Abstract
Background
The histopathological changes of Alzheimer's disease (AD) are detectable decades prior to its clinical expression. However, there is a need for an early, inexpensive, noninvasive diagnostic biomarker to detect specific Alzheimer pathology. Recently developed neuroimaging biomarkers show promising results, but these methods are expensive and cause radiation. Furthermore, the analysis of cerebrospinal fluid (CSF) biomarkers requires an invasive lumbar puncture. Saliva is an easily obtained body fluid, and a stable saliva biomarker would therefore be a promising candidate for a future method for diagnosing AD. The purpose of this systematic review was to investigate studies of biomarkers in saliva samples for the diagnosis of AD.
Methods
The included articles were identified through a literature search in PubMed and Google Scholar for all articles until November 1st, 2018, and furthermore, all reference lists of included articles were reviewed by hand. We included articles written in English investigating saliva from patients with AD and a control group.
Results
A total of 65 studies were identified, whereof 16 studies met the inclusion criteria and were included in the systematic review. A plethora of different biomarkers were investigated, and ten out of the sixteen studies showed a statistical significance in biomarkers between patients with AD and healthy, elderly controls, among these biomarkers for specific AD pathology (amyloid beta 1-42 (Aβ42) and tau).
Conclusion
Aβ42 and tau seem to be worthy candidates for future salivary biomarkers for AD, but other biomarkers such as lactoferrin and selected metabolites also have potential. More studies must be carried out with larger sample sizes and a standardization of the sampling and processing method. Factors such as diurnal variation, AD patients' decreased ability of oral self-care, and salivary flowrates must be taken into consideration.
1. Introduction
Alzheimer's disease (AD) is a neurodegenerative disease and is the leading cause of progressive dementia. It is estimated that 46.8 million people suffer from dementia worldwide, with the highest prevalences found in the older age groups (+65 years). By 2030, it is estimated that the prevalence will increase to approximately 74.7 million people, partly due to the increasing numbers of elderly people in the world [1]. AD is the cause of approximately 60% - 80% of dementia-related cases in people over 65, while it is only 30-40% in people under 65 years [2]. Cognitive deficits in AD progress with the duration of the disease caused by accelerating neurodegenerative processes. Formation of specific AD pathology, amyloid plaques between neurons and the accumulation of intracellular neurofibrillary tangles composed of tau, begin decades prior to the clinical expression of AD, and it is therefore essential to find a biomarker for early preclinical diagnosis and treatment monitoring. The biomarker sampling and analysis must be easy to perform, inexpensive, and noninvasive. Currently, an analysis of the cerebrospinal fluid (CSF) is used to aid the diagnosis of AD, which later in the disease course has good diagnostic precision [3]. Changes in the CSF's content of tau, phosphorylated tau, and amyloid beta 1-42 (Aβ42) can at this point be detected in most patients [4]. There is currently no disease-modifying treatment available for AD, but numerous trials are ongoing, especially in presymptomatic or early symptomatic stages of the disease [5]. Very recently, the monoclonal antibody, Aducanumab, was abandoned in phase III due to an analysis which concluded that Aducanumab would not be able to slow the cognitive decline by decreasing the production and aggregation of Aβ. Furthermore, it was reported that antiamyloid agents might not have a clinical effect in the symptomatic stages of the disease [6, 7]. Therefore, it is essential to develop a sensitive and noninvasive method for early diagnosis and monitoring, so a potentially disease-modifying intervention can be initiated in the presymptomatic or prodromal phase and thereby delay the onset of AD or modify the disease course.
Saliva is an easily obtained body fluid, and studies have reported that proteins from the central nervous system (CNS) are excreted into the saliva [8]. Many parts of the body are affected by AD, among these parts of the autonomic nervous system (ANS), including the brain stem, the hypothalamus, the cerebral neocortex, the insular cortex, and locus coeruleus. In addition, studies have reported that AD degenerates nerve terminals in the cholinergic system, which regulates the cardiovascular system and the ANS and that this alteration already can be seen in the preclinical phase of the disease [9]. The submandibular, the sublingual, and the parotid glands, which are the main salivary glands in the mouth, secrete saliva in response to cholinergic innervation from the glossopharyngeal cranial nerve and the facial cranial nerve, controlled by ANS. Consequently, an alteration in the ANS, as seen in AD, could affect the saliva production and composition, and this altered composition might thereby mirror pathological changes in the CNS [10]. In addition, studies have shown that most blood biomarkers can also be found in saliva, and it has been reported that proteins from the blood can pass into the saliva via passive diffusion, active transport, or microfiltration [8, 10, 11]. Furthermore, it has been suggested that some AD biomarkers, such as Aβ42, are expressed or produced in the salivary glands [10, 12, 13]. As a result, a saliva sample could be a valid alternative to CSF or blood, because the saliva sampling is easy to perform, inexpensive, and noninvasive. A valid and reproducible saliva biomarker would therefore be preferable over other present biomarkers. For this reason, the purpose of this systematic review was to review the studies of biomarkers obtained in saliva for AD diagnosis.
2. Methods
2.1. Eligibility Criteria
Studies selected for review included original, full-text articles published in English, investigating biomarkers for AD in saliva. Studies must include saliva samples from AD patients and a control group.
2.2. Search
The original studies were identified through a literature search in PubMed and Google Scholar for all relevant articles up until November 1st, 2018. The filters “English” and “humans” were applied, and the following keywords were used for the search: (Saliva) AND diagnos∗ AND (Alzheimer OR AD) AND (biomarker). Furthermore, all reference lists of identified studies were reviewed by hand.
2.3. Study Selection and Data Extraction
By screening the titles and the abstracts based on the eligibility criteria listed above, studies were selected for further data extraction. The selected original, full-text articles were reviewed independently by the first and last author according to a developed data extraction sheet, including biomarker identification, age and gender for AD patients and controls, inclusion and exclusion criteria, diagnostic criteria, analytical method, blinding of analysts, and statistics for the biomarkers involved in the study.
3. Results
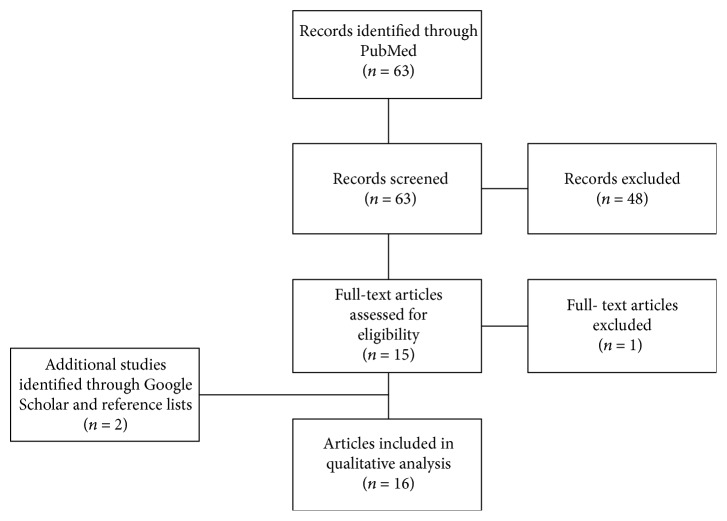
The study selection process is seen in Figure 1. The initial literature search in PubMed identified 63 studies. The titles and the abstracts from all identified studies were screened, and 48 studies were excluded, either due to irrelevance or because the studies did not fulfil the inclusion criteria. The remaining fifteen original studies were evaluated in full, and fourteen studies met the inclusion criteria and were therefore included in the qualitative analysis. In addition, a literature search in Google Scholar and a review of the reference lists of all included studies identified two further studies, which were included in the qualitative analysis. Altogether, a total of sixteen original, full-text articles were included in the systematic review.
Figure 1.
Flowchart of the literature search and the study selection.
3.1. Preanalytical Variables
Sampling and processing methods varied among studies. Seven studies required fasting prior to saliva sample collection [14–20], and eight studies required rinsing of the mouth before the saliva sample collection [14–18, 21–23]. In nearly all of the studies, the saliva collection was performed as unstimulated by spitting or drooling directly into a tube, except for one study where the sample collection was executed with a Salivette [24]. In three studies, the method for the saliva collection was not described [12, 13, 18]. The volume of saliva sample collected varied from 1 mL to around 5 mL.
3.2. Saliva Biomarkers
Biomarkers from the sixteen included articles were divided into the following categories: β-amyloid, tau, acetylcholinesterase, and other biomarkers. Table 1 shows an overview of the studies. Statistically significant difference in biomarker concentrations between the patients with AD and the control group was found in ten out of sixteen studies and will be described below.
Table 1.
An overview of the subjects and on the results of the included articles.
| References | Biomarkers investigated | Methods used to analyze biomarkers | Number of subjects | Age of subjects | Sex of subjects | Result biomarkers |
|---|---|---|---|---|---|---|
| Sabbagh et al. [25] | Aβ42 | ELISA | AD: n = 15 Healthy controls: n = 7 |
Mean age AD: 77.8 ± 1.8 Healthy controls: 60.4 ± 4.7 |
Male/female AD: 7/8 Healthy controls: 2/5 |
↑ Aβ42, p value AD/control < 0.05 |
|
| ||||||
| Huan et al. [14] | Metabolites | LC-MS | Two studies (discovery and validation) Discovery AD: n = 22 aMCI: n = 25 Healthy controls: n = 35 Validation AD: n = 7 aMCI: n = 10 Healthy controls: n = 10 |
Two studies (discovery and validation) Discovery Mean age AD: 77.09 aMCI: 70.4 Healthy controls: 69.94 Validation Mean age AD: 70.11 aMCI: 71.5 Healthy controls: 71.4 |
Two studies (discovery and validation) Discovery Male/female AD: 6/16 aMCI: 10/15 Healthy controls: 13/22 Validation Male/female AD: 2/5 aMCI: 5/5 Healthy controls: 5/5 |
Methylguanosine, histidylphenylalanine, choline-cytidine AD/healthy controls: AUC (discovery and validation) = 1.00 Sensitivity: 100% Specificity: 100% p value < 0.01 Amino-dihydroxybenzene, glucosyl-galactosyl-hydroxylysine-H2O, aminobytyric acid + H2 AD/aMCI: AUC (discovery and validation) = 1.00 Sensitivity: 100% Specificity: 100% p value < 0.01 Phenylalanylproline, phenylalanylphenylalanine, urocanic acid AD/healthy controls: AUC discovery: 0.820 AUC validation: 0.814 Sensitivity: 71.4% Specificity: 90.0% p value < 0.01 Alanylphenylalanine, phenylalanylproline AD/aMCI: AUC discovery: 0.881 AUC validation: 0.786 Sensitivity: 71.4% Specificity: 80.0% p value < 0.01 |
|
| ||||||
| Ashton et al. [15] | t-tau | SIMOA | AD: n = 53 aMCI: n = 68 Healthy controls: n = 160 |
Mean age AD: 81.4 ± 6.6 aMCI: 79.8 ± 7.4 Healthy controls: 78.0 ± 6.7 |
Male/female AD: 23/30 aMCI: 33/35 Healthy controls: 66/94 |
↑ t-tau, although not statistically significant, p value AD/aMCI/healthy controls = 0.219 Correlation with ventricular volume for AD: p value = 0.045 |
|
| ||||||
| Pekeles et al. [26] | t-tau p-tau |
Western blot | Two studies (round one and round two) Round one AD: n = 46 aMCI: n=55 Healthy controls: n = 47 Round two AD: n = 41 FTD: n = 16 Healthy older controls: n = 44 Neurological patients (other than dementia): n = 12 Young healthy controls: n = 76 |
Two studies (round one and round two) Round one Median age (IQR) AD: 80 (9) aMCI: 78 (14) Healthy controls: 73 (6) Round two Median age (IQR) AD: 80 (8) FTD: 71.5 (10) Healthy older controls: 72 (7) Neurological patients (other than dementia): 55 (11) Young healthy controls: 32 (22) |
Two studies (round one and round two) Round one Male/female AD: 24/22 aMCI: 23/32 Healthy controls: 15/32 Round two Male/female AD: 17/24 FTD: 11/5 Healthy older controls: 14/30 Neurological patients (other than dementia): 5/7 Young healthy controls: 31/45 |
Two studies (round one and round two) Round one ↑ p-tau/t-tau at phosphorylation site: S396, S404, and the combination of S400, T403, and T404, p value < 0.05 Round two ↑ median p-tau/t-tau at phosphorylation site: S396, p value AD/healthy older controls < 0.05 Sensitivity S396: 73% Specificity S396: 50% Sensitivity S404: 83% Specificity S404: 30% |
|
| ||||||
| McGeeR et al. [13] | Aβ42 | ELISA | AD: n = 23 Low controls: n = 25 High controls (risk for AD): n = 6 |
Mean age AD: 71.3 Low controls: 54.2 High controls (risk for AD): 69 |
Male/female AD: 8/5 Low controls: 17/8 High controls (risk of AD): 3/3 |
↑ Aβ42 (AD > high controls (risk for AD) > low controls) p value AD/low controls/high controls < 0.001 |
|
| ||||||
| Bakhtiari et al. [16] | AchE activity | Ellman's colorimetric method | AD: n = 15 Healthy controls: n=15 |
Mean age AD: 78.4 Healthy controls: 71 |
Male/female AD: 9/6 Healthy controls: 7/8 |
↑ AchE activity, p value AD/control = 0.25 |
|
| ||||||
| Carro et al. [27] | Lactoferrin | ELISA | Two studies (discovery and validation) Discovery AD: n = 80 aMCI: n = 44 Healthy controls: n = 91 PD: n = 59 Validation AD: n = 36 aMCI: n = 15 Healthy controls: n = 40 |
Two studies (discovery and validation) Discovery Mean age AD: 76.2 ± 5.33 aMCI: 75.16 ± 5.13 Healthy controls: 73.7 ± 6.88 PD: 69.5 ± 8.6 Validation Mean age AD: 80.67 ± 8.67 aMCI: 68.93 ± 6.12 Healthy controls: 66.78 ± 7.33 |
Two studies (discovery and validation) Discovery Male/female AD: 31/49 aMCI: 19/25 Healthy controls: 32/59 PD: 27/32 Validation Male/female AD: 13/23 aMCI: 10/5 Healthy controls: 15/25 |
Two studies (discovery and validation) Discovery ↓ lactoferrin, p value AD/healthy controls < 0.001, p value aMCI/healthy controls < 0.001 Validation Sensitivity: 100%, specificity: 100% Correlation with CSF Aβ42: p value < 0.001 Correlation with CSF t-tau: p value < 0.001 Correlation with MMSE in AD and aMCI: p value < 0.001 |
|
| ||||||
| Yilmaz et al. [17] | Metabolites (propionate, acetone) | Proton NMR spectroscopy | AD: n = 9 aMCI: n = 8 Healthy controls: n = 12 |
Mean age AD: 85 ± 7 aMCI: 83 ± 5 Healthy controls: 82 ± 8 |
Male/female AD: 3/6 aMCI: 3/5 Healthy controls: 4/8 |
↑ propionate, p value AD/aMCI/healthy controls = 0.034 Regression model for propionate and acetone AD/healthy controls: AUC = 0.871 Sensitivity: 90.9 % Specificity: 84.2% |
|
| ||||||
| Lee et al. [12] | Aβ42 | ELISA | AD+pre-AD: n = 10 Healthy controls: n = 26 PD: n = 1 |
Mean age AD+pre-AD: 70.1 Healthy controls+PD (control): 54.6 |
Male/female AD+pre-AD: 3/7 Healthy controls+PD (control): 18/9 |
↑ Aβ42, p value AD/control < 0.001 |
|
| ||||||
| Liang et al. [18] | Spinganine-1-phosphate Ornithine Phenyllactic acid Inosine 3-dehydrocarnitine Hypoxanthine |
FUPLC-MS | AD: n=256 Healthy controls: n = 218 |
Mean age AD: 78.6 ± 6.8 Healthy controls: 77.9 ± 5.6 |
Male/female AD: 124/132 Healthy controls: 102/116 |
↑ spinganine-1-phosphate, p value < 0.01 Sensitivity: 99.4% Specificity: 98.2% ↑ ornithine, p value < 0.01 Sensitivity: 81.9% Specificity: 90.7% ↑ phenyllactic acid, p value < 0.01 ↓ inosine, p value < 0.01 ↓ 3-dehydrocarnitine, p value < 0.01 ↓ hypoxanthine, p value < 0.01 |
|
| ||||||
| Lau et al. [19] | Trehalose Aβ42 t-tau p-tau |
Trehalose: EG-IDFET biosensor Aβ42, t-tau, p-tau: ELISA |
AD: n = 20 Healthy controls: n = 20 PD: n = 20 |
Mean age AD: 72.5 ± 7.68 Healthy controls: 66.1 ± 7.79 PD: 73 ± 8.07 |
Male/female AD: 8/12 Healthy controls: 9/11 PD: 5/15 |
Trehalose concentration: AD > PD > healthy controls Aβ42: not detected t-tau: no significant differences ↑ p-tau |
|
| ||||||
| Kim et al. [21] | Aβ42 Aβ40 |
Immunoassay with nanobeads | AD: n = 28 Healthy controls: n = 17 |
N.A. | N.A. | ↑ Aβ42 (statistically significant) ↑ Aβ40 (not statistically significant) |
|
| ||||||
| Shi et al. [24] | Aβ42 t-tau p-tau |
ELISA (Luminex assay) | AD: n = 21 Healthy controls: n = 38 |
Mean age AD: 68.8 Healthy controls: 69 |
Male/female AD: 10/11 Healthy controls: 19/19 |
Aβ42: not detected ↑ t-tau ↑ p-tau ↑ p-tau/t-tau in AD patients, p value < 0.05 |
|
| ||||||
| Bermejo-Pareja et al. [20] | Aβ42 Aβ40 |
ELISA | AD: n = 70 (mild: n = 29, moderate: n = 24, severe: n = 17) Healthy controls: n = 56 PD: n = 51 |
Mean age AD: 77.2 (60-91) Healthy controls: 74.35 (64-85) PD: 72.96 (60-93) |
Male/female AD: 21/49 Healthy controls: 17/39 PD: 26/25 |
Aβ42: ↑ concentration in mild and moderate AD p value mild AD = 0.043 p value AD/healthy control < 0.05 Sensitivity: 16% Specificity: 93% Interaction with age: p value = 0.016 Interaction with gender: p value = 0.002 Aβ40: ↑ concentration in AD and PD |
|
| ||||||
| Boston et al. [22] | AchE | Ellman's colorimetric method | AD: n = 15 Healthy controls: n = 13 VaD: n = 13 |
Mean age AD: 83.5 (71.3-95.8) Healthy controls: 80.8 (70.8-92.1) VaD: 81.8 (71.3-93.3) |
Male/female AD: 5/10 Healthy controls: 7/6 VaD: 9/4 |
No significant difference |
|
| ||||||
| Sayer et al. [23] | AchE activity | Ellman's colorimetric method | AD responders: n = 22 AD nonresponders: 14 Healthy controls: n = 11 |
Mean age AD responders: 75 (60-86) AD nonresponders: 75 (65-87) Healthy controls: 71 (64-91) |
Male/female AD responders: 7/15 AD nonresponders: 4/10 Healthy controls: 6/5 |
↓ AchE activity, p value nonresponders/healthy controls < 0.005 Interaction with age in controls: p value < 0.001 |
AD: Alzheimer's disease. aMCI: amnestic mild cognitive impairment. PD: Parkinson disease. FTD: frontotemporal dementia. Aβ42: amyloid beta 1-42. Aβ40: amyloid beta 1-40. p-tau: phosphorylated tau. t-tau: total tau. AchE: acetylcholinesterase. CSF: cerebrospinal fluid. MMSE: minimental state examination. ELISA: enzyme-linked immunosorbent assay. SIMOA: single molecule array. LC-MS: liquid chromatography mass spectrometry. FUPLC-MS: fast ultraperformance liquid chromatography mass spectrometry. EG-IDFET biosensor: extended gate ion-sensitive field-effect transistor biosensor. ↑: increased levels of the biomarker in patients with AD. ↓: decreased levels of the biomarker in patients with AD. AUC: area under the curve. N.A.: not available.
3.2.1. β-Amyloid
Aβ42 and Aβ40 in saliva were investigated in seven studies, which altogether included 187 subjects with AD, 72 subjects with Parkinson disease (PD), and 195 healthy controls. In four studies, increased Aβ42 levels in patients with AD were detected with enzyme-linked immunosorbent assay (ELISA) [12, 13, 20, 25]. In addition, one of these studies found an interaction with age (p value = 0.016) and an interaction with gender (p value = 0.002) [20]. An additional study used an immunoassay with nanobeads to detect an increased Aβ42 level with statistical significance, but no p value was provided [21]. Two other studies also used ELISA but did not detect Aβ42 in the saliva samples [19, 24]. Two studies reported no statistical significance on Aβ40 concentrations [20, 21].
3.2.2. Tau
Phosphorylated tau (p-tau) and total tau (t-tau) in saliva were investigated in four studies, which altogether included 181 subjects with AD, 123 subjects with amnestic mild cognitive impairment (aMCI), twenty subjects with PD, sixteen subjects with frontotemporal dementia (FTD), and 317 healthy controls. An increased p-tau/t-tau ratio in AD patients was identified with ELISA (p value < 0.05) in one study [24]. Furthermore, one of the studies reported an increased p-tau/t-tau ratio using a Western blot analyzing phosphorylation sites S396, S404, T404, and a combination of S400 and T403 (p value < 0.05) and an increased median p-tau/t-tau ratio at phosphorylation site S396 (p value < 0.05) [26]. In the two remaining studies, ELISA [19] and single molecule array (SIMOA) [15] were used to detect p-tau and t-tau. Although both p-tau and t-tau levels were described as increased in the two studies, no statistical significance was reported.
3.2.3. Acetylcholinesterase (AchE) Activity
AchE activity in saliva was investigated in three studies, which altogether included 66 subjects with AD, thirteen subjects with vascular dementia (VaD), and 39 healthy controls. All three studies were performed by Ellman's colorimetric method. In one study, decreased AchE activity was identified in patients with AD (p value < 0.005), and an interaction with age in healthy controls was reported (p value < 0.001) [23]. In contrast, increased AchE activity was reported in one study but with no statistical significance [16]. No statistically significant difference between patients with AD and healthy controls was found in the last study [22].
3.2.4. Other Biomarkers
Other biomarkers in saliva (lactoferrin, selected metabolites, and trehalose) were investigated in five studies, which altogether included 430 subjects with AD, 102 subjects with aMCI, 79 subjects with PD, and 426 healthy controls. In one study, decreased levels of lactoferrin were detected with ELISA both in AD patients compared to healthy controls (p value < 0.001) and in aMCI patients compared to healthy controls (p value < 0.001). In addition, the study identified a positive correlation with CSF Aβ42 and t-tau (p value < 0.001) and a positive correlation with minimental state examination (MMSE) in AD patients compared to aMCI patients (p < 0.001) [27].
Within the group of metabolites, one study detected increased levels of propionate among AD patients (p value < 0.034) [17] by using the proton NMR spectroscopy, while another study reported increased levels of spinganine-1-phosphate, ornithine, and phenyllactic acid (p value < 0.01) and decreased levels of inosine, 3-dehydrocarnithine, and hypoxanthine (p value < 0.01) by using the fast ultraperformance liquid chromatography mass spectrometry (FUPLC-MS) [18]. The third study used liquid chromatography mass spectrometry (LC-MS) for investigating metabolites in saliva. The study found a statistically significant difference (p value < 0.01) between AD patients and the healthy control group in methylguanosine, histidylphenylalanine, choline-cytidine, phenylalanylproline, phenylalanylphenylalanine, and urocanic acid. The study also found a statistically significant difference (p value < 0.01) between AD patients and aMCI patients in the metabolites: amino-dihydroxybenzene, glucosyl-galactosyl-hydroxylysine-H2O, aminobytyric acid + H2, alanylphenylalanine, and phenylalanylproline [14].
Finally, increased levels of trehalose in AD patients as compared to controls were found in one study by using an extended gate ion-sensitive field-effect transistor biosensor (EG-IDFET biosensor) but with no statistical significance [19].
4. Discussion
Biomarkers obtained from the CSF is a well-established method used to detect AD pathology, but the procedure is invasive and complications and adverse effects are frequently encountered [28]. Simultaneously, early diagnosis is essential for the prognosis, disease monitoring, and treatment of AD [29]. As a result, it is crucial to find a method that is easy and safe to perform, inexpensive, and noninvasive and is able to detect biomarkers in the presymptomatic phase. The purpose of this systematic review was to assess the existing literature on salivary biomarkers for AD. Although the exact source that excretes biomarkers from the CNS into the saliva is still undefined, saliva has shown to be a valid candidate for detection of biomarkers in AD. Studies have reported that most of the compounds found in blood can also be found in saliva, and consequently, it has been suggested that compounds from the blood can pass into the saliva via passive diffusion, active transport, or microfiltration [8, 10, 11]. Furthermore, it has been proposed that biomarkers are excreted directly from the axons of the glossopharyngeal cranial nerve and the facial cranial nerve that stimulate the salivary glands. In addition, it may be possible that biomarkers are expressed or produced in the salivary glands [10]. Studies have shown that, for example, Aβ42 is produced by all organs, which can serve as the explanation to why Aβ42 is increased in saliva when it is decreased in the CSF of patients with AD [12, 13].
The results obtained on salivary Aβ42 indicate that Aβ42 is a good candidate for a future salivary biomarker. Five out of seven studies reported increased levels of salivary Aβ42 in AD patients [12, 13, 20, 21, 25], while two studies did not detect Aβ42 [19, 24]. Common to all studies were a small sample size, and for that reason, further studies must be carried out with more participants. Two studies reported on statistical insignificant difference in concentrations of Aβ40 [20, 21], resulting in a less reliable salivary biomarker when compared to Aβ42. Further studies should also investigate p-tau and t-tau as salivary biomarkers. Only two out of four studies found a statistically significant increase [24, 26] in p-tau and t-tau, although the remainder of the studies also reported nonsignificantly increased levels in AD [15, 19]. The lack of concordance between the four studies might be due to the fact that four different techniques were used to analyze the saliva samples or because of a lack of sensitivity of the assays used. For that reason, a standardization of sampling and analytical methods must be performed in order to confirm the validity of p-tau and t-tau as salivary biomarkers. The results found on AchE activity in AD patients indicate that AchE activity is not a promising salivary biomarker for early detection of the disease [16, 22, 23], although AchE activity could serve as an indicator for the pathology of AD or as an indicator of the degeneration of the cholinergic system. Bakhtiari et al. [16] suggests that further studies must investigate the AchE activity according to the severity of the patients' disease (mild, moderate, and severe) and furthermore consider a standardization of the study design. In addition, it should be considered that different variables, such as sex, AchE inhibitor therapy, delirium, and stress, can affect AchE activity [16, 30, 31].
Besides these main biomarkers for AD, other biomarkers in saliva were investigated. The results found by Carro et al. [27] indicate that lactoferrin is a promising candidate for a future salivary biomarker for AD. Lactoferrin is an iron-binding glycoprotein, and it is one of the most important antimicrobial peptides in saliva. Lactoferrin increases the activity of leukocytes, it is bacteriocidic, and by functioning as an antioxidant, it can protect the body against free radicals (ROS). Furthermore, lactoferrin is antiviral by inhibiting viral receptors, which results in an inhibited binding between virus and healthy cells. Studies have shown that bacteria and viruses are involved in the pathology of AD by altering the permeability of the blood-brain barrier and thereby facilitating an overproduction and aggregation of Aβ42 [27, 32, 33]. For that reason, more studies must be performed in order to verify if decreased levels of lactoferrin can serve as an early, salivary biomarker for AD. In one study, trehalose was examined. Trehalose is a sugar molecule, and it is believed to be associated with physiological and metabolic changes in the body and therefore the pathophysiology of AD [19]. In addition to trehalose, four exploratory studies examined a plethora of different metabolites using four different unbiased analytical methods for AD biomarker discovery. Many of the metabolites were either significantly increased or decreased in AD patients [14, 17, 18]. Therefore, more studies must be conducted in order to verify which metabolites reflect the pathology of AD best and subsequently investigate if the results are reproducible.
Salivary biomarkers for the diagnosis of AD are still a new research area in need of more studies. Aβ42, tau, lactoferrin, and different metabolites seem to be worthy candidates for future salivary biomarkers for AD. However, the source that excretes the biomarkers into the saliva is still undefined. Based on the results of this systematic review, many of the included studies used different detection techniques. It is therefore essential to standardize the sampling, processing, and analytical methods with the purpose of investigating the reproducibility of the results. In addition, larger sample sizes must be considered, and thereby the reference intervals of the biomarker's concentration can be assessed. Another aspect, which should be taken into consideration, is diurnal variation of the biomarkers. Six out of the sixteen included full-text articles took the possible diurnal variation into account [15, 16, 18, 20, 22, 26], which is essential to avoid that a normal circadian rhythm affects the results. Furthermore, AD patients' decreased ability of self-care raises the question if a decreased oral health or hygiene could affect the detection of the biomarkers. It should also be taken into account that studies have shown AD patients have a decreased saliva production either due to side effects of the medication (antidepressants and antipsychotics) or due to the pathology of the disease [34]. Therefore, future studies must evaluate biomarker levels taking salivary flowrates and the oral health or hygiene into account to ascertain the clinical usefulness of saliva for early diagnosis of AD.
Acknowledgments
This study was supported by Absalonfonden and Lundbeckfonden. The Danish Dementia Research Centre is supported by grants from the Danish Ministry of Health (J No. 2007-12143-112, project 59506/J, No. 0901110, and project 34501) and the Danish Health Foundation (J No. 2007B004).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Martin Prince A. World Alzheimer Report 2015: the global impact of dementia: an analysis of prevalence, incidence, cost and trends. Alzheimer's Disease International; 2015. [Google Scholar]
- 2.What is Alzheimer’s | Alzheimer’s Association. November 2018, https://www.alz.org/alzheimers-dementia/what-is-alzheimers.
- 3.Simonsen A. H., Herukka S. K., Andreasen N., et al. Recommendations for CSF AD biomarkers in the diagnostic evaluation of dementia. Alzheimer's & Dementia. 2017;13(3):274–284. doi: 10.1016/j.jalz.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Fiandaca M. S., Kapogiannis D., Mapstone M., et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimer's & Dementia. 2015;11(6):600–607.e1. doi: 10.1016/j.jalz.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings J., Lee G., Ritter A., Zhong K. Alzheimer’s disease drug development pipeline: 2018. Alzheimer's & Dementia. 2018;4:195–214. doi: 10.1016/j.trci.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panza F., Lozupone M., Dibello V., et al. Are antibodies directed against amyloid-β (Aβ) oligomers the last call for the Aβ hypothesis of Alzheimer’s disease? Immunotherapy. 2019;11(1):3–6. doi: 10.2217/imt-2018-0119. [DOI] [PubMed] [Google Scholar]
- 7.Biogen/Eisai Halt Phase 3 Aducanumab Trials | ALZFORUM. April 2019, https://www.alzforum.org/news/research-news/biogeneisai-halt-phase-3-aducanumab-trials.
- 8.Spielmann N., Wong D. T. Saliva: diagnostics and therapeutic perspectives. Oral Diseases. 2011;17(4):345–354. doi: 10.1111/j.1601-0825.2010.01773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Femminella G. D., Rengo G., Komici K., et al. Autonomic dysfunction in Alzheimer’s disease: tools for assessment and review of the literature. Journal of Alzheimer's Disease. 2014;42(2):369–377. doi: 10.3233/JAD-140513. [DOI] [PubMed] [Google Scholar]
- 10.Farah R., Haraty H., Salame Z., Fares Y., Ojcius D. M., Said Sadier N. Salivary biomarkers for the diagnosis and monitoring of neurological diseases. Biomedical Journal. 2018;41(2):63–87. doi: 10.1016/j.bj.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jasim H., Carlsson A., Hedenberg-Magnusson B., Ghafouri B., Ernberg M. Saliva as a medium to detect and measure biomarkers related to pain. Scientific Reports. 2018;8(1):p. 3220. doi: 10.1038/s41598-018-21131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee M., Guo J. P., Kennedy K., McGeer E. G., McGeer P. L. A method for diagnosing Alzheimer’s disease based on salivary amyloid-β protein 42 levels. Journal of Alzheimer's Disease. 2017;55(3):1175–1182. doi: 10.3233/JAD-160748. [DOI] [PubMed] [Google Scholar]
- 13.McGeer P. L., Guo J. P., Lee M., Kennedy K., McGeer E. G. Alzheimer’s disease can be spared by nonsteroidal anti-inflammatory drugs. Journal of Alzheimer's Disease. 2018;62(3):1219–1222. doi: 10.3233/JAD-170706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huan T., Tran T., Zheng J., et al. Metabolomics analyses of saliva detect novel biomarkers of Alzheimer’s disease. Journal of Alzheimer's Disease. 2018;65(4):1401–1416. doi: 10.3233/JAD-180711. [DOI] [PubMed] [Google Scholar]
- 15.Ashton N. J., Ide M., Schöll M., et al. No association of salivary total tau concentration with Alzheimer’s disease. Neurobiology of Aging. 2018;70:125–127. doi: 10.1016/j.neurobiolaging.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Bakhtiari S., Moghadam N. B., Ehsani M., Mortazavi H., Sabour S., Bakhshi M. Can salivary acetylcholinesterase be a diagnostic biomarker for Alzheimer? Journal of Clinical and Diagnostic Research. 2017;11(1):ZC58–ZC60. doi: 10.7860/JCDR/2017/21715.9192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yilmaz A., Geddes T., Han B. S., et al. Diagnostic biomarkers of Alzheimer’s disease as identified in saliva using 1H NMR-based metabolomics. Journal of Alzheimer's Disease. 2017;58(2):355–359. doi: 10.3233/JAD-161226. [DOI] [PubMed] [Google Scholar]
- 18.Liang Q., Liu H., Zhang T., Jiang Y., Xing H., Zhang A. H. Metabolomics-based screening of salivary biomarkers for early diagnosis of Alzheimer’s disease. RSC Advances. 2015;5(116):96074–96079. doi: 10.1039/C5RA19094K. [DOI] [Google Scholar]
- 19.Lau H.-C., Lee I. K., Ko P. W., et al. Non-invasive screening for Alzheimer’s disease by sensing salivary sugar using Drosophila cells expressing gustatory receptor (Gr5a) immobilized on an extended gate ion-sensitive field-effect transistor (EG-ISFET) biosensor. PLoS One. 2015;10(2, article e0117810) doi: 10.1371/journal.pone.0117810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bermejo-Pareja F., Antequera D., Vargas T., Molina J. A., Carro E. Saliva levels of Abeta1-42 as potential biomarker of Alzheimer’s disease: a pilot study. BMC Neurology. 2010;10(1):p. 108. doi: 10.1186/1471-2377-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim C.-B., Choi Y. Y., Song W. K., Song K. B. Antibody-based magnetic nanoparticle immunoassay for quantification of Alzheimer’s disease pathogenic factor. Journal of Biomedical Optics. 2014;19(5, article 051205) doi: 10.1117/1.jbo.19.5.051205. [DOI] [PubMed] [Google Scholar]
- 22.Boston P. F., Gopalkaje K., Manning L., Middleton L., Loxley M. Developing a simple laboratory test for Alzheimer’s disease: measuring acetylcholinesterase in saliva - a pilot study. International Journal of Geriatric Psychiatry. 2008;23(4):439–440. doi: 10.1002/gps.1882. [DOI] [PubMed] [Google Scholar]
- 23.Sayer R., Law E., Connelly P. J., Breen K. C. Association of a salivary acetylcholinesterase with Alzheimer’s disease and response to cholinesterase inhibitors. Clinical Biochemistry. 2004;37(2):98–104. doi: 10.1016/j.clinbiochem.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Shi M., Sui Y. T., Peskind E. R., et al. Salivary tau species are potential biomarkers of Alzheimer’s disease. Journal of Alzheimer's Disease. 2011;27(2):299–305. doi: 10.3233/JAD-2011-110731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabbagh M. N., Shi J., Lee M., et al. Salivary beta amyloid protein levels are detectable and differentiate patients with Alzheimer’s disease dementia from normal controls: preliminary findings. BMC Neurology. 2018;18(1):p. 155. doi: 10.1186/s12883-018-1160-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pekeles H., Qureshi H. Y., Paudel H. K., Schipper H. M., Gornistky M., Chertkow H. Development and validation of a salivary tau biomarker in Alzheimer’s disease. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring. 2019;11:53–60. doi: 10.1016/j.dadm.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carro E., Bartolomé F., Bermejo-Pareja F., et al. Early diagnosis of mild cognitive impairment and Alzheimer’s disease based on salivary lactoferrin. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring. 2017;8:131–138. doi: 10.1016/j.dadm.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costerus J. M., Brouwer M. C., van de Beek D. Technological advances and changing indications for lumbar puncture in neurological disorders. The Lancet Neurology. 2018;17(3):268–278. doi: 10.1016/S1474-4422(18)30033-4. [DOI] [PubMed] [Google Scholar]
- 29.DeKosky S. T., Marek K. Looking backward to move forward: early detection of neurodegenerative disorders. Science. 2003;302(5646):830–834. doi: 10.1126/science.1090349. [DOI] [PubMed] [Google Scholar]
- 30.Jackson T. A., Moorey H. C., Sheehan B., Maclullich A. M. J., Gladman J. R., Lord J. M. Acetylcholinesterase activity measurement and clinical features of delirium. Dementia and Geriatric Cognitive Disorders. 2017;43(1-2):29–37. doi: 10.1159/000452832. [DOI] [PubMed] [Google Scholar]
- 31.Das A., Rai D., Dikshit M., Palit G., Nath C. Nature of stress: differential effects on brain acetylcholinesterase activity and memory in rats. Life Sciences. 2005;77(18):2299–2311. doi: 10.1016/j.lfs.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 32.Lactoferrin H. ab108882 Lactoferrin Human ELISA Kit. Abcam; 2018. [Google Scholar]
- 33.Welling M. M., Nabuurs R. J. A., van der Weerd L. Potential role of antimicrobial peptides in the early onset of Alzheimer’s disease. Alzheimer's & Dementia. 2015;11(1):51–57. doi: 10.1016/j.jalz.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 34.Ship J. A., DeCarli C., Friedland R. P., Baum B. J. Diminished submandibular salivary flow in dementia of the Alzheimer type. Journal of Gerontology. 1990;45(2):M61–M66. doi: 10.1093/geronj/45.2.M61. [DOI] [PubMed] [Google Scholar]