Abstract
The understanding of manganese (Mn) biology, in particular its cellular regulation and role in neurological disease, is an area of expanding interest. Mn is an essential micronutrient that is required for the activity of a diverse set of enzymatic proteins (e.g., arginase and glutamine synthase). Although necessary for life, Mn is toxic in excess. Thus, maintaining appropriate levels of intracellular Mn is critical. Unlike other essential metals, cell-level homeostatic mechanisms of Mn have not been identified. In this review, we discuss common forms of Mn exposure, absorption, and transport via regulated uptake/exchange at the gut and blood-brain barrier and via biliary excretion. We present the current understanding of cellular uptake and efflux as well as subcellular storage and transport of Mn. In addition, we highlight the Mn-dependent and Mn-responsive pathways implicated in the growing evidence of its role in Parkinson’s disease and Huntington’s disease. We conclude with suggestions for future focuses of Mn health-related research.
Keywords: neurodevelopment, cofactor, blood-brain barrier, metal transport, homeostasis, intracellular trafficking
INTRODUCTION
Manganese (Mn) is an essential metal that is critical for human health. It is the fifth most abundant metal and twelfth most abundant element overall on earth. Mn is found in a variety of ores, oxides, carbonates, and silicates. Erosion naturally distributes Mn into air, soil, and waterways, and it ultimately enters into our food supply. Legumes, rice, nuts, and whole grains contain the highest Mn levels, and Mn is also found in seafood, seeds, chocolate, tea, leafy green vegetables, spices, and some fruits such as pineapple and acai (14). Mn is also present in nutritional supplements and vitamins commonly taken on a daily basis. Due to the numerous sources of Mn in food, sufficient Mn levels are easily attained. This is important because Mn is involved in several significant physiological processes, including development, reproduction, immune function, energy metabolism, and antioxidant defenses.
Whereas the major source of Mn exposure is dietary, occupational exposures to Mn can produce toxic sequelae. Mn is used in a wide range of industrial processes and commercial products, including steel and stainless steel production and formation of aluminum alloys. Mn is also incorporated into fungicides, such as maneb and mancozeb. Proper levels of Mn are necessary for life, but excessive levels of Mn may result in a neurotoxic condition known as manganism. This condition resembles Parkinson’s disease (PD), sharing similar cognitive, motor, and emotional deficits (13, 236). Mn exposure has not been linked with cancer or damage to the heart, kidney, liver, skin, blood, or stomach. The nervous system is the primary target for excessive Mn.
Mn toxicity may also arise from certain medical conditions. Mn is present in significant concentrations in both total parenteral nutrition (TPN) and infant and neonatal formulas. These solutions contain many trace elements necessary for life support but may allow for the accumulation of high levels of Mn when given for prolonged periods of time (4, 140, 259). This may be harmful for the development of the child, especially in the central nervous system, which has a long period of development before reaching maturity. Individuals suffering from liver failure or hepatic encephalopathy can develop Mn toxicity, as Mn is excreted in the bile (308). Finally, iron (Fe) deficiency, one of the most common nutritional deficiencies in the world, can result in Mn toxicity. As Fe and Mn compete for similar transport proteins, decreased Fe levels leads to an accumulation of Mn to toxic levels over time (97, 262).
It is therefore essential that Mn levels in the body be properly maintained. This is achieved through mechanisms for absorption, distribution, storage, and excretion of the metal. In this review, we discuss the importance of Mn for physiological functions, sources of Mn exposure, Mn toxicity, cellular and subcellular Mn transport, Mn-responsive pathways, and the impact of Mn on neuronal health.
MANGANESE IS AN ESSENTIAL METAL
Essentiality
Mn is an essential metal for the human diet, as it is required for proper immune function, regulation of blood sugar and cellular energy, reproduction, digestion, bone growth, blood coagulation and hemostasis, and defense against reactive oxygen species (ROS). The beneficial effects of Mn are due to the incorporation of the metal into metalloproteins. The functions carried out by Mn metalloproteins include oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases. Additionally, Mn is incorporated into arginase, glutamine synthetase, phosphoenolpyruvate decarboxylase, pyruvate carboxylase, and Mn superoxide dismutase enzymes. Tissue contents in mammals are in the range of 0.3–2.9 μg Mn/g wet tissue weight (10), making Mn one of the most common metals found in tissues. More commonly, Mn levels in humans are reported as either g Mn consumed per day or blood concentration of Mn, as these measures are the least invasive to assess. There is no formal recommended dietary allowance for Mn; however, the US National Research Council has established an estimated safe and adequate dietary intake of 2–5 mg/day for adults (114). The Institute of Medicine’s Dietary Reference Intake for Mn cites approximately 2 mg/day as adequate intake for adults, and 1.2–1.5 mg/day for children. The Dietary Reference Intake also lists 9–11 mg/day Mn as the upper tolerable limit likely to pose no risk of adverse effects for adults, and 2–6 mg/day Mn for children, varying with age (183).
Deficiency
Due to the numerous dietary sources of Mn, Mn deficiency is exceptionally rare and has not been reported in the literature under nonexperimental settings. Inadequate dietary intake of Mn results in impaired growth, poor bone formation and skeletal defects, abnormal glucose tolerance, and altered lipid and carbohydrate metabolism (147, 232). Men experimentally placed on Mn-depleted diets developed a transient skin rash on their torsos and had decreased serum cholesterol levels (103). Additionally, blood calcium, phosphorus, and alkaline phosphatase levels were also elevated in the men on an Mn-deficient diet, which may indicate increased bone remodeling. Insufficient Mn levels have been shown to negatively affect reproductive health and development. Consumption of <1 mg Mn/day leads to altered mood and increased pain during the premenstrual phase of the estrous cycle (211). Decreased birth weight has been observed in children whose mothers had lower than average blood Mn levels (<16.9 μg Mn/L maternal blood) (43, 88). Low levels of Mn in children (<8.154 μg/L) have also been associated with lower color scores in the Stroop Color-Word Test, a measure of cognitive flexibility and processing speed (23).
Biological Sources of Mn
Diet and water.
The average American diet allows for intake of Mn between 0.9 and 10 mg Mn/kg/day, with an average around 5 mg Mn/kg. Whole grains (wheat germ, oats, and bran), rice, and nuts (hazelnuts, almonds, and pecans) contain the highest levels of Mn (30 mg Mn/kg). Chocolate, tea, mussels, clams, legumes, fruit, leafy vegetables (e.g., spinach), seeds (e.g., flax, sesame, pumpkin, sunflower, and pine nuts) and spices (e.g., chili powder, cloves, and saffron) are also rich in Mn. Dietary supplements and vitamins are another source of Mn, some of which contain up to 20 mg Mn. Mn is taken as a supplement for a variety of conditions, including osteoarthritis and osteoporosis (61, 161, 213). The concentration of Mn in drinking water varies by location, ranging between 1 and 100 μg/l. The US Environmental Protection Agency has set 50 μg/l as the maximum allowable Mn concentration in drinking water.
Milk and infant formulas.
Milk is an important source of Mn for infants. Human milk contains approximately 3–10 μg Mn/l, which is sufficient to prevent Mn deficiency. Commercial infant formulas contain significantly higher levels of Mn, with cow’s milk–based formulas containing 30–50 μg Mn/l and soy-based formulas containing 200–300 μg Mn/l (173). Similarly, a study investigating infant solid foods found levels of Mn over 100-fold higher than levels in cow’s milk (171). Very little is known about infant absorption of Mn; however, there is no evidence that the high Mn content of infant foods is associated with Mn toxicity. Adults are able to absorb 8% of the Mn from human milk, but only 2% from cow’s milk, and <1% from soy-based formulas (63). It is unknown whether infant absorption of Mn is similar to that of adults. Interestingly, in a study examining serum levels of Mn in infants fed either human milk (4.1 μg Mn/l) or infant formula (303 μg Mn/l), there was no significant difference in serum Mn levels (295). This suggests that the homeostatic mechanisms required for proper Mn levels are in place and functioning in neonates.
Airborne exposure.
Mn is used for multiple industrial purposes, including steel and stainless steel production, formation of aluminum alloys, purification of oxygen and chlorine, and also as a component of fungicides. Many of the individuals exposed to Mn in the occupational setting inhale Mn as a result of creating fine dusts from welding or smelting (51, 219). Unlike ingested Mn, inhaled Mn does not pass through the liver but can be directly transported into the brain by olfactory or trigeminal presynaptic nerve ending transport (6, 81). In the brain, Mn disrupts dopamine, serotonin, and glutamine signaling (192, 264) and can lead to the development of manganism (13, 236). Inhaled Mn additionally can cause cough, acute bronchitis, and decreased lung function (233). This, however, is due to lung irritation and inflammation that may be produced from inhaling any metal dusts and is not specific to Mn (233).
Environmentally, Mn can be deposited for inhalation by humans from automobile exhaust. Methylcyclopentadienyl Mn tricarbonyl (MMT) is an antiknock additive in nonleaded gasoline that contains roughly 24.4% Mn by weight. The combustion of MMT in the automobile engine emits 15% of the Mn in MTT as Mn phosphate and/or sulfate particles, with a mass mean aerodynamic diameter of 0.5 to 1.0 μm (76). Studies performed in Canada, where MTT had been used for 10 years prior to the sampling, found average total atmospheric concentrations of Mn in Montreal from 27 to 50 ng Mn/m3 and 21.5 ng Mn/m3 in Toronto (174, 210), which was similar to the typical urban ambient air concentration of 33 ng Mn/m3 (14). Similar results were also reported from experiments conducted in Indianapolis (209). Although the contribution of MMT combustion to environmental exposure to Mn appears to be minimal, the impact of long-term exposure to low levels of MMT combustion products requires further investigation.
Parenteral exposure.
The risk of Mn toxicity deriving from TPN is well documented and has recently been reviewed (241). Although Mn is supplemented in TPN to prevent depletion of endogenous Mn stores, there have been no cases reported of Mn deficiency (no changes in blood Mn observed) in patients receiving TPN without Mn supplementation (102). Mn content varies in TPN administered, with a range of 0.18 μmol/d (0.01 mg/d) to 40 μmol/d (2.2 mg/d) (241). Part of this variability is due to Mn also being a contaminant of TPN solutions. With such a range of TPN Mn content in use, toxicity to Mn has been observed in adults receiving >500 μg/d and in pediatric patients receiving >40 μg/kg/d (241). Additionally, duration of TPN treatment is associated with increased blood and brain concentrations of Mn (4, 140, 259). Current guidelines recommend monitoring patients for Mn toxicity if they receive TPN longer than 30 days. Long-term TPN can lead to the development of biliary stasis or obstructive jaundice (89, 113, 242), which can lead to increased Mn levels due to the importance of bile in the elimination of Mn. Additionally, patients who receive TPN may already have an increased risk for Mn toxicity. Infirm neonates receiving TPN are extremely vulnerable, as young individuals absorb more Mn and excrete fewer stools (10, 146, 312). Patients with compromised liver function also need to be monitored for Mn toxicity when receiving TPN.
MANGANESE ABSORPTION, TRANSPORT IN BLOOD, AND EXCRETION
Absorption
Adult humans absorb approximately 3–5% of ingested Mn. Radiolabeled 54Mn uptake studies show differential uptake of Mn based on gender; for a meal containing 1 mg Mn, adult males absorb 1.35 ± 0.51%, whereas adult females absorb 3.55 ± 2.11% (67, 93). Ingested Mn is readily absorbed in the intestine; however, molecular mechanisms of Mn uptake are not well characterized. Studies using the Caco-2 intestinal cell line describe a biphasic uptake process in which Mn transport achieves a steady-state condition after a brief period of equilibration between intracellular and extracellular components (157). It is thought that Mn can enter cells either through passive diffusion or active transport via the divalent metal transporter 1 (DMT1) (21, 108). The existence of an active transport mechanism was initially identified from in vivo studies using rat intestinal perfusions that showed intestinal Mn uptake was saturable and likely involved a high-affinity, low-capacity active transport mechanism (106). DMT1 is a membrane-associated transport protein that uses the proton gradient across the cell membrane to translocate several divalent metals into the cell, including Mn, Fe, and Cu (108). Both Fe and Mn are first-row transition metals with similar atomic masses, radii, and electron structure allowing for shared transport mechanisms. Therefore, altered Fe concentrations have been shown to influence the amount of Mn absorbed (107, 126). Additionally, studies using rat brush border membrane vesicles have also defined lactoferrin receptor-mediated uptake of Mn (65). Other dietary components alter Mn absorption, such as phytates, ascorbic acid, and polyphenols (62, 267).
The absorption of Mn is governed by many factors. The gastrointestinal (GI) tract responds to dietary Mn levels to regulate Mn uptake. High Mn intake, either through dietary or environmental exposure, causes the GI tract to absorb less Mn while the liver increases metabolism and biliary and pancreatic excretion increases (30, 67, 77). Age is a significant determinant of Mn absorption. Younger individuals absorb and retain higher levels of Mn than adults (146, 312), most likely because their requirements for Mn are much higher than those for adults. This is especially true for infants. TPN used in the treatment of severely ill or premature infants is usually supplemented with a trace element solution that contains small amounts of Mn. However, unlike the absorption of Mn from milk, which must traverse the GI tract, the intravenous exposure to Mn-supplemented TPN solutions bypasses GI tract absorption. Consequently, nearly 100% of Mn is available for absorption, which can lead to toxic conditions.
Transport in Blood
The mechanism by which Mn enters the bloodstream upon exiting the GI tract is poorly understood; however, it is known that distribution to tissues by plasma is quick. The estimated half-life for Mn to leave plasma is 1 min (162). Mn is distributed from plasma to the liver (30% of total Mn), kidney (5%), pancreas (5%), colon (1%), urinary system (0.2%), bone (0.5%), brain (0.1%), erythrocytes (0.02%), and the remaining 58.18% to the remaining soft tissues (162). The liver, pancreas, bone, kidney, and brain retain Mn more than other tissues and have the highest Mn concentrations in the body (10). This is likely due to the essential nature of Mn in energy production and the high energy demands of these tissues.
Mn speciation is important in influencing how Mn is bound and transported in the blood (225, 305). Mn can exist in 11 different oxidation states; the most commonly ingested forms include Mn2+ and Mn3+. Mn2+ is the most predominant form found in the human body, although the exact percentage of the total Mn pool that consists of Mn3+ is unknown (243). Mn3+ is highly reactive and will undergo disproportionation to Mn2+ and Mn4+ unless stabilized in a complex with a ligand (305). Interestingly, Mn2+ can be oxidized in the blood to Mn3+ by ceruloplasmin (141). Mn2+ is bound by several species in the blood for transport and distribution. These include albumin (84% of total Mn2+), hexahydrated ion (6%), bicarbonate (6%), citrate (2%), and transferrin (Tf) (1%) (128, 200). Albumin is the major protein bound to Mn2+ (99, 217), with a KD of 0.63 × 10−4 M−1. Tf is a circulating Fe-binding protein that has affinity for Mn in both the Mn2+ and Mn3+ states (64). Tf-mediated transport of Mn3+ allows for delivery of Mn3+ to neuronal tissues similar to Fe3+Tf transport, though Mn3+Tf transport is noticeably slower and occurs to a lesser degree than other Mn transport processes (123).
Biliary Excretion
Turnover of the ingested Mn is rapid, showing a mean retention of 10 days after ingestion (63). In the liver, excess Mn is conjugated to bile and passed to the intestine for fecal excretion (67, 245). Small amounts of bile-Mn conjugates are reabsorbed in enterohepatic circulation (245). Trace amounts of Mn can also be detected in urine, sweat, and breast milk (47, 67).
MANGANESE DISTRIBUTION AND CONCENTRATIONS IN THE CENTRAL NERVOUS SYSTEM
Transport Across the Blood-Brain Barrier
Mn can cross the blood-brain barrier (BBB); however, the mechanisms of influx and efflux of Mn across the BBB are not yet clearly understood. Several carrier proteins may actively or passively facilitate Mn influx across the BBB, although their identities are uncertain. A major route of Mn ion influx across the BBB may be mediated by Tf, the iron-carrying plasma protein (11). Mn citrate, in addition to Mn ion and the Mn-Tf complex, crosses the BBB, most likely via carrier-mediated transport (56). Store-operated calcium channels have also been implicated in brain Mn influx (58). The role of DMT1 has been debated, but studies of Mn uptake in the rat model do not support the hypothesis that DMT1 plays a primary role in Mn transport across the BBB (57). Studies using an in vitro model of the BBB suggest that Mn is actively transported across the BBB via a process that depends on time, temperature, and pH (95). In human brain microvascular endothelial cells, inhibition of iron accumulation by Mn points to the expression of an Mn-sensitive divalent cation transporter at the plasma membrane of BBB capillary endothelial cells (181). It is unlikely that Mn flux across the BBB can be attributed to one transport system; rather, studies suggest that this process occurs through a number of coinciding mechanisms(94). A recent study suggests that the blood-cerebrospinal fluid (CSF) barrier, rather than the blood-brain barrier, may in fact be the chief interface for Mn uptake in the brain (25). Even less is known about Mn efflux across the blood-CSF barrier; however, evidence points to diffusion as the major mechanism of Mn efflux (306). Recent studies suggest that Mn may alter the permeability of the BBB, contributing to its own toxic effect and that of other cytotoxins (78, 188).
Distribution in the Central Nervous System
Mn levels in the human brain have been found to be highest in the putamen, caudate nucleus, and globus pallidus and lowest in the pons and medulla. Human Mn brain levels, especially in the putamen, globus pallidus, and middle temporal gyrus, were found to positively correlate with age. Additionally, significantly increased Mn levels were observed in Parkinson’s diseased brain, particularly in the putamen, along with decreased Mn levels in the superior and middle temporal gyrus and globus pallidus (223). In Wilson’s disease murine brain and postmortem human brain tissue taken from patients with Alzheimer’s disease (AD) and dementia with Lewy bodies, Mn levels were not found to be significantly altered (3, 24). Magnetic resonance and X-ray fluorescence have indicated significant accumulation of Mn in the hippocampus, brain stem and midbrain, basal ganglia, and thalamus as well as the choroid plexus and olfactory bulbs following subchronic Mn exposure (92, 230, 231). On the subcellular level, Mn has long been thought to accumulate primarily in brain mitochondria, from which it has been shown to efflux very slowly (109, 168); however, in more recent investigations of intracellular distribution, Mn has been shown to accumulate mainly in the nuclei of cultured choroidal epithelial and brain endothelium cells and in the nuclei and cytoplasm of cultured dopaminergic (DAergic) neurons upon exposure (142). Furthermore, in neurons and astrocytes of the striatum and globus pallidus, Mn levels were found to be lowest in the mitochondria compared to the cytoplasm, where levels were intermediate, and the heterochromatin and nucleolus, where the highest levels were found. However, in the same study, after chronic Mn treatment the rate of Mn increase was higher in the mitochondria of these cells than in the nuclei, with astrocytes sequestering more Mn than neurons (194). Subcellular distribution of Mn has yet to be indisputably characterized.
Mn speciation and oxidation state may play an important role in its uptake and distribution in the central nervous system, as Mn-citrate has been shown to predominate in the CSF, and Mn3+ exposures have been shown to result in higher concentrations of Mn in the brain than have Mn2+ exposures (185, 224). Indeed, recently the subcellular distribution and speciation of Mn within pheochromocytoma (PC12) cells, an immortalized cell line with neural crest origins treated with various Mn compounds, was examined (39). Differential toxicities and subcellular distributions were observed depending on the chemical form of Mn exposed to the cells. PC12 cells exposed to Mn2O3 demonstrated normal Mn3+ particles within the cytoplasm with little toxicity, presumably due to its insolubility. For cells treated with MnCl2, MnSO4, and other organic compounds, Mn2+ was observed mainly in the Golgi apparatus (39). Mode of Mn delivery to the brain may mediate patterns of accumulation, as evidenced by the differential distribution across brain regions of injected Mn versus Mn released from peripheral tissues such as the liver (273). An investigation of low-level Mn exposure via drinking water showed significant levels of Mn deposited in several brain regions including the olfactory bulb, cortex, striatum, globus pallidus, and hippocampus (152).
The normal, physiological concentration of Mn in the human brain is estimated to be 5.32–14.03 ng Mn/mg protein (20.0–52.8 mM Mn), whereas 15.96–42.09 ng Mn/mg protein (60.1–158.4 mM Mn) is the estimated pathophysiological threshold (27). A variety of factors may affect Mn accumulation and distribution, thereby altering Mn homeostasis and toxicity. In a study of chromium (VI) stress, a twofold increase in brain Mn levels accompanied increased Cr concentrations (75). Metabolic stress may alter Mn distribution in tissues, as suggested by a recent study that found decreased levels of Mn in the brain stem and frontal lobe after strenuous exercise relative to control conditions and moderate exercise (83). Dietary iron levels may have an impact on levels of Mn accumulation in the brain (96). Ceruloplasmin, a plasma protein involved in the oxidation and mobilization of iron, may also affect the distribution of Mn in brain tissues (141). These studies point to related mechanisms of Mn and Fe homeostasis. 3-D elemental bioimaging, which has revealed elevated levels of Mn in the anterior pretectal nucleus, deep mesencephalic nucleus, and medial geniculate nucleus in a Parkinson’s disease model, also showed a high concentration of Mn in the region of the needle track where brain tissue was lesioned by injection of the neurotoxin 6-hydroxydopamine, suggesting that trauma may also lead to alterations in Mn distribution (127).
REGULATION OF CELLULAR AND SUBCELLULAR LEVELS
The mechanisms and details of intracellular Mn transport and storage are under active investigation. Most of the known transporters involved in transport of Mn into and within cells of the brain (including neurons and glia) are nonselective and also transport other essential metals (see Table 1 and Figure 1). As such, the known Mn transporters cannot explain how intracellular Mn concentrations are selectively maintained without simultaneously strongly influencing the concentrations of other metals. Further, aside from a few notable exceptions [e.g., secretory pathway Ca2+-ATPase isoform 1 (SPCA1)], the manner by which known Mn transporters regulate uptake and efflux of Mn into the cells, versus the subcellular distribution of Mn, is not well established. Indeed, Mn is the only essential metal for which cellular homeostatic processes are not defined, despite the presence of numerous Mn-dependent enzymes throughout the cell. New research to generate novel chemical tools to probe mechanisms of Mn transport was performed via a high-throughput screening approach (154). A study using a mouse striatal neural cell line identified 41 small molecules that are capable of significantly increasing or decreasing intracellular Mn content in a concentration-dependent manner (154). Understanding the targets of these molecules may improve our understanding of cellular and intracellular Mn trafficking and the regulation of Mn homeostasis.
Table 1.
Known manganese (Mn) transporters
Abbreviations: AtP13A2, ATPase type 13A2; DMT1, divalent metal transporter 1; HCO3, bicarbonate; HIP14/HIP14L, huntingtin-interacting protein 14/14-like; NCX, sodium-calcium exchanger; SLC, solute carrier; SPCA1, secretory pathway Ca2+-ATPase isoform 1.
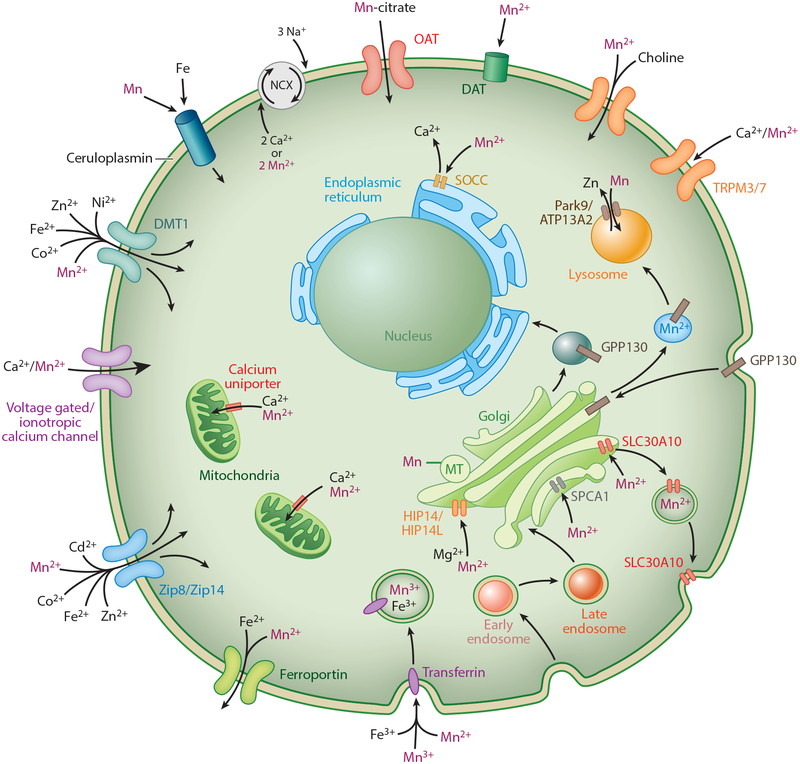
Figure 1.
Known transporters and channels permeable to Mn within the brain and their cellular localizations. Other metals with affinities for each transporter are also listed. Figure is not drawn to scale. Abbreviations: DAT, dopamine transporter; ER; endoplasmic reticulum; Gln, glutamine; Glu, glutamate; GS, glutamine synthetase; Mn, manganese; MT, metallothionein; NCX, sodium-calcium exchanger; OAT, organic anion transporter; SOCC, store-operated calcium channel.
Mechanisms of Cellular Mn Uptake
DMT1 and the transferrin system.
The divalent metal transporter [DMT1; previously known as NRAMP2, DCT1, or solute carrier (SLC)11A2] was given its name for its ability to transport several metal cations such as Co2+, Fe2+, Mn2+, Ni2+, Pb2+, and Zn2+ (100, 121). Its function was first discovered in Belgrade rats, a breed lacking a functional DMT1 transporter, which is demarcated by microcytic anemia and impaired transport of Fe and Mn (98, 121). Numerous studies have demonstrated that DMT1 is capable of transporting Mn2+ (12, 15, 21, 84, 108, 289), and despite reports of its presence in the BBB (116), the choroid plexus (289), and in cells of the basal ganglia (137), where the highest amounts of Mn collect following exposure (34, 293), others still question the role that DMT1 plays in Mn transport in the brain. The pH that is required for Mn to be taken up into cells seems to be different than the pH at which DMT1 operates (57), and the mere existence of DMT1 in capillary endothelial cells has also been questioned (34, 290). Despite having less than 1% of functional DMT1, Belgrade rats have the same concentration of Mn in the brain as wild-type rats (57). This and other studies (25, 55) at least suggest that DMT1 is not the major transporter of Mn in the brain. Recently, Seo and colleagues (249) noted that Mn accumulation increases in both in vitro and in vivo neural models following Fe depletion, concurrent with the upregulation of DMT1 (249). An alternative explanation to mediate conflicting results is that the presence of Fe diminishes the transport of Mn through a receptor independent of DMT1.
Although most biological free Mn appears to exist and be transported in its divalent state, a significant portion of Mn is transported as Mn3+ through a Tf-mediated mechanism (11, 64, 72, 123, 145). It was originally proposed that Mn was oxidized to a trivalent state and loaded onto Tf via the oxidase protein ceruloplasmin; however, Jursa & Smith (141) did not find any difference between control and a ceruloplasminemic mouse in trivalent Mn bound to Tf. As a side note, this study did find that mice lacking ceruloplasmin did distribute Mn throughout the body in a distribution that retained more Mn. The authors note an interaction of high Mn levels and greater oxidative stress in these mice (141). This suggests that ceruloplasmin may have a capacity to influence Mn toxicity. A more recent study described these knockout ceruloplasmin mice as developing Parkinsonism within the first six months of life (16). This phenotype could be reversed by treatment with the iron chelator deferiprone or intravenous injection of ceruloplasmin.
Much like the case of DMT1, the mechanism by which Tf transports Mn is similar to its normal function of transporting Fe. Though it has been shown that Mn3+ can still compete with Fe3+ for Tf transport (123), the former transport occurs at a much slower rate. Despite the similarities of Mn and Fe in their biological activity, these metals differ in their preferred oxidation states, where Fe is much more stable in its trivalent form, and Mn in its divalent form. The oxidative potential of Mn3+ is also stronger than that of Fe3+, so following its deposition by Tf, Mn3+ may do unknown oxidative damage and contribute to Mn toxicity when in excess inside the cell (123). Studies using Tf-deficient mice note different distributions of Mn in several organs but no changes from normal Mn concentrations in the brain, suggesting that Tf is not the primary Mn transporter in the brain(72).
Because of the size of the Tf complex, Mn or Fe bound to Tf must be bound to a Tf receptor (TfR) and endocytosed in order to cross the plasma membrane. It has been suggested that the TfR works in combination with DMT1 in a mechanism where the pH is lowered in the endosome via vacuolar H+-ATPase, causing the release of Mn from Tf and reduction to Mn2+. DMT1 then is able to transport H+ and Mn in its divalent state into the cytoplasm (123).
Zinc transporters.
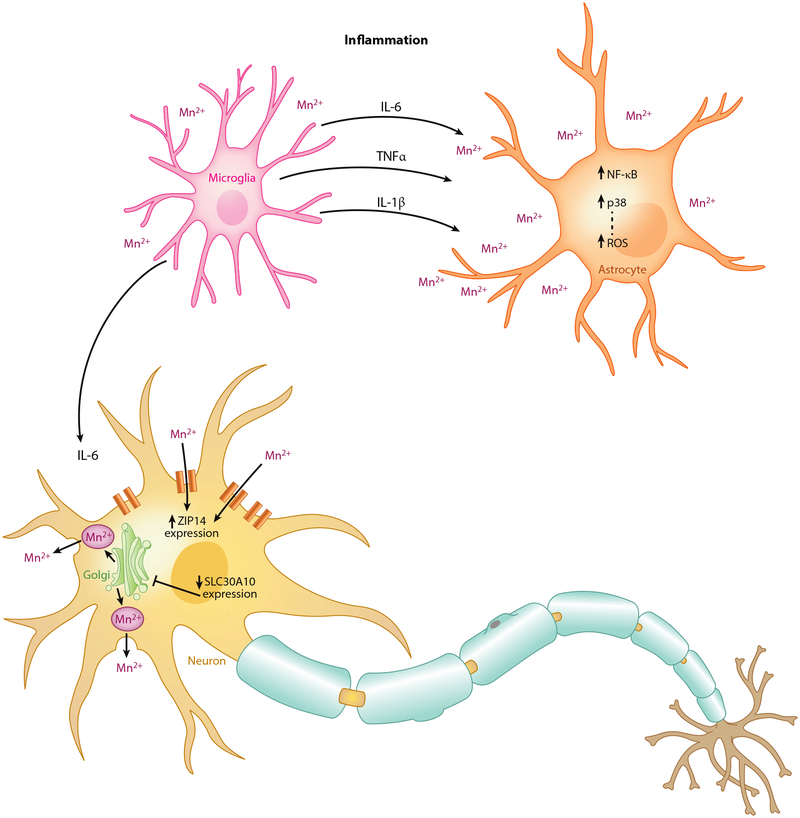
The SLC39 family of proteins is characterized by its variety of zinc transporters, but three in particular have been implicated in the regulation of Mn in cells. ZIP8 and ZIP14 are most closely related, both acting as divalent ion/bicarbonate (HCO3−) symporters that drive metals across a HCO3− gradient (130). When expressed in HEK 293T cells or Xenopus oocytes, they are capable of transporting several divalent ions such as Co, Fe, Cd, and of course Zn (287). The transport activities of Mn through these two proteins are not negligible, but their affinities are significantly lower than the metals just listed. Models studying ZIP8 and ZIP14 have had inconsistent results regarding the magnitude of Mn transport. Nevertheless, rat basophilic leukemia (RBL)-2H3 cells grown to be Mn resistant show marked suppression of ZIP8 expression (104). Knockdown of ZIP14 has shown reduction of Mn uptake in SH-SY5Y (105). Stimulating inflammatory conditions with interleukin (IL)-6 stimulates the uptake of Mn while concurrently upregulating ZIP14 and downregulating SLC30A10.
Citrate, choline, dopamine, and calcium transporters.
Crossgrove and colleagues (56) found that Mn citrate was able to cross the BBB at rates much faster than predicted for diffusion. This suggests that a mechanism exists, at the very least in situ, for transport of Mn bound to citrate across the BBB and plasma membrane. The rates of Mn citrate transport were also significantly faster than those of Mn alone, indicating that it may be a major mechanism of Mn transport (56) and facilitated perhaps through the organic ion transporter or the monocarboxylate transporter (305). Suwalsky and colleagues (272) noted that exposure of Mn citrate to the erythrocyte membrane induces far less structural damage than ionic Mn alone, arguably due to citrate’s metal-chelating abilities. For this reason, and because of the large availability of citrate in serum compared to Mn (185), it would be logical that citrate is a reasonable source of Mn for the cell. However, the reality of citrate playing a meaningful role in Mn transport has not yet been further tested.
Another possible yet unconfirmed significant route of Mn entrance into the brain is through the choline transporter. Exposure to Mn has been shown to inhibit choline uptake in perfused rodent brain by nearly 50% within in situ preparation (172). More recently, Bagga & Patel (17) reported that chronic Mn exposure in mice was associated with decreased levels of choline in the hypothalamus and thalamus. These areas were also marked by a reduction in glutamate, N-acetyl aspartate. Gamma-aminobutyric acid (GABA)ergic disruption was damaged only in the basal ganglia.
Considering the numerous connections of Mn with Parkinsonian disorders (see section titled Mn Excess in Neurological Disease), it is not surprising that Mn interacts with and is possibly transported by the dopamine transporter (DAT). Based on the observations that chronic exposure to Mn produces PD-like symptoms but spares the DAergic cells of the substantia nigra (206), it has been suggested that the mechanism would likely be acting at the presynaptic terminal, deactivating DAT (235). Indeed, the amphetamine-induced release of dopamine is prevented by Mn (119). It has also been observed that the presence of Mn induces the internalization of DAT in transfected HEK cells (238).
Mn has often been used as a tool to observe the functionality of other transporters that transport divalent ions. For this reason, several calcium channels have been identified to be permeable to Mn, but their contribution to normal Mn transport, storage, and homeostasis has not been adequately assessed. Examples of these include transient receptor potential (TRP) cation channels, such as TRPM3 (115) and TRPM7 (228). Voltage-gated L-type Ca2+ channels (175, 176), ionotropic glutamate receptor channels (143), and store-operated calcium channels (58, 120, 175, 228, 301) also have reported permeability to Mn.
Mechanisms of Cellular Mn Efflux
SLC30A10.
Originally described as a zinc transporter (also called ZnT10) based on its family classification, SLC30A10 can be distinguished from other zinc transporters through analysis of its amino acid structure (253). Immunohistochemical staining has indicated the localization of SLC30A10 at the plasma membrane and also throughout the secretory pathway, including the Golgi system and endosomes (215). Transfections of the human SLC30A10 gene into Mn-sensitive yeast cells reversed the obstructed growth phenotype when exposed to Mn. Consistent with the support of SLC30A10 as an Mn transporter, inducing mutations into this gene reverted the cells back to their original Mn-sensitive phenotype (280). Similar studies in Caenorhabditis elegans, HeLa cells, and primary cultures of mouse midbrain neurons have shown that expression of SLC30A10 yields protection from toxic Mn concentrations, and this effect is reversed when the gene is mutated (165).
Sodium-calcium exchanger.
The permeability of the sodium-calcium exchanger (NCX) to Mn2+ was first demonstrated in myocardial cells as a surrogate to study Ca2+ efflux (285, 286). More recently, the inhibition of the NCX channel for 24 hours was shown to increase cellular Mn levels in immortalized mouse striatal neuroprogenitors (276). Efflux through NCX is a proposed dominant mechanism of Ca2+ efflux following an action potential (9, 19, 284); given the similar chemical properties of Mn2+ and Ca2+, it is reasonable to postulate that Mn2+ can efflux the plasma membrane in the place of Ca2+ in exchange for the influx of three Na+ ions, all ions along their concentration gradients. The alteration of neuronal Mn content by NCX inhibition may act through blockage of Mn efflux; however, additional studies are needed to determine the role of NCX under normal Mn neuronal homeostatic conditions.
Ferroportin.
A third transporter of iron, ferroportin (FPN; also known as IREG1 or MTP1), allows for the efflux of both Fe and Mn from the cell (177, 304). Mn is capable of inducing FPN mRNA expression in a dose-dependent manner (167, 304). Also understood as a compensatory mechanism, exposure to Mn or Fe has been shown to change FPN localization in the choroid plexus at the blood-CSF barrier (289). Recently, Mitchell and colleagues (190) failed to reproducibly identify a difference of Mn efflux in Xenopus oocytes expressing FPN compared to those without FPN expression; an actual decrease of efflux of Mn in cells expressing FPN was found in some cases. It is not yet understood whether this particular study accounts for the decreased accumulation of Mn in the FPN-expressing cells as a reason for decreased efflux, as seen in a previous study using Xenopus oocytes (177). Regardless, the role of FPN as an exporter of Mn has already been recognized in mouse brain in vivo (304), and its association remains much less controversial than an Mn role with DMT1 in the brain.
Regulation of Subcellular Distribution and Storage
Park9/ATP13A2.
Park9, also known as ATP13A2, is a P-type ATPase primarily found in the neurons of the substantia nigra and is a putative cation shuttle across the lysosomal membranes (110, 222, 244, 274). The evidence supporting Park9 transport of Mn comes from studies showing that deletion of the yeast homolog Ypk9 yields sensitivity to toxicity of heavy metals including Mn (244). Similarly, a protective effect from Mn is seen when overexpressed in mammalian cell lines or rat primary cell cultures (274). The mechanism behind the protective effect of Park9 is not understood, but it has been proposed to help sequester toxic metals into vacuoles or to function as a Zn/Mn pump as described by Kong and colleagues (150). A recent review (282) has criticized the proposal of Park9 as a metal transporter and suggests that it acts as a flippase aiding in vesicle creation and fusion. Whether or not this is true, it is clear that Park9 in some fashion influences the proper movement of metals within the endosome/lysosome transport process.
SPCA1 and GPP130.
SPCA1 is a Ca2+/Mn2+ ATPase expressed highly in the brain on the surface of the Golgi membrane that transports cytosolic Mn2 and Ca2+ into the Golgi lumen (189, 198). By this mechanism, Ca2+ can be stored safely, and excess Mn2+ can be removed from the cytosol and exported through the secretory pathway. SPCA1 can transport one Mn2+ or Ca2+ ion at a time per hydrolyzed ATP. Considering its high affinity to Mn, equally comparable only to Ca (73, 74), SPCA1 is recognized as one of only two critical regulators of Mn known to date, the other being SLC30A10. Loss of function in the SPCA1 yeast homologue PMR1 leads to hypersensitivity to Mn toxicity (292). In humans, the specificity to Mn is even higher than for the yeast protein (277). The null mutant of SPCA1 in mice is lethal, with heterozygous mice having increased rates of apoptosis and demonstrating larger Golgi with diminished leaflets (204). Rats exposed to chronic MnCl2 (30 mg/kg i.p. daily for 30 days) had twofold increased expression of SPCA in the mitochondrial proteome in the brain (310), in what can be assumed as a compensatory detoxification process. However, higher concentrations of Mn2+ exposure (1 mM) in cultured mouse neurons and glia have been shown to inhibit Ca2+ ATPase activity of SPCA1 to approximately 50% of vehicle without influencing expression (252). The failure to see changes in SPCA1 expression are likely due to a timing difference (30 days as compared to 6 hours) or in vivo/in vitro differences, but what can be observed is that SPCA1 can be oversaturated by Mn, and toxicity will result from presumably blocking normal Ca2+ sequestration. On a systems level, a significant amount of detoxification of Mn2+ may occur in the liver. Knockdown of SPCA1 in HEK293T cells limited growth and decreased viability following Mn2+ exposure (164). Overexpression of SPCA1 in these cells allowed for increased Mn2+ tolerance. Similarly, a mutation to increase the pore size of SPCA1 in yeast resulted in a hyperactive transporter with increased Mn2+ efflux and Mn2+ tolerance (197).
SPCA1 mRNA expression and protein show only modest increases across postnatal development in the hippocampus, cortex, and cerebellum of mice; however, SPCA ATPase activity steadily increases over this developmental period (250). This discrepancy has been speculated to be due to differences in unknown activators of SPCA1 rather than SPCA2—a second isoform of SPCA that shares 64% of homology with SPCA1 (298) but whose expression in the brain is a contentious issue (250, 283, 299); it has been reported to have little or no ATPase activity (296). The relative amount of brain SPCA1 expression compared to other tissues has been unclear. Wootton and colleagues (296) found that SPCA1 mRNA expression and protein levels were highest in the rat brain, testis, and germ cells compared to rat heart, lung, kidney, and liver, consistent with older data (124) from a study prior to the identification of SPCA1’s function, which also found rat RNA levels of SPCA1 to be higher in the brain than in any other tissues tested. Regardless, expression of SPCA1 has been identified in neuronal, astroglial, ependymal, and oligodendroglial, but not microglial, cells (198). The subcellular distribution of SPCA1 is predominantly reported in the Golgi (184, 226, 251, 283), although the exact subsection is uncertain, and strangely, the amount of SPCA1 is not correlated with the amount of Golgi present in the cells (296).
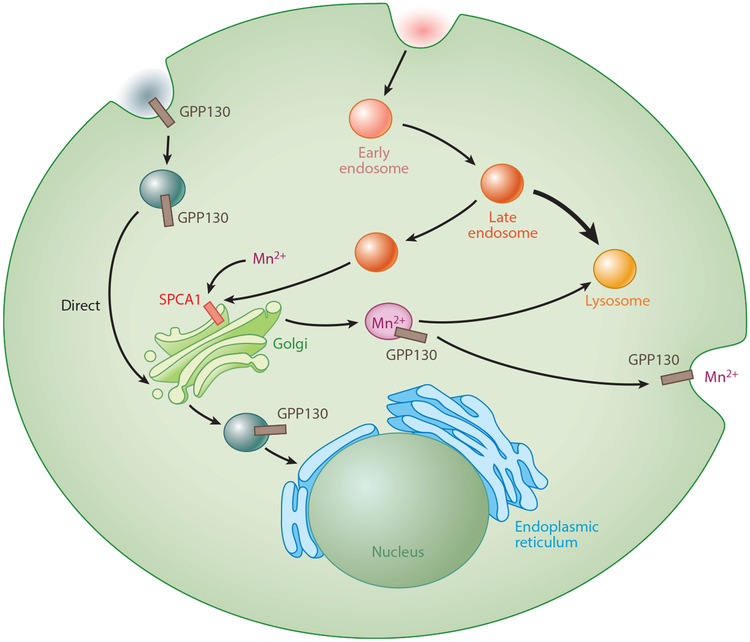
The important discovery that Mn exposure induces the cis-Golgi glycoprotein (GPP)130 to traffic from the Golgi to multivesicular bodies and then to lysosomes for degradation (196) has defined a molecular and biological sensor for Mn that may be involved in Mn homeostatic regulation (Figure 2). The normal function of GPP130 appears to involve the trafficking of vesicle directly from the endosomes to the Golgi, bypassing late endosomes and prelysosomes (214). Its sensitivity to Mn is delicate and specific to Mn rather than other metals. This mechanism has been recorded in neuronal cell lines, and the degradation of GPP130 from Mn exposure has been demonstrated in vivo as well (180). Recently, Tewari and colleagues (275) helped to elucidate this sorting mechanism by discovering that GPP130 binds to Mn, inducing oligomerization of the protein. SPCA1 is required for Mn2+ to reach the Golgi lumen and bind to GPP130, which provides a putative mechanism by which cells regulate excess cytosolic Mn. Increased cytosolic Mn may be pumped through SPCA1 to the Golgi lumen, where it binds to GPP130 and induces its oligomerization, resulting in sorting to the oligomer and secretion of Mn from the cell.
Figure 2.
Mechanism of Mn detection via SPCA1 and GPP130. Endocytosed vesicles containing GPP130 are typically sorted directly to the late Golgi. SPCA1 transports Mn into the Golgi complex. In the presence of Mn and GPP130, vesicles are either guided to lysosomes, where Mn is then sequestered, or are brought to the membrane, where Mn is then exocytosed. Abbreviations: GPP, cis-Golgi glycoprotein; Mn, manganese; SPCA1, secretory pathway Ca2+-ATPase isoform 1.
Metallothionein.
The roles that metallothioneins (MTs) have in the storage, detoxification, or transport of Mn, as is the case of other metals, is unknown. Metallothioneins are Golgi-localized low-molecular-weight proteins that bind metals at the thiol groups of the cysteine-rich residues. The literature on the relationship of Mn and MT is very sparse. Astrocyte exposure to Mn is known to decrease MT mRNA in a dose-dependent fashion, most likely due to a shift in metal metabolism (84). MT is induced in the liver of mice following Mn exposure; however, the metals bound to the induced MT were found to be mostly Zn rather than Mn. Interestingly, this induction appears to be completely dependent on IL-6 production; mice lacking IL-6 did not have any MT response from Mn exposure (149).
MANGANESE NEURONAL HEALTH
Brain Physiology and Function
Mn is essential for brain physiology and biology via its role as a cofactor in numerous enzymatic processes. Furthermore, several Mn-responsive pathways have been identified that may play a role in regulation of Mn cellular homeostasis and protection against neurotoxicity due to excess levels of Mn. It is likely that Mn, like other essential metals, must be carefully regulated to ensure proper health. The previously described high levels of Mn that occur normally in the brain argue for a particular role of Mn in brain physiology and function. Here we consider Mn-related neurobiological processes and the consequences of insufficient and excessive brain Mn levels. Given the diverse set of cellular processes contingent on the sufficient but controlled collection of Mn, the cellular demand for Mn is likely to change throughout development. Using small molecules that perturb cellular Mn transport, a recent study showed that developing mesencephalic DAergic precursors alter their mechanisms of Mn transport between key neural developmental stages (154).
Mn-Dependent and Mn-Activated Enzymes
Arginase is an Mn-dependent enzyme that catalyzes the hydrolysis of arginine to ornithine in the urea cycle. The two isozymes of arginase, ARG1 and ARG2, have the same function but differ in their expression. ARG1, which has been found at higher levels in the brain, is found primarily in the cytoplasm, whereas ARG2 is found primarily in the mitochondria (307). ARG2 is expressed in neurons and glial cells of most brain structures, with particularly high levels of expression found in the neocortex, corpus callosum, putamen, and ventral striatum (29). Whereas metal requirements for arginases may differ between species, optimal catalytic function of human ARG1 specifically depends upon Mn2+ (60, 144). Arginase is expressed in endothelial cells, where it controls the production of nitric oxide by mediating the bioavailability of arginine (22, 303). The function of arginase in the brain has not been fully characterized, although studies suggest neuroprotective effects. Depletion of arginine by ARG1 promotes neuronal survival through inhibition of nitric oxide synthesis (87) and protein synthesis. Arginase also plays a role in the neural immune response, contributing to neuronal protection and regeneration through microglial activation pathways (48) and polyamine synthesis pathways (71, 155).
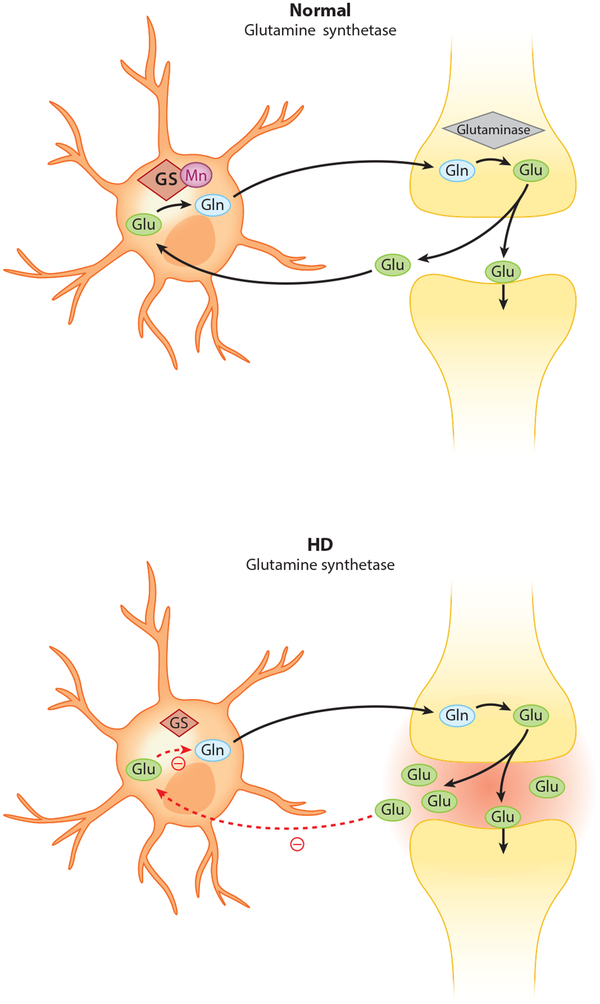
Glutamine synthetase (GS) is also a very prevalent and significant Mn-dependent enzyme found primarily in astrocytes, where it catalyzes the conversion of the neurotransmitter glutamate (Glu) into glutamine (Gln), which can be released and subsequently taken up by neurons for use in the synthesis of glutamate (129, 132, 202). Extracellular Glu is neurotoxic at high levels; accordingly, inhibited Glu uptake by astrocytes contributes to Glu neurotoxicity (314). This process is illustrated in Figure 3. Efficient Glu uptake relies on GS activity, indicating an important role for GS in normal synaptic function as well as a neuroprotective effect of this enzyme against Glu-induced excitotoxicity and neurodegeneration (111, 254, 313). The finding that elevated levels of extracellular Glu increase GS expression and inhibit glial Glu transporters supports this conclusion (163). Studies suggest that Mn neurotoxicity is associated with impaired Glu-Gln homeostatic mechanisms in the brain. Mn inhibits Gln transport into astrocytes (187, 258). Counterintuitively, Mn exposure has been found to reduce GS activity in the brain, particularly in the striatum and globus pallidus (195).
Figure 3.
Glutamine synthetase activity is diminished in Huntington’s disease (HD). Glutamine synthetase is an Mn-dependent enzyme. Efficient glutamate uptake by astrocytes relies on glutamine synthetase. In HD, where cellular Mn is reduced, impaired glutamine synthetase activity inhibits glutamate uptake. Abbreviations: Gln, glutamine; Glu, glutamate; GS, glutamine synthetase; Mn, manganese.
Mn-dependent superoxide dismutase/superoxide dismutase 2 (MnSOD/SOD2) is an antioxidant mitochondrial metalloenzyme that protects cells from oxidative stress by catalyzing the dismutation of superoxide anion radical to H2O2 and molecular oxygen, regulating cellular redox status and ROS generation (33). By reducing oxidative stress, MnSOD has an important role in mediating physiological and pathological neuronal apoptosis and neurodegeneration (148, 255). Overexpression of MnSOD in AD-model brains has been shown to reduce oxidative stress (79). Although Mn is required for the catalytic function of MnSOD, iron can also bind with high affinity to the Mn binding site of MnSOD (191). Mn supplementation has been shown to increase MnSOD activity in human lymphocytes (66).
Also among critical enzymes requiring Mn are pyruvate carboxylase and protein serine/threonine phosphatase-1 (PP1) (297). Pyruvate carboxylase is an Mn-dependent enzyme that converts pyruvate to the TCA cycle intermediate, oxaloacetate (41, 186, 247, 248). Pyruvate carboxylase deficiency can lead to accumulation of lactate in the bloodstream, causing lactic acidosis (193). PP1 is needed to dephosphorylate serine and threonine residues because it directly dephosphorylates AKT to regulate the AKT signaling transductions for cell survival and differentiation (300). PP1 has also been shown to directly dephosphorylate the apoptotic regulator p53, thereby regulating the p53-dependent apoptotic pathway and promoting cell survival (166).
Mn-Responsive Pathways
ATM-p53 signaling pathway.
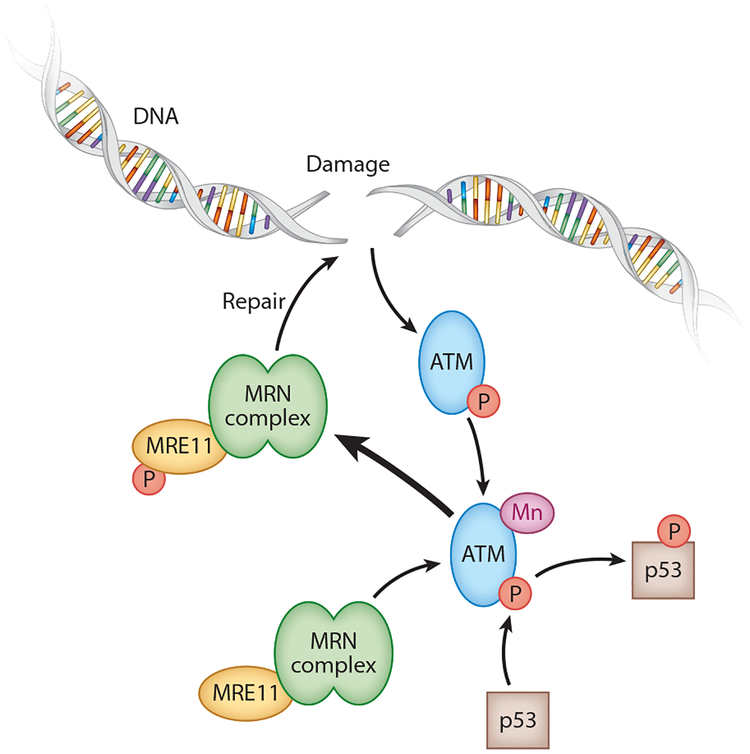
Ataxia telangiectasia mutated (ATM) is an Mn-activated serine/threonine protein kinase involved in the cellular response to DNA damage and is mutated in Ataxia telangiectasia, a neurodegenerative autosomal recessive disorder with impaired mitochondrial function and deficient mitochondrial DNA repair, indicating the importance of ATM for mitochondrial function in the brain (5, 256). ATM has been shown to phosphorylate p53, H2AX, and other targets in response to increased Mn levels, DNA damage, and oxidative stress (18, 125, 276). Ionizing radiation activates ATM to phosphorylate p53 in an Mn-dependent manner (38). ATP has also been shown to activate ATM to phosphorylate p53 and other downstream substrates via an autophosphorylation mechanism that is specific to ATM kinase (151). Mn is required for phosphorylation by ATM of proteins involved in DNA damage pathways and cell cycle checkpoints, including p53 (41). This process is illustrated in Figure 4. During activation of ATM via double-stranded DNA breakage, the DNA damage sensor complex MRN (Mre11, Rad50, Nbs1) is necessary. Mre11 has Mn-dependent exonuclease activity necessary for processing strand ends during nonhomologous end-joining repair. This nuclease activity requires Mn and cannot be substituted by magnesium or calcium (135, 208, 278). However, nuclease-activity-dead Mre11 mutants still undergo MRN complex binding to double-stranded breaks and Mre11-dependent ATM phosphorylation (169). A loss of Mn would likely result in stalled double-stranded breakage repair, providing a longer active time to phosphorylate ATM and thus increasing its DNA damage response in cells.
Figure 4.
Mn-ATM-p53 pathway. Following doubled-stranded DNA damage, ATM is activated by Mn to phosphorylate p53 and Mre11 of the MRN complex. The MRN complex is then able to repair the DNA damage. Abbreviations: ATM, ataxia telangiectasia mutated; Mn, manganese; MRN, Mre11, Rad50, Nbs1.
Increased levels of p53 have been found in cortical neurons and glial cells from Mn-exposed nonhuman primates, and analysis of gene expression changes in cortical tissue from these Mn-exposed animals has revealed a prominent role of p53 in Mn-induced alterations in gene expression (117, 118). K-homology splicing regulator protein, a regulatory protein involved in neuronal apoptotic signaling, is upregulated in Mn-exposed striatum along with p53, providing further evidence of the role of p53 in Mn neurotoxicity (257). Recently, a major p53 response to Mn exposure was found in mouse striatal cells and human neuroprogenitors. Activation of ATM kinase activity was shown to be sensitive to Mn at neurologically relevant concentrations, and inhibitors of ATM kinase decreased Mn-dependent p53 phosphorylation, confirming ATM-p53 as a significant Mn response pathway (276).
Inflammatory pathways.
The neurotoxicity mechanism of Mn may in part be due to a resulting glial activation and neuroinflammatory response (Figure 5). Proinflammatory cytokines such as IL-6, IL-1β, and tumor necrosis factor-alpha are induced by endotoxins such as lipopolysaccharide but potentiated in the presence of Mn (309). Cytokine toxicity potentiated by Mn has been shown to depend upon the presence of astrocytes (263). This is thought to be mediated by the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and p38, reflecting the way in which their pharmacological inhibition blocks this effect (53, 91). However, microglia are capable of releasing these cytokines in response to Mn alone (91, 170). The increased presence of microglia in the basal ganglia relative to any other areas throughout the brain (156) in part can explain the sensitivity of the area to Mn toxicity. PD patients and animal models do demonstrate increased activation of microglia in these areas (59).
Figure 5.
Inflammatory response induced by Mn. Mn exposure leads to the production of NF-κB and p38 in astrocytes, which increases reactive oxygen species. Inflammatory markers (IL-6, IL-1β, and TNF-α) are produced by microglia following Mn exposure. In the presence of these markers and Mn, neurons have been found to increase expression of the Mn-permeable ZIP14 and decrease expression of the Mn exporter SLC30A10. Abbreviations: IL-1β/6, interleukin-1β/6; Mn, manganese; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; ROS, reactive oxygen species; SLC, solute carrier; TNFα; tumor necrosis factor-alpha.
Continued glial cell activation can cause damage primarily by generating harmful ROS (discussed further below) and reactive nitrogen species such as nitric oxide (NO), but the overproduction of cytokines can lead to a cascade of further glial activation in the surrounding areas. Recently, it was noted that IL-6 could induce uptake of Mn while also upregulating the Mn-permeable ZIP14 zinc channels and downregulating the Mn-exporter SLC30A10 (105). Whether this can explain the potentiating effects of Mn with IL-6 and other cytokines is not yet clear.
Neuropathological Consequences of Altered Mn Biology
Mn insufficiency in neurological disease.
No known neurological conditions have been medically established to be due to Mn insufficiency. However, recent research on Huntington’s disease (HD) has identified a deficiency in neuronal Mn handling associated with decreased neuronal Mn levels under both basal and elevated Mn exposure conditions (28, 178, 266, 276, 291, 293, 294). Although additional studies are needed to establish the relationship between alterations in Mn homeostasis and HD pathogenesis, several lines of evidence point to a potential role. For example, many critical Mn-dependent enzymes of the brain show diminished levels of activity in HD patients and other models of HD (36, 44, 45). Of particular note, intracellular Mn levels are reduced in the striatum of HD patients and other HD models (234). Mn-dependent activation of ATM signaling is impaired in both mouse and human neuronal models of HD and rescued by restoration of intracellular Mn levels (276). Also, impairment of ATM-dependent repair pathways of DNA double-strand breaks have been reported in irradiated HD human fibroblasts, suggesting that ATM may be implicated in HD pathology (90). Consistent with the hypothesis of decreased Mn transport, HD cells have an increased tolerance to Mn toxicity (294). The mechanism by which mutant Huntingtin (HTT) protein impairs Mn transport is unknown. However, the change of Mn levels in one cellular model of HD has been shown to be independent of Tf or DMT1 activity, despite the known alterations of iron homeostasis in HD (293). Of note, expression of ferroportin is increased and Tf decreased in the cortex and striatum in mouse models of HD (42). Taking into consideration how iron concentrations are elevated in striatal neurons in HD, this may be a compensatory mechanism.
Dietary Mn deficiency has been found to decrease arginase activity and increase the activity of nitric oxide synthases (31, 82). Intracellular depletion of arginine by arginase may be neuroprotective (86, 160). Whereas inhibition of ARG1 leads to increased NO production, which contributes to neuronal death, inhibition of NO by ARG1 contributes to neuronal survival, as demonstrated by a surviving subpopulation of motor neurons deprived of trophic factors (87). Altered arginase activity (dependent on Mn as described above) has been implicated in the progression of HD. A urea cycle deficiency has been reported in HD. Increased ammonia and citrulline levels point to decreased arginase activity in these models (45). Neuronal nitric oxide synthase and dietary L-arginine, the NO precursor, have been shown to mediate the time of symptom onset in HD (68,69). Diminished GS activity has also been observed in HD brain (20, 40). Interestingly, decreased astrocytic GS expression and activity have been observed in the AD brain, especially in late stages of the disease (153, 205, 229).
Partial MnSOD deficiency has been shown to increase sensitivity to malonate and 3-nitropropionic acid, and both toxicants have been used to generate animal models for HD (8, 32, 246). Decreased MnSOD activity from Mn deficiency may cause damage to the mitochondrial membrane via increased lipid peroxidation from free radicals (311). Mitochondrial injury resulting from MnSOD deficiency contributes to neurodegeneration (158). Loss of MnSOD activity has been shown to increase neuronal sensitivity to the mitochondrial toxin 3-nitropropionic acid, which leads to an HD-like neurodegenerative phenotype, with degeneration of the medium spiny neurons of the striatum (caudate/putamen) in animal models and humans (8). 3-Nitropropionic acid has also been shown to disturb glutaminergic and DAergic signaling in part by mediating the activity of serine/threonine phosphatases (199). The PPM/PP2C family of protein phosphatases may regulate DAergic signaling in HD through AKT dephosphorylation of Htt protein in the G protein–dependent signaling pathway of the striatal dopamine D2 receptor (179).
Alteration of neuronal Mn homeostasis in HD could also occur via the interaction of Htt with huntingtin-interacting protein (HIP)14 and HIP14-like (HIP14L). Before their recognition as transporters for Mg2+ and other divalent metals such as Mn2+ (112, 216), HIP14 and HIP14L were recognized as a required protein for the proper palmitoylation and thus proper cellular distribution of Htt and several synaptic localized proteins (139, 203, 240, 260, 302). Down-regulation of HIP14 leads to increased inclusion in cells expressing either wild-type or mutant Htt (302). Taking into consideration the connections between HD and altered Mn biology, a putative transporter of Mn that has an imperative role in proper Htt trafficking and proper response to Htt is a compelling link. Mice deficient in either HIP14 or HIP14L have been reported to have behavioral, motor, and neuropathological features (261, 271). These proteins are found primarily in neurons within the Golgi complex, post-Golgi vesicles, and subplasmic membrane (112, 203). Importantly, HIP14L is capable of palmitoylating HIP14, and HIP14 is capable of palmitoylating itself. The presence of wild-type Htt potentiates this effect, and mutant Htt is incapable of this potentiation, thus providing a potential mechanism by which Mn transport is altered in HD (see below) (138). However, additional studies are needed to examine the functional relationship between HIP14/HIP14L and Mn biology.
Mn excess in neurological disease.
Manganism/Mn poisoning/Mn-induced Parkinsonism occurs primarily among industrial workers, such as welders, who are chronically exposed to Mn fumes or dust, the neurotoxic effects of which cause a PD-like syndrome. Chelating treatment with CaNa2 ethylenediaminetetraacetic acid (EDTA), along with sufficiently early removal from Mn exposure, has been found to effectively ameliorate symptoms in some workers with Mn-induced Parkinsonism, although irreversible cases have been reported (131). The link between Mn-induced Parkinsonism and PD has been debated, with some patient studies reporting considerable overlap between the pathophysiology and clinical characteristics of Mn-induced Parkinsonism and PD, except age of onset (218, 220). Significantly elevated levels of Mn have been found in the serum and CSF of PD patients (2, 136). Mn-exposed nonhuman primates with motor abnormalities have dysfunctional DAergic systems. Positron emission tomography imaging of nonhuman primate brains has revealed impaired DA transmission, a feature of PD, in the striatum of Mn-exposed animals (118, 119). Positron emission tomography imaging of asymptomatic Mn-exposed welders with 6-[18F]fluoro-L-dopa (FDOPA) also points to nigrostriatal DAergic dysfunction, but with a pattern of uptake inconsistent with that observed in patients with idiopathic PD (52). Mn exposure at subtoxic levels has been found to amplify the neurotoxic effects of 1-methyl-4-phenylpyridinium, which also contributes to a PD-like syndrome through the induction of ROS formation (288).
The E3 ligase, parkin, when overexpressed in SHSY5Y cells, is responsible for degrading 1B-DMT1 isoforms (239). This degradation was protective against Mn toxicity. Parkin protein, encoded by PARK2, a gene linked to PD pathogenesis, has been shown to accumulate in Mn-treated DAergic cells. Parkin may be critical for the attenuation of Mn-induced neuronal cell death, as overexpression of this protein has been found to protect DAergic neurons from the neurotoxic effects of Mn (133). In response to Mn exposure, human lymphocytes lacking active parkin have demonstrated greater mitochondrial dysfunction and enhanced apoptotic signaling (237). Workers who are repeatedly exposed to Mn through welding fumes are at risk for Parkinsonism. PARK genes may modulate the DAergic neurotoxicity of Mn-containing welding fumes, as exposure to Mn via welding fumes has been shown to cause mitochondrial dysfunction and alter DAergic PARK protein expression (265).
ATP13A2 (encoded by PARK9, introduced above) has been shown to protect cells from Mn toxicity by mediating intracellular Mn concentrations, an effect not observed in ATP13A2 mutants (274). Variants in the ATP13A2 gene (described above) have been reported to be a risk factor for Mn neurotoxicity in humans (227). Mutations of ATP13A2 are known to cause a Parkinsonianlike disease known as Kufor-Rakeb syndrome (221, 279, 281). Increasing expression of ATP13A2 shows a protective effect against alpha-synuclein, and conversely, reduction of ATP13A2 results in elevated alpha-synuclein accumulation.
Strong evidence for the association of Mn and Parkinsonism is seen from the recent literature describing the selective cell-surface exporter of Mn identified as SLC30A10 (165). The phenotype and age of onset observed in human patients with mutations in SLC30A10 varies greatly, even within families with the same inherited mutation. The symptoms include hypermanganesemia, polycythemia, dystonia, chronic hepatic disease, cirrhosis, motor neuropathy, and behavioral disturbances with differing severity (70). These symptoms have a large overlap with patients with PD; some studies have even found an increased occurrence of Parkinsonism with sufferers of cirrhosis (up to 21%) (35, 201). Consistent with this idea, patients presenting with Parkinsonian symptoms who have decreased ability to excrete biliary Mn2+ suffer from Mn2+-induced neurotoxicity (1, 35). It is important to note that affected individuals did not have any loss of striatal DAergic neurons in the striatum, which is the hallmark pathological feature in PD (159, 215, 280). Lechpammer and colleagues (159) describe in detail the pathology of the last 14 years of the life of one patient suffering from a mutation of SLC30A10; following the patient’s death at age 38, Mn levels in the basal ganglia were elevated 16-fold in comparison with control levels. There was marked loss of neurons in the medial and lateral globus pallidus, increased activation of microglia and astrocytes, myelin loss, and spongiosis. Although SLC30A10 has been shown to transport Zn and other cations other than Mn, it is remarkable that patients with mutations in SLC30A10 do not exhibit changes in concentrations in any other trace metals tested so far in the brain. It is also interesting that expression of SLC30A10 has been found to be significantly reduced (twofold) in the frontal cortex of patients with AD, an observation that is consistent in mouse models of AD (26).
Mitochondria-mediated toxicity is thought to occur in part by Mn2+ interfering with Ca2+ activation of ATP, leaving an energy deficit (122). Mn has been shown to induce apoptosis-promoting caspase-12. Not only does Mn induce apoptosis, but the presence of dopamine also potentiates apoptosis. This may in part explain the selective death of DAergic neurons in the striatum following Mn exposure. Cytochrome c and caspase-1, 3, 8, and 9 release also occurs following Mn exposure (46). These data suggest that the neuronal apoptosis is a response to excessive Mn levels in the brain.
Cellular redox pathways and neuronal oxidative stress.
Production of ROS following excessive Mn exposure is a canonical response seen in vitro and in vivo (207). The disabling of antioxidant defenses by Mn is also well characterized (168). Recent work has shown that the toxicity of Mn is largely dependent upon the redox state, as glutathione levels can inversely predict toxicity upon Mn exposure (269). Neuronal cell exposure to Mn induces oxidative damage to DNA, but this can be reversed with glutathione treatment (268). Antioxidant treatment in chronically exposed rodents reverses not only toxicity but also motor deficits and signaling pathways activated by oxidative stress (49). The signaling pathways associated with oxidative stress and Mn include PI3/Akt (49, 50, 182), protein kinase C, ERK1/2, p38, and JNK (37, 54, 134). Interestingly, the phosphorylation of DARPP-32 at Thr34 is induced by Mn, which allows DARPP-32 to act as an inhibitor to PP1 (50). The production of ROS is thought to be the cause of nitric oxide synthase and NF-κB induction (212). The autoxidation of dopamine by Mn is also well documented (270). Monoamine oxidase activity is increased as a result of increased ROS, and this leads to a decrease in dopamine in the striatal cells affected. It has been noted that the depletion of dopamine is independent of the ROS generation, as dopamine depletion occurs prior to ROS detection. Glutamate uptake by the glutamate/aspartate transporter is inhibited by Mn, leading to higher extracellular glutamate (85). Expression of GABA transporters is reduced with excess Mn, leading to increased extracellular GABA (7, 80, 101).
CONCLUSION
Mn is essential to human health. Given that Mn is required for a number of physiological functions but is toxic at excessive levels, mechanisms of Mn homeostasis are critically important. Exposure to Mn is mainly dietary and occupational. Although ingestion is the major route for exposure, Mn can also be inhaled, especially in certain industrial settings. Normally high levels of Mn in the brain suggest that Mn plays a particularly important role in brain physiology and function and argue for the importance of elucidating mechanisms of Mn homeostasis. A number of candidates for cellular Mn uptake have been identified and investigated, including DMTI, the Tf system, and zinc, citrate, choline, dopamine, and calcium transporters. Likely candidates for cellular efflux of Mn include SLC30A10, the sodium-calcium exchanger, and ferroportin. Regulation of subcellular distribution and storage has been attributed to Park9/ATP13A2, SPCA1, GPP130, and metallothioneins.
Mn is incorporated into a number of enzymes that are important for brain physiology and function. These include arginase, glutamine synthetase, MnSOD, pyruvate carboxylase, and protein serine/threonine phosphatase-1. Mn-responsive pathways have been identified, including the ATM-p53 pathway. Impaired neuronal Mn handling has been observed in HD. Excessive Mn levels are associated with manganism/PD, but the link between these conditions remains unclear. Impaired Mn homeostasis may alter the activity of Mn-dependent enzymes and Mn-sensitive pathways, contributing to neurotoxicity and the pathophysiology of neurodegenerative disorders, including HD and PD.
SUMMARY POINTS.
Manganese (Mn) is essential for human neuronal health but is toxic in high concentrations, creating the need for tight regulation.
Regulation mechanisms for Mn at the cellular level currently remain unidentified.
Mn exposure targets several signaling pathways, including inflammatory cascades and the p53-ATM pathway, that may contribute to its toxicity.
Excess Mn in the striatum is associated with Parkinson’s disease and Parkinsonian-like disorders, whereas deficient Mn in the same brain areas is strongly associated with Huntington’s disease.
Several nonspecific ion channels and transporters are semipermeable to Mn, although the channel(s) or transporter(s) responsible for the deficiency of Mn seen in Huntington’s disease has not yet been revealed.
FUTURE ISSUES.
Further study is needed to clearly define the mechanisms of Mn uptake and distribution in blood and tissues (e.g., ceruloplasmin). Additional studies are needed to clearly characterize the homeostatic mechanisms that regulate Mn and determine which of the transporters described here, if any, play the most significant roles in cellular Mn transport, distribution, and storage. Further research is needed to explain how alterations in neuronal Mn homeostasis affect Mn transporters, Mn-dependent enzymes, and Mn-responsive pathways to contribute to the pathogenesis and progression of devastating disorders.
ACKNOWLEDGMENTS
The authors were supported by a grant from the National Institutes of Health, National Institute of Environmental Health Sciences (NIEHS), R01 ES10563.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Aggarwal A, Vaidya S, Shah S, Singh J, Desai S, Bhatt M. 2006. Reversible Parkinsonism and T1W pallidal hyperintensities in acute liver failure. Mov. Disord 21:1986–90 [DOI] [PubMed] [Google Scholar]
- 2.Ahmed SS, Santosh W. 2010. Metallomic profiling and linkage map analysis of early Parkinson’s disease: a new insight to aluminum marker for the possible diagnosis. PLOS ONE 5:e11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akatsu H, Hori A, Yamamoto T, Yoshida M, Mimuro M, et al. 2012. Transition metal abnormalities in progressive dementias. Biometals 25:337–50 [DOI] [PubMed] [Google Scholar]
- 4.Alves G, Thiebot J, Tracqui A, Delangre T, Guedon C, Lerebours E. 1997. Neurologic disorders due to brain manganese deposition in a jaundiced patient receiving long-term parenteral nutrition. J. Parenter. Enteral Nutr 21:41–45 [DOI] [PubMed] [Google Scholar]
- 5.Ambrose M, Goldstine JV, Gatti RA. 2007. Intrinsic mitochondrial dysfunction in ATM-deficient lymphoblastoid cells. Hum. Mol. Genet 16:2154–64 [DOI] [PubMed] [Google Scholar]
- 6.Andersen ME, Gearhart JM, Clewell HJ 3rd. 1999. Pharmacokinetic data needs to support risk assessments for inhaled and ingested manganese. Neurotoxicology 20:161–71 [PubMed] [Google Scholar]
- 7.Anderson JG, Fordahl SC, Cooney PT, Weaver TL, Colyer CL, Erikson KM. 2008. Manganese exposure alters extracellular GABA, GABA receptor and transporter protein and mRNA levels in the developing rat brain. Neurotoxicology 29:1044–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andreassen OA, Ferrante RJ, Dedeoglu A, Albers DW, Klivenyi P, et al. 2001. Mice with a partial deficiency of manganese superoxide dismutase show increased vulnerability to the mitochondrial toxins malonate, 3-nitropropionic acid, and MPTP. Exp. Neurol 167:189–95 [DOI] [PubMed] [Google Scholar]
- 9.Antoons G, Mubagwa K, Nevelsteen I, Sipido KR. 2002. Mechanisms underlying the frequency dependence of contraction and [Ca2+]i transients in mouse ventricular myocytes. J. Physiol 543:889–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aschner JL, Aschner M. 2005. Nutritional aspects of manganese homeostasis. Mol. Aspects Med 26:353–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aschner M, Aschner JL. 1990. Manganese transport across the blood-brain barrier: relationship to iron homeostasis. Brain Res. Bull 24:857–60 [DOI] [PubMed] [Google Scholar]
- 12.Aschner M, Gannon M. 1994. Manganese (Mn) transport across the rat blood-brain barrier: saturable and transferrin-dependent transport mechanisms. Brain Res. Bull 33:345–49 [DOI] [PubMed] [Google Scholar]
- 13.Aschner M, Guilarte TR, Schneider JS, Zheng W. 2007. Manganese: recent advances in understanding its transport and neurotoxicity. Toxicol. Appl. Pharmacol 221:131–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ATSDR (Agency Toxic Subst. Dis. Regist.). 2008. Toxicological profile for manganese Atlanta, GA: ATSDR; http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=102&tid=23 [PubMed] [Google Scholar]
- 15.Au C, Benedetto A, Anderson J, Labrousse A, Erikson K, et al. 2009. SMF-1, SMF-2 and SMF-3 DMT1 orthologues regulate and are regulated differentially by manganese levels in C. elegans. PLOS ONE 4:e7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayton S, Lei P, Duce JA, Wong BXW, Sedjahtera A, et al. 2013. Ceruloplasmin dysfunction and therapeutic potential for Parkinson disease. Ann. Neurol 73:554–59 [DOI] [PubMed] [Google Scholar]
- 17.Bagga P, Patel AB. 2012. Regional cerebral metabolism in mouse under chronic manganese exposure: implications for manganism. Neurochem. Int 60:177–85 [DOI] [PubMed] [Google Scholar]
- 18.Banin S, Moyal L, Shieh S, Taya Y, Anderson CW, et al. 1998. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281:1674–77 [DOI] [PubMed] [Google Scholar]
- 19.Bassani JW, Bassani RA, Bers DM. 1994. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J. Physiol 476:279–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Behrens PF, Franz P, Woodman B, Lindenberg KS, Landwehrmeyer GB. 2002. Impaired glutamate transport and glutamate-glutamine cycling: downstream effects of the Huntington mutation. Brain 125:1908–22 [DOI] [PubMed] [Google Scholar]
- 21.Bell JG, Keen CL, Lonnerdal B. 1989. Higher retention of manganese in suckling than in adult rats is not due to maturational differences in manganese uptake by rat small intestine. J. Toxicol. Environ. Health 26:387–98 [DOI] [PubMed] [Google Scholar]
- 22.Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, et al. 2003. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation 108:2000–6 [DOI] [PubMed] [Google Scholar]
- 23.Bhang SY, Cho SC, Kim JW, Hong YC, Shin MS, et al. 2013. Relationship between blood manganese levels and children’s attention, cognition, behavior, and academic performance—a nationwide crosssectional study. Environ. Res 126:9–16 [DOI] [PubMed] [Google Scholar]
- 24.Boaru SG, Merle U, Uerlings R, Zimmermann A, Weiskirchen S, et al. 2014. Simultaneous monitoring of cerebral metal accumulation in an experimental model of Wilson’s disease by laser ablation inductively coupled plasma mass spectrometry. BMC Neurosci 15:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bornhorst J, Wehe CA, Huwel S, Karst U, Galla HJ, Schwerdtle T. 2012. Impact of manganese on and transfer across blood-brain and blood-cerebrospinal fluid barrier in vitro. J. Biol. Chem 287:17140–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bosomworth HJ, Adlard PA, Ford D, Valentine RA. 2013. Altered expression of ZnT10 in Alzheimer’s disease brain. PLOS ONE 8:e65475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowman AB, Aschner M. 2014. Considerations on manganese (Mn) treatments for in vitro studies. Neurotoxicology 41:141–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowman AB, Kwakye GF, Herrero Hernandez E, Aschner M. 2011. Role of manganese in neurodegenerative diseases. J. Trace Elements Med. Biol 25:191–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braissant O, Gotoh T, Loup M, Mori M, Bachmann C. 1999. L-arginine uptake, the citrulline-NO cycle and arginase II in the rat brain: an in situ hybridization study. Mol. Brain Res 70:231–41 [DOI] [PubMed] [Google Scholar]
- 30.Britton AA, Cotzias GC. 1966. Dependence of manganese turnover on intake. Am. J. Physiol 211:203–6 [DOI] [PubMed] [Google Scholar]
- 31.Brock AA, Chapman SA, Ulaman EA, Wu G. 1993. Dietary manganese deficiency decreases rat hepatic arginase activity. J. Nutr 124:340–44 [DOI] [PubMed] [Google Scholar]
- 32.Brouillet E 2014. The 3-NP model of striatal neurodegeneration. Curr. Protoc. Neurosci 67:9.48.1–14 [DOI] [PubMed] [Google Scholar]
- 33.Buettner GR, Ng CF, Wang M, Rodgers VGJ, Schafer FQ. 2006. A new paradigm: manganese superoxide dismutase influences the production of H2O2 in cells and thereby their biological state. Free Radic. Biol. Med 41:1338–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burdo JR, Menzies SL, Simpson IA, Garrick LM, Garrick MD, et al. 2001. Distribution of divalent metal transporter 1 and metal transport protein 1 in the normal and Belgrade rat. J. Neurosci. Res 66:1198–207 [DOI] [PubMed] [Google Scholar]
- 35.Burkhard PR, Delavelle J, Pasquier RD, Spahr L. 2003. Chronic Parkinsonism associated with cirrhosis: a distinct subset of acquired hepatocerebral degeneration. Arch. Neurol 60:521–28 [DOI] [PubMed] [Google Scholar]
- 36.Butterworth J 1986. Changes in nine enzyme markers for neurons, glia, and endothelial cells in agonal state and Huntington’s disease caudate nucleus. J. Neurochem 47:583–87 [DOI] [PubMed] [Google Scholar]
- 37.Cai T, Che H, Yao T, Chen Y, Huang C, et al. 2011. Manganese induces tau hyperphosphorylation through the activation of ERK MAPK pathway in PC12 cells. Toxicol. Sci 119:169–77 [DOI] [PubMed] [Google Scholar]
- 38.Canman CE, Lim DS, Cimprich KA, Taya Y, Tamai K, et al. 1998. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 281:1677–79 [DOI] [PubMed] [Google Scholar]
- 39.Carmona A, Roudeau S, Perrin L, Veronesi G, Ortega R. 2014. Environmental manganese compounds accumulate as Mn(II) within the Golgi apparatus of dopamine cells: relationship between speciation, subcellular distribution, and cytotoxicity. Metallomics 6:822–32 [DOI] [PubMed] [Google Scholar]
- 40.Carter CJ. 1982. Glutamine synthetase activity in Huntington’s disease. Life Sci 31:1151–59 [DOI] [PubMed] [Google Scholar]
- 41.Chan DW. 2000. Purification and characterization of ATM from human placenta. A manganesedependent, Wortmannin-sensitive serine/threonine protein kinase. J. Biol. Chem 275:7803–10 [DOI] [PubMed] [Google Scholar]
- 42.Chen J, Marks E, Lai B, Zhang Z, Duce JA, et al. 2013. Iron accumulates in Huntington’s disease neurons: protection by deferoxamine. PLOS ONE 8:e77023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen L, Ding G, Gao Y, Wang P, Shi R, et al. 2014. Manganese concentrations in maternal-infant blood and birth weight. Environ. Sci. Pollut. Res. Int 21:6170–75 [DOI] [PubMed] [Google Scholar]
- 44.Chiang MC, Chen HM, Lai HL, Chen HW, Chou SY, et al. 2009. The A2A adenosine receptor rescues the urea cycle deficiency of Huntington’s disease by enhancing the activity of the ubiquitin-proteasome system. Hum. Mol. Genet 18:2929–42 [DOI] [PubMed] [Google Scholar]
- 45.Chiang MC, Chen HM, Lee YH, Chang HH, Wu YC, et al. 2007. Dysregulation of C/EBPαby mutant Huntingtin causes the urea cycle deficiency in Huntington’s disease. Hum. Mol. Genet 16:483–98 [DOI] [PubMed] [Google Scholar]
- 46.Chun HS, Lee HH, Son JH. 2001. Manganese induces endoplasmic reticulum (ER) stress and activates multiple caspases in nigral dopaminergic neuronal cells, SN4741. Neurosci. Lett 316:5–8 [DOI] [PubMed] [Google Scholar]
- 47.Cohn JR, Emmett EA. 1978. The excretion of trace metals in human sweat. Ann. Clin. Lab. Sci 8:270–75 [PubMed] [Google Scholar]
- 48.Colton CA, Mott RT, Sharpe H, Xu Q, Van Nostrand WE, Vitek MP. 2006. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J. Neuroinflammation 3:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cordova FM, Aguiar AS Jr, Peres TV, Lopes MW, Goncalves FM, et al. 2013. Manganese-exposed developing rats display motor deficits and striatal oxidative stress that are reversed by Trolox. Arch. Toxicol 87:1231–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cordova FM, Aguiar AS Jr, Peres TV, Lopes MW, Goncalves FM, et al. 2012. In vivo manganese exposure modulates Erk, Akt and Darpp-32 in the striatum of developing rats, and impairs their motor function. PLOS ONE 7:e33057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Criswell SR, Perlmutter JS, Huang JL, Golchin N, Flores HP, et al. 2012. Basal ganglia intensity indices and diffusion weighted imaging in manganese-exposed welders. Occup. Environ. Med 69:437–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Criswell SR, Perlmutter JS, Videen TO, Moerlein SM, Flores HP, et al. 2011. Reduced uptake of [18F]FDOPA PET in asymptomatic welders with occupational manganese exposure. Neurology 76:1296–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crittenden PL, Filipov NM. 2008. Manganese-induced potentiation of in vitro proinflammatory cytokine production by activated microglial cells is associated with persistent activation of p38 MAPK. Toxicol. In Vitro 22:18–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crittenden PL, Filipov NM. 2011. Manganese modulation of MAPK pathways: effects on upstream mitogen activated protein kinase kinases and mitogen activated kinase phosphatase-1 in microglial cells.J. Appl. Toxicol 31:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crossgrove J, Zheng W. 2004. Manganese toxicity upon overexposure. NMR Biomed 17:544–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crossgrove JS, Allen DD, Bukaveckas BL, Rhineheimer SS, Yokel RA. 2003. Manganese distribution across the blood-brain barrier I. Evidence for carrier-mediated influx of manganese citrate as well as manganese and manganese transferrin. Neurotoxicology 24:3–13 [DOI] [PubMed] [Google Scholar]
- 57.Crossgrove JS, Yokel RA. 2004. Manganese distribution across the blood-brain barrier. III. The divalent metal transporter-1 is not the major mechanism mediating brain manganese uptake. Neurotoxicology 25:451–60 [DOI] [PubMed] [Google Scholar]
- 58.Crossgrove JS, Yokel RA. 2005. Manganese distribution across the blood-brain barrier. IV. Evidence for brain influx through store-operated calcium channels. Neurotoxicology 26:297–307 [DOI] [PubMed] [Google Scholar]
- 59.Czlonkowska A, Kohutnicka M, Kurkowska-Jastrzebska I, Czlonkowski A. 1996. Microglial reaction in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) induced Parkinson’s disease mice model. Neurodegeneration 5:137–43 [DOI] [PubMed] [Google Scholar]
- 60.D’Antonio EL, Hai Y, Christianson DW. 2012. Structure and function of non-native metal clusters in human arginase I. Biochemistry 51:8399–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Das A Jr, Hammad TA. 2000. Efficacy of a combination of FCHG49 glucosamine hydrochloride, TRH122 low molecular weight sodium chondroitin sulfate and manganese ascorbate in the management of knee osteoarthritis. Osteoarthritis Cartilage 8:343–50 [DOI] [PubMed] [Google Scholar]
- 62.Davidsson L, Cederblad A, Lonnerdal B, Sandstrom B. 1991. The effect of individual dietary components on manganese absorption in humans. Am. J. Clin. Nutr 54:1065–70 [DOI] [PubMed] [Google Scholar]
- 63.Davidsson L, Cederblad A, Lonnerdal B, Sandstrom B. 1989. Manganese absorption from human milk, cow’s milk, and infant formulas in humans. Am. J. Dis. Child 143:823–27 [DOI] [PubMed] [Google Scholar]
- 64.Davidsson L, Loennerdal B, Sandstroem B, Kunz C, Keen CL. 1989. Identification of transferrin as the major plasma carrier protein for manganese introduced orally or intravenously or after in vitro addition in the rat. J. Nutr 119:1461–64 [DOI] [PubMed] [Google Scholar]
- 65.Davidsson LA, Lonnerdal B. 1989. Fe-saturation and proteolysis of human lactoferrin: effect on brushborder receptor-mediated uptake of Fe and Mn. Am. J. Physiol 257:G930–34 [DOI] [PubMed] [Google Scholar]
- 66.Davis CD, Greger JL. 1992. Longitudinal changes of manganese-dependent superoxide dismutase and other indexes of manganese and iron status in women. Am. J. Clin. Nutr 55:747–52 [DOI] [PubMed] [Google Scholar]
- 67.Davis CD, Zech L, Greger JL. 1993. Manganese metabolism in rats: an improved methodology for assessing gut endogenous losses. Proc. Soc. Exp. Biol. Med 202:103–8 [DOI] [PubMed] [Google Scholar]
- 68.Deckel AW, Tang V, Nuttal D, Gary KA, Elder R. 2002. Altered neuronal nitric oxide synthase expression contributes to disease progression in Huntington’s disease transgenic mice. Brain Res 939:76–86 [DOI] [PubMed] [Google Scholar]
- 69.Deckel AW, Volmer P, Weiner R, Gary KA, Covault J, et al. 2000. Dietary arginine alters time of symptom onset in Huntington’s disease transgenic mice. Brain Res 875:187–95 [DOI] [PubMed] [Google Scholar]
- 70.Delnooz CC, Wevers RA, Quadri M, Clayton PT, Mills PB, et al. 2013. Phenotypic variability in a dystonia family with mutations in the manganese transporter gene. Mov. Disord 28:685–86 [DOI] [PubMed] [Google Scholar]
- 71.Deng K, He H, Qiu J, Lorber B, Bryson JB, Filbin MT. 2009. Increased synthesis of spermidine as a result of upregulation of arginase I promotes axonal regeneration in culture and in vivo. J. Neurosci 29:9545–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dickinson TK, Devenyi AG, Connor JR. 1996. Distribution of injected ion 59 and manganese 54 in hypotransferrinemic mice. J. Lab. Clin. Med 128:270–78 [DOI] [PubMed] [Google Scholar]
- 73.Dode L, Andersen JP, Raeymaekers L, Missiaen L, Vilsen B, Wuytack F. 2005. Functional comparison between secretory pathway Ca2+/Mn2+-ATPase (SPCA) 1 and sarcoplasmic reticulum Ca2+-ATPase (SERCA) 1 isoforms by steady-state and transient kinetic analyses. J. Biol. Chem 280:39124–34 [DOI] [PubMed] [Google Scholar]
- 74.Dode L, Andersen JP, Vanoevelen J, Raeymaekers L, Missiaen L, et al. 2006. Dissection of the functional differences between human secretory pathway Ca2+/Mn2+-ATPase (SPCA) 1 and 2 isoenzymes by steady-state and transient kinetic analyses. J. Biol. Chem 281:3182–89 [DOI] [PubMed] [Google Scholar]
- 75.Doker S, Mounicou S, Dogan M, Lobinski R. 2010. Probing the metal-homeostatis effects of the administration of chromium(VI) to mice by ICP MS and size-exclusion chromatography-ICP MS. Metallomics 2:549–55 [DOI] [PubMed] [Google Scholar]
- 76.Dorman DC, Struve MF, Clewell HJ 3rd, Andersen ME. 2006. Application of pharmacokinetic data to the risk assessment of inhaled manganese. Neurotoxicology 27:752–64 [DOI] [PubMed] [Google Scholar]
- 77.Dorman DC, Struve MF, James RA, McManus BE, Marshall MW, Wong BA. 2001. Influence of dietary manganese on the pharmacokinetics of inhaled manganese sulfate in male CD rats. Toxicol. Sci 60:242–51 [DOI] [PubMed] [Google Scholar]
- 78.dos Santos AP, Milatovic D, Au C, Yin Z, Batoreu MC, Aschner M. 2010. Rat brain endothelial cells are a target of manganese toxicity. Brain Res 1326:152–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dumont M, Wille E, Stack C, Calingasan NY, Beal MF, Lin MT. 2009. Reduction of oxidative stress, amyloid deposition, and memory deficit by manganese superoxide dismutase overexpression in a transgenic mouse model of Alzheimer’s disease. FASEB J 23:2459–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dydak U, Jiang YM, Long LL, Zhu H, Chen J, et al. 2011. In vivo measurement of brain GABA concentrations by magnetic resonance spectroscopy in smelters occupationally exposed to manganese. Environ. Health Perspect 119:219–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Elder A, Gelein R, Silva V, Feikert T, Opanashuk L, et al. 2006. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ. Health Perspect 114:1172–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ensunsa JL, Symons JD, Lanoue L, Schrader HR, Keen CL. 2004. Reducing arginase activity via dietary manganese deficiency enhances endothelium-dependent vasorelaxation of rat aorta. Exp. Biol. Med 229:1143–53 [DOI] [PubMed] [Google Scholar]
- 83.Ergen K, Ince H, Duzova H, Karakoc Y, Emre MH. 2013. Acute effects of moderate and strenuous running on trace element distribution in the brain, liver, and spleen of trained rats. Balkan Med. J 30:105–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Erikson K, Aschner M. 2002. Manganese causes differential regulation of glutamate transporter (GLAST) taurine transporter and metallothionein in cultured rat astrocytes. Neurotoxicology 23:595–602 [DOI] [PubMed] [Google Scholar]
- 85.Erikson KM, Suber RL, Aschner M. 2002. Glutamate/aspartate transporter (GLAST), taurine transporter and metallothionein mRNA levels are differentially altered in astrocytes exposed to manganese chloride, manganese phosphate or manganese sulfate. Neurotoxicology 23:281–88 [DOI] [PubMed] [Google Scholar]
- 86.Esch F, Lin K, Hills A, Baraban JM, Chatterjee S, et al. 1998. Purification of a multipotent antideath activity from bovine liver and its identification as arginase: nitric oxide-independent inhibition of neuronal apoptosis. J. Neurosci 18:4083–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Estevez AG, Sahawneh MA, Lange PS, Bae N, Egea M, Ratan RR. 2006. Arginase 1 regulation of nitric oxide production is key to survival of trophic factor-deprived motor neurons. J. Neurosci 26:8512–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eum JH, Cheong HK, Ha EH, Ha M, Kim Y, et al. 2014. Maternal blood manganese level and birth weight: a MOCEH birth cohort study. Environ. Health 13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fallon EM, Le HD, Puder M. 2010. Prevention of parenteral nutrition-associated liver disease: role of omega-3 fish oil. Curr. Opin. Organ Transplant 15:334–40 [DOI] [PubMed] [Google Scholar]
- 90.Ferlazzo ML, Sonzogni L, Granzotto A, Bodgi L, Lartin O, et al. 2014. Mutations of the Huntington’s disease protein impact on the ATM-dependent signaling and repair pathways of the radiation-induced DNA double-strand breaks: corrective effect of statins and bisphosphonates. Mol. Neurobiol 49:1200–11 [DOI] [PubMed] [Google Scholar]
- 91.Filipov NM, Seegal RF, Lawrence DA. 2005. Manganese potentiates in vitro production of proinflammatory cytokines and nitric oxide by microglia through a nuclear factor kappa B-dependent mechanism. Toxicol. Sci 84:139–48 [DOI] [PubMed] [Google Scholar]
- 92.Finkelstein Y, Zhang N, Fitsanakis VA, Avison MJ, Gore JC, Aschner M. 2008. Differential deposition of manganese in the rat brain following subchronic exposure to manganese: a T1-weighted magnetic resonance imaging study. Israel Med. Assoc. J 10:793–98 [PMC free article] [PubMed] [Google Scholar]
- 93.Finley JW, Johnson PE, Johnson LK. 1994. Sex affects manganese absorption and retention by humans from a diet adequate in manganese. Am. J. Clin. Nutr 60:949–55 [DOI] [PubMed] [Google Scholar]
- 94.Fitsanakis VA, Piccola G, Aschner JL, Aschner M. 2006. Characteristics of manganese (Mn) transport in rat brain endothelial (RBE4) cells, an in vitro model of the blood-brain barrier. Neurotoxicology 27:60–70 [DOI] [PubMed] [Google Scholar]
- 95.Fitsanakis VA, Piccola G, Aschner JL, Aschner M. 2005. Manganese transport by rat brain endothelial (RBE4) cell-based Transwell model in the presence of astrocyte conditioned media. J. Neurosci. Res 81:235–43 [DOI] [PubMed] [Google Scholar]
- 96.Fitsanakis VA, Zhang N, Anderson JG, Erikson KM, Avison MJ, et al. 2008. Measuring brain manganese and iron accumulation in rats following 14 weeks of low-dose manganese treatment using atomic absorption spectroscopy and magnetic resonance imaging. Toxicol. Sci 103:116–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fitsanakis VA, Zhang N, Avison MJ, Erikson KM, Gore JC, Aschner M. 2011. Changes in dietary iron exacerbate regional brain manganese accumulation as determined by magnetic resonance imaging. Toxicol. Sci 120:146–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. 1998. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. PNAS 95:1148–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Foradori AC, Bertinchamps A, Gulibon JM, Cotzias GC. 1967. The discrimination between magnesium and manganese by serum proteins. J. Gen. Physiol 50:2255–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Forbes JR, Gros P. 2003. Iron, manganese, and cobalt transport by Nramp1 (Slc11a1) and Nramp2 (Slc11a2) expressed at the plasma membrane. Blood 102:1884–92 [DOI] [PubMed] [Google Scholar]
- 101.Fordahl SC, Anderson JG, Cooney PT, Weaver TL, Colyer CL, Erikson KM. 2010. Manganese exposure inhibits the clearance of extracellular GABA and influences taurine homeostasis in the striatum of developing rats. Neurotoxicology 31:639–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Frankel D 1993. Supplementation of trace elements in parenteral nutrition: rationale and recommendations. Nutr. Res 13:583–96 [Google Scholar]
- 103.Friedman BJ, Freeland-Graves JH, Bales CW, Behmardi F, Shorey-Kutschke RL, et al. 1987. Manganese balance and clinical observations in young men fed a manganese-deficient diet. J. Nutr 117:133–43 [DOI] [PubMed] [Google Scholar]
- 104.Fujishiro H, Ohashi T, Takuma M, Himeno S. 2013. Suppression of ZIP8 expression is a common feature of cadmium-resistant and manganese-resistant RBL-2H3 cells. Metallomics 5:437–44 [DOI] [PubMed] [Google Scholar]
- 105.Fujishiro H, Yoshida M, Nakano Y, Himeno S. 2014. Interleukin-6 enhances manganese accumulation in SH-SY5Y cells: implications of the up-regulation of ZIP14 and the down-regulation of ZnT10. Metallomics 6:944–49 [DOI] [PubMed] [Google Scholar]
- 106.Garcia-Aranda JA, Wapnir RA, Lifshitz F. 1983. In vivo intestinal absorption of manganese in the rat.J. Nutr 113:2601–7 [DOI] [PubMed] [Google Scholar]
- 107.Garcia SJ, Gellein K, Syversen T, Aschner M. 2007. Iron deficient and manganese supplemented diets alter metals and transporters in the developing rat brain. Toxicol. Sci 95:205–14 [DOI] [PubMed] [Google Scholar]
- 108.Garrick MD, Dolan KG, Hobinski C, Ghio AJ, Higgins D, et al. 2003. DMT1: a mammalian transporter for multiple metals. Biometals 16:41–54 [DOI] [PubMed] [Google Scholar]
- 109.Gavin CE, Gunter KK, Gunter TE. 1990. Manganese and calcium efflux kinetics in brain mitochondria. Biochem. J 266:329–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gitler AD, Chesi A, Geddie ML, Strathearn KE, Hamamichi S, et al. 2009. Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat. Genet 41:308–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gorovitz RA, Avisar N, Shaked I, Vardimon L. 1997. Glutamine synthetase protects against neuronal degeneration in injured retinal tissue. PNAS 94:7024–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Goytain A, Hines RM, Quamme GA. 2008. Huntingtin-interacting proteins, HIP14 and HIP14L, mediate dual functions, palmitoyl acyltransferase and Mg2+ transport. J. Biol. Chem 283:33365–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Graham MF, Tavill AS, Halpin TC, Louis LN. 1984. Inhibition of bile flow in the isolated perfused rat liver by a synthetic parenteral amino acid mixture: associated net amino acid fluxes. Hepatology 4:69–73 [DOI] [PubMed] [Google Scholar]
- 114.Greger JL. 1998. Dietary standards for manganese: overlap between nutritional and toxicological studies.J. Nutr 128:368–71S [DOI] [PubMed] [Google Scholar]
- 115.Grimm C, Kraft R, Sauerbruch S, Schultz G, Harteneck C. 2003. Molecular and functional characterization of the melastatin-related cation channel TRPM3. J. Biol. Chem 278:21493–501 [DOI] [PubMed] [Google Scholar]
- 116.Gruenheid S, Canonne-Hergaux F, Gauthier S, Hackam DJ, Grinstein S, Gros P. 1999. The iron transport protein NRAMP2 is an integral membrane glycoprotein that colocalizes with transferrin in recycling endosomes. J. Exp. Med 189:831–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guilarte TR. 2010. APLP1, Alzheimer’s-like pathology and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. Neurotoxicology 31:572–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guilarte TR, Burton NC, Verina T, Prabhu VV, Becker KG, et al. 2008. Increased APLP1 expression and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. J. Neurochem 105:1948–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Guilarte TR, Chen MK, McGlothan JL, Verina T, Wong DF, et al. 2006. Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Exp. Neurol 202:381–90 [DOI] [PubMed] [Google Scholar]
- 120.Guilbert A, Gautier M, Dhennin-Duthille I, Haren N, Sevestre H, Ouadid-Ahidouch H. 2009. Evidence that TRPM7 is required for breast cancer cell proliferation. Am. J. Physiol. Cell Physiol 297:C493–502 [DOI] [PubMed] [Google Scholar]
- 121.Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, et al. 1997. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 338:482–88 [DOI] [PubMed] [Google Scholar]
- 122.Gunter TE, Gavin CE, Aschner M, Gunter KK. 2006. Speciation of manganese in cells and mitochondria: a search for the proximal cause of manganese neurotoxicity. Neurotoxicology 27:765–76 [DOI] [PubMed] [Google Scholar]
- 123.Gunter TE, Gerstner B, Gunter KK, Malecki J, Gelein R, et al. 2013. Manganese transport via the transferrin mechanism. Neurotoxicology 34:118–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gunteski Hamblin AM, Clarke DM, Shull GE. 1992. Molecular cloning and tissue distribution of alternatively spliced mRNAs encoding possible mammalian homologues of the yeast secretory pathway calcium pump. Biochemistry 31:7600–8 [DOI] [PubMed] [Google Scholar]
- 125.Guo ZK, Kozlov S, Lavin MF, Person MD, Paull TT. 2010. ATM activation by oxidative stress. Science 330:517–21 [DOI] [PubMed] [Google Scholar]
- 126.Hansen SL, Trakooljul N, Liu HC, Moeser AJ, Spears JW. 2009. Iron transporters are differentially regulated by dietary iron, and modifications are associated with changes in manganese metabolism in young pigs. J. Nutr 139:1474–79 [DOI] [PubMed] [Google Scholar]
- 127.Hare DJ, George JL, Grimm R, Wilkins S, Adlard PA, et al. 2010. Three-dimensional elemental bioimaging of Fe, Zn, Cu, Mn and P in a 6-hydroxydopamine lesioned mouse brain. Metallomics 2:745–53 [DOI] [PubMed] [Google Scholar]
- 128.Harris WR, Chen Y. 1994. Electron paramagnetic resonance and difference ultraviolet studies of Mn2+ binding to serum transferrin. J. Inorg. Biochem 54:1–19 [DOI] [PubMed] [Google Scholar]
- 129.Hassel B, Bachelard H, Jones P, Fonnum F, Sonnewald U. 1997. Trafficking of amino acids between neurons and glia in vivo. Effects of inhibition of glial metabolism by fluoroacetate. J. Cereb. Blood Flow Metab 17:1230–38 [DOI] [PubMed] [Google Scholar]
- 130.He L, Girijashanker K, Dalton TP, Reed J, Li H, et al. 2006. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: characterization of transporter properties. Mol. Pharmacol 70:171–80 [DOI] [PubMed] [Google Scholar]
- 131.Herrero Hernandez E, Discalzi G, Valentini C, Venturi F, Chio A, et al. 2006. Follow-up of patients affected by manganese-induced Parkinsonism after treatment with CaNa2EDTA. Neurotoxicology 27:333–39 [DOI] [PubMed] [Google Scholar]
- 132.Hertz LY, Yu A, Svenneby G, Kvamme E, Fosmark H, Schousboe A. 1980. Absence of preferential glutamine uptake into neurons—an indication of a net transfer of TCA constituents from nerve endings to astrocytes? Neurosci. Lett 16:103–9 [DOI] [PubMed] [Google Scholar]
- 133.Higashi Y, Asanuma M, Miyazaki I, Hattori N, Mizuno Y, Ogawa N. 2004. Parkin attenuates manganeseinduced dopaminergic cell death. J. Neurochem 89:1490–97 [DOI] [PubMed] [Google Scholar]
- 134.Hirata Y, Furuta K, Miyazaki S, Suzuki M, Kiuchi K. 2004. Anti-apoptotic and pro-apoptotic effect of NEPP11 on manganese-induced apoptosis and JNK pathway activation in PC12 cells. Brain Res 1021:241–47 [DOI] [PubMed] [Google Scholar]
- 135.Hopfner K, Karcher A, Craig L, Woo TT, Carney JP, Tainer JA. 2001. Structural biochemistry and interaction architecture of the DNA double-strand break repair Mre11 nuclease and Rad50-ATPase. Cell 105:473–85 [DOI] [PubMed] [Google Scholar]
- 136.Hozumi I, Hasegawa T, Honda A, Ozawa K, Hayashi Y, et al. 2011. Patterns of levels of biological metals in CSF differ among neurodegenerative diseases. J. Neurol. Sci 303:95–99 [DOI] [PubMed] [Google Scholar]
- 137.Huang E, Ong WY, Connor JR. 2004. Distribution of divalent metal transporter-1 in the monkey basal ganglia. Neuroscience 128:487–96 [DOI] [PubMed] [Google Scholar]
- 138.Huang K, Sanders SS, Kang R, Carroll JB, Sutton L, et al. 2011. Wild-type HTT modulates the enzymatic activity of the neuronal palmitoyl transferase HIP14. Hum. Mol. Genet 20:3356–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Huang K, Yanai A, Kang R, Arstikaitis P, Singaraja RR, et al. 2004. Huntingtin-interacting protein HIP14 is a palmitoyl transferase involved in palmitoylation and trafficking of multiple neuronal proteins. Neuron 44:977–86 [DOI] [PubMed] [Google Scholar]
- 140.Iinuma Y, Kubota M, Uchiyama M, Yagi M, Kanada S, et al. 2003. Whole-blood manganese levels and brain manganese accumulation in children receiving long-term home parenteral nutrition. Pediatr. Surg. Int 19:268–72 [DOI] [PubMed] [Google Scholar]
- 141.Jursa T, Smith DR. 2009. Ceruloplasmin alters the tissue disposition and neurotoxicity of manganese, but not its loading onto transferrin. Toxicol. Sci 107:182–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kalia K, Jiang W, Zheng W. 2008. Manganese accumulates primarily in nuclei of cultured brain cells. Neurotoxicology 29:466–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kannurpatti SS, Joshi PG, Joshi NB. 2000. Calcium sequestering ability of mitochondria modulates influx of calcium through glutamate receptor channel. Neurochem. Res 25:1527–36 [DOI] [PubMed] [Google Scholar]
- 144.Kanyo ZF, Scolnick LR, Ash DE, Christianson DW. 1996. Structure of a unique binuclear manganese cluster in arginase. Nature 383:554–57 [DOI] [PubMed] [Google Scholar]
- 145.Keefer RC, Barak AJ, Boyett JD. 1970. Binding of manganese and transferrin in rat serum. Biochim. Biophys. Acta 221:390–93 [DOI] [PubMed] [Google Scholar]
- 146.Keen CL, Bell JG, Lonnerdal B. 1986. The effect of age on manganese uptake and retention from milk and infant formulas in rats. J. Nutr 116:395–402 [DOI] [PubMed] [Google Scholar]
- 147.Keen CL, Ensunsa JL, Watson MH, Baly DL, Donovan SM, et al. 1999. Nutritional aspects of manganese from experimental studies. Neurotoxicology 20:213–23 [PubMed] [Google Scholar]
- 148.Keller JN, Kindy MS, Holtsberg FW, St Clair DK, Yen HC, et al. 1998. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J. Neurosci 18:687–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kobayashi K, Kuroda J, Shibata N, Hasegawa T, Seko Y, et al. 2007. Induction of metallothionein by manganese is completely dependent on interleukin-6 production. J. Pharmacol. Exp. Ther 320:721–27 [DOI] [PubMed] [Google Scholar]
- 150.Kong SM, Chan BK, Park JS, Hill KJ, Aitken JB, et al. 2014. Parkinson’s disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes α-Synuclein externalization via exosomes. Hum. Mol. Genet 23:2816–33 [DOI] [PubMed] [Google Scholar]
- 151.Kozlov S 2003. ATP activates ataxia-telangiectasia mutated (ATM) in vitro. Importance of autophosphorylation. J. Biol. Chem 278:9309–17 [DOI] [PubMed] [Google Scholar]
- 152.Krishna S, Dodd CA, Hekmatyar SK, Filipov NM. 2014. Brain deposition and neurotoxicity of manganese in adult mice exposed via the drinking water. Arch. Toxicol 88:47–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kulijewicz-Nawrot M, Sykova E, Chvatal A, Verkhratsky A, Rodriguez JJ. 2013. Astrocytes and glutamate homoeostasis in Alzheimer’s disease: a decrease in glutamine synthetase, but not in glutamate transporter1, in the prefrontal cortex. ASN Neuro 5:273–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kumar KK, Lowe EW Jr, Aboud AA, Neely MD, Redha R, et al. 2014. Cellular manganese content is developmentally regulated in human dopaminergic neurons. Sci. Rep 4:6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kuo HS, Tsai MJ, Huang MC, Chiu CW, Tsai CY, et al. 2011. Acid fibroblast growth factor and peripheral nerve grafts regulate Th2 cytokine expression, macrophage activation, polyamine synthesis, and neurotrophin expression in transected rat spinal cords. J. Neurosci 31:4137–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lawson LJ, Perry VH, Dri P, Gordon S. 1990. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39:151–70 [DOI] [PubMed] [Google Scholar]
- 157.Leblondel G, Allain P. 1999. Manganese transport by Caco-2 cells. Biol. Trace Element Res 67:13–28 [DOI] [PubMed] [Google Scholar]
- 158.Lebovitz RM, Zhang H, Vogel H, Cartwright JJ, Dionne L, et al. 1996. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. PNAS 93:9782–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lechpammer M, Clegg MS, Muzar Z, Huebner PA, Jin LW, Gospe SM Jr. 2014. Pathology of inherited manganese transporter deficiency. Ann. Neurol 75:608–12 [DOI] [PubMed] [Google Scholar]
- 160.Lee J, Ryu H, Ferrante RJ, Morris SM Jr, Ratan RR. 2003. Translational control of inducible nitric oxide synthase expression by arginine can explain the arginine paradox. PNAS 100:4843–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Leffler CT, Philippi AF, Leffler SG, Mosure JC, Kim PD. 1999. Glucosamine, chondroitin, and manganese ascorbate for degenerative joint disease of the knee or low back: a randomized, double-blind, placebo-controlled pilot study. Mil. Med 164:85–91 [PubMed] [Google Scholar]
- 162.Leggett RW. 2011. A biokinetic model for manganese. Sci. Total Environ 409:4179–86 [DOI] [PubMed] [Google Scholar]
- 163.Lehmann C, Bette S, Engele J. 2009. High extracellular glutamate modulates expression of glutamate transporters and glutamine synthetase in cultured astrocytes. Brain Res 1297:1–8 [DOI] [PubMed] [Google Scholar]
- 164.Leitch S, Feng M, Muend S, Braiterman LT, Hubbard AL, Rao R. 2011. Vesicular distribution of secretory pathway Ca2+-ATPase isoform 1 and a role in manganese detoxification in liver-derived polarized cells. Biometals 24:159–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Leyva-Illades D, Chen P, Zogzas CE, Hutchens S, Mercado JM, et al. 2014. SLC30A10 is a cell surfacelocalized manganese efflux transporter, and Parkinsonism-causing mutations block its intracellular trafficking and efflux activity. J. Neurosci 34:14079–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li DW, Liu JP, Schmid PC, Schlosser R, Feng H, et al. 2006. Protein serine/threonine phosphatase-1 dephosphorylates p53 at Ser-15 and Ser-37 to modulate its transcriptional and apoptotic activities. Oncogene 25:3006–22 [DOI] [PubMed] [Google Scholar]
- 167.Li X, Xie J, Lu L, Zhang L, Zhang L, et al. 2013. Kinetics of manganese transport and gene expressions of manganese transport carriers in Caco-2 cell monolayers. Biometals 26:941–53 [DOI] [PubMed] [Google Scholar]
- 168.Liccione JJ, Maines MD. 1988. Selective vulnerability of glutathione metabolism and cellular defense mechanisms in rat striatum to manganese. J. Pharmacol. Exp. Ther 247:156–61 [PubMed] [Google Scholar]
- 169.Limbo O, Moiani D, Kertokalio A, Wyman C, Tainer JA, Russell P. 2012. Mre11 ATLD17/18 mutation retains Tel1/ATM activity but blocks DNA double-strand break repair. Nucleic Acids Res 40:11435–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu M, Cai T, Zhao F, Zheng G, Wang Q, et al. 2009. Effect of microglia activation on dopaminergic neuronal injury induced by manganese, and its possible mechanism. Neurotox. Res 16:42–49 [DOI] [PubMed] [Google Scholar]
- 171.Ljung K, Palm B, Grander M, Vahter M. 2011. High concentrations of essential and toxic elements in infant formula and infant foods—a matter of concern. Food Chem 127:943–51 [DOI] [PubMed] [Google Scholar]
- 172.Lockman PR, Roder KE, Allen DD. 2001. Inhibition of the rat blood brain barrier choline transporter by manganese chloride. J. Neurochem 79:588–94 [DOI] [PubMed] [Google Scholar]
- 173.Lonnerdal B 1994. Nutritional aspects of soy formula. Acta Paediatr. Suppl 402:105–8 [DOI] [PubMed] [Google Scholar]
- 174.Loranger S, Zayed J. 1997. Environmental contamination and human exposure to airborne total and respirable manganese in Montreal. J. Air Waste Manag. Assoc 47:983–89 [DOI] [PubMed] [Google Scholar]
- 175.Loutzenhiser K, Loutzenhiser R. 2000. Angiotensin II-induced Ca2+ influx in renal afferent and efferent arterioles differing roles of voltage gated and store operated Ca2+ entry. Circ. Res 87:551–57 [DOI] [PubMed] [Google Scholar]
- 176.Lucaciu CM, Dragu C, Copăescu L, Morariu VV. 1997. Manganese transport through human erythrocyte membranes. An EPR study. Biochim. Biophys. Acta 1328:90–98 [DOI] [PubMed] [Google Scholar]
- 177.Madejczyk MS, Ballatori N. 2012. The iron transporter ferroportin can also function as a manganese exporter. Biochim. Biophys. Acta 1818:651–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Madison JL, Wegrzynowicz M, Aschner M, Bowman AB. 2012. Disease-toxicant interactions in manganese exposed Huntington disease mice: early changes in striatal neuron morphology and dopamine metabolism. PLOS ONE 7:e31024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Marion S, Urs NM, Peterson SM, Sotnikova TD, Beaulieu JM, et al. 2014. Dopamine D2 receptor relies upon PPM/PP2C protein phosphatases to dephosphorylate huntingtin protein. J. Biol. Chem 289:11715–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Masuda M, Braun-Sommargren M, Crooks D, Smith DR. 2013. Golgi phosphoprotein 4 (GPP130) is a sensitive and selective cellular target of manganese exposure. Synapse 67:205–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.McCarthy RC, Kosman DJ. 2012. Mechanistic analysis of iron accumulation by endothelial cells of the BBB. Biometals 25:665–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.McDougall SA, Der-Ghazarian T, Britt CE, Varela FA, Crawford CA. 2011. Postnatal manganese exposure alters the expression of D2L and D2S receptor isoforms: relationship to PKA activity and Akt levels. Synapse 65:583–91 [DOI] [PubMed] [Google Scholar]
- 183.Institute of Medicine. 2001. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc Washington, DC: Natl. Acad. Press; [PubMed] [Google Scholar]
- 184.Micaroni M, Perinetti G, Berrie CP, Mironov AA. 2010. The SPCA1 Ca2+ pump and intracellular membrane trafficking. Traffic 11:1315–33 [DOI] [PubMed] [Google Scholar]
- 185.Michalke B, Berthele A, Mistriotis P, Ochsenkuhn-Petropoulou M, Halbach S. 2007. Manganese species from human serum, cerebrospinal fluid analyzed by size exclusion chromatography-, capillary electrophoresis coupled to inductively coupled plasma mass spectrometry. J. Trace Elem. Med. Biol 21(Suppl.1):4–9 [DOI] [PubMed] [Google Scholar]
- 186.Midvan AS, Scrutton MC, Utter MF. 1966. Pyruvate carboxylase: VII. A possible role for tightly bound manganese. J. Biol. Chem 241:3488–98 [PubMed] [Google Scholar]
- 187.Milatovic D, Yin Z, Gupta RC, Sidoryk M, Albrecht J, et al. 2007. Manganese induces oxidative impairment in cultured rat astrocytes. Toxicol. Sci 98:198–205 [DOI] [PubMed] [Google Scholar]
- 188.Mishra G, Shukla R, Hasan M, Khanna SK, Das M. 2009. Potentiation of neurotoxicity of Lathyrus sativus by manganese: alterations in blood-brain barrier permeability. Toxicol. Mech. Methods 19:318–26 [DOI] [PubMed] [Google Scholar]
- 189.Missiaen L, Raeymaekers L, Dode L, Vanoevelen J, Van Baelen K, et al. 2004. SPCA1 pumps and Hailey-Hailey disease. Biochem. Biophys. Res. Commun 322:1204–13 [DOI] [PubMed] [Google Scholar]
- 190.Mitchell CJ, Shawki A, Ganz T, Nemeth E, Mackenzie B. 2014. Functional properties of human ferroportin, a cellular iron exporter reactive also with cobalt and zinc. Am. J. Physiol. Cell Physiol 306:C450–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mizuno K, Whittaker MM, Bachinger HP, Whittaker JW. 2004. Calorimetric studies on the tight binding metal interactions of Escherichia coli manganese superoxide dismutase. J. Biol. Chem 279:27339–44 [DOI] [PubMed] [Google Scholar]
- 192.Moberly AH, Czarnecki LA, Pottackal J, Rubinstein T, Turkel DJ, et al. 2012. Intranasal exposure to manganese disrupts neurotransmitter release from glutamatergic synapses in the central nervous system in vivo. Neurotoxicology 33:996–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mochel F, DeLonlay P, Touati G, Brunengraber H, Kinman RP, et al. 2005. Pyruvate carboxylase deficiency: clinical and biochemical response to anaplerotic diet therapy. Mol. Genet. Metab 84:305–12 [DOI] [PubMed] [Google Scholar]
- 194.Morello M, Canini A, Mattioli P, Sorge RP, Alimonti A, et al. 2008. Sub-cellular localization of manganese in the basal ganglia of normal and manganese-treated rats: an electron spectroscopy imaging and electron energy-loss spectroscopy study. Neurotoxicology 29:60–72 [DOI] [PubMed] [Google Scholar]
- 195.Morello M, Zatta P, Zambenedetti P, Martorana A, D’Angelo V, et al. 2007. Manganese intoxication decreases the expression of manganoproteins in the rat basal ganglia: an immunohistochemical study. Brain Res. Bull 74:406–15 [DOI] [PubMed] [Google Scholar]
- 196.Mukhopadhyay S, Bachert C, Smith DR, Linstedt AD. 2010. Manganese-induced trafficking and turnover of the cis-Golgi glycoprotein GPP130. Mol. Biol. Cell 21:1282–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mukhopadhyay S, Linstedt AD. 2011. Identification of a gain-of-function mutation in a Golgi P-type ATPase that enhances Mn2+ efflux and protects against toxicity. PNAS 108:858–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Murin R, Verleysdonk S, Raeymaekers L, Kaplan P, Lehotsky J. 2006. Distribution of secretory pathway Ca2+ ATPase (SPCA1) in neuronal and glial cell cultures. Cell. Mol. Neurobiol 26:1355–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Napolitano M, Centonze D, Gubellini P, Rossi S, Spiezia S, et al. 2004. Inhibition of mitochondrial complex II alters striatal expression of genes involved in glutamatergic and dopaminergic signaling: possible implications for Huntington’s disease. Neurobiol. Dis 15:407–14 [DOI] [PubMed] [Google Scholar]
- 200.Nischwitz V, Berthele A, Michalke B. 2008. Speciation analysis of selected metals and determination of their total contents in paired serum and cerebrospinal fluid samples: an approach to investigate the permeability of the human blood-cerebrospinal fluid-barrier. Anal. Chim. Acta 627:258–69 [DOI] [PubMed] [Google Scholar]
- 201.Noone ML, Kumar P, Ummer K, Achambat L, Salam KA. 2008. Cirrhosis presenting as Parkinsonism. Ann. Indian Acad. Neurol 11:179–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Norenberg MD. 1979. Distribution of glutamine synthetase in the rat central nervous system. J. Histochem. Cytochem 27:756–62 [DOI] [PubMed] [Google Scholar]
- 203.Ohyama T, Verstreken P, Ly CV, Rosenmund T, Rajan A, et al. 2007. Huntingtin-interacting protein 14, a palmitoyl transferase required for exocytosis and targeting of CSP to synaptic vesicles. J. Cell Biol 179:1481–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Okunade GW, Miller ML, Azhar M, Andringa A, Sanford LP, et al. 2007. Loss of the Atp2c1 secretory pathway Ca2+-ATPase (SPCA1) in mice causes Golgi stress, apoptosis, and midgestational death in homozygous embryos and squamous cell tumors in adult heterozygotes. J. Biol. Chem 282:26517–27 [DOI] [PubMed] [Google Scholar]
- 205.Olabarria M, Noristani HN, Verkhratsky A, Rodriguez JJ. 2011. Age-dependent decrease in glutamine synthetase expression in the hippocampal astroglia of the triple transgenic Alzheimer’s disease mouse model: mechanism for deficient glutamatergic transmission? Mol. Neurodegener 6:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Olanow CW, Good PF, Shinotoh H, Hewitt KA, Vingerhoets F, et al. 1996. Manganese intoxication in the rhesus monkey: a clinical, imaging, pathologic, and biochemical study. Neurology 46:492–98 [DOI] [PubMed] [Google Scholar]
- 207.Parenti M, Rusconi L, Cappabianca V, Parati EA, Groppetti A. 1988. Role of dopamine in manganese neurotoxicity. Brain Res 473:236–40 [DOI] [PubMed] [Google Scholar]
- 208.Paull TT, Gellert M. 1998. The 3 to 5 exonuclease activity of Mre11 facilitates repair of DNA doublestrand breaks. Mol. Cell 1:969–79 [DOI] [PubMed] [Google Scholar]
- 209.Pellizzari ED, Clayton CA, Rodes CE, Mason RE, Piper LL, et al. 2001. Particulate matter and manganese exposures in Indianapolis, Indiana. J. Expo. Anal. Environ. Epidemiol 11:423–40 [DOI] [PubMed] [Google Scholar]
- 210.Pellizzari ED, Clayton CA, Rodes CE, Mason RE, Piper LL, et al. 1999. Particulate matter and manganese exposures in Toronto, Canada. Atmos. Environ 33:721–34 [DOI] [PubMed] [Google Scholar]
- 211.Penland JG, Johnson PE. 1993. Dietary calcium and manganese effects on menstrual cycle symptoms. Am. J. Obstet. Gynecol 168:1417–23 [DOI] [PubMed] [Google Scholar]
- 212.Prabhakaran K, Ghosh D, Chapman GD, Gunasekar PG. 2008. Molecular mechanism of manganese exposure-induced dopaminergic toxicity. Brain Res. Bull 76:361–67 [DOI] [PubMed] [Google Scholar]
- 213.Price CT, Langford JR, Liporace FA. 2012. Essential nutrients for bone health and a review of their availability in the average North American diet. Open Orthop. J 6:143–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Puri S, Bachert C, Fimmel CJ, Linstedt AD. 2002. Cycling of early Golgi proteins via the cell surface and endosomes upon lumenal pH disruption. Traffic 3:641–53 [DOI] [PubMed] [Google Scholar]
- 215.Quadri M, Federico A, Zhao T, Breedveld GJ, Battisti C, et al. 2012. Mutations in SLC30A10 cause parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. Am. J. Hum. Genet 90:467–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Quamme GA. 2010. Molecular identification of ancient and modern mammalian magnesium transporters. Am. J. Physiol. Cell Physiol 298:C407–29 [DOI] [PubMed] [Google Scholar]
- 217.Rabin O, Hegedus L, Bourre J, Smith QR. 1993. Rapid brain uptake of manganese(II) across the bloodbrain barrier. J. Neurochem 61:509–17 [DOI] [PubMed] [Google Scholar]
- 218.Racette BA, Antenor JA, McGee-Minnich L, Moerlein SM, Videen TO, et al. 2005. [18F]FDOPA PET and clinical features in parkinsonism due to manganism. Mov. Disord 20:492–96 [DOI] [PubMed] [Google Scholar]
- 219.Racette BA, Criswell SR, Lundin JI, Hobson A, Seixas N, et al. 2012. Increased risk of parkinsonism associated with welding exposure. Neurotoxicology 33:1356–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Racette BA, McGee-Minnich L, Moerlein SM, Mink JW, Videen TO, Perimutter JS. 2001. Weldingrelated parkinsonism. Neurology 56:8–12 [DOI] [PubMed] [Google Scholar]
- 221.Ramirez A, Heimbach A, Grundemann J, Stiller B, Hampshire D, et al. 2006. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat. Genet 38:1184–91 [DOI] [PubMed] [Google Scholar]
- 222.Ramonet D, Podhajska A, Stafa K, Sonnay S, Trancikova A, et al. 2012. PARK9-associated ATP13A2 localizes to intracellular acidic vesicles and regulates cation homeostasis and neuronal integrity. Hum. Mol. Genet 21:1725–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ramos P, Santos A, Pinto NR, Mendes R, Magalhaes T, Almeida A. 2014. Anatomical region differences and age-related changes in copper, zinc, and manganese levels in the human brain. Biol. Trace Element Res 161:190–201 [DOI] [PubMed] [Google Scholar]
- 224.Reaney SH, Bench G, Smith DR. 2006. Brain accumulation and toxicity of Mn(II) and Mn(III) exposures. Toxicol. Sci 93:114–24 [DOI] [PubMed] [Google Scholar]
- 225.Reaney SH, Kwik-Uribe CL, Smith DR. 2002. Manganese oxidation state and its implications for toxicity. Chem. Res. Toxicol 15:1119–26 [DOI] [PubMed] [Google Scholar]
- 226.Reinhardt TA, Lippolis JD, Shull GE, Horst RL. 2004. Null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2 impairs calcium transport into milk. J. Biol. Chem 279:42369–73 [DOI] [PubMed] [Google Scholar]
- 227.Rentschler G, Covolo L, Haddad AA, Lucchini RG, Zoni S, Broberg K. 2012. ATP13A2 (PARK9) polymorphisms influence the neurotoxic effects of manganese. Neurotoxicology 33:697–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Riccio A, Mattei C, Kelsell RE, Medhurst AD, Calver AR, et al. 2002. Cloning and functional expression of human short TRP7, a candidate protein for store-operated Ca2+ influx. J. Biol. Chem 277:12302–9 [DOI] [PubMed] [Google Scholar]
- 229.Robinson SR. 2000. Neuronal expression of glutamine synthetase in Alzheimer’s disease indicates a profound impairment of metabolic interactions with astrocytes. Neurochem. Int 36:471–82 [DOI] [PubMed] [Google Scholar]
- 230.Robison G, Zakharova T, Fu S, Jiang W, Fulper R, et al. 2012. X-ray fluorescence imaging: a new tool for studying manganese neurotoxicity. PLOS ONE 7:e48899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Robison G, Zakharova T, Fu S, Jiang W, Fulper R, et al. 2013. X-ray fluorescence imaging of the hippocampal formation after manganese exposure. Metallomics 5:1554–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Rodriguez-Rodriguez E, Bermejo LM, Lopez-Sobaler AM, Ortega RM. 2011. An inadequate intake of manganese may favour insulin resistance in girls. Nutr. Hosp 26:965–70 [DOI] [PubMed] [Google Scholar]
- 233.Roels H, Lauwerys R, Buchet JP, Genet P, Sarhan MJ, et al. 1987. Epidemiological survey among workers exposed to manganese: effects on lung, central nervous system, and some biological indices. Am. J. Ind. Med 11:307–27 [DOI] [PubMed] [Google Scholar]
- 234.Rosas HD, Chen YI, Doros G, Salat DH, Chen NK, et al. 2012. Alterations in brain transition metals in Huntington disease: an evolving and intricate story. Arch. Neurol 69:887–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Roth J, Ponzoni S, Aschner M. 2013. Manganese homeostasis and transport. Met. Ions Life Sci 12:169–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Roth JA. 2006. Homeostatic and toxic mechanisms regulating manganese uptake, retention, and elimination. Biol. Res 39:45–57 [DOI] [PubMed] [Google Scholar]
- 237.Roth JA, Ganapathy B, Ghio AJ. 2012. Manganese-induced toxicity in normal and human B lymphocyte cell lines containing a homozygous mutation in parkin. Toxicol. In Vitro 26:1143–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Roth JA, Li Z, Sridhar S, Khoshbouei H. 2013. The effect of manganese on dopamine toxicity and dopamine transporter (DAT) in control and DAT transfected HEK cells. Neurotoxicology 35:121–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Roth JA, Singleton S, Feng J, Garrick M, Paradkar PN. 2010. Parkin regulates metal transport via proteasomal degradation of the 1B isoforms of divalent metal transporter 1. J. Neurochem 113:454–64 [DOI] [PubMed] [Google Scholar]
- 240.Sanders SS, Mui KK, Sutton LM, Hayden MR. 2014. Identification of binding sites in Huntingtin for the Huntingtin interacting proteins HIP14 and HIP14L. PLOS ONE 9:e90669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Santos D, Batoreu C, Mateus L, Marreilha Dos Santos AP, Aschner M. 2014. Manganese in human parenteral nutrition: considerations for toxicity and biomonitoring. Neurotoxicology 43:36–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sax HC, Talamini MA, Brackett K, Fischer JE. 1986. Hepatic steatosis in total parenteral nutrition: failure of fatty infiltration to correlate with abnormal serum hepatic enzyme levels. Surgery 100:697–704 [PubMed] [Google Scholar]
- 243.Scheuhammer AM, Cherian MG. 1985. Binding of manganese in human and rat plasma. Biochim. Biophys. Acta 840:163–69 [DOI] [PubMed] [Google Scholar]
- 244.Schmidt K, Wolfe DM, Stiller B, Pearce DA. 2009. Cd2+, Mn2+, Ni2+ and Se2+ toxicity to Saccharomyces cerevisiae lacking YPK9p the orthologue of human ATP13A2. Biochem. Biophys. Res. Commun 383:198–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Schroeder HA, Balassa JJ, Tipton IH. 1996. Essential trace metals in man: manganese, a study in homeostasis. J. Chron. Dis 19:545–71 [DOI] [PubMed] [Google Scholar]
- 246.Schulz JB, Matthews RT, Klockgether T, Dichgans J, Beal MF. 1997. The role of mitochondrial dysfunction and neuronal nitric oxide in animal models of neurodegenerative diseases. Mol. Cell Biochem 174:193–97 [PubMed] [Google Scholar]
- 247.Scrutton MC, Utter MF. 1965. Pyruvate carboxylase: III. Some physical and chemical properties of the highly purified enzyme. J. Biol. Chem 240:1–9 [PubMed] [Google Scholar]
- 248.Scrutton MC, Utter MF, Mildvan AS. 1966. Pyruvate carboxylase: VI. The presence of tightly bound manganese. J. Biol. Chem 241:3480–87 [PubMed] [Google Scholar]
- 249.Seo YA, Li Y, Wessling-Resnick M. 2013. Iron depletion increases manganese uptake and potentiates apoptosis through ER stress. Neurotoxicology 38:67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Sepulveda MR, Marcos D, Berrocal M, Raeymaekers L, Mata AM, Wuytack F. 2008. Activity and localization of the secretory pathway Ca2+-ATPase isoform 1 (SPCA1) in different areas of the mouse brain during postnatal development. Mol. Cell Neurosci 38:461–73 [DOI] [PubMed] [Google Scholar]
- 251.Sepulveda MR, Vanoevelen J, Raeymaekers L, Mata AM, Wuytack F. 2009. Silencing the SPCA1 (secretory pathway Ca2+-ATPase isoform 1) impairs Ca2+ homeostasis in the Golgi and disturbs neural polarity. J. Neurosci 29:12174–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Sepulveda MR, Wuytack F, Mata AM. 2012. High levels of Mn2+ inhibit secretory pathway Ca2+/Mn2+- ATPase (SPCA) activity and cause Golgi fragmentation in neurons and glia. J. Neurochem 123:824–36 [DOI] [PubMed] [Google Scholar]
- 253.Seve M, Chimienti F, Devergnas S, Favier A. 2004. In silico identification and expression of SLC30 family genes: an expressed sequence tag data mining strategy for the characterization of zinc transporters’ tissue expression. BMC Genomics 5:32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Shaked I, Ben-Dror I, Vardimon L. 2002. Glutamine synthetase enhances the clearance of extracellular glutamate by the neural retina. J. Neurochem 83:574–80 [DOI] [PubMed] [Google Scholar]
- 255.Shan X, Chi L, Ke Y, Luo C, Qian S, et al. 2007. Manganese superoxide dismutase protects mouse cortical neurons from chronic intermittent hypoxia-mediated oxidative damage. Neurobiol. Dis 28:206–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Sharma NK, Lebedeva M, Thomas T, Kovalenko OA, Stumpf JD, et al. 2014. Intrinsic mitochondrial DNA repair defects in ataxia telangiectasia. DNA Repair (Amst.) 13:22–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Shi S, Zhao J, Yang L, Nie X, Han J, et al. 2015. KHSRP participates in manganese-induced neurotoxicity in rat striatum and PC12 cells. J. Mol. Neurosci 55:454–65 [DOI] [PubMed] [Google Scholar]
- 258.Sidoryk-Wegrzynowicz M, Lee E, Albrecht J, Aschner M. 2009. Manganese disrupts astrocyte glutamine transporter expression and function. J. Neurochem 110:822–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Siepler JK, Nishikawa RA, Diamantidis T, Okamoto R. 2003. Asymptomatic hypermanganesemia in long-term home parenteral nutrition patients. Nutr. Clin. Pract 18:370–73 [DOI] [PubMed] [Google Scholar]
- 260.Singaraja RR, Hadano S, Metzler M, Givan S, Wellington CL, et al. 2002. HIP14, a novel ankyrin domain-containing protein, links huntingtin to intracellular trafficking and endocytosis. Hum. Mol. Genet 11:2815–28 [DOI] [PubMed] [Google Scholar]
- 261.Singaraja RR, Huang K, Sanders SS, Milnerwood AJ, Hines R, et al. 2011. Altered palmitoylation and neuropathological deficits in mice lacking HIP14. Hum. Mol. Genet 20:3899–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Smith EA, Newland P, Bestwick KG, Ahmed N. 2012. Increased whole blood manganese concentrations observed in children with iron deficiency anaemia. J. Trace Elem. Med. Biol 27:65–69 [DOI] [PubMed] [Google Scholar]
- 263.Spranger M, Schwab S, Desiderato S, Bonmann E, Krieger D, Fandrey J. 1998. Manganese augments nitric oxide synthesis in murine astrocytes: a new pathogenetic mechanism in manganism? Exp. Neurol 149:277–83 [DOI] [PubMed] [Google Scholar]
- 264.Sriram K, Jefferson AM, Lin GX, Afshari A, Zeidler-Erdely PC, et al. 2014. Neurotoxicity following acute inhalation of aerosols generated during resistance spot weld-bonding of carbon steel. Inhal. Toxicol 26:720–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sriram K, Lin GX, Jefferson AM, Roberts JR, Wirth O, et al. 2010. Mitochondrial dysfunction and loss of Parkinson’s disease-linked proteins contribute to neurotoxicity of manganese-containing welding fumes. FASEB J 24:4989–5002 [DOI] [PubMed] [Google Scholar]
- 266.Stansfield KH, Bichell TJ, Bowman AB, Guilarte TR. 2014. BDNF and huntingtin protein modifications by manganese: implications for striatal medium spiny neuron pathology in manganese neurotoxicity.J. Neurochem 131:655–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Stef DS, Gergen I. 2012. Effect of mineral-enriched diet and medicinal herbs on Fe, Mn, Zn, and Cu uptake in chicken. Chem. Cent. J 6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Stephenson AP, Schneider JA, Nelson BC, Atha DH, Jain A, et al. 2013. Manganese-induced oxidative DNA damage in neuronal SH-SY5Y cells: attenuation of thymine base lesions by glutathione and N-acetylcysteine. Toxicol. Lett 218:299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Stredrick DL, Stokes AH, Worst TJ, Freeman WM, Johnson EA, et al. 2004. Manganese-induced cytotoxicity in dopamine-producing cells. Neurotoxicology 25:543–53 [DOI] [PubMed] [Google Scholar]
- 270.Subhash MN, Padmashree TS. 1990. Regional distribution of dopamine beta-hydroxylase and monoamine oxidase in the brains of rats exposed to manganese. Food Chem. Toxicol 28:567–70 [DOI] [PubMed] [Google Scholar]
- 271.Sutton LM, Sanders SS, Butland SL, Singaraja RR, Franciosi S, et al. 2013. Hip14l-deficient mice develop neuropathological and behavioural features of Huntington disease. Hum. Mol. Genet 22:452–65 [DOI] [PubMed] [Google Scholar]
- 272.Suwalsky M, Villena F, Sotomayor CP. 2010. Mn2+ exerts stronger structural effects than the Mn-citrate complex on the human erythrocyte membrane and molecular models. J. Inorg. Biochem 104:55–61 [DOI] [PubMed] [Google Scholar]
- 273.Takeda AJ, Sawashita J, Okada S. 1998. Manganese concentration in rat brain: manganese transport from the peripheral tissues. Neurosci. Lett 242:45–48 [DOI] [PubMed] [Google Scholar]
- 274.Tan J, Zhang T, Jiang L, Chi J, Hu D, et al. 2011. Regulation of intracellular manganese homeostasis by Kufor-Rakeb syndrome-associated ATP13A2 protein. J. Biol. Chem 286:29654–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Tewari R, Jarvela T, Linstedt AD. 2014. Manganese induces oligomerization to promote downregulation of the intracellular trafficking receptor used by Shiga toxin. Mol. Biol. Cell 25:3049–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Tidball AM, Bryan MR, Uhouse MA, Kumar KK, Aboud AA, et al. 2014. A novel manganese-dependent ATM-p53 signaling pathway is selectively impaired in patient-based neuroprogenitor and murine striatal models of Huntington’s disease. Hum. Mol. Genet 24:1929–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Ton VK, Mandal D, Vahadji C, Rao R. 2002. Functional expression in yeast of the human secretory pathway Ca2+, Mn2+-ATPase defective in Hailey-Hailey disease. J. Biol. Chem 277:6422–27 [DOI] [PubMed] [Google Scholar]
- 278.Trujillo KM, Yuan SSF, Lee EY-HP, Sung P. 1998. Nuclease activities in a complex of human recombination and DNA repair factors Rad50, Mre11, and p95. J. Biol. Chem 273:21447–50 [DOI] [PubMed] [Google Scholar]
- 279.Tsunemi T, Krainc D. 2014. Zn2+ dyshomeostasis caused by loss of ATP13A2/PARK9 leads to lysosomal dysfunction and alpha-synuclein accumulation. Hum. Mol. Genet 23:2791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Tuschl K, Clayton PT, Gospe SM Jr, Gulab S, Ibrahim S, et al. 2012. Syndrome of hepatic cirrhosis, dystonia, polycythemia, and hypermanganesemia caused by mutations in SLC30A10, a manganese transporter in man. Am. J. Hum. Genet 90:457–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Ugolino J, Fang S, Kubisch C, Monteiro MJ. 2011. Mutant Atp13a2 proteins involved in parkinsonism are degraded by ER-associated degradation and sensitize cells to ER-stress induced cell death. Hum. Mol. Genet 20:3565–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.van Veen S, Sorensen DM, Holemans T, Holen HW, Palmgren MG, Vangheluwe P. 2014. Cellular function and pathological role of ATP13A2 and related P-type transport ATPases in Parkinson’s disease and other neurological disorders. Front. Mol. Neurosci 7:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Vanoevelen J, Dode L, Van Baelen K, Fairclough RJ, Missiaen L, et al. 2005. The secretory pathway Ca2+/Mn2+-ATPase 2 is a Golgi-localized pump with high affinity for Ca2+ ions. J. Biol. Chem 280:22800–8 [DOI] [PubMed] [Google Scholar]
- 284.Varro A, Negretti N, Hester SB, Eisner DA. 1993. An estimate of the calcium content of the sarcoplasmic reticulum in rat ventricular myocytes. Pflugers Arch 423:158–60 [DOI] [PubMed] [Google Scholar]
- 285.Waghorn B, Schumacher A, Liu J, Jacobs S, Baba A, et al. 2011. Indirectly probing Ca2+ handling alterations following myocardial infarction in a murine model using T1-mapping manganese-enhanced magnetic resonance imaging. Magn. Reson. Med 65:239–49 [DOI] [PubMed] [Google Scholar]
- 286.Waghorn B, Yang Y, Baba A, Matsuda T, Schumacher A, et al. 2009. Assessing manganese efflux using SEA0400 and cardiac T1-mapping manganese-enhanced MRI in a murine model. NMR Biomed 22:874–81 [DOI] [PubMed] [Google Scholar]
- 287.Wang CY, Jenkitkasemwong S, Duarte S, Sparkman BK, Shawki A, et al. 2012. ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. J. Biol. Chem 287:34032–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Wang R, Zhu X. 2003. Subtoxic concentration of manganese synergistically potentiates 1-methyl-4phenylpyridinium-induced neurotoxicity in PC12 cells. Brain Res 961:131–38 [DOI] [PubMed] [Google Scholar]
- 289.Wang X, Miller DS, Zheng W. 2008. Intracellular localization and subsequent redistribution of metal transporters in a rat choroid plexus model following exposure to manganese or iron. Toxicol. Appl. Pharmacol 230:167–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wang XS, Ong WY, Connor JR. 2001. A light and electron microscopic study of the iron transporter protein DMT-1 in the monkey cerebral neocortex and hippocampus. J. Neurocytol 30:353–60 [DOI] [PubMed] [Google Scholar]
- 291.Wegrzynowicz M, Holt HK, Friedman DB, Bowman AB. 2012. Changes in the striatal proteome of YAC128Q mice exhibit gene-environment interactions between mutant Huntingtin and manganese.J. Proteome Res 11:1118–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Wei Y, Chen J, Rosas G, Tompkins DA, Holt PA, Rao R. 2000. Phenotypic screening of mutations in Pmr1, the yeast secretory pathway Ca2+/Mn2+-ATPase, reveals residues critical for ion selectivity and transport. J. Biol. Chem 275:23927–32 [DOI] [PubMed] [Google Scholar]
- 293.Williams BB, Kwakye GF, Wegrzynowicz M, Li D, Aschner M, et al. 2010. Altered manganese homeostasis and manganese toxicity in a Huntington’s disease striatal cell model are not explained by defects in the iron transport system. Toxicol. Sci 117:169–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Williams BB, Li D, Wegrzynowicz M, Vadodaria BK, Anderson JG, et al. 2010. Disease-toxicant screen reveals a neuroprotective interaction between Huntington’s disease and manganese exposure. J. Neurochem 112:227–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Wilson DC, Tubman TR, Halliday HL, McMaster D. 1992. Plasma manganese levels in the very low birth weight infant are high in early life. Biol. Neonate 61:42–46 [DOI] [PubMed] [Google Scholar]
- 296.Wootton LL, Argent CC, Wheatley M, Michelangeli F. 2004. The expression, activity and localisation of the secretory pathway Ca2+-ATPase (SPCA1) in different mammalian tissues. Biochim. Biophys. Acta 1664:189–97 [DOI] [PubMed] [Google Scholar]
- 297.Wozniak-Celmer E, Oldziej S, Ciarkowski J. 2001. Theoretical models of catalytic domains of protein phosphatases 1 and 2A with Zn2+ and Mn2+ metal dications and putative bioligands in their catalytic centers. Acta Biochim. Pol 48:35–52 [PubMed] [Google Scholar]
- 298.Wuytack F, Raeymaekers L, Missiaen L. 2002. Molecular physiology of the SERCA and SPCA pumps. Cell Calcium 32:279–305 [DOI] [PubMed] [Google Scholar]
- 299.Xiang M, Mohamalawari D, Rao R. 2005. A novel isoform of the secretory pathway Ca2+,Mn2+-ATPase, hSPCA2, has unusual properties and is expressed in the brain. J. Biol. Chem 280:11608–14 [DOI] [PubMed] [Google Scholar]
- 300.Xiao L, Gong LL, Yuan D, Deng M, Zeng XM, et al. 2010. Protein phosphatase-1 regulates Akt1 signal transduction pathway to control gene expression, cell survival and differentiation. Cell Death Differ 17:1448–62 [DOI] [PubMed] [Google Scholar]
- 301.Xu SZ, Zeng F, Boulay G, Grimm C, Harteneck C, Beech DJ. 2005. Block of TRPC5 channels by 2aminoethoxydiphenyl borate: a differential, extracellular and voltage-dependent effect. Br. J. Pharmacol 145:405–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Yanai A, Huang K, Kang R, Singaraja RR, Arstikaitis P, et al. 2006. Palmitoylation of huntingtin by HIP14 is essential for its trafficking and function. Nat. Neurosci 9:824–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Yang J, Gonon AT, Sjoquist PO, Lundberg JO, Pernow J. 2013. Arginase regulates red blood cell nitric oxide synthase and export of cardioprotective nitric oxide bioactivity. PNAS 110:15049–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Yin Z, Jiang H, Lee ES, Ni M, Erikson KM, et al. 2010. Ferroportin is a manganese-responsive protein that decreases manganese cytotoxicity and accumulation. J. Neurochem 112:1190–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Yokel RA. 2009. Manganese flux across the blood-brain barrier. Neuromol. Med 11:297–310 [DOI] [PubMed] [Google Scholar]
- 306.Yokel RA, Crossgrove JS, Bukaveckas BL. 2003. Manganese distribution across the blood-brain barrier. II. Manganese efflux from the brain does not appear to be carrier mediated. Neurotoxicology 24:15–22 [DOI] [PubMed] [Google Scholar]
- 307.Yu H, Iyer RK, Kern RM, Rodriguez WI, Grody WW, Cederbaum SD. 2001. Expression of arginase isozymes in mouse brain. J. Neurosci. Res 66:406–22 [DOI] [PubMed] [Google Scholar]
- 308.Zeron HM, Rodriguez MR, Montes S, Castaneda CR. 2011. Blood manganese levels in patients with hepatic encephalopathy. J. Trace Elements Med. Biol 25:225–29 [DOI] [PubMed] [Google Scholar]
- 309.Zhang P, Lokuta KM, Turner DE, Liu B. 2010. Synergistic dopaminergic neurotoxicity of manganese and lipopolysaccharide: differential involvement of microglia and astroglia. J. Neurochem 112:434–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Zhang S, Fu J, Zhou Z. 2005. Changes in the brain mitochondrial proteome of male Sprague-Dawley rats treated with manganese chloride. Toxicol. Appl. Pharmacol 202:13–17 [DOI] [PubMed] [Google Scholar]
- 311.Zidenberg-Cherr S, Keen CL, Lönnerdal B, Hurley LS. 1983. Superoxide dismutase activity and lipid peroxidation in the rat: developmental correlations affected by manganese deficiency. J. Nutr 113:2498–504 [DOI] [PubMed] [Google Scholar]
- 312.Zlotkin SH, Atkinson S, Lockitch G. 1995. Trace elements in nutrition for premature infants. Clin. Perinatol 22:223–40 [PubMed] [Google Scholar]
- 313.Zou J, Wang YX, Dou FF, Lu HZ, Ma ZW, et al. 2010. Glutamine synthetase down-regulation reduces astrocyte protection against glutamate excitotoxicity to neurons. Neurochem. Int 56:577–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Zou JY, Crews FT. 2005. TNFα potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NFκB inhibition. Brain Res 1034:11–24 [DOI] [PubMed] [Google Scholar]