Abstract
BACKGROUND
Cell number and viability are important in cord blood (CB) transplantation. While 10% dimethyl sulfoxide (DMSO) is the standard medium, adding a starch to freezing medium is increasingly utilized as a cytoprotectant for the thawing process. Similar to hetastarch, pentastarch has the advantages of faster renal clearance and less effect on the coagulation system.
STUDY DESIGN AND METHODS
We compared a lower DMSO concentration (5%) containing pentastarch with 10% DMSO and performed cell viability assay, colony-forming units (CFUs), and transplantation of CB cells in NOD/SCID IL2Rγnull mice.
RESULTS
CB cells in 5% DMSO/pentastarch had similar CD34+, CD3+, and CD19+ cell percentages after thawing as fresh CB cells. CB cells in 5% DMSO/pentastarch had higher viability (83.3 ± 9.23%) than those frozen in 10% DMSO (75.3 ± 11.0%, p < 0.05). We monitored cell viability postthaw every 30 minutes. The mean loss in the first 30 minutes was less in the 5% DMSO/pentastarch group. At the end of 3 hours, the viability decreased by a mean of 7.75% for the 5% DMSO/pentastarch and 17.5% for the 10% DMSO groups. CFUs were similar between the two cryopreserved groups. Frozen CB cells engrafted equally well in IL2Rγnull mice compared to fresh CB cells up to 24 weeks, and CB cells frozen in 5% DMSO/pentastarch engrafted better than those in 10% DMSO.
CONCLUSION
Our data indicate that the lower DMSO concentration with pentastarch represents an improvement in the CB cryopreservation process and could have wider clinical application as an alternate freezing medium over 10% DMSO.
Hematopoietic stem cell (HSC) transplantation represents a curative approach to treat many blood diseases. Umbilical cord blood (CB) has been developed as an alternative source of HSCs because of the ease of collection, wide availability, and less reported graft-versus-host disease.1–3 The lower cell dose per CB unit has limited single CB units to children and young adults and attempts to target 2.5 × 107 to 3.0 × 107 total nucleated cells per kilogram of recipient weight4 by larger CB volumes collection or double CB units5,6 have emerged as the accepted routine practice in CB transplantation for adults. The cryopreservation and thawing process, however, reduces viability and cell number, thus improving the freezing method would benefit CB transplantation.
While the defined freezing rate through programmed freezers,7,8 the optimal cell concentration,9,10 and new containers for storage11,12 have been incorporated in current cryopreservation protocols, freezing medium is one area where further improvement can be made. Ten percent dimethyl sulfoxide (DMSO) has been widely used as the standard medium.13–17 However, when DMSO is infused with HSCs, DMSO has been commonly reported to cause nausea, vomiting, hypertension, and abdominal cramps.18 There are other associated cardiovascular,19 respiratory,20,21 central nervous system,22,23 renal, and hemolytic24 side effects. This infusional toxicity appears to be dose related,19 and any attempt to decrease the volume of DMSO infused into recipients would be desirable.
Membrane stabilizers such as hydroxylethyl starch (HES) have been combined with DMSO to successfully cryopreserve peripheral blood–derived stem cells or marrow stem cells.25–27 HES are linking glucose units with hydroxyethyl groups attached to different carbon positions of the hexose ring (C2, C3, and/or C6). Clinically available HES solutions in the United States include pentastarch and hetastarch and have several clinical indications: 1) colloid volume expander as an alternative to albumin or plasma in patients who are critically ill,28 with preeclampsia,29 or before cesarean sections;30,312) red blood cell (RBC) sedimentation to reduce the volume of CB cells;32,33 and 3) cryopreservation.
Pentastarch is a five hydroxyethyl starch similar to hetastarch, but with fewer hydroxyethyl groups (molar substitution ratio of 0.45 vs. 0.70) resulting in a lower mean molecular weight (264,000 vs. 450,000). This reduction leads to enhanced enzymatic hydrolysis, faster renal elimination (initial intravascular half-life of 2.5 hr vs. 25.5 hr for hetastarch) and causes less thrombocytopenia and dilutional effect on coagulation factors.34 These beneficial characteristics of pentastarch have prompted us to compare the combination of reduced DMSO (5%) and pentastarch to the standard 10% as the cryopreservation medium for routine use, by evaluating in vitro viability assays, short-term progenitors by colony-forming unit (CFU) assays, and in vivo xenotransplant model.
MATERIALS AND METHODS
Collection of CB units
CB units were collected from normal full-term deliveries and transported in a controlled container under room temperature conditions to the cell processing laboratory. Written informed consent was obtained before deliveries using an institutional review board–approved protocol 01-H-0122 (same as 01-DK-0122) at the National Institutes of Health (Bethesda, MD). This study is registered at http://www.clinicaltrials.gov (NCT 00012545). The CB units were collected ex utero, using sterile techniques and collection bags containing citrate phosphate dextrose (Pall Corp., East Hills, NY). CB units without clots and with volume of 50 mL or more were processed within 24 hours of collection.
CB preparation, cryopreservation, and thawing
CB units were weighted and nucleated cells enumerated using a hematology analyzer (Cell-Dyn Sapphire, Abbott Diagnostics, Santa Clara, CA). The CB units were then separated into mononuclear cells (MNCs) using the manual density gradient procedure. If a CB unit was more than 100 mL, then a buffy coat procedure35 was used to reduce the number of RBCs and volume. The CB was then centrifuged at 419 × g for 10 to 30 minutes at room temperature (20–24°C). The top buffy coat layer was expressed off. Ten-milliliter aliquots of CB white blood cells (WBCs) were pipetted into 50-mL conical tubes, diluted to 40 mL with Hank’s balanced salt solution (HBSS), and underlayed with 10 mL of lymphocyte separation medium (density 1.077, Ficoll-Paque Plus, Amersham Biotech, Piscataway, NJ). The conical tubes were centrifuged for 25 minutes at 835 × g at room temperature. The majority of the upper supernatant was removed and discarded. Using a serologic pipette, the MNC layer was removed and transferred to a newly labeled 50-mL conical tube. The conical tube was filled with HBSS and mixed well. The tube was centrifuged for 10 minutes at 677 × g. The supernatant was removed and the cells were resuspended in 10 mL of HBSS. The MNCs were enumerated using a hematology analyzer (Cell-Dyn 3500, Abbott) and divided equally into three aliquots.
One aliquot was given for a fresh infusion into NOD/SCID IL2Rγnul1 (Fresh); one aliquot for cryopreservation in 10% DMSO, Plasmalyte-A, heparin, and dornase alfa (DNAse; 10% DMSO); and one aliquot for cryopreservation in 5% DMSO with 6% pentastarch, 25% human albumin, heparin, and DNAse (5% DMSO/pentastarch). These latter aliquots were cryopreserved in 5 mL vials (Nunc A/S CryoTube vials, Roskilde, Denmark), and placed in a controlled-rate freezer (Kryo Save Integra 750 Plus, Planer PLC, Middlesex, UK) at a programmed cooling rate of −1°C per minute to −120°C.7,8,36 At −120°C the CB vials were removed and placed in long-term storage in the vapor phase of a liquid nitrogen permanent storage tank. The cryopreserved CBs were stored for a minimum of 10 days.
CB units were thawed rapidly by immersing each tube in a 37°C water bath until the disappearance of small ice crystals. DMSO or pentastarch was removed by washing the nucleated cells twice with phosphate-buffered saline (PBS). RBCs were removed using ammonium chloride potassium lysis buffer (Quality Biological, Gaithersburg, MD).
In vitro characterization of CB samples
Viability of each CB sample was determined using 7-aminoactinomycin-D (7-AAD)37 on a flow cytometer (FACSCanto, BD Biosciences, San Jose, CA). CD45+ cells that excluded 7-AAD (and were therefore judged as viable) were analyzed for CD34+, CD19+, and CD3+ (all BD Biosciences). The percentage of viable cells expressing each marker was calculated by dividing the number of viable cells expressing the marker by the total cells in the gated population. The two-sample t test was used to compare viability of fresh and cryopreserved cells.
Granulocyte-macrophage (GM)–CFUs, burst-forming units–erythroid (BFUs-E), and granulocyte-erythrocyte-monocyte-macrophage (GEMM)–CFUs were assayed in a commercially available methylcellulose culture medium containing a combination of recombinant colony-stimulating factors (MethoCult 4434, Stem Cell Technologies, Vancouver, British Columbia, Canada). Duplicate 1-mL cultures, each containing 1 × 105 MNCs, were plated in 35-mm gridded petri dishes and incubated at 37°C in a humidified atmosphere in 5% CO2. The total number of CFUs-GM, BFUs-E, and CFUs-GEMM were enumerated on Day 14 by using an inverted microscope, and data were analyzed by one-way analysis of variance (ANOVA).
Transplantation for NOD/LtSz-scid IL2Rgnull mice
Male NOD.Cg-Prkdcscid IL2rgtm1Wjl/Sz/J (NOD/LtSz-scid/IL2Rγnull, NOD/SCID IL2Rγnull,) mice were purchased from Jackson Laboratory (Bar Harbor, ME) and have been reported elsewhere.38,39 Recipient mice were 7 to 10 weeks old at the time of transplant and were handled according to the animal care and use protocol approved by the Animal Care and Use Committee at the National Institutes of Health. Our humanized xenograft mouse model has previously been described40 and was performed with modification of the busulfan dose: 35 mg/kg busulfan (Busulfex, Otsuka Pharmaceutical, Rockville, MD) was diluted with PBS (Biofluids, Rockville, MD) to a final volume 500 μL and injected into recipient mice intraperitoneally at least 24 hours before cell infusion.
CD34 enrichment was performed on aliquots of CB MNCs by positive CD34 antibody and streptavidin microbead selection (Miltenyi Biotec, Auburn, MA). Cells passed through the MACS column twice, and the purity was assessed by flow cytometry. A total of 2 × 106 fresh CB CD34+ cells were infused in recipient mice on the same day of positive selection; an equal cell dose was cryopreserved in 10% DMSO (10% DMSO CB CD34+). After 10 days, the two frozen aliquots (10% DMSO CB MNCs and 10% DMSO CB CD34+ cells) were thawed in a 37°C water bath, washed twice with PBS, and injected via tail vein41 (Fig. 2). Two-way ANOVA was used to compare engraftment by CD34+ selected cells, nonselected MNCs, and the two different freezing methods. The interaction effect between type of cells and freezing methods was also examined.
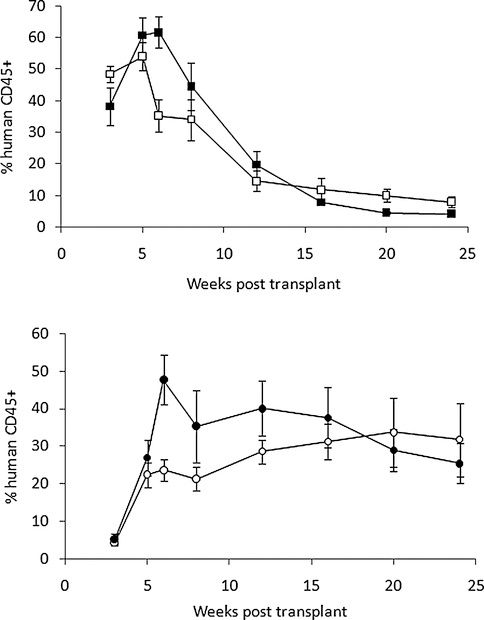
Fig. 2.
Percentage of human cell engraftment in NOD/SCID ILZRγnull mice. CB MNCs (2 × 107 cells per mouse, top) or CD34-se1ected cells (CD34+, 1 × 106 per mouse, bottom) were infused (□, ○) fresh or (■, ●) after being frozen in 10% DMSO. Peripheral blood samples were assayed for human cells periodically. There were five mice per group; error bars represent standard error of the means.
Since CB units are rarely selected before cryopreservation, we compared CB MNCs infused in NOD/SCID mice before, after 10% DMSO, or after 5% DMSO/pentastarch. Then 1.2 × 106 or 2 × 106 cells per mouse were diluted with PBS to a final volume of 500 μL and infused intravenously via tail vein 24 hours after preconditioning with 35 mg/kg busulfan. Peripheral blood samples were collected from mice tail veins, and RBCs were removed by ammonium chloride potassium lysing buffer as described earlier. The remaining nucleated cells were stained with fluorescein isothiocyanate–conjugated anti-human CD45 antibody and phycoerythrin–conjugated anti-mouse CD45 antibody (BD Biosciences). The percentages of human CD45+ cells were analyzed by flow cytometry (Fig. 3), and two-sample t test was used to compare the two different freezing methods.
Fig. 3.
Comparison of two freezing methods. Since most CB units are stored unselected, we infused CB MNCs fresh (■, 2 × 107, n = 6), after being frozen with 10% DMSO (□, 2 × 107 n = 7), or after 5% DMSO/pentastarch ( , 2 × 107 n = 6). The mean percentages of human CD45+ cells are plotted with respect to time after transplant. When t test was used to compare the two freezing methods, 5% DMSO/pentastarch had higher human cell engraftment at 6 weeks (p = 0.027, oneway ANOVA) and 12 weeks (p = 0.042, one-way ANOVA).
, 2 × 107 n = 6). The mean percentages of human CD45+ cells are plotted with respect to time after transplant. When t test was used to compare the two freezing methods, 5% DMSO/pentastarch had higher human cell engraftment at 6 weeks (p = 0.027, oneway ANOVA) and 12 weeks (p = 0.042, one-way ANOVA).
RESULTS
Characterization of CB units
Sixteen CB units were collected and processed within 24 hours of collection (Fig. S1, available as supporting information in the online version of this paper). The first 4 units were used to optimize experiments, 4 units for cell viability after thawing experiment (Fig. 1), 4 units for transplantation of fresh and frozen CB cells (Fig. 2), and 4 units for comparing the two cryopreservation methods (Fig. 3). Their volumes ranged from 50 to 172 mL, and their hematologic variables are shown in Table 1A. We then assessed the WBC subset of fresh and cryopreserved CB cells (Table 1B). The percentage of cell viability by 7-AAD staining was 98.3 ± 1.2% in the fresh group, 75.3 ± 11.0% in the 10% DMSO group, and 83.3 ± 9.2% in the 5% DMSO/pentastarch group (Table 2). CD34+ cells constituted 0.9% of the fresh CB nucleated cells; this percentage decreased to 0.78% after 5% DMSO/pentastarch and to 0.61% after 10% DMSO, although these modest decreases were not significant. CD19+ cells represented 9.96% of the fresh CB cells, 10.7% after 5% DMSO/pentastarch, and 10.6% after 10% DMSO. CD3+ cells comprised 40.5% of the fresh CB products; this percentage decreased to 31.4% after 5% DMSO/pentastarch (p = 0.07) and to 27.7% after 10% DMSO cryopreservation (p = 0.003).
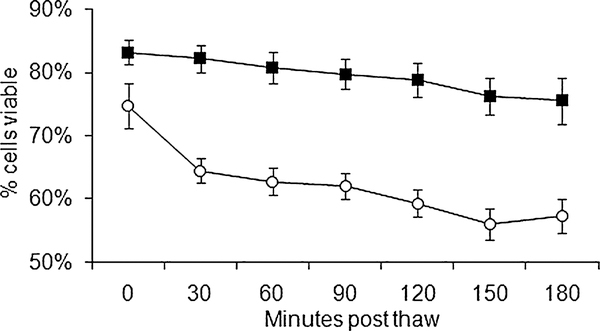
Fig. 1.
Cell viability after thawing. CB samples (n = 4) cryopreserved in (○) 10% DMSO or (■) 5% DMSO/pentastarch were thawed and maintained at room temperature. A small aliquot was sampled every 30 minutes and analyzed for 7-AAD by flow cytometry. The percentage of viable cells (Y-axis) is presented with respect to time postthaw (X-axis). The error bars represent standard errors of the mean. There was a significant difference (p < 0.05) between 10% DMSO and 5% DMSO/pentastarch at time points 30 minutes and onward.
TABLE 1A.
Blood counts of CB units (n = 16)*
| Volume (mL) | WBC count (×109 cells/L) | Hemoglobin (g/dL) | Hematocrit (%) | PLT count (×109 cells/L) |
|---|---|---|---|---|
| 113 ± 37 | 10.9 ± 4.2 | 11.6 ± 1.9 | 35.8 ± 6.1 | 223 ± 77 |
All percentages are means ± SDs.
TABLE 1B.
WBC lineage (%) of CB units*
| Fresh (n = 16) | 10% DMSO* (n = 13) | 5% DMSO/pentastarch† (n = 13) | |
|---|---|---|---|
| CD34+ | 0.91 ± 0.7 | 0.61 ± 0.5 | 0.75 ± 0.5 |
| CD3+ | 40.5 ± 8.3 | 27.7 ± 11.1 | 31.4 ± 12.7 |
| CD19+ | 9.96 ± 2.7 | 10.7 ± 2.7 | 10.6 ± 3.0 |
All percentages are means ± SDs and are derived from viable cells.
Samples analyzed immediately after thawing.
TABLE 2.
The number of CFU from CB products*
| Fresh (n = 16) | 10% DMSO (n = 13) | 5% DMSO/pentastarch (n = 13) | |
|---|---|---|---|
| Viability | 98.2 ± 1.2 | 75.3 ± 10.7 | 83.3 ± 8.9 |
| CFU-GM | 68.6 ± 10.0 | 49.5 ± 4.0 | 49.9 ± 5.8 |
| BFU-E | 61.1 ± 9.4 | 59.3 ± 6.4 | 66.5 ± 7.1 |
| CFU-GEMM | 0.4 ± 0.1 | 0.72 ± 0.3 | 0.4 ± 0.2 |
| Total | 130.0 ± 17.6 | 88.9 ± 12.9 | 94.8 ± 14.8 |
Mean number of colonies ± standard errors of the mean are presented. A total of 1 × 105 MNCs per mL were plated in Methocult semisolid medium and the CFUs were enumerated after 14 days of culture. Comparison of CFU-GM and CFU-E was not significant.
Viability of CB cells postthawing
To assess the viability of cells with respect to time after thaw, we monitored the viability by flow cytometry from the two frozen products (10% DMSO or 5% DMSO/pentastarch) every 30 minutes up to 3 hours postthawing (n = 4, Fig. 1). The 5% DMSO/pentastarch group had more viable cells from 30 minutes to 180 minutes postthawing (p < 0.05). The mean of loss of viability at first 30 minutes was 10.3 ± 6.1% in 10% DMSO and 1.25 ± 6.3% in 5% DMSO/pentastarch (p < 0.05). Interestingly, the loss of viability in the 5% DMSO/pentastarch group was small and gradual, while the 10% DMSO group had a substantial decrease within the first 30 minutes. At the end of 3 hours, the viability of the 10% DMSO group decreased from 74.8 ± 4.6% to 57.0 ± 8.6% (mean loss of 17.5%), and the 5% DMSO/pentastarch group from 83.3 ± 8.7% to 75.5 ± 7.6% (mean loss of 7.75%). These results suggest that the addition of pentastarch is not toxic and is better at preserving the CB cells postthaw.
To further assess the differences in the proliferative capacity of short-term progenitors, CFU assays were performed (Table 2). Overall all the CB products provided similar numbers of total, myeloid (CFU-GM), or erythroid (BFU-E) colonies. The 10% DMSO group appeared to have a lower mean number of CFUs, but this was not significant, when compared to the fresh or 5% DMSO/pentastarch groups.
Comparison of two CB freezing methods
We then performed transplantation of cryopreserved CB cells into NOD/SCID IL2Rγnull mice to test if the observed in vitro differences would lead to better human cell engraftment. First, we tested if standard cryopreservation with 10% DMSO would alter the duration or kinetics of human cell engraftment. We transplanted human CB MNCs (2 × 107) or selected CB CD34+ (1 × 106) cells into each recipient mouse (n = 5 per group) with or without cryopreservation (Fig. 2). The freshly infused cells were 99 ± 0% viable, while the CB cells after 10% DMSO cryopreservation had 77.5 ± 2.5% viability. This difference in viability did not lower human cell engraftment in the CB MNC or selected CB CD34+ mice up to 24 weeks posttransplantation (Fig. 2). In mice that received unselected cells, human cell engraftment peaked at 6 weeks post–HSC infusion and gradually declined over the next 4 to 5 months. In contrast, in mice that received CD34+ cells, human cell engraftment increased gradually and began to plateau after 6 weeks. At Week 6, mice that received cryopreserved cells had higher percentages of human cells (p < 0.05, t test). After 12 weeks, this difference was no longer present, but the CD34+ groups had consistently higher human cell engraftment than unselected groups (two-way ANOVA, p < 0.05).
Since most CB units are stored without CD34+ selection, we compared the two freezing methods using CB MNCs. Prior work by Abrahamsen and coworkers42 showed that 5% DMSO may be a better cryopreservant than 10% DMSO; thus we chose 5% DMSO and added pentastarch as the experimental group to compare with fresh CB and 10% DMSO frozen CB cells. The mean viability of transplanted cells was 98.8 ± 0.5% in the fresh cohort, 81.8 ± 8.7% in the 10% DMSO cohort, and 87.3 ± 6.7% in 5% DMSO/pentastarch cohort. As expected, the human cell chimerism peaked at 6 weeks and decreased gradually thereafter. The 5% DMSO/pentastarch group had higher human cell engraftment at 6 weeks (p = 0.027, one-way ANOVA) and at 12 weeks (p = 0.042). However, these differences were no longer present at 16 weeks (Fig. 3). These results indicated that fresh and cryopreserved CB MNCs engraft equally well in this xenograft model. When we used a lower cell dose, 1.2 × 107 CB MNCs per mouse, there was expected lower human cell engraftment all throughout the monitoring period. The mice in the 10% DMSO group had less than 5% human cell engraftment, and the mice in the 5% DMSO/pentastarch group all died before reaching 16 weeks, so no direct comparison was available (Fig. S2, available as supporting information in the online version of this paper).
DISCUSSION
We first assessed the WBC subset of fresh and cryopreserved CB cells. CD34+ and CD19+ cells constituted approximately 1 and 10% of the fresh CB nucleated cells, respectively; these percentages did not significantly decrease after freezing in either medium. CD19+ cells appeared to be the least affected, and CD3+ cells, most affected by either cryopreservation. While CB stem cells generally engraft well, the low percentage of CD34+ and CD3+ cells might predict a higher percentage of graft failure, compared to marrow-derived or G-CSF-mobilized peripheral blood–derived HSCs in nonmyeloablative transplants.
We then assessed the proliferative potential of CB cells by cell viability and CFU assays and further tested if the differences were significant in xenotransplantation studies. The addition of pentastarch to a lower percentage (5%) of DMSO offers protection of CB cells over the standard 10% DMSO. The 5% DMSO/pentastarch group had 83% of viable cells at the time of thaw, compared to 75% of the 10% DMSO group (p < 0.05). At 30 and up to 180 minutes after thaw, the 5% DMSO/pentastarch group had a higher percentage of viable cells. These percentages were consistent with our prior clinical trial data where we cryopreserved G-CSF–mobilized peripheral blood stem cells (81.4 ± 4.8% in the 5% DMSO/pentastarch group and 76.8 ± 8.1% in the 10% DMSO group).43
While 10% DMSO is the widely accepted cryopreservation medium,13,14,17 our results corroborate with other reports that lower percentages of DMSO may be at least equivalent or even superior.15,42,44 When cryopreserved CB cells were cultured in the semisolid media, the 5% DMSO/pentastarch group yielded a mean of 96 colonies per 105 cells plated, and the 10% DMSO group, 89 colonies. Although the difference in CFU was not significant, our observations suggest that the lower DMSO concentration with pentastarch is equivalent to 10% DMSO in protecting CB cells during the freezing process. Our data are comparable to other reports that the addition of a second agent, trehalose,45 pentastarch,46 or dextran,47 improves the post-thaw recovery of frozen CB cells by possibly protecting cells through the thawing process.
We then compared engraftment potential of fresh and cryopreserved CB cells. As expected,40 mice that received unselected CB MNCs had higher engraftment up to 6 weeks posttransplantation, but mice that received the selected CB CD34+ cells had higher human engraftment beyond 12 weeks. Interestingly, there were no appreciable differences between fresh and cryopreserved (10% DMSO) CB cells. This observation is similar with a prior report by Nicol and coworkers14 where frozen CB cells yielded fewer CFUs, but derived similar numbers of granulocyte-macrophage colonies in the long term culture-initiating cells, and similar numbers of CD34+ cells up to 12 weeks. Our xenograft results extended the current experience17,48 where the kinetics of human cell engraftment were characterized for 24 weeks posttransplant (Fig. 2). Using 2 × 107 unselected CB MNCs for transplantation led to modest long-term human cell engraftment of 5% to 10%. When we used 1.2 × 107 unselected CB MNCs, the mice in the 10% DMSO group had less than 5% human cell engraftment, suggesting that lower unselected CB MNC doses would not be sufficient to compare the two cryopreservation methods. Our results are consistent with those of a prior study by Zhang and colleagues49 where 5 × 106 unselected CB MNCs were infused in NOD/SCID mice and yielded about 5% human cell engraftment in murine peripheral blood. It is important to note that in this xenotransplantation model, peripheral blood engraftment is typically severalfold lower than engraftment in the marrow or spleen. Thus using even lower cell doses suggests that the difference between the two freezing media would be small and difficult to detect with confidence. Limiting dilution transplantation with CB CD34+ cells would be the next logical step to directly compare 10% DMSO and 5% DMSO with pentastarch. Since CB units are typically stored without CD34+ selection, we chose to compare the two freezing methods using unselected CB cells.
Five percent has previously been shown to be the minimal DMSO concentration, above which viability and recovery of CB cells are preserved.15 The addition of HES (disaccharides or hetastarch) has been hypothesized to offer additional protection during the postthaw recovery of nucleated cells with greater CFUs, long term culture-initiating cells, and short-term NOD/SCID human cell engraftment.45,46,49 Our xenograft transplant experiment extended the posttransplant monitoring period and showed that the 5% DMSO/pentastarch group had better human cell chimerism up to 12 weeks. These preclinical data complement a Phase III clinical trial in which patients who received G-CSF–mobilized HSCs frozen in 5% DMSO with HES had faster neutrophil recovery, but similar platelet (PLT) recovery and transfusion requirement as patients who received G-CSF–mobilized HSCs frozen in 10% DMSO.27 Additionally, our own clinical experience of cryopreserving G-CSF-mobilized HSCs in sickle cell trait individuals demonstrated 5% DMSO/pentastarch was superior to 10% DMSO.43 Thus there are accumulating preclinical and clinical data to support the addition of HES offering more cryoprotection over the standard 10% DMSO. Among the different preparations of HES, pentastarch has the advantages of having a lower molecular weight, faster systemic clearance, and lower toxicity over hetastarch.50
In summary, we have made important progress in optimizing the freezing medium, which has clinical implications. Our 5% DMSO with pentastarch improves cell viability after the CB thawing procedure, preserves the short-term CB progenitors where the proliferative potential matches to that of fresh CB cells, and achieves better short-term human cell engraftment over the standard 10% DMSO in xenotransplantation model.
Supplementary Material
Fig. S1. Flow diagram of the experiments performed with 16 human cord blood units
Fig. S2. Comparison of 2 freezing methods using 2 cell doses. Since most cord blood units are stored unselected, we infused CB mononuclear cells. The average percentages of human CD45 positive cells are plotted with respect to time after transplant. The first graph used a lower cell dose (1.2 × 107, n = 6 in all groups), and second graph used a higher cell dose (2 × 107) as seen in Fig. 3, fresh (n = 6), after frozen with 10% DMSO (n = 7), or after 5% DMSO/pentastarch (n = 6).
ACKNOWLEDGMENTS
We appreciate the statistical help by Richard Chen. This work is supported by the intramural research program of the National Institute of Diabetes, Digestive, and Kidney diseases and the National Heart, Lung, and Blood Institute at NIH.
ABBREVIATIONS
- 7-AAD
7-aminoactinomycin-D
- CB
cord blood
- GEMM
granulocyte-erythrocyte-monocyte-macrophage
Footnotes
CONFLICT OF INTEREST
The authors have no financial conflict of interest to declare.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article:
REFERENCES
- 1.Wagner JE, Rosenthal J, Sweetman R, Shu XO, Davies SM, Ramsay NK, McGlave PB, Sender L, Cairo MS. Successful transplantation of HLA-matched and HLA-mismatched umbilical cord blood from unrelated donors: analysis of engraftment and acute graft-versus-host disease. Blood 1996;88:795–802. [PubMed] [Google Scholar]
- 2.Gluckman E, Rocha V, Boyer-Chammard A, Locatelli F, Arcese W, Pasquini R, Ortega J, Souillet G, Ferreira E, Laporte JP, Fernandez M, Chastang C. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med 1997;337: 373–81. [DOI] [PubMed] [Google Scholar]
- 3.Laughlin MJ, Barker J, Bambach B, Koc ON, Rizzieri DA, Wagner JE, Gerson SL, Lazarus HM, Cairo M, Stevens CE, Rubinstein P, Kurtzberg J. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med 2001;344:1815–22. [DOI] [PubMed] [Google Scholar]
- 4.Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio AR, Berkowitz RL, Cabbad M, Dobrila NL, Taylor PE, Rosenfield RE, Stevens CE. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med 1998;339:1565–77. [DOI] [PubMed] [Google Scholar]
- 5.Barker JN, Weisdorf DJ, Wagner JE. Creation of a double chimera after the transplantation of umbilical-cord blood from two partially matched unrelated donors. N Engl J Med 2001;344:1870–1. [DOI] [PubMed] [Google Scholar]
- 6.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, McGlave PB, Miller JS, Verfaillie CM, Wagner JE. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood 2005;105:1343–7. [DOI] [PubMed] [Google Scholar]
- 7.Lewis JP, Passovoy M, Conti SA, McFate PA, Trobaugh FE Jr. The effect of cooling regimens on the transplantation potential of marrow. Transfusion 1967;7:17–32. [DOI] [PubMed] [Google Scholar]
- 8.Ketheesan N, Whiteman C, Malczewski AB, Hirst RG, La Brooy JT. Effect of cryopreservation on the immunogenicity of umbilical cord blood cells. Transfus Apher Sci 2004; 30:47–54. [DOI] [PubMed] [Google Scholar]
- 9.Aird W, Labopin M, Gorin NC, Antin JH. Long-term cryopreservation of human stem cells. Bone Marrow Transplant 1992;9:487–90. [PubMed] [Google Scholar]
- 10.Silberstein LE, Jefferies LC. Placental-blood banking—a new frontier in transfusion medicine. N Engl J Med 1996; 335:199–201. [DOI] [PubMed] [Google Scholar]
- 11.Yang H, Zhao H, Acker JP, Liu JZ, Akabutu J, McGann LE. Effect of dimethyl sulfoxide on post-thaw viability assessment of CD45+ and CD34+ cells of umbilical cord blood and mobilized peripheral blood. Cryobiology 2005;51:165–75. [DOI] [PubMed] [Google Scholar]
- 12.Mericka P, Schustr P, Vins M, Dudek A, Vávra L, Cervinka M, Rondiak J. Containers for freezing and storage of bone marrow stem cells. Sb Ved Pr Lek Fak Karlovy Univerzity Hradci Kralove 1991;34:367–87. [PubMed] [Google Scholar]
- 13.Campos L, Roubi N, Guyotat D. Definition of optimal conditions for collection and cryopreservation of umbilical cord hematopoietic cells. Cryobiology 1995;32:511–5. [DOI] [PubMed] [Google Scholar]
- 14.Nicol A, Nieda M, Donaldson C, Denning-Kendall P, Bradley B, Hows J. Analysis of cord blood CD34+ cells purified after cryopreservation. Exp Hematol 1995;23:1589–94. [PubMed] [Google Scholar]
- 15.Donaldson C, Armitage WJ, Denning-Kendall PA, Nicol AJ, Bradley BA, Hows JM. Optimal cryopreservation of human umbilical cord blood. Bone Marrow Transplant 1996;18: 725–31. [PubMed] [Google Scholar]
- 16.Shlebak AA, Marley SB, Roberts IA, Davidson RJ, Goldman JM, Gordon MY. Optimal timing for processing and cryopreservation of umbilical cord haematopoietic stem cells for clinical transplantation. Bone marrow Transplant 1999; 23:131–6. [DOI] [PubMed] [Google Scholar]
- 17.Broxmeyer HE, Srour EF, Hangoc G, Cooper S, Anderson SA, Bodine DM. High-efficiency recovery of functional hematopoietic progenitor and stem cells from human cord blood cryopreserved for 15 years. Proc Natl Acad Sci U S A 2003;100:645–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zambelli A, Poggi G, Da Prada G, Pedrazzoli P, Cuomo A, Miotti D, Perotti C, Preti P, Robustelli della Cuna G. Clinical toxicity of cryopreserved circulating progenitor cells infusion. Anticancer Res 1998;18:4705–8. [PubMed] [Google Scholar]
- 19.Davis JM, Rowley SD, Braine HG, Piantadosi S, Santos GW. Clinical toxicity of cryopreserved bone marrow graft infusion. Blood 1990;75:781–6. [PubMed] [Google Scholar]
- 20.Benekli M, Anderson B, Wentling D, Bernstein S, Czuczman M, McCarthy P. Severe respiratory depression after dimethylsulphoxide-containing autologous stem cell infusion in a patient with AL amyloidosis. Bone Marrow Transplant 2000;25:1299–301. [DOI] [PubMed] [Google Scholar]
- 21.Miniero R, Vai S, Giacchino M, Giubellino C, Madon E. Severe respiratory depression after autologous bone marrow infusion. Haematologica 1992;77:98–9. [PubMed] [Google Scholar]
- 22.Hequet O, Dumontet C, El Jaafari-Corbin A, Salles G, Espinouse D, Arnaud P, Thieblemont C, Bouafia F, Coiffier B. Epileptic seizures after autologous peripheral blood progenitor infusion in a patient treated with high-dose chemotherapy for myeloma. Bone Marrow Transplant 2002;29: 544. [DOI] [PubMed] [Google Scholar]
- 23.Higman MA, Port JD, Beauchamp NJ Jr, Chen AR. Reversible leukoencephalopathy associated with re-infusion of DMSO preserved stem cells. Bone Marrow Transplant 2000; 26:797–800. [DOI] [PubMed] [Google Scholar]
- 24.Burger J, Gilmore MJ, Jackson B, Prentice HG. Acute haemoglobinaemia associated with the reinfusion of bone marrow buffy coat for autologous bone marrow transplantation. Bone Marrow Transplant 1991;7:322–4. [PubMed] [Google Scholar]
- 25.Halle P, Tournilhac O, Knopinska-Posluszny W, Kanold J, Gembara P, Boiret N, Rapatel C, Berger M, Travade P, Angielski S, Bonhomme J, Deméocq F. Uncontrolled-rate freezing and storage at −80 degrees C, with only 3.5-percent DMSO in cryoprotective solution for 109 autologous peripheral blood progenitor cell transplantations. Transfusion 2001;41:667–73. [DOI] [PubMed] [Google Scholar]
- 26.Katayama Y, Yano T, Bessho A, Deguchi S, Sunami K, Mahmut N, Shinagawa K, Omoto E, Makino S, Miyamoto T, Mizuno S, Fukuda T, Eto T, Fujisaki T, Ohno Y, Inaba S, Niho Y, Harada M. The effects of a simplified method for cryopreservation and thawing procedures on peripheral blood stem cells. Bone Marrow Transplant 1997;19:283–7. [DOI] [PubMed] [Google Scholar]
- 27.Rowley SD, Feng Z, Chen L, Holmberg L, Heimfeld S, MacLeod B, Bensinger WI. A randomized phase III clinical trial of autologous blood stem cell transplantation comparing cryopreservation using dimethylsulfoxide vs dimethylsulfoxide with hydroxyethylstarch. Bone Marrow Transplantat 2003;31:1043–51. [DOI] [PubMed] [Google Scholar]
- 28.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K; German Competence Network Sepsis (SepNet). Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008;358:125–39. [DOI] [PubMed] [Google Scholar]
- 29.Ganzevoort W, Rep A, Bonsel GJ, Fetter WP, van Sonderen L, DeVries JI, Wolf H; PETRA Investigators. A randomised controlled trial comparing two temporising management strategies, one with and one without plasma volume expansion, for severe and early onset pre-eclampsia. BJOG 2005;112:1358–68. [DOI] [PubMed] [Google Scholar]
- 30.Riley ET, Cohen SE, Rubenstein AJ, Flanagan B. Prevention of hypotension after spinal anesthesia for cesarean section: six percent hetastarch versus lactated Ringer’s solution. Anesth Analg 1995;81:838–42. [DOI] [PubMed] [Google Scholar]
- 31.French GW, White JB, Howell SJ, Popat M. Comparison of pentastarch and Hartmann’s solution for volume preloading in spinal anaesthesia for elective caesarean section. Br J Anaesth 1999;83:475–7. [DOI] [PubMed] [Google Scholar]
- 32.Yang H, Acker JP, Abley D, McGann LE, Akabutu J. Highefficiency volume reduction of cord blood using pentastarch. Bone Marrow Transplant 2001;27:457–61. [DOI] [PubMed] [Google Scholar]
- 33.Solves P, Mirabet V, Planelles D, Blasco I, Perales A, Carbonell-Uberos F, Soler MA, Roig R. Red blood cell depletion with a semiautomated system or hydroxyethyl starch sedimentation for routine cord blood banking: a comparative study. Transfusion 2005;45:867–73. [DOI] [PubMed] [Google Scholar]
- 34.Strauss RG, Pennell BJ, Stump DC. A randomized, blinded trial comparing the hemostatic effects of pentastarch versus hetastarch. Transfusion 2002;42:27–36. [DOI] [PubMed] [Google Scholar]
- 35.Areman EM, Deeg HJ, Sacher RA. Bone marrow and stem cell processing: a manual of current techniques. Philadelphia (PA): FA Davis Co; 1992. [Google Scholar]
- 36.Berz D, McCormack EM, Winer ES, Colvin GA, Quesenberry PJ. Cryopreservation of hematopoietic stem cells. Am J Hematol 2007;82:463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lecoeur H, Ledru E, Prevost MC, Gougeon ML. Strategies for phenotyping apoptotic peripheral human lymphocytes comparing ISNT, annexin-V and 7-AAD cytofluorometric staining methods. J Immunol Methods 1997;209:111–23. [DOI] [PubMed] [Google Scholar]
- 38.Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, Watanabe T, Akashi K, Shultz LD, Harada M. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood 2005;106:1565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, Greiner DL, Handgretinger R. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol 2005;174:6477–89. [DOI] [PubMed] [Google Scholar]
- 40.Hayakawa J, Hsieh MM, Uchida N, Phang O, Tisdale JF. Busulfan produces efficient human cell engraftment in NOD/LtSz-Scid IL2Rgamma(null) mice. Stem Cells 2009; 27:175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayakawa J, Migita M, Ueda T, Shimada T, Fukunaga Y. Generation of a chimeric mouse reconstituted with green fluorescent protein-positive bone marrow cells: a useful model for studying the behavior of bone marrow cells in regeneration in vivo. Int J Hematol 2003;77:456–62. [DOI] [PubMed] [Google Scholar]
- 42.Abrahamsen JF, Rusten L, Bakken AM, Bruserud O. Better preservation of early hematopoietic progenitor cells when human peripheral blood progenitor cells are cryopreserved with 5 percent dimethylsulfoxide instead of 10 percent dimethylsulfoxide. Transfusion 2004;44:785–9. [DOI] [PubMed] [Google Scholar]
- 43.Kang EM, Areman EM, David-Ocampo V, Fitzhugh C, Link ME, Read EJ, Leitman SF, Rodgers GP, Tisdale JF. Mobilization, collection, and processing of peripheral blood stem cells in individuals with sickle cell trait. Blood 2002;99: 850–5. [DOI] [PubMed] [Google Scholar]
- 44.Liseth K, Abrahamsen JF, Bjorsvik S, Grottebo K, Bruserud O. The viability of cryopreserved PBPC depends on the DMSO concentration and the concentration of nucleated cells in the graft. Cytotherapy 2005;7:328–33. [DOI] [PubMed] [Google Scholar]
- 45.Sasnoor LM, Kale VP, Limaye LS. Supplementation of conventional freezing medium with a combination of catalase and trehalose results in better protection of surface molecules and functionality of hematopoietic cells. J Hematother Stem Cell Res 2003;12:553–64. [DOI] [PubMed] [Google Scholar]
- 46.Solves P, Mirabet V, Planelles D, Carbonell-Uberos F, Roig R. Influence of volume reduction and cryopreservation methodologies on quality of thawed umbilical cord blood units for transplantation. Cryobiology 2008;56:152–8. [DOI] [PubMed] [Google Scholar]
- 47.Stylianou J, Vowels M, Hadfield K. Novel cryoprotectant significantly improves the post-thaw recovery and quality of HSC from CB. Cytotherapy 2006;8:57–61. [DOI] [PubMed] [Google Scholar]
- 48.Yasutake M, Zheng Y, Inoue-Nagamura T, Kagawa E, Tokushima Y, Terashima S, Takahashi TA. CID-repopulating activity of human umbilical cord blood-derived hematopoietic stem and/or progenitor cells in a nonobese diabetic/Shi-SCID mice serial xenotransplantation model and immune cell activities in vitro: a comparative study of the filter method and the hydroxyethyl starch method. Transfusion 2005;45:1899–908. [DOI] [PubMed] [Google Scholar]
- 49.Zhang XB, Li K, Yau KH, Tsang KS, Fok TF, Li CK, Lee SM, Yuen PM. Trehalose ameliorates the cryopreservation of cord blood in a preclinical system and increases the recovery of CFUs, long-term culture-initiating cells, and nonobese diabetic-SCID repopulating cells. Transfusion 2003; 43:265–72. [DOI] [PubMed] [Google Scholar]
- 50.London MJ, Ho JS, Triedman JK, Verrier ED, Levin J, Merrick SH, Hanley FL, Browner WS, Mangano DT. A randomized clinical trial of 10% pentastarch (low molecular weight hydroxyethyl starch) versus 5% albumin for plasma volume expansion after cardiac operations. J Thorac Cardiovasc Surg 1989;97:785–97. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Flow diagram of the experiments performed with 16 human cord blood units
Fig. S2. Comparison of 2 freezing methods using 2 cell doses. Since most cord blood units are stored unselected, we infused CB mononuclear cells. The average percentages of human CD45 positive cells are plotted with respect to time after transplant. The first graph used a lower cell dose (1.2 × 107, n = 6 in all groups), and second graph used a higher cell dose (2 × 107) as seen in Fig. 3, fresh (n = 6), after frozen with 10% DMSO (n = 7), or after 5% DMSO/pentastarch (n = 6).