n = 1489; MeanAG = 7.94, SE = 1.18, n = 900; and MeanGG = 19.62, SE = 4.86, n = 151; post-hoc comparisons: AA vs. AG, p = 0.843, R2 = 0.0005, AA vs. GG, p = 3.67E-5, R2 = 0.02 and AG vs. GG, p = 0.001, R2 = 0.013.
Association of cotinine level and self-reported smoking behavior
Higher cotinine levels correlated with a higher number of self-reported cigarettes (Spearman's ρ = 0.33, p = 3.7 × 10−53), thus validating self-reported smoking. Mean cotinine levels in ng/ml (SD) for each category of smokers were: Never-Tried = 0.73 (2.73), Ever-Tried = 15.18 (46.51), Smokers = 28.13 (61.64) and Weekly-Smokers = 64.97 (82.51). One-way ANOVA revealed significant difference across lifetime smoking categories (F(3,3319) = 25.09, p = 4.80 × 10−16, R2 = 0.022). Stratification of mean plasma cotinine levels according to rs2236709 genotypes indicated a significant positive association with the minor G-allele [F(2,2537) = 9.64, p = 6.76 × 10−5, R2 = 0.003) (Fig. 2C, Supplementary Table 4), thus confirming the association of rs2236709 with self-reported smoking.
Rs2236709 influences on striatal DRD2 expression
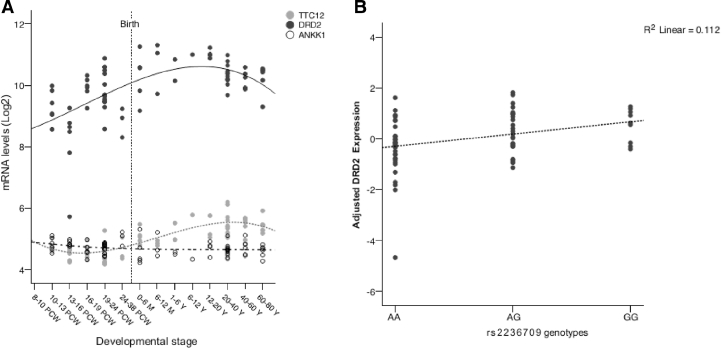
Rs2236709 is located within TTC12 and is in LD with the other genes in the locus, ANKK1 and DRD2 (see Supplementary-Material Fig. 1). To investigate which of these three genes contributed to the effects of rs2236709 on smoking, we analysed their expression in post-mortem human brain samples. Fig. 3A shows expression of TTC12, ANKK1 and DRD2 in the striatum across the lifetime. It was indicated that DRD2 mRNA levels in the striatum increased steadily during the foetal stages and reached a peak in adolescence, while ANKK1 and TTC12 expression levels were low overall. We performed association analyses to investigate the effects of rs2236709 on striatal DRD2, TTC12 and ANKK1 expression and included developmental stage as a covariate. The minor G-allele of rs2236709 was associated with increased expression of DRD2 in the striatum (r = 0.39, p = 0.013, df = 37; Fig. 3B). Neither expression of TTC12 nor that of ANKK1 was influenced by rs2236709 genotypes (p = 0.176 and p = 0.244, respectively). There was no association of gender (p = 0.627) or RNA integrity (p = 0.296) with DRD2 gene expression.
Fig. 3.
(A) Expression of DRD2, TTC12 and ANKK1 in the human striatum across the lifetime. The x-axis represents different stages of fetal and postnatal development, as indicated. PCW = post-conception weeks, M = months, Y = years. (B) Striatal expression of DRD2 corrected for effects of developmental stage and stratified by rs2236709 genotypes. The dotted line visualizes the linear regression against the number of minor G-alleles (nAA = 22, nAG = 16 and, nGG = 5).
As there is, to our knowledge, no other dataset contains expression levels of DRD2 in the striatum and rs2236709 genotype data, we independently replicated the association between DRD2 expression levels and rs2236709 in cortical brain tissue samples of European ethnicity (Heinzen et al., 2008), and found the association to be significant (puncorrected = 0.003, pcorrected = 0.049, corrected for the number of DRD2 probes).
Association of rs2236709 genotype and ventral striatum BOLD response during reward anticipation
As our results suggest that altered dopaminergic transmission in reward-related brain areas (e.g., the ventral striatum) may underlie the association of rs2236709 with smoking, we further measured associations of this variant with BOLD response during reward anticipation in the ventral striatum of 1236 IMAGEN participants. Due to a skewed allele distribution of rs2236709 (nAA = 706, nAG = 467, nGG = 90), we pooled heterozygotes and GG-homozygotes (G-carriers) and compared them to AA-homozygotes. Our results show a significantly higher BOLD response in the left ventral striatum of G-carriers vs. AA-homozygotes [F(1,1261) = 5.803, puncorrected = 0.016, pcorrected = 0.032, R2 = 0.005, Supplementary-Materials), indicating an association of the risk allele (G) with greater reward sensitivity and increased DRD2 expression. No significant associations of rs2236709 genotypes with BOLD response in the right ventral striatum (p = 0.237) were observed.
Association between risk taking, rs2236709, and ventral striatum BOLD response during reward anticipation
As we expected a positive association between genetic risk for smoking and risk taking, this association was tested one-tailed. Risk taking positively associated with risk allele (G) of rs2236709 (r = 0.056, p = 0.033), and ventral striatal BOLD response (left: r = 0.033, p = 0.112; right: r = 0.049, p = 0.034). Risk taking also significantly associated with the number of occasions of lifetime cigarette smoking (r = 0.056, p = 0.034).
Discussion
The current study carried out a neurobehavioral characterization of the TTC12-ANKK1-DRD2 gene-cluster and smoking in adolescence using four large datasets from different European countries. Our result demonstrated that this gene-locus exerts its effect on smoking behaviors such as smoking initiation and frequency already in the very early stages of nicotine abuse. To attain a mechanistic understanding of the associations of the minor G-allele of rs2236709 with both increased self-reported nicotine intake and higher cotinine levels in adolescents, we found that the genetic risk factor rs2236709 regulates DRD2 gene expression, and is also associated with activation of ventral striatum during reward anticipation and risk taking, a behaviour associated with drug initiation.
DRD2 is a key molecular determinant of reward sensitivity (Sweitzer et al., 2012), which reaches its peak expression in the striatum during adolescence. High DRD2 expression increases sensitivity to rewarding stimuli, thus increases sensitivity to the effects of substances (DiNieri et al., 2011) as well as risk taking (Cocker et al., 2012). This combination increases vulnerability for addictive behaviors (Leyton and Vezina, 2014). Thus, enhanced DRD2 expression in adolescents is likely to be one component of risk for substance abuse and smoking in particular. Whereas DRD2 receptor availability is reduced during the compulsive stages of substance addiction (Johnson and Kenny, 2010), early substance use has been linked to increased DRD2 expression levels in the ventral striatum (Koob and Volkow, 2009), suggesting a differential regulation of DRD2 in adolescents experimenting with drugs compared to established nicotine dependence in adults.
Our results suggest that carriers of rs2236709 G-risk allele are at a particularly high risk of nicotine abuse, presumably due to an allele-specific increase in DRD2 expression, in carriers of the G-risk allele found in human post-mortem brain tissue retrieved from the striatum. While our cohorts have different ancestry the LD structure of the rs2236709 locus is similar, suggesting that the findings in the post-mortem human brains can be extended to all our cohorts. Concurrently, increased ventro-striatal response during reward anticipation was observed in carriers of the G-risk allele in functional neuroimaging analyses of 14 year-old IMAGEN participants. Together these findings suggest an enhanced (ventro-)striatal dopaminergic activity underlying the observed association. Our cognitive findings using the IMAGEN sample are in keeping with this interpretation, namely that carriers of the risk allele showed higher risk taking behavior, which in turn was associated with increased ventral striatal activation and with increased nicotine use.
We did not detect significant association between smoking frequency and ventral striatal activity in this sample of 14 year-old adolescents in which 72% had never smoked. This might be explained by insufficient exposure to cigarettes due to young age and/or reduced availability of cigarettes compared to older cohorts, such as NFBC1966 where only 32% adolescents had never smoked at that age.
Rs2236709 is localized in the second intron of the TTC12 gene locus and tags a haplotype that ranges from the promoter to the third exon of the TTC12 gene. While it is correlated with DRD2 expression in the striatum, it is not associated with the expression of TTC12 itself. However, it is well known that even Cis-acting gene expression can be regulated by genetic variations that are not immediately adjacent to the gene they are regulating (Kirsten et al., 2015).
This study has some limitations: (i) different cohorts applied different measures to assess smoking behaviour, which limited us from obtaining a continuous measure consistent across studies, but instead combined information into distinct groups. (ii) Smoking habits have been subject to changing societal and legal attitudes over the last decades. Data acquisition among the different cohorts spreads over several decades, which was not accounted for. (iii) Cotinine can also account for second hand smoking, moreover the half-life of cotinine being 16–18 h is much shorter than some of the self-reported smoking behaviours that measure longer tobacco use (Jarvis et al., 1988, Perez-Stable et al., 1995, Vartiainen et al., 2002). (iv) Genetic heterogeneity between studies should be noted as we pooled all samples of European ancestry due to limited data on adolescent smoking. Two cohorts (NFBC1966, NFBC1986) were of Finnish ancestry, and genetically distinct from the other two European cohorts (IMAGEN, ALSPAC), which might limit generalization of our findings. Notably, despite some differences of the minor allele frequency (MAF), the direction of the smoking-risk SNP effects is consistent across cohorts and I2 of rs2236709 indicated no heterogeneity across cohorts.
Our work proposes a neurobiological pathway to nicotine abuse in a developmental period characterized by both vulnerability to the effects of nicotine, and great therapeutic potential to prevent or overcome its abuse (Toumbourou et al., 2007). Noting the recent success of interventions targeting behavioral risk profiles (Conrod et al., 2013), our neurobehavioral characterization provided a basis for developing interventions targeting biological mechanisms underlying nicotine abuse.
Conflict of interest
Dr. Banaschewski has served as an advisor or consultant to Bristol-Myers Squibb, Desitin Arzneimittel, Eli Lilly, Medice, Novartis, Pfizer, Shire, UCB, and Vifor Pharma; he has received conference attendance support, conference support, or speaking fees from Eli Lilly, Janssen McNeil, Medice, Novartis, Shire, and UCB; and he is involved in clinical trials conducted by Eli Lilly, Novartis, and Shire; the present work is unrelated to these relationships. Dr. Gallinat has received research funding from the German Federal Ministry of Education and Research, AstraZeneca, Eli Lilly, Janssen-Cilag, and Bristol-Myers Squibb; he has received speaking fees from AstraZeneca, Janssen-Cilag, and Bristol-Myers Squibb; the present work is unrelated to these relationships. The other authors report no financial interests or potential conflicts of interest.
Contributors
Authors C.M., F.D., U.B.,C.B., T.B., A.L.W.B., P.C., H.F., V.F., J.G., H.G., P.A.G, A.H., B.H., M.L., J.M., T.P., S.D., M.M., M.J., and G.S. designed the study and wrote the protocol. Authors C.M., F.D., B.R., T.J., M.K., G.K., P.C., F.C., J.P., M.H., J.B., A.B. and J.V. undertook the statistical analysis, and authors C.M., F.D., Y.Z., B.R., and G.S. wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Role of funding source
The IMAGEN consortium received support from the following sources: the European Union-funded FP6 Integrated Project IMAGEN (Reinforcement-related behaviour in normal brain function and psychopathology) (LSHM-CT-2007-037286), the FP7 projects IMAGEMEND (IMAging GEnetics for MENtal Disorders; 602450), AGGRESSOTYPE (602805) and MATRICS (603016), the Innovative Medicine Initiative Project EU-AIMS (115300-2), a Medical Research Council Programme Grant “Developmental pathways into adolescent substance abuse” (93558), and Consortium on Vulnerability to Externalizing Disorders and Addictions [c-VEDA] (MR/N000390/1), the Swedish funding agencies VR, FORTE and FORMAS, the Medical Research Council and the Wellcome Trust (Behavioural and Clinical Neuroscience Institute, University of Cambridge), the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London, the Bundesministerium für Bildung und Forschung (BMBF) grants 01GS08152, 01EV0711, eMED SysAlc 01ZX1311A and Forschungsnetz AERIAL, the Deutsche Forschungsgemeinschaft (DFG grants SM 80/7-1, SM 80/7-2, SFB 940/1), the National Institutes of Health, U.S.A. (Axon, Testosterone and Mental Health during Adolescence; RO1 MH085772-01A1) and by NIH Consortium grant U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centres of Excellence, as well as the NIHR academic Clinical Lecturer and NARSAD Young Investigator Award (FD).
Financial support for NFBC was received from the Academy of Finland (project grants 104781, 120315, 1110143), University Hospital Oulu, Biocenter, University of Oulu, Finland and NIH/NHLBI grant 5R01HL087679-02 through the STAMPEED program and NIH/NIMH grant 1RL1MH083268-01 and the current study is a component project of EU funded ENGAGE programme (HEALTH-F4-2007-201413). The DNA extractions, sample quality controls, biobank up-keeping and aliquotting were performed in the national Public Health Institute, Biomedicum Helsinki, Finland and supported financially by the Academy of Finland and Biocentrum Helsinki.
The UK Medical Research Council, the Wellcome Trust (grant no. 092731) and the University of Bristol provide core funding support for ALSPAC.
Acknowledgments
The IMAGEN consortium received support from the following sources: the European Union-funded FP6 Integrated Project IMAGEN (Reinforcementrelated behaviour in normal brain function and psychopathology) (LSHM-CT-2007-037286), the Horizon 2020 funded ERC Advanced Grant ‘STRATIFY’ (Brain network based stratification ofreinforcement-related disorders) (695313), ERANID (Understanding the Interplay between Cultural, Biological and Subjective Factors in Drug Use Pathways) (PR-ST-0416-10004), BRIDGET (JPND: BRain Imaging, cognition Dementia and next generation GEnomics) (MR/N027558/1), the FP7 projects IMAGEMEND(602450; IMAging GEnetics for MENtal Disorders) and MATRICS (603016), the Innovative Medicine Initiative Project EU-AIMS (115300-2), the Medical Research Council Grant 'c-VEDA’ (Consortium on Vulnerability to Externalizing Disorders and Addictions) (MR/N000390/1), the Swedish Research Council FORMAS, the Medical Research Council, the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London, the Bundesministeriumfür Bildung und Forschung (BMBF grants 01GS08152; 01EV0711; eMED SysAlc01ZX1311A; Forschungsnetz AERIAL 01EE1406A), the Deutsche Forschungsgemeinschaft (DFG grants SM 80/7-1, SM 80/7-2, SFB 940/1). Further support was provided by grants from: ANR (project AF12-NEUR0008-01-WM2NA, and ANR-12-SAMA-0004), the Fondation de France, the Fondation pour la Recherche Médicale, the Mission Interministérielle de Lutte-contre-les-Drogues-et-les-Conduites-Addictives (MILDECA), the Assistance-Publique-Hôpitaux-de-Paris and INSERM (interface grant), Paris Sud University IDEX 2012; the National Institutes of Health, Science Foundation Ireland (16/ERCD/3797), U.S.A. (Axon, Testosterone and Mental Health during Adolescence; RO1 MH085772-01A1), and by NIH Consortium grant U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centres of Excellence.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.euroneuro.2018.07.101.
Appendix. Supplementary materials
References
- Castelo-Branco G., Arenas E. Function of Wnts in dopaminergic neuron development. Neuro Deg. Dis. 2006;3:5. doi: 10.1159/000092086. [DOI] [PubMed] [Google Scholar]
- Cocker P.J., Dinelle K., Kornelson R., Sossi V., Winstanley C.A. Irrational choice under uncertainty correlates with lower striatal D2/3 receptor binding in rats. J. Neurosci. 2012;32:15450–15457. doi: 10.1523/JNEUROSCI.0626-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrod P.J., O'Leary-Barrett M., Newton N. Effectiveness of a selective, personality-targeted prevention program for adolescent alcohol use and misuse: A cluster randomized controlled trial. JAMA Psychiat. 2013;70:334–342. doi: 10.1001/jamapsychiatry.2013.651. [DOI] [PubMed] [Google Scholar]
- DiNieri J.A., Wang X., Szutorisz H., Spano S.M., Kaur J., Casaccia P., Dow-Edwards D., Hurd Y.L. Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biol. Psychiat. 2011;70:763–769. doi: 10.1016/j.biopsych.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducci F., Kaakinen M., Pouta A., Hartikainen A.L., Veijola J., Isohanni M., Charoen P., Coin L., Hoggart C., Ekelund J., Peltonen L., Freimer N., Elliott P., Schumann G., Jarvelin M.R. TTC12-ANKK1-DRD2 and CHRNA5-CHRNA3-CHRNB4 influence different pathways leading to smoking behavior from adolescence to mid-adulthood. Biol. Psychiat. 2011;69:650–660. doi: 10.1016/j.biopsych.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J., Yu Y., Weiss R., Brady K., Panhuysen C., Yang B.Z., Kranzler H.R., Farrer L. Haplotype spanning TTC12 and ANKK1, flanked by the DRD2 and NCAM1 loci, is strongly associated to nicotine dependence in two distinct American populations. Hum. Mol. Genet. 2006;15:3498–3507. doi: 10.1093/hmg/ddl426. [DOI] [PubMed] [Google Scholar]
- Golding J. Children of the nineties. A longitudinal study of pregnancy and childhood based on the population of Avon (ALSPAC) West Engl. Med. J. 1990;105:80–82. [PMC free article] [PubMed] [Google Scholar]
- Heinzen E.L., Ge D., Cronin K.D., Maia J.M., Shianna K.V., Gabriel W.N., Welsh-Bohmer K.A., Hulette C.M., Denny T.N., Goldstein D.B. Tissue-specific genetic control of splicing: implications for the study of complex traits. PLoS Biol. 2008;6:e1. doi: 10.1371/journal.pbio.1000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman A.I., DeVito E.E., Jensen K.P., Sofuoglu M. Pharmacogenetics of nicotine addiction: role of dopamine. Pharmacogenomics. 2014;15:221–234. doi: 10.2217/pgs.13.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Payne T.J., Ma J.Z., Beuten J., Dupont R.T., Inohara N., Li M.D. Significant association of ANKK1 and detection of a functional polymorphism with nicotine dependence in an African-American sample. Neuropsychopharmacology. 2009;34:319–330. doi: 10.1038/npp.2008.37. [DOI] [PubMed] [Google Scholar]
- Jarvis M.J., Russell M.A., Benowitz N.L., Feyerabend C. Elimination of cotinine from body fluids: implications for noninvasive measurement of tobacco smoke exposure. Am. J. Pub. Health. 1988;78:696–698. doi: 10.2105/ajph.78.6.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P.M., Kenny P.J. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat. Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel D.B., Schaffran C., Griesler P.C., Hu M.-C., Davies M., Benowitz N. Salivary cotinine concentration versus self-reported cigarette smoking: three patterns of inconsistency in adolescence. Nicotene Tob. Res. 2006;8:525–537. doi: 10.1080/14622200600672732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H.J., Kawasawa Y.I., Cheng F., Zhu Y., Xu X., Li M., Sousa A.M., Pletikos M., Meyer K.A., Sedmak G., Guennel T., Shin Y., Johnson M.B., Krsnik Z., Mayer S., Fertuzinhos S., Umlauf S., Lisgo S.N., Vortmeyer A., Weinberger D.R., Mane S., Hyde T.M., Huttner A., Reimers M., Kleinman J.E., Sestan N. Spatio-temporal transcriptome of the human brain. Nature. 2011;478:483–489. doi: 10.1038/nature10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskitalo K., Broms U., Heliovaara M., Ripatti S., Surakka I., Perola M., Pitkaniemi J., Peltonen L., Aromaa A., Kaprio J. Association of serum cotinine level with a cluster of three nicotinic acetylcholine receptor genes (CHRNA3/CHRNA5/CHRNB4) on chromosome 15. Hum. Mol. Genet. 2009;18:4007–4012. doi: 10.1093/hmg/ddp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsten H., Al-Hasani H., Holdt L., Gross A., Beutner F., Krohn K., Horn K., Ahnert P., Burkhardt R., Reiche K. Dissecting the genetics of the human transcriptome identifies novel trait-related trans-eqtls and corroborates the regulatory relevance of non-protein coding loci. Hum. Mol. Genet. 2015;24(16) doi: 10.1093/hmg/ddv194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Westdorp A., Kaiser E., Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Koob G.F., Volkow N.D. Neurocircuitry of addiction. Neuropsychopharmacology. 2009;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M., Vezina P. Dopamine ups and downs in vulnerability to addictions: a neurodevelopmental model. Trends Pharmacol. Sci. 2014;35:268–276. doi: 10.1016/j.tips.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon D.M., Wilson S.J., Child A., Geier C.F. Adolescent brain maturation and smoking: what we know and where we're headed. Neurosci. Biobehav. Rev. 2014;45:323–342. doi: 10.1016/j.neubiorev.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew K.P., Flay B.R., Mott J.A. Stages in the development of adolescent smoking. Drug Alcohol Depend. 2000;59(Suppl 1):S61–S81. doi: 10.1016/s0376-8716(99)00165-9. [DOI] [PubMed] [Google Scholar]
- Munafo M.R., Flint J. Dissecting the genetic architecture of human personality. Trends Cognit. Sci. 2011;15:395–400. doi: 10.1016/j.tics.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Nelson E.C., Lynskey M.T., Heath A.C., Wray N., Agrawal A., Shand F.L., Henders A.K., Wallace L., Todorov A.A., Schrage A.J., Saccone N.L., Madden P.A., Degenhardt L., Martin N.G., Montgomery G.W. ANKK1, TTC12, and NCAM1 Polymorphisms and Heroin Dependence: Importance of Considering Drug Exposure. JAMA Psychiat. 2013;70(3):1–9. doi: 10.1001/jamapsychiatry.2013.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation . World Health Organization; 2015. WHO Report On the Global Tobacco Epidemic 2015. [Google Scholar]
- Perez-Stable E.J., Benowitz N.L., Marin G. Is serum cotinine a better measure of cigarette smoking than self-report. Prevent. Med. 1995;24:171–179. doi: 10.1006/pmed.1995.1031. [DOI] [PubMed] [Google Scholar]
- Sabatti C., Service S.K., Hartikainen A.L., Pouta A., Ripatti S., Brodsky J., Jones C.G., Zaitlen N.A., Varilo T., Kaakinen M., Sovio U., Ruokonen A., Laitinen J., Jakkula E., Coin L., Hoggart C., Collins A., Turunen H., Gabriel S., Elliot P., McCarthy M.I., Daly M.J., Jarvelin M.R., Freimer N.B., Peltonen L. Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nature genetics. 2009;41:35–46. doi: 10.1038/ng.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann G., Loth E., Banaschewski T., Barbot A., Barker G., Büchel C., Conrod P., Dalley J., Flor H., Gallinat J. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol. Psychiat. 2010;15:1128–1139. doi: 10.1038/mp.2010.4. [DOI] [PubMed] [Google Scholar]
- Sweitzer M.M., Donny E.C., Hariri A.R. Imaging genetics and the neurobiological basis of individual differences in vulnerability to addiction. Drug Alcohol Depend. 2012;123(Suppl 1):S59–S71. doi: 10.1016/j.drugalcdep.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toumbourou J.W., Stockwell T., Neighbors C., Marlatt G.A., Sturge J., Rehm J. Adolescent health 4 – interventions to reduce harm associated with adolescent substance use. Lancet. 2007;369:1391–1401. doi: 10.1016/S0140-6736(07)60369-9. [DOI] [PubMed] [Google Scholar]
- Vaarasmaki M., Pouta A., Elliot P., Tapanainen P., Sovio U., Ruokonen A., Hartikainen A.L., McCarthy M., Jarvelin M.R. Adolescent manifestations of metabolic syndrome among children born to women with gestational diabetes in a general-population birth cohort. Am. J. Epidemiol. 2009;169:1209–1215. doi: 10.1093/aje/kwp020. [DOI] [PubMed] [Google Scholar]
- Van De Ven M.O., Greenwood P.A., Engels R.C., Olsson C.A., Patton G.C. Patterns of adolescent smoking and later nicotine dependence in young adults: a 10-year prospective study. Pub. Health. 2010;124:65–70. doi: 10.1016/j.puhe.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Vartiainen E., Seppala T., Lillsunde P., Puska P. Validation of self reported smoking by serum cotinine measurement in a community-based study. J. Epidemiol. Commun. Health. 2002;56:167–170. doi: 10.1136/jech.56.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink J.M., Willemsen G., Boomsma D.I. Heritability of smoking initiation and nicotine dependence. Behav. Genet. 2005;35:397–406. doi: 10.1007/s10519-004-1327-8. [DOI] [PubMed] [Google Scholar]
- Wittchen H.U., Behrendt S., Hofler M., Perkonigg A., Lieb R., Buhringer G., Beesdo K. What are the high risk periods for incident substance use and transitions to abuse and dependence? Implications for early intervention and prevention. Int. J. Methods Psychiatr. Res. 2008;17(Suppl 1):S16–S29. doi: 10.1002/mpr.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Lichtermann D., Lipsky R.H., Franke P., Liu X., Hu Y., Cao L., Schwab S.G., Wildenauer D.B., Bau C.H., Ferro E., Astor W., Finch T., Terry J., Taubman J., Maier W., Goldman D. Association of specific haplotypes of D2 dopamine receptor gene with vulnerability to heroin dependence in 2 distinct populations. Arch. Gen. Psychiat. 2004;61:597–606. doi: 10.1001/archpsyc.61.6.597. [DOI] [PubMed] [Google Scholar]
- Yacubian J., Glaescher J., Schroeder K., Sommer T., Braus D.F., Buechel C. Dissociable systems for gain- and loss-related value predictions and errors of prediction in the human brain. J. Neurosci. 2006;26:9530–9537. doi: 10.1523/JNEUROSCI.2915-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B.Z., Kranzler H.R., Zhao H., Gruen J.R., Luo X., Gelernter J. Association of haplotypic variants in DRD2, ANKK1, TTC12 and NCAM1 to alcohol dependence in independent case control and family samples. Hum. Mol. Genet. 2007;16:2844–2853. doi: 10.1093/hmg/ddm240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.