Abstract
Background
Some studies showed that microRNA-497 (miR-497) might act as a prognostic biomarker of cancer. However, the conclusion was not consistent. The aim of this study was to investigate the prognostic role of miR-497 in various carcinomas.
Methods
We systematically searched the databases of PubMed, Embase, Web of Science, Chinese National Knowledge Infrastructure (CNKI), and Wanfang Data to identify relevant studies. Two independent reviewers performed the data extraction and assessed the study quality. Hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) for overall survival (OS) and disease-free survival/relapse-free survival (DFS/RFS) were used to assess the associations between miR-497 expression and cancer prognosis.
Results
A total of 15 studies involving 1760 participants fulfilled the inclusion criteria. The lower level of miR-497 expression was significantly associated with shorter overall survival (HR = 2.19, 95% CI: 1.84-2.60). No significant association was found between miR-497 expression and DFS/RFS in various carcinomas (HR = 1.17, 95% CI: 0.53-2.57). Subgroup analyses by ethnicity and cancer type showed the consistent results.
Conclusion
Our studies suggested that miR-497 might be a prognostic biomarker in cancers. However, further multicenter prospective clinical researches are needed to confirm the association between miR-497 expression and cancer prognosis.
1. Introduction
MicroRNAs (miRNAs) are highly conserved, endogenous non-protein-encoded small molecules with lengths of 21 to 24 nucleotides which can bind to the target sequence of the 3′-untranslated region (3′-UTR) of the target mRNAs, causing degradation or translation inhibition of the target mRNAs at the posttranscriptional level [1, 2]. They can negatively regulate gene expression and play an important role in cancer biology, such as cell proliferation, invasion, angiogenesis, and immune evasion [3, 4]. In recent years, miRNAs have been considered as potential utility biomarkers for cancer prognosis owing to their robust expression patterns, stability within cancerous samples, and easy assessment by qRT-PCR [5, 6].
MicroRNA-497 (miR-497) belongs to the miR-15 superfamily, sharing the same 3′-UTR binding seed sequence AGCAGCA [7]. miR-497 was first reported in human breast cancer [8]. Subsequently, miR-497 downregulation has been demonstrated in various carcinomas, including hepatocellular carcinoma [9], adrenocortical carcinoma [10], and bladder cancer [11], suggesting that miR-497 has a tumor-suppressive role. In addition, many targets of miR-497 have been identified, such as WEE1, IGF-1R, and eIF4E [12–14]. Recently, some studies indicated that low miR-497 expression was significantly associated with poor prognosis in cancers, containing hepatocellular carcinoma [15, 16], renal cancer [17], and neuroblastoma [12]. However, many individual studies have small sample sizes and they have not reached consistent conclusions [15, 18–20]. Thus, the prognostic role of miR-497 in cancers remains unclear.
In this systematic review and meta-analysis, we summarized available data from the published studies to evaluate the role of miR-497 as a prognostic biomarker and to clarify the association between miR-497 expression and long-term survival and early prediction in various carcinomas.
2. Materials and Methods
2.1. Literature Search Strategy
We systematically searched the databases of PubMed, Embase, Web of Science, Chinese National Knowledge Infrastructure (CNKI), and Wanfang Data to identify relevant studies up to 15 October 2018. The following search strategies were used to retrieve articles in English or Chinese: “(miR497 OR miR-497 OR microRNA497 OR microRNA-497) AND (neoplasms OR cancer OR carcinoma) AND prognosis.” The reference lists of retrieved studies were also examined manually to identify potentially missing relevant studies.
2.2. Inclusion and Exclusion Criteria
Inclusion criteria are as follows: (1) the full-text article written in English or Chinese, (2) the subjects were patients with any type of carcinoma, (3) miR-497 expression measured in tumor tissue, (4) evaluating the association between the miR-497 expression level and survival outcomes, including overall survival (OS), disease-free survival (DFS), and relapse-free survival (RFS), (5) reporting hazard ratio (HR) with 95% confidence interval (95% CI) or survival curves, and (6) studies based on the same population, with only the latest study included.
Exclusion criteria are as follows: (1) reviews, letters, case reports, and conference reports and (2) lacking key information about survival outcomes.
2.3. Data Extraction
Two reviewers extracted data independently from eligible studies. The following information was extracted: the first author, publication year, country, ethnicity of patients, number of cases, cancer type, tumor stage, sample type, detection method, follow-up and cut-off values, HR, and the corresponding 95% CI of miR-497 for OS, DFS, and RFS. If obtaining directly is not possible, data were extracted by survival curves and calculated following Tierney et al.'s method [21]. HR was measured by comparing low expression with high expression (high expression as the reference). HR > 1 indicates a poor prognosis in the low-expression group.
Disagreements were resolved through comprehensive discussion and examined by a third investigator.
2.4. Quality Assessment
The quality of each included study was assessed according to the Newcastle-Ottawa Scale (NOS) criteria for cohort studies [22]. The NOS criteria include three aspects: selection, comparability, and outcome. Nine points is the highest score, and more than six points is high quality [23, 24].
The assessments were processed independently by two reviewers and the final score was achieved by consensus.
2.5. Statistical Analysis
Statistical analyses were performed using Review Manager 5.3 (Cochrane Collaboration, Oxford, UK) and Stata 14.0 (Stata Corp., College Station, TX, USA), and all tests were 2 tailed. HRs and corresponding 95% CIs extracted directly or by survival curves were used to calculate the pooled HR by the generic inverse variance method. The significance of pooled HR was calculated by the Z-test, and P < 0.05 was regarded as statistically significant. Heterogeneity between studies was tested by the Q test and I2 statistics. If Pheterogeneity < 0.10 or I2 > 50% (heterogeneity existed), the random effects model was applied to calculate pooled HR and meta-regression was further used to explore sources of heterogeneity. If not, the fixed effects model was applied. The subgroup analyses were conducted by ethnicity and cancer type. Sensitivity analyses were performed by omitting each study at a time to assess the consistency and stability of the pooled results. We evaluated potential publication bias by funnel plots and further used Begg's test (rank correlation test) [25] and Egger's test (weighted linear regression test) [26] to quantitatively evaluate the publication bias. If publication bias existed, the trim and fill method [27] was used to adjust the results.
3. Results
3.1. Summary of the Included Studies
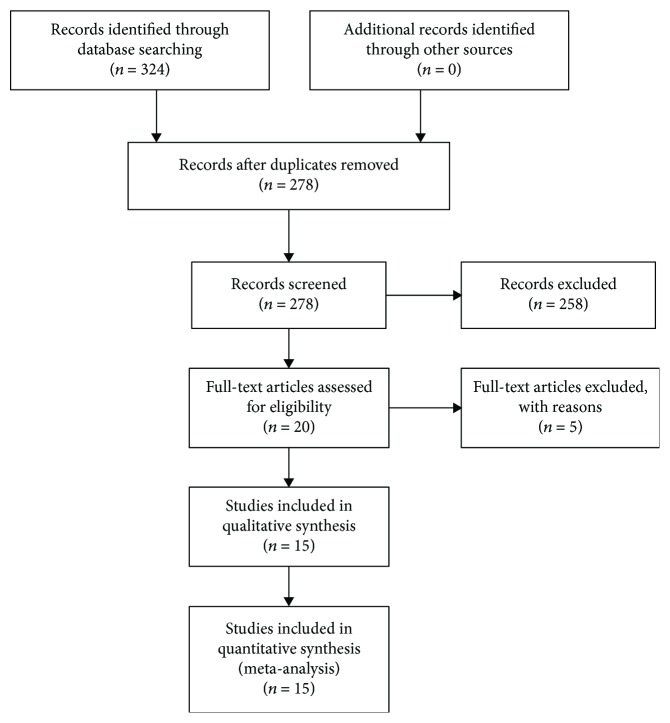
324 studies were initially identified through database searching. 278 studies were further reviewed after duplicates were removed. According to the inclusion and exclusion criteria, 258 studies were excluded after screening titles and abstracts, 20 full-text articles were further assessed for eligibility. One retracted article [28], three articles without sufficient data [29–31], and one article [32] which brought great clinical heterogeneity were further excluded. Finally, 15 eligible studies were included in the meta-analysis [12–20, 33–38], including 15 for OS [12–20, 33–38] and 4 for DFS/RFS [13, 15, 33, 37] (Figure 1).
Figure 1.
Flow diagram of systematic literature search.
The included studies encompassed a total of 1317 patients with OS data and 443 patients with DFS/RFS data from China, Ireland, and Austria, published from 2012 to 2017. The patients could be divided into Asian or Caucasian by their ethnic background. The types of carcinomas included hepatocellular carcinoma (HCC), cervical cancer, neuroblastoma, ovarian cancer, pancreatic cancer, osteosarcoma, breast cancer, gliomas, non-small-cell lung cancer (NSCLC), renal cancer, gastric cancer (GC), diffuse large B-cell lymphoma (DLBCL), colorectal cancer, and Ewing sarcoma. All studies used tissue specimens. Quantitative real-time polymerase chain reaction (qRT-PCR) was conducted in all 15 studies. All of the follow-up time was more than 60 months. The cut-off values were different, most with median or mean. HR and the corresponding 95% CI were obtained directly in 8 studies, and others were extracted and calculated by survival curves.
All studies included in this meta-analysis were cohort studies and assessed based on the NOS. The scores ranged from 6 to 8, and the average score was 7.13. The details of characteristics and the NOS scores were summarized in Table 1.
Table 1.
Characteristics of studies included in the meta-analysis.
| Author | Year | Country | Ethnicity | Number | Cancer type | Stage | Sample | Method | Follow-up (months) | Cut-off | Survival analysis | Hazard ratio | NOS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OS | DFS/RFS | |||||||||||||
| Zhang et al. [15] | 2016 | China | Asian | 86 | 86 | HCC | I-IV (TNM) | Frozen tissue | qRT-PCR | 60 | Median | OS/DFS | HR/SC | 8 |
| Luo et al. [13] | 2013 | China | Asian | 60 | 60 | Cervical cancer | I-II (FIGO) | Frozen tissue | qRT-PCR | 60 | Mean (0.44) | OS/DFS | HR/SC | 8 |
| Creevey et al. [12] | 2013 | Ireland | Caucasian | 143 | 143 | Neuroblastoma | 1-4 s (INSS) | Frozen tissue | qRT-PCR | 60 | First Quartile | OS | SC | 6 |
| Wang et al. [33] | 2014 | China | Asian | 96 | 96 | Ovarian cancer | I-IV (TNM) | Frozen tissue | qRT-PCR | 60 | NR | OS/RFS | SC | 8 |
| Xu et al. [34] | 2014 | China | Asian | 87 | / | Pancreatic cancer | I-IV (TNM) | Frozen tissue | qRT-PCR | 87 | Score | OS | HR/SC | 8 |
| Liu et al. [18] | 2016 | China | Asian | 240 | / | Breast cancer | I-III (TNM) | Frozen tissue | qRT-PCR | 80 | Median (1.60) | OS | HR/SC | 7 |
| Feng et al. [35] | 2016 | China | Asian | 110 | / | Gliomas | I-IV (TNM) | Frozen tissue | qRT-PCR | 60 | Median (1.73) | OS | HR/SC | 7 |
| Huang et al. [36] | 2015 | China | Asian | 51 | / | NSCLC | I-III (TNM) | Frozen tissue | qRT-PCR | 60 | Median | OS | SC | 8 |
| Zhao et al. [17] | 2015 | China | Asian | 86 | / | Renal cancer | T1-T4 (TNM) | Frozen tissue | qRT-PCR | 60 | Mean | OS | HR/SC | 7 |
| Li et al. [14] | 2014 | China | Asian | 97 | / | GC | I-IV (TNM) | Frozen tissue | qRT-PCR | 60 | Median | OS | SC | 6 |
| Troppan et al. [37] | 2015 | Austria | Caucasian | 58 | 58 | DLBCL | I-IV (TNM) | Frozen tissue | qRT-PCR | 120 | Median | OS/DFS | HR/SC | 6 |
| Song et al. [19] | 2017 | China | Asian | 46 | / | Osteosarcoma | I-III (TNM) | Frozen tissue | qRT-PCR | 72 | Mean | OS | SC | 7 |
| Wang et al. [38]∗ | 2012 | China | Asian | 57 | / | Colorectal cancer | I-IV (TNM) | Frozen tissue | qRT-PCR | 60 | N/T = 2 | OS | SC | 7 |
| Zhang [20]∗ | 2016 | China | Asian | 39 | / | Ewing sarcoma | NR | Frozen tissue | qRT-PCR | 90 | NR | OS | SC | 6 |
| Xie and Wang [16]∗ | 2017 | China | Asian | 61 | / | HCC | I-IV (TNM) | Frozen tissue | qRT-PCR | 60 | Median (0.24) | OS | HR/SC | 8 |
OS: overall survival; DFS: disease-free survival; RFS: relapse-free survival; HCC: hepatocellular carcinoma; NSCLC: non-small-cell lung cancer; GC: gastric cancer; DLBCL: diffuse large B-cell lymphoma; TNM: tumor node metastasis; FIGO: International Federation of Gynaecology and Obstetrics staging criteria; INSS: International neuroblastoma staging system; NR: not reported; qRT-PCR: quantitative real-time PCR; HR: hazard ratio; SC: survival curve; NOS: Newcastle-Ottawa Scale. ∗Written in Chinese.
3.2. miR-497 Expression Level and OS
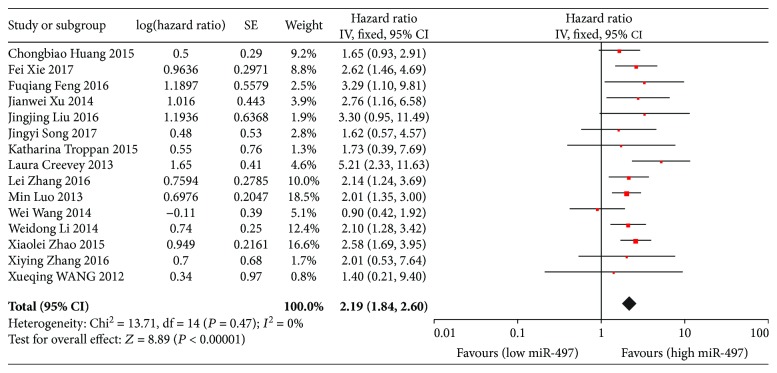
15 studies evaluated the association of miR-497 expression levels and OS, and the pooled HR was 2.19 (95% CI: 1.84-2.60), which indicated that the lower level of miR-497 expression was associated with shorter overall survival (Figure 2). Subgroup analyses by ethnicity showed that low miR-497 expression was significantly associated with poor OS in both Asian and Caucasian patients (Asian: HR = 2.10, 95% CI: 1.76-2.51; Caucasian: HR = 4.06, 95% CI: 2.00-8.24). Further subgroup analyses by cancer type also indicated that significant associations were observed in hepatocellular carcinoma and other cancers (HCC: HR = 2.35, 95% CI: 1.58-3.50; other cancers: HR = 2.15, 95% CI: 1.78-2.60). (Table 2).
Figure 2.
Forest plots of the relationship between the miR-497 expression level and OS. The squares and horizontal lines represent the HR and 95% CI, respectively. The area of the squares reflects the weight of each study. The diamond represents the pooled HR and 95% CI. OS: overall survival; CI: confidence interval; SE: standard error; df: degrees of freedom; miR: microRNA.
Table 2.
Main results of pooled HRs in the meta-analysis.
| Comparisons | Heterogeneity test | Pooled HR (95% CI) | Hypothesis test | No. of studies | |||
|---|---|---|---|---|---|---|---|
| Q | P | I 2 (%) | Z | P | |||
| OS | |||||||
| Total | 13.71 | 0.47 | 0 | 2.19 (1.84, 2.60) | 8.89 | <0.001 | 15 |
| Ethnicity | |||||||
| Asian | 8.94 | 0.71 | 0 | 2.10 (1.76, 2.51) | 8.19 | <0.001 | 13 |
| Caucasian | 1.62 | 0.20 | 38 | 4.06 (2.00, 8.24) | 3.89 | <0.001 | 2 |
| Cancer type | |||||||
| HCC | 0.25 | 0.62 | 0 | 2.35 (1.58, 3.50) | 4.21 | <0.001 | 2 |
| Other cancers | 13.30 | 0.35 | 10 | 2.15 (1.78, 2.60) | 7.84 | <0.001 | 13 |
| DFS/RFS | |||||||
| Total | 12.92 | 0.005 | 77 | 1.17 (0.53, 2.57) | 0.39 | 0.69 | 4 |
| Ethnicity | |||||||
| Asian | 9.99 | 0.007 | 80 | 1.42 (0.55, 3.67) | 0.73 | 0.47 | 3 |
| Caucasian | — | — | — | 0.63 (0.27, 1.47) | 1.07 | 0.29 | 1 |
OS: overall survival; DFS: disease-free survival; RFS: relapse-free survival; HCC: hepatocellular carcinoma; HR: hazard ratio; CI: confidence interval.
3.3. miR-497 Expression Level and DFS/RFS
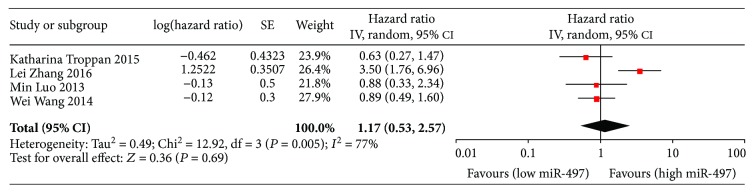
Data on DFS/RFS were available in 4 studies, and the pooled result showed no statistical association between miR-497 expression and early predicted survival (HR = 1.17, 95% CI: 0.53-2.57) (Figure 3). Subgroup analyses by ethnicity also showed negative results in both Asian and Caucasian patients (Asian: HR = 1.42, 95% CI: 0.55-3.67; Caucasian: HR = 0.63, 95% CI: 0.27-1.47). (Table 2).
Figure 3.
Forest plots of the relationship between the miR-497 expression level and DFS/RFS. The squares and horizontal lines represent the HR and 95% CI, respectively. The area of the squares reflects the weight of each study. The diamond represents the pooled HR and 95% CI. DFS: disease-free survival; RFS: relapse-free survival; CI: confidence interval; SE: standard error; df: degrees of freedom; miR: microRNA.
3.4. Meta-Regression Analysis
To explore the source of heterogeneity of overall survival, we used meta-regression to evaluate the possible covariates including ethnicity, sample size (median 86 as the boundary), cancer type, NOS (mean 7.13 as the boundary), and cut-off. Univariate and multivariate analyses both showed that all the above covariates were not the sources of heterogeneity (P > 0.05). It was indicated that the pooled results were not affected by above covariates (Table 3).
Table 3.
The results of meta-regression analysis of OS.
| Covariates | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| Exp(b) | 95% CI | P | Exp(b) | 95% CI | P | Adjusted P | |
| Ethnicity | 1.93 | 0.87-4.32 | 0.100 | 1.70 | 0.70-4.11 | 0.208 | 0.617 |
| Sample size | 1.19 | 0.81-1.74 | 0.352 | 1.01 | 0.62-1.63 | 0.981 | 1.000 |
| Cancer type | 1.09 | 0.67-1.80 | 0.703 | 1.43 | 0.72-2.84 | 0.272 | 0.725 |
| NOS | 0.77 | 0.53-1.13 | 0.167 | 0.74 | 0.43-1.30 | 0.259 | 0.705 |
| Cut-off | 0.99 | 0.67-1.46 | 0.955 | 0.91 | 0.56-1.46 | 0.658 | 0.984 |
Adjusted P was calculated by the Monte Carlo permutation test for meta-regression. CI: confidence interval; NOS: Newcastle-Ottawa Scale; OS: overall survival.
3.5. Sensitivity Analysis and Publication Bias
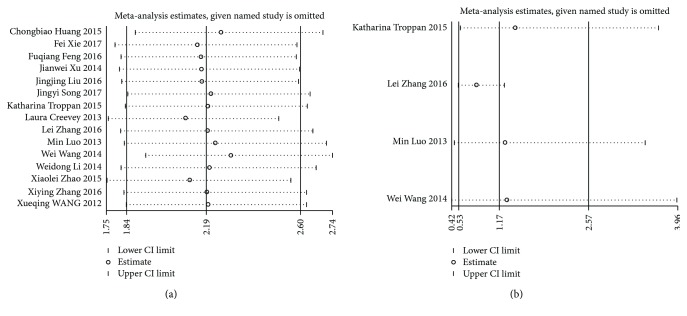
Sensitivity analysis showed that the pooled HRs in OS and DFS/RFS were not significantly influenced by omitting the individual study (Figure 4).
Figure 4.
(a) Sensitivity analysis for OS. (b) Sensitivity analysis for DFS/RFS. OS: overall survival; DFS: disease-free survival; RFS: relapse-free survival; CI: confidence interval.
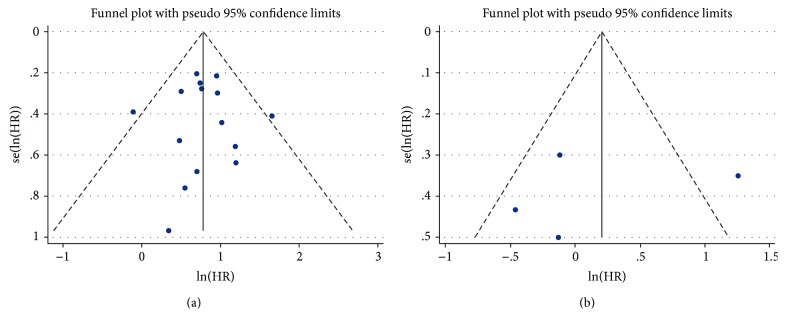
The shape of the funnel plot did not indicate visual evidence of the asymmetry (Figure 5). Begg's test and Egger's test both showed no significant publication bias detected (P > 0.05) (Table 4).
Figure 5.
(a) Funnel plot for publication bias analysis of OS. (b) Funnel plot for publication bias analysis of DFS/RFS. OS: overall survival; DFS: disease-free survival; RFS: relapse-free survival; HR: hazard ratio.
Table 4.
Publication bias of miR-497 for Begg's test and Egger's test.
| Comparisons | Begg's test | Egger's test | |||
|---|---|---|---|---|---|
| Z | P | t | P | 95% CI | |
| OS | 0.20 | 0.843 | 0.02 | 0.983 | -1.398477-1.427367 |
| DFS/RFS | -0.34 | 1.000 | -0.33 | 0.775 | -30.04166-25.80952 |
OS: overall survival; DFS: disease-free survival; RFS: relapse-free survival; CI: confidence interval.
4. Discussion
Results of this meta-analysis, for the first time, showed that the lower level of miR-497 expression was associated with shorter overall survival but not significantly associated with DFS/RFS in patients with a variety of carcinomas. Subgroup analyses by ethnicity and cancer type showed the consistent results. Sensitivity analyses which were performed by omitting each study at a time did not alter the results. Both analyses indicated that the results of this meta-analysis were stable and reliable.
The main reason for the poor survival of cancer is invasion and metastasis [39]. miR-497 functions mainly as a tumor suppressor, and overexpression of miR-497 suppresses cell proliferation and induces apoptosis in HCC [15] and pancreatic cancer [34] and inhibits migration and invasion in cervical cancer [13] and breast cancer [18]. In addition, miR-497 overexpression was found to initiate G0/G1 cell phase arrest of MCF-7 breast cancer cells [40] and block the G1/S transition of gastric cancer cells [14]. Conversely, miR-497 downregulation contributed to angiogenesis in HCC [41] and ovarian cancer [42]. Knockdown of miR-497 increased cell growth and invasion in NSCLC [43] and induced osteosarcoma cell chemoresistance [44]. Increasing evidences indicated that miR-497 expression might be associated with cancer progression, the current meta-analysis confirmed that it could serve as a long-term prognostic biomarker.
According to the NCI Dictionary of Cancer Terms, DFS and RFS are the same outcomes which are defined as “the length of time after primary treatment for a cancer ends that the patient survives without any signs or symptoms of that cancer.” Due to the included studies that used DFS or RFS to evaluate early tumor relapse, we combined the two indices to evaluate the early predictive value of miR-497. However, only 4 related studies with a relatively small sample size were included in this meta-analysis, so the result was less reliable to some extent. To confirm whether the miR-497 expression can predict the early tumor relapse or not, more well-designed clinical researches with larger sample sizes should be carried out in the future.
Obvious heterogeneity was discovered when we conducted analysis of the miR-497 expression level and DFS/RFS. In order to find out the origin of heterogeneity, we performed the subgroup and sensitivity analyses. When we omitted the study of Zhang et al. [15], there was no significant heterogeneity observed, so this study was the main source of heterogeneity. Noteworthily, the results of Zhang et al. [15] showed that the cancer-specific survival of HCC was not consistent with the total pooled results. It might suggest that miR-497 could predict the early relapse of HCC, which needed further confirmation.
Although this meta-analysis suggested that miR-497 had clinical utility for the prediction of prognosis in patients with cancers, several issues should be considered about its clinical application. First, it is very important to determine a clear definition of the cut-off value of miR-497 expression. But the lack of abundant miR-497 expression data and the variability in different ethnic populations make it difficult to set a standard cut-off value. Second, several other miRNA prognostic biomarkers of cancers have been reported nowadays, such as miR-210 [45], miR-218 [46], miR-29 [47], and miR-214 [48]. The prediction power of a panel of miRNAs may be stronger than a single miR-497, so using a set of miRNAs or a single miR-497 as predictive factors should be carefully considered. Third, circulating miRNAs represent a class of ideal biomarkers for cancer prognosis, they exhibit higher stability in body fluids and can be extracted and measured noninvasively [49]; so, could we use miR-497 in serum or plasma in place of tissue as prognostic biomarker? However, only one study [50] reported that miR-497 could serve as a potential serum biomarker for the prognosis of osteosarcoma. More relevant studies should be conducted to investigate the association between serum miR-497 expression and cancer prognosis.
This study has several limitations. First, the cut-off value of miR-497 expression was various in original studies, including median, mean, and others, lacking of a golden standard and a clear definition. Second, because survival data of some eligible studies could not be obtained directly by multivariate cox regression, the data extracted from survival curves might not exclude the influence of some potential confounding factors; these calculated HRs and corresponding 95% CIs might also bring several tiny errors. Third, heterogeneity between some studies still existed, although we used several statistical methods to minimize the effect of the heterogeneity, including the random effects model, subgroup analysis, and meta-regression.
In conclusion, the current meta-analysis demonstrated that miR-497 expression was significantly associated with long-term survival, not significantly associated with early prediction. It might suggest that detected miR-497 expression could predict overall survival of cancer patients in the future clinical application. More multicenter prospective clinical researches should be conducted to confirm the association between miR-497 expression and cancer prognosis.
Acknowledgments
This work was supported by the Natural Science Foundation of Fujian Province of China (grant number 2016J01417) and the Science and Technology Foundation of Fuzhou City (grant number 2015-S-143-19).
Contributor Information
Yongyi Zeng, Email: lamp197311@126.com.
Jingfeng Liu, Email: drjingfeng@126.com.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- 1.Bartel D. P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Pasquinelli A. E. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nature Reviews Genetics. 2012;13(4):271–282. doi: 10.1038/nrg3162. [DOI] [PubMed] [Google Scholar]
- 3.Kasinski A. L., Slack F. J. Epigenetics and genetics. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nature Reviews Cancer. 2011;11(12):849–864. doi: 10.1038/nrc3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stahlhut C., Slack F. J. MicroRNAs and the cancer phenotype: profiling, signatures and clinical implications. Genome Medicine. 2013;5(12):p. 111. doi: 10.1186/gm516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayes J., Peruzzi P. P., Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends in Molecular Medicine. 2014;20(8):460–469. doi: 10.1016/j.molmed.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Ferracin M., Veronese A., Negrini M. Micromarkers: miRNAs in cancer diagnosis and prognosis. Expert Review of Molecular Diagnostics. 2010;10(3):297–308. doi: 10.1586/erm.10.11. [DOI] [PubMed] [Google Scholar]
- 7.Finnerty J. R., Wang W. X., Hebert S. S., Wilfred B. R., Mao G., Nelson P. T. The miR-15/107 group of microRNA genes: evolutionary biology, cellular functions, and roles in human diseases. Journal of Molecular Biology. 2010;402(3):491–509. doi: 10.1016/j.jmb.2010.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan L. X., Huang X. F., Shao Q., et al. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008;14(11):2348–2360. doi: 10.1261/rna.1034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie Y., Wei R. R., Huang G. L., Zhang M. Y., Yuan Y. F., Wang H. Y. Checkpoint kinase 1 is negatively regulated by miR-497 in hepatocellular carcinoma. Medical Oncology. 2014;31(3):p. 844. doi: 10.1007/s12032-014-0844-4. [DOI] [PubMed] [Google Scholar]
- 10.Ozata D. M., Caramuta S., Velazquez-Fernandez D., et al. The role of microRNA deregulation in the pathogenesis of adrenocortical carcinoma. Endocrine-Related Cancer. 2011;18(6):643–655. doi: 10.1530/ERC-11-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du M., Shi D., Yuan L., et al. Circulating miR-497 and miR-663b in plasma are potential novel biomarkers for bladder cancer. Scientific Reports. 2015;5(1, article 10437) doi: 10.1038/srep10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creevey L., Ryan J., Harvey H., et al. MicroRNA-497 increases apoptosis in MYCN amplified neuroblastoma cells by targeting the key cell cycle regulator WEE1. Molecular Cancer. 2013;12(1):p. 23. doi: 10.1186/1476-4598-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo M., Shen D., Zhou X., Chen X., Wang W. MicroRNA-497 is a potential prognostic marker in human cervical cancer and functions as a tumor suppressor by targeting the insulin-like growth factor 1 receptor. Surgery. 2013;153(6):836–847. doi: 10.1016/j.surg.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Li W., Jin X., Deng X., Zhang G., Zhang B., Ma L. The putative tumor suppressor microRNA-497 modulates gastric cancer cell proliferation and invasion by repressing eIF4E. Biochemical and Biophysical Research Communications. 2014;449(2):235–240. doi: 10.1016/j.bbrc.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L., Yu Z., Xian Y., Lin X. microRNA-497 inhibits cell proliferation and induces apoptosis by targeting YAP1 in human hepatocellular carcinoma. FEBS Open Bio. 2016;6(2):155–164. doi: 10.1002/2211-5463.12032. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Xie F., Wang W. The expression of MicroRNA-497 in hepatocellular carcinoma and its relationship with clinical features and survival rate. Chinese Journal of Microcirculation. 2017;27(1):47–50+53. [Google Scholar]
- 17.Zhao X., Zhao Z., Xu W., Hou J., Du X. Down-regulation of miR-497 is associated with poor prognosis in renal cancer. International Journal of Clinical and Experimental Pathology. 2015;8(1):758–764. [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J., Zhou Y., Shi Z., et al. microRNA-497 modulates breast cancer cell proliferation, invasion, and survival by targeting SMAD7. DNA and Cell Biology. 2016;35(9):521–529. doi: 10.1089/dna.2016.3282. [DOI] [PubMed] [Google Scholar]
- 19.Song J., Wu X., Liu F., et al. Long non-coding RNA PVT1 promotes glycolysis and tumor progression by regulating miR-497/HK2 axis in osteosarcoma. Biochemical and Biophysical Research Communications. 2017;490(2):217–224. doi: 10.1016/j.bbrc.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X. The Expression of EZH2 and miR-127 in Chinese Ewing Sarcoma Patients and the Correlation to Prognosis. Shandong University; 2016. [Google Scholar]
- 21.Tierney J. F., Stewart L. A., Ghersi D., Burdett S., Sydes M. R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8(1):p. 16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European Journal of Epidemiology. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 23.Liu W., Zhou J. G., Sun Y., Zhang L., Xing B. C. Hepatic resection improved the long-term survival of patients with BCLC stage B hepatocellular carcinoma in Asia: a systematic review and meta-analysis. Journal of Gastrointestinal Surgery. 2015;19(7):1271–1280. doi: 10.1007/s11605-015-2811-6. [DOI] [PubMed] [Google Scholar]
- 24.Wang L., Liu Z., Liu X., Zeng Y., Liu J. The hepatectomy efficacy of huge hepatocellular carcinoma and its risk factors: a meta analysis. Medicine. 2017;96(52, article e9226) doi: 10.1097/MD.0000000000009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Begg C. B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 26.Egger M., Smith G. D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duval S., Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang S., Li H., Wang J., Wang D. Expression of microRNA-497 and its prognostic significance in human breast cancer. Diagnostic Pathology. 2013;8(1):p. 172. doi: 10.1186/1746-1596-8-172. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Zhang Y., Zhang Z., Li Z., et al. MicroRNA-497 inhibits the proliferation, migration and invasion of human bladder transitional cell carcinoma cells by targeting E2F3. Oncology Reports. 2016;36(3):1293–1300. doi: 10.3892/or.2016.4923. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Yixiao W. B. Association of miR-497 and prognosis in patients with renal cell carcinoma and its effects on proliferation, apoptosis and invasion of human renal cell carcinoma cell line 786-0. Chinese Journal of Pathophysiology. 2016;32(11):1979–1983. [Google Scholar]
- 31.Zhong H., Yang J., Zhang B., et al. LncRNA GACAT3 predicts poor prognosis and promotes cell proliferation in breast cancer through regulation of miR-497/CCND2. Cancer Biomarkers. 2018;22(4):787–797. doi: 10.3233/CBM-181354. [DOI] [PubMed] [Google Scholar]
- 32.Liang L. MicroRNA-497 Expression, Prognosis and Regulation of Invasion and Metastasis in Hepatocellular Carcinoma. Fudan University; 2013. [Google Scholar]
- 33.Wang W., Ren F., Wu Q., et al. MicroRNA-497 inhibition of ovarian cancer cell migration and invasion through targeting of SMAD specific E3 ubiquitin protein ligase 1. Biochemical and Biophysical Research Communications. 2014;449(4):432–437. doi: 10.1016/j.bbrc.2014.05.053. [DOI] [PubMed] [Google Scholar]
- 34.Xu J., Wang T., Cao Z., et al. MiR-497 downregulation contributes to the malignancy of pancreatic cancer and associates with a poor prognosis. Oncotarget. 2014;5(16):6983–6993. doi: 10.18632/oncotarget.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng F., Kuai D., Wang H., et al. Reduced expression of microRNA-497 is associated with greater angiogenesis and poor prognosis in human gliomas. Human Pathology. 2016;58:47–53. doi: 10.1016/j.humpath.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 36.Huang C., Ma R., Yue J., Li N., Li Z., Qi D. MiR-497 suppresses YAP1 and inhibits tumor growth in non-small cell lung cancer. Cellular Physiology and Biochemistry. 2015;37(1):342–352. doi: 10.1159/000430358. [DOI] [PubMed] [Google Scholar]
- 37.Troppan K., Wenzl K., Pichler M., et al. miR-199a and miR-497 are associated with better overall survival due to increased chemosensitivity in diffuse large B-cell lymphoma patients. International Journal of Molecular Sciences. 2015;16(8):18077–18095. doi: 10.3390/ijms160818077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X., Shi Q., Wang J., Ma H., Zhou X. Study on expression and clinicopathologic characteristics of miR-195 and miR-497 in colorectal cancers. Journal of Medical Postgraduates. 2012;25(11):1163–1167. [Google Scholar]
- 39.Gupta G. P., Massague J. Cancer metastasis: building a framework. Cell. 2006;127(4):679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Shen L., Li J., Xu L., et al. miR-497 induces apoptosis of breast cancer cells by targeting Bcl-w. Experimental and Therapeutic Medicine. 2012;3(3):475–480. doi: 10.3892/etm.2011.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan J. J., Zhang Y. N., Liao J. Z., et al. MiR-497 suppresses angiogenesis and metastasis of hepatocellular carcinoma by inhibiting VEGFA and AEG-1. Oncotarget. 2015;6(30):29527–29542. doi: 10.18632/oncotarget.5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang W., Ren F., Wu Q., Jiang D., Li H., Shi H. MicroRNA-497 suppresses angiogenesis by targeting vascular endothelial growth factor A through the PI3K/AKT and MAPK/ERK pathways in ovarian cancer. Oncology Reports. 2014;32(5):2127–2133. doi: 10.3892/or.2014.3439. [DOI] [PubMed] [Google Scholar]
- 43.Gu A., Lu J., Wang W., Shi C., Han B., Yao M. Role of miR-497 in VEGF-A-mediated cancer cell growth and invasion in non-small cell lung cancer. The International Journal of Biochemistry & Cell Biology. 2016;70:118–125. doi: 10.1016/j.biocel.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 44.Shao X. J., Miao M. H., Xue J., Xue J., Ji X. Q., Zhu H. The down-regulation of MicroRNA-497 contributes to cell growth and cisplatin resistance through PI3K/Akt pathway in osteosarcoma. Cellular Physiology and Biochemistry. 2015;36(5):2051–2062. doi: 10.1159/000430172. [DOI] [PubMed] [Google Scholar]
- 45.Li M., Ma X., Li M., et al. Prognostic role of microRNA-210 in various carcinomas: a systematic review and meta-analysis. Disease Markers. 2014;2014:10. doi: 10.1155/2014/106197.106197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duan F., Wang K., Dai L., et al. Prognostic significance of low microRNA-218 expression in patients with different types of cancer: evidence from published studies. Medicine. 2016;95(37, article e4773) doi: 10.1097/MD.0000000000004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qi Y., Huang Y., Pang L., et al. Prognostic value of the microRNA-29 family in multiple human cancers: a meta-analysis and systematic review. Clinical and Experimental Pharmacology & Physiology. 2017;44(4):441–454. doi: 10.1111/1440-1681.12726. [DOI] [PubMed] [Google Scholar]
- 48.Feng Y., Duan F., Liu W., Fu X., Cui S., Yang Z. Prognostic value of the microRNA-214 in multiple human cancers: a meta-analysis of observational studies. Oncotarget. 2017;8(43):75350–75360. doi: 10.18632/oncotarget.17642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng G. Circulating miRNAs: roles in cancer diagnosis, prognosis and therapy. Advanced Drug Delivery Reviews. 2015;81:75–93. doi: 10.1016/j.addr.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Pang P. C., Shi X. Y., Huang W. L., Sun K. miR-497 as a potential serum biomarker for the diagnosis and prognosis of osteosarcoma. European Review for Medical and Pharmacological Sciences. 2016;20(18):3765–3769. [PubMed] [Google Scholar]