Abstract
Cytokines are required for γ-retroviral transduction of human CD34+ cells. However, cytokines may reduce engraftment of CD34+ cells and may not be necessary for their lentiviral transduction. We sought to optimize transduction and engraftment of human CD34+ cells using lentiviral vectors. Single 24 h transduction of human CD34+ cells with human immunodeficiency virus type 1 (HIV1)-based lentiviral vectors in media containing stem cell factor (SCF), FMS-like tyrosine kinase 3 (FLT3) ligand, thrombopoietin (each 100 ng ml−1) and 10% fetal bovine serum was compared with various cytokine conditions during ex vivo culture and assayed using humanized xenograft mice for 6 months after transplantation. Serum-free media improved transduction efficiency of human CD34+ cells. Interleukin-3 (20 ng ml−1) had little effect on transduction efficiency or engraftment. Threefold higher cytokine mixture (each 300 ng ml−1) reduced engraftment of CD34+ cells. SCF alone (100 ng ml−1) proved insufficient for maintaining engraftment ability and reduced transduction efficiency. Short-term prestimulation had little effect on transduction efficiency or engraftment, yet 24 h prestimulation showed higher transduction efficiency, higher gene expression levels and lower engraftment. In summary, 24 h prestimulation followed by single 24-h lentiviral transduction in serum-free media with SCF, FLT3 ligand and thrombopoietin yields high transduction efficiency to engrafting human CD34+ cells, and is applicable in human clinical gene therapy trials.
Keywords: hematopoietic stem cell, human CD34+ cell, humanized xenograft mouse, lentiviral vector, transduction efficiency
INTRODUCTION
Gene therapy targeting hematopoietic stem cells (HSCs) has proven effective in treating hereditary immunodeficiency diseases,1,2 using γ-retroviral vectors to transfer a corrective gene. Hemoglobin disorders, such as thalassemia and sickle cell disease (SCD), remain promising candidates for HSC gene therapy. The development of human immunodeficiency virus type 1 (HIV1)-based lentiviral vector systems proved pivotal in directing human β-globin expression among erythroid progeny of transduced HSCs, and lentiviral vectors based on HIV1 have shown efficacy in mouse models of β-thalassemia and SCD.3–8 More recently, lentiviral vectors have proven effective in a clinical trial in human beings with a rare form of brain demyelination;9 however, moderate benefits were also suggested in a single recipient of genetically modified HSCs for the treatment of thalassemia.10 Unfortunately, for disorders such as the thalassemias and hemoglobinopathies, high gene transfer rates and expression levels will be required for reliable benefit, thus more efficient transduction strategies that promote high gene expression rates should be developed for human HSCs.
Humanized xenograft mouse models were developed to evaluate the long-term repopulating potential of human HSCs and have become an indispensable tool for human HSC research.11 We previously showed efficient human cell engraftment in non-obese diabetic/severe combined immunodeficiency/interleukin (IL)-2 receptor γ-chain null (NOD/SCID/IL-2Rγnull) mice after non-myeloablative busulfan conditioning, with increased engraftment of human HSCs and survival compared with previously developed humanized mouse models.12,13 This humanized xenograft mouse model has also proven successful for the evaluation of HIV1 infection of human lymphocytes in vivo14 and transgene expression in reconstituted peripheral blood cells after human HSC transduction and transplantation.15–18
The array and concentration of cytokines used to support the ex vivo culture are important factors in attaining efficient transduction and engraftment of human HSCs. Cytokine exposure on fibronectin-coated plates has proven essential for HSC culture in vitro to maintain stem cells during transduction.19–22 HSC transduction with γ-retroviral vectors requires cytokine exposure23 as retroviral vectors require cell cycle transit, whereas HIV1-based vectors are able to transduce non-dividing cells, such as HSCs.24 Thus, cytokines’ exposure may be not necessary for lentiviral transduction of human HSCs. Furthermore, overstimulation may induce cell differentiation and reduce engraftment of HSCs. In this study, we sought to evaluate the effects of cytokines on lentiviral transduction efficiency and engraftment of human HSCs using a humanized xenograft mouse model.
RESULTS
Serum and interleukin-3 stimulation reduces CD34 expression rates
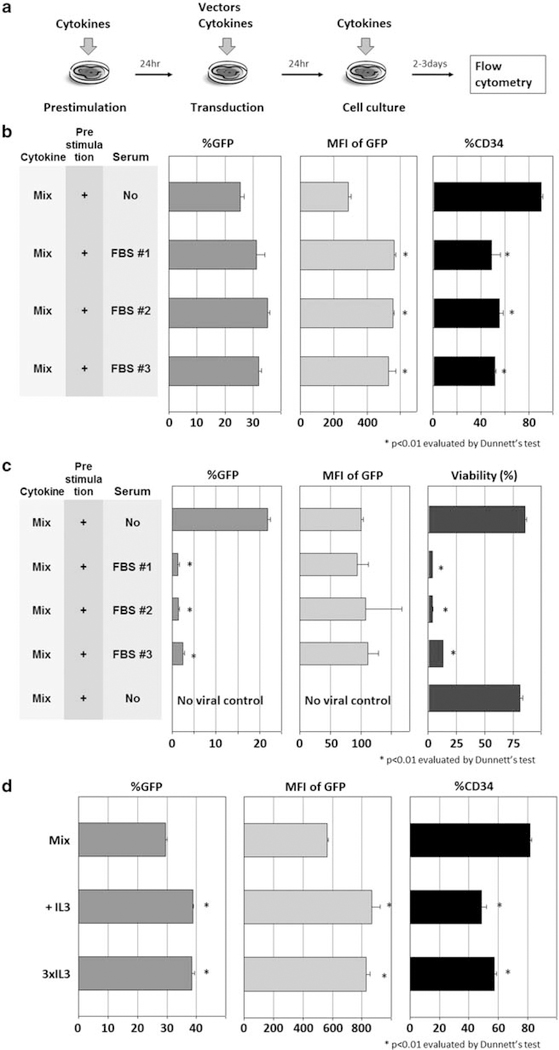
Human CD34+ cells were cultured with cytokines for 24 h without viral vector (prestimulation) and then transduced with cytokines using an enhanced green fluorescent protein (GFP)-expressing HIV1-based lentiviral vector for an additional 24 h (transduction; Figure 1a). To evaluate whether fetal bovine serum (FBS) has effects on human CD34+ cell transduction, we compared three lot numbers of FBS (nos. 1, 2 and 3) in Dulbecco’s modified Eagle’s medium (DMEM) or serum-free X-VIVO10 media for efficiency of human CD34+ cell transduction. Both media contained 100 ng ml−1 each of stem cell factor (SCF), FMS-like tyrosine kinase 3 ligand (FLT3L) and thrombopoietin (TPO). At a multiplicity of infection (MOI) of 5, serum-containing media yielded higher transduction rates (P<0.05 in FBS no. 2), higher intensities of GFP (P<0.01 in FBS nos. 1, 2 and 3), but lower rates of CD34+ expression (P<0.01 in FBS nos. 1, 2 and 3; Figure 1b). At an MOI of 50, 10% FBS conditions yielded much lower transduction rates (P<0.01 in FBS nos. 1, 2 and 3), as most of the cells died after transduction in serum-containing media (P<0.01 in FBS nos. 1, 2 and 3; Figure 1c). These data show small variations in FBS lot numbers from the same provider, and that serum with high vector MOI increased toxicity during human CD34+ cell transduction.
Figure 1.
Effects of additional serum or IL-3 on human CD34+ cell transduction. (a) As in our basic condition, we cultured human CD34+ cells with cytokines alone for 24h (prestimulation), with cytokines and a GFP-expressing HIV1-based lentiviral vector for an additional 24h (transduction). (b) To evaluate whether FBS has effects on human CD34+ cell transduction, we performed transduction at an MOI of 5 in DMEM with 10% FBS or serum-free X-VIVO10 medium, and we analyzed GFP expression rates (%GFP), mean fluorescence intensity (MFI) of GFP and CD34+ rates in bulk cells (%CD34). (c) To evaluate the effects of FBS at high MOI, we transduced human CD34+ cells at an MOI of 50, and analyzed %GFP, MFI of GFP and cell viability (Trypan blue stain). (d) To evaluate effects of supplemental IL-3 to the 100 ng ml−1 cytokine mixture (Mix) in serum-free X-VIVO10 medium, we compared Mix, addition of 20 ng ml−1 IL-3 (+IL-3) and addition of 60 ng ml−1 IL-3 (3×IL-3).
We then evaluated the effects of additional IL-3 in X-VIVO10 media containing 100 ng ml−1 each of SCF, FLT3L and TPO on human CD34+ cell transduction (Figure 1d). Both 20 ng ml−1 IL-3 (3×IL-3) and 60 ng ml−1 IL-3 (3×IL-3) yielded higher transduction rates (P<0.01) and higher intensities of GFP (P<0.01), but lower CD34+ rates (P<0.01). These data suggest that IL-3 supplementation has similar effects as FBS in inducing CD34+ cell differentiation.
Twenty-four hour prestimulation increases transduction efficiency in human CD34+ cells
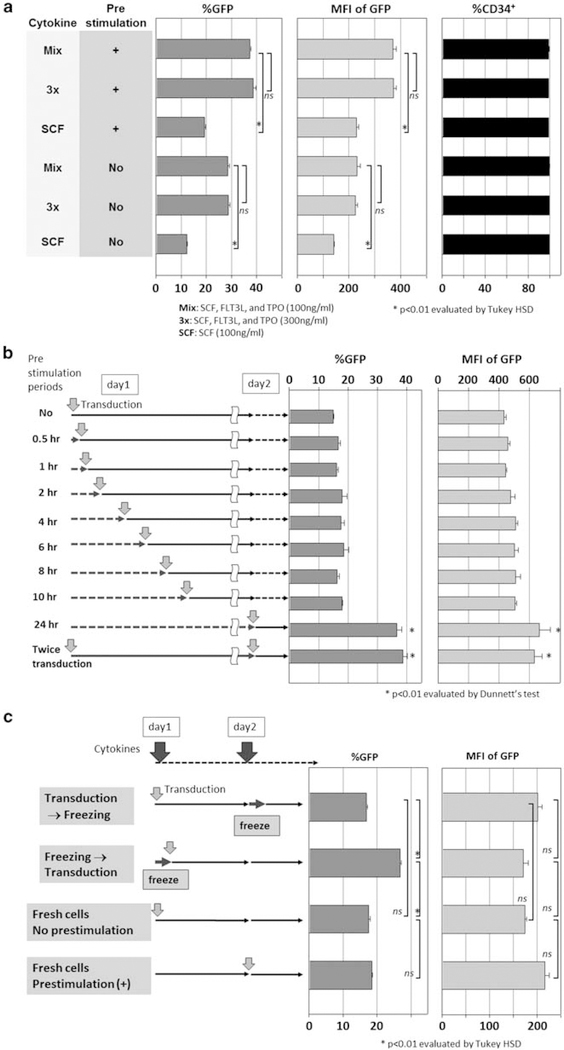
Human CD34+ cells were cultured and transduced with lentiviral vector at an MOI of 50 in serum-free media containing various cytokine combinations (Figure 1a). We tested a threefold increased cytokine concentration mixture (3×, 300 ng ml−1 each of SCF, FLT3L and TPO) and SCF alone (100 ng ml−1). The 3× cytokine mixture neither increased transduction rates nor intensities of GFP over the 1× cytokine mixture (Mix, 100 ng ml−1 each of SCF, FLT3L and TPO). Furthermore, cells exposed to SCF alone (100 ng ml−1) showed lower transduction rates (P<0.01) and intensities of GFP (P<0.01). Importantly, the CD34+ rates remained high in all serum-free conditions (Figure 2a). On the other hand, cells exposed to cytokines for 24 h before lentiviral transduction (prestimulation) yielded higher transduction rates (P<0.01) and intensities of GFP (P<0.01) than cells that were not prestimulated (Figure 2a). These data show that prestimulation is an important component in the transduction procedure. Higher cytokine concentration (that is, 3×) did not provide better transduction efficiency, and SCF alone was inferior to cytokine mixture of SCF, FLT3L and TPO (Mix).
Figure 2.
Effects of cytokine concentration and duration of cytokine prestimulation on human CD34+ cell transduction. (a) To evaluate effects of cytokine concentrations in various conditions, we compared lentiviral transduction between no prestimulation and 24h prestimulation; in 100 ng ml−1 cytokine mixture of SCF, FLT3L and TPO (Mix), threefold higher concentrated cytokine mixture (3×) and SCF alone (SCF, 100 ng ml−1). (b) To evaluate the duration of prestimulation, we compared no prestimulation, short-term (0.5–10h) and 24h prestimulation. Single transduction after 24h prestimulation was also compared with double transductions before and after 24h prestimulation. (c) Fresh CD34+ cells were first transduced with and without prestimulation. CD34+ cells were then transduced before and after freezing, both without prestimulation.
We then evaluated the length of prestimulation, comparing from 0.5 to 10 h to the 24h prestimulation (Figure 2b). Twenty-four hour prestimulation yielded the highest transduction rates (P<0.01) and GFP intensities (P<0.01). Interestingly, no prestimulation and less than 10 h of prestimulation all yielded similar transduction rates (P=NS) and GFP intensities, suggesting that shorter duration prestimulation is insufficient. Two viral exposures, before and after 24 h prestimulation, also did not improve transduction rates or intensity of GFP, compared with the single transduction after 24 h prestimulation (P=NS).
Although frozen CD34+ cells were used in most of our experiments, we hypothesized that fresh CD34+ cells might not require prestimulation. We thus designed four conditions to test the effects of cryopreservation: fresh CD34+ cell transduction with or without prestimulation; cryopreservation of CD34+ cells before or after transduction (Figure 2c); prestimulation did not improve transduction rates among fresh CD34+ cells; and transduction after freezing, however, yielded the highest GFP expression (P<0.01).
Effects of various transduction conditions on human cell engraftment in xenografted mice
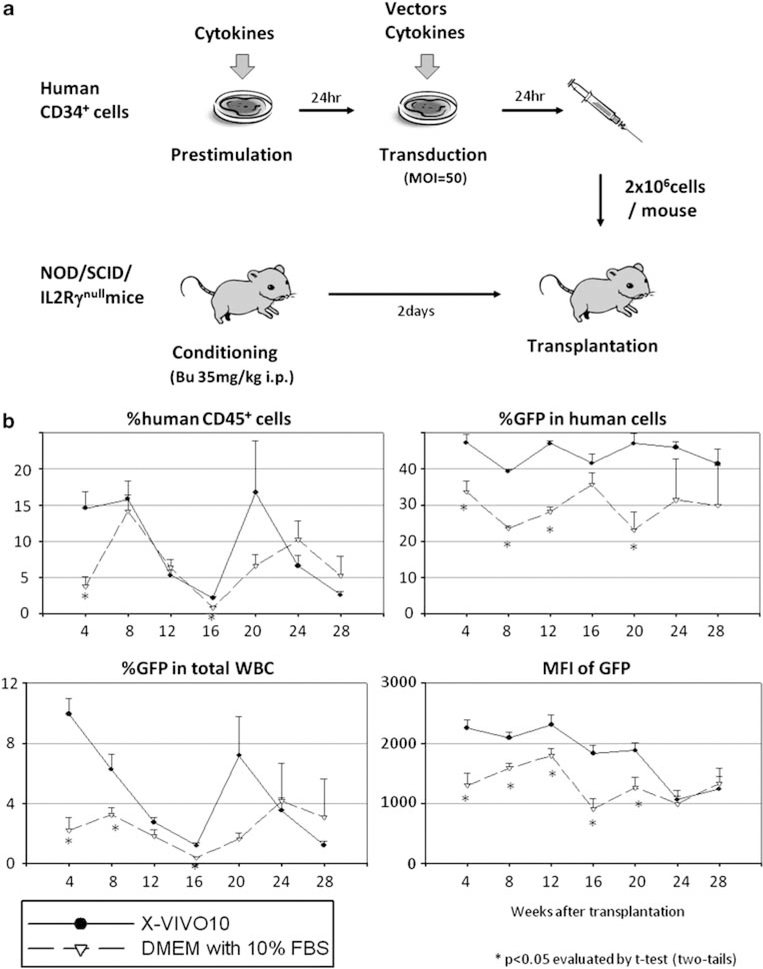
To evaluate the engraftment potential of transduced human hematopoietic stem/progenitor cells, we performed transplantation with transduced CD34+ cells using our humanized xenograft mouse model (Figure 3a). We tested various transduction conditions in xenograft mice: media with and without serum (Figure 3b); with and without supplemental IL-3 (Supplementary Figure 1); 100 ng ml−1 mixture, 3× cytokines and SCF alone (Figure 4); with and without prestimulation (Figure 5); and 4 vs 24h of prestimulation (Supplementary Figure 2). The results are summarized in Table 1.
Figure 3.
Effects of serum on human CD34+ cell transduction in xenograft mice. (a) Human CD34+ cell transduction was performed using a 24h prestimulation with cytokines and an additional 24h of fresh media containing the same cytokines and viral vector addition. (b) Human CD34+ cells were transduced with serum-free X-VIVO10 media or DMEM with 10% FBS, and transplanted into our xenograft mouse model.
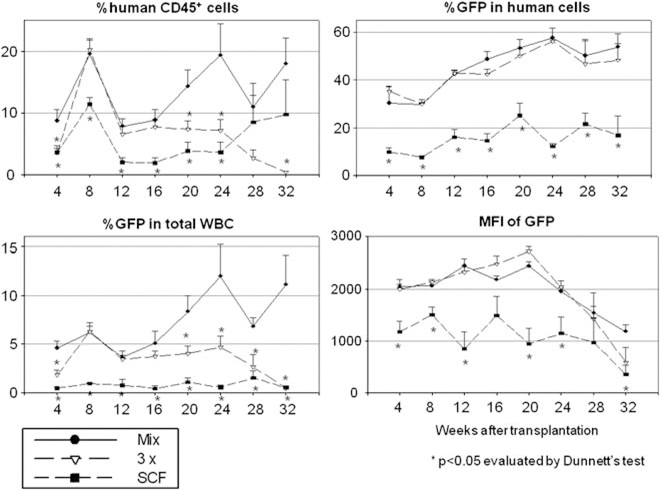
Figure 4.
Effects of cytokine concentration on human CD34+ cell transduction in xenograft mice. Human CD34+ cells were transduced with a 100ng ml−1 cytokine mixture (Mix), a threefold higher mixture (3×) or SCF alone (SCF) ex vivo and infused into sublethally conditioned NOD/SCID/IL2Rγnull mice.
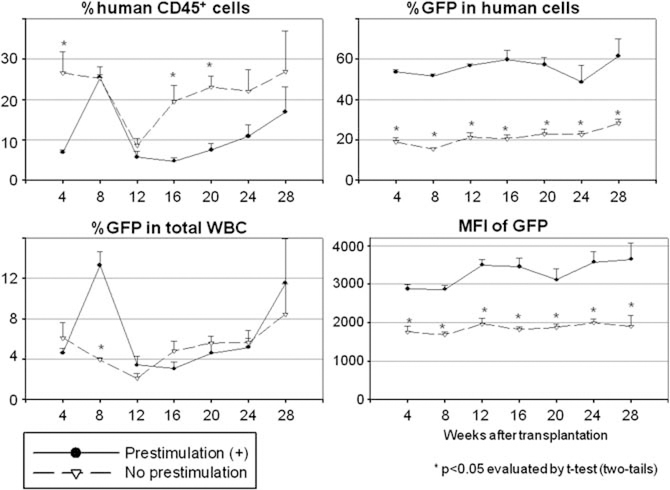
Figure 5.
Effects of cytokine prestimulation on human CD34+ cell transduction in xenograft mice. Transduced human CD34+ cells were infused in xenograft mouse model after ex vivo CD34+ cell transduction with or without 24 h prestimulation.
Table 1.
Summary of results from various transduction conditions on human CD34+ cells in xenograft mice between 20 and 28 weeks after HSC transplantation
| Human chimerism | Transduction rates in human cells | Transduction rates in total WBCs | |
|---|---|---|---|
| Mix | Reference | Reference | Reference |
| 3× | − | ± | − |
| SCF | − | − | − |
| No prestimulation | + | − | ± |
| 4 h prestimulation | ± ~ + | ± ~ − | ± |
| + IL-3 | ± | ± | ± |
| 10% FBS | ± | − | ± |
Abbreviations: FBS, fetal bovine serum; HSC, hematopoietic stem cell; IL, interleukin; SCF, stem cell factor; WBCs, white blood cells; +, increase; −, decrease; ±, no change.
When serum-free X-VIVO10 medium was compared with serum-containing medium (DMEM with 10% FBS) using xenografted mice (Figure 3b and Table 1), serum-containing medium exhibited lower transduction rates among human cells (P<0.05 in 4, 8, 12 and 20 weeks after SCT) and lower intensities of GFP (P<0.05 in 4, 8, 12, 16 and 20 weeks after SCT). However, there was no significant difference in the levels of human chimerism detected between the two groups. These data suggest that FBS decreases transduction efficiency among human stem/progenitor cells. We also evaluated the 100 ng ml−1 cytokine mixture (100 ng ml−1 each of SCF, FLT3L and TPO) with supplemental IL-3 (20 ng ml−1; +IL-3) during ex vivo transduction (Table 1 and Supplementary Figure 1). There was no significant difference in human chimerism, transduction rates or intensities of GFP between the two groups, suggesting that the addition of IL-3 has minimal effects on human stem/progenitor cell transduction.
We next compared cytokines at 100 ng ml−1 mixture (Mix), 3× cytokines and SCF alone (Figure 4 and Table 1). The recipients of cells cultured in the 3× cytokine mixture group showed lower long-term human chimerism levels (P<0.05 in 20, 24 and 32 weeks after SCT), but similar transduction rates among human cells (NS in all time points), and similar intensities of GFP (NS in all time points), compared with those in the 100 ng ml−1 cytokine mix group (Mix). The SCF-alone group showed lower human chimerism levels (P<0.05 in 4–24 weeks after SCT), lower transduction rates (P<0.05 in all time points) and lower intensities of GFP (P<0.05 in 4–12, 20, 24 and 32 weeks after SCT). These data suggest that the 3× higher cytokine mixture reduces engraftment ability among CD34+ cells, and that SCF alone is insufficient to maintain stem/progenitor cells or increase transduction efficiency.
Twenty-four hours of cytokine prestimulation is optimal for ex vivo human CD34+ cell transduction
We next compared ex vivo transduction conditions between no prestimulation (No prestimulation) and 24 h prestimulation (Prestimulation (+)) using our xenograft mice (Figure 5 and Table 1). The 24 h prestimulation group showed lower human chimerism levels (P<0.05 in 4, 16 and 20 weeks after SCT), but higher transduction rates (P<0.05 in all time points) and higher intensities of GFP (P<0.05 in all time points) among human cells. When transduction with 4 h prestimulation was compared with that of 24 h prestimulation (Table 1 and Supplementary Figure 1), the 4h prestimulation group showed a tendency towards higher human chimerism levels at 24 and 28 weeks after transplantation, but lower transduction rates at 16, 20 and 24 weeks. However, there was no statistically significant difference between the two groups.
DISCUSSION
HSC transplantation is an ideal platform for delivering stem cell therapies, such as ex vivo cell manipulation for gene therapy applications. Although ex vivo cell culture is a necessary step for the therapeutic modification of HSCs, the most appropriate culture conditions may vary depending on the vector type, the therapeutic gene to be transferred and the underlying genetic disorder. In this study, we focused on variables important in ex vivo human HSC transduction using HIV1-based lentiviral vectors, and evaluated repopulating ability in the humanized xenograft mouse model.
One measure with proven efficacy in improving gene transfer rates to repopulating cells is optimization of ex vivo culture conditions.25,26 Our best method thus far for γ-retroviral vectors includes the cytokines of SCF, FLT3L and TPO in the presence of FBS. These conditions proved unsatisfactory for our lentiviral system, and we thus began to explore other combinations. One earlier study showed that 10% FBS had no significant effect on ex vivo transduction efficiency among rhesus CD34+ cells transduced with an amphotropic pseudo-typed retroviral vectors, evaluated by in vivo marking levels in peripheral blood cells after transplantation.27 Another study reported among human CD34+ cells, the addition of 10% FBS reduced transduction efficiency with vesicular stomatitis virus glycoprotein-pseudotyped HIV1 vectors using a 2-day prestimulation and 2-day transduction at an MOI of 9.28 Our in vitro data showed that 10% FBS induced higher %GFP, but lower CD34+ rates for 4–5 day cultures at an MOI of 5 (Figure 1b), and it decreased %GFP and cell viability at an MOI of 50 (Figure 1c). In addition, 10% FBS did not change human chimerism 6 months after transplantation (Figure 3b and Table 1). These data suggest that FBS induces expansion of progenitor cells, but reduces transduction efficiency for HSCs. It is possible that transduced progenitor cells might expand at low MOI, but die at high MOI owing to excessive transduction. Furthermore, FBS has disadvantages for clinical usage, such as infection risks and immune reactions caused by the development of antibodies to FBS.29 Taken together, the use of serum is beneficial in γ-retroviral vectors, but likely has a limited role in eventual clinical gene therapy trials using lentiviral vectors.
Previously, the addition of IL-3 was shown to increase mouse hematopoietic progenitor cell numbers, evaluated by colony-forming unit assay30 and colony-forming unit-spleen assays,31 whereas IL-3 exposure for 7 days decreased the reconstituting ability of mouse HSCs.30 Human CD34+ cells lost long-term repopulating ability under culture conditions containing IL-3, evaluated by long-term culture-initiating cell assay.32 On the other hand, IL-3 increased transduction efficiency with retroviral vectors for mouse HSCs (2-day prestimulation and 2-day transduction, on a producer cell line), evaluated by long-term transgene expression in vivo.31 In human CD34+ cells, the addition of IL-3 increased transduction efficiency with HIVl-based lentiviral vectors (1-day prestimulation and 2-day transduction), evaluated by in vitro culture.28 In our data herein, the addition of IL-3 increased %GFP, but reduced CD34+ expression after 4–5 days culture in vitro (Figure 1d), yet no change in human chimerism or %GFP was observed (1-day prestimulation and 1-day transduction) when followed for 6 months in vivo (Table 1 and Supplementary Figure 1). These data suggest that IL-3 induces cell expansion and higher transduction rates of early progenitor cells, but does not change transduction efficiency or the repopulating ability of HSCs after a 2-day culture. Thus, similar to FBS, the role of IL-3 in clinical gene therapy trials using lentiviral vectors may also be limited.
Previous work by others has reported superior maintenance, even expansion, of human CD34+ CD38− cells in a medium, including 300 ng ml−1 SCF, 300 ng ml−1 FLT3L and 60 ng ml−1 IL-3, compared with that observed for 30-fold lower concentration of same cytokines, evaluated by the long-term culture-initiating cell assay in non-transduced human cells.33 However, when we compared a 100 ng ml−1 cytokine mixture of SCF, FLT3L and TPO (Mix), a 3× higher concentration of cytokines and SCF alone in ex vivo transduction in the gene transfer experiments, the 100 ng ml−1 cytokine mixture (Mix) and 3× cytokines had equivalent transduction efficiency and CD34+ rates in vitro (Figure 2a). These three conditions were then evaluated in humanized mice, where 100ngrnl−1 cytokine mixture (Mix) yielded the best human cell engraftment and transduction rates (Figure 4 and Table 1). In another study, 2 days of ‘rest’ in SCF alone after a standard 4 days of transduction of rhesus CD34+ cells with γ-retroviral vectors increased in vivo marking levels compared with that observed for no SCF stimulation after retroviral transduction of CD34+ cells.34 These results also suggested that maintenance in SCF alone might be sufficient for HSC culture using lentiviral vectors that do not require cell cycle transit for transduction. Those earlier data are in contrast with our current results where SCF alone both decrease the transduction efficiency and repopulating ability of human HSCs assayed in vivo at 4–6 month post-transplantation (Figure 4 and Table 1).
Cytokine prestimulation has historically been utilized to recruit quiescent HSCs into the active cell cycle, and to thus increase transduction efficiency with γ-retroviral vectors as these vectors require active replication of targeted cells at the time of transduction.31,35,36 Prestimulation may not be necessary for HSC transduction with lentiviral vectors because lentiviral vector can transduce both proliferating and non-proliferating cells.37 However, in previous data, cytokine prestimulation improved human CD34+ cell transduction with lentiviral vectors, evaluated by colony-forming unit assay and long-term culture-initiating cell assay.28,38 We thus examined the effects of prestimulation duration and showed that > 10 h of cytokine exposure before lentiviral transduction yielded the highest in vitro GFP expression (Figure 2b). When no prestimulation and 24h prestimulations were compared directly in vivo, mice in the 24 h prestimulation group showed lower human chimerism levels, higher transduction efficiencies and higher GFP intensities (Figure 5 and Table 1). Mice transplanted with HSCs subjected to a 4h prestimulation had a tendency to show higher human chimerism levels and lower transduction efficiency, compared with those of the 24 h prestimulation (Table 1 and Supplementary Figure 2). Collectively, our data support the hypothesis that prolonged cytokine prestimulation increases transduction efficiency (%GFP), but at the expense of reduced HSC repopulating ability (%human CD45). In addition, these data also show that 24 h prestimulation can increase transgene expression levels (intensity of GFP), an important observation for gene therapy targets like SCD, because a high expression (intensity) of β- or γ-globin will be required to overcome the pathological globin. Similarly, an 18 h prestimulation showed higher reconstitution ability of human CD34+ cells with lentiviral transduction compared with 36 and 96 h prestimulation.39 Indeed, the struggle to develop transduction methods that preserve engraftment while improving HSC transduction remains. In an attempt to improve engraftment of genetically modified cells, we and others previously explored the ex vivo expansion of transduced CD34+ cells, with a surprising and significant reduction in engraftment, despite expansion rates of up to 100-fold,20,40,41 suggesting that any period of ex vivo culture can result in reduced engraftment rates. Even with our best conditions to date, ex vivo expanded cells engraft at rates that approach equivalence to the non-expanded control fraction.19 These data corroborate with our current data and suggest the optimal time of ex vivo culture designated as prestimulation for maximum transgene expression is 18–24 h. Thus, the balance between the positive effects of prestimulation on transduction rates and transgene expression levels and the negative effect on reconstitution ability rest on the desired level of transgene expression: 10–24 h of prestimulation would favor higher levels of transgene expression necessary for globin disorders, and shorter duration of prestimulation might be sufficient in the immunodeficiency disorders where lower levels of transgene expression and higher reconstituting ability would suffice.
Interestingly, prestimulation had a negligible effect on transduction of fresh CD34+ cells (Figure 2c). It is possible that fresh CD34+ cells were already under the influence of endogenous cytokines induced by granulocyte colony-stimulating factor mobilization before apheresis, rendering prestimulation unnecessary. Frozen cells might also transiently lose expression of HIV1 entry restriction factors, such as TRIM5a and APOBEC3G, thereby increasing transduction efficiency.42,43 In addition, we previously showed that cryopreservation does not reduce repopulating ability of human CD34+ cells evaluated by 6-month follow-up in humanized mice.44 These in vitro data reassured that freezing selected CD34+ cells before or after transduction do not yield inferior transgene expression, compared with fresh cells. The preferred combination of fresh vs frozen CD34+ cells and freezing before vs after transduction can therefore be determined by the logistical considerations where the clinical trials are conducted, such as staffing, locations of the cell processing center and hospital, and the need to transport genetically modified cells.
We evaluated correlations between in vitro data and in vivo mouse data at the 4–6 months post-transplant stage. The CD34+ rates in vitro had no correlation with the human chimerism in vivo. The 3× higher cytokine concentration mixture, SCF alone and 24 h prestimulation decreased engraftment of human CD34+ cells even though CD34+ rates in vitro did not change. Lower CD34+ rates in vitro were not caused by reduction of the stemness, such as addition of IL-3 or FBS, suggesting expansion of early progenitor cells.
In summary, human HSCs lose repopulating ability when exposed to a prolonged duration or a threefold higher cytokine concentration mixture. Although encouraging results exist for γ-retroviral vectors, SCF alone was insufficient for maintaining HSCs and increasing transduction rates using our lentiviral vector system. FBS and IL-3 have the equivalent effect of improving in vitro transduction efficiency, but reducing CD34 expression. Our best conditions for lentiviral vector-mediated human CD34+ cell transduction include a 24 h prestimulation, followed by a 24 h vector exposure, both in the presence of the cytokines of SCF, FLT3L and TPO (each 100 ng ml−1) in serum-free X-VIVO10 media. All considered, these observations provide important information for the design of future clinical gene therapy trials.
MATERIALS AND METHODS
Lentiviral vector preparation
Self-inactivating HIV1-based lentiviral vectors were prepared as described previously.45–47 We performed co-transfection of 293T cells with vector plasmid (pCL20c MpGFP), Gag/Pol plasmid (pCAG-KGPl.1R), Rev/Tat plasmid (pCAG4-RTR2) and vesicular stomatitis virus glycoprotein envelope plasmid (pCAGGS-vesicular stomatitis virus glycoprotein), which were kindly provided by Dr Arthur Nienhuis.45 The lentiviral vector expressed GFP under the control of a murine stem cell virus long-term repeat promoter.
Human CD34+ cell transduction
Human CD34+ cells were enriched from peripheral blood cells, which after mobilization by granulocyte colony-stimulating factor under a protocol approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Disease and were stored in a −180 °C freezer as described previously.47 Frozen human CD34+ cells were quickly thawed in a 37 °C water bath and cultured on fibronectin- (RetroNectin; Takara, Shiga, Japan) coated plates using two types of culture media with various combinations of cytokines. The DMEM (Mediatech Inc., Manassas, VA, USA) including 10% FBS or serum free X-VIVO10 (Lonza, Basel, Switzerland) were used. Cytokines included SCF (R&D Systems, Minneapolis, MN, USA), FLT3L (R&D Systems), TPO (R&D Systems) and IL-3 (R&D Systems). After various periods of prestimulation in culture, media were changed to fresh media with identical conditions, but with lentiviral vectors added at an MOI of 5 or 50. Prestimulation periods ranged from 0 to 24 h. At 24 h after transduction, culture media were changed and the cells were cultured for an additional 2–3 days, after which GFP expression was evaluated by FACSCalibur (BD Biosciences, Franklin Lakes, NJ, USA). CD34+ rates were detected by human CD34-phycoerythrin-conjugated antibody (clone 563; BD Biosciences). All in vitro experiments were carried out in triplicate.
Humanized xenograft mouse model
We used male NOD/SCID/IL-2Rγnull mice (NOD.Cg-PrkdcscidIL2rgttm1Wjl/SzJ; Jackson Laboratory, Bar Harbor, ME, USA) that were 6–8 weeks old, following the guidelines set out by the Public Health Services Policy on Humane Care and Use of Laboratory Animals under a protocol approved by the Animal Care and Use Committee of the National Institute of Diabetes and Digestive and Kidney Diseases. Mice were injected intraperitoneally with sublethal dose of busulfan, 35mgkg−1 (Busulfex; PDL BioPharma, Redwood City, CA, USA), 2 days before transplantation. Human CD34+ cells (2×106 cells per mouse) were transduced with lentiviral vectors at an MOI of 50 with various cytokine conditions, and injected into the tail vein. Following transplantation, peripheral blood samples were obtained to evaluate GFP expression among human CD45+ cells (human CD45-PE antibody, clone HI30; BD Biosciences) over a period of 6 months. Three to five mice were evaluated for each group at all experimental time points.
Statistical analysis
Statistical analyses were performed using JMP 7 software (SAS Institute Inc., Cary, NC, USA). Various types of cytokine conditions were evaluated by Dunnett’s test (one-way analysis of variance for one control) or Tukey’s HSD (honestly significant difference) test (one-way analysis of variance for all). Two types of conditions were evaluated by the Student’s f-test. Standard errors of the mean are shown as error bars in all figures.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the intramural research program of the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK) at the National Institutes of Health (NIH).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on Gene Therapy website (http://www.nature.com/gt)
References
- 1.Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay JP et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med 2002; 346: 1185–1193. [DOI] [PubMed] [Google Scholar]
- 2.Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med 2009; 360: 447–458. [DOI] [PubMed] [Google Scholar]
- 3.May C, Rivella S, Callegari J, Heller G, Gaensler KM, Luzzatto L et al. Therapeutic haemoglobin synthesis in beta-thalassaemic mice expressing lentivirus-encoded human beta-globin. Nature 2000; 406: 82–86. [DOI] [PubMed] [Google Scholar]
- 4.Imren S, Payen E, Westerman KA, Pawliuk R, Fabry ME, Eaves CJ et al. Permanent and panerythroid correction of murine beta thalassemia by multiple lentiviral integration in hematopoietic stem cells. Proc Natl Acad Sci USA 2002; 99: 14380–14385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levasseur DN, Ryan TM, Pawlik KM, Townes TM. Correction of a mouse model of sickle cell disease: lentiviral/antisickling beta-globin gene transduction of unmobilized, purified hematopoietic stem cells. Blood 2003; 102: 4312–4319. [DOI] [PubMed] [Google Scholar]
- 6.Pawliuk R, Westerman KA, Fabry ME, Payen E, Tighe R, Bouhassira EE et al. Correction of sickle cell disease in transgenic mouse models by gene therapy. Science 2001; 294: 2368–2371. [DOI] [PubMed] [Google Scholar]
- 7.Persons DA, Allay ER, Sabatino DE, Kelly P, Bodine DM, Nienhuis AW. Functional requirements for phenotypic correction of murine beta-thalassemia: implications for human gene therapy. Blood 2001; 97: 3275–3282. [DOI] [PubMed] [Google Scholar]
- 8.Rivella S, May C, Chadburn A, Riviere I, Sadelain M. A novel murine model of Cooley anemia and its rescue by lentiviral-mediated human beta-globin gene transfer. Blood 2003; 101: 2932–2939. [DOI] [PubMed] [Google Scholar]
- 9.Slaets H, Hendriks JJ, Van den Haute C, Coun F, Baekelandt V, Stinissen P et al. CNS-targeted LIF expression improves therapeutic efficacy and limits autoimmune-mediated demyelination in a model of multiple sclerosis. Mol Ther 2010; 18: 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F et al. Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature 2010; 467: 318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol 2007; 7: 118–130. [DOI] [PubMed] [Google Scholar]
- 12.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood 2002; 100: 3175–3182. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood 2005; 106: 1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe S, Terashima K, Ohta S, Horibata S, Yajima M, Shiozawa Y et al. Hematopoietic stem cell-engrafted NOD/SCID/IL2Rgamma null mice develop human lymphoid systems and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood 2007; 109: 212–218. [DOI] [PubMed] [Google Scholar]
- 15.Roesler J, Brenner S, Bukovsky AA, Whiting-Theobald N, Dull T, Kelly M et al. Third-generation, self-inactivating gp91(phox) lentivector corrects the oxidase defect in NOD/SCID mouse-repopulating peripheral blood-mobilized CD34+ cells from patients with X-linked chronic granulomatous disease. Blood 2002; 100: 4381–4390. [DOI] [PubMed] [Google Scholar]
- 16.Piacibello W, Bruno S, Sanavio F, Droetto S, Gunetti M, Ailles L et al. Lentiviral gene transfer and ex vivo expansion of human primitive stem cells capable of primary, secondary, and tertiary multilineage repopulation in NOD/SCID mice. Nonobese diabetic/severe combined immunodeficient. Blood 2002; 100: 4391–4400. [DOI] [PubMed] [Google Scholar]
- 17.Naumann N, De Ravin SS, Choi U, Moayeri M, Whiting-Theobald N, Linton GF et al. Simian immunodeficiency virus lentivector corrects human X-linked chronic granulomatous disease in the NOD/SCID mouse xenograft. Gene Therapy 2007; 14: 1513–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyoshi H, Smith KA, Mosier DE, Verma IM, Torbett BE. Transduction of human CD34+ cells that mediate long-term engraftment of NOD/SCID mice by HIV vectors. Science 1999; 283: 682–686. [DOI] [PubMed] [Google Scholar]
- 19.Sellers SE, Tisdale JF, Agricola BA, Donahue RE, Dunbar CE. The presence of the carboxy-terminal fragment of fibronectin allows maintenance of non-human primate long-term hematopoietic repopulating cells during extended ex vivo culture and transduction. Exp Hematol 2004; 32: 163–170. [DOI] [PubMed] [Google Scholar]
- 20.Tisdale JF, Hanazono Y, Sellers SE, Agricola BA, Metzger ME, Donahue RE et al. Ex vivo expansion of genetically marked rhesus peripheral blood progenitor cells results in diminished long-term repopulating ability. Blood 1998; 92: 1131–1141. [PubMed] [Google Scholar]
- 21.Kiem HP, Andrews RG, Morris J, Peterson L, Heyward S, Allen JM et al. Improved gene transfer into baboon marrow repopulating cells using recombinant human fibronectin fragment CH-296 in combination with interleukin-6, stem cell factor, FLT-3 ligand, and megakaryocyte growth and development factor. Blood 1998; 92: 1878–1886. [PubMed] [Google Scholar]
- 22.Williams DA, Rios M, Stephens C, Patel VP. Fibronectin and VLA-4 in haematopoietic stem cell-microenvironment interactions. Nature 1991; 352: 438–441. [DOI] [PubMed] [Google Scholar]
- 23.Dunbar CE. Gene transfer to hematopoietic stem cells: implications for gene therapy of human disease. Annu Rev Med 1996; 47: 11–20. [DOI] [PubMed] [Google Scholar]
- 24.Bukrinsky MI, Haggerty S, Dempsey MP, Sharova N, Adzhubel A, Spitz L et al. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature 1993; 365: 666–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abonour R, Williams DA, Einhorn L, Hall KM, Chen J, Coffman J et al. Efficient retrovirus-mediated transfer of the multidrug resistance 1 gene into autologous human long-term repopulating hematopoietic stem cells. Nat Med 2000; 6: 652–658. [DOI] [PubMed] [Google Scholar]
- 26.Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 2000; 288: 669–672. [DOI] [PubMed] [Google Scholar]
- 27.Kluge KA, Bonifacino AC, Sellers S, Agricola BA, Donahue RE, Dunbar CE. Retroviral transduction and engraftment ability of primate hematopoietic progenitor and stem cells transduced under serum-free versus serum-containing conditions. Mol Ther 2002; 5: 316–322. [DOI] [PubMed] [Google Scholar]
- 28.Millington M, Arndt A, Boyd M, Applegate T, Shen S. Towards a clinically relevant lentiviral transduction protocol for primary human CD34 hematopoietic stem/progenitor cells. PLoS One 2009; 4: e6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selvaggi TA, Walker RE, Fleisher TA. Development of antibodies to fetal calf serum with arthus-like reactions in human immunodeficiency virus-infected patients given syngeneic lymphocyte infusions. Blood 1997; 89: 776–779. [PubMed] [Google Scholar]
- 30.Yonemura Y, Ku H, Hirayama F, Souza LM, Ogawa M. Interleukin 3 or interleukin 1 abrogates the reconstituting ability of hematopoietic stem cells. Proc Natl Acad Sci USA 1996; 93: 4040–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bodine DM, Karlsson S, Nienhuis AW. Combination of interleukins 3 and 6 preserves stem cell function in culture and enhances retrovirus-mediated gene transfer into hematopoietic stem cells. Proc Natl Acad Sci USA 1989; 86: 8897–8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piacibello W, Gammaitoni L, Bruno S, Gunetti M, Fagioli F, Cavalloni G et al. Negative influence of IL3 on the expansion of human cord blood in vivo long-term repopulating stem cells. J Hematother Stem Cell Res 2000; 9: 945–956. [DOI] [PubMed] [Google Scholar]
- 33.Zandstra PW, Conneally E, Petzer AL, Piret JM, Eaves CJ. Cytokine manipulation of primitive human hematopoietic cell self-renewal. Proc Natl Acad Sci USA 1997; 94: 4698–4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunbar CE, Takatoku M, Donahue RE. The impact of ex vivo cytokine stimulation on engraftment of primitive hematopoietic cells in a non-human primate model. Ann NY Acad Sci 2001; 938: 236–244; [DOI] [PubMed] [Google Scholar]
- 35.Miller DG, Adam MA, Miller AD. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol 1990; 10: 4239–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karlsson S Treatment of genetic defects in hematopoietic cell function by gene transfer. Blood 1991; 78: 2481–2492. [PubMed] [Google Scholar]
- 37.Vigna E, Naldini L. Lentiviral vectors: excellent tools for experimental gene transfer and promising candidates for gene therapy. J Gene Med 2000; 2: 308–316. [DOI] [PubMed] [Google Scholar]
- 38.Geronimi F, Richard E, Redonnet-Vernhet I, Lamrissi-Garcia I, Lalanne M, Ged C et al. Highly efficient lentiviral gene transfer in CD34+ and CD34+/38—/lin— cells from mobilized peripheral blood after cytokine prestimulation. Stem Cells 2003; 21: 472–480. [DOI] [PubMed] [Google Scholar]
- 39.Higashimoto T, Grassman E, Cancelas JA, Malik P. Small but powerful, predicting the outcome of gene therapy trial using NSG Mice. Mol Ther 2010; 18 (Suppl 1): 203 (abstract 526). [Google Scholar]
- 40.Peters SO, Kittler EL, Ramshaw HS, Quesenberry PJ. Murine marrow cells expanded in culture with IL-3, IL-6, IL-11, and SCF acquire an engraftment defect in normal hosts. Exp Hematol 1995; 23: 461–469. [PubMed] [Google Scholar]
- 41.Herrera C, Sanchez J, Torres A, Bellido C, Rueda A, Alvarez MA. Early-acting cytokine-driven ex vivo expansion of mobilized peripheral blood CD34+ cells generates postmitotic offspring with preserved engraftment ability in non-obese diabetic/severe combined immunodeficient mice. Br J Haematol 2001; 114: 920–930. [DOI] [PubMed] [Google Scholar]
- 42.Malim MH. APOBEC proteins and intrinsic resistance to HIV-1 infection. Philos Trans R Soc Lond Ser B 2009; 364: 675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luban J Cyclophilin A, TRIM5, and resistance to human immunodeficiency virus type 1 infection. J Virol 2007; 81: 1054–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayakawa J, Joyal EG, Gildner JF, Washington KN, Phang OA, Uchida N et al. 5% dimethyl sulfoxide (DMSO) and pentastarch improves cryopreservation of cord blood cells over 10% DMSO. Transfusion 2010; 50: 2158–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanawa H, Kelly PF, Nathwani AC, Persons DA, Vandergriff JA, Hargrove P et al. Comparison of various envelope proteins for their ability to pseudotype lentiviral vectors and transduce primitive hematopoietic cells from human blood. Mol Ther 2002; 5: 242–251. [DOI] [PubMed] [Google Scholar]
- 46.Uchida N, Hanawa H, Dan K, Inokuchi K, Shimada T. Leukemogenesis of b2a2-type p210 BCR/ABL in a bone marrow transplantation mouse model using a lentiviral vector. J Nippon MedSch 2009; 76: 134–147. [DOI] [PubMed] [Google Scholar]
- 47.Uchida N, Washington KN, Hayakawa J, Hsieh MM, Bonifacino AC, Krouse AE et al. Development of an HIV1-based lentiviral vector that allows efficient transduction of both human and rhesus blood cells. J Virol 2009; 83: 9854–9862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.