Abstract
Objectives
Type 2 diabetes mellitus (T2DM) is associated with coronary artery calcification (CAC) which is an independent risk factor for cardiovascular events. Metformin is the first-line antidiabetic medication. We aimed to investigate the association between metformin use and CAC.
Methods
We included 369 patients with T2DM in this cross-sectional study. CAC scores, clinical characteristics, and antidiabetic drug prescription information of the patients were acquired. Baseline parameters were balanced for metformin and nonmetformin users using the propensity score matching (PSM) strategy.
Results
Among the 369 subjects who met our inclusion criteria, 288 subjects were included for further analysis after PSM. Metformin prescription rather than other antidiabetic medications was related to lower CAC scores (OR [95% CI] = 0.55 [0.34–0.90]; P = 0.018). Further multivariable logistic regression analysis demonstrated that metformin was negatively associated with CAC severity (OR [95% CI] = 0.58 [0.34–0.99]; P = 0.048), which was independent of age, BMI, eGFR, gender, cigarette smoking, duration of diabetes, hypertension, statin prescription, and number of nonmetformin antidiabetic agents. A subgroup analysis revealed a significant association between metformin and CAC scores in smokers (OR [95% CI] = 0.38 [0.16–0.93]; P = 0.035), but the association was not observed in never-smokers (OR [95% CI] = 0.72 [0.34–1.51]; P = 0.383).
Conclusions
Metformin usage was independently associated with lower CAC scores in T2DM patients. The negative correlation between CAC scores and metformin was most prominent in patients with a history of cigarette smoking.
1. Introduction
During the past decades, compelling evidence has demonstrated that coronary artery calcification (CAC) is an independent risk factor of cardiovascular events. Moreover, CAC is a challenge for percutaneous coronary intervention (PCI) and is linked with increased post-PCI events. However, there is no clinically proved therapy for vascular calcification [1]. Type 2 diabetes mellitus (T2DM) is deemed as a coronary artery disease (CAD) equivalent [2], which doubles or even triples the CAD incidence [3]. Moreover, T2DM patients tend to suffer from more calcified and diffuse coronary artery lesions, while having a blunted appreciation of ischemic episodes [4].
Imaging by computed tomography (CT) reveals that T2DM-affected individuals have extensive calcification of their vascular beds, which is reported as the CAC scores, reflecting significant cardiovascular disease burden [5, 6]. The CAC score has independent added value beyond traditional risk factors in predicting the outcome of major cardiovascular events, especially in asymptomatic patients [7]. In patients with an intermediate Framingham risk score, CAC scores less than 99, between 100 and 399, and more than 400 are related to 0.4%, 1.3%, and 2.4% of annual CAD death, respectively [8].
As the first-line antidiabetic therapy, recent studies indicate that metformin has highlighted effect on alleviating vascular calcification. We and other groups reported that metformin prevents vascular calcification via AMP-activated protein kinase (AMPK) activation [9, 10], and we identify that metformin prevents atherosclerotic calcification in mice [10]. Moreover, recent clinical data showed that metformin prescription was independently associated with a decreased level of lower-limb arterial calcification [11]. Thus, it is reasonable to hypothesize that metformin therapy may be associated with lower levels of CAC severity in T2DM patients. We therefore performed a cross-sectional study in a population of asymptomatic T2DM patients to evaluate the association between metformin use and CAC scores.
2. Materials and Methods
2.1. Study Design
This is a cross-sectional study conducted among in-hospital T2DM patients who underwent coronary artery CT for preoperative screening between June 1st, 2016, and May 31st, 2017, in the Second Affiliated Hospital, Zhejiang University School of Medicine. Those patients were candidates for noncardiac surgery including hip/knee replacement, lumbar surgery, radical resection of pulmonary carcinoma, and cerebral artery aneurysm intervention. Main inclusion criteria were (1) diagnosed as T2DM and took antidiabetic drugs regularly for at least 3 months and (2) antidiabetic drug prescription remained unchanged for the last 3 months. Main exclusion criteria were (1) a history of CAD or PCI or coronary artery bypass grafting or clinical presentation of CAD like chest pain and shortness of breath; (2) type 1 diabetes mellitus; and (3) glomerular filtration rate ≤ 30 mL/min measured by the CKD-EPI equation [12]. The study was conducted on the grounds of the Declaration of Helsinki and approved by the Ethics Committee of the Second Affiliated Hospital, Zhejiang University School of Medicine.
2.2. CAC Quantification
Coronary artery CT images were acquired with Siemens SOMATOM definition flash CT. A radiologist who was blinded to patients reread all CT scans and reassessed their CAC scores. CAC scores were calculated with the Agatston method [13], i.e., by multiplying the area with a density above 130 Hounsfield units (Hu) by a factor reflecting the maximum attenuation.
2.3. Data Collection
We collected fasting blood data within 7 days before the coronary artery CT scan, in avoidance of the interference of contrast-induced nephropathy. This work was accomplished by one clinician, who was blinded to patients' medical history. Hemoglobin A1c (HbA1c), serum creatinine, fasting glucose, calcium, phosphate, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG) results were collected. The CKD-EPI equation was adopted for estimating the glomerular filtration rate (eGFR).
2.4. Statistical Analyses
Data were presented as means ± standard deviation (SD) or median (25th and 75th percentiles) for continuous variables, as appropriate. Data were expressed as the number (percentage) for qualitative variables. Data were categorized into metformin and nonmetformin users. Major imbalances were found in age, BMI, history of hypertension, eGFR, and glucosidase inhibitor usage between metformin and nonmetformin users. Those imbalanced factors were used to perform propensity score matching (PSM) for patients in two groups. A multivariable logistic regression model including these variables was applied. Matching was performed using the nearest neighbor matching, with a default caliper of 0.1. The CAC score = 100 Agatston units was set as the cutoff point in accordance with the risk classification in ACCF/AHA 2007. The χ 2 test was used to compare categorical variables, and Student's t-test or the Mann–Whitney test was used for continuous variables between the two groups. The association between antidiabetic drugs and CAC scores was analyzed by univariate logistic regression. To assess the independence of this association, we performed a multivariable logistic regression that included both variables identified in univariate analyses and relevant clinical variables or demographic factors (age, BMI, gender, eGFR, duration of diabetes and hypertension, and cigarette smoking). Furthermore, we did a subgroup analysis among male and female patients, which demonstrated that there was no gender difference. Another subgroup analysis among patients with or without a history of smoking was processed. Multivariable logistic regression with the same adjustments was used in the subgroup analysis. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 22.0 (IBM, Armonk, New York).
3. Results
3.1. Demographic and Clinical Characteristics
Among the 656 subjects, we excluded 287 subjects who did not meet our inclusion criteria. The remaining 369 subjects were divided into the metformin group (n = 150) and the nonmetformin group (n = 219). The median CAC score in the metformin group was 8.05 (0-124.6), and the score was almost octupled in the nonmetformin group (61.6 (0-319.6), P = 0.005) (Figure 1). Major imbalances were spotted in age (P < 0.001), BMI (P = 0.005), history of hypertension (P = 0.011), eGFR (P < 0.001), and glucosidase inhibitor usage (P = 0.012) between metformin and nonmetformin users. We performed PSM of the imbalanced factors and excluded another 81 unmatched subjects. Demographic and clinical characteristics of metformin and nonmetformin users before and after PSM were presented in Table 1, and the flow chart of exclusion was presented in Supplemental Figure 1. The median CAC score in the metformin group after PSM was 8.05 (0-124.6), and the score in the nonmetformin group after PSM was 37.00 (0.00, 220.50), but P value is marginal (0.097) (Table 1, Figure 1).
Figure 1.
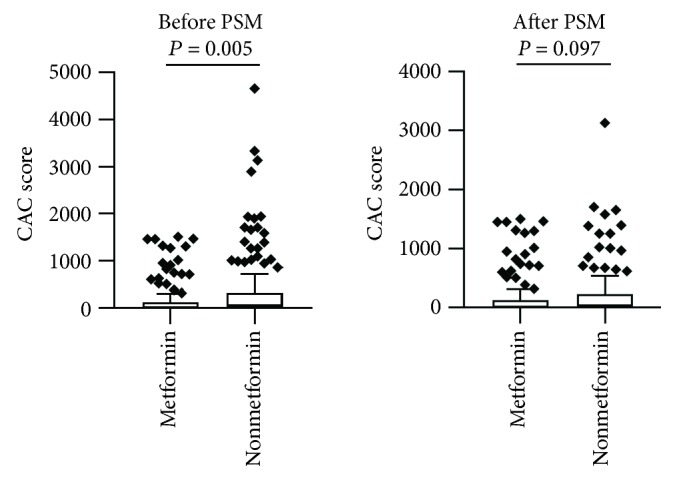
The comparison of CAC scores between patients treated with or without metformin. The Mann–Whitney test was used for the comparison between patients treated with or without metformin. The median CAC scores were 8.05 (0, 124.6) and 61.6 (0, 319.6), respectively (P = 0.005), before propensity score matching (PSM). And median CAC scores were 8.05 (0, 124.6) and 37.00 (0.00, 220.50) (P = 0.097) after matching.
Table 1.
The characteristics of included patients according to metformin usage.
| Variables | Metformin usage (before PSM) | Metformin usage (after PSM) | ||||
|---|---|---|---|---|---|---|
| Metformin (n = 150) | Nonmetformin (n = 219) | P value | Metformin (n = 150) | Nonmetformin (n = 138) | P value | |
| Age | 65.04 ± 9.47 | 70.03 ± 10.12 | <0.001 | 65.04 ± 9.47 | 66.91 ± 9.69 | 0.100 |
| BMI | 25.20 ± 3.36 | 24.18 ± 3.52 | 0.005 | 25.20 ± 3.36 | 24.69 ± 3.41 | 0.206 |
| Male gender | 96 (64.00%) | 132 (60.27%) | 0.469 | 96 (64.00%) | 85 (61.59%) | 0.673 |
| Smoking | 61 (40.67%) | 74 (33.79%) | 0.178 | 61 (40.67%) | 52 (37.68%) | 0.604 |
| DM duration | 10.00 (4.75, 15.00) | 8.00 (3.00, 15.00) | 0.119 | 10.00 (4.75, 15.00) | 7.00 (3.00, 15.00) | 0.039 |
| HTN | 93 (62.00%) | 163 (74.43%) | 0.011 | 93 (62.00%) | 98 (71.01%) | 0.106 |
| HTN duration | 6.00 (0.00, 15.00) | 10.00 (0.00, 20.00) | 0.605 | 6.00 (0.00, 15.00) | 5.25 (0.00, 15.00) | 0.513 |
| HbA1c (%) | 7.50 (6.70, 8.15) | 7.30 (6.78, 8.43) | 0.990 | 7.50 (6.70, 8.15) | 7.30 (6.80, 8.48) | 0.988 |
| eGFR (mL/min/1.73 m2) | 96.53 (90.49, 105.50) | 92.02 (81.63, 99.23) | <0.001 | 96.53 (90.49, 105.50) | 94.82 (85.60, 101.10) | 0.037 |
| FBG (mmol/L) | 6.64 (5.52, 8.57) | 7.11 (5.76, 8.70) | 0.480 | 6.64 (5.52, 8.57) | 7.04 (5.66, 8.94) | 0.395 |
| Ca (mmol/L) | 2.28 ± 0.16 | 2.25 ± 0.12 | 0.020 | 2.29 ± 0.16 | 2.26 ± 0.13 | 0.098 |
| P (mmol/L) | 1.15 ± 0.19 | 1.13 ± 0.20 | 0.429 | 1.15 ± 0.19 | 1.13 ± 0.20 | 0.361 |
| LDL-C (mmol/L) | 2.25 (1.61, 2.75) | 2.30 (1.72, 2.89) | 0.141 | 2.25 (1.61, 2.75) | 2.48 (1.75, 2.99) | 0.171 |
| HDL-C (mmol/L) | 1.05 (0.89, 1.28) | 1.09 (0.91, 1.27) | 0.835 | 1.05 (0.89, 1.28) | 1.13 (0.93, 1.29) | 0.153 |
| TG (mmol/L) | 1.46 (1.01, 2.15) | 1.39 (0.98, 1.89) | 0.219 | 1.46 (1.01, 2.15) | 1.56 (0.99, 1.92) | 0.900 |
| Sulfonylureas | 50 (33.33%) | 86 (39.27%) | 0.246 | 50 (33.33%) | 56 (40.58%) | 0.203 |
| Glinides | 28 (18.67%) | 27 (12.33%) | 0.093 | 28 (18.67%) | 20 (14.49%) | 0.429 |
| GI | 49 (32.67%) | 100 (45.66%) | 0.012 | 49 (32.67%) | 53 (38.41%) | 0.309 |
| TZD | 4 (2.67%) | 12 (5.48%) | 0.193 | 4 (2.67%) | 8 (5.80%) | 0.302 |
| DPP4 inhibitor | 2 (1.33%) | 5 (2.28%) | 0.511 | 2 (1.33%) | 2 (1.45%) | 0.675 |
| Insulin | 26 (17.33%) | 54 (24.65%) | 0.094 | 26 (17.33%) | 31 (24.65%) | 0.345 |
| Statins | 54 (36.00%) | 62 (28.31%) | 0.118 | 54 (36.00%) | 43 (22.46%) | 0.385 |
| Antiplatelets | 47 (31.33%) | 70 (31.96%) | 0.898 | 47 (31.33%) | 43 (31.16%) | 0.975 |
| CAC scores | 8.05 (0, 124.6) | 61.6 (0, 319.6) | 0.005 | 8.05 (0, 124.6) | 37.00 (0.00, 220.50) | 0.097 |
BMI: body mass index; DM: diabetes mellitus; GI: glucosidase inhibitors; HTN: hypertension; eGFR: estimated glomerular filtration rate; FBG: fasting blood glucose; Ca: calcium; P: phosphorus; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; TG: triglyceride; DPP4: dipeptidyl peptidase-4; TZD: thiazolidinediones; PSM: propensity score match.
In accordance with the risk classification in ACCF/AHA 2007, we categorized the subjects as CAC < 100 and CAC ≥ 100 to clarify the baseline differences between subjects with mild and moderate calcification (Table 2). Subjects in the higher CAC score group were significantly older and had longer history of hypertension. The higher CAC score was also related to lower eGFR. There were no significant intergroup differences for BMI, gender, smoking status, or relevant laboratory index including serum calcium, phosphate, fasting glucose, LDL-C, HDL-C, or TG. The data of HbA1c of 114 subjects were missing, but there was no intergroup difference in the remaining 255 subjects. Another interesting finding was that a higher percentage of subjects underwent statins or antiplatelet therapies, like aspirin and clopidogrel in the group with higher CAC scores (P < 0.05). The associations remained unchanged after PSM.
Table 2.
The characteristics of included patients according to CAC scores.
| Variables | CAC scores (before PSM) | CAC scores (after PSM) | ||||
|---|---|---|---|---|---|---|
| Score < 100 (n = 226) | Score ≥ 100 (n = 143) | P value | Score < 100 (n = 187) | Score ≥ 100 (n = 101) | P value | |
| Age | 65.65 ± 9.65 | 71.73 ± 9.83 | <0.001 | 64.15 ± 9.25 | 69.24 ± 9.41 | <0.001 |
| BMI | 24.57 ± 3.47 | 24.63 ± 3.53 | 0.886 | 24.96 ± 3.47 | 24.18 ± 3.52 | 0.987 |
| Male gender | 143 (63.27%) | 85 (59.44) | 0.460 | 119 (63.64%) | 62 (61.39%) | 0.706 |
| Smoking | 77 (34.07%) | 58 (40.56) | 0.207 | 68 (36.36%) | 45 (44.55%) | 0.174 |
| DM duration | 9.50 (4.00, 5.00) | 10.00 (3.00,15.00) | 0.814 | 9.00 (4.00, 15.00) | 10.00 (3.00, 15.00) | 0.993 |
| HTN | 149 (65.93%) | 107 (74.83) | 0.071 | 119 (63.64%) | 72 (71.29%) | 0.190 |
| HTN duration | 5.00 (0.00, 15.00) | 10.00 (0.00, 20.00) | 0.002 | 5.00 (0.00, 15.00) | 8.00 (0.00, 15.00) | 0.087 |
| HbA1c (%) | 7.4 (6.7, 8.3) | 7.4 (6.8, 8.2) | 0.790 | 7.40 (6.70-8.25) | 7.45 (6.90-8.20) | 0.456 |
| eGFR (mL/min/1.73 m2) | 95.70 (88.02, 104.88) | 91.75 (81.47, 98.46) | <0.001 | 97.40 (89.74, 105.80) | 92.46 (85.55, 99.03) | <0.001 |
| FBG (mmol/L) | 7.04 (5.91, 8.90) | 6.82 (5.44, 8.39) | 0.220 | 7.00 (5.93-8.94) | 6.82 (5.38-8.35) | 0.205 |
| Ca (mmol/L) | 2.26 ± 0.13 | 2.26 ± 0.16 | 0.963 | 2.27 ± 0.12 | 2.27 ± 0.18 | 0.924 |
| P (mmol/L) | 1.13 ± 0.19 | 1.16 ± 0.21 | 0.151 | 1.13 ± 0.19 | 1.15 ± 0.21 | 0.399 |
| LDL-C (mmol/L) | 2.27 (1.70, 2.85) | 2.25 (1.60, 2.82) | 0.603 | 2.23 (1.71-2.88) | 2.27 (1.58-2.97) | 0.848 |
| HDL-C (mmol/L) | 1.09 (0.81, 1.28) | 1.03 (0.89, 1.27) | 0.267 | 1.11 (0.92-1.29) | 1.03 (0.88-1.27) | 0.158 |
| TG (mmol/L) | 1.41 (0.99, 2.04) | 1.39 (0.97, 1.88) | 0.497 | 1.50 (1.00-2.06) | 1.56 (0.98-2.00) | 0.995 |
| Metformin | 107 (47.34%) | 43 (30.07%) | 0.001 | 107 (57.22%) | 43 (42.57%) | 0.018 |
| Sulfonylureas | 82 (36.28%) | 54 (37.76%) | 0.774 | 68 (36.36%) | 38 (37.62%) | 0.832 |
| Glinides | 33 (14.60%) | 22 (15.38%) | 0.837 | 31 (16.58%) | 17 (16.83%) | 0.956 |
| GI | 85 (37.61%) | 64 (44.76%) | 0.173 | 65 (34.76%) | 37 (36.63%) | 0.751 |
| TZD | 10 (4.42%) | 6 (4.29%) | 0.916 | 7 (3.74%) | 5 (4.95%) | 0.857 |
| DPP4 inhibitor | 5 (2.21%) | 2 (1.40%) | 0.711 | 4 (2.14%) | 0 (0.00%) | 0.341 |
| Insulin | 47 (20.80%) | 33 (23.07%) | 0.605 | 36 (19.25%) | 21 (20.79%) | 0.754 |
| Statins | 62 (27.43%) | 54 (37.76%) | 0.037 | 53 (28.34%) | 44 (43.56%) | 0.009 |
| Antiplatelets | 59 (26.11%) | 58 (40.56%) | 0.004 | 48 (25.67%) | 42 (41.58%) | 0.005 |
BMI: body mass index; DM: diabetes mellitus; GI: glucosidase inhibitors; HTN: hypertension; eGFR: estimated glomerular filtration rate; FBG: fasting blood glucose; Ca: calcium; P: phosphorus; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; TG: triglyceride; DPP4: dipeptidyl peptidase-4; TZD: thiazolidinediones; PSM: propensity score match.
Notably, the CAC scores of 146 subjects were 0. In order to investigate the characteristics of subjects with CAC = 0, we classified subjects into CAC = 0, 0 < CAC < 100, and CAC ≥ 100 (Supplemental Table 1). Metformin was used in 46.58%, 49.37%, and 30.07% subjects in CAC = 0, 0 < CAC < 100, and CAC ≥ 100 groups, respectively. The intergroup difference was also significant (P = 0.004).
3.2. Association between Antidiabetic Therapy and CAC Severity
As per Table 2, metformin usage had a significant difference between CAC < 100 and ≥100 groups. Interestingly, 47.3% of subjects were treated with metformin in the CAC < 100 group, while the proportion was 30.1% in the ≥100 group, indicating that metformin usage corresponded to the lower CAC scores. Subjects in the groups of CAC < 100 and ≥100, respectively, took 1.16 ± 0.69 and 1.26 ± 0.76 kinds of nonmetformin antidiabetic drugs, which were not significantly different between the two groups (P = 0.230). The estimation association remained unchanged after PSM (Table 2).
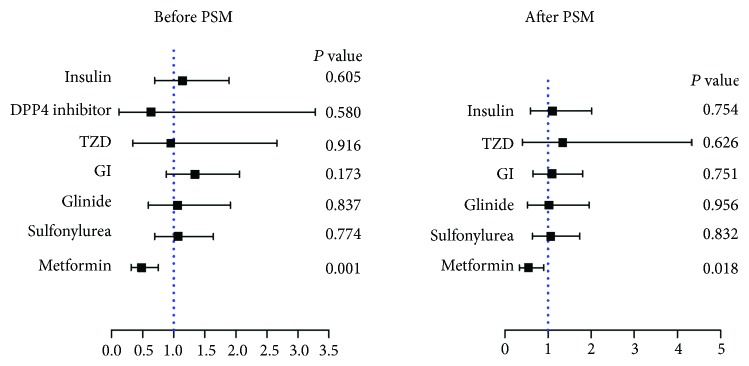
Univariate logistic regression was further applied, with CAC score = 100 as the cutoff point. The result confirmed that metformin prescription was related to lower CAC scores (OR [95% CI] = 0.55 [0.34–0.90]; P = 0.018). In contrast, the usage of other antidiabetic medicines did not affect the CAC scores (Figure 2, Supplemental Table 2).
Figure 2.
The association between metformin and other antidiabetic medicines and CAC scores. Metformin was negatively related to the CAC score before (OR [95% CI] = 0.48 [0.31–0.75]; P = 0.001) and after propensity score matching (OR [95% CI] = 0.55 [0.34–0.90]; P = 0.018). BMI: body mass index; DM: diabetes mellitus; HTN: hypertension; eGFR: estimated glomerular filtration rate; NMA: nonmetformin antidiabetic agents; PSM: propensity score match. Since the DPP4 inhibitor user was 0 among subjects with CAC ≥ 100 after propensity score matching, we omitted this data accordingly.
3.3. Independent Association between Metformin and CAC Severity
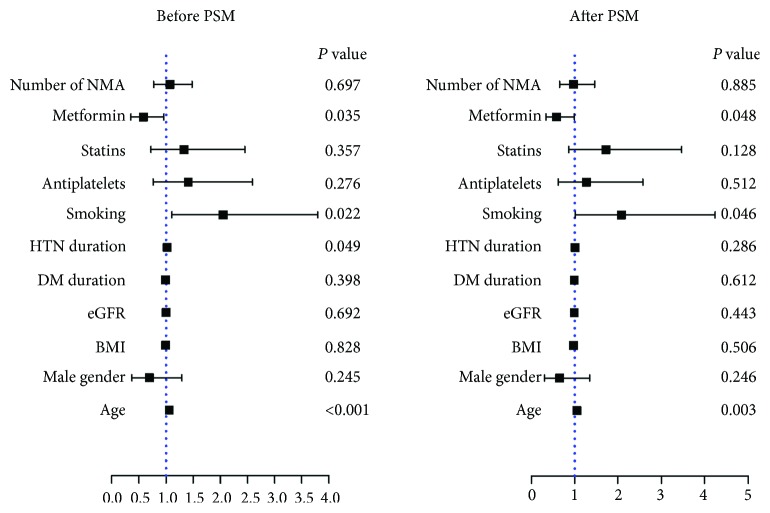
Univariate logistic regression analysis was adopted to recognize factors that were statistically significant between CAC < 100 and ≥100. Among all the clinical characteristics, we identified eGFR (P < 0.001), metformin (P = 0.018), statins (P = 0.009), and antiplatelet drug prescription (P = 0.005) as predictive factors. Since combinations of medication might impact the outcome, we also took the number of nonmetformin antidiabetic drugs into consideration. Those factors together with demographic characteristics including age, BMI, male gender, smoking, and duration of diabetes and hypertension were reevaluated in a multivariable logistic regression analysis. The result indicated that metformin was still significantly associated with CAC severity (OR [95% CI] = 0.58 [0.35–0.96]; P = 0.035) (Figure 3, Supplemental Table 3). The association between metformin and CAC scores was consistent after PSM (OR [95% CI] = 0.58 [0.34–0.99]; P = 0.048, Figure 3, Supplemental Table 3).
Figure 3.
The independent association of metformin and other factors with CAC scores. The multivariable logistic regression analysis demonstrated that metformin was negatively associated with CAC severity before (OR [95% CI] = 0.58 [0.35–0.96]; P = 0.035) and after propensity score matching (OR [95% CI] = 0.58 [0.34–0.99]; P = 0.048), while smoking (OR [95% CI] = 2.08 [1.01–4.24]; P = 0.046) and age (OR [95% CI] = 1.05 [1.02–1.09]; P = 0.003) were positively associated with CAC severity. BMI: body mass index; DM: diabetes mellitus; HTN: hypertension; eGFR: estimated glomerular filtration rate; NMA: nonmetformin antidiabetic agents; PSM: propensity score match.
3.4. Association between Metformin and CAC Severity in Smokers or Never-Smokers
Cigarette smoking was positively related to CAC scores (after PSM, OR [95% CI] = 2.08 [1.01-4.24]; P = 0.046). The interaction between smoking and metformin use was not significant (P = 0.371). Since smoking was the only modifiable factor that was associated with CAC scores, we decided to take a further look at this factor. One hundred and thirty-five subjects with a history of cigarette smoking were included. Notably, in our study, all smokers were male. In order to rule out gender difference, we first did a sensitivity analysis by looking at the association between metformin and CAC scores in both genders. A CAC score = 100 was set as the cutoff point in the analysis. After adjustment of the predictive factors and demographic characteristics, metformin was not related to CAC in male (OR [95% CI] = 0.64 [0.34–1.20]; P = 0.162) and female (OR [95% CI] = 0.42 [0.17–1.03]; P = 0.059) subgroups.
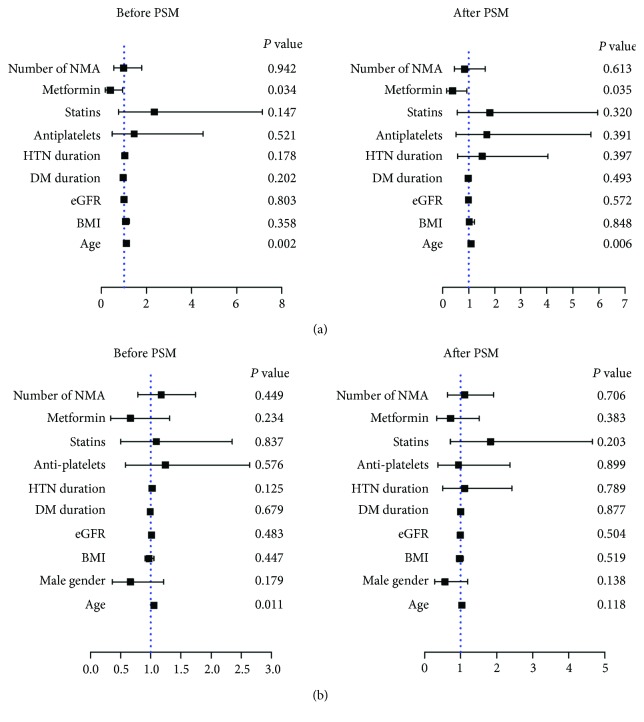
We proceeded to perform subgroup analysis in subjects with or without a history of smoking. Multivariable logistic regression revealed a significant association between metformin and CAC scores in smokers (OR [95% CI] = 0.38 [0.16–0.93]; P = 0.035) (Figure 4(a), Supplemental Table 4), but the association diminished in never-smokers (OR [95% CI] = 0.72 [0.34–1.51]; P = 0.383) (Figure 4(b), Supplemental Table 5).
Figure 4.
The relationship of CAC scores and metformin usage in smokers and never-smokers. Multivariable logistic regression revealed a significant association between metformin and CAC scores in male smokers before (OR [95% CI] = 0.40 [0.17–0.94]; P = 0.034) and after propensity score matching (PSM, OR [95% CI] = 0.38 [0.16–0.93]; P = 0.035) (a), but the association was not observed in never-smokers (OR [95% CI] = 0.72 [0.34–1.51]; P = 0.383) (b). BMI: body mass index; DM: diabetes mellitus; HTN: hypertension; eGFR: estimated glomerular filtration rate; NMA: nonmetformin antidiabetic agents.
4. Discussion
In the present study, we investigated the association of metformin on CAC severity in T2DM populations. The major finding is that metformin is linked to a lower level of CAC scores in T2DM patients, especially in those with a history of cigarette smoking.
Metformin has been established for its unshakeable status in T2DM therapy as it reduces the morbidity and mortality of macrovascular complications [14]. Long-term metformin therapy is related to a 33% reduction in myocardial infarction [15]. In the CAMERA study, investigators also explored the effect of metformin on nondiabetic CAD. Even though metformin reduced the HbA1c level and improved insulin resistance, there was no difference in carotid intima-media thickness (cIMT) progression between the metformin and placebo groups [16]. However, the result was confounded by the small sample size and the use of statins, which made the window of opportunity for cIMT improvement rather small. We believe that the undergoing large randomized clinical trial GLINT might bring good news on the use of metformin in CAD patients without T2DM.
So far, the mechanism of metformin in CAD still needs to be explored. A randomized placebo-controlled trial conducted in 50 HIV-infected subjects with metabolic syndrome demonstrated that metformin reduces CAC progression [17], but the validity of the result in general diabetic population was hampered by the small sample size and HIV infection status in the study. Moreover, a recent study indicated that metformin treatment had protective effect on coronary atherosclerosis in male prediabetic population, but the result was inconsistent in female subjects [6]. However, in the present study, we found that there was no gender difference in the association between metformin and CAC. This might be explained by the difference in participants' age. In the former study, the average of female subjects was 49.1 ± 9.3, including 38% premenopausal, while the average age of female was 69.3 ± 9.8 in our study. It would be interesting to further investigate the association of metformin and CAC in premenopausal diabetic patients.
As mentioned, the current antidiabetic regimen was defined as drugs that were taken regularly without modification in prescription for at least 3 months. It was of interest that metformin rather than other antidiabetic agents was associated with lower CAC scores in our study. Since we did not have the information of antidiabetic medications they received in the past, subjects who discontinued metformin before the study gave rise to time-related bias. Yet, this bias tightened the association between metformin and CAC scores. It was because some patients in the nonmetformin group had metformin before the study, which would weaken the difference in the CAC scores between the metformin and nonmetformin groups in the study. A number of clinical studies had shown that metformin had cardiovascular protective effects independent of glucose-lowering effects [6, 18]. Since the CAC score is a well-accepted risk factor for cardiovascular events, the preventive effect of metformin on CAD, may at least partially, attributes to its role in reducing CAC.
The mechanisms by which metformin might result in lower CAC are still not fully understood. Our group revealed that metformin prevents atherosclerotic calcification via the activation of AMPK and subsequent Runx2 degradation [10]. Cao, et al. reported that metformin upregulated endothelial nitric oxide synthase (eNOS) in vascular smooth muscle cells to prevent calcification [9]. However, other mechanisms may also be involved, such as proautophagy, antioxidative stress, and anti-inflammation via both AMPK-dependent and AMPK-independent pathways [19].
It is noteworthy that metformin is profoundly linked with lower CAC scores in smoking subjects in the light of our study. Cigarette smoking is a well-established risk factor that is modifiable for CAD. The Heinz Nixdorf Recall study reported that smoking was also positively associated with CAC scores [20]. In another large retrospective cohort study in T2DM patients, researchers found that metformin is associated with lower risk of CAD and mortality in current and former smokers [21]. Smoking releases numerous toxic chemicals and free radicals, exposing the coronary artery to excess oxidative stress, while vascular calcification is also attributed to oxidative stress [22]. Metformin may exhibit its beneficial role in CAC due to its antioxidative stress properties [23] as well as its role in upregulating some components of the antioxidant defense system [24]. Nevertheless, the specific underlying mechanism is yet to be elucidated.
The limitations of our study include (1) the cross-sectional design, (2) involvement of relatively small numbers of subjects, (3) the missing data of HbA1c that hampered the interpretation of blood glucose control over the CAC severity, and (4) inability to exclude other interfering factors like lifestyle intervention. A prospective randomized controlled trial is needed to overcome these limitations. It would be interesting to examine the type and dose of antidiabetic medications, the CAC scores, and if possible, the change in the CAC scores.
To sum up, our results reveal that metformin prescription rather than other antidiabetic agents is negatively and independently associated with CAC severity in T2DM patients. We also witnessed a markedly negative association of metformin and CAC scores in smokers. The findings emphasize the use of metformin in all T2DM populations, especially those patients with a history of smoking.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81670390 to Zhejun Cai and No. 81501298 to Jia Wei), the Zhejiang Provincial Natural Science Foundation of China (No. LQ19H020006 to Yi Lu and No. LY19H040012 to Jia Wei), and the Fundamental Research Funds for the Central Universities (2019QNA7036 to Zhejun Cai).
Contributor Information
Zhejun Cai, Email: caizhejun@zju.edu.cn.
Meixiang Xiang, Email: xiangmx@zju.edu.cn.
Data Availability
The clinical data used to support the findings of this study are restricted by the Ethics Committee of the Second Affiliated Hospital, Zhejiang University School of Medicine, in order to protect patient privacy. Data are available from the corresponding author for researchers who meet the criteria for access to confidential data.
Conflicts of Interest
The authors declare that there is no conflict of interest.
Authors' Contributions
Yi Lu and Yidong Wang contributed equally.
Supplementary Materials
Supplemental Figure 1: the flow chart of the patient inclusion and exclusion. Supplemental Table 1: the characteristics of included patients according to CAC scores. Supplemental Table 2: the association between metformin and other antidiabetic medicine and CAC scores. Supplemental Table 3: the independent association of metformin and other factors with CAC scores. Supplemental Table 4: the subgroup analysis in patients with a history of smoking. Supplemental Table 5: the subgroup analysis in patients without a history of smoking.
References
- 1.Nakahara T., Dweck M. R., Narula N., Pisapia D., Narula J., Strauss H. W. Coronary artery calcification: from mechanism to molecular imaging. JACC: Cardiovascular Imaging. 2017;10(5):582–593. doi: 10.1016/j.jcmg.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106(25):3143–3421. doi: 10.1161/circ.106.25.3143. [DOI] [PubMed] [Google Scholar]
- 3.Kannel W. B., McGee D. L. Diabetes and cardiovascular risk factors: the Framingham study. Circulation. 1979;59(1):8–13. doi: 10.1161/01.CIR.59.1.8. [DOI] [PubMed] [Google Scholar]
- 4.Norhammar A., Malmberg K., Diderholm E., et al. Diabetes mellitus: the major risk factor in unstable coronary artery disease even after consideration of the extent of coronary artery disease and benefits of revascularization. Journal of the American College of Cardiology. 2004;43(4):585–591. doi: 10.1016/j.jacc.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal S., Cox A. J., Herrington D. M., et al. Coronary calcium score predicts cardiovascular mortality in diabetes: diabetes heart study. Diabetes Care. 2013;36(4):972–977. doi: 10.2337/dc12-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg R. B., Aroda V. R., Bluemke D. A., et al. Effect of long-term metformin and lifestyle in the diabetes prevention program and its outcome study on coronary artery calcium. Circulation. 2017;136(1):52–64. doi: 10.1161/CIRCULATIONAHA.116.025483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Detrano R., Guerci A. D., Carr J. J., et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. New England Journal of Medicine. 2008;358(13):1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 8.Greenland P., Bonow R. O., Brundage B. H., et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. Journal of the American College of Cardiology. 2007;49(3):378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Cao X., Li H., Tao H., et al. Metformin inhibits vascular calcification in female rat aortic smooth muscle cells via the AMPK-eNOS-NO pathway. Endocrinology. 2013;154(10):3680–3689. doi: 10.1210/en.2013-1002. [DOI] [PubMed] [Google Scholar]
- 10.Cai Z., Ding Y., Zhang M., et al. Ablation of adenosine monophosphate-activated protein kinase α1 in vascular smooth muscle cells promotes diet-induced atherosclerotic calcification in vivo. Circulation Research. 2016;119(3):422–433. doi: 10.1161/CIRCRESAHA.116.308301. [DOI] [PubMed] [Google Scholar]
- 11.Mary A., Hartemann A., Liabeuf S., et al. Association between metformin use and below-the-knee arterial calcification score in type 2 diabetic patients. Cardiovascular Diabetology. 2017;16(1):p. 24. doi: 10.1186/s12933-017-0509-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey A. S., Stevens L. A., Schmid C. H., et al. A new equation to estimate glomerular filtration rate. Annals of Internal Medicine. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agatston A. S., Janowitz W. R., Hildner F. J., Zusmer N. R., Viamonte M., Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. Journal of the American College of Cardiology. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-T. [DOI] [PubMed] [Google Scholar]
- 14.Inzucchi S. E., Bergenstal R. M., Buse J. B., et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 15.Holman R. R., Paul S. K., Bethel M. A., Matthews D. R., Neil H. A. W. 10-year follow-up of intensive glucose control in type 2 diabetes. New England Journal of Medicine. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 16.Preiss D., Lloyd S. M., Ford I., et al. Metformin for non-diabetic patients with coronary heart disease (the CAMERA study): a randomised controlled trial. The Lancet Diabetes & Endocrinology. 2014;2(2):116–124. doi: 10.1016/S2213-8587(13)70152-9. [DOI] [PubMed] [Google Scholar]
- 17.Fitch K., Abbara S., Lee H., et al. Effects of lifestyle modification and metformin on atherosclerotic indices among HIV-infected patients with the metabolic syndrome. AIDS. 2012;26(5):587–597. doi: 10.1097/QAD.0b013e32834f33cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valencia W. M., Palacio A., Tamariz L., Florez H. Metformin and ageing: improving ageing outcomes beyond glycaemic control. Diabetologia. 2017;60(9):1630–1638. doi: 10.1007/s00125-017-4349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pryor R., Cabreiro F. Repurposing metformin: an old drug with new tricks in its binding pockets. Biochemical Journal. 2015;471(3):307–322. doi: 10.1042/BJ20150497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehmann N., Möhlenkamp S., Mahabadi A. A., et al. Effect of smoking and other traditional risk factors on the onset of coronary artery calcification: results of the Heinz Nixdorf Recall study. Atherosclerosis. 2014;232(2):339–345. doi: 10.1016/j.atherosclerosis.2013.11.045. [DOI] [PubMed] [Google Scholar]
- 21.Paul S. K., Klein K., Majeed A., Khunti K. Association of smoking and concomitant metformin use with cardiovascular events and mortality in people newly diagnosed with type 2 diabetes. Journal of Diabetes. 2016;8(3):354–362. doi: 10.1111/1753-0407.12302. [DOI] [PubMed] [Google Scholar]
- 22.Al-Aly Z. Phosphate, oxidative stress, and nuclear factor-κB activation in vascular calcification. Kidney International. 2011;79(10):1044–1047. doi: 10.1038/ki.2010.548. [DOI] [PubMed] [Google Scholar]
- 23.Faure P., Rossini E., Wiernsperger N., Richard M. J., Favier A., Halimi S. An insulin sensitizer improves the free radical defense system potential and insulin sensitivity in high fructose-fed rats. Diabetes. 1999;48(2):353–357. doi: 10.2337/diabetes.48.2.353. [DOI] [PubMed] [Google Scholar]
- 24.Anedda A., Rial E., Gonzalez-Barroso M. M. Metformin induces oxidative stress in white adipocytes and raises uncoupling protein 2 levels. The Journal of Endocrinology. 2008;199(1):33–40. doi: 10.1677/JOE-08-0278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: the flow chart of the patient inclusion and exclusion. Supplemental Table 1: the characteristics of included patients according to CAC scores. Supplemental Table 2: the association between metformin and other antidiabetic medicine and CAC scores. Supplemental Table 3: the independent association of metformin and other factors with CAC scores. Supplemental Table 4: the subgroup analysis in patients with a history of smoking. Supplemental Table 5: the subgroup analysis in patients without a history of smoking.
Data Availability Statement
The clinical data used to support the findings of this study are restricted by the Ethics Committee of the Second Affiliated Hospital, Zhejiang University School of Medicine, in order to protect patient privacy. Data are available from the corresponding author for researchers who meet the criteria for access to confidential data.