Abstract
Background
Distant metastasis of early gastric cancer is a rare subgroup and poorly understood. The present study is aimed at summarizing the clinicopathological characteristics, prognosis, and management of clinical T1N0M1 (cT1N0M1) gastric cancer.
Method
Between 2004 and 2015, patients diagnosed with cT1N0M1 gastric cancer were retrospectively analyzed using the Surveillance, Epidemiology, and End Results (SEER) database.
Results
A total of 1093 cT1N0M1 gastric cancer patients were identified. 49 patients (4.5%) received cancer-directed surgery, and 113 patients (10.4%) were managed with radiotherapy. Compared with the other stage IV diseases, a relatively high proportion of black population (19.9% vs. 15.8%), patients older than 60 years (63.1% vs. 57.8%), and adenocarcinoma (59.5% vs. 55.9%) were observed in the cT1N0M1 gastric cancer subgroup. Besides that, patients with cT1N0M1 had the characteristics of less poor differentiated or undifferentiated (54.3% vs. 61.7%). Patients with cT1N0M1 had worse cancer-specific survival (CSS) and overall survival (OS) as compared to the other metastatic gastric cancer patients (CSS: p = 0.002, OS: p = 0.001 for log-rank test). Intriguingly, patients with cT1N0M1 had poor prognosis as compared to patients with cT1N+M1 (CSS: p = 0.015, OS: p = 0.007 for log-rank test). The 3-year and 5-year CSS for patients with cT1N0M1 were 5.7% and 4.0%, respectively. The addition of surgery resulted in improved CSS (p < 0.001 for log-rank test) while radiotherapy was not associated with CSS (p = 0.756 for log-rank test) in patients with cT1N0M1. A multivariate Cox analysis showed that surgery (HR = 0.378, 95% CI: 0.255-0.562) and patients younger than 60 (HR = 0.745, 95% CI: 0.647-0.858) years were independent protective factors for these subgroup patients.
Conclusion
Patients with cT1N0M1 gastric cancer had distinctive clinicopathological characteristics and presented poor prognosis. Knowledge of these differences contributes to guiding clinical evaluation for metastatic gastric cancer patients. More aggressive therapeutic strategy should be highlighted for this subgroup.
1. Introduction
Gastric cancer (GC) is one of the most common cancers worldwide and is responsible for over 1,000,000 new diagnosed cases and an estimated 783,000 deaths in 2018. Besides that, GC remains the third leading cause of cancer mortality [1]. Systemic treatment of GC has remarkably improved the long-term survival of GC patients, especially in early ones, which 5-year survival rate can reach more than 90% [2, 3]. However, the overall prognosis of advanced GC remains very poor, especially in stage IV GC patients with the 5-year survival rate being about 10% [4].
General prognostic factors of GC including depth of wall invasion, lymph node or distant metastasis status, age, and genetic factors have been well recognized [5–7]. Nomograms were built and validated on the basis of prognostic factors for predicting the overall survival or disease-free survival in different GC subgroups with guiding optimal therapy [8–10]. Normally, the deeper the tumor infiltrates and the more lymph node metastasizes, the worse the prognosis of GC patients is. A previous study reported that the survival rates of patients with pT1, pT2, pT3, and pT4 stage tumors were 89.3%, 72.4%, 36.9%, and 23.7%, respectively [5]. And higher lymph node ratios are significantly associated with a shorter overall survival [6]. According to the 8th AJCC staging system, stage IV includes the TanyNanyM1, T1-3N3M0, T4N1-2M0, and T4N3M0 groups. A previous study reported that the survival rate of patients with subclassification IVa gastric cancers was significantly higher than that of patients with subclassification IVb ones [11].
The diagnostic rate of early GC (EGC) has increased in recent years, possibly due to a combination of increased screening and improved diagnostic techniques. EGC is defined as lesion confined to the mucosa and submucosa regardless of status of lymph node with favorable prognosis [12]. Very rare cases of EGC developed to distant metastasis (T1NXM1). But the prognosis of EGC with distant metastasis is poor defined because of the limited cases. Most people may think that T1N0M1 GC patients with mild gastric wall invasion should have better prognosis than T1NanyM1 and other M1 (T2-4NanyM1) patients. Is it justified? In order to address this question, in the present study, we delineated clinicopathological characteristics and prognosis of clinical T1N0M1 (cT1N0M1) gastric cancer using the Surveillance, Epidemiology, and End Results (SEER) database, to develop a clinicopathological risk score that can be used preoperatively to determine the risk of cT1N0M1 patients.
2. Material and Methods
2.1. Data Collection
Patients with metastatic GC were included from the SEER database (2004-2015). Of these, 1093 patients presented stage cT1N0M1. This study was approved by the Institutional Review Board of the Second Affiliated Hospital of Nanjing Medical University. The inclusion criteria were summarized as follows: the site code represented “stomach (143),” patients with distant metastases (M1) according to American Joint Committee on Cancer 7th edition, GC was diagnosed by positive histology or cytology, GC was the only type of primary cancer, and information about cancer-specific survival (CSS) and overall survival (OS) months was clear.
The following data were extracted: gender, age at diagnosis, marital status, race, histologic type, differentiation status, T stage, N stage, surgery, radiation, survival months, CSS, and OS. CSS was defined as the time from the date of diagnosis to the date of death caused by GC.
2.2. Statistical Analysis
The differences between groups were determined by using the χ2 test. The Kaplan-Meier method was utilized to analyze CSS and OS. The difference was identified with log-rank test. Multivariate analyses were performed to recognize the independent prognostic factors for CSS. All statistical analyses were performed with SPSS 25.0.
3. Results
3.1. Characteristics of Patients
A total of 13,253 metastatic GC patients were identified. 1093 patients were diagnosed at stage cT1N0M1. In this subgroup, 49 (4.5%) received cancer-directed surgery and 113 (10.4%) were managed with radiotherapy. 351 patients with records of definite organ metastases were available. 291 patients suffered isolated organ involvement and 60 patients experienced multiple organ metastases. The most commonly single involved site is the liver (53%), followed by the bone (16.8%), the lung (12.3%), and the brain (0.9%). Compared with the other stage IV diseases, a relatively high proportion of black population (19.9% vs. 15.8%), patients older than 60 years (63.1% vs. 57.8%), and signet ring cell carcinoma (59.5% vs. 55.9%) were observed in the cT1N0M1 GC subgroup. Besides that, patients with cT1N0M1 had the characteristics of less poor differentiated or undifferentiated (54.3% vs. 61.7%). No differences were observed in terms of marital status, sex, and metastatic sites. The details are summarized in Table 1.
Table 1.
Clinical characteristics of patients with metastatic gastric cancer according to different clinical stages.
| Variable | cT1N0M1 | Other M1 | p value | ||
|---|---|---|---|---|---|
| Number | % | Number | % | ||
| Age | 0.001 | ||||
| >60 | 690 | 63.1 | 7032 | 57.8 | |
| ≤60 | 403 | 36.9 | 5128 | 42.2 | |
| Sex | 0.262 | ||||
| Male | 603 | 55.2 | 6922 | 56.9 | |
| Female | 490 | 44.8 | 5238 | 43.1 | |
| Marital status | 0.269 | ||||
| Unmarried | 464 | 42.5 | 4878 | 40.1 | |
| Married | 582 | 53.2 | 6784 | 55.8 | |
| Unknown | 47 | 4.3 | 498 | 4.1 | |
| Race | <0.001 | ||||
| White | 719 | 65.8 | 8015 | 65.9 | |
| Black | 217 | 19.9 | 1919 | 15.8 | |
| Others | 157 | 14.4 | 2226 | 18.3 | |
| Histologic type | 0.026 | ||||
| Adenocarcinoma | 650 | 59.5 | 6803 | 55.9 | |
| Signet ring cell carcinoma | 295 | 27.0 | 3369 | 27.7 | |
| Others | 148 | 13.5 | 1988 | 16.3 | |
| Differentiation | <0.001 | ||||
| Well and moderate | 231 | 21.1 | 1768 | 14.5 | |
| Poor and undifferentiated | 593 | 54.3 | 7499 | 61.7 | |
| Unknown | 269 | 24.6 | 2893 | 23.8 | |
| Metastatic site | 0.193 | ||||
| Bone | 59 | 16.8 | 400 | 13.4 | |
| Brain | 3 | 0.9 | 35 | 1.2 | |
| Liver | 186 | 53.0 | 1705 | 57.3 | |
| Lung | 43 | 12.3 | 291 | 9.8 | |
| Multiple organs | 60 | 17.1 | 547 | 18.4 | |
3.2. Survival Outcomes
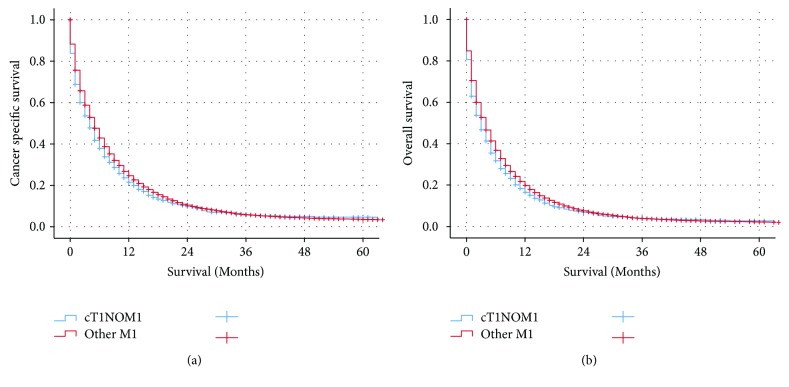
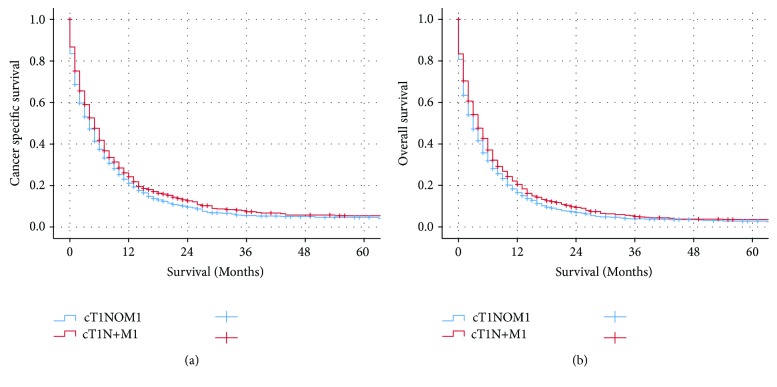
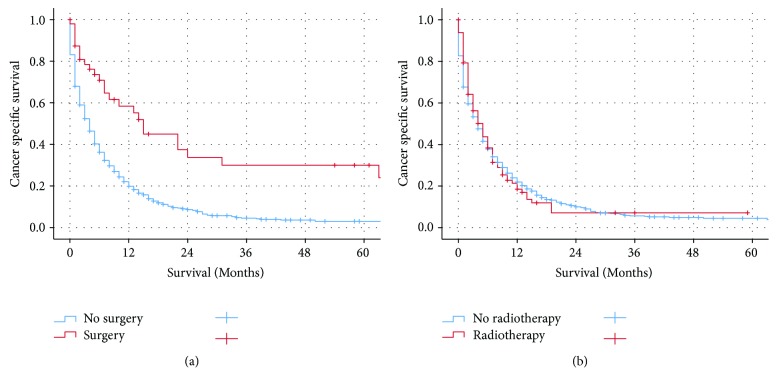
Median CSS for GC patients with stage cT1N0M1, the other stage IV patients, and cT1N+M1 were 4, 5, and 5 months, respectively. Patients with cT1N0M1 had worse cancer-specific survival (CSS) and overall survival (OS) as compared to the other metastatic GC patients (CSS: p = 0.002, OS: p = 0.001 for log-rank test) (Figure 1). Besides that, patients with cT1N0M1 had poor prognosis as compared to patients with cT1N+M1 (CSS: p = 0.015, OS: p = 0.007 for log-rank test) (Figure 2). The 3-year and 5-year CSS for patients with cT1N0M1 were 5.7% and 4.0%, respectively. The 3-year and 5-year CSS for patients with other M1 were 5.8% and 3.5%, respectively. The 3-year and 5-year CSS for patients with cT1N+M1 were 7.4% and 4.9%, respectively. The addition of surgery resulted in improved CSS (p < 0.001 for log-rank test) while radiotherapy was not associated with CSS (p = 0.756 for log-rank test) in GC patients with cT1N0M1 (Figure 3).
Figure 1.
The Kaplan-Meier survival curves revealed that patients with cT1N0M1 had worse cancer-specific survival and overall survival as compared to the other metastatic gastric cancer patients.
Figure 2.
The Kaplan-Meier survival curves revealed that patients with cT1N0M1 had worse cancer-specific survival and overall survival as compared to patients with cT1N+M1.
Figure 3.
The Kaplan-Meier survival curves revealed that the addition of surgery resulted in improved CSS while radiotherapy was not associated with cancer-specific survival in patients with cT1N0M1.
In Cox multivariate regression analysis, surgery (HR = 0.378, 95% CI: 0.255-0.562) and patients younger than 60 years (HR = 0.745, 95% CI: 0.647-0.858) were independent protective factors for this subgroup patients (Table 2).
Table 2.
Univariate and multivariate analysis for cancer-specific survival in cT1N0M1 gastric cancer.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Median survival time (month) | p value | HR (95% CI) | p value | |
| Age | <0.001 | |||
| >60 | 3 | Reference | ||
| ≤60 | 6 | 0.745 (0.647-0.858) | <0.001 | |
| Sex | 0.179 | NI | ||
| Male | 4 | |||
| Female | 4 | |||
| Marital status | 0.034 | NI | ||
| Unmarried | 3 | |||
| Married | 5 | |||
| Unknown | / | / | ||
| Race | 0.011 | NI | ||
| White | 4 | |||
| Black | 4 | |||
| Others | / | |||
| Histologic type | 0.221 | NI | ||
| Adenocarcinoma | 4 | |||
| Signet ring cell carcinoma | 4 | |||
| Others | 6 | |||
| Differentiation | 0.308 | NI | ||
| Well and moderate | 4 | |||
| Poor and undifferentiated | 4 | |||
| Unknown | / | / | ||
| Surgery | <0.001 | |||
| No/unknown | 4 | Reference | ||
| Yes | 15 | 0.378 (0.255-0.562) | <0.001 | |
| Radiation | 0.960 | NI | ||
| No/unknown | 4 | |||
| Yes | 4 | |||
NI: not included in multivariate survival analysis.
4. Discussion
Over the past decades, risk factors of lymph node metastasis in EGC have been well established and scholars have reached consensus on endoscopic submucosal dissection and endoscopic mucosal resection for EGC [13–16]. However, EGC with distant metastasis has been rarely described. Only scattered case reports presented the limited characteristics of this rare situation, and the incidence is about 0.14% [17–20]. To our knowledge, this is the first retrospective study reported clinicopathological characteristics and prognosis of cT1N0M1 GC with large sample size.
Our study demonstrated that surgery improved the prognosis of cT1N0M1 GC patients while radiotherapy did not. This rare entity is consistent with the general stage IV GC in terms of palliative surgery [21]. A previous review has interpreted the survival benefit of gastrectomy compared to nonoperative treatment for stage IV GC [22]. For cT1N0M1 GC, surgery should be taken into account in a proper way. Further studies are needed to establish optimized regimes for the management of this rare entity.
The classic progressive pattern of GC refers to spreading to nearby tissues and perigastric or distant lymph nodes and metastasizing to distant organs. GC seldom presents distant metastases within stage T3. Our study demonstrated that GC patients with cT1N0M1 had worse prognosis as compared to the other stage IV GC patients including stage cT1N+M1. cT1N0M1 GC skipped lymph node involved and directly metastasized to distant organs. We hypothesized that this subgroup is associated with more aggressive tumor behaviors and predicts poor prognosis. Similarly, compared with the other metastatic GC, tumors with mild gastric wall invasion and negative lymph nodes represent more aggressive malignancies with a distinct biology. Unexpectedly, a relatively less proportion of signet ring cell carcinoma and more well-differentiated patients in the cT1N0M1 GC subgroup complicated this rare entity. Precision mechanisms and distinct biology merit further investigation. Collectively, this rare entity requires more intensive intervention and follow-up due to dismal prognosis.
In our present study, the proportion of EGC distant metastasis seems to be higher than previous literature reports [23]. Only 49 (4.5%) patients received cancer-directed surgery with clear pathological outcomes, and the others were diagnosed with clinical stage. We speculate that ultrasound gastroscopy and CT as the main clinical stages for GC may underestimate the clinical T stage. Some patients with stage T2 are often mistaken for stage T1 [24]. However, it remains true that tumors with mild gastric wall invasion metastasizing to distant organs predicted an extremely poor prognosis.
Additionally, there are several limitations in our study. Firstly, stage IV consists of heterogeneous subgroups including TanyNanyM1, T1-3N3M0, T4N1-2M0, and T4N3M0. In the present study, we only compared the prognosis of cT1N0M1 with other stage IV ones (cT1N+M1 and cT2-4NanyM1), but not with T1-3N3M0, T4N1-2M0, and T4N3M0, respectively. Secondly, as mentioned above, the clinical TNM stage of GC patients was determined by imaging results with a gap compared with the pathological TNM stage. The evidences for the diagnosis of distant metastasis are sometimes insufficient. Finally, the study was designed based on the condition of USA population, and the conclusions should thus be extended to other ethnic groups with caution.
In conclusion, we first evaluated clinicopathological characteristics and prognosis of cT1N0M1 GC with large sample size. Our results showed that GC patients with cT1N0M1 had worse CSS and OS as compared to the other M1 GC patients, and patients with cT1N0M1 had poor prognosis as compared to patients with cT1N+M1. Sometimes small tumors go big. Knowledge of these differences is conducive to guiding clinical evaluation for metastatic GC patients and highlighting more aggressive therapeutic strategy.
Acknowledgments
This work was supported by the General Project of Nanjing Medical Technology Development Fund (YKK17208) and the Science and Technology Development Fund of Nanjing Medical University (2016NJMU040).
Contributor Information
Xiang Ma, Email: maxiang_fighting@163.com.
Chi Huang, Email: huangchi1026@126.com.
Data Availability
The datasets are available in the SEER repository and can be obtained from https://seer.cancer.gov.
Disclosure
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Authors' Contributions
Jianbo Han, Junhao Tu, and Chaoyang Tang contributed equally to this work.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z., Ma L., Zhang X. M., Zhou Z. X. Long-term outcomes after D2 gastrectomy for early gastric cancer: survival analysis of a single-center experience in China. Asian Pacific Journal of Cancer Prevention. 2014;15(17):7219–7222. doi: 10.7314/APJCP.2014.15.17.7219. [DOI] [PubMed] [Google Scholar]
- 3.Choi I. J., Lee J. H., Kim Y. I., et al. Long-term outcome comparison of endoscopic resection and surgery in early gastric cancer meeting the absolute indication for endoscopic resection. Gastrointestinal Endoscopy. 2015;81(2):333–341.e1. doi: 10.1016/j.gie.2014.07.047. [DOI] [PubMed] [Google Scholar]
- 4.Yagi Y., Seshimo A., Kameoka S. Prognostic factors in stage IV gastric cancer: univariate and multivariate analyses. Gastric Cancer. 2000;3(2):71–80. doi: 10.1007/PL00011699. [DOI] [PubMed] [Google Scholar]
- 5.Lu J., Huang C. M., Zheng C. H., et al. Consideration of tumor size improves the accuracy of TNM predictions in patients with gastric cancer after curative gastrectomy. Surgical Oncology. 2013;22(3):167–171. doi: 10.1016/j.suronc.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Zhu J., Xue Z., Zhang S., et al. Integrated analysis of the prognostic role of the lymph node ratio in node-positive gastric cancer: a meta-analysis. International Journal of Surgery. 2018;57:76–83. doi: 10.1016/j.ijsu.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Ma X., Yang C., Tang R., et al. Association between LMP2 and LMP7 gene polymorphisms and the risk of gastric cancer: a case-control study. Oncology Letters. 2015;10(1):509–517. doi: 10.3892/ol.2015.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Z. F., Lu J., Wang W., et al. Development and external validation of a simplified nomogram predicting individual survival after R0 resection for gastric cancer: an international, multicenter study. Annals of Surgical Oncology. 2018;25(8):2383–2390. doi: 10.1245/s10434-018-6551-1. [DOI] [PubMed] [Google Scholar]
- 9.Chen S., Chen X., Nie R., et al. A nomogram to predict prognosis for gastric cancer with peritoneal dissemination. Chinese Journal of Cancer Research. 2018;30(4):449–459. doi: 10.21147/j.issn.1000-9604.2018.04.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pietrantonio F., Barretta F., Fanotto V., et al. Estimating survival probabilities of advanced gastric cancer patients in the second-line setting: the gastric life nomogram. Oncology. 2018;95(6):344–352. doi: 10.1159/000491753. [DOI] [PubMed] [Google Scholar]
- 11.An Q., Song X., Li Y., Shi X. Y. Prognostic significance of subclassification of stage IV gastric cancer according to pTNM categories. Hepato-Gastroenterology. 2010;57(102-103):1325–1329. [PubMed] [Google Scholar]
- 12.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14(2):113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 13.Yang H. J., Kim S. G., Lim J. H., et al. Predictors of lymph node metastasis in patients with non-curative endoscopic resection of early gastric cancer. Surgical Endoscopy. 2015;29(5):1145–1155. doi: 10.1007/s00464-014-3780-7. [DOI] [PubMed] [Google Scholar]
- 14.Lee J. H., Nam B. H., Ryu K. W., et al. Tumor differentiation is not a risk factor for lymph node metastasis in elderly patients with early gastric cancer. European Journal of Surgical Oncology. 2014;40(12):1771–1776. doi: 10.1016/j.ejso.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 15.Lee J. H., Choi I. J., Han H. S., et al. Risk of lymph node metastasis in differentiated type mucosal early gastric cancer mixed with minor undifferentiated type histology. Annals of Surgical Oncology. 2015;22(6):1813–1819. doi: 10.1245/s10434-014-4167-7. [DOI] [PubMed] [Google Scholar]
- 16.Ono H., Yao K., Fujishiro M., et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Digestive Endoscopy. 2016;28(1):3–15. doi: 10.1111/den.12518. [DOI] [PubMed] [Google Scholar]
- 17.Sakurai K., Muguruma K., Murata A., et al. Early gastric cancer with suspected brain metastasis arising eight years after curative resection: a case report. BMC Research Notes. 2014;7(1):p. 818. doi: 10.1186/1756-0500-7-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anagnostopoulos G., Sakorafas G. H., Kostopoulos P., Margantinis G., Tsiakos S., Pavlakis G. Early (mucosal) gastric cancer with synchronous osteosclerotic bone metastases: a case report. European Journal of Cancer Care. 2010;19(4):554–557. doi: 10.1111/j.1365-2354.2007.00847.x. [DOI] [PubMed] [Google Scholar]
- 19.Hwang E. J., Jang J. Y., Kim Y. W., et al. A case of early gastric cancer with solitary metastasis to the pleura. Clinical Endoscopy. 2013;46(6):666–670. doi: 10.5946/ce.2013.46.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiomi M., Kamisako T., Yutani I., et al. Two cases of histopathologically advanced (stage IV) early gastric cancers. Tumori Journal. 2001;87(3):191–195. doi: 10.1177/030089160108700315. [DOI] [PubMed] [Google Scholar]
- 21.Smith J. K., Hill J. S., Ng S. C., McDade T. P., Shah S. A., Tseng J. F. Potential benefit of resection for stage IV gastric cancer: a national survey. Journal of Gastrointestinal Surgery. 2010;14(11):1660–1668. doi: 10.1007/s11605-010-1351-3. [DOI] [PubMed] [Google Scholar]
- 22.Lasithiotakis K., Antoniou S. A., Antoniou G. A., Kaklamanos I., Zoras O. Gastrectomy for stage IV gastric cancer. A systematic review and meta-analysis. Anticancer Research. 2014;34(5):2079–2085. [PubMed] [Google Scholar]
- 23.Lawrence M., Shiu M. H. Early gastric cancer. Twenty-eight-year experience. Annals of Surgery. 1991;213(4):327–334. doi: 10.1097/00000658-199104000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pongpornsup S., Neungton P., Chairoongruang S., Apisamrnthanarak P. Diagnostic performance of multidetector computed tomography (MDCT) in evaluation for peritoneal metastasis in gastric cancer. Journal of the Medical Association of Thailand. 2014;97(8):863–869. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets are available in the SEER repository and can be obtained from https://seer.cancer.gov.