Abstract
Purpose:
Dysphagia is a common problem after stroke that is often associated with tongue weakness. However, the physiological mechanisms of post-stroke tongue muscle weakness and optimal treatments have not been established. To advance understanding of physiological mechanisms of post stroke dysphagia, we sought to validate the unilateral transient middle cerebral artery occlusion (MCAO) rat model of ischemic stroke as a translational model of post stroke dysphagia. Our goal was to establish clinically relevant measures and chronicity of functional deficits; criteria that increase the likelihood that findings will translate to the clinic. We hypothesized that MCAO would cause tongue weakness and functional swallowing changes.
Methods:
Maximum voluntary tongue forces and videofluoroscopic swallowing studies were collected in 8-week old male Sprague-Dawley rats prior to receiving either a left MCAO (N = 10) or sham (N = 10) surgery. Tongue forces and VFSS were reassessed at 1 and 8 weeks post-surgery.
Results:
Maximum voluntary tongue force, bolus area, and bolus speed were significantly reduced in the MCAO group at the 1 and 8-week timepoints.
Conclusion:
Clinically relevant changes to swallowing and tongue force support the use of the MCAO rat model as a translational model of post stroke dysphagia. This model will allow for future studies to improve our understanding of the physiology contributing to these functional changes as well as the impact of therapeutic interventions on physiological targets and function.
Keywords: Stroke, Swallowing, Dysphagia, Rat, Model, Tongue
1. Introduction
Dysphagia occurs in 20–80% of people following stroke (Arnold et al., 2016; Bath et al., 2018; Suntrup-Krueger et al., 2017) and is associated with poor health outcomes and increased health care costs (Attrill et al., 2018; Cohen et al., 2016; Geeganage et al., 2012; Rofes et al., 2018). Tongue strengthening, neuromuscular stimulation, and other approaches may have potential to improve swallowing function (Park et al., 2015; Kim et al., 2017; Steele et al., 2016; Rogus-Pulia and Robbins, 2013; Cabib et al., 2016), but more evidence is needed to established a standard treatment for post stroke dysphagia (Bath et al., 2018; Cohen et al., 2016; Suntrup-Krueger et al., 2018).
For any potential therapy, there is a wide range of possible treatment parameters that require optimization. However, the necessary clinical studies to perform such optimization are often limited by small sample sizes, variability in lesion characteristics, lack of controls, and restrictions on the type and number of swallowing assessments that can be safely performed. To provide an ethical framework for asking good clinical questions, an animal model of post stroke dysphagia would allow preclinical testing of hypotheses for later study in randomized clinical trials. In addition, an animal model would advance our understanding of the physiological mechanisms of post stroke dysphagia (German et al., 2017).
Unilateral ischemia, the most common type of stroke (Mozaffarian et al., 2016), frequently results in dysphagia (Barer, 1989; Marian et al., 2017; Remesso et al., 2011; Robbins et al., 1993; Wilmskoetter et al., 2018) and contralateral tongue weakness, which can be observed as deviation of the tongue towards the contralateral side when protruded (Umapathi et al., 2000). The middle cerebral artery occlusion (MCAO) rat model of stroke induces a unilateral transient focal ischemia and has been widely used for decades to study the neural effects of ischemia (Bederson et al., 1986; Macrae, 2011; Tamura et al., 1981). The MCAO rat model is potentially useful for studying post stroke dysphagia because MCAO rats under anesthesia exhibit a delay in swallowing initiation and a reduced number of swallows in response to an infusion of water (Sugiyama et al., 2014). Reductions in tongue protrusion (Gulyaeva et al., 2003) and licking efficiency (Ahmed et al., 2017) have also been reported in this model. However, clinically relevant measures are needed to improve translation from animal models to human studies (Pankevich et al., 2013). Therefore, we sought to validate this animal model by determining whether it exhibits clinically relevant changes in swallowing and tongue function and whether these deficits persist chronically, as chronicity of functional impairment has been identified as a key feature of human stroke that must be incorporated in an animal model to avoid translational failure (Corbett et al., 2015).
The tongue has a prominent role in the oral phase of swallowing including bolus formation, manipulation, and propulsion. Reduced tongue forces occur after stroke in humans (Clark Heather M., 2003; Hori et al., 2005), have been directly related to swallowing problems (Hirota et al., 2010; Konaka et al., 2010; Lee et al., 2016), and are targeted by several promising interventions (Kim et al., 2017; Park et al., 2015; Robbins et al., 2007; Steele et al., 2016). Key features of post stroke dysphagia that have been reported in videofluoroscopic swallowing studies (VFSS), considered the gold standard of clinical swallowing diagnostic tools, include weaker tongue bolus propulsion forces, reduced bolus speed, delayed closure of the laryngeal vestibule, prolonged transit times, delayed hyoid excursion, and pharyngeal bolus residue (Oommen et al., 2011; Terré and Mearin, 2006; Vilardell et al., 2017; Wilmskoetter et al., 2019a).
Our primary hypothesis was that MCAO in the rat would reduce voluntary tongue force at both 1 week and 2 months after ischemia. We also hypothesized that tongue pressing rate, a secondary measure of lingual function, would be reduced by MCAO. Additionally, we sought to identify altered swallowing function by using VFSS. We hypothesized that MCAO rats would develop changes to swallowing function assessed by VFSS including reductions in bolus speed, bolus area, swallowing rate, and mastication rate. Previous studies found these rat VFSS measures to be successful at detecting deficits in swallowing function with age and neurological deficits (Kletzien et al., 2019; Russell et al., 2013). Body weight was also assessed as weight loss with MCAO has frequently been reported (Palmer et al., 2001; Parkkinen et al., 2013; Yuan et al., 2014).
2. Results
Repeated measures MANOVA results indicated a significant interaction between timepoint (Baseline, 1 week, and 8 weeks) and treatment group (MCAO and Sham) across all 7 dependent variables (Pillai’s Trace = 0.681, F(14,62) = 2.289, p = 0.013). Multivariate contrasts indicated significant differences between MCAO and Sham groups at the 1 week (Pillai’s Trace = 0.751, F(7,12) = 5.182, p = 0.006) and 8 week (Pillai’s Trace = 0.823, F(7,12) = 7.988, p = 0.001) time points, but not at baseline (Pillai’s Trace = 0.173, F(7,12) = 0.359, p = 0.910). The MANOVA results were followed up with univariate repeated measures ANOVA time-treatment interaction contrasts for each dependent variable.
2.1. Weight
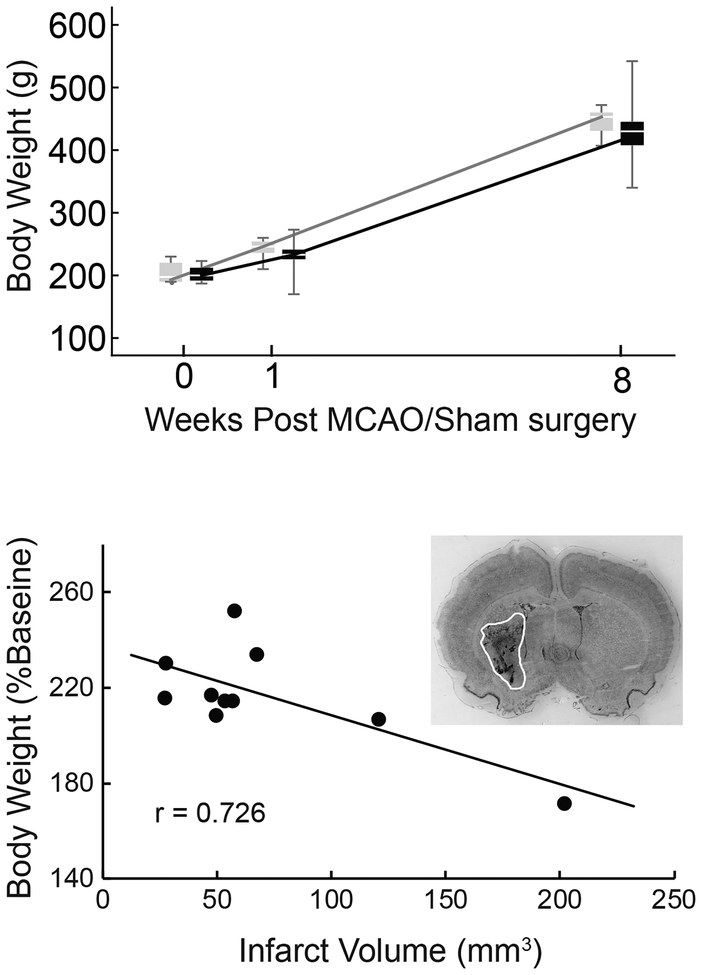
Body weight increased significantly in both groups over time (Figure 1, Top; 1-week and 8-weeks vs baseline: F(1,18) = 35.886, p < 0.001; F(1,18) = 679.072, p < 0.001). No significant difference between groups was found. In the MCAO group, weight at the 8-week time point was moderately negatively correlated with infarct volume (Figure 1, Bottom). Rats aged from 8 weeks at the time of surgery to 16 weeks at the completion of the study. This corresponds to a steep portion of the Sprague-Dawley growth curve (Brower et al., 2015), and weights increased over time accordingly. Growth may be a confounding factor in comparing many measures to baseline, therefore we focused primarily on MCAO versus Sham comparisons.
Fig. 1.
Body weight and infarct volume. Both stroke (black boxes) and sham (gray boxes) groups gained weight over time (p < 0.001), however no significant weight differences were found between MCAO and Sham (Top). Bodyweight at the 8-week time point, normalized to baseline, was negatively correlated with infarct volume (Pearson’s r = - 0.726, p = 0.017); the animals with the largest infarcts had gained the least weight. Inset: Representative image of a Cresyl violet stained brain section used to calculate infarct volume (infarct area outlined).
2.2. Maximum voluntary tongue force
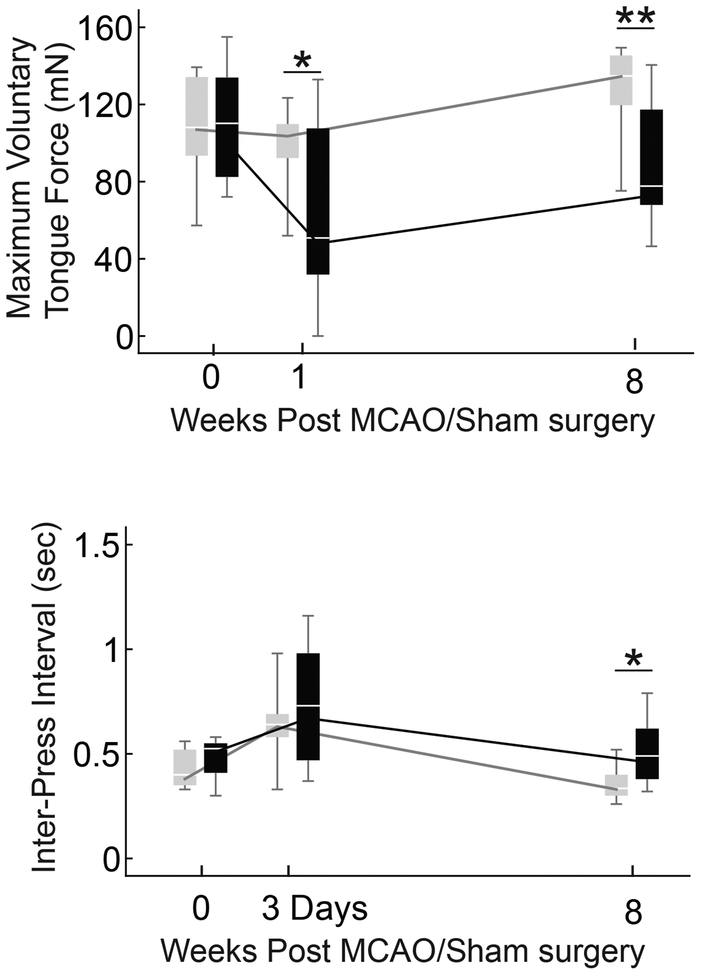
Our primary hypothesis was that MCAO would cause reduced tongue force. Maximum voluntary tongue force in the MCAO group was significantly less than the Sham group at 1 and 8 weeks (Figure 2; F(1,18) = 5.286, p = 0.034 and F(1,18) = 11.717, p = 0.003 respectively). The inter-press interval was collected at 3-days and 8-weeks after surgery. Only the 8-week time point was significantly different between the MCAO and Sham groups, with the MCAO group’s inter-press interval longer than the Sham group (Figure 2; F(1,18) = 6.515, p = 0.02).
Fig. 2.
Tongue force and inter-press interval. Asterisks indicate significant differences between the MCAO (black) and sham (gray) groups. The MCAO groups tongue force was significantly lower at 1 and 8 weeks (p = 0.034, 0.003), and was significantly reduced from baseline at both time points (p < 0.001, p = 0.011). The sham group was not significantly different than baseline at 1-week but was significantly larger than baseline at 8 weeks (p = 0.355, p = 0.007). At 3 days post-surgery the inter-press interval of both groups was slower than baseline (MCAO p = 0.003, Sham p = 0.015). At 8 weeks, neither group was significantly different from baseline (MCAO p = 0.33, Sham p = 0.15), but the MCAO group was significantly slower than sham at the 8-week time point (p = 0.02).
2.3. Videofluoroscopic swallowing assessments
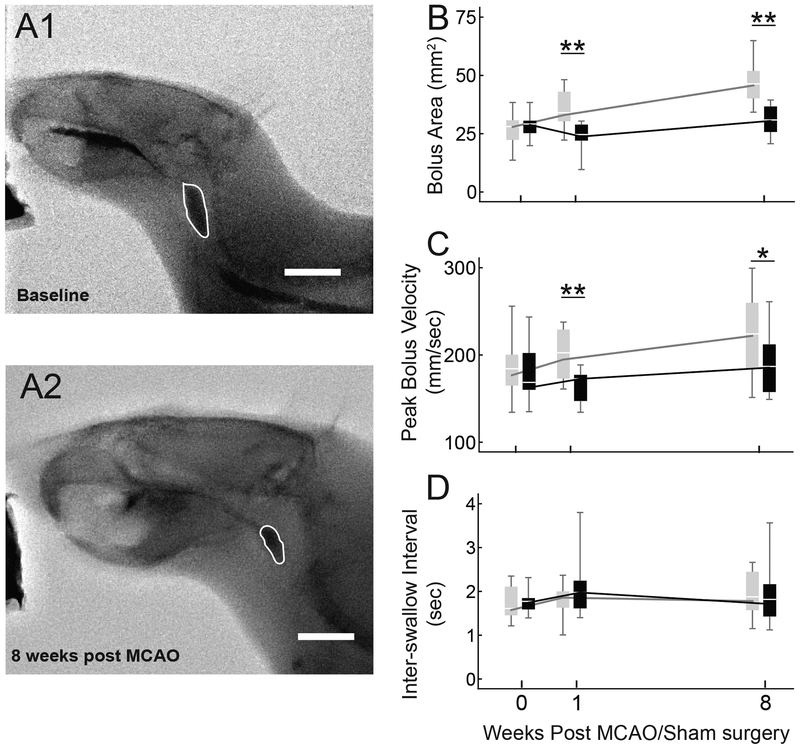
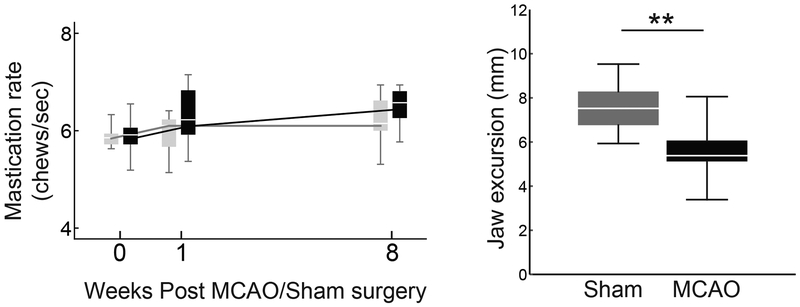
Average bolus area was significantly smaller in the MCAO vs. Sham group at both 1 and 8 weeks (Figure 3A and 3B; F(1,18) = 13.646, p = 0.002; F(1,18) = 18.680, p < 0.001). Peak bolus speed was reduced in the MCAO group (Figure 3C; F(1,18) = 9.839, p = 0.006 and F(1,18) = 4.515, p = 0.048 at 1 and 8 weeks). No significant differences between groups were found for inter-swallow interval (Figure 3D) or mastication rate (Figure 4), however the jaw excursion during chewing was significantly smaller in the MCAO group (Figure 4; MCAO = 5.62±1.3mm, Sham = 7.59±1.06mm; t(18) = −3.71, p = 0.0017).
Fig. 3.
Videofluoroscopic swallowing study detected reduced bolus area and peak speed after MCAO. Example videofluoroscopic images of swallowing in the same rat before and 8 weeks after MCAO (A1 and A2), with the bolus outlined in white. B: The MCAO group (black) swallowed smaller boluses at both 1 and 8 weeks after MCAO (p = 0.002, p < 0.001). At both time points peak bolus speed was lower in the MCAO group (C; p = 0.006, p = 0.048). No significant differences were found between groups for inter-swallow interval (D).
Fig. 4.
Mastication rate and jaw excursion. Mastication rate did not significantly differ between the MCAO (black) and Sham (gray) groups at any time point (Left). The jaw excursion distance was significantly less in the MCAO group (Right panel, data from week 8 shown).
3. Discussion
The middle cerebral artery occlusion rat model of stroke exhibited both acute and chronic reductions in voluntary tongue force, bolus area, and bolus speed. These results model clinically relevant symptoms of post stroke dysphagia and support the use of the MCAO rat model to improve our understanding of the physiology contributing to these functional changes and the impact of therapeutic interventions on physiological targets and function.
3.1. Tongue force and function
The tongue has a critical role in swallowing, and stroke frequently results in lingual weakness and discoordination (Daniels et al., 1999; Hirota et al., 2010; Hori et al., 2005; Konaka et al., 2010; Lee et al., 2016). In human patients, dysphagia has been associated with lingual weakness following stroke (Konaka et al., 2010) and correlated with deficits in several aspects of oral processing, including bolus formation, oral transit time, and premature bolus loss (Lee et al., 2016). Our results in the rat are consistent with these human findings. Specifically, we found that following induction of the stroke model, maximum voluntary tongue force was significantly less in the MCAO group versus the Sham group at both the 1 and 8-week time points (Figure 2).
We also evaluated the rate at which the rat pressed a disk with its tongue to dispense water at a minimal force setting, measured as the inter-press interval. This interval was significantly longer in the MCAO group at the 8-week time point, but not at 3 days after surgery (Figure 2). Both groups showed an increase from baseline inter-press interval at the 3-day time point, suggesting this time point did not allow for sufficient recovery time after surgery and any effects from ischemia cannot be separated from general post-surgery effects. The slower pressing rate in the MCAO group at 8-weeks indicates an additional aspect of lingual motor dysfunction that can be assessed in this model.
3.2. VFSS
We found the average bolus area swallowed was significantly smaller after MCAO (Figure 3). It is not clear from the literature whether humans also self-select smaller boluses after stroke because clinical swallow studies typically use pre-measured boluses. With pre-measured boluses, the prevalence of unsafe and fractional swallows has been shown to increase with large boluses (Vilardell et al., 2017). Intake of smaller boluses throughout a meal would require a greater number of swallows if meal size is held constant. In this case, fatigue could be a limiting factor in meeting nutritional needs. In fact, a study of aged individuals with dysphagic symptoms found reduced tongue pressures to be associated with taking a longer time to consume meals, and long meal times were also associated with consuming less total food, a risk for malnutrition (Namasivayam et al., 2016). Small boluses may also lengthen the pharyngeal delay time, defined as the delay from when the bolus passes the ramus of the mandible to the onset of laryngeal elevation, which may increase the risk of airway penetration (Park et al., 2016). Therefore, bolus size in the rat model may be an easily assessed measure of impaired swallowing with implications for swallowing efficiency and safety.
In humans with post stroke dysphagia, unsafe swallows were reported to have significantly slower bolus velocities than safe swallows (Vilardell et al., 2017). A corollary to this finding was observed in the rat MCAO model in that mean peak bolus speed was significantly lower than in the Sham group. Low bolus velocities would likely result in longer pharyngeal transit times, which have been shown to be associated with aspiration (Li Bingjie et al., 2010) and other abnormal VFSS findings after stroke (Kang et al., 2011). Thus, the MCAO rat model VFSS captures functional swallowing changes that are analogous to clinical syndromes.
Mastication rate was not significantly different between the Sham and MCAO groups, however mastication was accomplished with reduced jaw excursion after ischemia (Figure 4) and may be another indicator of reduced feeding efficiency or a compensatory mechanism. Human studies have reported reduced chewing efficiency after stroke, assessed by a two color gum mixing test (Schimmel et al., 2017).
3.3. Limitations
A limitation of this study was that we were unable to sufficiently visualize the hyoid and other laryngeal structures of interest that are typically available in a human VFSS analysis. Future studies may be able to take advantage of higher resolution small animal videofluoroscopic imaging equipment or radiopaque markers to track additional structures of interest (Lever et al., 2015b, 2015a). The current study found significant differences in swallowing function using a mixture of peanut butter and barium, but an additional improvement to the VFSS protocol may be to consider the impact of different consistencies on swallowing after MCAO, as a viscosity-dependent impact on swallowing safety and efficacy after stroke has been reported (Vilardell et al., 2017). Increased transit times and stage transitions have been reported in swallowing after stroke, yet the inter-swallow interval in the MCAO model was not significantly different than sham (Figure 3D). It is likely that this measure was not sensitive to changes in swallowing duration due to large variability in behavior between swallows, as has been previously reported (Russell et al., 2013). A higher frame rate may allow for the detection of timing differences within individual swallows.
Another limitation of this study was the young age of the rats. We used 8-week old rats which correspond to a weight range frequently used for MCAO studies. On average, rats doubled in weight over the course of this study due to growth, which made it difficult to make meaningful comparisons to baseline measures. The small size of the rats at the early time points also resulted in more limited resolution on the VFSS. Most importantly, stroke most commonly occurs in aged individuals (Mozaffarian et al., 2016), and future studies will seek to include age as a factor in pursuing the mechanisms contributing to the development of post stroke dysphagia and its treatment. The MCAO group was compared with a sham-surgery group to control for surgical effects on lingual function and swallowing, however alternative surgical approaches may be less invasive (Hill and Nemoto, 2014) and should be considered for future studies.
Unilateral stroke of either cerebral hemisphere can produce dysphagia (Daniels et al., 1996; Li et al., 2009; Robbins and Levine, 1988; Wilmskoetter et al., 2018). Many studies have attempted to determine differential effects of stroke location on swallowing function and have reported mixed results (Daniels et al., 1999; Dehaghani et al., 2016; Marian et al., 2017; Robbins et al., 1993; Steinhagen et al., 2009; Suntrup et al., 2015; Theurer et al., 2008). We elected to study left MCAO as some studies have suggested that infarcts in the left hemisphere may impact the oral phase of swallowing (Cola et al., 2010; Irie and Lu, 1995; Li et al., 2009; Robbins et al., 1993; Robbins and Levine, 1988), and we were interested in modeling post stroke lingual weakness. However, oral impairment has been reported with lesions of either hemisphere (Daniels et al., 1999; Wilmskoetter et al., 2018) and recent studies have associated right hemispheric ischemia with more severe dysphagia and pharyngeal impairment (Suntrup- Krueger et al., 2017; Wilmskoetter et al., 2019b). Occlusion of the right MCA in this model should be evaluated in future studies.
3.5. Conclusions
We found clinically relevant changes to swallowing and tongue force that support the use of the MCAO rat model as a translational model of post stroke dysphagia. Ongoing studies aim to determine the impact of MCAO on structural, biochemical, and contractile lingual muscle properties and the relationships between altered muscle physiology and functional swallowing changes. Therapeutic interventions for dysphagia targeting the lingual muscles, such as tongue exercise and neuromuscular stimulation, have previously been investigated in rat models (Connor et al., 2009; Kletzien et al., 2013; Johnson and Connor, 2011; Connor et al., 2013), and could be applied to this stroke model to improve our understanding of their impact on the neuromuscular swallowing system after stroke.
4. Methods
4.1. Animals
Experiments were approved by the University of Wisconsin School of Medicine and Public Health Animal Care and Use Committee. All applicable international, national, and institutional guidelines for the care and use of animals were followed. Twenty-five 6-week old male Sprague-Dawley rats were obtained from Charles River Laboratories two weeks prior to MCAO/sham surgery to allow for acclimation, tongue press training, and baseline testing. Rats were randomized into MCAO and Sham groups. Fifteen rats underwent MCAO; two died after surgery and 3 were removed from analysis when no infarct was detected (N = 10 MCAO, 10 Sham).
4.2. Maximum voluntary tongue force
Following previously described procedures (Connor et al., 2009; Cullins et al., 2018; Kletzien et al., 2013; Schaser et al., 2016), rats were progressively water restricted over one week, after which water restriction was maintained at 3 hours of water access per day (Toth and Gardiner, 2000). Thirsty rats were acclimated to a custom designed operandum and learned to press a disk with their tongue for a water reward. During the second day of acclimation, water was dispensed at a minimal threshold of 2 mN and tongue presses were recorded to calculate the inter-press interval. After two days of acclimation, maximum voluntary tongue force was determined by challenging the rats with increasing force thresholds for receipt of a water reward. The reward threshold was incremented by 2 mN after each successful tongue press. This process was repeated over the course of 3 days to account for learning and motivational changes, and the average of the 10 highest forces was used to determine an individual rat’s maximum voluntary tongue force. Force tests were repeated at 1- and 8-week time points.
4.3. Videofluoroscopy
As previously described (Russell et al., 2013), a mixture of peanut butter and barium sulfate (Varibar Thin Honey) was placed on a platform in the rat’s home cage. During ad libitum feeding of the peanut butter mixture, video was obtained at 30 frames per second on a C-ARM fluoroscope model OEC 9800 (GE Medical Systems-OEC, Salt Lake City, UT). Only swallows with a clear sagittal view were analyzed. ImageJ (Schindelin et al., 2012) was used to assess swallowing measures including bolus area, bolus speed, mastication rate, and jaw excursion. Mean bolus area for each rat was calculated from 9–15 swallows. The bolus area was determined by tracing the bolus 1 frame before the bolus entered the upper esophageal sphincter (UES). The UES is typically near vertebrae C4 and the bolus slows down and darkens as it enters the UES.
Peak bolus speed and mastication rate were averaged from 3 swallows from each rat, based on a previous study (Russell et al., 2013). The head of the bolus was tracked from initiation of the swallow, determined by tongue base retraction propelling the bolus past the vallecula, until the bolus reached the UES, typically 5–6 frames. The distance traveled by the bolus between frames was determined by calculating the change in the location of the head of the bolus relative to a stable anatomical marker on the skull, the external occipital protuberance, to account for any overall movement of the rat. Peak speed typically occurred in the first two frames after initiation of the swallow. Jaw excursion was measured as the difference between the distance from the hard pallet to the mandible at jaws closed and jaws opened.
4.4. Middle cerebral artery occlusion and sham surgeries
Rats were anesthetized with isoflurane and a midline neck incision exposed the common carotid artery, external carotid artery (ECA), and internal carotid artery (ICA). After controlling blood flow with sutures and clips, a monofilament suture (Doccol Corporation) was inserted in the transected ECA and advanced through the ICA approximately 17–20mm, occluding the middle cerebral artery (MCA). Occlusion was maintained for 90 minutes, then the suture was withdrawn to restore blood flow. Sham surgery included all steps except MCA occlusion. Body temperature was maintained during and after surgery with heating pads. Buprenorphine-SR and topical lidocaine were administered for analgesia.
4.5. Infarct volume
After the rats were euthanized, brains were collected and snap frozen. Brains were sectioned coronally at 50 μm on a cryostat (Leica) and stained with Cresyl violet. Slides were scanned (Epson V500) and ImageJ was be used to measure the infarct volume.
4.6. Statistical Analysis
To account for multiple dependent variables, a repeated measures MANOVA was used to assess the following dependent variables: weight, tongue force, inter-press interval, bolus area, peak bolus speed, mastication rate, and inter-swallow interval. The Pillai’s trace test statistics are reported. MANOVA was followed by individual repeated measures ANOVAs. Rats aged from 8 weeks at the time of surgery to 16 weeks at the completion of the study. This corresponds to a steep portion of the Sprague-Dawley growth curve (Brower et al., 2015), and weights increased over time accordingly (Figure 1). Growth may be a confounding factor in comparing many measures to baseline, therefore we focused primarily on MCAO versus Sham comparisons. An independent T-test was used to compare jaw excursion between groups at the 8-week time point as a follow up on the mastication rate results.
Highlights.
MCAO in the rat results in acute and chronic lingual weakness
Changes in swallowing after MCAO include reduced bolus size and speed
MCAO can be used to model post stroke dysphagia in the rat
Funding
This study was funded by the following NIH grants: F32HD094527, R01DC008149, R01DC01435, and R37CA225608.
Abbreviations:
- MCAO
middle cerebral artery occlusion
- VFSS
videofluoroscopic swallowing study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Ahmed J, Dwyer DM, Farr TD, Harrison DJ, Dunnett SB, Trueman RC, 2017. Lickometry: A novel and sensitive method for assessing functional deficits in rats after stroke. J. Cereb. Blood Flow Metab. 37, 755–761. 10.1177/0271678X16684141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold M, Liesirova K, Broeg-Morvay A, Meisterernst J, Schlager M, Mono M-L, El-Koussy M, Kägi G, Jung S, Sarikaya H, 2016. Dysphagia in Acute Stroke: Incidence, Burden and Impact on Clinical Outcome. PLOS ONE 11, e0148424 10.1371/journal.pone.0148424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attrill S, White S, Murray J, Hammond S, Doeltgen S, 2018. Impact of oropharyngeal dysphagia on healthcare cost and length of stay in hospital: a systematic review. BMC Health Serv. Res 10.1186/s12913-018-3376-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barer D, 1989. The natural history and functinoal consequences of dysphagia after hemispheric stroke. Jounral Neurol. Neurosurg. Psychiatry 236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath PM, Lee HS, Everton LF, 2018. Swallowing therapy for dysphagia in acute and subacute stroke. Cochrane Database Syst. Rev 10.1002/14651858.CD000323.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H, 1986. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. stroke 17, 472–476. [DOI] [PubMed] [Google Scholar]
- Brower M, Grace M, Kotz CM, Koya V, 2015. Comparative analysis of growth characteristics of Sprague Dawley rats obtained from different sources. Lab. Anim. Res 31, 166–173. 10.5625/lar.2015.31.4.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib C, Ortega O, Kumru H, Palomeras E, Vilardell N, Alvarez-Berdugo D, Muriana D, Rofes L, Terre R, Mearin F, Clave P, 2016. Neurorehabilitation strategies for poststroke oropharyngeal dysphagia: from compensation to the recovery of swallowing function: Poststroke dysphagia rehabilitation. Ann. N. Y. Acad. Sci 1380, 121–138. 10.1111/nyas.13135 [DOI] [PubMed] [Google Scholar]
- Clark Heather M, 2003. Neuromuscular Treatments for Speech and Swallowing. Am. J. Speech Lang. Pathol 12, 400–415. 10.1044/1058-0360(2003/086) [DOI] [PubMed] [Google Scholar]
- Cohen DL, Roffe C, Beavan J, Blackett B, Fairfield CA, Hamdy S, Havard D, McFarlane M, McLauglin C, Randall M, Robson K, Scutt P, Smith C, Smithard D, Sprigg N, Warusevitane A, Watkins C, Woodhouse L, Bath PM, 2016. Post-stroke dysphagia: A review and design considerations for future trials. Int. J. Stroke 11, 399–411. 10.1177/1747493016639057 [DOI] [PubMed] [Google Scholar]
- Cola MG, Daniels SK, Corey DM, Lemen LC, Romero M, Foundas AL, 2010. Relevance of Subcortical Stroke in Dysphagia. Stroke 41, 482–486. 10.1161/STROKEAHA.109.566133 [DOI] [PubMed] [Google Scholar]
- Connor NP, Russell JA, Jackson MA, Kletzien H, Wang H, Schaser AJ, Leverson GE, Zealear DL, 2013. Tongue muscle plasticity following hypoglossal nerve stimulation in aged rats. Muscle Nerve 47, 230–240. 10.1002/mus.23499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor NP, Russell JA, Wang H, Jackson MA, Mann L, Kluender K, 2009. Effect of tongue exercise on protrusive force and muscle fiber area in aging rats. J. Speech Lang. Hear. Res 52, 732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett D, Jeffers M, Nguemeni C, Gomez-Smith M, Livingston-Thomas J, 2015. Lost in translation, in: Progress in Brain Research Elsevier, pp. 413–434. 10.1016/bs.pbr.2014.12.002 [DOI] [PubMed] [Google Scholar]
- Cullins MJ, Krekeler BN, Connor NP, 2018. Differential impact of tongue exercise on intrinsic lingual muscles. The Laryngoscope 128, 2245–2251. 10.1002/lary.27044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels SK, Brailey K, Foundas AL, 1999. Lingual Discoordination and Dysphagia following Acute Stroke: Analyses of Lesion Localization. Dysphagia 14, 85–92. 10.1007/PL00009592 [DOI] [PubMed] [Google Scholar]
- Daniels SK, Foundas AL, Iglesia GC, Sullivan MA, 1996. Lesion site in unilateral stroke patients with dysphagia. J. Stroke Cerebrovasc. Dis 6, 30–34. 10.1016/S1052-3057(96)80023-1 [DOI] [PubMed] [Google Scholar]
- Dehaghani S, Yadegari F, Asgari A, Chitsaz A, Karami M, 2016. Brain regions involved in swallowing: Evidence from stroke patients in a cross-sectional study. J. Res. Med. Sci 21, 45 10.4103/1735-1995.183997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeganage C, Beavan J, Ellender S, Bath PM, 2012. Interventions for dysphagia and nutritional support in acute and subacute stroke, in: The Cochrane Collaboration (Ed.), Cochrane Database of Systematic Reviews John Wiley & Sons, Ltd, Chichester, UK. [DOI] [PubMed] [Google Scholar]
- German RZ, Crompton AW, Gould FDH, Thexton AJ, 2017. Animal Models for Dysphagia Studies: What Have We Learnt So Far. Dysphagia 32, 73–77. 10.1007/s00455-016-9778-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyaeva N, Thompson C, Shinohara N, Lazareva N, Onufriev M, Stepanichev M, Moiseeva Y, Fliss H, Hakim AM, 2003. Tongue protrusion: a simple test for neurological recovery in rats following focal cerebral ischemia. J. Neurosci. Methods 125, 183–193. 10.1016/S0165-0270(03)00056-6 [DOI] [PubMed] [Google Scholar]
- Hill JW, Nemoto EM, 2014. Transient middle cerebral artery occlusion with complete reperfusion in spontaneously hypertensive rats. MethodsX 1, 283–291. 10.1016/j.mex.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota N, Konaka K, Ono T, Tamine K, Kondo J, Hori K, Yoshimuta Y, Maeda Y, Sakoda S, Naritomi H, 2010. Reduced Tongue Pressure Against the Hard Palate on the Paralyzed Side During Swallowing Predicts Dysphagia in Patients With Acute Stroke. Stroke 41, 2982–2984. 10.1161/STROKEAHA.110.594960 [DOI] [PubMed] [Google Scholar]
- Hori K, Ono T, Iwata H, Nokubi T, Kumakura I, 2005. Tongue pressure against hard palate during swallowing in post-stroke patients: Tongue pressure in post-stroke patients. Gerodontology 22, 227–233. 10.1111/j.1741-2358.2005.00089.x [DOI] [PubMed] [Google Scholar]
- Irie H, Lu CC, 1995. Dynamic evaluation of swallowing in patients with cerebrovascular accident. Clin. Imaging 19, 240–243. 10.1016/0899-7071(94)00060-P [DOI] [PubMed] [Google Scholar]
- Johnson AM, Connor NP, 2011. Effects of electrical stimulation on neuromuscular junction morphology in the aging rat tongue. Muscle Nerve 43, 203–211. 10.1002/mus.21819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SH, Kim D-K, Seo K-M, Seo J-H, 2011. Usefulness of Videofluoroscopic Swallow Study with Mixed Consistency Food for Patients with Stroke or Other Brain Injuries. J. Korean Med. Sci 26, 425–430. 10.3346/jkms.2011.26.3.425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HD, Choi JB, Yoo SJ, Chang MY, Lee SW, Park JS, 2017. Tongue-to-palate resistance training improves tongue strength and oropharyngeal swallowing function in subacute stroke survivors with dysphagia. J. Oral Rehabil 44, 59–64. 10.1111/joor.12461 [DOI] [PubMed] [Google Scholar]
- Kletzien H, Cullins MJ, Connor NP, 2019. Age-related alterations in swallowing biomechanics. Exp. Gerontol 118, 45–50. 10.1016/j.exger.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kletzien H, Russell JA, Leverson GE, Connor NP, 2013. Differential effects of targeted tongue exercise and treadmill running on aging tongue muscle structure and contractile properties. J. Appl. Physiol 114, 472–481. 10.1152/japplphysiol.01370.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konaka K, Kondo J, Hirota N, Tamine K, Hori K, Ono T, Maeda Y, Sakoda S, Naritomi H, 2010. Relationship between Tongue Pressure and Dysphagia in Stroke Patients. Eur. Neurol 64, 101–107. 10.1159/000315140 [DOI] [PubMed] [Google Scholar]
- Lee JH, Kim H-S, Yun DH, Chon J, Han YJ, Yoo SD, Kim DH, Lee SA, Joo HI, Park J, Kim JC, Soh Y, 2016. The Relationship Between Tongue Pressure and Oral Dysphagia in Stroke Patients. Ann. Rehabil. Med 40, 620 10.5535/arm.2016.40.4.620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever TE, Braun SM, Brooks RT, Harris RA, Littrell LL, Neff RM, Hinkel CJ, Allen MJ, Ulsas MA, 2015a. Adapting Human Videofluoroscopic Swallow Study Methods to Detect and Characterize Dysphagia in Murine Disease Models. J. Vis. Exp. JoVE 10.3791/52319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever TE, Brooks RT, Thombs LA, Littrell LL, Harris RA, Allen MJ, Kadosh MD, Robbins KL, 2015b. Videofluoroscopic Validation of a Translational Murine Model of Presbyphagia. Dysphagia 30, 328–342. 10.1007/s00455-015-9604-7 [DOI] [PubMed] [Google Scholar]
- Bingjie Li, Tong Zhang, Xinting Sun, Jianmin Xu, Guijun Jiang, 2010. Quantitative videofluoroscopic analysis of penetration-aspiration in post-stroke patients. Neurol. India 58, 42–47. 10.4103/0028-3886.60395 [DOI] [PubMed] [Google Scholar]
- Li S, Luo C, Yu B, Yan B, Gong Q, He C, He L, Huang X, Yao D, Lui S, Tang H, Chen Q, Zeng Y, Zhou D, 2009. Functional magnetic resonance imaging study on dysphagia after unilateral hemispheric stroke: a preliminary study. J. Neurol. Neurosurg. Psychiatry 80, 1320–1329. 10.1136/jnnp.2009.176214 [DOI] [PubMed] [Google Scholar]
- Macrae I, 2011. Preclinical stroke research - advantages and disadvantages of the most common rodent models of focal ischaemia: Animal models of focal cerebral ischaemia. Br. J. Pharmacol 164, 1062–1078. 10.1111/j.1476-5381.2011.01398.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marian T, Schröder JB, Muhle P, Claus I, Riecker A, Warnecke T, Suntrup-Krueger S, Dziewas R, 2017. Pharyngolaryngeal Sensory Deficits in Patients with Middle Cerebral Artery Infarction: Lateralization and Relation to Overall Dysphagia Severity. Cerebrovasc. Dis. Extra 7, 130–139. 10.1159/000479483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin E, Go A, Arnett DK, Blaha MJ, Cushman M, Das SR, Sarah de Ferranti MD, Després J-P, Fullerton HJ, others, 2016. AHA statistical Update. Circulation 133, e38–e360. 10.1161/CIR.0000000000000350 [DOI] [PubMed] [Google Scholar]
- Namasivayam AM, Steele CM, Keller H, 2016. The effect of tongue strength on meal consumption in long term care. Clin. Nutr 35, 1078–1083. 10.1016/j.clnu.2015.08.001 [DOI] [PubMed] [Google Scholar]
- Oommen ER, Kim Y, McCullough G, 2011. Stage Transition and Laryngeal Closure in Poststroke Patients with Dysphagia. Dysphagia 26, 318–323. 10.1007/s00455-010-9314-0 [DOI] [PubMed] [Google Scholar]
- Palmer GC, Peeling J, Corbett D, Bigio MRD, Hudzik TJ, 2001. T2-Weighted MRI Correlates with Long-Term Histopathology, Neurology Scores, and Skilled Motor Behavior in a Rat Stroke Model. Ann. N. Y. Acad. Sci 939, 283–296. 10.1111/j.1749-6632.2001.tb03636.x [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Wizemann TM, Altevogt BM, others, 2013. Improving the Utility and Translation of Animal Models for Nervous System Disorders: Workshop Summary National Academies Press. [PubMed] [Google Scholar]
- Park J-S, Kim H-J, Oh D-H, 2015. Effect of tongue strength training using the Iowa Oral Performance Instrument in stroke patients with dysphagia. J. Phys. Ther. Sci 27, 3631–3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J-W, Sim G-J, Yang D-C, Lee K-H, Chang J-H, Nam K-Y, Lee H-J, Kwon B-S, 2016. Increased Bolus Volume Effect on Delayed Pharyngeal Swallowing Response in Post-stroke Oropharyngeal Dysphagia: A Pilot Study. Ann. Rehabil. Med 40, 1018–1023. 10.5535/arm.2016.40.6.1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkinen S, Ortega FJ, Kuptsova K, Huttunen J, Tarkka I, Jolkkonen J, 2013. Gait Impairment in a Rat Model of Focal Cerebral Ischemia. Stroke Res. Treat 2013 10.1155/2013/410972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remesso GC, Fukujima MM, Chiappetta AL de ML, Oda AL, Aguiar AS, Oliveira, A. de SB, do Prado GF, 2011. Swallowing disorders after ischemic stroke. Arq. Neuropsiquiatr 69, 785–789. [DOI] [PubMed] [Google Scholar]
- Robbins J, Kays SA, Gangnon RE, Hind JA, Hewitt AL, Gentry LR, Taylor AJ, 2007. The Effects of Lingual Exercise in Stroke Patients With Dysphagia. Arch. Phys. Med. Rehabil 88, 150–158. 10.1016/j.apmr.2006.11.002 [DOI] [PubMed] [Google Scholar]
- Robbins J, Levine RL, 1988. Swallowing after unilateral stroke of the cerebral cortex: Preliminary experience. Dysphagia 3, 11–17. 10.1007/BF02406275 [DOI] [PubMed] [Google Scholar]
- Robbins J, Levine RL, Maser A, Rosenbek JC, Kempster GB, 1993. Swallowing after unilateral stroke of the cerebral cortex. Arch. Phys. Med. Rehabil 74, 1295–1300. [DOI] [PubMed] [Google Scholar]
- Rofes L, Muriana D, Palomeras E, Vilardell N, Palomera E, Alvarez-Berdugo D, Casado V, Clavé P, 2018. Prevalence, risk factors and complications of oropharyngeal dysphagia in stroke patients: A cohort study. Neurogastroenterol. Motil 30, e13338 10.1111/nmo.13338 [DOI] [PubMed] [Google Scholar]
- Rogus-Pulia N, Robbins J, 2013. Approaches to the Rehabilitation of Dysphagia in Acute Poststroke Patients. Semin. Speech Lang 34, 154–169. 10.1055/s-0033-1358368 [DOI] [PubMed] [Google Scholar]
- Russell JA, Ciucci MR, Hammer MJ, Connor NP, 2013. Videofluorographic Assessment of Deglutitive Behaviors in a Rat Model of Aging and Parkinson Disease. Dysphagia 28, 95–104. 10.1007/s00455-012-9417-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaser AJ, Ciucci MR, Connor NP, 2016. Cross-activation and detraining effects of tongue exercise in aged rats. Behav. Brain Res 297, 285–296. 10.1016/j.bbr.2015.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmel M, Voegeli G, Duvernay E, Leemann B, Müller F, 2017. Oral tactile sensitivity and masticatory performance are impaired in stroke patients. J. Oral Rehabil 44, 163–171. 10.1111/joor.12482 [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A, 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele CM, Bayley MT, Peladeau-Pigeon M, Nagy A, Namasivayam AM, Stokely SL, Wolkin T, 2016. A Randomized Trial Comparing Two Tongue-Pressure Resistance Training Protocols for Post-Stroke Dysphagia. Dysphagia 31, 452–461. 10.1007/s00455-016-9699-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhagen V, Grossmann A, Benecke R, Walter U, 2009. Swallowing Disturbance Pattern Relates to Brain Lesion Location in Acute Stroke Patients. Stroke 40, 1903–1906. 10.1161/STROKEAHA.108.535468 [DOI] [PubMed] [Google Scholar]
- Sugiyama N, Nishiyama E, Nishikawa Y, Sasamura T, Nakade S, Okawa K, Nagasawa T, Yuki A, 2014. A Novel Animal Model of Dysphagia Following Stroke. Dysphagia 29, 61–67. 10.1007/s00455-013-9481-x [DOI] [PubMed] [Google Scholar]
- Suntrup S, Kemmling A, Warnecke T, Hamacher C, Oelenberg S, Niederstadt T, Heindel W, Wiendl H, Dziewas R, 2015. The impact of lesion location on dysphagia incidence, pattern and complications in acute stroke. Part 1: dysphagia incidence, severity and aspiration. Eur. J. Neurol 22, 832–838. 10.1111/ene.12670 [DOI] [PubMed] [Google Scholar]
- Suntrup‐Krueger S, Kemmling A, Warnecke T, Hamacher C, Oelenberg S, Niederstadt T, Heindel W, Wiendl H, Dziewas R, 2017. The impact of lesion location on dysphagia incidence, pattern and complications in acute stroke. Part 2: Oropharyngeal residue, swallow and cough response, and pneumonia. Eur. J. Neurol 24, 867–874. 10.1111/ene.13307 [DOI] [PubMed] [Google Scholar]
- Suntrup-Krueger S, Minnerup J, Muhle P, Claus I, Schröder JB, Marian T, Warnecke T, Kalic M, Berger K, Dziewas R, 2018. The Effect of Improved Dysphagia Care on Outcome in Patients with Acute Stroke: Trends from 8-Year Data of a Large Stroke Register. Cerebrovasc. Dis 45, 101–108. 10.1159/000487811 [DOI] [PubMed] [Google Scholar]
- Tamura A, Graham DI, McCulloch J, Teasdale GM, 1981. Focal cerebral ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion. J. Cereb. Blood Flow Metab 1, 53–60. [DOI] [PubMed] [Google Scholar]
- Terré R, Mearin F, 2006. Oropharyngeal dysphagia after the acute phase of stroke: predictors of aspiration. Neurogastroenterol. Motil 18, 200–205. 10.1111/j.1365-2982.2005.00729.x [DOI] [PubMed] [Google Scholar]
- Theurer JA, Johnston JL, Taves DH, Bach D, Hachinski V, Martin RE, 2008. Swallowing after Right Hemisphere Stroke: Oral versus Pharyngeal Deficits La déglutition après un accident vasculaire cérébral à l’hémisphère droit: déficiences et pharyngées 32, 9. [Google Scholar]
- Toth LA, Gardiner TW, 2000. Food and Water Restriction Protocols: Physiological and Behavioral Considerations. Contemp Top Anim Sci 39, 9–17. [PubMed] [Google Scholar]
- Umapathi T, Venketasubramanian N, Leck KJ, Tan CB, Lee WL, Tjia H, 2000. Tongue deviation in acute ischaemic stroke: a study of supranuclear twelfth cranial nerve palsy in 300 stroke patients. Cerebrovasc. Dis 10, 462–465. [DOI] [PubMed] [Google Scholar]
- Vilardell N, Rofes L, Arreola V, Martin A, Muriana D, Palomeras E, Ortega O, Clave P, 2017. Videofluoroscopic assessment of the pathophysiology of chronic poststroke oropharyngeal dysphagia. Neurogastroenterol. Motil 29, e13111 10.1111/nmo.13111 [DOI] [PubMed] [Google Scholar]
- Wilmskoetter J, Bonilha L, Martin-Harris B, Elm JJ, Horn J, Bonilha HS, 2019a. Mapping acute lesion locations to physiological swallow impairments after stroke. NeuroImage Clin 22, 101685 10.1016/j.nicl.2019.101685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmskoetter J, Bonilha L, Martin-Harris B, Elm JJ, Horn J, Bonilha HS, 2019b. Mapping acute lesion locations to physiological swallow impairments after stroke. NeuroImage Clin 22, 101685 10.1016/j.nicl.2019.101685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmskoetter J, Martin-Harris B, Pearson WG, Bonilha L, Elm JJ, Horn J, Bonilha HS, 2018. Differences in swallow physiology in patients with left and right hemispheric strokes. Physiol. Behav 194, 144–152. 10.1016/j.physbeh.2018.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F, Lin X, Guan Y, Mu Z, Chen K, Wang Y, Yang G-Y, 2014. Collateral circulation prevents masticatory muscle impairment in rat middle cerebral artery occlusion model. J. Synchrotron Radiat 21, 1314–1318. 10.1107/S1600577514016130 [DOI] [PubMed] [Google Scholar]