Abstract
Volatile general anesthetics are used commonly in adults and children, yet their mechanisms of action are complex and the changes in single unit firing and synaptic activity that underlie the broad decreases in neuronal activity induced by these drugs have not been well characterized. Capturing such changes throughout the anesthesia process is important for comparing the effects of different anesthetics and gaining a better understanding of their mechanisms of action and their impact on different brain regions. Using chronically implanted electrodes in the rabbit somatosensory cortex, we compared the effects of two common general anesthetics, isoflurane and sevoflurane, on cortical neurons. Single unit activity and local field potentials (LFP) were recorded continuously before and during anesthetic delivery at 1 MAC, as well as during recovery. Our findings show that, although isoflurane and sevoflurane belong to the same class of volatile general anesthetics, their effects upon cortical single units and LFP were quite different. Overall, the suppression of neuronal firing was greater and more uniform under sevoflurane. Moreover, the changes in LFP frequency bands suggest that effect of anesthesia upon beta oscillations does not necessarily depend on the level of single unit activity, but rather on the changes in GABA/glutamate neurotransmission induced by each drug.
Keywords: Local field potentials, Beta oscillations, Volatile anesthetics, Single-neuron activity
1. Introduction
General anesthetics are used commonly in the course of surgery, imaging and other medical procedures in adults and children, yet the mechanisms responsible for their effects, as well as the neuronal changes that underlie them, are not entirely understood (Shushruth 2013). These drugs are known to affect the brain by decreasing firing frequency of a majority of neurons, primarily via interaction with GABA receptors but also via other receptors as well as potassium channels.
Isoflurane and sevoflurane, which are the most commonly used general anesthetics, have a similar effect on GABA receptors (Nishikawa and Harrison 2003) but differ in their effects on the glutamatergic system. Vinje et al. (Vinje et al. 2002) measured glutamate release fluorometrically in vitro using cerebral cortex preparations and found that sevoflurane reduced calcium-dependent glutamate release in a dose-dependent manner. Sevoflurane concentrations of 1.5, 2.5 and 4.0% reduced glutamate release by 58, 69 and 94%, respectively. Using a similar approach, Larsen et al. (Larsen et al. 1998) showed that isoflurane reduced calcium-dependent glutamate release in an inversely dose-dependent manner. Isoflurane concentrations of 0.5, 1.5 and 3.0% reduced glutamate release by 56, 43 and 36%, respectively. These findings suggest that at high doses isoflurane anesthesia has the potential to preserve excitatory activity to a greater degree than sevoflurane and therefore we would expect these two anesthetics to induce different degrees of suppression of neuronal firing.
In addition to their effects on receptors, it has been shown that volatile anesthetics act on two-pore-domain K+ channels (Patel et al. 1999; Franks and Lieb 1988) and can affect both the presynaptic and postsynaptic membranes. It is not clear, however, if sevoflurane and isoflurane have significantly different effects on the different types of these channels (Chae et al. 2010; Yao et al. 2017; Luethy et al. 2017).
Little is known about the effect of isoflurane and sevoflurane on the local field potentials which reflect primarily synaptic activity (Buzsaki, Anastassiou, and Koch 2012). A number of studies using EEG, which is believed to provide information about the same processes as LFP (Buzsaki, Anastassiou, and Koch 2012), have described the effects of individual anesthetics (Hight et al. 2017; Constant et al. 1999; Hagihira 2015). Typically, under general anesthesia the slower delta band increases whereas the faster gamma band, associated with inhibition/activation processes (for review see (Herrmann et al. 2016)), decreases. However, the direction of the anesthesia-induced changes in the beta band has not been consistent across published studies in the literature. Thus, it remains unclear whether the differential effect of these drugs on the glutamatergic system would be reflected in the LFP bands, as well as if single unit firing and LFP bands change in parallel under the conditions of anesthesia. In order to understand these differential effects, it is important to record both single unit activity and LFP in the same brain area throughout the awake and anesthetized states.
In this study we used chronically implanted electrodes in the somatosensory cortex to record continuously before and during anesthesia with either sevoflurane or isoflurane, as well as during recovery. This brain region represents one of the main components of the thalamocortical circuits which are important for anesthesia-induced loss of consciousness/unresponsiveness (Alkire et al., 2000), and the state of somatosensation is an indicator of depth of anesthesia. The somatosensory cortex has been examined previously in experiments using propofol (Ishizawa et al. 2016), and due to the uniformity of the effects of anesthesia on cerebral cortex (Ishizawa et al. 2016), it should provide a good representation of the behavior of the cortex as a whole.
Our results indicate that at an equivalent concentration of 1 MAC sevoflurane and isoflurane anesthesia produced significantly different effects upon single unit firing with sevoflurane producing greater suppression of single unit activity. Furthermore, the changes in LFP induced by each drug suggest that the effect of anesthesia upon the beta band does not depend on the level of single unit activity, but rather on the GABA/glutamate ratio, which is affected differently by these anesthetics.
2. Methods
2.1. Animal preparation.
Four adult female Dutch-belted rabbits (9-months old, 2–3 kg) were used in accordance with the National Institutes of Health guidelines and protocols approved by the NorthShore University HealthSystem Research Institute Institutional Animal Care and Use Committee. Rabbits were housed in standard stainless steel cages with water and food ad lib. Animals were chronically implanted with electrodes, as reported previously (Aksenov et al. 2015). For this procedure, animals were anesthetized with a mixture of ketamine (60 mg/kg) and xylazine (10 mg/kg). The recording assembly consisted of a silica tube (Polymicro Technologies, Phoenix, AZ) containing a bundle of four 25μm diameter gold-silver alloy microwires with formvar insulation (California Fine Wire, Grover Beach, CA). These electrodes terminated at different levels within a distance of 100 μm. The microwires were connected to a small 6-pin connector that was embedded in dental acrylic. A 150 μm silver wire was placed between the skull and dura mater for grounding. During surgery, lambda was positioned 1.5 mm below bregma and the stereotaxic coordinates were as follows: anterior-posterior was 2 mm dorsal to bregma, medial-lateral was 6 mm from midline, and dorsal-ventral was under visual control. After implantation the electrode assembly was cemented to the skull using dental acrylic and nylon support screws. After recovery from surgery each subject was habituated for at least 3–5 days prior to the experiments. The rabbits were restrained by means of a cloth sleeve and secured in an acrylic imaging cradle by Velcro straps. The electrodes were advanced to layer IV and their location was confirmed using anatomical MR imaging.
2.2. Experimental design.
All experiments were performed beginning at least 14 days after surgery. The animal preparation has been described previously (Wyrwicz et al. 2000). Briefly, each rabbit was secured in a cloth bag and the head was fixed to an acrylic cradle. For the experiments the rabbits were placed inside the magnet wearing earplugs and the lights were off. Rabbits were habituated to this environment prior to experiments. Spontaneous respiration was measured using a pressure pad/respiration transducer (TSD110) (Biopac Systems, Inc, Goleta, CA, USA). Single unit activity and LFP was recorded continuously, first in the awake state for 2 min. Rabbits then were anesthetized with either isoflurane (Piramal Enterprises Limited, Kohir Mandal, Andhra Pradesh, India) or sevoflurane (AbbVie Inc., North Chicago, IL, USA) at 1 MAC via a custom mask using calibrated vaporizers (Drager Vapor 19.1). The level of 1 MAC was reported previously in rabbits and was on average 2% isoflurane (Drummond 1985) and 4% sevoflurane (Yin, Yan, and Zhu 2012). Prior to experiments, we used toe pinch test across the entire duration of the experiment to confirm that the anesthesia level of 1 MAC does not substantially change even if the respiration rate varies. Anesthesia was delivered for 6 min in air, then for 6 min in 80% oxygen, and finally in air again for 6 min. Anesthetic delivery was tested in air as well as in combination with 80% O2 because anesthesia is often delivered in higher-than-air oxygen concentration and it has been shown that hyperoxia can affect neuronal activity in the awake state (Sheng et al. 2017). Anesthesia was then stopped and recordings continued during the period of recovery for 20 minutes. Each rabbit was exposed to isoflurane and sevoflurane on different days (at least 3 days between experiments) using this paradigm.
2.3. Electrophysiological recording.
The multiple signals from the microwires were fed through a miniature preamplifier to a multichannel differential amplifier system (Neuralynx Inc, Bozeman, Montana, USA). The signals were amplified, band-pass–filtered (300Hz to 3 kHz for single units and 1–150 Hz for local field potentials), and digitized (32 kHz/channel) using a Neuralynx data acquisition system. Unit discrimination was performed offline using threshold detection followed by a cluster analysis of individual action potential wave shapes using Neuralynx analysis software. After setting the threshold using CscSpikeExtractor, the data were converted into a SpikeSort 3D datafile. The criteria for the threshold was 3× an estimate of the standard deviation of the background noise (Quiroga, Nadasdy, and Ben-Shaul 2004). Spike sorting was performed automatically, using KlustaKwik, which is incorporated into SpikeSort 3D. KlustaKwik analysis was followed by manual adjustments of the clusters. We evaluated the interspike interval to ensure that it is no less than 1 ms. We attempted to sort single units into pyramidal and interneuron cell groups based on the criteria described by Swadlow in the somatosensory cortex of awake rabbits (Swadlow 1989). It was shown that spontaneous firing rate for interneurons was greater than 2 spikes/s and the majority of interneurons had an action potential duration less than 0.6 ms, whereas efferent neurons did not have an action potential duration less than 0.5 ms. Since we could not be sure that we were recording the same single units across days between isoflurane and sevoflurane experiments, we analyzed single units separately for isoflurane and sevoflurane. LFP data were analyzed using Sigview spectrum analyzer FFT-based signal analysis software (SignalLab e.K., Germany). For each phase of exposure, the data were analyzed using the last minute of recording prior to the change in anesthesia or inspired oxygen concentration.
Electrode locations were confirmed by MRI using a 9.4T imaging spectrometer (BioSpec 94/30USR, Bruker Biospin MRI GmbH) operating at 1H frequency of 400MHz. The spectrometer was equipped with an actively-shielded gradient coil (BFG-240–150-S-7, Research Resonance, Inc., Billerica, MA, USA). A single-turn, 20mm-diameter circular RF surface coil was used for both transmission and reception. Prior to each experiment, anatomical images were acquired using a multislice gradient echo pulse sequence with a TR of 1.5 s, a TE of 10 ms, a 30mm×30mm FOV, and a matrix size of 128×128, corresponding to an in-plane resolution of 234 μm × 234 μm.
2.4. Statistical analysis.
Single unit firing was averaged during the last minute of each phase of the experiment (i.e., pre-anesthesia, anesthesia in air, 80% O2, and air once again, and then recovery), and these values were used for statistical comparison. The recovery of individual single units was evaluated 20 min after the end of anesthesia. A unit was considered recovered if the spiking rate reached the mean minus 2×SEM of single unit activity in the awake state. Paired t-test was used to analyze changes in single unit activity, LFP power spectra and respiration. One-way ANOVA (Statistica, StatSoft, Tulsa, OK) was used to compare differences in the normalized single unit activity between isoflurane and sevoflurane since the same set of single units cannot be recorded in experiments separated by days. Values were normalized to the pre-anesthesia level. The data are presented as mean +/− SEM unless otherwise specified.
3. Results
Each anesthetic produced distinct effects upon single unit activity in the cortex. Figure 1 shows the responses of selected single units before, during anesthesia and recovery to illustrate the range of temporal behaviors that were observed for each drug. In all cases neuronal firing decreased rapidly and reached a plateau within 5 minutes from the start of anesthetic delivery. Isoflurane anesthesia (Fig. 1a) typically resulted in less suppression of neuronal firing compared to sevoflurane (Fig. 1b). For both anesthetics most units recovered to their pre-anesthesia levels (11 of 16 units for isoflurane and 16 of 20 units for sevoflurane) within 20 minutes after the end of drug delivery, although the recovery was smoother in the case of sevoflurane. However, some cells (Fig. 1c,d) showed a slower rate of recovery (5 units for isoflurane and 4 units for sevoflurane). In addition, a subset of recorded units were less affected by each drug (Fig. 1e,f) in terms of suppression of neuronal firing (for isoflurane 3 units did not decrease below 40% of the initial level during the second time point (i.e., the first exposure to anesthesia in air) and for sevoflurane 5 units did not decrease below 20% of initial level during second time point). No difference in firing frequency was observed for anesthetic delivery in air vs. 80% oxygen.
Figure 1:
Temporal behavior of selected single units. Examples of individual single units were chosen to illustrate the differences in suppression and recovery that were observed. The bars show the single unit firing averaged for each minute of the experiment before anesthesia, during anesthetic delivery in air (yellow shaded) and 80% O2 (red shaded), and during recovery. In all cases neuronal activity decreased to a steady-state plateau within the first 5 minutes of drug delivery. Most single units recovered nearly to the pre-anesthesia level within approximately 20 minutes. For most recorded cells isoflurane (a) produced a shallower suppression of neuronal firing and a less consistent recovery compared to sevoflurane (b). Some units (c, d) did not completely recover to the pre-anesthesia level after the end of anesthetic delivery. A subset of units for both isoflurane (e) and sevoflurane (f) showed a considerably smaller decrease in neuronal firing under anesthesia.
Figure 2 shows the firing rates for all recorded single units at each phase of the experiment for groups corresponding to isoflurane (Fig. 2a,c) and sevoflurane (Fig. 2b,d). The log scale (Figure 2 c–d) is presented to visualize more clearly the behavior of the slow-firing single units. The values represent the averaged single unit firing rate during the last minute of recording for each phase. In the pre-anesthesia phase recorded cells displayed a range of firing rates, up to approximately 7–8 spikes/s; the distribution of firing rates was similar for the isoflurane and sevoflurane groups. Four interneurons were assigned for isoflurane (the average action potential duration for these cells was 0.31±0.03 ms) and five for sevoflurane (the average action potential duration was 0.34±0.02 ms). During anesthesia both drugs decreased the firing rate for all units to below approximately 2 spikes/s. The anesthesia effects on interneurons did not depend on their firing rate in the awake state. The magnitude of decrease was not proportional to the initial firing rate, and no difference was evident between anesthetic delivered in air vs. 80% O2. The suppression of single unit firing was greater and more uniform under sevoflurane. Notably, during sevoflurane anesthesia 4 out of 20 recorded single units showed no firing for 2 or more minutes, whereas for isoflurane no units ceased firing for more than 1 minute. Following the end of sevoflurane delivery, most single units recovered nearly to their pre-anesthesia firing rates after 20 minutes. For isoflurane, in contrast, the recovery was less consistent, with a number of cells remaining well below their initial firing rates.
Figure 2:
Effect of anesthesia on single unit firing. The firing rates of all recorded single units are shown for isoflurane (a) and sevoflurane (b), corresponding to the last minute of each phase before, during and after anesthesia. Cells are arranged from lowest to highest firing rate. Interneurons are marked with a black circle on the top of the figure. The colored lines serve as a visual guide to illustrate the level of activity across all units for each phase. In the awake state prior to anesthesia, both groups show a similar distribution of single unit activity. Neuronal activity decreased for all cells during anesthesia in air and 80% O2, to a degree that was not necessarily proportional to the initial firing rate. The logarithmic scale shows changes in low-frequency single units in greater detail (c, d). Note that zero values for firing rate were replaced with 0.01 to prevent missing points on the log scale. For each phase of the experiment single unit firing was averaged across all recorded cells for isoflurane (e) and sevoflurane (f) groups. The graph shows the average for all cells as well as average of interneurons (IN) and pyramidal cells (PC) separately. Means were calculated from averaging the firing rates during the last minute of each phase, and were normalized to the pre-anesthesia level. For both drugs, single unit activity decreased significantly and remained at a steady-state level under anesthesia, and then returned to near the pre-anesthesia level within 20 minutes. The decrease in firing under sevoflurane was significantly greater than under isoflurane. There was no significant difference between anesthesia delivery in air or 80% O2 for either drug, with the exception of an ongoing decrease for PC under sevoflurane. Asterisks indicate significant changes between the awake state and the initial phase of anesthesia, as well as the final phase of anesthesia and recovery.
The mean single unit activity was compared at each phase for both anesthetics (Figure 2 e–f) to assess quantitatively the changes in neuronal firing. The values were calculated at the plateau level by averaging the single unit firing during the last minute of recording for each phase and were normalized to the pre-anesthesia level. Isoflurane (Fig. 2e) greatly decreased mean single unit activity (N=16) to 24±5.3% of the awake level (p<0.001) when delivered in air. Delivery in 80% oxygen produced no further change in single unit activity. Activity significantly recovered (p<0.001) to 91±22.6% of the pre-anesthesia level 20 minutes after the end of isoflurane delivery, at which point activity was not significantly different from the pre-anesthesia level.
Sevoflurane delivered in air (Fig. 2f) produced a deeper suppression of single unit activity than isoflurane. Mean single unit activity decreased to 11±3.2% of the awake level (p<0.0005), and like isoflurane increasing the inspired oxygen concentration to 80% resulted in no further change in single unit activity. However, the activity of pyramidal cells continued to decrease during the course of sevoflurane. Activity was 14.1±4.4% of the awake level at the second time point, 10.13±3.2% at the third time point and 5.7±1.8% at the fourth time point (p<0.044 comparing the third and fourth time points and p<0.003 comparing second and fourth time points). Comparing the last time points of anesthesia for each anesthetic, one-way ANOVA showed that sevoflurane decreased single unit activity significantly more than isoflurane (F= 15.38, p< 0.0004). Twenty minutes after the end of sevoflurane delivery single unit activity significantly recovered (p<0.0007) to 93±24.6% of the pre-anesthesia level, which is not statistically different from the pre-anesthesia level.
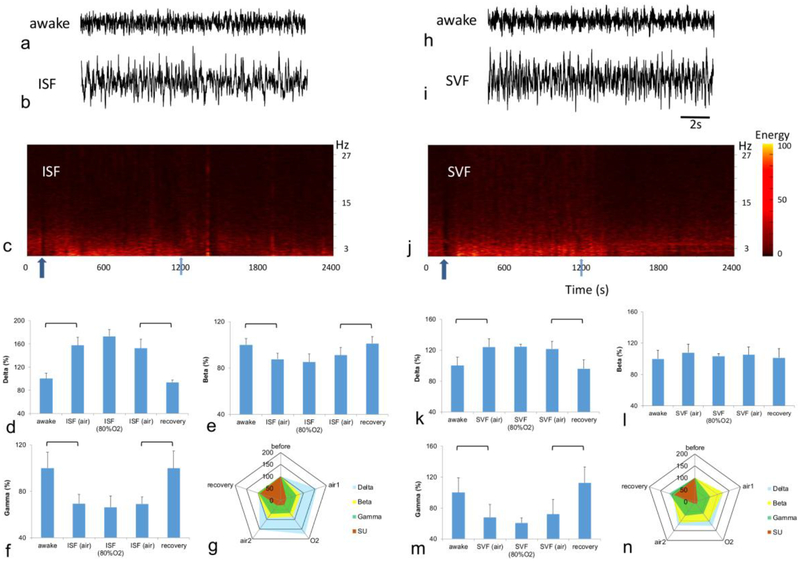
Both anesthetics affected the power spectra of LFP (Fig. 3). Examples of individual recordings in the awake state and during anesthesia are shown for isoflurane (Fig. 3a,b) and sevoflurane (Fig. 3h,i) as well as spectrograms (Fig. 3c,j). Isoflurane increased delta oscillations to 157±13.9% of the awake level (p<0.019, Fig. 3d) and decreased gamma oscillations to 69±8.2% of the awake level (p<0.025, Fig. 3f). Beta oscillations also decreased mainly due to changes in the high frequency beta range. The decrease in this range was 85±4.3% of the awake level (p<0.018, Fig. 3e). Alpha and theta bands did not change. Increasing the inspired oxygen concentration to 80% resulted in no further change in any frequency range.
Figure 3:
Effect of anesthesia on LFP. The examples of LFP recording are shown for both isoflurane (ISF) (a, b) and sevoflurane (SVF) (h, i). Note that both drugs visibly increased low-frequency activity. The power spectra of LFP are shown for both isoflurane (c) and sevoflurane (j). Arrows indicate the onset (dark blue) and termination (light blue) of anesthesia delivery. Under isoflurane, anesthesia delta oscillations (d) increased, whereas beta (e) and gamma (f) oscillations decreased. The circular plot (g) compares changes in averaged single unit activity with changes in LFP band over all five timepoints of isoflurane. Sevoflurane produced significant changes in delta (k) and gamma oscillations (m). No change was recorded in beta oscillations (l) under sevoflurane. There was no significant difference between anesthesia delivery in the air or 80% O2 for either drug. The circular plot (n) compares changes in averaged single unit activity with changes in LFP band over all five timepoints of sevoflurane. Brackets indicate statistical significance.
Sevoflurane increased delta oscillations to 124±10.6% of the awake level (p<0.017, Fig. 3k) and decreased gamma oscillations to 67±16.6% of the awake level (p<0.022, Fig. 3m). In contrast to isoflurane, beta oscillations were not affected, and no decrease was observed (Fig. 3l). Alpha and theta bands did not change. Increasing the inspired oxygen concentration to 80% produced no further change in any frequency range. The circular plots emphasize the difference of isoflurane and sevoflurane effects on single units relative to the changes in LFP bands (Fig. 3g, n).
The respiratory rate decreased for both anesthetics within 1 min after the start of delivery. The respiratory rate was 59.5±5.9 cpm before isoflurane delivery and decreased to 42.5±3.2 cpm during delivery of isoflurane in air (p<0.01). During delivery in 80% O2 the respiratory rate was 37.7±4.4 cpm. During subsequent delivery of isoflurane in air the respiratory rate was 44±3.6 cpm. For sevoflurane the respiratory rate was 63.5±7.4 cpm in the pre-anesthesia phase and decreased to 41.5±4.0 cpm during delivery in air (p<0.02). During delivery in 80% O2 the respiratory rate decreased to 31.7±3.5 cpm (p<0.015). For subsequent delivery of sevoflurane in air the respiratory rate was 40±4.6 cpm (p<0.02). Respiration fully returned to normal within 10 min of the end of delivery for both anesthetics.
4. Discussion
Although there was some variation in the response of individual single units for each drug, as illustrated in Figure 1, our results show that for most cells sevoflurane produced a greater suppression of single unit activity than isoflurane, which was reflected in the mean firing rates during anesthesia. These findings are in agreement with previous in vitro studies (Vinje et al. 2002; Larsen et al. 1998) which showed that at high doses isoflurane anesthesia preserves excitatory activity to a greater degree than sevoflurane due to different effects on glutamatergic receptors. The recorded single units show a range of spontaneous firing rates reflecting the functional diversity of cortical neurons (Douglas and Martin 2004). Our findings demonstrate that the suppression of neuronal firing by the two anesthetics does not depend on the initial firing rate. However, the magnitude of suppression was greater for the cells with higher firing frequencies which may reflect variation in the density of GABA receptors on neurons even within the same brain area (Christie, Miralles, and De Blas 2002). Despite its deeper level of suppression, sevoflurane also produced a visibly faster recovery compared to isoflurane. It has been shown that sevoflurane has a lower solubility than isoflurane and consequently a faster uptake and elimination (Stachnik 2006), which likely explains this behavior.
Our results show that both isoflurane and sevoflurane increase slower delta oscillations and decrease gamma oscillations, which was expected based on previous EEG findings in the literature (Hagihira 2015; Purdon et al. 2015), but that only isoflurane decreases beta waves. Previous studies reported both decreases (Freye, Bruckner, and Latasch 2004; Schwender et al. 1998; Sitdikova et al. 2014) and increases (Akeju et al. 2014) in the power of beta oscillations induced by volatile anesthetics. Benzodiazepines with GABA-receptor modulation properties have been shown to increase beta activity (van Lier et al. 2004), and it has been suggested that the power of beta waves is associated with GABA neurotransmission. However, it has been shown recently that beta oscillations can be increased by the glutamate antagonist ketamine (Anderson et al. 2017). Since both isoflurane and sevoflurane are believed to have similar effects on GABA neurotransmission (Nishikawa and Harrison 2003) but different glutamatergic effects, our results indicate that beta oscillations may reflect the ratio of GABA to glutamate, which is affected differently by isoflurane and sevoflurane, and not merely GABA alone. Alternatively, these changes in the LFP bands could reflect the differential effects of isoflurane and sevoflurane on two-pore-domain potassium channels. However, a physiological difference in their effects has not been clearly established by direct comparison.
Multiple studies have reported the burst-suppression phenomenon under deep levels of anesthesia (Hudetz and Imas 2007; Hartikainen, Rorarius, Makela, Perakyla, et al. 1995; Sitdikova et al. 2014; Hartikainen, Rorarius, Makela, Yli-Hankala, et al. 1995). We did not observe this phenomenon. The absence of this activity is likely related to the 1 MAC anesthesia level, as burst-suppression typically requires more than 1 MAC (Akrawi et al. 1996; Williams et al. 2016; Hartikainen, Rorarius, Makela, Yli-Hankala, et al. 1995). Moreover, burst-suppression occurs due to external stimulation (Hudetz and Imas 2007; Kroeger and Amzica 2007), which was largely excluded by our experimental setup.
As the alveolar concentration of anesthesia is determined by the inspired amount of anesthetic within a given time, it depends upon the rate of respiration, which can change according to the anesthetic concentration as well as the accompanying concentration of oxygen when mechanical ventilation is not employed. For our experiments the respiration rate decreased within 1 minute of the start of anesthesia delivery for both drugs along with single unit activity. Surgical protocols often involve the delivery of anesthesia at a higher-than-air oxygen concentration to avoid hypoxia, and no difference was observed in the steady-state suppression of single units during anesthesia in air vs. 80% O2, despite the differences in spontaneous respiration. Thus, under these conditions, any changes in brain oxygenation associated with anesthesia exposure did not appear to impact single unit firing or LFP during the time frame of the experiments. The fact that the neuronal activity remained unchanged despite the decrease in respiration rate during 80% O2 indicates that respiratory regulation by carotid bodies and the respiratory center (Prabhakar and Semenza 2015) is at least partially preserved during isoflurane and sevoflurane anesthesia.
In conclusion, differences between isoflurane and sevoflurane in their effects on neuronal activity have been observed previously using in vitro electrophysiology and reported from human clinical EEG studies. This study is the first to compare these two anesthetics in vivo during continuous recording of cortical single units and LFP during the awake, anesthesia and complete recovery phases. Sevoflurane produced deep, uniform suppression of neuronal firing and, in contrast to isoflurane, did not affect LFP beta oscillations despite its greater decrease in single unit firing. This behavior suggests that beta oscillations do not necessarily reflect the level of neuronal firing and instead may depend not only on GABA neurotransmission but also on the GABA/glutamate neurotransmission ratio, which will be different for these two anesthetics.
Funding
This work was supported by National Institute of General Medical Sciences (R01GM112715)
Footnotes
Conflict of interest
All authors declared no conflict of interest.
References
- Akeju O, Westover MB, Pavone KJ, Sampson AL, Hartnack KE, Brown EN, and Purdon PL. 2014. ‘Effects of sevoflurane and propofol on frontal electroencephalogram power and coherence’, Anesthesiology, 121: 990–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akrawi WP, Drummond JC, Kalkman CJ, and Patel PM. 1996. ‘A comparison of the electrophysiologic characteristics of EEG burst-suppression as produced by isoflurane, thiopental, etomidate, and propofol’, J Neurosurg Anesthesiol, 8: 40–6. [DOI] [PubMed] [Google Scholar]
- Aksenov DP, Li L, Miller MJ, Iordanescu G, and Wyrwicz AM. 2015. ‘Effects of anesthesia on BOLD signal and neuronal activity in the somatosensory cortex’, J Cereb Blood Flow Metab, 35: 1819–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson PM, Jones NC, O’Brien TJ, and Pinault D. 2017. ‘The N-Methyl d-Aspartate Glutamate Receptor Antagonist Ketamine Disrupts the Functional State of the Corticothalamic Pathway’, Cereb Cortex, 27: 3172–85. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Anastassiou CA, and Koch C. 2012. ‘The origin of extracellular fields and currents--EEG, ECoG, LFP and spikes’, Nat Rev Neurosci, 13: 407–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae YJ, Zhang J, Au P, Sabbadini M, Xie GX, and Yost CS. 2010. ‘Discrete change in volatile anesthetic sensitivity in mice with inactivated tandem pore potassium ion channel TRESK’, Anesthesiology, 113: 1326–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie SB, Miralles CP, and De Blas AL. 2002. ‘GABAergic innervation organizes synaptic and extrasynaptic GABAA receptor clustering in cultured hippocampal neurons’, J Neurosci, 22: 684–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constant I, Dubois MC, Piat V, Moutard ML, McCue M, and Murat I. 1999. ‘Changes in electroencephalogram and autonomic cardiovascular activity during induction of anesthesia with sevoflurane compared with halothane in children’, Anesthesiology, 91: 1604–15. [DOI] [PubMed] [Google Scholar]
- Douglas RJ, and Martin KA. 2004. ‘Neuronal circuits of the neocortex’, Annu Rev Neurosci, 27: 419–51. [DOI] [PubMed] [Google Scholar]
- Drummond JC 1985. ‘MAC for halothane, enflurane, and isoflurane in the New Zealand white rabbit: and a test for the validity of MAC determinations’, Anesthesiology, 62: 336–8. [DOI] [PubMed] [Google Scholar]
- Franks NP, and Lieb WR. 1988. ‘Volatile general anaesthetics activate a novel neuronal K+ current’, Nature, 333: 662–4. [DOI] [PubMed] [Google Scholar]
- Freye E, Bruckner J, and Latasch L. 2004. ‘No difference in electroencephalographic power spectra or sensory-evoked potentials in patients anaesthetized with desflurane or sevoflurane’, Eur J Anaesthesiol, 21: 373–8. [DOI] [PubMed] [Google Scholar]
- Hagihira S 2015. ‘Changes in the electroencephalogram during anaesthesia and their physiological basis’, Br J Anaesth, 115 Suppl 1: i27–i31. [DOI] [PubMed] [Google Scholar]
- Hartikainen K, Rorarius M, Makela K, Perakyla J, Varila E, and Jantti V. 1995. ‘Visually evoked bursts during isoflurane anaesthesia’, Br J Anaesth, 74: 681–5. [DOI] [PubMed] [Google Scholar]
- Hartikainen K, Rorarius M, Makela K, Yli-Hankala A, and Jantti V. 1995. ‘Propofol and isoflurane induced EEG burst suppression patterns in rabbits’, Acta Anaesthesiol Scand, 39: 814–8. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Struber D, Helfrich RF, and Engel AK. 2016. ‘EEG oscillations: From correlation to causality’, Int J Psychophysiol, 103: 12–21. [DOI] [PubMed] [Google Scholar]
- Hight D, Voss LJ, Garcia PS, and Sleigh J. 2017. ‘Changes in Alpha Frequency and Power of the Electroencephalogram during Volatile-Based General Anesthesia’, Front Syst Neurosci, 11: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudetz AG, and Imas OA. 2007. ‘Burst activation of the cerebral cortex by flash stimuli during isoflurane anesthesia in rats’, Anesthesiology, 107: 983–91. [DOI] [PubMed] [Google Scholar]
- Ishizawa Y, Ahmed OJ, Patel SR, Gale JT, Sierra-Mercado D, Brown EN, and Eskandar EN. 2016. ‘Dynamics of Propofol-Induced Loss of Consciousness Across Primate Neocortex’, J Neurosci, 36: 7718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger D, and Amzica F. 2007. ‘Hypersensitivity of the anesthesia-induced comatose brain’, J Neurosci, 27: 10597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen M, Valo ET, Berg-Johnsen J, and Langmoen IA. 1998. ‘Isoflurane reduces synaptic glutamate release without changing cytosolic free calcium in isolated nerve terminals’, Eur J Anaesthesiol, 15: 224–9. [PubMed] [Google Scholar]
- Luethy A, Boghosian JD, Srikantha R, and Cotten JF. 2017. ‘Halogenated Ether, Alcohol, and Alkane Anesthetics Activate TASK-3 Tandem Pore Potassium Channels Likely through a Common Mechanism’, Mol Pharmacol, 91: 620–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa K, and Harrison NL. 2003. ‘The actions of sevoflurane and desflurane on the gamma-aminobutyric acid receptor type A: effects of TM2 mutations in the alpha and beta subunits’, Anesthesiology, 99: 678–84. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Lesage F, Fink M, Romey G, and Lazdunski M. 1999. ‘Inhalational anesthetics activate two-pore-domain background K+ channels’, Nat Neurosci, 2: 422–6. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, and Semenza GL. 2015. ‘Oxygen Sensing and Homeostasis’, Physiology (Bethesda), 30: 340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdon PL, Sampson A, Pavone KJ, and Brown EN. 2015. ‘Clinical Electroencephalography for Anesthesiologists: Part I: Background and Basic Signatures’, Anesthesiology, 123: 937–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroga RQ, Nadasdy Z, and Ben-Shaul Y. 2004. ‘Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering’, Neural Comput, 16: 1661–87. [DOI] [PubMed] [Google Scholar]
- Schwender D, Daunderer M, Klasing S, Finsterer U, and Peter K. 1998. ‘Power spectral analysis of the electroencephalogram during increasing end-expiratory concentrations of isoflurane, desflurane and sevoflurane’, Anaesthesia, 53: 335–42. [DOI] [PubMed] [Google Scholar]
- Sheng M, Liu P, Mao D, Ge Y, and Lu H. 2017. ‘The impact of hyperoxia on brain activity: A resting-state and task-evoked electroencephalography (EEG) study’, PLoS One, 12: e0176610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shushruth S 2013. ‘Exploring the neural basis of consciousness through anesthesia’, J Neurosci, 33: 1757–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitdikova G, Zakharov A, Janackova S, Gerasimova E, Lebedeva J, Inacio AR, Zaynutdinova D, Minlebaev M, Holmes GL, and Khazipov R. 2014. ‘Isoflurane suppresses early cortical activity’, Ann Clin Transl Neurol, 1: 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan MH, Conard PF, Karsunky PK, and Gross JB. 1996. ‘Sevoflurane versus isoflurane: induction and recovery characteristics with single-breath inhaled inductions of anesthesia’, Anesth Analg, 82: 528–32. [DOI] [PubMed] [Google Scholar]
- Stachnik J 2006. ‘Inhaled anesthetic agents’, Am J Health Syst Pharm, 63: 623–34. [DOI] [PubMed] [Google Scholar]
- Swadlow HA. 1989. ‘Efferent neurons and suspected interneurons in S-1 vibrissa cortex of the awake rabbit: receptive fields and axonal properties’, J Neurophysiol, 62: 288–308. [DOI] [PubMed] [Google Scholar]
- van Lier H, Drinkenburg WH, van Eeten YJ, and Coenen AM. 2004. ‘Effects of diazepam and zolpidem on EEG beta frequencies are behavior-specific in rats’, Neuropharmacology, 47: 163–74. [DOI] [PubMed] [Google Scholar]
- Vinje ML, Moe MC, Valo ET, and Berg-Johnsen J. 2002. ‘The effect of sevoflurane on glutamate release and uptake in rat cerebrocortical presynaptic terminals’, Acta Anaesthesiol Scand, 46: 103–8. [DOI] [PubMed] [Google Scholar]
- Williams DC, Aleman MR, Brosnan RJ, Fletcher DJ, Holliday TA, Tharp B, Kass PH, Steffey EP, and LeCouteur RA. 2016. ‘Electroencephalogram of Healthy Horses During Inhaled Anesthesia’, J Vet Intern Med, 30: 304–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrwicz AM, Chen N-K, Li L, Weiss C, and Disterhoft JF. 2000. ‘fMRI of visual system activation in the conscious rabbit’, Magn Res Med, 44: 474–78. [DOI] [PubMed] [Google Scholar]
- Yao C, Li Y, Shu S, Yao S, Lynch C, Bayliss DA, and Chen X. 2017. ‘TASK channels contribute to neuroprotective action of inhalational anesthetics’, Sci Rep, 7: 44203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Yan M, and Zhu T. 2012. ‘Minimum alveolar concentration of sevoflurane in rabbits with liver fibrosis’, Anesth Analg, 114: 561–5. [DOI] [PubMed] [Google Scholar]