Abstract
Magnetic resonance imaging (MRI) is a noninvasive medical imaging modality that is routinely used in clinics, providing anatomical information with micron resolution, soft tissue contrast, and deep penetration. Exogenous contrast agents increase image contrast by shortening longitudinal (T1) and transversal (T2) relaxation times. Most of the T1 agents used in clinical MRI are based on paramagnetic lanthanide complexes (largely Gd-based). In moving to translatable formats of reduced toxicity, greater chemical stability, longer circulation times, higher contrast, more controlled functionalisation and additional imaging modalities, considerable effort has been applied to the development of nanoparticles bearing paramagnetic ions. This review summarises the most relevant examples in the synthesis and biomedical applications of paramagnetic nanoparticles as contrast agents for MRI and multimodal imaging. It includes the most recent developments in the field of production of agents with high relaxivities, which are key for effective contrast enhancement, exemplified through clinically relevant examples.
1. Introduction
Noninvasive imaging techniques support disease diagnosis and pathological characterisation with ease in a comparatively safe manner [1, 2]. These techniques include nuclear imaging modalities such as positron emission tomography (PET), single photon emission computed tomography (SPECT), computed tomography (CT), optical imaging (OI), ultrasound (US), and magnetic resonance imaging (MRI). All these present individual distinct advantages, as well as limitations which need to be considered within the aims of the specific study. MRI is routinely utilised because of its associated intrinsic high spatial resolution, deep tissue penetration, and three-dimensional anatomical information [3]. It is, thus, widely employed in clinics for the diagnosis and prognosis of a broad range of disease states. Despite this, MRI suffers from inherently low sensitivity; hence, exogenous contrast agents are applied to overcome this obstacle by shortening the relaxation times of bulk water [4]. Contrast agents not only enhance image contrast but also support multimodal imaging. They can be classified into two different groups depending on the operational mode. T1, also called positive contrast agents, shortens longitudinal relaxation times and brightens the accumulation area [5]. T2, or negative contrast agents, conversely shortens transversal relaxation times and darkens the immediate and surrounding area. From metal complexes to nanoparticles, different formulations have been employed as contrast agents for MRI with most of these based on the use of highly paramagnetic ions such as Gd3+, Mn2+, and Fe3+ [6]. They are usually utilised as coordination complexes with acyclic or cyclic chelate agents to reduce associated toxicity of the free metal ion. Although these molecular agents have been extensively used, paramagnetic metallic complexes have problems related to their fast excretion [7]. In terms of targeting and adding additional imaging modes, the use of low molecular weight lanthanide complexes is limited by high synthetic demand and the possibility of undesirable side reactions occurring during the synthesis. For these reasons, paramagnetic contrast agent research has focused on the development of nanoparticulate forms over the last few years [8, 9]. Paramagnetic nanoparticles present several advantages over traditional coordination complexes. Composition is readily tuneable as is size and shape. Magnetic characteristics are improved by geometric local density effects rendering markedly higher T1 and/or T2 relaxometric values than the corresponding coordination complexes. In addition, control over associated pharmacokinetics enables an increase in blood circulation time [10]. Ultimately, most nanoparticulate platforms are cleared by the reticuloendothelial system (RES) with the action of macrophages, such as Kupffer cells, generating uptake from the bloodstream to the liver and spleen. The rate of this process, known as opsonization, depends on the size of the nanoparticle and its chemical coating. Nanoparticles below 5–7 nm in diameter are able to pass through the kidney glomerulus triggering fast excretion through urine. Larger particles are excreted by the RES route with kinetics that can be tuned through the surface chemistry. The protein corona that develops on these particles during circulation is key to this and can be controlled by pegylation, for example, or a polymer coating such that blood circulation time is optimised [11]. This has a direct role in MRI acquisition, where a greater acquisition time increases signal-to-noise and consequently “image quality.” Another important feature within the use of nanoparticles is their high surface-to-volume ratio, supporting a high ligand (protein, antibody, and peptide) payload as well as tuneable and potentially accessible internal volume [12]. These features enable combination with drugs such that targeted, imaging-based, diagnosis may be followed by simultaneous therapy specific to that condition, known as theranostics [13].
Many different approaches have been used to develop paramagnetic nanoparticles for MRI, a few of which will be represented herein. In general, one of two synthetic strategies is followed: (1) formation of nanoparticles with the paramagnetic ion incorporated into the nanostructured framework and (2) postfunctionalisation of the particles with a lanthanide coordination complex. The first approach has been largely based on the synthesis of stoichiometric or nonstoichiometric nanoparticulate metal oxides such as Gd2O3, MnO, Mn3O4, Dy2O3, or γ-Fe2O3. This approach also includes NaGdF4, KGdF4, β-NaDyF4, NiFe2O4, and ZnFe2O4 nanoparticles as well as others [14–19]. The second strategy has been developed with a number of supporting nanoparticle scaffolds (silica, gold, micelles, polymers, and semiconducting quantum dots) which are subsequently doped with DTPA, DOTA, or derivatives [20–24]. This review focuses on the synthesis of Gd3+, Mn2+, Dy3+, and Ho3+ nanoparticles and their biomedical application as contrast agents for MRI and multimodal imaging.
2. Paramagnetic Ion-Based Relaxivity
The relaxivity value, in mM−1·s−1, quantifies the ability of a contrast agent to promote contrast in MRI. In paramagnetic ions, two different mechanisms should be considered, both an inner-sphere and an outer-sphere mechanism, respectively:
| (1) |
The inner-sphere mechanism is predominant in paramagnetic contrast agents. It represents the influence of the paramagnetic ion on highly local water protons and is sensitive to the chemical water exchange between the first coordination sphere (inner-sphere) of the paramagnetic ion and bulk water. In terms of longitudinal relaxation rate, a range of different factors are considered within the inner-sphere contribution as described in the following equation:
| (2) |
where Pm refers to the mole fraction of the paramagnetic ion, q is the number of water molecules coordinated to the metal centre (hydration number), T1m the relaxation time of the water protons bounded to the ion, and τM is the bound residence lifetime. By considering equation (2) and Solomon–Bloembergen–Morgan (SBM) theory, three important parameters must be taken into consideration in agent design: hydration number (q), residence lifetime (τM), and the rotational correlation time (τR) [25]. The hydration number is usually equal to 1 in kinetically stable lanthanide complexes though a broad range of chelate agents with larger hydration numbers (and higher longitudinal relaxivities) have also been reported [26, 27]. An increase in hydration can come with the cost of stability (either chemical or signal output) [4]. Residence lifetimes should scale inversely with strength of the magnetic field and optimal values have been found to be in the range of 1–30 ns [28]. In addition, it has been shown that τR governs the relaxivity when τM is in the optimal range. The best relaxometric performance is typically observed when τR is the range of a few nanoseconds [29].
Gadolinium (Gd3+) and manganese (Mn2+) are the most used paramagnetic T1 contrast agents for MRI. Gd3+ has 7 unpaired electrons in the 4f subshell and a high associated spin quantum number (S = 7/2). Mn2+ contains 5 unpaired electrons in its valence d orbitals and hence also a high spin quantum number (S = 5/2). Both of them present high magnetic moments, symmetric orbital ground states, large longitudinal electronic relaxation times (∼10−8 s), and fast water exchange kinetics [30, 31].
Although paramagnetic ions are more commonly used as T1 contrast agents, there are examples, such as Dy3+ and Ho3+, which present notable T2 contrast. In contrast to Mn and Gd, Dy3+ and Ho3+ have highly anisotropic ground states with substantial spin-orbit and Zeeman effects [32]. Therefore, these ions show very short electronic relaxation times (∼10−13 s) that are accompanied by high effective magnetic moment (μeff Dy3+ = 10.6 μB). Due to these properties, Dy3+ and Ho3+ coordination complexes are found to affect primarily the transversal relaxivity (T2 contrast) with relaxivities increasing with magnetic field strength by a Curie relaxation mechanism. The latter points to facilitating an application of Dy3+/Ho3+ molecular complexes in ultrahigh field MRI [33, 34].
It has been reported that the inner-sphere contribution governs the transverse relaxation of bulk water in these complexes where slow water exchange results in better relaxivities [35, 36]. Very recently, it has been demonstrated that the incorporation of Dy-DOTA complexes into silica nanoparticles changes the relaxation mechanism towards that associated with an enhanced Curie outer-sphere contribution [20].
3. Paramagnetic Nanoparticles
A very broad range of nanoparticulate MRI contrast agents have been reported. This review will focus on a few of these (Table 1), namely, those associated with the paramagnetic ions Gd3+, Mn2+, Dy3+, and Ho3+, since they have been extensively studied and often show striking MRI performance.
Table 1.
Examples of nanoparticle-based paramagnetic contrast agents for magnetic resonance imaging included in this review.
| Material | Size (nm) | r 1 (mM−1·s−1) | r 2 (mM−1·s−1) | Magnetic field (T) | Reference |
|---|---|---|---|---|---|
| Gd-Si-DTTA | 37 | 19.7 | 60.0 | 3 | [37] |
| MSN-DTTA-Gd | 75 | 28.8 | 65.5 | 3 | [38] |
| Gd-DOTA-MSNs | 66.3 ± 6.6 | 33.6 ± 1.3 | — | 7 | [39] |
| CeOx : Gd9% | 3.8 | 13.4 | 25.8 | 1.41 | [40] |
| GO-DOTA-Gd | 20–50 | 14.2 | — | 11.7 | [41] |
| iGd-TMV-Si | 300 × 18 nm, 4 nm internal channel | 29.7 | — | 1.41 | [42] |
| Gd-DND | 4.9 | 33.4 | 332.0 | 8 | [43] |
| Hsp DTPA-Gd nanocage | 37.1 | 47.4 | — | 1.5 | [44] |
| DOTA(Gd)-Fe@Fe3O4 | 258 | 7.2 | 109.4 | 0.5 | [45] |
| Gd-M-dots | 14.58 | 23.4 | 123.3 | 3 | [46] |
| Gd2O3 | 1.0 | 9.9 | 10.5 | 1.5 | [15] |
| Silica-Gd2O3 | 91.5 | 30.8 | — | 0.55 | [47] |
| Gd2O3 : Eu3+ | 7.4 ± 0.3 | 34.3 | — | 3 | [48] |
| QDs@DTDTPA-Gd | 24.7 ± 2.7 | 9.9 | — | 3 | [49] |
| A-C-dots@Ce6 | 98 ± 10 | 32.1 | — | 3 | [50] |
| GBCAs-BP | 70 | 15.4 | 73.5 | 3 | [51] |
| GDO(Gd + Dy) | 1.0 | 6.0 | 40.0 | 1.5 | [52] |
| PEG-MnO | 1.9 | 12.9 | 60.3 | 3 | [53] |
| MnO | 7 | 0.4 | 1.7 | 3 | [16] |
| Mn3O4@CF | ~70 | 3.5 | — | 1.41 | [17] |
| MnIO | 17.3 | 57.8 ± 6.5 | 306.3 ± 15.2 | 0.5 | [54] |
| OA-PL-HMON | — | 1.1 | 9.2 | 3 | [55] |
| NCP@peg-AA | 78.6 ± 5.4 | 11.6 | 19.7 | 3 | [56] |
| NPs-dopa-PEG-DOTA/RGD | 26.4 ± 7.5 | — | 267.5 | 7 | [57] |
| 64Cu-NOTA-FA-FI-PEG-PEI-Ac-Mn3O4 | 476.5 ± 13.5 | 1.0 | — | 0.5 | [58] |
| Mn-LDH | 48.0 ± 1.8 | 9.5 at pH 5.0 1.2 at pH 7.4 |
— | 16.4 | [59] |
| Fe3O4@C@MnO2 | 150 | 5.3 at pH 5 2.2 at pH 7.4 |
364.2 at pH 5 442.4 at pH 7.4 |
3 | [60] |
| MnO@Au NCs | 45.0 ± 5.1 | 2.4 at pH 5.4 1.2 at pH 7.4 |
— | 7 | [61] |
| Dy-MSNs-L | 166.2 ± 1.9 | — | 143.5 ± 8.2 | 11.7 | [20] |
| Dy(DOTA)-Cy7.5-TMV-PEG-DGEA | 300 × 18 nm, 4 nm internal channel | — | 399.0 | 9.4 | [62] |
| DyF3 | 100.35 | 0.9 | 380.4 | 9.4 | [63] |
| D2O3 (D-glucuronic acid coating) | 3.2 | 0.008 | 65.0 | 1.5 | [18] |
| NaDyF4 | 20.3 | 0.3 | 101.0 | 9.4 | [19] |
| Dy-doped MnCO3 | 9.27 ± 0.72 | 4.5 | — | 7 | [64] |
| Dy2O3 : Tb3+ | 3.0 ± 0.3 | — | 2.2 | 7 | [65] |
| PEG-NaGdF4:Dy | — | 5.2 | 10.6 | 9.4 | [66] |
| SiO2@Gd2O3 : Dy3+ | 101.5 | 30.2 | — | 0.55 | [67] |
| NaHoF4 | 28.9 | 0.6 | 222.6 | 7 | [68] |
| PEG-Ho2O3 | 80–90 | — | 23.5 | 1.5 | [69] |
| HoF3 | 94.3 | 0.6 | 608.4 | 9.4 | [63] |
3.1. Gadolinium
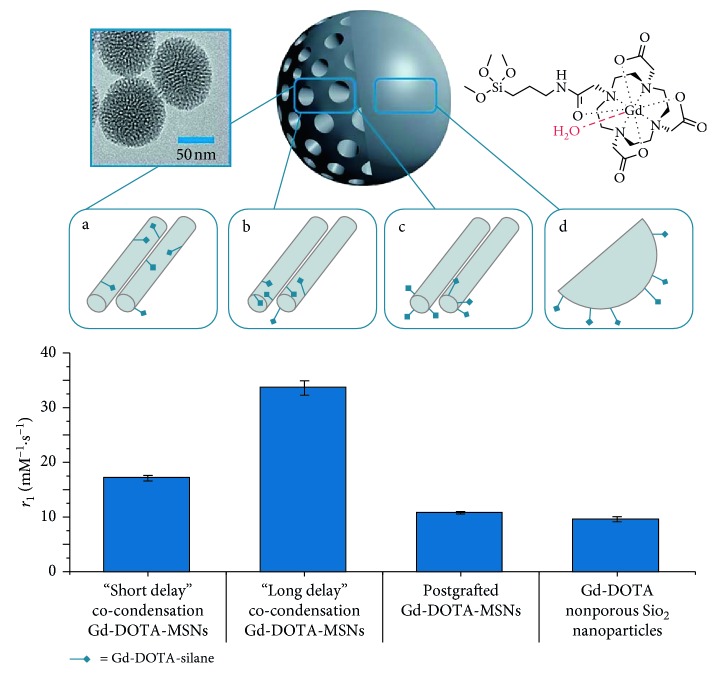
A broad range of approaches have been applied to the incorporation of Gd chelates into nanoparticles, some of which are included here, since they enable high levels of chemical tailoring [70]. Of them, gadolinium-doped silica nanoparticles have been extensively reported. A study by Rieter et al., for example, utilised a luminescent [Ru(bpy)3]Cl3 core to which a coating of a silylated Gd complex was applied [37]. This produced stable particles with r1 values markedly greater than conventional molecular Gd chelates. This work was then taken further to produce mesoporous silica nanoparticles (MSNs) with even higher relaxivity values in an approach that has subsequently been adopted by many research groups [38]. Other work has demonstrated that the location of the Gd chelate within the MSNs greatly influences its relaxometric properties. The highest relaxivities have been specifically reported to occur when synthesis is by a long delay co-condensation process with r1 value of 33.6 ± 1.3 mM−1·s−1, higher than any previously reported Gd-DOTA silica nanoparticles and 20 times larger than free Gd-DOTA (Figure 1) [39]. These particles were then biotinylated showing that the large relaxivity of the particles was essentially unchanged on external biomodification but was then reversibly gateable on subsequent protein recognition [72].
Figure 1.
Typical transmission electron microscope image and schematic representation of Gd-DOTA-MSNs (66.3 ± 6.6 nm) prepared using (a) “short delay” co-condensation, where functionalities are internalised deeply in the structure (r1 = 17.14 ± 0.49 mM−1·s−1), (b) “long delay” co-condensation, where functionalities are internalised nearer to the porous openings (r1 = 33.57 ± 1.29 mM−1·s−1), and (c) postgrafting, where functionalities are loaded on external surfaces (r1 = 10.77 ± 0.22 mM−1·s−1). (d) Postgrafted Gd-DOTA-non-porous silica nanoparticles (r1 = 19.56 ± 0.47 mM−1·s−1) (reproduced from [71]).
Graphene oxide (GO) has also been used as a scaffold to integrate Gd-DOTA moieties. In one study, GO was first pegylated, functionalised with DOTA, and then metallated with Gd3+. These nanoparticles presented a large r1 value of 14.2 mM−1·s−1 measured at 11.7 T [41]. Another interesting scaffold for Gd-DOTA is the use of the tobacco mosaic virus (TMV). TMV is a rod-like plant virus formed by 2130 identical coat proteins assembled into a 300 × 18 nm hollow tube with a 4 nm pore channel. Gd-DOTA was loaded into the interior channel by a copper-catalysed azide-alkyne cycloaddition reaction yielding r1 = 10.9 mM−1·s−1. Surprisingly, this relaxivity increased to 29.7 mM−1·s−1 when the surface of the TMV was functionalised with silica [42].
In recent work, cerium oxide nanoparticles have been utilised to produce Gd-cerium nanoparticles with antioxidant capabilities. These nanoparticles, between 3 and 5 nm in size, rendered longitudinal relaxivities around 7–13 mM−1·s−1 [40]. Gd3+-impregnated nanodiamonds have also been used to produce CAs. These particles have been synthesised via an unusual detonation synthesis where an oxygen deficient trinitrotoluene/1,3,5-trinitroperhydro-1,3,5-triazine (TNT/RDX) mixture is detonated under an inert atmosphere. The nanodiamonds, formed in the early stages of the detonation under high pressure, reportedly are 4–5 nm in size with a narrow size distribution. These were then modified with Gd3+ at the surface carboxylic groups generating unprecedented transverse relaxivity (r2 = 332 mM−1·s−1) and corresponding r1 = 33.4 mM−1·s−1; the relaxivity mechanisms that are responsible for this high r2 value warrant further analysis [43]. Another interesting example is a report of the synthesis of protein-based nanocages. These were further functionalised with maleimide-DTPA-Gd3+ using a cysteine residue of the protein with the relaxometric properties analysed for four nanocages with different sizes (from 16.8 to 37.1 nm). The data revealed that higher r1 values (up to 47 mM−1·s−1 at 1.5 T) were obtained in larger nanocages due to the reduction in the tumbling rate of associated water molecules [44].
Gadolinium oxides are the most utilised alternatives to Gd chelates, where it has been found that decreasing particle diameter results in a progressive trend towards higher relaxivities. For instance, Park et al. showed that the highest relaxivities were obtained for nanoparticles synthesised with an average diameter of d = 1–2.5 nm [15]. It should be noted, however, that ultrasmall Gd2O3 have been found to form deposits in the brain and consequently there is a compromise between limiting the toxicity of the particles while maximising imaging potency. A study by Yin et al. produced silica nanoparticles coated in a Gd2O3 nanoshell of varying thicknesses. By systematically changing the thickness of the silica shell, the variations in relaxivity values could be investigated and demonstrated that a thinner shell resulted in larger r1 values [47].
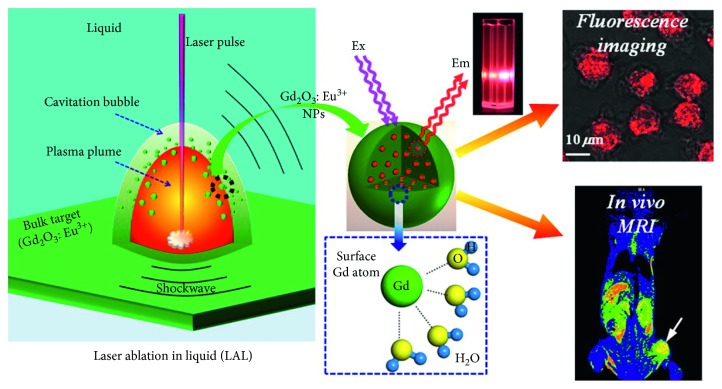
A broad number of dual modal paramagnetic particle systems have been developed [73]. Gd2O3 nanoparticles for fluorescence/MR imaging have, for example, been reported and doped with Eu3+ to produce Gd2O3 : Eu3+ particles. Fluorescence imaging here mediated by Gd3+ absorbing a photon and moving to a 6IJ excited state. Energy transfer between this state to the highly unstable 5D0 state of Eu3+ is accompanied by the emission of a photon on which the electrons return to the ground state of Eu3+. This coupled with a high r1 = 34.3 mM−1·s−1 results in an efficient bimodal imaging probe (Figure 2) [48]. CuInS2/ZnS quantum dots have also been reported as good candidates for fluorescence/MRI bimodal imaging. These QDs were conjugated with a derivative compound of DTPA for further chelation with Gd3+. The composition of these QDs enhanced near-infrared fluorescence (NIRF) and MR imaging with a moderate r1 = 9.91 mM−1·s−1 [49]. A recent approach has described the use of carbon dots (CDs) decorated with Gd3+ for fluorescence/MRI. In this work, CDs with surface carboxylic groups were obtained via a microwave synthesis. Gd3+ was then used to mediate a spherical assembly of the CDs by intercluster electrostatic linkages (-COO−-Gd3+-−OOC). This formulation showed a fluorescence enhancement with increasing Gd3+ concentration and a high r1 value of 32.1 mM−1·s−1 [50].
Figure 2.
Figure illustrating the dual-modal imaging properties of Gd2O3 : Eu3+ nanoparticles (reprinted (adapted) with permission from [48]).
A dual MR/CT imaging agent based on surface functionalised bisphosphonate (BP) gadolinium oxide nanoparticles has been developed. Gd2O3 nanoparticles were synthesised via the polyol method and encapsulated into a mesoporous silica shell by addition of glycidyloxipropyl trimethoxysilane (GPTES). The final formulation presented an r1 = 15.41 mM−1·s−1 with r2/r1 = 4.77 at 3 T [51].
In addition to contrast agents enabling multiple imaging modalities, dual T1-T2 contrast agents can provide enhanced MRI imaging capabilities. Though it is sequence dependent, T2 agents are, in general, natively disadvantaged since negative contrast generated by the contrast agent can easily be confused with natural artefacts coming from calcification or internal bleeding [74]. One approach providing T1-T2 contrast reported by Tirusew et al. used mixed Gd-Dy oxide nanoparticles [52]. Both gadolinium and dysprosium have high magnetic moments with the former promoting a shortening of longitudinal relaxation and dysprosium promoting the transverse relaxation. In an alternative approach, iron oxide nanoparticles were used instead of dysprosium to enhance T2 contrast. In recent work, for example, Gd-labelled Fe@Fe3O4 particles have been reported with relaxivity values of r1 = 7.2 mM−1·s−1 and r2 = 109.4 mM−1·s−1 at 0.5 T [45]. Another example utilises biologically inspired ultrasmall (<10 nm) melanin nanoparticles loaded with Gd-DOTA as a T1-T2 dual-modal contrast agent. One of the active components, melanin, is a biological pigment that is paramagnetic in nature and chelates metal ions very strongly. Its use has thus been proposed as a promising alternative to traditional iron oxide or silica-based particles [46].
3.2. Manganese
Manganese nanoparticles have been extensively researched as possible T1 contrast agents with reduced toxicity (compared to that of gadolinium) but however suffer from low native r1 relaxivities [53, 75]. Much effort has been invested in increasing relaxivity and biocompatibility through the use of derived nanoparticulate systems. For instance, MnO nanoparticles have been encapsulated in polyethylene glycol showing no significant toxicity [16]. More recently, PEG-functionalised Mn3O4 nanoparticles have been encapsulated in a mesoporous, biocompatible carbon framework, with the latter enabling the water access required while apparently reducing Mn cation loss [17]. Other works conducted by Zhao et al. substituted Fe2+ ions on the surface of magnetite particles with Mn2+ to produce particles with T1-weighted imaging capabilities [54]. On increasing the extent of doping of Mn2+ on the surface, an element with both a longer electronic relaxation time and greater paramagnetism than Fe2+, there was a corresponding increase in r1.
Hollow Mn3O4 nanoparticles have been used in a study of the effect of surface functionalisation on relaxometric properties. It was observed, for example, that, with carboxylic acid functionalised ligands, there was a corresponding increase in r1. This was proposed to be due to the induction of ferromagnetic spins between free surface spins [55]. Coordination polymers (NCP) of nanometric size (78.6 ± 5.4 nm) have been loaded with Mn2+ to produce efficient T1 contrast agents. In this case, organic bridging ligands are used to produce a self-assembly process with Mn2+. These nanoparticles displayed high Mn loading of up to 13.3 ± 4 wt.% with a maximum r1 value of 11.6 mM−1·s−1. The increase in the relaxivity was assigned to a reduction in the tumbling rates [56].
There has been a growing interest in the development of environmentally responsive MR agents [76]. One major class of these are pH responsive contrast agents which offer potential value in resolving the low pH microenvironment associated with tumour tissue [76]. Manganese-based double-layered hydroxide nanoparticles were the first reported ultrasensitive pH-responsive Mn-based contrast agent for T1 MRI imaging, showing a 6-fold increase in longitudinal relaxivity values in acidic media (pH = 5) than at pH = 7.4, a switch assigned to the unique structure of Mn ions in the double-layered hydroxide [59]. Another reported pH responsive nanoparticulate example has been the synthesis of Fe3O4@C@MnO2 nanoparticles. Here, iron oxide nanoparticles were coated with a carbon layer and reacted with KMnO4 to produce MnO2 nanosheets on the outer carbon shells. In a protic environment, these MnO2 nanosheets are reduced to Mn2+ The increased concentration of paramagnetic Mn2+ ions in solution is accompanied by a corresponding increase in T1 relaxation [60]. Manganese monoxide nanocomposites functionalised with porous gold nanoclusters have also been used as pH-responsive probes. In this work, it was suggested that the gold nanoclusters sterically hinder the release Mn2+ from the particles, consequently providing delayed T1 contrast and a longer diagnostic window. They also allow the system to function as a multimodal probe with photoacoustic and X-ray CT imaging modalities additionally supported [61].
3.3. Dysprosium
Dysprosium is one possible alternative to conventional gadolinium-based contrast agents and acts as an effective T2 contrast agent through its high magnetic moment (the largest of the lanthanides) and short (∼10−13 s) electronic relaxation time. Many approaches have been employed to produce dysprosium-modified nanoparticles, most recently MSNs that incorporate Dy-DOTA chelates in the outer pore channel producing particles with high r2 values of 143.5 ± 8.2 mM−1·s−1 at 11.7 T, some 20 times larger in magnitude than the molecular analogue [20]. Even higher T2 relaxivities have been reported by loading Dy-DOTA chelates into the cavity of the tobacco mosaic virus (r2 values of 326 mM−1·s−1 at 7 T and 399 mM−1·s−1 at 9 T) [62]. Dysprosium oxide nanoparticles and dysprosium fluoride have also been proposed as promising agents. For example, González-Mancebo et al. produced DyF3 rhombus-shaped nanoparticles with an average size of 110 × 50 nm. These nanoparticles present a remarkable r2 of 380.4 mM−1·s−1 when measured at 9.4 T, ascribed to strong outer-sphere effects where the diffusion correlation time (τD) is affected by the effective radius of the nanoparticles [63]. Dysprosium oxide nanoparticles and dysprosium hydroxide nanorods have also been described as good candidates for T2 contrast agents. Research by Kattel et al. synthesised d-glucuronic acid-coated ultrasmall Dy2O3 nanoparticles with an average size of 3.2 nm and Dy(OH)3 nanorods with an average size of 20 × 300 nm. The first formulation showed an r2 = 65.0 mM−1·s−1 whilst the nanorods possessed a higher value (r2 = 181.57 mM−1·s−1 at 1.5 T) due to their greater size [18]. β-NaDyF4 nanoparticles have shown potential as ultrahigh field magnetic resonance imaging (9.4 T) agents with high r2 values [19]. Another formulation based on the synthesis of MnCO3 nanoparticles doped with Dy exhibited reported r1 values higher than those typical of MnO NPs [64].
There are numerous reports of dual-modal contrast nanoparticulate agents containing dysprosium. Tb3+-doped Dy2O3 nanoparticles have, for example, been reported to possess both MRI and optical imaging modalities since Tb3+ emits across the range of 489 nm to 619 nm, the brightest region being ∼545 nm (green) [65]. PEGylated NaGdF4 : Dy nanoprobes as both dual-modal T1-T2 and MRI/CT agents were produced by Jin et al. T1-weighted contrast is provided by the presence of Gd3+ ions with T2-weighted contrast promoted by the doped Dy3+ ions, which also facilitates the use of the nanoprobe for CT imaging [66]. Dysprosium has been used to dope Gd2O3 nanoparticles resulting in dual-modal MR/fluorescence imaging [67]. These particles contain a silica core encapsulated by a Gd2O3 : Dy3+ nanoshell. Altering the thickness of this shell changed the relaxometric properties of the system with the highest r1 values being reported for the thinnest (2 nm) shells.
3.4. Holmium
Holmium is another paramagnetic lanthanide with a highly effective magnetic moment and short electronic relaxation time. The first example of single Ho3+-doped upconversion nanoparticles for T2-weighted MRI was reported by Ni et al., focusing on Ho3+-doped NaYbF4 with surface phospholipid-PEGylation. Incorporation of both Yb3+ as a sensitizer and Ho3+ as an activator facilitates upconversion such that optical emission in the visible region is possible. In conjunction with efficient r2 relaxation, this example is an effective dual-modal contrast agent [77]. NaHoF4 nanoparticles have also been shown to be effective for T2-weighted MRI with reported values of r2 = 222.6 mM−1·s−1 at 7 T [68], as have holmium oxide nanoparticles. [69] The highest reported r2 values for holmium, and indeed any of the examples mentioned in this review, are for rhombus-like HoF3 nanoparticles produced by González-Mancebo et al. with r2 = 608.4 at 9.4 T.
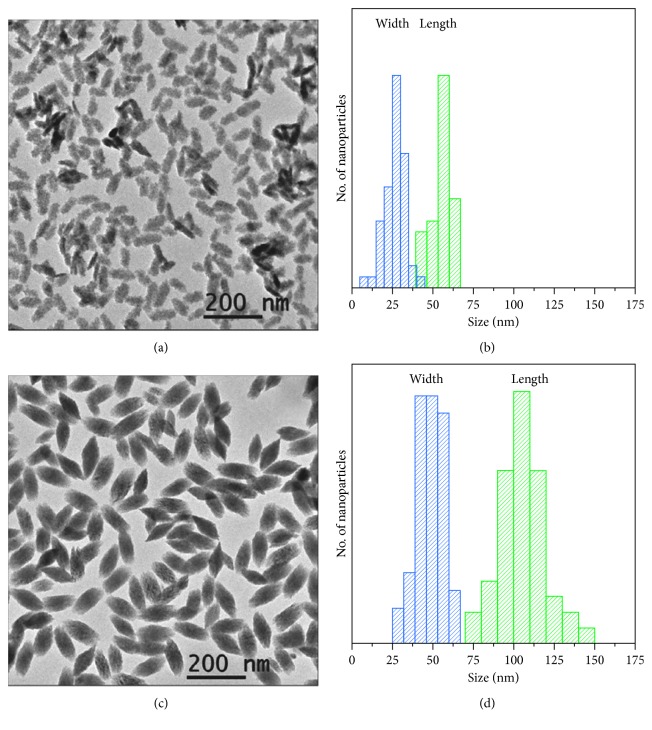
In this work, two different HoF3 formulations were synthesised by homogeneous precipitation in ethylene glycol. Particle size and shape was notably tuneable with Ho(NO3)3 rendering ellipsoid-like nanoparticles (so-called HoF-el) and 70 × 30 nm in size (Figures 3(a) and 3(b)). An alternative with Ho(CH3CO2)3 as the precursor formed rhombus-like nanoparticles (so-called HoF-rh) with an average size of 110 × 50 nm (Figures 3(c) and 3(d)). Although HoF-el displayed large r2 value of 350.0 mM−1·s−1 at high magnetic fields (9.4 T), an unprecedented r2 value of 608.4 mM−1·s−1 was reported for HoF-rh indicating not only a size dependency (increased magnetisation of the larger particles) but also pronounced geometric effects [63].
Figure 3.
TEM images and size distribution plots obtained from TEM images of (a, b) HoF-el and (c, d) HoF-rh NPs (reprinted (adapted) with permission from [63]).
4. Multimodal Imaging and Theranostic Applications
Paramagnetic nanoparticles have applications in responsive MRI, targeted imaging, cell tracking, multimodal imaging, and as part of a theranostic platform. Here, some relevant and interesting examples reported across the last few years are described.
Very recently, Gd3+ complexes have been integrated into protein, calcium phosphate, polymeric, gold, and bismuth based nanoparticles. These have been utilised as nanotheranostics tools for multimodal imaging and in cancer therapy but also for the chemical imaging of neurotransmitters [78–83]. In 2018, Gd2O3 nanoparticles were applied to the targeted imaging of integrins for cancer diagnostics, cell labelling studies, and the multimodal imaging of calcium phosphate bone cement [51, 84, 85]. Gd3+ has been immobilized into metallofullerenes for MRI and photothermal therapy at tumour sites, within cerium oxide nanoparticles (CeNP) as a promising antioxidant theranostic agent, within leukosomes with enhanced activity towards activated endothelium cells, and on carbon dots for imaging-guided radiotherapy of tumours [40, 86–88].
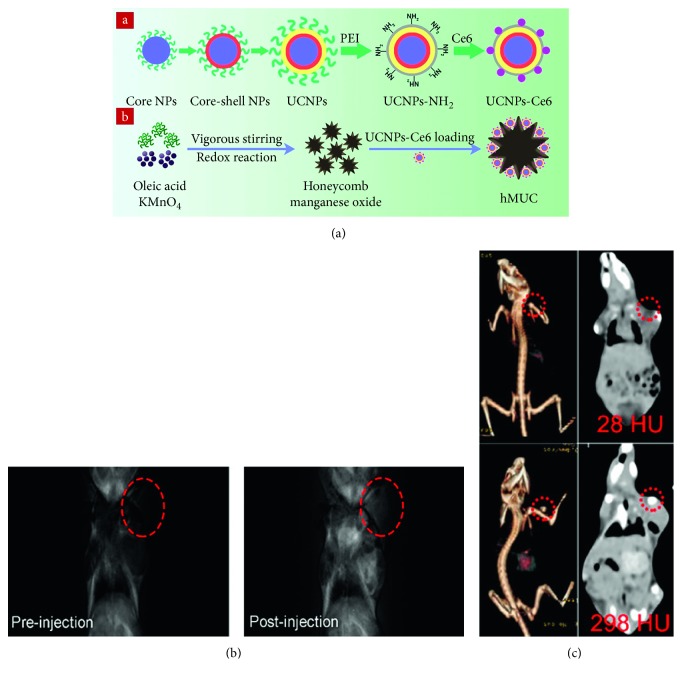
Liu et al. reported multifunctional redox/pH responsive MnO2 nanoparticles for cancer theranostics [89]. Based on honeycomb MnO2 nanoparticles (hMnO2), Sun et al. have developed pH/H2O2 responsive nanoparticles loaded with the photosensitiser chlorin e6 (Ce6). These upconverting nanoparticles, denoted as hMUC (Figure 4(a)), support high in vivo MRI T1 contrast within tumours (Figure 4(b)). The presence of high Z-elements also facilitated CT imaging (Figure 4(c)). Finally, the particles were able to produce reactive oxygen species (ROS) through action of the Ce6 photosensitiser enabling tumour treatment by photodynamic therapy (PDT) [90].
Figure 4.
(a) Schematic illustration for the synthesis of UCNPs-Ce6 and hMUC. (b) In vivo T1-weighted MR images of a tumour-bearing mouse before (left) and after (right) intravenous injection of hMUC. (c) In vivo CT images of a tumour-bearing mouse before (upper) and after (lower) intratumour injection (reprinted (adapted) with permission from [90]).
Similar methodologies have been reported in enabling the pH and/or redox responsive diagnosis and photodynamic therapy of tumours using MnO2 nanoparticles coupled with gold nanocages, copper sulfide nanostructures, or iron oxide nanoparticles [91–94].
Wang et al. have recently reported the synthesis of holmium-doped hollow silica nanospheres to create multifunctional theranostic nanoparticles. These were subsequently conjugated with a prostate stem cell antigen (PSCA) monoclonal antibody for targeted bimodal US/MRI of tumours as well as combined sonodynamic and hypoxia activated therapy [95]. Another application of multifunctional nanoparticles for dual-modal imaging is reported by Li et al., where ternary-doped (fluorine, ytterbium, and holmium) hydroxyapatite nanoparticles were used for multimodal imaging/tracking of hydroxyapatite in hard tissue repair [96]. Pegylated NaHoF4 nanoparticles have also been employed for single MR and MR/CT dual-modality imaging applications [68, 97].
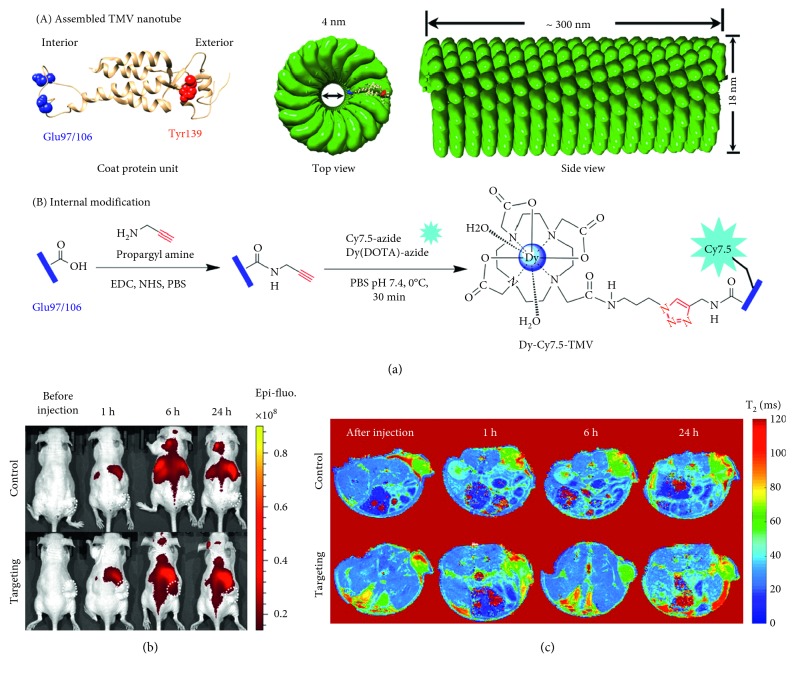
A notable example is reported by Hu et al. where dysprosium-modified tobacco mosaic virus nanoparticles were used for the bimodal MR/NIRF imaging of prostate cancer. In this work, internal glutamic acid residues were exploited to functionalise the nanoparticles with Dy-DOTA-azide and a NIRF dye Cy7.5-azide by a copper-catalysed azide-alkyne click chemistry cycloaddition (Figure 5(a)). Then, the external surface was conjugated with a DGEA peptide that binds to a specific integrin on the surface of PC-3 prostate cancer cells. The final formulation was able to produce targeted NIRF imaging in nude mice (Figure 5(b)), displaying strong T2 contrast as supported by a high transverse relaxivity (399 mM−1·s−1 at 9.4 T) (Figure 5(c)) [62].
Figure 5.
(a) Structure of the tobacco mosaic virus (TMV) nanoparticle's coat protein with surface-exposed residues highlighted as internal glutamic acid (blue) and external tyrosine (red) and the structure of the assembled capsid/strategy for internal modification. (b) Near-infrared fluorescence (NIRF) imaging of subcutaneous PC-3 (α2β1) prostate tumours in athymic nude mice (n = 3) before and 1, 6, and 24 h after the intravenous injection of Dy-Cy7.5-TMV-mPEG (control group) or Dy-Cy7.5-TMV-DGEA (targeting group). (c) In vivo T2-mapping MRI of subcutaneous PC-3 (α2β1) prostate tumours in athymic nude mice (n = 3) obtained before and 1, 6, and 24 h after the intravenous injection of Dy-Cy7.5-TMV-mPEG (control group) and Dy-Cy7.5-TMV-DGEA (targeting group) (reprinted (adapted) with permission from [62]).
5. Conclusions
Paramagnetic contrast agents present themselves as valuable tools for a broad range of potent MRI applications. Although traditional paramagnetic coordination complexes have been extensively applied, control over both thermodynamic and kinetic stability, pharmacokinetics, biodistribution, and imaging potency is limited. There are a wide variety of accessible synthetic procedures to develop nanoparticles conjugated with paramagnetic ions with a control over composition, size, and shape. This supports facile management of stability, pharmacokinetics, and biodistribution. Associated paramagnet hydration, water exchange kinetics, or residence lifetime can be tuned, all at high levels of local loading. The ability to readily integrate additional imaging modes and the employment of multivalent vectors across comparatively high particle surface areas make these yet more promising. To date, these constructs have been applied to the targeted multimodal molecular imaging, of cancer, cardiovascular, and neurological diseases as well as drug delivery, photodynamic, and sonodynamic therapy. One would fully expect the chemical richness available here to be truly impactful over the next decade.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Frangioni J. V. New technologies for human cancer imaging. Journal of Clinical Oncology. 2008;26(24):4012–4021. doi: 10.1200/jco.2007.14.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanz J., Fayad Z. A. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451(7181):953–957. doi: 10.1038/nature06803. [DOI] [PubMed] [Google Scholar]
- 3.Geethanath S., Vaughan J. T., Jr. Accessible magnetic resonance imaging: a review. Journal of Magnetic Resonance Imaging. 2019 doi: 10.1002/jmri.26638. In press. [DOI] [PubMed] [Google Scholar]
- 4.De León-Rodríguez L. M., Martins A. F., Pinho M. C., Rofsky N. M., Sherry A. D. Basic MR relaxation mechanisms and contrast agent design. Journal of Magnetic Resonance Imaging. 2015;42(3):545–565. doi: 10.1002/jmri.24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bao Y., Sherwood J. A., Sun Z. Magnetic iron oxide nanoparticles as T1 contrast agents for magnetic resonance imaging. Journal of Materials Chemistry C. 2018;6(6):1280–1290. doi: 10.1039/c7tc05854c. [DOI] [Google Scholar]
- 6.Xiao Y.-D., Paudel R., Liu J., Ma C., Zhang Z.-S., Zhou S.-K. MRI contrast agents: classification and application (review) International journal of molecular medicine. 2016;38(5):1319–1326. doi: 10.3892/ijmm.2016.2744. [DOI] [PubMed] [Google Scholar]
- 7.Bottrill M., Kwok L., Long N. J. Lanthanides in magnetic resonance imaging. Chemical Society Reviews. 2006;35(6):557–571. doi: 10.1039/b516376p. [DOI] [PubMed] [Google Scholar]
- 8.Na H. B., Hyeon T. Nanostructured T1 MRI contrast agents. Journal of Materials Chemistry. 2009;19(35):6267–6273. doi: 10.1039/b902685a. [DOI] [Google Scholar]
- 9.Peng E., Wang F., Xue J. M. Nanostructured magnetic nanocomposites as MRI contrast agents. Journal of Materials Chemistry B. 2015;3(11):2241–2276. doi: 10.1039/c4tb02023e. [DOI] [PubMed] [Google Scholar]
- 10.Ernsting M. J., Murakami M., Roy A., Li S.-D. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. Journal of Controlled Release. 2013;172(3):782–794. doi: 10.1016/j.jconrel.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoo J.-W., Chambers E., Mitragotri S. Factors that control the circulation time of nanoparticles in blood: challenges, solutions and future prospects. Current Pharmaceutical Design. 2010;16(21):2298–2307. doi: 10.2174/138161210791920496. [DOI] [PubMed] [Google Scholar]
- 12.Han X., Xu K., Taratula O., Farsad K. Applications of nanoparticles in biomedical imaging. Nanoscale. 2019;11(3):799–819. doi: 10.1039/c8nr07769j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva C. O., Pinho J. O., Lopes J. M., Almeida A. J., Gaspar M. M., Reis C. Current trends in cancer nanotheranostics: metallic, polymeric, and lipid-based systems. Pharmaceutics. 2019;11(1):p. 22. doi: 10.3390/pharmaceutics11010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu F., Zhao Y. S. Inorganic nanoparticle-based T1 and T1/T2 magnetic resonance contrast probes. Nanoscale. 2012;4(20):6235–6243. doi: 10.1039/c2nr31865b. [DOI] [PubMed] [Google Scholar]
- 15.Park J. Y., Baek M. J., Choi E. S., et al. Paramagnetic ultrasmall gadolinium oxide nanoparticles as advanced T1 MRI contrast agent: account for large longitudinal relaxivity, optimal particle diameter, and in vivo T1 MR images. ACS Nano. 2009;3(11):3663–3669. doi: 10.1021/nn900761s. [DOI] [PubMed] [Google Scholar]
- 16.Na H. B., Lee J. H., An K., et al. Development of a T1 contrast agent for magnetic resonance imaging using MnO nanoparticles. Angewandte Chemie International Edition. 2007;46(28):5397–5401. doi: 10.1002/anie.200604775. [DOI] [PubMed] [Google Scholar]
- 17.Deka K., Guleria A., Kumar D., et al. Mesoporous 3D carbon framework encapsulated manganese oxide nanoparticles as biocompatible T1 MR imaging probe. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2018;539:229–236. doi: 10.1016/j.colsurfa.2017.12.030. [DOI] [Google Scholar]
- 18.Kattel K., Park J. Y., Xu W., et al. Paramagnetic dysprosium oxide nanoparticles and dysprosium hydroxide nanorods as T2 MRI contrast agents. Biomaterials. 2012;33(11):3254–3261. doi: 10.1016/j.biomaterials.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Das G. K., Johnson N. J. J., Cramen J., et al. NaDyF4 nanoparticles as T2 contrast agents for ultrahigh field magnetic resonance imaging. Journal of Physical Chemistry Letters. 2012;3(4):524–529. doi: 10.1021/jz201664h. [DOI] [PubMed] [Google Scholar]
- 20.Zheng X.-Y., Pellico J., Khrapitchev A. A., Sibson N. R., Davis J. J. Dy-DOTA integrated mesoporous silica nanoparticles as promising ultrahigh field magnetic resonance imaging contrast agents. Nanoscale. 2018;10(45):21041–21045. doi: 10.1039/c8nr07198e. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y., Chen S., Li H., et al. Engineered paramagnetic graphene quantum dots with enhanced relaxivity for tumor imaging. Nano Letters. 2018;19(1):441–448. doi: 10.1021/acs.nanolett.8b04252. [DOI] [PubMed] [Google Scholar]
- 22.Litti L., Rivato N., Fracasso G., et al. A SERRS/MRI multimodal contrast agent based on naked Au nanoparticles functionalized with a Gd(III) loaded PEG polymer for tumor imaging and localized hyperthermia. Nanoscale. 2018;10(3):1272–1278. doi: 10.1039/c7nr07398d. [DOI] [PubMed] [Google Scholar]
- 23.Babic A., Vorobiev V., Trefalt G., et al. MRI micelles self-assembled from synthetic gadolinium-based nano building blocks. Chemical Communications. 2019;55(7):945–948. doi: 10.1039/c8cc08875f. [DOI] [PubMed] [Google Scholar]
- 24.Craciun I., Gunkel-Grabole G., Belluati A., Palivan C. G., Meier W. Expanding the potential of MRI contrast agents through multifunctional polymeric nanocarriers. Nanomedicine. 2017;12(7):811–817. doi: 10.2217/nnm-2016-0413. [DOI] [PubMed] [Google Scholar]
- 25.Villaraza A. J. L., Bumb A., Brechbiel M. W. Macromolecules, dendrimers, and nanomaterials in magnetic resonance imaging: the interplay between size, function, and pharmacokinetics. Chemical Reviews. 2010;110(5):2921–2959. doi: 10.1021/cr900232t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang B., Cheng L., Duan B., et al. Gadolinium complexes of diethylenetriamine-N-oxide pentaacetic acid-bisamide: a new class of highly stable MRI contrast agents with a hydration number of 3. Dalton Transactions. 2019;48(5):1693–1699. doi: 10.1039/c8dt04478c. [DOI] [PubMed] [Google Scholar]
- 27.Datta A., Raymond K. N. Gd−Hydroxypyridinone (HOPO)-based high-relaxivity magnetic resonance imaging (MRI) contrast agents. Accounts of Chemical Research. 2009;42(7):938–947. doi: 10.1021/ar800250h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Werner E. J., Datta A., Jocher C. J., Raymond K. N. High-relaxivity MRI contrast agents: where coordination chemistry meets medical imaging. Angewandte Chemie International Edition. 2008;47(45):8568–8580. doi: 10.1002/anie.200800212. [DOI] [PubMed] [Google Scholar]
- 29.Granato L., Vander Elst L., Henoumont C., Muller R. N., Laurent S. Optimizing water exchange rates and rotational mobility for high-relaxivity of a novel Gd-DO3A derivative complex conjugated to inulin as macromolecular contrast agents for MRI. Chemistry & Biodiversity. 2018;15(2) doi: 10.1002/cbdv.201700487.e1700487 [DOI] [PubMed] [Google Scholar]
- 30.Boros E., Gale E. M., Caravan P. MR imaging probes: design and applications. Dalton Transactions. 2015;44(11):4804–4818. doi: 10.1039/c4dt02958e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gale E. M., Atanasova I. P., Blasi F., Ay I., Caravan P. A manganese alternative to gadolinium for MRI contrast. Journal of the American Chemical Society. 2015;137(49):15548–15557. doi: 10.1021/jacs.5b10748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norek M., Peters J. A. MRI contrast agents based on dysprosium or holmium. Progress in nuclear magnetic resonance spectroscopy. 2011;59(1):64–82. doi: 10.1016/j.pnmrs.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Vander Elst L., Zhang S., Sherry A. D., Laurent S., Botteman F., Muller R. N. Dy-complexes as high field T2 contrast agents. Academic Radiology. 2002;9(2):S297–299. doi: 10.1016/s1076-6332(03)80208-8. [DOI] [PubMed] [Google Scholar]
- 34.Pereira G. A., Peters J. A., Almeida Paz F. A., Rocha J., Geraldes C. F. G. C. Evaluation of [Ln(H2cmp)(H2O)] metal organic framework materials for potential application as magnetic resonance imaging contrast agents. Inorganic Chemistry. 2010;49(6):2969–2974. doi: 10.1021/ic9025014. [DOI] [PubMed] [Google Scholar]
- 35.Vander Elst L., Roch A., Gillis P., et al. Dy-DTPA derivatives as relaxation agents for very high field MRI: the beneficial effect of slow water exchange on the transverse relaxivities. Magnetic Resonance in Medicine. 2002;47(6):1121–1130. doi: 10.1002/mrm.10163. [DOI] [PubMed] [Google Scholar]
- 36.Caravan P., Greenfield M. T., Bulte J. W. M. Molecular factors that determine Curie spin relaxation in dysprosium complexes. Magnetic Resonance in Medicine. 2001;46(5):917–922. doi: 10.1002/mrm.1277. [DOI] [PubMed] [Google Scholar]
- 37.Rieter W. J., Kim J. S., Taylor K. M. L., et al. Hybrid silica nanoparticles for multimodal imaging. Angewandte Chemie International Edition. 2007;46(20):3680–3682. doi: 10.1002/anie.200604738. [DOI] [PubMed] [Google Scholar]
- 38.Taylor K. M. L., Kim J. S., Rieter W. J., An H., Lin W., Lin W. Mesoporous silica nanospheres as highly efficient MRI contrast agents. Journal of the American Chemical Society. 2008;130(7):2154–2155. doi: 10.1021/ja710193c. [DOI] [PubMed] [Google Scholar]
- 39.Davis J. J., Huang W.-Y., Davies G.-L. Location-tuned relaxivity in Gd-doped mesoporous silica nanoparticles. Journal of Materials Chemistry. 2012;22(43):22848–22850. doi: 10.1039/c2jm35116a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eriksson P., Tal A. A., Skallberg A., et al. Cerium oxide nanoparticles with antioxidant capabilities and gadolinium integration for MRI contrast enhancement. Scientific Reports. 2018;8(1):p. 6999. doi: 10.1038/s41598-018-25390-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang M., Liu X., Huang J., et al. Ultrasmall graphene oxide based T1 MRI contrast agent for in vitro and in vivo labeling of human mesenchymal stem cells. Nanomedicine: Nanotechnology, Biology and Medicine. 2018;14(7):2475–2483. doi: 10.1016/j.nano.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 42.Bruckman M. A., Randolph L. N., Gulati N. M., Stewart P. L., Steinmetz N. F. Silica-coated Gd(DOTA)-loaded protein nanoparticles enable magnetic resonance imaging of macrophages. Journal of Materials Chemistry B. 2015;3(38):7503–7510. doi: 10.1039/c5tb01014d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panich A. M., Salti M., Goren S. D., et al. Gd(III)-grafted detonation nanodiamonds for MRI contrast enhancement. Journal of Physical Chemistry C. 2019;123(4):2627–2631. doi: 10.1021/acs.jpcc.8b11655. [DOI] [Google Scholar]
- 44.Kawano T., Murata M., Kang J.-H., et al. Ultrasensitive MRI detection of spontaneous pancreatic tumors with nanocage-based targeted contrast agent. Biomaterials. 2018;152:37–46. doi: 10.1016/j.biomaterials.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 45.Wang K., An L., Tian Q., Lin J., Yang S. Gadolinium-labelled iron/iron oxide core/shell nanoparticles as T1-T2 contrast agent for magnetic resonance imaging. RSC Advances. 2018;8(47):26764–26770. doi: 10.1039/c8ra04530e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu L., Hong S. H., Sun Y., et al. Dual T1 and T2 weighted magnetic resonance imaging based on Gd3+ loaded bioinspired melanin dots. Nanomedicine: Nanotechnology, Biology and Medicine. 2018;14(6):1743–1752. doi: 10.1016/j.nano.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 47.Yin J., Chen D., Zhang Y., Li C., Liu L., Shao Y. MRI relaxivity enhancement of gadolinium oxide nanoshells with a controllable shell thickness. Physical Chemistry Chemical Physics. 2018;20(15):10038–10047. doi: 10.1039/c8cp00611c. [DOI] [PubMed] [Google Scholar]
- 48.Liu J., Tian X., Luo N., et al. Sub-10 nm monoclinic Gd2O3:Eu3+ nanoparticles as dual-modal nanoprobes for magnetic resonance and fluorescence imaging. Langmuir. 2014;30(43):13005–13013. doi: 10.1021/la503228v. [DOI] [PubMed] [Google Scholar]
- 49.Yang Y., Lin L., Jing L., Yue X., Dai Z. CuInS2/ZnS quantum dots conjugating Gd(III) chelates for near-infrared fluorescence and magnetic resonance bimodal imaging. ACS Applied Materials & Interfaces. 2017;9(28):23450–23457. doi: 10.1021/acsami.7b05867. [DOI] [PubMed] [Google Scholar]
- 50.Liu Y., Zhi X., Hou W., et al. Gd3+-Ion-induced carbon-dots self-assembly aggregates loaded with a photosensitizer for enhanced fluorescence/MRI dual imaging and antitumor therapy. Nanoscale. 2018;10(40):19052–19063. doi: 10.1039/c8nr05886e. [DOI] [PubMed] [Google Scholar]
- 51.Mastrogiacomo S., Kownacka A. E., Dou W., et al. Bisphosphonate functionalized gadolinium oxide nanoparticles allow long-term MRI/CT multimodal imaging of calcium phosphate bone cement. Advanced Healthcare Materials. 2018;7(19) doi: 10.1002/adhm.201800202.e1800202 [DOI] [PubMed] [Google Scholar]
- 52.Tirusew T., Wenlong X., Md Wasi A., et al. Nanotechnology. 2015;26:p. 365102. [Google Scholar]
- 53.Li J., Wu C., Hou P., Zhang M., Xu K. One-pot preparation of hydrophilic manganese oxide nanoparticles as T1 nano-contrast agent for molecular magnetic resonance imaging of renal carcinoma in vitro and in vivo. Biosensors and Bioelectronics. 2018;102:1–8. doi: 10.1016/j.bios.2017.10.047. [DOI] [PubMed] [Google Scholar]
- 54.Zhao Z., Sun C., Bao J., et al. Surface manganese substitution in magnetite nanocrystals enhances T1 contrast ability by increasing electron spin relaxation. Journal of Materials Chemistry B. 2018;6(3):401–413. doi: 10.1039/c7tb02954c. [DOI] [PubMed] [Google Scholar]
- 55.Lee J., Kumari N., Kim S. M., et al. Anchoring ligand-effect on bright contrast-enhancing property of hollow Mn3O4 nanoparticle in T1-weighted magnetic resonance imaging. Chemistry of Materials. 2018;30(12):4056–4064. doi: 10.1021/acs.chemmater.8b00854. [DOI] [Google Scholar]
- 56.Liu D., He C., Poon C., Lin W. Theranostic nanoscale coordination polymers for magnetic resonance imaging and bisphosphonate delivery. Journal of Materials Chemistry B. 2014;2(46):8249–8255. doi: 10.1039/c4tb00751d. [DOI] [PubMed] [Google Scholar]
- 57.Shi X., Shen L. Integrin αvβ3 receptor targeting PET/MRI dual-modal imaging probe based on the 64Cu labeled manganese ferrite nanoparticles. Journal of Inorganic Biochemistry. 2018;186:257–263. doi: 10.1016/j.jinorgbio.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 58.Zhu J., Li H., Xiong Z., et al. Polyethyleneimine-coated manganese oxide nanoparticles for targeted tumor PET/MR imaging. ACS Applied Materials & Interfaces. 2018;10(41):34954–34964. doi: 10.1021/acsami.8b12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li B., Gu Z., Kurniawan N., Chen W., Xu Z. P. Manganese-based layered double hydroxide nanoparticles as a T1-MRI contrast agent with ultrasensitive pH response and high relaxivity. Advanced Materials. 2017;29(29) doi: 10.1002/adma.201700373.1700373 [DOI] [PubMed] [Google Scholar]
- 60.Duan B., Wang D., Wu H., et al. Core-shell structurized Fe3O4@C@MnO2 nanoparticles as pH responsive T1-T2∗ dual-modal contrast agents for tumor diagnosis. ACS Biomaterials Science & Engineering. 2018;4(8):3047–3054. doi: 10.1021/acsbiomaterials.8b00287. [DOI] [PubMed] [Google Scholar]
- 61.Liu Y., Lv X., Liu H., et al. Porous gold nanocluster-decorated manganese monoxide nanocomposites for microenvironment-activatable MR/photoacoustic/CT tumor imaging. Nanoscale. 2018;10(8):3631–3638. doi: 10.1039/c7nr08535d. [DOI] [PubMed] [Google Scholar]
- 62.Hu H., Zhang Y., Shukla S., Gu Y., Yu X., Steinmetz N. F. Dysprosium-modified tobacco mosaic virus nanoparticles for ultra-high-field magnetic resonance and near-infrared fluorescence imaging of prostate cancer. ACS Nano. 2017;11(9):9249–9258. doi: 10.1021/acsnano.7b04472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.González-Mancebo D., Becerro A. I., Rojas T. C., Garcia-Martin M. L., de la Fuente J. M., Ocana M. HoF3 and DyF3 nanoparticles as contrast agents for high-field magnetic resonance imaging. Particle & Particle Systems Characterization. 2017;34(10) doi: 10.1002/ppsc.201700116.1700116 [DOI] [Google Scholar]
- 64.Shi X., Liu K., Wang T., Zheng S., Gu W., Ye L. Formation mechanism of dysprosium-doped manganese carbonate nanoparticles by thermal decomposition. RSC Advances. 2016;6(101):99339–99345. doi: 10.1039/c6ra20347g. [DOI] [Google Scholar]
- 65.Das G. K., Zhang Y., D’Silva L., et al. Single-phase Dy2O3: Tb3+ nanocrystals as dual-modal contrast agent for high field magnetic resonance and optical imaging. Chemistry of Materials. 2011;23(9):2439–2446. doi: 10.1021/cm2003066. [DOI] [Google Scholar]
- 66.Jin X., Fang F., Liu J., et al. An ultrasmall and metabolizable PEGylated NaGdF4: Dy nanoprobe for high-performance T1/T2-weighted MR and CT multimodal imaging. Nanoscale. 2015;7(38):15680–15688. doi: 10.1039/c5nr04065e. [DOI] [PubMed] [Google Scholar]
- 67.Yin J., Li C., Chen D., et al. Structure and dysprosium dopant engineering of gadolinium oxide nanoparticles for enhanced dual-modal magnetic resonance and fluorescence imaging. Physical Chemistry Chemical Physics. 2017;19(7):5366–5376. doi: 10.1039/c6cp06712c. [DOI] [PubMed] [Google Scholar]
- 68.Ni D., Zhang J., Bu W., et al. PEGylated NaHoF4 nanoparticles as contrast agents for both X-ray computed tomography and ultra-high field magnetic resonance imaging. Biomaterials. 2016;76:218–225. doi: 10.1016/j.biomaterials.2015.10.063. [DOI] [PubMed] [Google Scholar]
- 69.Atabaev T. S., Shin Y. C., Song S. J., Han D. W., Hong N. H. Toxicity and T2-weighted magnetic resonance imaging potentials of holmium oxide nanoparticles. Nanomaterials. 2017;7(8):p. 219. doi: 10.3390/nano7080216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cao Y., Xu L., Kuang Y., Xiong D., Pei R. Gadolinium-based nanoscale MRI contrast agents for tumor imaging. Journal of Materials Chemistry B. 2017;5(19):3431–3461. doi: 10.1039/c7tb00382j. [DOI] [PubMed] [Google Scholar]
- 71.Karaman D. S., Sarparanta M. P., Rosenholm J. M., Airaksinen A. J. Multimodality imaging of silica and silicon materials in vivo. Advanced Materials. 2018;30(24) doi: 10.1002/adma.201703651.e1703651 [DOI] [PubMed] [Google Scholar]
- 72.Huang W.-Y., Davies G.-L., Davis J. J. High signal contrast gating with biomodified Gd doped mesoporous nanoparticles. Chemical Communications. 2013;49(1):60–62. doi: 10.1039/c2cc37545a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang L., Liu R., Peng H., Li P., Xu Z., Whittaker A. K. The evolution of gadolinium based contrast agents: from single-modality to multi-modality. Nanoscale. 2016;8(20):10491–10510. doi: 10.1039/c6nr00267f. [DOI] [PubMed] [Google Scholar]
- 74.Wei H., Bruns O. T., Kaul M. G., et al. Exceedingly small iron oxide nanoparticles as positive MRI contrast agents. Proceedings of the National Academy of Sciences. 2017;114(9):2325–2330. doi: 10.1073/pnas.1620145114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pan D., Schmieder A. H., Wickline S. A., Lanza G. M. Manganese-based MRI contrast agents: past, present, and future. Tetrahedron. 2011;67(44):8431–8444. doi: 10.1016/j.tet.2011.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davies G.-L., Kramberger I., Davis J. J. Environmentally responsive MRI contrast agents. Chemical Communications. 2013;49(84):9704–9721. doi: 10.1039/c3Ccc44268c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ni D., Bu W., Zhang S., et al. Single Ho3+-doped upconversion nanoparticles for high-performance T2-weighted brain tumor diagnosis and MR/UCL/CT multimodal imaging. Advanced Functional Materials. 2014;24(42):6613–6620. doi: 10.1002/adfm.201401609. [DOI] [Google Scholar]
- 78.Wang L., Lin H., Chi X., et al. A self-assembled biocompatible nanoplatform for multimodal mr/fluorescence imaging assisted photothermal therapy and prognosis analysis. Small. 2018;14(35) doi: 10.1002/smll.201801612.e1801612 [DOI] [PubMed] [Google Scholar]
- 79.Zhang N.-N., Yu R.-S, Xu M., et al. Visual targeted therapy of hepatic cancer using homing peptide modified calcium phosphate nanoparticles loading doxorubicin guided by T1 weighted MRI. Nanomedicine: Nanotechnology, Biology and Medicine. 2018;14(7):2167–2178. doi: 10.1016/j.nano.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 80.Luo Y., Kim E. H., Flask C. A., Clark H. A. Nanosensors for the chemical imaging of acetylcholine using magnetic resonance imaging. ACS Nano. 2018;12(6):5761–5773. doi: 10.1021/acsnano.8b01640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou J., Li T., Zhang C., Xiao J., Cui D., Cheng Y. Charge-switchable nanocapsules with multistage pH-responsive behaviours for enhanced tumour-targeted chemo/photodynamic therapy guided by NIR/MR imaging. Nanoscale. 2018;10(20):9707–9719. doi: 10.1039/c8nr00994e. [DOI] [PubMed] [Google Scholar]
- 82.Yang W., Wu X., Dou Y., et al. A human endogenous protein exerts multi-role biomimetic chemistry in synthesis of paramagnetic gold nanostructures for tumor bimodal imaging. Biomaterials. 2018;161:256–269. doi: 10.1016/j.biomaterials.2018.01.050. [DOI] [PubMed] [Google Scholar]
- 83.Wu B., Lu S.-T., Yu H., et al. Gadolinium-chelate functionalized bismuth nanotheranostic agent for in vivo MRI/CT/PAI imaging-guided photothermal cancer therapy. Biomaterials. 2018;159:37–47. doi: 10.1016/j.biomaterials.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 84.Kuang Y., Cao Y., Liu M., et al. Geometrical confinement of gadolinium oxide nanoparticles in poly(ethylene glycol)/arginylglycylaspartic acid-modified mesoporous carbon nanospheres as an enhanced T1 magnetic resonance imaging contrast agent. ACS Applied Materials & Interfaces. 2018;10(31):26099–26107. doi: 10.1021/acsami.8b09709. [DOI] [PubMed] [Google Scholar]
- 85.Wang F. H., Bae K., Huang Z. W., Xue J. M. Two-photon graphene quantum dot modified Gd2O3 nanocomposites as a dual-mode MRI contrast agent and cell labelling agent. Nanoscale. 2018;10(12):5642–5649. doi: 10.1039/c7nr08068a. [DOI] [PubMed] [Google Scholar]
- 86.Wang S., Zhou Z., Yu G., et al. Gadolinium metallofullerene-polypyrrole nanoparticles for activatable dual-modal imaging-guided photothermal therapy. ACS Applied Materials & Interfaces. 2018;10(34):28382–28389. doi: 10.1021/acsami.8b09670. [DOI] [PubMed] [Google Scholar]
- 87.Martinez J. O., Molinaro R., Hartman K. A., et al. Biomimetic nanoparticles with enhanced affinity towards activated endothelium as versatile tools for theranostic drug delivery. Theranostics. 2018;8(4):1131–1145. doi: 10.7150/thno.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Du F., Zhang L., Zhang L., et al. Engineered gadolinium-doped carbon dots for magnetic resonance imaging-guided radiotherapy of tumors. Biomaterials. 2017;121:109–120. doi: 10.1016/j.biomaterials.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 89.Liu J. J., Chen Q., Zhu W. W., et al. Nanoscale-coordination-polymer-shelled manganese dioxide composite nanoparticles: a multistage Redox/pH/H2O2-responsive cancer theranostic nanoplatform. Advanced Functional Materials. 2017;27(10) doi: 10.1002/adfm.201605926.1605926 [DOI] [Google Scholar]
- 90.Sun Q., He F., Sun C., et al. Honeycomb-satellite structured pH/H2O2-responsive degradable nanoplatform for efficient photodynamic therapy and multimodal imaging. ACS Applied Materials & Interfaces. 2018;10(40):33901–33912. doi: 10.1021/acsami.8b10207. [DOI] [PubMed] [Google Scholar]
- 91.Liang R., Liu L., He H., et al. Oxygen-boosted immunogenic photodynamic therapy with gold nanocages@manganese dioxide to inhibit tumor growth and metastases. Biomaterials. 2018;177:149–160. doi: 10.1016/j.biomaterials.2018.05.051. [DOI] [PubMed] [Google Scholar]
- 92.Cao Y., Meng X., Wang D., et al. Intelligent MnO2/Cu2–xS for multimode imaging diagnostic and advanced single-laser irradiated photothermal/photodynamic therapy. ACS Applied Materials & Interfaces. 2018;10(21):17732–17741. doi: 10.1021/acsami.8b05050. [DOI] [PubMed] [Google Scholar]
- 93.Yang G., Zhang R., Liang C., et al. Manganese dioxide coated WS2@Fe3O4 /sSiO2 nanocomposites for pH-responsive MR imaging and oxygen-elevated synergetic therapy. Small. 2018;14(2) doi: 10.1002/smll.201702664. [DOI] [PubMed] [Google Scholar]
- 94.Yang G., Xu L., Chao Y., et al. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nature Communications. 2017;8(1):p. 902. doi: 10.1038/s41467-017-01050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang Y., Liu Y., Wu H., Zhang J., Tian Q., Yang S. Functionalized holmium-doped hollow silica nanospheres for combined sonodynamic and hypoxia-activated therapy. Advanced Functional Materials. 2019;29(3) doi: 10.1002/adfm.201805764.1805764 [DOI] [Google Scholar]
- 96.Li X. Y., Zou Q., Chen L., Li W. A ternary doped single matrix material with dual functions of bone repair and multimodal tracking for applications in orthopedics and dentistry. Journal of Materials Chemistry B. 2018;6:6047–6056. doi: 10.1039/c8tb02041h. [DOI] [PubMed] [Google Scholar]
- 97.Zhang X., Blasiak B., Marenco A. J., Trudel S., Tomanek B., van Veggel F. C. J. M. Design and regulation of NaHoF4 and NaDyF4 nanoparticles for high-field magnetic resonance imaging. Chemistry of Materials. 2016;28(9):3060–3072. doi: 10.1021/acs.chemmater.6b00264. [DOI] [Google Scholar]